Abstract
Echinocandin drugs target the fungal enzyme β-(1,3)-glucan synthase (GS), which is required for the synthesis of cell wall component β-(1,3)-d-glucan. They are first-line therapy for Candida infections but are increasingly used as second-line therapy for Aspergillus infections. Resistance to echinocandins has been mainly studied in Candida and occurs due to mutations in FKS genes encoding GS. In our recent report, we identified a novel mechanism of echinocandin resistance in Aspergillus fumigatus. We showed that caspofungin exposure modifies GS, rendering it insensitive to echinocandins. This mechanism of resistance involved alteration of the GS lipid microenvironment and was mediated via an off-target effect on mitochondria leading to increased reactive oxygen species (ROS). We hypothesized that caspofungin-induced ROS alters the lipid composition around GS, changing its conformation and making it insensitive to echinocandins. In this commentary, we review both fks1-dependent and fks1-independent mechanisms of echinocandin resistance in A fumigatus. We believe this new resistance mechanism is also conserved among Candida spp. with implications for drug tolerance and/or resistance. Furthermore, we propose that ROS acts as a signaling molecule regulating lipid biogenesis, which impacts the structure-function of membrane proteins with implications for other types of drug-target interactions.
Keywords: echinocandin resistance, tolerance, lipid microenvironment
The mold pathogen Aspergillus fumigatus is a primary cause of invasive aspergillosis resulting in life-threatening infections among immunocompromised patients. Azole antifungal drugs, which target the ergosterol biosynthesis pathway, are first-line therapy for the treatment of Aspergillus infections and are fungicidal. In contrast, fungistatic echinocandins are second-line therapy for Aspergillus infections and are used in treating patients who are refractory to primary azole therapy. Currently, there are 3 Food and Drug Administration (FDA)-approved echinocandin drugs: caspofungin, micafungin, and anidulafungin. The rising global incidence of azole resistance in A fumigatus has prompted an expanded use of echinocandins as therapy to treat Aspergillus infections. 1 As drug resistance emerges, there is a need to better understand underlying mechanism(s).
Known Mechanism of Clinical Echinocandin Resistance
Resistance to echinocandins is mostly observed in Candida spp., where it is known that mutations in hot-spot regions of FKS genes confer resistance. 2 These mutations cause amino acid substitutions in the target enzyme glucan synthase, which alter drug-binding affinity, leading to drug resistance and clinical failures. Mutations in FKS hot-spot regions confer cross-resistance to all echinocandin class drugs. Due to limited usage of echinocandins in treating Aspergillus infections, little is known about the mechanisms of echinocandin resistance in A fumigatus. We recently reported the first clinical case of an echinocandin-resistant A fumigatus isolated from a patient with chronic pulmonary aspergillosis 3 and demonstrated that the isolate had a mutation in the conserved hot-spot region of the fks1 gene, comparable to what is seen with Candida resistance.
A Novel Mechanism of Clinical Echinocandin Resistance
Among a pool of clinical isolates of A fumigatus isolated from patients with chronic pulmonary aspergillosis who failed echinocandin therapy, there were many isolates that did not contain mutations in the fks1 gene, suggesting the presence of a novel resistance mechanism. We recently described this novel resistance mechanism 4 and believe that it could become more common as echinocandin therapy for aspergillosis increases. In our report, we detected a modified form of glucan synthase, which was formed in cells during exposure to caspofungin and also showed a dose-dependent insensitivity to echinocandins in vitro. Importantly, caspofungin exposure during growth induced an altered lipid composition in the plasma membrane resulting in a change in the microenvironment of glucan synthase that rendered it drug insensitive. Finally, we identified an off-target effect of caspofungin in cells involving mitochondrial reactive oxygen species (ROS) production, which we proposed acts as a cellular signal for altered lipid biosynthesis. Collectively, these results have led us to propose a model whereby caspofungin induces increases in mitochondrial ROS leading to changes in lipid composition in the membrane microenvironment of glucan synthase. The new lipid milieu alters the enzyme drug-binding affinity, either directly or indirectly, which ultimately leads to drug resistance.
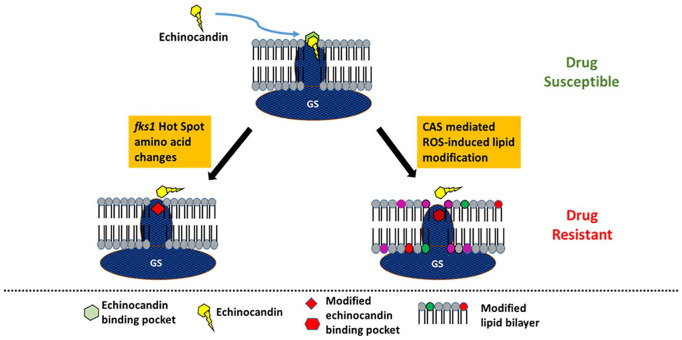
In summary, both fks1 mutation-dependent and fks1 mutation-independent mechanisms of echinocandin resistance can lead to clinical failure of Aspergillus infections. The 2 kinds of clinical resistance to echinocandins are summarized in Figure 1.
Figure 1.
Diagrammatic representation of fks mutant-dependent and mutant-independent mechanisms of echinocandin resistance in A fumigatus.
Source: Adapted from Satish et al. 4
Off-Target Effect of Caspofungin-Mitochondrial ROS Production
Although echinocandins are suggested to act from the outside of the cell, one study in Candida albicans showed that caspofungin is taken up by cells through a high-affinity facilitated-diffusion transporter. 5 Since caspofungin enters the cytoplasm, it allows for possible off-target interactions of the drug within the cell. A study to understand physiological mechanisms of caspofungin-induced cell death in C albicans showed that the drug also kills cells by causing both cellular apoptosis and necrosis. 6 Furthermore, the cells showed ROS production on exposure to caspofungin. Another study in C albicans showed that exposure to caspofungin triggers activation of glutathione reductase (GLR1), superoxide dismutase (SOD2), and mitochondrial processing protease (MAS1) genes, all of which are known to be associated with detoxification of cells during periods of oxidative stress. 7 Furthermore, in vitro studies have shown that addition of ROS scavengers, such as N-acetyl-l-cysteine and Vitamin C, to C albicans cells treated with echinocandins made the cells less susceptible to caspofungin. 8
In our study, we measured ROS production in A fumigatus strains using a fluorescent dye-based assay and observed that only caspofungin, but not other echinocandins, induced prominent ROS production in cells. 4 Furthermore, addition of mitochondrial inhibitor antimycin A significantly reduced ROS levels, indicating that caspofungin-induced ROS is mitochondrial in origin. Importantly, neutralizing ROS with scavengers blocked induction of downstream drug resistance. Most recently, preliminary data obtained in C albicans, Candida glabrata, and Candida auris also showed similar caspofungin-specific mitochondrial induction of ROS effects with lipid alterations that alter target properties (data not shown). The implications of this work, which are ongoing, are significant for potential cell tolerance and drug resistance in Candida. 9
ROS as a Signaling Molecule Altering Lipid Composition in Fungal Plasma Membrane
ROS, a group of chemical species comprising free radicals such as superoxide, hydroxyl radical, and singlet oxygen, and nonradical species such as hydrogen peroxide, show chemical reactivity that is not observed with molecular oxygen. Historically, they are known to be detrimental for cell survival, as they have the capacity to damage cellular components such as nucleic acids, proteins, and lipids. Classically, ROS are viewed as molecules employed by host immune cells for fighting microbial pathogens. However, more recent studies indicate that ROS can also be beneficial and are involved in growth and differentiation of cells. 10 In addition, they also act as secondary messengers and modify various signaling molecules that could help maintain normal physiologic functions and even promote cell survival.11-14 ROS can react with cysteine residues in proteins and oxidize them, causing structural changes within the proteins and altering their functions. This in turn can affect downstream transcription, phosphorylation, and other important signaling events.15-17 Cancer cells maintain a high intracellular level of ROS, which favors cell proliferation via several pathways including mitogen-activated protein kinases (MAPKs).16,18 In our study, we report a similar beneficial role of ROS in A fumigatus in the presence of drug. Our model suggests that enhanced ROS production induces a modified lipid composition with increased abundance of specific lipids (dihydrosphingosine and phytosphingosine) around glucan synthase, altering enzyme conformation and leading to echinocandin resistance. 4 How ROS brings about increased abundance of these lipids is yet to be understood. We hypothesize that ROS acts as a signaling molecule enhancing transcription of certain lipid biogenesis genes. We also speculate that clinically resistant isolates of A fumigatus 4 are genetically more sensitive to changes in ROS levels, perhaps due to mutation(s) in lipid biogenesis genes or ROS-sensitive transcription factors. This hypothesis is being investigated in greater detail because of its clinical significance.
Role of Plasma Membrane Lipids in Drug Resistance
Over the last several years, the role of lipids in development of microbial drug resistance has gained considerable attention. Numerous studies have reported that changes in plasma membrane lipid composition can affect the localization and functioning of key membrane proteins, including those involved in drug binding and trafficking. 19 The concept of modulating lipid composition to alter protein function has gained traction and is referred to as membrane lipid therapy. 19 Sphingolipids form an important component of fungal cell membrane, and in the past decade, several studies have focused on specific interactions between sphingolipids and membrane proteins. The 3’-hydroxyl group and the amide nitrogen in sphingolipids represent hydrogen-bond donors and acceptors, allowing these sphingolipids to form a network with other sphingolipids. 20 This constrained intramolecular hydrogen-bonded network leads to a reduction in the molecular area per sphingolipid, making them rigid and altering the membrane fluidity. In addition, sphingolipid-based regions have increased membrane thickness. 21 The abundance of polar sphingolipid head groups in these regions allows for changes in the interaction between transmembrane domains of membrane proteins and sphingolipids.
The importance of sphingolipids in normal cell function has implications for cancer therapy, drug resistance, and immune presentation. About 2 decades ago, researchers first showed that standard of care treatments, such as chemotherapeutics and radiation, modulate sphingolipid metabolism to increase endogenous ceramides, which kill cancer cells. 22 Strikingly, resistance to these treatments has also been linked to altered sphingolipid metabolism, favoring lipid species that ultimately lead to cell survival. As cancer cells routinely display increased growth properties and escape from cell death, it has been suggested that enzymes involved in sphingolipid synthesis or catabolism may be altered in cancer cells. 23 Sphingolipids also have implications in development of drug resistance in fungal species. In one study, laboratory and clinical strains of C glabrata showed an interesting phenotype of resistance to caspofungin but sensitivity to micafungin.24,25 These “CRS-MIS” strains showed elevated dihydrosphingosine and phytosphingosine levels, and the study hypothesized that these changes in sphingolipids differentially modulated the binding affinities of caspofungin and micafungin to their target. Our study also found increased sphingolipids in the microenvironment of glucan synthase enzyme, altering its conformation and binding affinity to echinocandins, eventually leading to resistance. 4 A recent study in C glabrata emphasized the importance of phosphatidylinositol 3,5-bisphosphate, a low abundance lipid molecule, in the maintenance of cell wall chitin, caspofungin tolerance, survival within host cells, and virulence. 26 Another study in C auris, an emerging pathogenic fungus, showed higher abundance of sphingoid bases in its plasma membrane, including higher levels of phytosphingosine compared to C albicans strains. 27 This study also suggests that increased sphingolipid content may be related to increased resistance to antifungals typical of C auris. The authors mention that these lipids are important for the assembly of membrane platforms containing drug efflux pumps. Collectively, there is an increasing recognition for the important role of lipid composition in microbial resistance. Nevertheless, the role of altered lipid composition is clearly complex and requires further investigation.
In conclusion, we have identified a novel mechanism of echinocandin resistance in A fumigatus and have proposed that it is relevant to other fungal species and may be conserved at a higher level. The clinical impact of this mechanism is likely to become more significant, as the number of patients with Aspergillus infections being treated with echinocandins expands. For Candida spp. where echinocandins are already used as first-line therapy, the role of this mechanism in tolerance and potential drug escape is just emerging.
Acknowledgments
We thank Erika Shor for her critical review of the manuscript.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the U.S. National Institutes of Health (AI109025) and Astellas Pharma (Reference Center for Molecular Evaluation of Drug Resistance to Echinocandin and Triazole Antifungal Drugs) to D.S.P. D.S.P. receives funding from the U.S. National Institutes of Health and contracts from Astellas, Scynexis, Cidara, and Amplyx. He serves on advisory boards for Astellas, Cidara, Amplyx, Scynexis, Matinas, and N8 Pharmaceuticals. In addition, D.S.P. has an issued U.S. patent concerning echinocandin resistance.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Both authors contributed equally to this manuscript.
ORCID iD: David S Perlin  https://orcid.org/0000-0002-1268-5524
https://orcid.org/0000-0002-1268-5524
References
- 1. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 2. Perlin DS. Echinocandin resistance in Candida. Clin Infect Dis. 2015;61:S612-S617. doi: 10.1093/cid/civ791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jimenez-Ortigosa C, Moore C, Denning DW, Perlin DS. Emergence of echinocandin resistance due to a point mutation in the fks1 gene of Aspergillus fumigatus in a patient with chronic pulmonary aspergillosis. Antimicrob Agents Chemother. 2017;61:e01277-17. doi: 10.1128/AAC.01277-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satish S, Jimenez-Ortigosa C, Zhao Y, et al. Stress-induced changes in the lipid microenvironment of β-(1,3)-d-glucan synthase cause clinically important echinocandin resistance in Aspergillus fumigatus. mBio. 2019;10(3):e00779-19. doi: 10.1128/mBio.00779-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paderu P, Park S, Perlin DS. Caspofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob Agents Chemother. 2004;48:3845-3849. doi: 10.1128/AAC.48.10.3845-3849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hao B, Cheng S, Clancy CJ, Nguyen MH. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother. 2013;57:326-332. doi: 10.1128/AAC.01366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly J, Rowan R, McCann M, Kavanagh K. Exposure to caspofungin activates Cap and Hog pathways in Candida albicans. Med Mycol. 2009;47:697-706. doi: 10.3109/13693780802552606. [DOI] [PubMed] [Google Scholar]
- 8. Wurtele H, Tsao S, Lépine G, et al. Modulation of histone H3 lysine 56 acetylation as an antifungal therapeutic strategy. Nat Med. 2010;16:774-780. doi: 10.1038/nm.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Healey KR, Perlin DS. Fungal resistance to echinocandins and the MDR Phenomenon in Candida glabrata. J Fungi (Basel). 2018;4(3):e105. doi: 10.3390/jof4030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158-167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bae Y S, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32:491-509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 14. Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472-481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 15. Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8-13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Truong TH, Carroll KS. Redox regulation of protein kinases. Crit Rev Biochem Mol Biol. 2013;48:332-356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 19. Escribá PV, Busquets X, Inokuchi J, et al. Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res. 2015;59:38-53. doi: 10.1016/j.plipres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20. Holthuis JC, Pomorski T, Raggers RJ, Sprong H, Van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev. 2001;81:1689-1723. doi: 10.1152/physrev.2001.81.4.1689. [DOI] [PubMed] [Google Scholar]
- 21. Niemela PS, Hyvonen MT, Vattulainen I. Atom-scale molecular interactions in lipid raft mixtures. Biochim Biophys Acta. 2009;1788:122-135. doi: 10.1016/j.bbamem.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 22. Shaw J, Costa-Pinheiro P, Patterson L, Drews K, Spiegel S, Kester M. Novel sphingolipid-based cancer therapeutics in the personalized medicine era. Adv Cancer Res. 2018;140:327-366. doi: 10.1016/bs.acr.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molino S, Tate E, McKillop WM, Medin JA. Sphingolipid pathway enzymes modulate cell fate and immune responses. Immunotherapy. 2017;9:1185-1198. doi: 10.2217/imt-2017-0089. [DOI] [PubMed] [Google Scholar]
- 24. Healey KR, Katiyar SK, Castanheira M, Pfaller MA, Edlind TD. Candida glabrata mutants demonstrating paradoxical reduced caspofungin susceptibility but increased micafungin susceptibility. Antimicrob Agents Chemother. 2011;55:3947-3949. doi: 10.1128/AAC.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Healey KR, Katiyar SK, Raj S, Edlind TD. CRS-MIS in Candida glabrata: sphingolipids modulate echinocandin-FKS interaction. Mol Microbiol. 2012;86: 303-313. doi: 10.1111/j.1365-2958.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choudhary DK, Bhakt P, Kaur R. Essential role for the phosphatidylinositol 3,5-bisphosphate synthesis complex in caspofungin tolerance and virulence in Candida glabrata. Antimicrob Agents Chemother. 2019;63:e00886-19. doi: 10.1128/AAC.00886-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zamith-Miranda D, Heyman HM, Cleare LG, et al. Multi-omics signature of Candida auris, an emerging and multidrug-resistant pathogen. mSystems. 2019; 63(8): e00886-19. doi: 10.1128/AAC.00886-19. [DOI] [PMC free article] [PubMed] [Google Scholar]