Abstract
Introduction
Repeated use of functional electrical stimulation can promote functional recovery in individuals with neurological paralysis. We designed garments able to deliver functional electrical stimulation.
Methods
Shirts and pants containing electrodes knitted with a conductive yarn were produced. Electrodes were moistened with water before use. Stimulation intensity at four thresholds levels (sensory, movement, full range of motion, and maximal), stimulation comfort, and electrical properties of the interface were tested in one able-bodied subject with garment electrodes and size-matched conventional gel electrodes. The pants and shirt were then used to explore usability and design limitations.
Results
Compared to gel electrodes, fabric electrodes had a lower sensory threshold (on forearm muscles) but they had a higher maximal stimulation threshold (for all tested muscles). The stimulation delivery was comfortable when the garment electrodes were recently moistened; however, as the electrodes dried (within 9 to 18 min) stimulation became unpleasant. Inconsistent water content in the fabric electrodes caused inconsistent intensity thresholds and inconsistent voltage necessary to apply a desired stimulation current. Garments’ tightness and impracticality of electrode lead necessitate further design improvement.
Conclusions
Fabric electrodes offer a promising alternative to gel electrodes. Further work involving people with paralysis is required to overcome the identified challenges.
Keywords: Assistive technology, design requirements, electrical stimulation, functional textile, spinal cord injury rehabilitation, stroke rehabilitation
Introduction
Neuro-muscular electrical stimulation (NMES) has been used in rehabilitation for more than 50 years. 1 NMES produces artificial contractions of muscles by applying sequences of short and low-energy pulses to the peripheral nerves. 2 NMES has been shown beneficial for knee ligament surgery, 3 prevention and management of venous diseases, 4 muscle weakness with advanced progressive disease (e.g. chronic respiratory disease, chronic heart failure, thoracic cancer), 5 critically ill patients confined to bed,6,7 and neurorehabilitation after spinal cord injury (SCI) or stroke. 8
Functional electrical stimulation (FES) is the use of NMES to execute a functional movement (e.g. grasping, reaching, standing, and walking). It can be delivered in an open-loop (pre-programmed) fashion, 9 or in a closed-loop fashion. 10 FES can be supervised by a therapist, 11 unsupervised, 12 or supervised remotely.13,14 It can be used as a long-term assistive device or as a short-term therapeutic intervention (i.e. FES therapy), particularly for management of neurological paralysis.2,15
It is well established that early and intensive physical activity is an effective tool to enhance neurological and functional recovery after SCI16–19 and stroke.20–22 Most FES rehabilitation studies with significant clinical benefits required between 12 and 72 h of FES therapy delivered over 8 to 16 weeks.9,23–30 The present tendency, however, is to reduce the length of stay in post-acute rehabilitation,31–36 resulting in a progressive transition from hospital inpatient care to home-based care after SCI and stroke.37–39 The present changes in healthcare services are partly dictated by the anticipated growth of the aging population and progression of chronic diseases and disabilities.40,41 For example, years of life with disability related to ischemic stroke increased worldwide by 35% between 2006 and 2016. 42 There is thus a clinical, social, and economic challenge in providing intensive neurorehabilitation, including FES, while length of stay in subacute care is being reduced.
The solutions for FES delivery are: (i) separated disposable gel electrodes and off-the-shelf stimulator, (ii) electrode arrays with a specific stimulator-controller, (iii) external neuroprosthesis (an apparatus adding mechanical support to gel electrodes and integrating a stimulator), and (iv) implanted neuroprosthesis. Disposable surface self-adhesive gel electrodes are commonly used by clinicians, widely available at a moderate cost, and allow ad hoc positioning. 43 Their limitations include the time, dexterity, and anatomical knowledge required for set-up, and the necessity to secure the electrodes on the skin with wrap or tape. A recent review on electrode arrays 44 identified three types of material: plastic substrate, embroidered silver-coated fibers, and screen printing on fabric. Electrodes arrays are designed to give better selectivity, but require considerable time to calibrate the system and select the optimal electrode configuration for different movements, which has to be done daily. It also requires hydrogel to deliver FES comfortably and additional instrumentation to allow the stimulator to select the proper combinations of electrodes. External neuroprostheses, such as a foot drop stimulator positioned below the knee 45 or a hand stimulator positioned on the forearm 46 have the advantage of portability and specificity for a given function (i.e. walking or grasping) but their cost can be prohibitive, and wearing them can be cumbersome while lacking versatility. Implanted neuroprostheses have the advantage of the selectivity of muscle activation but the disadvantage of requiring a surgical procedure and similar amount of time to done and doff the external components of the system as the surface stimulation systems (7–10 min).
A panel of consumer experts with mobility impairments highlighted that the most important aspects of assistive devices are effectiveness, operability, dependability, affordability and flexibility. 47 Present solutions for FES appear to fulfill some of these requirements partially, but not all of them simultaneously. One potential solution to address these expectations is a garment-based stimulation technology. The advantage of garments over presently used technology is the possibility to customize it for a particular individual, i.e. to fit a particular body shape, or a specific need. Garments can also be mass-produced at a relatively low cost to make the device affordable to a larger population. Delivery of FES with fabric electrodes made of a conductive yarn has been attempted with promising results, although they required water 48 or gel44,49 to deliver comfortable stimulation. One of these methods used a textile made of a “silvered” thread to produce a rectangular electrode which had to be wetted before applying stimulation. 48 Today, silver-coated textiles are used to record bio-signal (e.g. electrocardiography and electromyography) and to deliver transcutaneous electrical nerve stimulation (TENS, a pain-relieving sensory stimulation). To the best of our knowledge, no garment-based FES system has reached the stage of clinical readiness yet.
Based on our 20+ years of experience with FES, our laboratory, in collaboration with the industrial partner Myant Inc. (Toronto, Canada), conceptualized and designed FES-garments for delivery of NMES and FES. The FES-garments presented here aim to solve some of the aforementioned limitations by improving ease of donning/doffing, comfort and discretion of wearing the device, durability, and versatility of use. This paper presents design strategies and proof of concept for the proposed FES-garments.
Method
II-A – Design phase
The FES-garments were produced by a knitting technician at Myant Inc. with an automatic circular knitting machine. The electrodes employ Myant Inc. patented technology of seamlessly knitting electrodes within the garment that ultimately produce a structure with high conformity to the body. The garments were knitted with nylon and LYCRA® (elastane) yarns for the non-conductive parts, and with a nylon yarn coated with Ag–AgCl and LYCRA® for the conductive parts (the FES electrodes). We chose a combination of nylon and LYCRA® yarns because it provides superior stretch and compression compared to cotton or nylon alone, which ensures sufficient compression of the garment on the skin during use, a critical point for the effectiveness and safety of FES. Additionally, Nylon-Lycra fabric is hydrophobic by nature, which reduces the moistening of the garment that is in between the electrodes (i.e. where the garment should not be wet to avoid electrical shorting of the electrodes). The Ag–AgCl-coated conductive yarn, inspired from previous application for embroidered electrode arrays, 44 was specifically selected to resist multiple washing cycles with standard consumer-grade laundry machines and detergent. The conductive and non-conductive parts were knitted together and merged during the building of the garment, without sewing. The pieces of fabric produced by the circular knitting machine were then used to produce long-sleeve shirts and leggings.
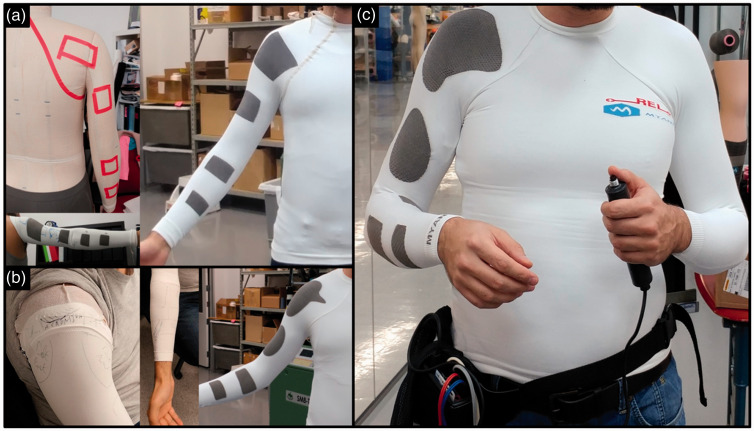
To position the electrodes on the FES-shirt (four channels, eight electrodes: finger flexors, finger extensors, biceps and anterior deltoid, triceps and posterior deltoid) an iterative approach was used (Figure 1). The following process was followed: (1) measurement of length and perimeters of the upper-limbs and trunk (as if a custom-made shirt was being produced); (2) positioning of the electrodes based on anatomical knowledge, and production of an FES-shirt; (3) refining of the position and size of fabric electrodes through outlining on a blank sleeve, and production of a shirt mimicking the outlined electrodes; (4) redrawing of the electrodes contour on the previous prototype to correct errors due to perspective and stretching bias; (5) production of the final prototype based on these corrections; and (6) leads attached and positioned on the inside of the garment, with an interface to the stimulator’s leads at the bottom edge of the shirt.
Figure 1.
Multiple iterations in the production of the four-channel FES-shirt: (a) Initial positioning of the electrodes and first prototype; (b) Drawing of ad hoc electrodes on pieces of garment and second prototype; (c) Final prototype, connected here to a Compex Motion Stimulator worn on the hip. FES: functional electrical stimulation.
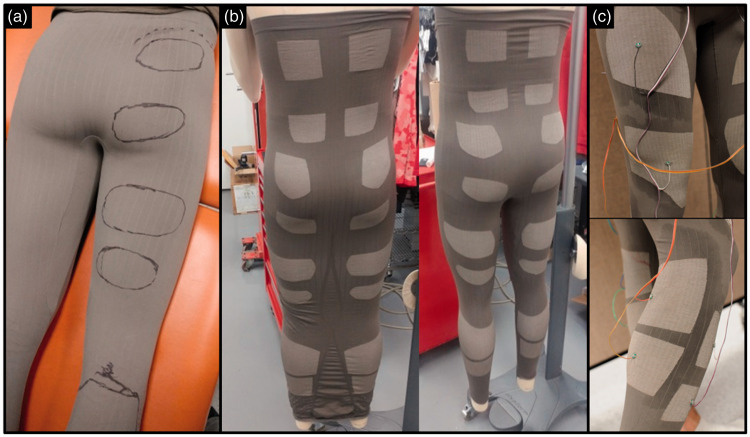
To improve the speed and reduce cost of prototypes production, the design approach was changed for the FES-pants (28 electrodes, 14 channels: ankle plantar- and dorsi-flexors, knee flexors and extensors, gluteus maximus, rectus abdominis, erector lumbar spinae (Figure 2)). The following process was followed: (1) standard medium-size legging-type pants were produced with the same knitting machine (with non-conductive yarn only, hence cheaper and quicker to design), with a regular pattern of thin vertical and horizontal lines knitted to form a visible grid; (2) the desired positions of the electrodes were outlined with a marker directly on the fabric while worn by the subject (hence stretched); (3) using the grid as landmarks, the position of each electrode, drawn in “three-dimensions” on the subject, was reproduced on the two-dimensions knitting-model of the un-stretched garment initially used; (4) production of the FES-pants with the electrodes made of conductive yarn in the locations previously outlined; and (5) connection and routing of the wires on the outside of the garment. This process required producing only one blank pair of leggings before producing a satisfactory pair of FES-pants.
Figure 2.
Single iteration for production of the 14-channel FES-pants; (a) Drawing of electrodes outline for each targeted muscles on a meshed-blank garment; (b) Production of the garment with the circular knitting machine and sewing of legs; (c) Testing of the wired FES-pants (quadriceps electrodes above, triceps electrodes below). FES: functional electrical stimulation.
II-B – Comparison of gel and fabric electrodes
One able-bodied individual consented to test the ability of the garment to deliver FES.
II-B-1. Comparison of intensity thresholds for gel and fabric electrodes during NMES
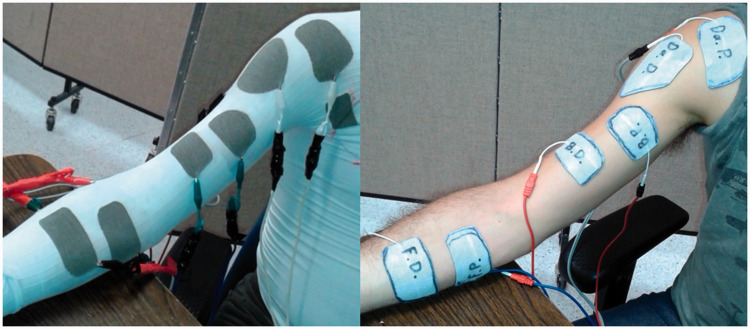
The grid-based design described above was used to create a second FES-shirt with nine pairs of electrodes. For this experiment, six of these pairs were used to stimulate the flexor and extensor muscles of the fingers, elbow and shoulder joints. While the subject was wearing the FES-shirt, the shape and size of the fabric electrodes were traced on commercially available self-adhesive gel electrodes (ValuTrode, 5 cm × 9 cm or 7.5 cm × 13 cm, Axelgaard, USA), which were then cut out to have the same size and shape as the fabric electrodes (Figure 3). Picture and measurements of the fabric electrodes position with respect to body landmark were taken to reproduce their position with the gel electrodes.
Figure 3.
Set-up for intensity thresholds identification with fabric (left) and gel (right) electrodes.
A four-channel programmable current-controlled stimulator (Compex Motion, Compex SA, Switzerland) 50 was used to deliver NMES through garment and self-adhesive gel electrodes. Negative stimulation pulses were 300 µs long, balanced, asymmetric, biphasic, alternate, and delivered at frequency of 30 Hz. The stimulation intensity was manually ramped up in one mA steps to identify four thresholds for each pair of electrodes:
the sensory threshold (eliciting a consistent buzzing sensation under the electrodes),
the movement threshold (where a movement of the limb was observed, not against gravity),
the full range-of-motion threshold (intensity fully mobilizing the limb, against gravity),
the maximal threshold (where the subject could not tolerate further increase of intensity).
Each time one of the first three thresholds was identified, the stimulation was turned off and on again for confirmation, and lower intensities were tested to ensure that they could not qualify for this threshold. Similarly, for maximal threshold, the stimulation was turned off and on again to verify that the discomfort was not due solely to muscle cramping (due to prolonged tetanic contraction), in which case, higher stimulation intensities were tested.
Each pair of electrodes was tested four times to evaluate the consistency of intensity thresholds. The fabric electrodes were connected to the stimulator leads with alligator clips and regularly moistened with tap water by applying a wet sponge on the outside of the garment. The transcutaneous electrical stimulation questionnaire (TESQ) was used as a guide for the subjective description of the stimulations delivered with the FES-shirt. The TESQ is a modified form of the short-term McGill pain questionnaire, with 14 different sensations related to cutaneous, deep and general sensory receptors. 48
Observations on the practical aspects in using both systems (FES-garment and gel electrodes) were also recorded. In particular, the time for donning and doffing was recorded. Donning time was recorded between a “Go” signal, with the participant having all the equipment before him, and the moment he was ready to initiate stimulations. Doffing time was recorded between a “Go” signal and the moment all equipment were taken off the participant and put away on the table in front of him.
Results for intensity thresholds for gel and fabric electrodes during NMES
The ranges of intensity for each threshold and muscle are presented in Table 1. Fabric electrodes had lower sensory thresholds than gel electrodes on the forearm (stimulation was felt at a slightly lower intensity) but not on elbow and shoulder muscles. Fabric electrodes required higher intensity to induce a visible limb motion for triceps and posterior deltoid muscles but not for other muscles. Threshold differences for full range of motion were inconsistent across muscles: three muscles required higher stimulation with gel electrodes and three muscles required higher intensity with fabric electrodes. Fabric electrodes allowed for higher maximal stimulation on all muscles (i.e. the participant tolerated stimulation at higher intensity with the fabric electrodes, regardless of the contraction elicited).
Table 1.
Intensity at sensory, movement, full range of motion, and maximal stimulation thresholds of stimulations (in mA) delivered with fabric electrodes of a FES-shirt and with size-matched conventional gel electrodes.
| Sensory threshold [mA] |
Movement threshold [mA] |
Full motion threshold [mA] |
Maximal stimulation threshold [mA] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscles | Fabric min–max | Gel min–max | Δ | Fabric min–max | Gel min–max | Δ | Fabric min–max | Gel min–max | Δ | Fabric min–max | Gel min–max | Δ |
| Finger/wrist flexors | 3–9 | 7–8 | –4 | 8–11 | 8–11 | –1 | 12–16 | 17–19 | –4 | 25–31 | 20–30 | 2.5 |
| Finger/wrist extensors | 8–12 | 15–16 | –3.5 | 13–21 | 15–17 | 1 | 21–26 | 29–39 | –9.5 | 40–46 | 38–45 | 3.5 |
| Biceps brachialis | 5–8 | 6–8 | 0 | 6–19 | 7–9 | 1 | 22–34 | 34–37 | –3.5 | 43–54 | 42–45 | 5 |
| Triceps brachialis | 5–8 | 6–7 | 0 | 13–20 | 10–11 | 8 | 24–30 | 23–26 | 3.5 | 34–40 | 30–36 | 3.5 |
| Anterior deltoid | 5–8 | 7–8 | 0 | 14–15 | 16–16 | –1 | 39–47 | 43–44 | 1.5 | 49–65 | 53–56 | 5.5 |
| Posterior deltoid | 4–6 | 4–5 | 0.5 | 14–17 | 8–10 | 5.5 | 21–31 | 21–27 | 4 | 35–46 | 36–39 | 4 |
| Average intensity ±SD | 7 ±3 | 8 ±4 | −1 ±2 | 14 ±4 | 12 ±3 | 2 ±4 | 28 ±10 | 30 ±9 | −1 ±5 | 43 ±11 | 39 ±10 | 4 ±1 |
| Average range | 3.5 | 1.2 | 5.8 | 1.7 | 7.5 | 4.2 | 9.3 | 4.8 | ||||
min-max: minimal and maximal intensity (four trials); Δ: Difference between the medians of electrodes’ thresholds (negative value indicates greater intensity with gel electrode); SD: standard deviation. Average range: mean of the differences between minimal and maximal value of each muscle, expressing variability.
Note: Values are the minimal, maximal and range of stimulation intensities for the four trials.
The reasons for not increasing intensity beyond maximal threshold were the muscle contraction sensation (deep cramping sensation, 1/3 of tests, mostly on the forearm), the unpleasant electricity sensation (superficial vibration, buzzing, 1/3 of tests, mostly on the upper arm), or a combination of both sensations (1/3 of tests). The reasons for not increasing intensity beyond maximal threshold were similar with gel and fabric electrodes for all muscles, with the exception of the posterior deltoid electrodes where electricity sensation was limiting for fabric electrodes, while a combination of sensations (contraction and electricity) was limiting for gel electrodes.
The variability of stimulation intensities – as measured by the difference between minimal and maximal value for a given muscle and a given threshold (range, Table 1) – was greater at each threshold for the fabric electrodes than for the gel electrode. As an example, the sensory threshold for the finger flexors ranged from 3 to 9 mA (range = 6 mA) with the fabric electrodes but only from 7 to 8 mA (range = 1 mA) for the gel electrodes, making the latter more consistent (less variable). This was presumably because we could not control exactly the amount of moisture delivered to the fabric electrodes or the rate of evaporation and skin absorption of the water.
Similarly, the quality of the stimulation sensation with fabric electrodes as estimated with the TESQ varied significantly depending on the level of moisture in the electrodes. When the electrode was recently moistened, sensations were predominantly of deep contraction (“cramping,” “throbbing,” “gnawing”); conversely, when the fabric electrodes were drier, sensations were predominantly superficial (“prickling,” “hot,” “tender,” “splitting”).
Results for usability of gel and fabric electrodes during NMES
The practical aspects identified for both set-ups were that the electrodes were fairly stable with the FES-garments, with homogeneous pressure ensured by the elasticity of the knitted garment. Because gel electrodes tended to peel off and wrinkle during motion, due to stretching of the skin and bulking of the muscles, they had to be covered with a foam wrap on the arm and forearm, and taped on the shoulder. Additionally, the gel electrode leads required to be taped onto the skin during the stimulation-induced motions to ensure they did not catch on furniture and objects around the subject.
In the present study, the position of the electrodes was selected based on commonly used electrode location for stimulation of each muscular group. As with gel electrodes, the position of the fabric electrodes was not necessarily ideal at first, so the position was fine-tuned by moving the garment based on the effect of FES, until it allowed functional movement without undesired stimulation (e.g. wrist and finger extension without ulnar or radial deviation). Repositioning of multiple gel electrodes can be necessary when the therapist wants to execute another FES task: it is a lengthy operation that requires removing the tape/wrap to pull them off the skin (potentially tearing hairs), repositioning them, and re-tapping/wrapping. Because of the FES-garments’ elasticity, fabric electrodes could be repositioned by pulling the sleeve up, down or sideway, as necessary, without difficulty. Additionally, the electrodes for hand, elbow and shoulder movements were all positioned at once when donning the FES-shirt, even if only part of them were being used.
Additionally, positioning self-adhesive gel electrodes requires strength and dexterity, unavailable to some individuals with paralysis, while donning the FES-garment may be done independently, requiring little strength and dexterity. Furthermore, the self-adhesive electrodes cannot be easily and accurately positioned on the posterior aspect of the body by the subject themselves, while the FES-shirt only required to be adjusted on the body until all electrodes were positioned as desired.
Because the fabric electrodes need to be individually moistened, the total set-up time of the FES-shirt (4.5 min for the shirt in Figure 6, done independently) was similar to that of the gel electrodes (5 min for four pairs of electrodes, done with assistance). The removal time was much shorter as it required only doffing the shirt (20 s), while gel electrodes needed to be individually taken off the skin (sometimes unpleasantly pulling skin and hairs) and reposition on their plastic support if they were to be reused (3.5 min). Also, the gel electrodes and tape can leave residue on the skin (on stimulation site and fingers), while for the FES-shirt no cleaning is required after use.
Figure 6.
Drink-like FES sequence delivered with the FES-shirt. FES: functional electrical stimulation.
II-B-2. Comparison of voltage applied with gel and fabric electrodes during NMES
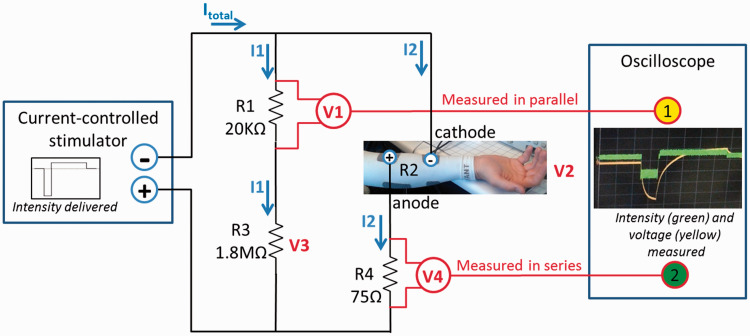
An oscilloscope (DSO6012A, Two-channel, 100 MHz, 2 GSA/s, Agilent Technologies, USA) was used to measure and record voltage in series and in parallel with the electrodes (Figure 4) to estimate the voltage and the current applied to the skin in two different set-ups: fabric electrodes embedded in a FES-shirt and self-adhesive gel electrodes.
Figure 4.
Diagram of the electrical circuit used to estimate voltage (V2) and intensity (I2) of the stimulations delivered to the electrodes. Channel 1 of the oscilloscope measured and recorded the voltage across R1, a resistor in parallel with the electrodes (V1, to estimate voltage across electrodes). Channel 2 measured the voltage across R4, a resistor in series with the electrodes (V4, to estimate the current amplitude going through the electrodes).
The FES-shirt described in Part I was used to stimulate the finger/wrist flexors, and two gel electrodes were cut out similarly as in experiment II-B-1. The measurement was done with the fabric electrodes first. Electrodes were moistened only once at the beginning of the experiment. Stimulation was applied and measured every 2–4 min until stimulations were not tolerated due to the drying of the electrodes (stimulation was turned off between measurements). Then the same set-up was used for the gel electrodes, for the same duration. All stimulations were delivered at 20 mA, which was sufficient to generate finger and wrist flexion, with the same stimulator and pulses characteristic as in previous experiment. This current-controlled stimulator aimed at maintaining the target intensity (20 mA) by constantly adjusting the voltage. In that setting, the distal electrode was used as a cathode to favor the activation of the finger flexors and relatively limit the activation of the wrist flexors.
The measured voltages were filtered using a moving average filter (five sample windows = 2.5 µs). 51 The intensity (I2) and voltage (V2) of the stimulations delivered to the electrodes were calculated based on (i) the voltage measured in parallel and in series of the electrodes (V1 and V4), (ii) Ohm’s law (V = I × R; intensities of current are the same in series; total voltage are the same in parallel), and (iii) the known values of resistors R1, R3 and R4 (Figure 4)
Results for measure of voltage applied with gel and fabric electrodes during NMES
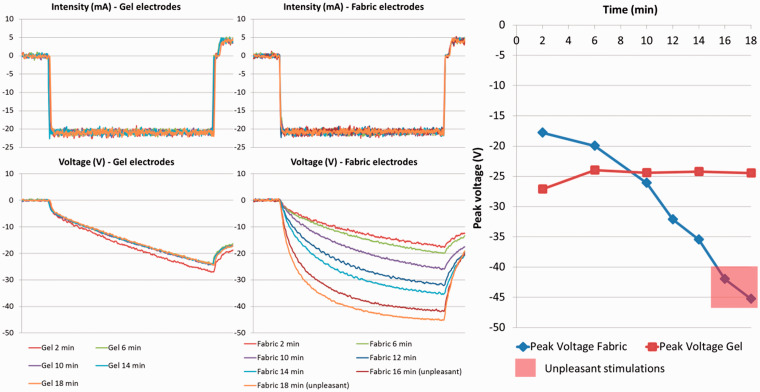
Stimulation with the fabric electrodes on finger flexor at 20 mA produced an effective closing of the finger, with a sensation of deep contraction, until 14 min after applying water on the electrodes. The stimulation applied 16 min after applying water was mildly unpleasant, and the one at 18 min was too unpleasant to continue (superficial sensations as defined in the TESQ). The measurements were consequently stopped with the fabric electrodes and the measurements with the gel electrodes done until 18 min after positioning them on the skin (Figure 5).
Figure 5.
Left: Intensity (mA) and voltage (V) of the stimulation pulses measured with gel and fabric electrodes along time (time between fall and rise of the pulse = 300 µs). Right: Peak voltage of the stimulation pulses for fabric and gel electrodes, over time.
The voltage necessary to apply the 20 mA pulses was consistent across the 18 min for the gel electrodes, while this voltage regularly increased (in absolute value) for the fabric electrodes as the garment became dry (Figure 5). From 2 to 6 min after the fabric electrodes was moistened, the absolute voltage required to apply stimulations was less than for gel electrodes. Contrarily, 12 min after applying water onward, the peak voltage necessary to deliver the pulses was higher than those of gel electrodes (Figure 5). The last two stimulations delivered with fabric electrodes (16 and 18 min after applying water) exhibit the highest peak voltage and were the two unpleasant stimulations.
Finally, the shape of the voltage curves along pulse delivery evidence a shift toward square voltage variation, with the negative slope becoming steeper as the fabric electrodes became drier.
II-C – Proof of concept with functional movements
The two garments presented in Design above, the FES-shirt and FES-pants, were used to generate clinically meaningful FES exercises.
II-C-1. Drink-like motion with the FES-shirt
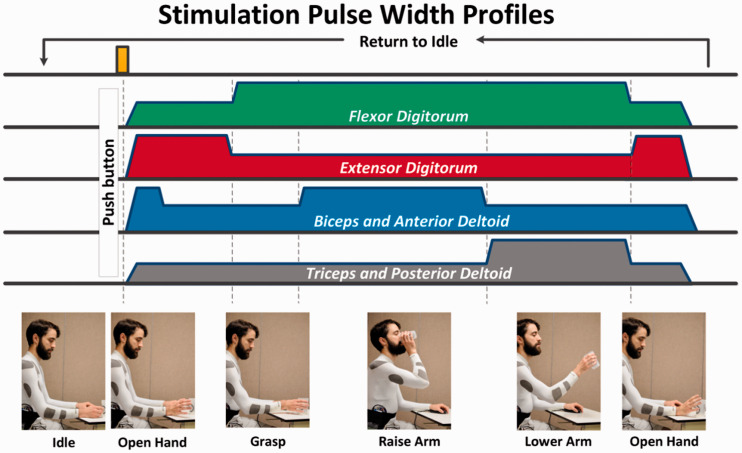
The Four-channel FES-shirt was used to produce a repetitive drink-like motion: lifting and opening hand → closing hand → rising hand at head level → bring hand back down → opening hand (Figure 6). The FES sequence lasted 6s, followed by 6 s of rest with no stimulation. Stimulations were delivered with the same stimulator and pulse parameters as previously described. Anterior deltoid, posterior deltoid, biceps, and triceps were stimulated together to produce an upper-limb flexion and extension (Figure 6).
Because stimulations were delivered in an open-loop fashion on an able-bodied individual trying not to move voluntarily, a baseline level of co-contraction was delivered to antagonist muscle to soften the movement (prevent abrupt motions), e.g. during upper-limb flexion, the shoulder and elbow extensor received pulses at 50% of their pulses width (150 µs) to prevent the hand from rising too fast. This experimental choice would not necessarily apply to an individual with paralysis, where the sequence of stimulations would be defined by the therapist based on the clinical examination (e.g. strength, endurance, spasticity of agonist and antagonist) and the objective of the task.
After donning the shirt, the electrodes were moistened by applying tap water with a wet sponge. Stimulations intensities were set at the last known level allowing execution the full range of motion with that FES-shirt. During the first minute (i.e. the first five stimulation cycles), intensities and garment position were adjusted between stimulations so that the movement obtained was satisfactory. The stimulation-rest cycle was repeated until stimulation was perceived as uncomfortable by the subject.
Results for upper-limb FES proof of concept
Stimulation intensities were 18 mA on finger flexors, 28 mA on biceps and anterior deltoid, 22 mA on triceps and posterior deltoid, and 24 mA on finger extensors. Movements were generated as expected, although the finger flexor stimulation also produced an unintended wrist flexion.
Stimulations were stopped after 18 min and 54 s, i.e., after the 94th sequence of stimulation, when stimulation on finger extensors was deemed too unpleasant to continue. The change in stimulation sensation was progressive, with prickling sensation appearing slowly from 10 min onwards, and the buzzing feeling became more pronounced as the electrodes became drier, getting mildly unpleasant after 15 min.
The subject needed approximately 4.5 min to don the FES-shirt and moisten the eight electrodes on their right side with their left hand. Removing the FES-shirt required about 20 s. No assistance was required at any point for set-up or delivery of FES. The elasticity of the garment made it comfortable to wear and move in, although its compression made it difficult to put on. Contrary to what happens with leads used with gel electrodes, the integrated leads of the FES-shirt allow more freedom of motion. One potential drawback of this FES-shirt was that the leads were between the garment and the skin, which required caution when donning the garment and left a temporary contact mark on the skin after use, which could be problematic when used by individuals with sensory deficits and/or at risk of skin break-down.
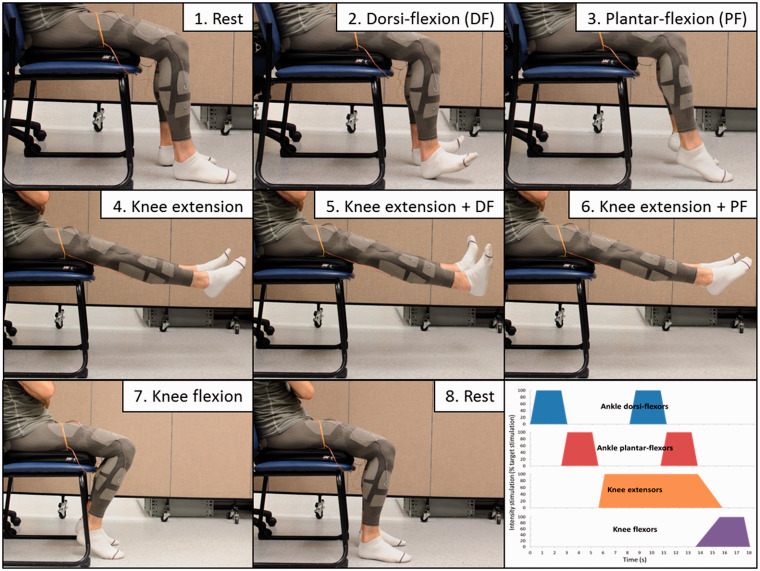
II-C-2. Leg mobilization with the FES-pants
The FES-pants were used to produce a repetitive mobilization of knee and ankle muscles and joints on both legs simultaneously with the subject seated on a chair: ankle dorsi-flexion → ankle plantar flexion → prolonged knee extension, with successively ankle dorsi- and plantar flexion → knee flexion (Figure 7). Two stimulators were used, delivering synchronously the same pulses as previously described, for a total of eight channels (16 electrodes), targeting quadriceps, hamstrings, triceps surae, and foot dorsi-flexors (tibialis anterior and toe extensors). The FES sequence lasted 18 s, followed by 18 s of rest with no stimulation. Electrical pulses were delivered to the fabric electrodes through thin flexible sheathed steel wires terminated by a small metal plate sewn to the outside of the garment. The wires were running loosely, unsecured except for the attachment on the electrode itself, on the outside of the garment and were connected to the stimulators.
Figure 7.
Leg mobilization FES sequence delivered with the FES-pants. FES: functional electrical stimulation.
After donning the FES-pants, electrodes were moistened by applying tap water with a wet sponge. Before starting the sequential FES stimulation, the intensity thresholds were identified as in Part II-1 (sensory, movement, full range-of-motion, and maximal). For the sequential FES, stimulations intensities were set initially at full range-of-motion threshold. Water was briefly re-applied on each electrode just before starting the FES sequence. The stimulation-rest cycle was repeated until stimulation was perceived as uncomfortable by the subject. Intensities were adjusted between stimulations so that the movement obtained was satisfactory (e.g. dorsi-flexion in knee extension required higher intensity than in knee flexion, and quadriceps fatigue required higher intensity during the last testing cycles).
Results for lower-limb FES proof of concept
Donning the FES-pants was fast and easy (<30 s) because the legging-style pants were very elastic, and most of the donning time was used to position adequately the electrodes on the anterior thigh and shank (aligning electrodes on muscles contours). However, making the 16 large electrodes moistened required more time (about 5 min). Doffing the FES-pants took about 20 s.
Intensity thresholds identified for the FES-pants are detailed in Table 2. The maximal stimulation intensity was limited by the cramping sensation produced by FES, except for the right ankle dorsi-flexors for which the limitation to intensity increase was a combination of contraction and skin sensations. The FES stimulation-rest cycles were repeated for 9 min (15 cycles of 36 s), and stopped because the stimulation on the right ankle dorsi-flexors became too unpleasant.
Table 2.
Intensity at sensory, movement, full range of motion, and maximal stimulation thresholds of stimulations delivered with the FES pants (in mA).
| Muscle site | Sensory threshold (mA) | Motor threshold (mA) | Full motion threshold (mA) | Maximal stimulation (mA) |
|---|---|---|---|---|
| Ankle dorsi-flexor left | 16 | 24 | 50 | 65 |
| Ankle dorsi-flexor right | 15 | 27 | 43 | 65 |
| Ankle plantar-flexor left | 20 | 39 | 61 | 72 |
| Ankle plantar-flexor right | 13 | 25 | 56 | 76 |
| Quadriceps left | 10 | 34 | 52 | 80 |
| Quadriceps right | 11 | 26 | 63 | 85 |
| Hamstrings left | 7 | 25 | 33 | 61 |
| Hamstrings right | 6 | 23 | 43 | 53 |
FES: functional electrical stimulation.
Similar to what happens with self-adhesive gel electrodes, the wires were partially hanging from the garment, with the risk of catching on something and damaging the connection between the wire and the fabric electrode. Contrary to the FES-shirt, the elasticity of the FES-pants and the outside wiring made donning the garment particularly easy. The tendency of the FES-pants, however, was to slip downward (three cm, approximately) when walking. All those practical aspects will be taken into consideration during further developments of the FES-garments.
Discussion
This paper presents the rationale, design, and proofs of concept of wearable garments able to deliver FES (FES-shirt and FES-pants). Several advantages, limitations, and development perspectives were identified and are discussed hereafter. The observed advantages of the proposed FES-garments were efficacy, simplicity, independence, and potential versatility of use. The limitations observed with the proposed FES-garments were compression, lead management, need for repeated water application, low selectivity and relatively basic open-loop stimulation. Possible solutions are discussed for each of these issues.
In terms of efficacy, it was shown here, in an able-bodied subject, that, as long as the electrodes were moistened, the performance of the proposed FES-garment was comparable to that of standard self-adhesive gel electrodes regarding comfort of stimulations and contraction produced. This makes them a potential alternative to conventional gel electrodes. Integration of other electrode designs might even allow to eliminate the required moistening and apply FES with dry electrodes. 52
The simplicity of use of these garments could save significant time compared to using self-adhesive gel electrodes with separate leads. This gain in time will likely increase as the number of targeted muscles increases. While the FES protocols tested targeted only four and eight muscles (with one and two stimulators, respectively), our latest prototypes have significantly more electrodes. Together with advanced multi-channel stimulators, which now have 8 to 16 stimulation channels,53,54 this could significantly broaden the types of therapy and functional movement available to people with paralysis in the future.
The potential independence in using these FES-garments could be of great importance to people with motor impairments. 47 End-users with residual hand function able to dress independently will likely be able to put on these garments, providing that the garment design is adapted to their needs and limitations. 55 For end-users relying on caregiver assistance for daily dressing, it is likely that they would need similar assistance for FES-garments. For people aiming to practice FES, the electrode set-up could make the device usable independently, or at least without the need for a specialized therapist (e.g. in a home setting).
The multiple custom-sized fabric-based electrodes in the proposed FES-garments offer the potential for a versatile device able to apply NMES or FES in diverse situations. For example, the large electrodes use in the pants could allow for intensive NMES sessions aiming at muscle-mass gain and increase in blood circulation, which could result in significant health benefits. 56 The same FES-pants could be used for cycling or rowing exercises to engage the entire lower-limbs and trunk, which could have positive impact on cardiovascular function, muscle mass, and even bone density.57–60 The FES-shirt and FES-pants tested in this study were able to produce clinically meaningful sequences of movements for duration compatible with clinical use (9–18 min in a row without re-applying water). Using these FES-garments to train movements while trying to voluntary realize these movement (i.e. FES therapy) may help individuals with paralysis regain certain voluntary movement and function. 8 Another possible use for the FES-pants could be to replace compression stockings: while being much less tight (thus easier to don and doff), they might be able to ensure a regular venous blood return to the heart, with the advantage of using muscles instead of relying on high passive compression. Indeed, NMES, by activating muscles, create a “call” for blood flow, which increases microcirculation in the lower-limbs. 56 Simultaneously, contractions (and possibly stretching) of the large muscle groups (triceps, quadriceps) create a pump-effect increasing venous circulation towards the heart.4,61
One of the observed limitations of the FES-garments presented here is their compression (tightness). Depending on the functional level of the user, putting the garments on may be difficult and/or require assistance from a caregiver. For individuals who usually do not require dressing assistance, this issue could be detrimental to the usability and acceptance of the garment. For individuals who usually require dressing assistance, the garment should be as easy to don as possible to avoid additional burden on caregivers. Additionally, for certain FES applications, it is necessary to reposition the electrodes on the body (e.g. to train different type of grasp), which should be made easy by these garments.
The four-channel FES-shirt was described as very tight, but the electrodes were extremely stable on the skin, while the FES-pants were much more elastic and easy to don but tended to slip downwards. There is thus a trade-off to optimize between easiness to don and reliability of electrodes position. This can be achieved by developing specific designs that facilitate donning and doffing. To identify optimal design solutions, the relevance of these FES garments is being investigated with clinicians and potential end-users through focus-group discussions.47,62 This qualitative study should help refine the FES-garments design based on needs and expectations of end-users. 63 Additionally, we are working with cloth designers, material engineers, and end-users to find ways to optimize the trade-off between compression and easiness to don the garments. To date, the solutions tested include zipper, magnetic hooks, tightening bands, uneven compression (garment looser where no electrodes present, possibly made of a different material) and overall design (e.g. a “bolero-style” shirt (sleeves only) to suppress the need to pass their head through the hole).
The management of leads for the two garments tested was only partly satisfactory: having leads inside the garment made donning more difficult and left contact marks (indentations) on the skin. Leads positioned on the outside limited mobility almost in the same way as when using gel electrodes. To overcome this issue, the new generation of FES-garments now has a dual-layer: the inside layer corresponds to the present garments with the stimulation electrodes, the leads running on top, and the outside layer protects the wires and isolate the conductive electrodes from the outside. The dual layer may also limit water evaporation and thus prolong the possible time of consecutive FES delivery.
The need to apply water on the outside of the electrode before delivering stimulations was manageable, but not optimal. This was relatively time intensive (1–5 min depending on the number of electrodes) but because the tested garments were thin, they dried in 9–18 min (depending on the humidity of the room during use), making stimulation unpleasant. In the present stage of development, a user would have to reapply water every 5–15 min if they want to use FES for a long period. Current research focus on maintaining moisture longer, improving delivery of water to the electrodes, as well as interfacing the conductive fabric electrodes with a material that would not require water.64,65 The challenge of such material would be to ensure easy donning and doffing, comfortable prolonged wearing, and sweat evacuation when not in use.
Because large electrodes were used for the forearm, the selectivity of finger flexor muscles stimulation was limited and wrist flexors were unintentionally stimulated. This problem is well known and several strategies, such as electrode arrays and motion sensors, 44 were attempted to limit this problem. Electrode arrays can also be used to limit fatigue by applying spatially distributed sequential stimulation, 66 particularly in the lower-limbs where large muscles are stimulated to generate strength for long periods of time (e.g. standing, biking, and rowing). Also, stimulations studied here were simple open-loop FES protocols. The FES-garments could be instrumented directly with wearable motion sensors (e.g. inertial measurement units, bending, pressure or stretching sensors, cameras) to allow for closed-loop controlled FES system. This would allow more sophisticated intervention with tasks such as grasping, 67 walking, 68 sitting, 10 standing, 69 or cycling. 70
It should be noted that using array of electrodes and/or closed-loop FES requires a more complex stimulation, instrumentation and control system 71 than what was presented here (i.e. open-loop stimulation). Such increase in complexity might affect affordability and usability of the device. Identifying end-users’ needs through interview and questionnaires might be a good way to guide those industrial choices.
One limitation of this proof-of-concept study is that tests were conducted with a single able-bodied participant. Although this is a standard preliminary step in any development of medical technology, it is not possible to demonstrate here that there was no active participation or bias from that participant. To overcome this limitation, FES-garments would need to be tested in individuals with significant paralysis and in groups of volunteers. A pilot clinical trial involving individuals who had an SCI or a stroke to test the safety, feasibility and efficacy of these FES-garments is presently in the recruitment phase.
Conclusion
This study presented the design, feasibility, and opportunity for improvement of garments delivering FES: the FES-shirt and the FES-pants. The elasticity of the knitted fabric ensured contact of the electrodes on the skin, allowing stimulations comparable to conventional gel electrode so long as the fabric electrodes were wet. These garments are now being tested clinically (pilot trial) and investigated qualitatively (focus groups) with the help of individuals with paralysis due to stroke or SCI.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Milos R Popovic is CTO of MyndTec Inc., a company manufacturing stimulators for functional electrical stimulation. Milad Alizadeh-Meghrazi is Director of Research and Development at Myant Inc., a company manufacturing textile-based technologies. Bastien Moineau is now an intern at Myant Inc., as part of a Mitacs Accelerate post-doctoral fellowship with the University of Toronto.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Bastien Moineau was supported by post-doctoral fellowships from Spinal Cord Injury Ontario and AGE-WELL, a Network of Centre of Excellence of the Government of Canada. Milos R Popovic's laboratory was supported by a donation from Dean Connor and Maris Uffelmann.
Guarantor
BM and MRP.
Contributorship
BM and MAM designed the electrodes shape and positions. MAM and Myant Inc developed the knitting techniques, garment form factors, and textile-electronic connections. BM designed the stimulation sequences. BM and CMC performed data collection. BM, CMC, MAM and MRP wrote the manuscript.
Acknowledgements
Authors are thankful to Gabriel Stefan and Monica Nealis for their contribution to the garment design, and to Naaz Kapadia and Martha Garcia-Garcia for their assistance in data collection.
References
- 1.Liberson W, Holmquest H, Scot D, et al. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 1961; 42: 101–105. [PubMed] [Google Scholar]
- 2.Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 2007; 35: 562–590. [DOI] [PubMed] [Google Scholar]
- 3.Hauger AV, Reiman MP, Bjordal JM, et al. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc 2018; 26: 399–410. [DOI] [PubMed]
- 4.Williams KJ, Ravikumar R, Gaweesh AS, et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv Exp Med Biol 2017; 906: 377–386. [DOI] [PubMed] [Google Scholar]
- 5.Jones S, Man WDC, Gao W, et al. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 2016; 10: 1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segers J, Hermans G, Bruyninckx F, et al. Feasibility of neuromuscular electrical stimulation in critically ill patients. J Crit Care 2014; 29: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 7.Ojima M, Takegawa R, Hirose T, et al. Hemodynamic effects of electrical muscle stimulation in the prophylaxis of deep vein thrombosis for intensive care unit patients: a randomized trial. J Intensive Care 2017; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai MK, Marquez-Chin C and Popovic MR. Why is functional electrical stimulation therapy capable of restoring motor function following severe injury to the central nervous system? In: Tuszynski M. (eds) Translational Neuroscience. Boston, MA: Springer, 2016, pp.479–498.
- 9.Popovic MR, Kapadia N, Zivanovic V, et al. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011; 25: 433–442. [DOI] [PubMed] [Google Scholar]
- 10.Murphy OJ, Audu LM, Lombardo ML, et al. Feasibility of closed-loop controller for righting seated posture after spinal cord injury. J Rehabil Res Dev 2014; 51: 747–760. [DOI] [PubMed] [Google Scholar]
- 11.Popovic MR, Thrasher TA, Adams ME, et al. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006; 44: 143–151. [DOI] [PubMed] [Google Scholar]
- 12.Kern H, Carraro U. Home-based functional electrical stimulation for long-term denervated human muscle: history, basics, results and perspectives of the Vienna rehabilitation strategy. Eur J Transl Myol 2014; 24: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buick AR, Kowalczewski J, Carson RG, et al. Tele-supervised FES-assisted exercise for hemiplegic upper limb. IEEE Trans Neural Syst Rehabil Eng 2016; 24: 79–87. [DOI] [PubMed] [Google Scholar]
- 14.Hermann VH, Herzog M, Jordan R, et al. Telerehabilitation and electrical stimulation: an occupation-based, client-centered stroke intervention. Am J Occup Ther 2010; 64: 73–81. [DOI] [PubMed] [Google Scholar]
- 15.Kapadia N, Popovic M. Functional electrical stimulation therapy for grasping in spinal cord injury: an overview. Top Spinal Cord Inj Rehabil 2011; 17: 70–76. [Google Scholar]
- 16.de Groot PCE, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord 2003; 41: 673–679. [DOI] [PubMed] [Google Scholar]
- 17.Panisset MG, Galea MP, El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal Cord 2016; 54: 1–9. [DOI] [PubMed] [Google Scholar]
- 18.Scivoletto G, Morganti B, Molinari M. Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study. Arch Phys Med Rehabil 2005; 86: 512–516. [DOI] [PubMed] [Google Scholar]
- 19.Sumida M, Fujimoto M, Tokuhiro A, et al. Early rehabilitation effect for traumatic spinal cord injury. Arch Phys Med Rehabil 2001; 82: 391–395. [DOI] [PubMed] [Google Scholar]
- 20.Harris JE, Eng JJ, Miller WC, et al. A self-administered graded repetitive arm supplementary program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke 2009; 40: 2123–2128. [DOI] [PubMed] [Google Scholar]
- 21.Maulden SA, Gassaway J, Horn SD, et al. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil 2005; 86(12 Suppl.): 34–40. [DOI] [PubMed] [Google Scholar]
- 22.Bernhardt J, Godecke E, Johnson L, et al. Early rehabilitation after stroke. Curr Opin Neurol 2017; 30: 48–54. [DOI] [PubMed] [Google Scholar]
- 23.Kapadia N, Masani K, Catharine Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med 2014; 37: 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia N, Nagai M, Zivanovic V, et al. Functional electrical stimulation therapy for recovery of reaching and grasping in severe chronic pediatric stroke patients. J Child Neurol 2014; 29: 493–499. [DOI] [PubMed] [Google Scholar]
- 25.Thrasher TA, Zivanovic V, McIlroy W, et al. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair 2008; 22: 706–714. [DOI] [PubMed] [Google Scholar]
- 26.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation (FES) may modify the poor prognosis of stroke survivors with severe motor loss of the upper extremity: a preliminary study. Am J Phys Med Rehabil 2008; 87: 627–636. [DOI] [PubMed] [Google Scholar]
- 27.Daly JJ, Zimbelman J, Roenigk KL, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair 2011; 25: 588–596. [DOI] [PubMed] [Google Scholar]
- 28.Baldi JC, Jackson RD, Moraille R, et al. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998; 36: 463–469. [DOI] [PubMed] [Google Scholar]
- 29.Griffin L, Decker MJ, Hwang JY, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009; 19: 614–622. [DOI] [PubMed] [Google Scholar]
- 30.Kapadia NM, Zivanovic V, Furlan J, et al. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs 2011; 35: 212–216. [DOI] [PubMed] [Google Scholar]
- 31.Ottenbacher KJ, Smith PM, Illig SB, et al. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA 2004; 292: 1687–1695. [DOI] [PubMed] [Google Scholar]
- 32.Martin S, Street A, Han L, et al. Have hospital readmissions increased in the face of reductions in length of stay? Evidence from England. Health Policy 2016; 120: 89–99. [DOI] [PubMed] [Google Scholar]
- 33.National Spinal Cord Injury Statistical Center. Spinal cord injury – facts and figures at a glance. Birmingham: Author, 2016.
- 34.Jang HJ, Park J, Shin H-I. Length of hospital stay in patients with spinal cord injury. Ann Rehabil Med 2011; 35: 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Y, Jiao Y, Hu R, et al. Reduction of length of stay and costs through the implementation of clinical pathways for stroke management in China. Stroke 2014; 45: 81–84. [DOI] [PubMed] [Google Scholar]
- 36.Wren M-A, Gillespie P, Smith S, et al. Towards earlier discharge, better outcomes, lower cost: stroke rehabilitation in Ireland, Dublin: Economic and Social Research Institute, 2014. [Google Scholar]
- 37.Santana S, Rente J, Neves C, et al. Early home-supported discharge for patients with stroke in Portugal: a randomised controlled trial. Clin Rehabil 2016; 1: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madaris LL, Onyebueke M, Liebman J, et al. SCI hospital in home program: bringing hospital care home for veterans with spinal cord injury. Nurs Adm Q 2016; 40: 109–114. [DOI] [PubMed] [Google Scholar]
- 39.Langstaff C, Martin C, Brown G, et al. Enhancing community-based rehabilitation for stroke survivors: creating a discharge link. Top Stroke Rehabil 2014; 21: 510–519. [DOI] [PubMed] [Google Scholar]
- 40.Dixon-Ibarra A, Krahn G, Fredine H, et al. Adults aging ‘with’ and ‘into’ paralysis: epidemiological analyses of demography and health. Disabil Health J 2016; 9: 575–583. [DOI] [PubMed] [Google Scholar]
- 41.He W, Goodkind D, Kowal P. An aging world: 2015 international population reports, Washington DC: U.S. Census Bureau, International Population Reports, 2016. [Google Scholar]
- 42.Abajobir AA, Abate KH, Abbafati C, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botter A, Oprandi G, Lanfranco F, et al. Atlas of the muscle motor points for the lower limb: implications for electrical stimulation procedures and electrode positioning. Eur J Appl Physiol 2011; 111: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 44.Koutsou AD, Moreno JC, del Ama AJ, et al. Advances in selective activation of muscles for non-invasive motor neuroprostheses. J Neuroeng Rehabil 2016; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailes AF, Caldwell C, Clay M, et al. An exploratory study of gait and functional outcomes after neuroprosthesis use in children with hemiplegic cerebral palsy. Disabil Rehabil 2016; 8288: 1–9. [DOI] [PubMed] [Google Scholar]
- 46.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair 2007; 21: 207–215. [DOI] [PubMed] [Google Scholar]
- 47.Batavia, Andrew I, Hammer GS. Toward the development of consumer-based criteria for the evaluation of assistive devices. J Rehabil Res 1990; 27: 425–436. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H, Lu Y, Chen W, et al. Stimulating the comfort of textile electrodes in wearable neuromuscular electrical stimulation. Sensors 2015; 15: 17241–17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssen TWJ, Raaijmakers K, Van Oost I, et al. Energy expenditure during rest and ADL increased by electrical stimulation-induced leg muscle activation, La Grande Motte, France: IFESS, 2016. [Google Scholar]
- 50.Keller T, Popovic MR, Pappas IPI, et al. Transcutaneous functional electrical stimulator “compex motion.” Artif Organs 2002; 26: 219–223. [DOI] [PubMed]
- 51.Vargas Luna JL, Krenn M, Cortés Ramírez JA, et al. Dynamic impedance model of the skin-electrode interface for transcutaneous electrical stimulation. PLoS One 2015; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang K, Meadmore K, Freeman C, et al. Development of user-friendly wearable electronic textiles for healthcare applications. Sensors 2018; 18: 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hebert DA, Bowen JM, Ho C, et al. Examining a new functional electrical stimulation therapy with people with severe upper extremity hemiparesis and chronic stroke: a feasibility study. Br J Occup Ther 2017; 80: 651–659. [Google Scholar]
- 54.Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord Off J Int Med Soc Paraplegia 2008; 46: 255–274. [DOI] [PubMed] [Google Scholar]
- 55.Kabel A, Dimka J, McBee-Black K. Clothing-related barriers experienced by people with mobility disabilities and impairments. Appl Ergon 2017; 59: 165–169. [DOI] [PubMed] [Google Scholar]
- 56.Smit CAJ, de Groot S, Stolwijk-Swuste JM, et al. Effects of electrical stimulation on risk factors for developing pressure ulcers in people with a spinal cord injury. Am J Phys Med Rehabil 2016; 95: 535–552. [DOI] [PubMed] [Google Scholar]
- 57.Deley G, Denuziller J, Casillas J-MM, et al. One year of training with FES has impressive beneficial effects in a 36-year-old woman with spinal cord injury. J Spinal Cord Med 2015; 0268: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbons RS, Stock CG, Andrews BJ, et al. The effect of FES-rowing training on cardiac structure and function: pilot studies in people with spinal cord injury. Spinal Cord 2016; 54: 822–829. [DOI] [PubMed] [Google Scholar]
- 59.Gibbons RS, Beaupre GS, Kazakia GJ. FES-rowing attenuates bone loss following spinal cord injury as assessed by HR-pQCT. Spinal Cord Ser Cases 2016; 2: 15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frotzler A, Coupaud S, Perret C, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 2008; 43: 169–176. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida T, Masani K, Sayenko DG, et al. Cardiovascular response of individuals with spinal cord injury to dynamic functional electrical stimulation under orthostatic stress. IEEE Trans Neural Syst Rehabil Eng 2013; 21: 37–46. [DOI] [PubMed] [Google Scholar]
- 62.Smith-Jackson TL, Nussbaum MA, Mooney AM. Accessible cell phone design: development and application of a needs analysis framework. Disabil Rehabil 2003; 25: 549–560. [DOI] [PubMed] [Google Scholar]
- 63.Shah SGS, Robinson I, AlShawi S. Developing medical device technologies from users’ perspectives: a theoretical framework for involving users in the development process. Int J Technol Assess Health Care 2009; 25: 514–521. [DOI] [PubMed] [Google Scholar]
- 64.Yang K, Freeman C, Torah R, et al. Screen printed fabric electrode array for wearable functional electrical stimulation. Sensors Actuat A Phys 2014; 213: 108–115. [Google Scholar]
- 65.Oh KW, Park HJ, Kim SH. Stretchable conductive fabric for electrotherapy. J Appl Polym Sci 2003; 88: 1225–1229. [Google Scholar]
- 66.Sayenko DG, Nguyen R, Popovic MR, et al. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources. Eur J Appl Physiol 2014; 114: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carpaneto J, Micera S, Zaccone F, et al. A sensorized thumb for force closed-loop control of hand neuroprostheses. IEEE Trans Neural Syst Rehabil Eng 2003; 11: 346–353. [DOI] [PubMed] [Google Scholar]
- 68.Braz GGP, Russold M, Davis GGM. Functional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristics. Neuromodul Technol Neural Interf 2009; 12: 180–190. [DOI] [PubMed] [Google Scholar]
- 69.Shimada Y, Sato K, Matsunaga T, et al. Closed-loop control using a stretch sensor for restoration of standing with functional electrical stimulation in complete paraplegia. Tohoku J Exp Med 2001; 193: 221–227. [DOI] [PubMed] [Google Scholar]
- 70.Kawai H, Bellman MJ, Downey RJ, et al. Tracking control for FES-cycling based on force direction efficiency with antagonistic bi-articular muscles. In: American control conference, Portland (OR), USA, 4 June-6 June 2014, pp.5484–5489. Piscataway, NJ: IEEE, pp.5484–5489. Piscataway: IEEE, 2014.
- 71.Lynch CL, Popovic MR. A comparison of closed-loop control algorithms for regulating electrically stimulated knee movements in individuals with spinal cord injury. IEEE Trans Neural Syst Rehabil Eng 2012; 20: 539–548. [DOI] [PubMed] [Google Scholar]