To the Editor:
First approved in 2010 for treatment of metastatic melanoma, immune checkpoint inhibitors (ICIs) are now approved for more than 17 cancer types, and it is estimated that 1 in 3 patients with cancer now qualify for ICIs.1 ICIs remove key regulators of T-cell function to unleash antitumor effects, but T-cell disinhibition can also cause immune-related adverse events in up to 60%–85% of patients.2 Though the epidemiology of ICI-induced acute kidney injury has been well characterized,3–5 there is extremely limited data on the long-term toxicities of ICIs. We sought to determine annual changes in kidney function in patients surviving at least 1 year by conducting a retrospective cohort study including all adults who received ICIs at Mass General Brigham in 2010–2018 with follow-up until December 31, 2019.
The primary outcome was a composite of new-onset CKD (defined by eGFR <60 mL/min/1.73 m2 for >90 days), a sustained ≥30% decline in eGFR from baseline for >90 days with no interim eGFR reflecting a <20% decline from baseline, or kidney replacement therapy (KRT).6 A ≥30% decline in eGFR was chosen based on data indicating this is predictive of future need for KRT.7 As a secondary outcome, we defined “rapid eGFR decline” as >3 mL/min/1.73 m2 per year decline, which is associated with kidney failure and adverse cardiovascular outcomes.8,9 Data on cancer type, ICI type and start date, comorbidities, medications, laboratory values, and prior nephrotoxic cancer therapies used were obtained from electronic medical records (Item S1).
Cox proportional hazard models were performed to identify predictors of the primary outcome; patients were censored at death or loss to follow-up. We examined traditional risk factors associated with CKD progression as well as cancer-related factors; final model selection was guided by a combination of clinical plausibility and Akaike and Bayesian information criteria.
We identified 5,934 adult patients who began treatment with ICIs in 2010–2018. After exclusion of 844 patients (14%) without baseline or at least 1 repeat creatinine, 72 (1%) who were enrolled in a placebo-controlled trial involving ICIs, and 14 (0.2%) with kidney failure, 5,004 (84%) remained. Of these, 2,563 (51%) survived ≥1 year and were included in the primary analysis. Baseline characteristics are shown in Table 1. Patients were followed for a median of 688 (interquartile range [IQR], 496–1,031) days and had a median of 20 (IQR, 13–29) creatinine measurements per year.
Table 1.
Baseline Characteristics
| Characteristic | All Patients (N = 5,004) | Patients Surviving ≥1 Year (n = 2,563) |
|---|---|---|
| Demographics | ||
| Age, years | 64 ± 13 | 63 ± 13 |
| Male sex | 2,699 (54%) | 1,377 (54%) |
| Race/ethnicity | ||
| White, non-Hispanic | 4,529 (91%) | 2,356 (92%) |
| Black, non-Hispanic | 112 (2%) | 47 (2%) |
| Asian | 157 (3%) | 59 (2%) |
| Hispanic | 51 (1%) | 28 (1%) |
| Other or unknown | 155 (3%) | 73 (3%) |
| Coexisting conditions a | ||
| Hypertension | 2,930 (59%) | 1,479 (58%) |
| Diabetes mellitus | 819 (16%) | 427 (17%) |
| Cirrhosis | 78 (2%) | 36 (1%) |
| Chronic obstructive pulmonary disease | 515 (10%) | 227 (9%) |
| Coronary artery disease | 1,168 (23%) | 605 (24%) |
| eGFR category | ||
| ≥90 mL/min/1.73 m2 | 1,956 (39%) | 959 (37%) |
| 60–89 mL/min/1.73 m2 | 2,249 (45%) | 1,195 (47%) |
| 45–59 mL/min/1.73 m2 | 745 (15%) | 384 (15%) |
| <45 mL/min/1.73 m2 | 52 (1%) | 25 (1%) |
| Baseline medications | ||
| ACEI/ARB | 1,390 (28%) | 670 (26%) |
| Uric acid-lowering agent | 243 (5%) | 93 (4%) |
| NSAIDs | 2,499 (50%) | 1,256 (49%) |
| Diuretics | 1,315 (26%) | 544 (21%) |
| Nephrotoxic antineoplastic therapies | 2,081 (42%) | 889 (35%) |
| Histamine 2 receptor blockade | 2,616 (52%) | 1,212 (47%) |
| Proton pump inhibitors | 2,127 (43%) | 929 (36%) |
| ICI class | ||
| PD1 | 3,720 (74%) | 1,846 (72%) |
| PDL1 | 519 (10%) | 283 (11%) |
| CTLA4 | 355 (7%) | 205 (8%) |
| Combination CTLA4/PD1 | 410 (8%) | 229 (9%) |
| Cancer type | ||
| Lung | 1,706 (34%) | 723 (28%) |
| Melanoma | 1,157 (23%) | 810 (32%) |
| Head and neck | 506 (10%) | 241 (9%) |
| Genitourinary | 365 (7%) | 241 (9%) |
| Gynecologic | 317 (7%) | 153 (6%) |
| Gastrointestinal | 243 (5%) | 74 (3%) |
| Blood | 196 (4%) | 98 (4%) |
| Breast | 162 (3) | 64 (3) |
| Sarcoma | 86 (2%) | 38 (1%) |
| Other | 266 (5%) | 119 (5%) |
Age given as mean ± standard deviation; all other values as count (%). Baseline comorbidities (defined using ICD-9/ICD-10 codes) and medications were determined at the time of ICI initiation. Nephrotoxic chemotherapies had been prescribed within 1 year prior to ICI start. Full list of nephrotoxic agents are shown in Table S3. Blood cancers included myeloma, lymphoma, and leukemia. Abbreviations and definitions: ACEI/ARB, angiotensin-converting enzyme inhibitor or angiotensin receptor blockade; CTLA4, cytotoxic T lymphocyte–associated antigen; eGFR, estimated glomerular filtration rate (calculated by CKD-EPI equation); ICI, immune checkpoint inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs; PD1, programmed death 1; PDL1, programmed death ligand 1.
Baseline comorbidities (defined using ICD-9/ICD-10 codes) and medications were determined at the time of ICI initiation.
Among the 2,563 patients, 334 (13%) developed the primary composite outcome (new-onset CKD, 30% eGFR decline, or KRT) at a median of 471 (IQR, 304–782) days after the first ICI dose; the incidence rate was 6.34 per 100 patient-years. The cumulative incidence of the primary composite outcome and death are shown in Fig 1A. The rate of the primary composite outcome by year survived post-ICI is shown in Fig S1. Independent predictors of the primary outcome included age (AHR per 5 years, 1.13 [95% CI, 1.07–1.20]) and proton pump inhibitor use (AHR, 1.38 [95% CI, 1.10–1.73]). ICI class was not associated with the primary outcome (Fig 1B, Table S1). Rapid eGFR decline was also common, affecting 35% of those who survived ≥3 years (Table S2). Analysis of eGFR slope pre- versus post-ICI initiation among patients who had ≥1 year of baseline data prior to ICI initiation and who survived ≥1 year following ICI initiation (n = 1,335) showed significantly faster eGFR decline after ICI initiation (1.4 vs 3.7 mL/min/1.73 m2 per year average decline prior to vs after ICI, P = 0.01, Fig S2).
Figure 1.
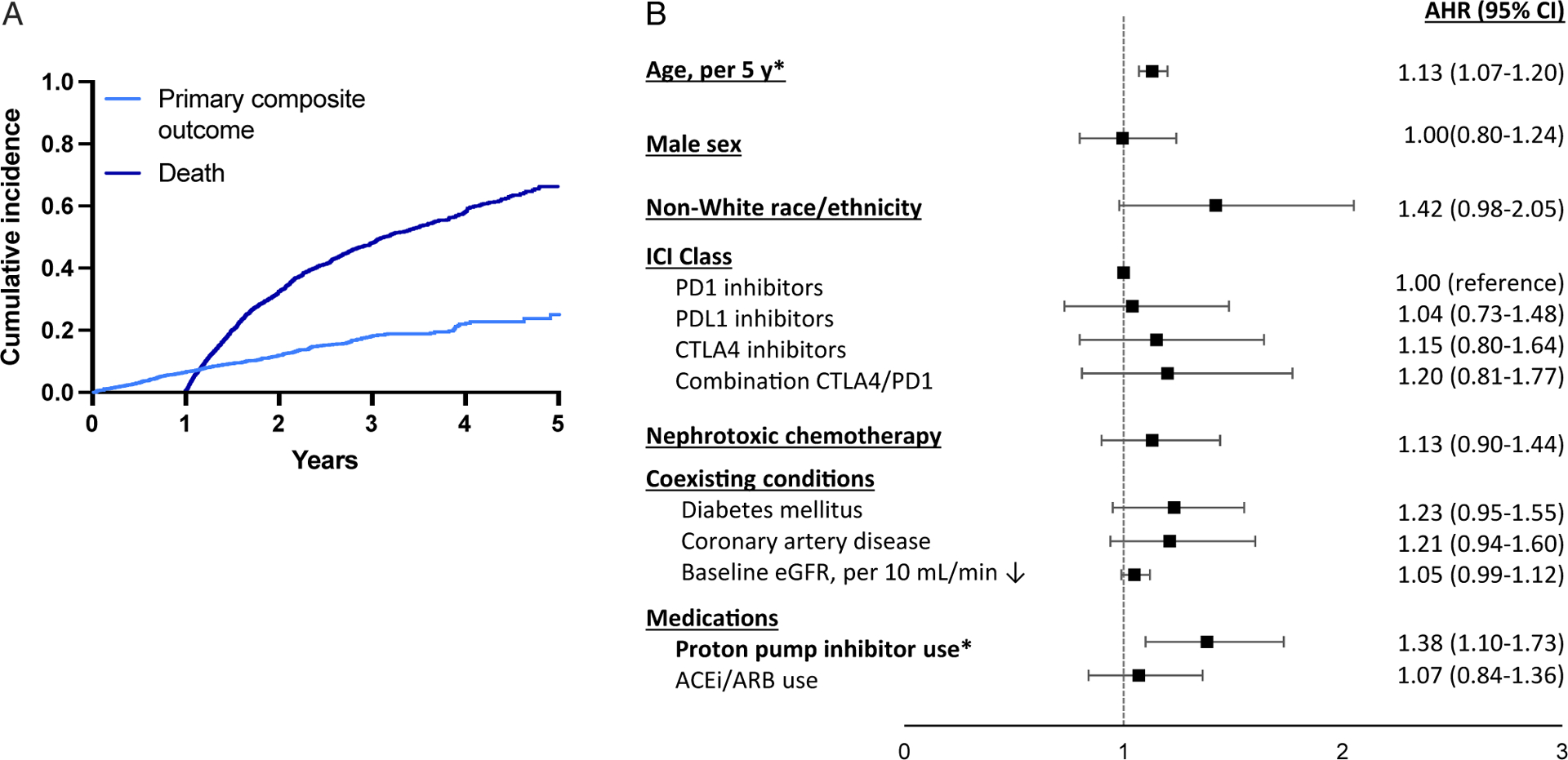
Cumulative incidence and predictors of new-onset chronic kidney disease (CKD) or a sustained 30% eGFR decline among 2,563 patients surviving ≥1 year. (A) Cumulative incidence function of new-onset CKD or a sustained 30% eGFR decline and death. (B) Cox proportional hazard model estimates of the association (as adjusted hazard ratio [AHR]) between baseline characteristics and the primary composite outcome of new-onset CKD or 30% decline in eGFR. Death determined by chart review or set as 45 days after the last laboratory datapoint in patients without laboratory studies for >6 months. Univariable model shown in Table S1. *Statistically significant.
This retrospective cohort study of kidney function in 2,563 patients who survived ≥1 year after treatment with ICIs shows that incident CKD or a clinically significant eGFR decline is common. The robustness of this finding is supported by the high incidence of rapid eGFR decline and results of multiple sensitivity analyses (Item S1, Table S4–S6). Our study is limited by the lack of a control group, which is absent because the very limited survival in many metastatic cancers prior to ICIs make it challenging to find a well-matched population with the potential for long-term survival. Using patients as their own controls by looking at pre- and posttreatment eGFR slope, we observed substantially faster eGFR decline after treatment. Because the rapid expansion of ICI use only began in 2015, the number of patients with >4 years follow-up is small; our findings will need to be validated in this and other cohorts with longer follow-up. Our study has important implications for patients treated with ICIs: a subset of survivors may experience significant eGFR decline that may contribute to long-term health consequences and may limit the use or dosage of other anticancer drugs and eligibility for clinical trial enrollment. We identified age and use of proton pump inhibitors as important risk factors for new-onset CKD and sustained 30% eGFR decline; both have previously been shown to be risk factors for AKI patients with cancer.3,10 Future studies will be needed to determine the pathophysiology of kidney function decline after ICIs and evaluate strategies to slow eGFR decline.
Supplementary Material
Support:
MES is supported by National Institutes of Health (NIH) K23 DK 117014 and the Claflin Distinguished Scholars Award. TGN is supported by NIH R01HL137562, R01HL130539, and K24HL150238, and, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg. DEL is supported by NIH R01HL144566 and R01DK125786. The NIH had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure:
LZ serves as a consultant for Merck. TGN has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, H3-Biomedicine, and AbbVie, outside of the current work. TGN also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to ICIs and has received grant funding from AstraZeneca. The remaining authors declare that they have no relevant financial interests.
Footnotes
Prior Presentation: A preprint version of this Research Letter was posted December 22, 2020 at medRxiv with doi 10.1101/2020.12.18.20248471.
References
- 1.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 3.Seethapathy H, Zhao S, Chute DF, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seethapathy H, Zhao S, Strohbehn IA, et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int Rep. 2020;5(10):1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. Clinical features and outcomes of immune checkpoint inhibitorassociated AKI: a multicenter study. J Am Soc Nephrol. 2020;31(2):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siew ED, Abdel-Kader K, Perkins AM, et al. Timing of recovery from moderate to severe AKI and the risk for future loss of kidney function. Am J Kidney Dis. 2020;75(2):204–213. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168(20):2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Coresh J, Ballew SH, et al. Past decline versus current eGFR and subsequent ESRD risk. J Am Soc Nephrol. 2016;27(8):2447–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitchlu A, McArthur E, Amir E, et al. Acute kidney injury in patients receiving systemic treatment for cancer: a population-based cohort study. J Natl Cancer Inst. 2019;111(7):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


