Abstract
The immune checkpoint inhibitors (ICIs), by targeting cytotoxic-T-lymphocyte-associated protein 4, programmed cell death 1 (PD-1), or PD-ligand 1, have dramatically changed the natural history of several cancers, including non–small cell lung cancer (NSCLC). There are unusual response manifestations (such as pseudo-progression, hyper-progression, and immune-related adverse events) observed in patients with ICIs because of the unique mechanisms of these agents. These specific situations challenge response and prognostic assessment to ICIs challenging. This review demonstrates how 18F-FDG PET/CT can help identify these unusual response patterns in a non-invasive and effective way. Then, a series of semi-quantitative parameters derived from 18F-FDG PET/CT are introduced. These indexes have been recognized as the non-invasive biomarkers to predicting the efficacy of ICIs and survival of NSCLC patients according to the latest clinical studies. Moreover, the current situation regarding the functional criteria based on 18F-FDG PET/CT for immunotherapeutic response assessment is presented and analyzed. Although the criteria based on 18F-FDG PET/CT proposed some resolutions to overcome limitations of morphologic criteria in the assessment of tumor response to ICIs, further researches should be performed to validate and improve these assessing systems. Then, the last part in this review displays the present status and a perspective of novel specific PET probes targeting key molecules relevant to immunotherapy in prediction and response assessment.
Keywords: PET/CT, FDG, immunotherapy, immune checkpoint inhibitors (ICI), lung cancer, NSCLC, response, prognosis
1 Introduction
Immune checkpoint inhibitors (ICIs), by targeting cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), or PD-ligand 1 (PD-L1), have yielded durable anti-tumor responses and long-term remissions in non–small cell lung cancer (NSCLC) and other cancer types. Consequently, the clinical importance and use of ICIs have skyrocketed.
ICIs reactivate the patient’s immune system through T lymphocytes against tumor cells. This mechanism differs from that of cytotoxic or targeted agents, which could result in the development of inflammation at the tumor sites and subsequent anti-tumor responses (Missailidis, 2008). These responses are of unique patterns, pseudo-progression, hyper-progression (HPD), long duration of response, and disease regression, which continue after treatment discontinuation, especially pseudo-progression and HPD, which can influence the treatment evaluation in many clinical cases and predict the entirely reverse prognosis. Moreover, such an increased immune system activity can activate T immune cells in a variety of normal tissues, causing autoimmune side effects, also known as immune-related adverse events (irAEs).
Therefore, the novel manifestations, previously seldom seen during conventional therapies and frequently described under immunotherapy, have challenged responsive and prognostic assessment to ICIs. Nevertheless, a substantial number of patients still do not derive benefit from checkpoint inhibitors. Clinical data showed that the responding rate of ICIs is closely correlated with biomarker expression in tumor specimens, for example, PD-L1 status (Borghaei et al., 2015; Herbst et al., 2016; Reck et al., 2016), tumor mutational burden (TMB) (Galvano et al., 2021), microsatellite instability-high (MSI-H) (Gregg et al., 2019; Zhao et al., 2019), and mismatch repair-deficient (MMR-deficient) (Gregg et al., 2019; Zhao et al., 2019). In particular, PD-L1 expression and TMB have been recognized as the valuable predictive factors for NSCLC patients with ICIs: a recent meta-analysis demonstrated that NSCLC patients with high PD-L1 expression did benefit the most from a single-agent ICI treatment in the first-line setting (Passiglia et al., 2021), and another meta-analysis resulted in a proven benefit in overall survival (OS) in favor of ICIs in the TMB-high NSCLC population (Galvano et al., 2021). MSI-H and MMR-deficient are proposed as the predictors for anti-PD-1/anti-PD-L1 immunotherapy efficacy in colorectal cancer (Zhao et al., 2019). Given that MSI-H lung cancers are extremely rare, MSI testing/MMR immunohistochemistry (IHC) is not considered as routine in lung cancer closely associated with high expression of PD-1/PD-L1 and shows durable responses to PD-1 blockade (Gregg et al., 2019). However, the heterogeneity within tumors limits the effectiveness of predictive value of these biomarkers based on IHC and next-generation sequencing (NGS) of tumor specimens. Additionally, since not all advanced NSCLC patients are suitable for tumor biopsy, biopsy specimens are not always readily available for the pathological assay. Thus, non-invasive biomarkers are urgently needed for the prediction of potential responsiveness of ICIs.
2-Deoxy-2-(18F)-fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) takes advantage of non-invasively evaluating the glucose metabolic level to determine the tumor burden all over the body. Parameters derived from 18F-FDG PET/CT, relevant to glucose metabolism on the molecular level, are demonstrated to be correlated with the treatment response and survival, which shows a potential capability of recognizing and tracing biomarkers related to responsiveness and prognosis. Therefore, 18F-FDG PET/CT has been widely used to determine the tumor stage and drug efficacy including the responsiveness of ICIs in NSCLC patients (Na et al., 2017; Liao et al., 2020).
Meanwhile, new positron molecular probes targeting key molecules associated with immunotherapy have been developed and would be used to improve the assessing specificity and effectiveness for NSCLC patients treated with immunotherapy.
Thus, this review summarizes the potential applications of 18F-FDG PET/CT as a non-invasive method for the prediction and tracking of responsiveness and prognosis of ICIs in NSCLC patients. A perspective of novel PET molecular probes in immunotherapy prediction and response assessment is also discussed.
2 Manuscript
2.1 18F-FDG PET/CT
2.1.1 Identification of Unusual Response Patterns
The mechanism of ICIs leads to unique manifestations during and after immunotherapy. Pseudo-progression and HPD belong to the unusual response patterns, which occur more frequently in ICIs than in other treatments (chemotherapy, target treatment, angiogenic inhibitors, etc.) (Iacovelli et al., 2015; Mellema et al., 2015; Barrón et al., 2018; Ferrara et al., 2018; Zhou et al., 2020).
Immune system-related pseudo-progression was first described in melanoma patients receiving ipilimumab (an anti-CTLA-4 antibody) in 2008. Pseudo-progression is described as a process with an increase in tumor size or metabolic tumor burden or the appearance of new lesions before the occurrence of a subsequent decrease or stability (Costa et al., 2021). Among patients with advanced NSCLC, the rate of pseudo-progression is 3–5% (Gettinger et al., 2015; Ferrara et al., 2018; Fujimoto et al., 2019), which is lower than that among patients with melanoma, for whom the equivalent rate was reported as up to 10% (Hodi et al., 2008). Besides the primary manifestations above (varying tumor size and metabolic tumor burden, emergence of new lesions), other rare forms of pseudo-progression include pleural or pericardial effusion (Kanazu et al., 2018; Song et al., 2019), intestinal perforation (Kim et al., 2019), and brain pseudo-progression (Melian et al., 2018). The certain mechanisms of pseudo-progression are unknown. There are some hypotheses trying to explain this pattern, including delayed and weaker activation of the immune system, immune cell infiltration, intracellular and vasogenic edema, and inflammation and intra-tumor hemorrhage (Fujimoto et al., 2019) (Gerwing et al., 2019). The chronic activation of immune system results in delay control of tumor growth, which can be shown as the elevation of FDG uptake in tumor sites with or without increase in tumor size and appearance of new lesions during the early period after administration of ICI agents. In contrast, immune cell infiltration and activation by checkpoint blockade antibodies influence the tumor immune microenvironment and then might promote the glycolysis of cancer cells, leading to the increase in FDG uptake at the early treatment phase (Tomita et al., 2018; Tomita et al., 2020). In terms of the diverse hypotheses for pseudo-progression, the mechanisms of glucose-metabolic elevation are completely different, but the FDG-uptake increase and the subsequent decrease or stability indeed can be observed through multiple PET/CT scans to help distinguish pseudo-progression from other responses. It is reported that pseudo-progression could occur between weeks 4 and 20 from baseline (Evangelista et al., 2019; Costa et al., 2021) and this time interval from baseline to pseudo-progression tends to be shorter than that from baseline to response. Additionally, patients with pseudo-progression would experience a markedly superior survival to those with normal response situations (Kurra et al., 2016; Solinas et al., 2017; Zhou et al., 2020). Thus, imaging reassessment is suggested after 4–8 weeks (never more than 12 weeks) (Costa et al., 2021).
HPD was depicted as a process of expansive growth or change in the rate of tumor progression that is grossly different from baseline, causing a detrimental effect on the patients. It is another atypical response pattern of ICIs and becomes a big challenge in clinical practices because of the higher incidence and worse prognosis. HPD’s molecular mechanisms are still unclear. Champiat et al. concluded the following probable explanations: 1) expansion of regulatory T cells; 2) exhaustion of compensatory T cells; 3) modulation of protumorigenic immune cell subsets; 4) oncogenic pathway activation; and 5) activation of aberrant inflammation (Champiat et al., 2018). The expansive growth and proliferation of tumors inevitably induce the raising of tumoral glucose consumption leading to the rapid and sustained increase in FDG uptake. The incidence of HPD in NSCLC patients is about 5–19.2% (Ferrara et al., 2018), and HPD could be apparently associated with a poor prognosis (Ferrara et al., 2018). Multi-center retrospective research with 406 advanced NSCLC patients demonstrated patients experiencing HPD treated with ICIs had significantly lower overall survival (OS) compared with patients with normal progressive disease (median OS, 3.4 months [95% confidence interval (CI), 2.8–7.5 months] vs. 6.2 months (95% CI, 5.3–7.9 months); hazard ratio, 2.18 [95% CI, 1.29–3.69]; P = 0.003). Nevertheless, it is difficult to differentiate HPD from other response patterns within 8 weeks of starting treatments and the second early imaging evaluation is proposed after this period (more than 8 weeks after immunotherapy) to properly adjust therapeutic programs (Costa et al., 2021).
In fact, there is no consensus on the definitions for pseudo-progression and HPD, and their molecular mechanisms are still unclear. The range of their incidences is quite wide, which reflects the heterogeneity of cancer subtypes and therapies (type of agents and dose).
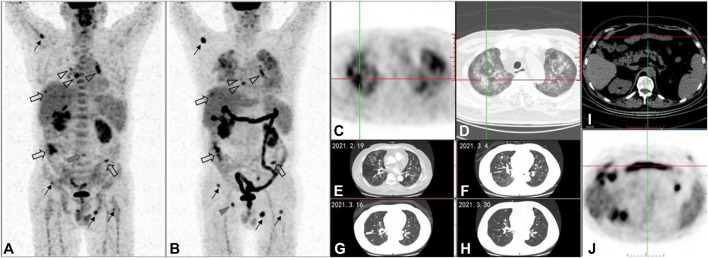
Reactivation of the immune system through the use of ICIs has led to the development of a new series of side effects named irAEs. The precise pathophysiology underlying irAEs is not clearly understood. Due to the whole immunity enhancement, irAEs can potentially occur in any system of the whole body and can vary in involved systems, incidence, and severity of grade according to each particular agent and its dose and tumor types. irAEs commonly manifest within the first 3 months of administration for most drugs and can also occur at any time during treatment, even after discontinuation of ICI therapy (Costa et al., 2021). These effects could be mistaken as the disease progression, including HPD resulting in inappropriate strategies. Although most irAEs are mild and can be managed through transient immunosuppression with corticosteroids, high-grade events often require hospitalization and specialized treatment because some of them are life-threatening. In addition, multiple studies reported that patients who experienced irAEs demonstrate marked improvements in progression-free survival (PFS), OS, and overall response rate compared to those lacking toxicity (Das and Johnson, 2019). Therefore, early and accurate identification of irAEs is essential to aid in the diagnosis and management of patients and to reduce associated morbidity. Some effects can be apparent on 18F-FDG PET/CT imaging. A correct interpretation of 18F-FDG PET/CT images could be complicated for imaging manifestations of the unusual patterns and adverse reactions. However, the whole-body scan is also essential to discover these situations and know their characteristic localizations, especially when some effects are not always associated with the presence of clinical symptoms and signs. Thus, PET imaging with 18F-FDG can ensure close clinical monitoring and medical intervention when necessary to avoid serious complications. In some situations, imaging features from 18F-FDG PET/CT can effectively improve the diagnosis of particular effects. Taking the intestinal adverse effect for example, after carefully considering and ruling out other possibilities such as inflammatory bowel disease or continuing metformin before undergoing PET/CT, diffuse or segmental FDG uptake in bowel with or without thickening wall could be recognized as the toxicity of ICIs (Figure 1). Additionally, multiple scans (before, during, and after treatment) based on assessment criteria (see 2.1.3) can further contribute to distinguishing among these situations. Table 1 displays the common irAEs, including the features in PET/CT to help to recognize these effects.
FIGURE 1.
A 58-year-old male patient with metastatic intrahepatic cholangiocarcinoma was treated with the third line of ipilimumab. After four cycles of treatment, compared with 18F-FDG PET/CT maximum intensity projection (MIP) image (A) before treatment, the posttreatment 18F-FDG PET/CT (B-D), (I,J) demonstrates the following: 1) multiple soft tissue density nodules in the peritoneum, which were larger in volume and higher in FDG uptake than before ((A,B) hollow arrows); 2) multiple nodules without FDG uptake in both lungs, some of which are larger in volume than before and some of which have no change compared with before (cannot be shown in MIP images- (A,B)); 3) the lymph nodes with increased FDG uptake in mediastinal areas and bilateral hilar, which were smaller in volume and FDG-uptake lower than before ((A,B) hollow triangles); and 4) multiple foci of increased FDG uptake in bones: the FDG-uptake degree in vertebral body of lumbar 4 was significantly lower than before ((A) gray triangle); the right acetabular lesion was the new lesion ((B) gray triangle); the remaining ostial lesions were higher than those before ((A,B) black line arrows). According to PERCIMT criteron: SMD was confirmed for the number of the newly FDG-positive lesions is less than 4. Meanwhile, in the light of imPERCIST5, PMD was also evaluated for the sum of SULpeak of the patient’s top five target lesions after treatment was more than 30% higher than that of the top five target lesions before treatment. As a result of clinical follow-up, the patient was confirmed PD and those lesion above were validated as metastases. PD was determined for the appearance of new lesion based on PECRIT. PMD was determined for new FDG-avid lesion at SCAN-2, which can be considered as UPMD in the line with iPERCIST. But the patient didn’t receive the next 18F-FDG PET/CT between days 21 and 28 after treatment or 4–8 weeks later, so it could not be evaluated according to PECRIT and iPERCIST. Follow-up results showed the PFS for ICI was 6 months. Additionally, this posttreatment PET/CT displayed: 1) newly patchy ground glass density foci in both lungs, with increased FDG uptake (C- PET axial image, D-CT axial image); 2) diffuse increased FDG uptake in ascending colon, transverse colon, descending colon, sigmoid colon and rectum (i-PET axial image) which is new compared with the previous one, and the corresponding colonic walls were not significantly thickened ((J)-CT axial image). The patchy ground glass shadows of both lungs gradually disappeared during chest CT follow-up ((E–H)-CT axial images). Combined with clinical information, the patchy ground glass shadows of both lungs were diagnosed as immunerelated pneumonia. Under the administration of MDT, the diffuse increase of glucose metabolism in the colon was considered as immune-related colitis.
TABLE 1.
Common immune-related adverse events.
| irAEs | Incidence in NSCLC | Symptoms, signs, and laboratory test | Susceptibility factors | Imaging features | Intervention |
|---|---|---|---|---|---|
| Gastrointestinal toxicity | Anti-PD-1: 8% (Davies and Duffield, 2017) | Diarrhea, abdominal pain, vomiting, fever, and hematochezia (Sosa et al., 2018) | Diffuse or segmental bowel wall thickening, with increased enhancement and FDG uptake (Costa et al., 2021) | Symptomatic treatment, including oral hydration, oral or intravenous corticosteroids, and immunosuppressive agents; discontinuation of ICIs in severe cases (Sosa et al., 2018) | |
| Dermatologic toxicity | Anti-PD-1: 9% (Davies and Duffield, 2017; Sosa et al., 2018) | Rash, pruritus, vitiligo, photosensitivity reactions, and xerosis cutis (Sosa et al., 2018) | Rarely shown at CT, MR, and PET/CT (Costa et al., 2021) | Symptomatic treatment, including topical corticosteroids and oral antihistamines (Sosa et al., 2018) | |
| Endocrine toxicity | |||||
| Thyroiditis | Anti-PD-1: hyperthyroidism 1–8%, hypothyroidism 4–9% (Davies and Duffield, 2017) | Asymptomatic or with symptoms and signs of short-term hyperthyroidism and subsequent temporary or permeant hypothyroidism (Sosa et al., 2018) | Female, more cycles of ICIs (Davies and Duffield, 2017) | Normal or diffuse FDG uptake (Costa et al., 2021) | Monitor thyroid function monthly or every two cycles during ICI; temporary or persistent hormone replacement therapy, but the ICI may be continued (Sosa et al., 2018) |
| Anti-PD-L1: hyperthyroidism 1%, hypothyroidism 4% (Davies and Duffield, 2017) | |||||
| Hypophysitis | Anti-PD-1: ≤ 1% (Sosa et al., 2018) | Fatigue, headache, and visual field changes; the abnormality of relevant hormone (Sznol et al., 2017) | Normal or diffuse FDG and uptake with or without the swollen size (Sznol et al., 2017) | Hormone replacement therapy, but the ICI may be continued (Sosa et al., 2018) | |
| Adrenalitis | Rare (only case report) (Sznol et al., 2017) | Fatigue, postural dizziness, orthostatic hypotension, anorexia, weight loss, and abdominal discomfort; the abnormality of relevant hormone (Sznol et al., 2017) | Bilateral mild and diffuse gland enlargement with FDG uptake (Sznol et al., 2017) | Stress-dose and emergency corticosteroid administration when PAI is confirmed; long-term glucocorticoid and mineralocorticoid replacement (Sznol et al., 2017) | |
| Hepatotoxicity | Anti-PD-1: 2% (Davies and Duffield, 2017) | Asymptomatic increase of ALT, AST, or total bilirubin (Sosa et al., 2018) | patients with prior autoimmune disease (Sosa et al., 2018) | Hepatomegaly, periportal edema, or heterogeneous liver enhancement, with or without diffuse FDG uptake (Costa et al., 2021) | Monitor transaminases and bilirubin twice a week during ICI. Oral corticosteroids when LFTs remain elevated after 1-2 weeks and re-starting the ICI once LFTs have improved and steroid has been tapered (Sosa et al., 2018) |
| Pancreatitis | 4% (Sosa et al., 2018) | Elevated levels of pancreatic enzymes with or without abdominal pain, nausea, and vomiting (Sosa et al., 2018) | Diffuse pancreatic enlargement with FDG uptake at PET with or without peripancreatic fat stranding (Costa et al., 2021). | Routine monitoring of amylase and lipase is not recommended; symptomatic mild elevations of these enzymes should not be treated (Sosa et al., 2018) | |
| Pulmonary toxicity | Anti-PD-1: 3–6%; anti-PD-L1: 4% (Davies and Duffield, 2017) | Highly variable in extent of severity (Sosa et al., 2018) | Male former smokers, previous lung radiation therapy, lung fibrosis (Davies and Duffield, 2017) | 1. COP: multifocal GGOs and consolidations with a predominantly peripheral distribution; 2. NSIP: mild GGOs with a tendency for peripheral distribution; 3. HP: diffuse mild GGOs and centrilobular nodules; 4. AIP/ARDS: diffuse GGOs, consolidations, and lung volume loss | Systemic treatment with corticosteroids and antibiotics and withholding ICI treatment with 2–4 grades (Sosa et al., 2018) |
| Asymptomatic or dry cough, fever, chest pain, progressive dyspnea, and fine inspiratory crackles (Sosa et al., 2018) | Such pulmonary opacities can show different intensities of FDG uptake with or without mediastinal lymphadenopathy and pleural effusions (Costa et al., 2021) | ||||
| Nephrotoxicity | Anti-PD-1: 1–3% (Davies and Duffield, 2017) | Asymptomatic elevation of creatinine (Sosa et al., 2018) | Rarely shown at CT, MR, and PET/CT. | Stopping any concomitant nephrotoxic drugs; evaluating etiologies; corticosteroid treatment and withholding ICI (Sosa et al., 2018) | |
| Neurological toxicity | Anti-PD-1: < 1% (Davies and Duffield, 2017) | Polyneuropathy, facial paralysis, optic neuritis, GBS, myasthenia gravis, transverse myelitis, encephalitis, and aseptic meningitis (Sosa et al., 2018) | Imaging examinations, especially MR to rule out metastasis (Sosa et al., 2018) | Steroid treatment; higher doses or other procedures (e.g., intravenous immunoglobulin for GBS) might be required for more severe toxicity (Sosa et al., 2018) | |
| Cardiotoxicity | Myocarditis 0.27% of anti-CTLA-4, 0.06% of anti-PD-1 (Sosa et al., 2018) | Heart failure, cardiomyopathy, heart block, myocardial fibrosis, myocarditis, pericarditis, cardiomyopathy, and arrhythmias (Sosa et al., 2018) | Abnormal signal could be detected by MR. | Consultation with cardiologists and treatment with steroids (Sosa et al., 2018) | |
| Musculoskeletal and rheumatologic toxicity | Arthralgia: 43%, myalgia: 21%, arthritis/tenosynovitis: 1–7%, myositis/fasciitis: <1% (Sosa et al., 2018) | Arthralgia, myalgia, arthritis/tenosynovitis, myositis/fasciitis, rheumatoid arthritis, polymyalgia rheumatica, lupus erythematosus, and Sjögren syndrome (Sosa et al., 2018) | Joint effusion, synovial thickening, or tendon/muscle edema with increased enhancement and FDG uptake (Costa et al., 2021) | Symptomatic or low-dose steroids (Sosa et al., 2018) | |
| Sarcoidosis or sarcoid-like reaction | 5–7% (Nishino et al., 2018) | Asymptomatic (Nishino et al., 2018) | FDG avid enlarged lymph nodes of mediastinum, bilateral hilar, neck and abdomen; seldom manifestation: perilymphatic pulmonary nodules, focal lung consolidation, splenic or hepatic nodule (Nishino et al., 2018) | Holding ICIs without any specific treatment (Kelly et al., 2021) | |
irAEs: immune-related adverse events; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; PD-1: programmed cell death 1; PD-L1: PD-ligand 1; CT: computed tomography; MR: magnetic resonance; PET/CT: positron emission tomography/computed tomography; ICIs: immune checkpoint inhibitors; FDG: fluorodeoxyglucose; PAI: primary adrenal insufficiency; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LFTs: liver function tests; COP: cryptogenic organizing pneumonia; GGOs, ground-glass opacities; NSIP: nonspecific interstitial pneumonia; HP: hypersensitivity pneumonitis; AIP/ARDS: acute interstitial pneumonia/acute respiratory distress syndrome; GBS: Guillain–Barre syndrome.
The identification of pseudo-progression, HPD, and irAEs challenges the immunotherapy in NSCLC. According to the demonstrations above, during the early period of immunotherapy, the alterations of FDG uptake based on pseudo-progression and HPD may be similar. However, their mechanisms of the FDG uptake are entirely different. Additionally, some irAEs could further confound the diagnosis. Taking the sarcoidosis or sarcoid-like reaction, for example, these FDG avid enlarged lymph nodes may be misdiagnosed as lymph node metastases. Thus, the finds in 18F-FDG PET/CT imaging need to be compared with those of the subsequent PET/CT scans to confirm the kind of response (in 2.1.3, the assessment criteria based on 18F-FDG PET/CT displayed how to use PET/CT scans to confirm the response status). The quantitative parameters based on 18F-FDG PET/CT could help in differentiation (the role of parameters from 18F-FDG PET/CT in differentiation is shown in 2.1.2). As mentioned above, the recommended imaging assessment time differs among pseudo-progression, HPD, and irAEs. There is no uniform recommendation optimum time yet when those responses or events could be differentiated well. Thus, the complexity of these responses and events leads to the discernment process requiring a comprehensive analysis combined with multiple information such as clinical symptoms, signs, laboratory examinations, and imaging manifestations, which can be well implemented by the model of the multidisciplinary team (MDT) including nuclear medicine physicians.
2.1.2 Parameters in Prognostic Evaluation for Immunotherapy
Generally, there are three imaging assessing approaches based on 18F-FDG PET/CT: visual interpretation and estimation of relative uptake, assessment of uptake over a defined time using semi-quantitative methods, and assessment of uptake from the time of injection to a defined endpoint using kinetic analysis (Shankar et al., 2006). Visual assessment is of subjectivity, and the results could vary among readers. Additionally, FDG accumulation is influenced by uptake time, blood glucose concentration, and partial-volume effects (Shankar et al., 2006). The visual comparison among different scans is unsuitable and less rigorous for evaluation. Full kinetic quantitative analysis can provide an absolute rate for FDG metabolism, which is independent of imaging time and contributes to the investigation of various components of glucose metabolism, such as transport and phosphorylation. Nevertheless, because of the complexity of such an approach, including patient compliance issues and the requirement for arterial blood sampling or dynamic imaging of a blood-pool structure to obtain a precise input function, the kinetic analysis has been used infrequently for treatment evaluation of malignancy (Weber et al., 1999).
The semi-quantitative method is a relatively objective approach to evaluate the metabolic tumoral status. Semi-quantitative parameters—standardized uptake value (SUV)—have been used to determine tracer uptake in attenuation-corrected PET images in 18F-FDG PET/CT, mainly representing the extent of glucose metabolism. However, it was reported that SUV based on 18F-FDG PET/CT is associated with molecules relevant to some signaling pathways, for instance, the mTOR pathway. Previous studies have shown SUV of FDG is significantly associated with PD-L1 expression (Takada et al., 2017), and the activation of the protein kinase B (AKT)–mTOR pathway increases PD-L1 protein expression in NSCLC (Kaira et al., 2014). Then SUV from 18F-FDG PET/CT could be relevant to the prognosis of immunotherapy in NSCLC.
The maximum SUV(SUVmax) is widely used in clinical practices because of its high repeatability. However, SUVmax, only representing the highest metabolic point in the volume of interest (VOI) determination, ignores the intra-tumoral and inter-tumoral heterogeneity. Other semi-quantitative parameters such as mean SUV (SUVmean), peak SUV(SUVpeak), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) can reflect more information on biological behaviors such as heterogeneity of tumors, therapeutic responsiveness, and prognosis. SUVmean was defined as the average SUV related to the tumor burden. SUVpeak was the highest average SUV calculated within a spherical VOI (usually 1-cm-diameter region). MTV represents the metabolically active portion of the tumor, obtained through several SUV-based segmentation techniques. TLG is the product of SUVmean x MTV. MTV and TLG, which consider volumetric information and reflect the whole-body tumor burden, have been more frequently proposed as prognostic factors for immunotherapy in NSCLC patients (Seban et al., 2020a; Seban et al., 2020b; Castello et al., 2020; Seban et al., 2020c; Polverari et al., 2020; Monaco et al., 2021). The latest relevant studies on 18F-FDG PET-derived parameters for predicting the response and prognosis of ICIs in NSCLC patients in the recent 3 years are displayed in Table 2. These research works demonstrated the value of baseline semi-quantitative parameters of 18F-FDG PET/CT in the responsiveness and prognosis of NSCLC patients treated with ICIs. However, under the semi-quantitative method, FDG accumulation is still affected by many technical, physiological, and pathological factors. Thus, the imaging-based comparative response evaluations during treatment for immune-treatment have been adopted.
TABLE 2.
The studies on the predictive value of semi-quantitative parameters for immunotherapy in NSCLC patients.
| Studies | Sample | ICIs | Outcomes | SUV | Design | Results |
|---|---|---|---|---|---|---|
| Monaco et al., 2021 (Monaco et al., 2021) | 92 NSCLC | Nivolumab, pembrolizumab, or atezolizumab | 1. Response | wbMTV, wbTLG, SUVmax, SUVmean | Retrospective study | 1. Patients who achieved disease control (CR, PR, SD) had significantly lower wbMTV median values than patients with PD (77 vs. 160.2, p = 0.039) |
| 2. OS | 2. Patients with lower wbMTV and wbTLG had improved OS compared to patients with higher MTV (p = 0.03) and TLG (p = 0.05) | |||||
| Seban et al., 2020 (Seban et al., 2020a) | 63 advanced NSCLC with a PD-L1 TBS ≥50% 63 | pembrolizumab | 1. LTB | SUVmax, SUVmean wbMTV wbTLG | Multi-center retrospective study | 1. In multivariate analyses, high wbMTV (>84 cm3) and high tumor SUV mean (>10.1) remained independent factors for predicting LTB (OR 0.2; p = 0.03 and OR 3.7; p = 0.04) and PFS (HR 2.2; p = 0.02 and HR 0.5; p = 0.045) |
| 2. PFS | 2. High wbMTV was significantly associated with poor OS (HR 3.1; p = 0.03) | |||||
| 3. OS | ||||||
| Polverariet al, 2020 (Polverari et al., 2020) | 57 advanced NCSLC | Pembrolizumab or nivolumab or atezolizumab | 1. PFS | MTV, TLG, SUVmax | Retrospective study | 1. MTV (p = 0.028) and TLG (p = 0.035) were significantly associated with progressive vs. non-progressive disease status (p = 0.035) |
| 2. OS | 2. TLG had a higher probability of failing immunotherapy | |||||
| 3. Response | ||||||
| Chardin D et al., 2020 (Chardin et al., 2020) | 75 NSCLC | Pembrolizumab or nivolumab | 1. OS | SUVmax, SUVpeak, MTV, TLG | Prospective study | 1. A high MTV and a high TLG were significantly associated with a lower OS (p < 0.001) |
| 2. ETD | 2. MTV and TLG could reliably predict ETD (area under the ROC curve = 0.76, 95% CI: 0.65 to 0.87 and 0.72, 95% CI: 0.62 to 0.84, respectively) | |||||
| Seban R et al., 2020 (Seban et al., 2020b) | 63 advanced NSCLC with a PD-L1 TBS ≥50% | Pembrolizumab | 1. PFS | wbMTV | Multi-center retrospective study | Patients have been grouped based on score combining the wbMTV and dNLR into the good, intermediate and poor |
| 2. OS | 1. Median OS was 17.9 months (14.6 not reached) for the good group vs. 13.8 (95%CI 8.4–18.9) and 6.6 (CI 2.0–11.2) months for the intermediate and poor groups, respectively | |||||
| 3. DCR | 2. Median PFS was 15.1 (95%CI 12.1–20.0) months for the good group vs. 5.2 (1.9–8.5) and 1.9 (95%CI 1.3–2.5) months for the intermediate and poor groups, respectively | |||||
| 4. ORR | 3. The poor prognosis group was associated with DCR and ORR (p < 0.05) | |||||
| Seban R D et al., 2020 (Seban et al., 2020c) | 80 advanced NSCLC | Pembrolizumab or nivolumab or atezolizumab | 1. PFS | Highest SUVmax of all lesions, wbMTV | Retrospective study | wbMTV > 75 cm3 combined with dNLR >3 were associated with shorter OS (HR 2.5, 95%CI 1.3–4.7 and HR 3.3, 95%CI 1.6–6.4) and absence of DCB (OR 0.3, 95%CI 0.1–0.9 and OR 0.4, 95%CI 0.2–0.9) |
| 2. OS | ||||||
| 3. DCB classification | ||||||
| Evangelista et al., 2019 (Evangelista et al., 2019) | 32 metastatic NSCLC | Nivolumab | Response | wbSUVmax wbMTV, wbTLG | Retrospective study | wbSUV max was significantly higher in patients without a response than those with a response to immunotherapy (median: 48.97 vs. 20.85; Student’s t-test: p = 0.002) |
Bold values indicates the statistically significant indicators of studies. NSCLC: non–small cell cancer; ICIs: immune checkpoint inhibitors; SUV: standardized uptake value; OS: overall survival; wbMTV: whole-body metabolic tumor volume; wbTLG: whole-body total lesion glycolysis; SUVmax: maximum SUV; SUVmean: mean SUV; CR: complete response; PR: partial response; SD: stable disease; PD-L1: programmed cell death ligand 1; LTB: long-term benefit; PFS: progression-free survival; OR: odds ratio; HR: hazard ratio; MTV: metabolic tumor volume; TLG: total lesion glycolysis; ETD: early treatment discontinuation; 95%CI: confidence interval; ROC: receiver operating characteristic; TBS: tumor proportion score; DCR: disease control rate; ORR: overall response rate; dNLR: neutrophils to lymphocytes ratio; DCB: disease clinical benefit; wbSUVmax: whole-body SUVmax.
It should be noted that SULpeak is consistently employed as the only semi-quantitative parameter in functional criteria (see 2.1.3). SUV can be normalized to body mass, lean body mass, or body surface area. SUV normalized to lean body mass is named SUL. SUV normalized to body mass is the commonest parameter in clinical practices but cannot consider the relatively lower 18F-FDG accumulation in fatty tissues (Wahl et al., 1992) and are more dependent on body habitus across populations than SUL and SUV corrected for body surface area (Evangelista et al., 2020). Moreover, normalization to body surface area or lean body mass potentially reduces the effect of weight loss (which may occur during therapy) on subsequent SUV determinations. In addition, lean body mass may be the better method because of the availability of sex-specific corrections (Sugawara et al., 1999). Peak standardized uptake values normalized by lean body mass (SULpeak) were the highest average SUL calculated within a spherical VOI in the site of the most metabolically active tumor manifestation and of high repeatability. Therefore, SULpeak has been used as an effective index for evaluating malignancies treatment, including immunotherapy.
2.1.3 Metabolic-Based Response Assessment Criteria
Imaging-based response assessment is essential for the clinical management of immunotherapy. The role of the assessment system is as follows: 1) identifying non-responders; 2) confirming the response patterns; and 3) detecting irAEs.
Morphologic criteria are widely adopted in clinical trials and practices. These anatomic/structural assessing systems determine therapeutic effectiveness on the ground of the change of target-lesion size and the presence of new lesions principally. However, these anatomic response criteria cannot timely discern response without lesion volumetric changes such as cavitation, cystic change, intra-tumoral hemorrhage, and reduction in vascularity, which might occur after the use of immunotherapeutic agents and are considered physiopathologic features of a specific response to them (Costa et al., 2021). For example, as quite extensively used anatomic response criteria, Response Evaluation Criteria in Solid Tumors version 1.0 or version 1.1 (RECIST 1.0 or RECIST 1.1) define tumor responses into a series of four bins of response [complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)]. Nevertheless, some tumor sizes shrink slowly, but patients live for a long period with SD evaluated by RECIST 1.0/1.1, so this stable-disease response could have highly beneficial outcomes (Benjamin et al., 2007; Llovet et al., 2008; Van den Abbeele, 2008). Thus, with such reductionism through RECIST, the potentially valuable information that may be important is lost (Wahl et al., 2009). An additional consideration for RECIST is that the response evaluation from the baseline and follow-up studies is based on a precise estimate, which requires repeatability and accuracy of the assessment. However, more misclassifications and variance in response are seen when a different reader assesses the baseline and follow-up studies (Erasmus et al., 2003; Wahl et al., 2009).
FDG uptake of malignant tissues depends on the tumor’s functional status, such as rate of glycolysis, expression of glucose transport, level of proliferation, and association with particular molecules. Thus, 18F-FDG is a marker of tumoral activity. In terms of the functional volume, metabolic extent, and whole-body burden of tumor, functional criteria with 18F-FDG PET/CT to assess immunotherapy responses have been adapted for lung cancer and melanoma patients. There are at least four FDG PET/CT criteria (Table 4):
TABLE 4.
Novel PET probes for immunotherapy.
| Probe | Target | Study | PET probe | Experiment stage (subject) | Characteristics |
|---|---|---|---|---|---|
| Anti-PD-1 antibody | PD-1-expressing tumor-infiltrating lymphocyte | Liu et al., 2021 (Liu et al., 2021) | 68Ga-NOTA-Nb109 | Preclinical experiment (cell and animal models bearing different tumors) | 68Ga-NOTA-Nb109 can specifically target endogenous PD-L1 and dynamic monitoring of the change of PD-L1 expression and could guide the immunotherapy and immunochemotherapy for refractory cancers |
| Kelly et al., 2021 (Kelly et al., 2021) | 89Zr-REGN3504 | Preclinical experiment (mice and monkeys) | 1.89Zr-REGN3504 specifically localized to spleen and lymph nodes in the PD-1/PD-L1 humanized mice | ||
| 2.89Zr-REGN3504 immuno-PET accurately detected a significant reduction in splenic PD-L1 positive cells following systemic treatment | |||||
| Niemeije et al., 2021 (Niemeijer et al., 2021) | 89Z-pembrolizumab | Clinical experiment (NSCLC patients) | A significant correlation between the grade of uptake of the traces and the response assessed after 3 months of nivolumab was observed | ||
| Li et al., 2020 (Li et al., 2021) | 89Zr-N-sucDf-pembrolizumab | Preclinical experiment (healthy cynomolgus monkeys) | Preferential uptake in the lymphoid tissues, including the lymph nodes, spleen, and tonsils, was shown | ||
| England et al., 2018 (England et al., 2018) | 89Zr-DF nivolumab | Preclinical experiment | There was highly specific binding of 89Zr-DF nivolumab to activated T-cell infiltrating tumors in humanized murine models | ||
| Cole et al., 2017 (Cole et al., 2017) | 89Zr-nivolumab (BMS-936558) | Preclinical experiment (healthy non-human primates) | A study of biodistribution and clearance of BMS-936558 in animals | ||
| Anti-PD-L1 antibody | PD-L1-expressing tumor cell | Laffon et al., 2021 (Laffon and Marthan, 2021) | 18F-BMS-986192 | Clinical experiment (NSCLC patients) | A quantitative research: the ratio of SUV normalized for body weight to plasma concentration might be probed as a complementary possible simplified parameter, that is correlated with Ki/(kb + λ) within 50–55 min after injection |
| Huisman et al., 2020 (Huisman et al., 2020) | 18F-BMS-986192 (anti-PD-L1 adnectin) | Clinical experiment (NSCLC patients), quantitative research | SUV normalized for body weight at 60 min after injection may be a relevant simplified parameter to quantify tumor uptake for baseline PET studies | ||
| Bridgwater et al., 2018 (Bridgwater et al., 2020) | 89Zr-Df-F (ab')2 | Preclinical experiment (Melanoma Mouse Mode) | PET/CT images clearly showed that 89Zr-Df-F (ab')2 possessed superior pharmacokinetics and imaging contrast over the radiolabeled full antibody, with much earlier and higher tumor uptake (5.5 times more at 2 h after injection) and much lower liver background (51% reduction at 2 h after injection) | ||
| Truillet et al., 2018 (Truillet et al., 2018) | 89Zr-C4 | Preclinical experiment | 1.89Zr-C4 can specifically detect antigen in human NSCLC and prostate cancer models endogenously expressing a broad range of PD-L1 | ||
| 2.89Zr-C4 detects mouse PD-L1 expression changes in immunocompetent mice, suggesting that endogenous PD-1/2 will not confound human imaging | |||||
| 3.89Zr-C4 could detect acute changes in tumor expression of PD-L1 due to standard of care chemotherapies | |||||
| Bensch et al., 2018 (Bensch et al., 2018) | 89Zr-atezolizumab | Clinical experiment (bladder cancer, NSCLC, or TNBC patients) | 1. Tumor uptake was generally high but heterogeneous, varying within and among lesions, patients, and tumor types. 2. Clinical responses in patients were better correlated with pretreatment PET signal than IHC- or ribonucleic acid-sequencing-based biomarkers | ||
| Xing et al., 2019 (Xing et al., 2019) | 99mTc-NM-01 | Clinical experiment (NSCLC patients) | 1. Intra-tumoral and inter-tumoral heterogeneity was observed | ||
| 2. Primary tumor: blood-pool ratios at 2 h correlated with IHC. | |||||
| Anti-CTLA-4 antibodies | CTLA-4-expressing activated T cells and some tumor cells | Ehlerding et al., 2019 (Ehlerding et al., 2019) | 64Cu-NOTA-ipilimumab-F (ab')2 | Preclinical experiment (mice) | PET imaging with both 64Cu-NOTA-ipilimumab and 64Cu-NOTA-ipilimumab-F (ab')2 was able to localize CTLA-4+ tissues |
| Ehlerding et al., 2017 (Ehlerding et al., 2017) | 64Cu-DOTA- ipilimumab | Preclinical experiment (mouse models of NSCLC) | 64Cu-DOTA- ipilimumab can correctly localize the tumor, but a link was found with the receptor on the cell surface rather than in the intracellular domain | ||
| Anti-interferon-γ | Activated lymphocytes inside tumor lesions | Gibson et al., 2018 (Gibson et al., 2018) | 89Zr-anti-IFN-γ | Preclinical experiment (mouse with mammary tumors) | The activation status of cytotoxic T cells is annotated by 89Zr-anti-IFN-γ PET, providing valuable non-invasive insight into the function of immune cells in situ |
| Protease granzyme B | Cytotoxic CD8+ T cells and natural killer cells | Larimer et al., 2017 (Larimer et al., 2017) | 68Ga-NOTA-GZP | Preclinical experiment (human melanoma specimens) | Granzyme B PET imaging can serve as a quantitatively useful predictive biomarker for efficacious responses to cancer immunotherapy |
| Interleukin-2 | Tumor/tissue infiltrating T lymphocytes | Markovic et al., 2018 (Markovic et al., 2018) | 99mTc-HYNIC-IL-2 | Clinical experiment (melanoma patients with ipilimumab or pembrolizumab) | 1. Safety and feasibility are verified |
| 2. Detect TIL and distinguish between true progression from HPD. |
PET: positron emission tomography; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; NSCLC: non–small cell lung cancer; SUV: standardized uptake value; CT: computed tomography; PET/CT: positron emission tomography/computed tomography; TNBC: triple-negative breast cancer; IHC: immunohistochemistry; CTLA-4: cytotoxic T-lymphocyte associated-protein 4; IFN: interferon; IL-2: interleukin-2; TIL: tumor lymphocyte infiltration; HPD: hyper-progression.
1) Early Prediction of Response to Immune Checkpoint Inhibitor therapy (PECRIT) (Cho et al., 2017): PECRIT is the combining functional and anatomic assessing system based on the RECIST 1.1 and PET Response Criteria in Solid Tumors (PERCIST), which requires two PET/CT scans (first scan: before therapy; second scan: between days 21 and 28 on therapy). According to PECRIT, initial SD, defined by RECIST 1.1, should be assessed by 18F-FDG PET/CT (SCAN 2) after 3–4 weeks. On SCAN 2, when the percent change in SULpeak per PERCIST criteria is more than 15.5%, the stable metabolic disease (SMD) will be confirmed, or when the percent change in SULpeak is less than or equal to 15.5%, this pattern will be confirmed as progressive metabolic disease (PMD). As shown in Table 3, PECRIT admits the initial increase in FDG uptake during the early immunotherapeutic period and could effectively distinguish pseudo-progression and real progression by the particular threshold value of SULpeak percent change (15.5%). Moreover, this evaluation criteria combined with morphologic and functional criteria to effectively reduce assessment cost. However, measurement of all tumor lesions, as suggested by this PECRIT, is not only time-consuming but also sometimes impossible, especially in patients with advanced disease from an aggressive malignancy, which may be associated with a high number of metastatic lesions and/or diffuse organ infiltration. In addition, although data from PECRIT original research demonstrated that sensitivity, specificity, and accuracy of this algorithm to predict response at 4 months after initiation of therapy were 100, 93.3, and 95.0%, respectively, PECRIT is just established through the study based on 20 advanced melanoma patients. Therefore the threshold value of SULpeak could be further validated or statistically calculated based on large samples with multiple cancer types.
TABLE 3.
18F-FDG PET/CT assessing criteria for immunotherapy.
| Criteria | CR/CMR | pR/PMR | SD/SMD | pD/PMD | Notes |
|---|---|---|---|---|---|
| PECRITCho et al., 2017 (Cho et al., 2017) | Disappearance of all target and non-target lesions without any new lesions. Any pathological lymph nodes must have a reduction in short axis to <10 mm. Determined by two observations not less than 4 weeks apart | At least a 30% decrease in the sum of maximum diameters of target lesions; no new lesions; no progression of disease | 1. Does not meet the criteria for CR, PR, or PD, taking the smallest sum of the maximum diameters of target lesions as referencesAnd | 1. Sum of the maximum diameter of lesions increased by >20% over the smallest achieved sum of maximum diameter. The appearance of one or more new lesions is always considered progressionOr | |
| 2. Then, 18F-FDG PET (SCAN 2) assessment is required after 3-4 weeks: i) when percent change in SULpeak per PERCIST criteria is more than 15.5%, the SMD will be confirmed | 2. SD/SMD; then, 18F-FDG PET (SCAN 2) assessment is required after 3-4 weeks: ii) when the percent change in SULpeak is less than or equal to 15.5%, this pattern will be confirmed as PMD. | ||||
| ≥4 months of clinical benefit | ≥6 months of clinical benefit | ≥6 months of clinical benefit | No clinical benefit | ||
| PERCIMT Anwar et al., 2018 (Anwar et al., 2018) | Disappearance of metabolically active lesions | Disappearance of some metabolically active lesions | Neither CR/CMR, pR/PMR, nor pD/PMD | 1.4 new lesions with functional size <1.0 cm | |
| 2.3 new lesions with functional size >1.0 cm | |||||
| 3.2 new lesions with functional size >1.5 cm | |||||
| iPERCIST Goldfarb et al., 2019 (Goldfarb et al., 2019) | Complete resolution of FDG uptake within the target lesion | 1. ≥30% decrease in the target tumor FDG SULpeakor | Neither CR/CMR, pR/PMR, nor pD/PMD | 1. ≥ 30% increase in FDG SULpeak or advent of new FDG-avid lesions (UPMD) | |
| 2. confirmed as PMR by SCAN-3 from UPMD at SCAN-2 | 2. Need to be confirmed by a third PET (second posttreatment scan) at 4–8 weeks later (CPMD); if progression is followed by PMR or SMD, the bar is reset | ||||
| (Clinical stability is considered when deciding whether treatment is continued after UPMD) | |||||
| Responders | Responders | Responders | Non-responders | ||
| imPERCIST5 Ito K et al., 2019 (Ito et al., 2019) | Complete resolution of FDG uptake within all lesions to a level of less than or equal to that of the mean liver activity and that is indistinguishable from the background (blood-pool uptake) | Reduction of at least 30% in the sum of SULpeak of all target lesions detected at baseline and an absolute drop of 0.8 SULpeak units | Neither CMR, PMR, nor PMD. | 1. Increase in at least 30% in the sum of SULpeak of all target lesions detected at baseline, and an absolute increase in 0.8 SULpeak units with exclusion of infection/treatment effect | Target lesion: up to five measurable target lesions, typically the five hottest lesions among all lesions, including new lesions, and no more than two per organ |
| Patients with appearance of new FDG-avid lesions must be confirmed 4–8 weeks later | |||||
| PMR or SMD with appearance of new FDG-avid lesions at 4–8 weeks must be reevaluated | PMR or SMD with appearance of new FDG-avid lesions at 4–8 weeks must be reevaluated |
18F-FDG: 2-deoxy-2-[18F]-fluoro-D-glucose; PET/CT: positron emission tomography/computed tomography; CR: complete response; CMR: complete metabolic response; PR: partial response; PMR: partial metabolic response; SD: stable disease; SMD: stable metabolic disease; PD: progressive disease; PMD: progressive metabolic disease; SULpeak: peak standardized uptake values normalized by lean body mass; FDG: fluorodeoxyglucose; UPMD: unconfirmed progressive metabolic disease; CPMD: confirmed progressive metabolic disease; HPD: hyper-progression.
2) PET Response Evaluation Criteria for Immunotherapy (PERCIMT) (Anwar et al., 2018): PERCIMT, which demands two (pretherapeutic and posttherapeutic) PET/CT scans, suggests the number of newly emerged FDG-avid lesions on posttherapy PET/CT as cut-off value to identify the treatment failure in patients under immunotherapy which is quite different from the other criteria. Compared to other criteria, PERCIMT simplifies the assessment protocol by focusing on the limited number of new lesions. In the original study of PERCIMT, an absolute number of four new 18F-FDG-avid lesions, irrespective of their size, gave a reliable indication of nonresponse to treatment, and when the cut-off of newly emerged FDG-avid lesions with functional size < 1.0 cm was four, this assessing system showed the high sensitivity (84%) and specificity (100%). However, this research was also limited to the relatively small number of patients (N = 41), single cancer type (metastatic melanoma), and monotherapy (ipilimumab).
3) Immune PERCIST (iPERCIST) (Goldfarb et al., 2019): iPERCIST, which was adapted from PERCIST and the immune RECIST (iRECIST), demands two or three PET/CT scans (pretreatment, 2 months later after immunotherapy and 4–8 weeks after the second scan when necessary). iPERCIST’s dual-time-point evaluation is designed to differentiate the unconfirmed progressive metabolic disease (UPMD) from the confirmed progressive metabolic disease (CPMD). Then, the UPMD (PMD at SCAN-2) needs to be reevaluated 4–8 weeks later at SCAN-3. The continuation of treatment after the first evaluation by SCAN-2 was according to the physician’s judgment, metabolic response and the deterioration of clinical status, as described in iRECIST. If this UPMD (PMD at SCAN-2) is still considered as UPMD at SCAN-3, the UPMD at SCAN-3 is finally confirmed as CPMD. Patients with complete metabolic response (CMR), partial metabolic response (PMR), stable metabolic disease (SMD), or UPMD followed by PMR or SMD were classified as responders. Patients with UPMD associated with clinical deterioration or UPMD followed by CPMD were classified as non-responders. iPERCIST more effectively avoids the interference from pseudo-progression and HPD as the third scan could be administrated. The original clinical trial of iPERCIST showed the reliable ability to identification for responsiveness: responders continued treatment for a mean of 10.7 months (range 3.8–26.3), OS was longer for responders than that for non-responders (19.9 vs. 3.6 months, log-rank P = 0.0003), and the 1-year survival rates were 94% for responders and 11% for non-responders (Goldfarb et al., 2019). Nevertheless, this original trial was also developed through small sample research (N = 28), single cancer type (NSCLC), and monotherapy (nivolumab, a PD-1 blocker), and the prognostic value of iPERCIST should be confirmed in large prospective multicentric studies.
4) Immunotherapy-modified PERCIST5 (imPERCIST5) (Ito et al., 2019): imPERCIST5 requires several PET/CT scans, and especially when PMR or SMD is with newly emerged lesions, several scans should be administrated to reevaluate. The key difference of imPERCIST5 lies in the interpretation of new lesions on the posttreatment scan. In imPERCIST5, tumor response was assessed by the change in the sum of SULpeak of up to five lesions. New lesions do not necessarily result in a scan to be classified as PMD, for the appearance of new lesions that resolved spontaneously and were probably inflammatory in nature. Thus, imPERCIST5 could efficiently discern real progression from pseudo-progression and irAEs. According to the original clinical analysis, imPERCIST remained prognostic (hazard ratio, 3.853; 95% confidence interval, 1.498–9.911; P = 0.005) (Ito et al., 2019). Of note, in the original study of imPERCIST5, whereas imPERCIST5 reduces overdiagnosis of progressive disease, new lesions in patients with PMR or SMD by imPERICST5 were eventually found to have metastases in 55% of the cases. Thus, if the patients have the decrease or no change in metabolism of existing target lesions as well as the appearance of new lesions, the prognosis appears indeterminate. Then biopsy should be considered before any change in treatment. imPERCIST5 was still produced based on a clinical study with only 60 metastatic melanoma patients treated with ipilimumab. A case study with assessment on PERCIMT and imPERCIST5 is provided in Figure 1.
These assessment criteria above discovered the limitations of current morphologic criteria, such as response without lesion volumetric changes and individual measurement differences among readers. Then, they all highlight the value of 18F-FDG PET/CT (e.g., the high repeatability and accuracy of measurement for semi-quantitative parameters among readers) in responsiveness evaluation and prognosis in malignancies with immunotherapy, and three of the four assessment systems suggested more than one posttreatment PET/CT scan. Furthermore, they proposed their own resolutions to overcome the limitations of anatomic criteria: PECRIT and iPERCIST focused on the overall metabolic level in the whole body; PERCIMT emphasized the number of the newly FDG-avid lesions; and imPERCIST5 stressed the limited target lesions’ FDG-uptake values. However, it must be admitted that these criteria are in diversity and just verified in small samples at present. Only one of them was developed from a study based on NSCLC patients. Thus, large-sample multi-center validation and further improvement are necessary in the future. Moreover, given the diversity of the metabolic level among NSCLC subtypes (glucose metabolism level of lung adenocarcinoma is lower than that of other NSCLC pathological subtypes (Lopci et al., 2016)), the dedicated evaluation criteria oriented to NSCLC or NSCLC subtypes should be developed. In addition, the increase in radiation dose and high cost of PET/CT scan also become the reasons restricting the application of assessment criteria based on 18F-FDG PET/CT. Above all, the lack of specificity of FDG is the main limitation for 18F-FDG PET/CT. It is reported that an increased consumption of glucose may be due either to lymphocytes’ activation, which reflects the adaptive host response to tumor and is associated with a more favorable outcome, or to neutrophils’ activation, promoting an inflammatory cascade leading to tumor progression and dismal prognosis (De Larco et al., 2004). Therefore, the posttherapeutic assessment is needed more than once to confirm the right status.
2.2 Specific PET Probes for Immunotherapy
New molecular probes arise that directly target the key molecules of immune checkpoint pathways and immune responses. They can not only image the PD-1/PDL-1/CTLA-4 status or lymphocyte infiltrations of tumor tissue and better understand the uptake and distribution of agents/molecules and their mechanisms, but also be useful to monitor patients initially presenting sensitivity to ICIs and then showing an acquired resistance after a variable period of time.
The novel PET probes and their research progression are presented in Table 4. Fundamental research demonstrated the high target specificity and fulfilling affinity and adequate tumor penetration of these probes. The tracers under clinical settings have authenticated the safety of humans. During the imaging experiments, the uptake of tracers has shown sufficient resolution, which can assess potential heterogeneity within each lesion and among lesions from the same patients. Furthermore, in some studies concerning the assessment of response to immunotherapy, significant correlations were displayed between the grade of uptake of tracers and the response. Beyond the scope of PET imaging, promising molecular structures can also be targeted using single-photon emission tomography ligands (Table 4) and provide another potential and feasible choice for response evaluation and prediction of prognosis in malignancies with immunotherapy. However, there are still limitations such as high hepatic and splenic uptake that affect the detection of abdominal lesions, long-circulating half-life, and radiation exposure for patients. In addition, most of them are still under the preclinical research phase, and only preliminary human studies have been carried out for some probes. Therefore, further research, improvement, and validation of specific probes to ICIs will continue in the future.
3 Conclusion
Immunotherapy represents a powerful approach if used in selected oncological populations, including NSCLC patients. At present, PET/CT with 18F-FDG is an effective tool in identifying atypical patterns and adverse effects associated with immunotherapy, evaluating response, and predicting prognosis for NSCLC patients with ICIs, especially adopting semi-quantitative parameters and functional assessing criteria. Novel PET probes targeting key molecules relevant to ICIs and treatment response provide the promising perspective to more specifically screen oncological population that will benefit from immunotherapy and continuously assess ICI response in the future.
Acknowledgments
The authors thank Dr. Xueqi Chen from department of Nuclear Medicine of Peking University First Hospital for providing the figures of a classical case.
Author Contributions
XL was responsible for outline design and writing articles. ML assisted in the writing of this review. RW designed the outline. JZ was responsible for the selection of the theme and revising the draft.
Funding
This article was supported by the Peking University First Hospital Youth Clinical Research Special project (2021CR04), Beijing TCM Science and Technology Foundation (JJ-2020-04), and Interdisciplinary clinical research project of Peking University First Hospital (2021CR32).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Anwar H., Sachpekidis C., Winkler J., Kopp-Schneider A., Haberkorn U., Hassel J. C., et al. (2018). Absolute Number of New Lesions on 18F-FDG PET/CT Is More Predictive of Clinical Response Than SUV Changes in Metastatic Melanoma Patients Receiving Ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 45, 376–383. 10.1007/s00259-017-3870-6 [DOI] [PubMed] [Google Scholar]
- Barrón F., Cardona A. F., Cardona A. F., Corrales L., Ramirez-Tirado L.-A., Caballe-Perez E., et al. (2018). Characteristics of Progression to Tyrosine Kinase Inhibitors Predict Overall Survival in Patients with Advanced Non-small Cell Lung Cancer Harboring an EGFR Mutation. J. Thorac. Dis. 10, 2166–2178. 10.21037/jtd.2018.03.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. S., Choi H., Macapinlac H. A., Burgess M. A., Patel S. R., Chen L. L., et al. (2007). We Should Desist Using RECIST, at Least in GIST. Jco 25, 1760–1764. 10.1200/JCO.2006.07.3411 [DOI] [PubMed] [Google Scholar]
- Bensch F., van der Veen E. L., Lub-de Hooge M. N., Jorritsma-Smit A., Boellaard R., Kok I. C., et al. et al (2018). 89Zr-atezolizumab Imaging as a Non-invasive Approach to Assess Clinical Response to PD-L1 Blockade in cancerZr-Atezolizumab Imaging as a Non-invasive Approach to Assess Clinical Response to PD-L1 Blockade in Cancer. Nat. Med. 24, 1852–1858. 10.1038/s41591-018-0255-8 [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D. R., Steins M., Ready N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373, 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgwater C., Geller A., Hu X., Burlison J. A., Zhang H.-G., Yan J., et al. (2020). 89Zr-Labeled Anti-PD-L1 Antibody Fragment for Evaluating In Vivo PD-L1 Levels in Melanoma Mouse ModelZr-Labeled Anti-PD-L1 Antibody Fragment for Evaluating In Vivo PD-L1 Levels in Melanoma Mouse Model. Cancer Biother. Radiopharm. 35, 549–557. 10.1089/cbr.2019.3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello A., Rossi S., Mazziotti E., Toschi L., Lopci E. (2020). Hyperprogressive Disease in Patients with Non-small Cell Lung Cancer Treated with Checkpoint Inhibitors: The Role of 18F-FDG PET/CT. J. Nucl. Med. 61, 821–826. 10.2967/jnumed.119.237768 [DOI] [PubMed] [Google Scholar]
- Champiat S., Ferrara R., Massard C., Besse B., Marabelle A., Soria J.-C., et al. (2018). Hyperprogressive Disease: Recognizing a Novel Pattern to Improve Patient Management. Nat. Rev. Clin. Oncol. 15, 748–762. 10.1038/s41571-018-0111-2 [DOI] [PubMed] [Google Scholar]
- Chardin D., Paquet M., Schiappa R., Darcourt J., Bailleux C., Poudenx M., et al. et al (2020). Baseline Metabolic Tumor Volume as a strong Predictive and Prognostic Biomarker in Patients with Non-small Cell Lung Cancer Treated with PD1 Inhibitors: a Prospective Study. J. Immunother. Cancer 8, e000645. 10.1136/jitc-2020-000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. Y., Lipson E. J., Im H.-J., Rowe S. P., Gonzalez E. M., Blackford A., et al. (2017). Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 58, 1421–1428. 10.2967/jnumed.116.188839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E. L., Kim J., Donnelly D. J., Smith R. A., Cohen D., Lafont V., et al. (2017). Radiosynthesis and Preclinical PET Evaluation of 89Zr-Nivolumab (BMS-936558) in Healthy Non-human Primates. Bioorg. Med. Chem. 25, 5407–5414. 10.1016/j.bmc.2017.07.066 [DOI] [PubMed] [Google Scholar]
- Costa L. B., Queiroz M. A., Barbosa F. G., Nunes R. F., Zaniboni E. C., Ruiz M. M., et al. (2021). Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. Radiographics 41, 120–143. 10.1148/rg.2021200093 [DOI] [PubMed] [Google Scholar]
- Das S., Johnson D. B. (2019). Immune-related Adverse Events and Anti-tumor Efficacy of Immune Checkpoint Inhibitors. J. Immunotherapy Cancer 7, 306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M., Duffield E. A. (2017). Safety of Checkpoint Inhibitors for Cancer Treatment: Strategies for Patient Monitoring and Management of Immune-Mediated Adverse Events. Itt 6, 51–71. 10.2147/ITT.S141577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Wuertz B. R. K., Furcht L. T. (2004). The Potential Role of Neutrophils in Promoting the Metastatic Phenotype of Tumors Releasing Interleukin-8. Clin. Cancer Res. 10, 4895–4900. 10.1158/1078-0432.CCR-03-0760 [DOI] [PubMed] [Google Scholar]
- Ehlerding E. B., Lee H. J., Jiang D., Ferreira C. A., Zahm C. D., Huang P., et al. (2019). Antibody and Fragment-Based PET Imaging of CTLA-4+ T-Cells in Humanized Mouse Models. Am. J. Cancer Res. 9, 53–63. [PMC free article] [PubMed] [Google Scholar]
- Ehlerding E. B., England C. G., Majewski R. L., Valdovinos H. F., Jiang D., Liu G., et al. (2017). ImmunoPET Imaging of CTLA-4 Expression in Mouse Models of Non-small Cell Lung Cancer. Mol. Pharmaceutics 14, 1782–1789. 10.1021/acs.molpharmaceut.7b00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England C. G., Jiang D., Ehlerding E. B., Rekoske B. T., Ellison P. A., Hernandez R., et al. (2018). 89Zr-labeled Nivolumab for Imaging of T-Cell Infiltration in a Humanized Murine Model of Lung cancerZr-Labeled Nivolumab for Imaging of T-Cell Infiltration in a Humanized Murine Model of Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 110–120. 10.1007/s00259-017-3803-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus J. J., Gladish G. W., Broemeling L., Sabloff B. S., Truong M. T., Herbst R. S., et al. (2003). Interobserver and Intraobserver Variability in Measurement of Non-small-cell Carcinoma Lung Lesions: Implications for Assessment of Tumor Response. Jco 21, 2574–2582. 10.1200/JCO.2003.01.144 [DOI] [PubMed] [Google Scholar]
- Evangelista L., Cuppari L., Menis J., Bonanno L., Reccia P., Frega S., et al. (2019). 18F-FDG PET/CT in Non-small-cell Lung Cancer Patients. Nucl. Med. Commun. 40, 802–807. 10.1097/MNM.0000000000001025 [DOI] [PubMed] [Google Scholar]
- Evangelista L., Sepulcri M., Pasello G. (2020). PET/CT and the Response to Immunotherapy in Lung Cancer. Crp 13, 177–184. 10.2174/1874471013666191220105449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara R., Mezquita L., Texier M., Lahmar J., Audigier-Valette C., Tessonnier L., et al. et al (2018). Hyperprogressive Disease in Patients with Advanced Non-small Cell Lung Cancer Treated with PD-1/pd-L1 Inhibitors or with Single-Agent Chemotherapy. Jama Oncol. 4, 1543–1552. 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto D., Yoshioka H., Kataoka Y., Morimoto T., Hata T., Kim Y. H., et al. (2019). Pseudoprogression in Previously Treated Patients with Non-small Cell Lung Cancer Who Received Nivolumab Monotherapy. J. Thorac. Oncol. 14, 468–474. 10.1016/j.jtho.2018.10.167 [DOI] [PubMed] [Google Scholar]
- Galvano A., Gristina V., Malapelle U., Pisapia P., Pepe F., Barraco N., et al. (2021). The Prognostic Impact of Tumor Mutational burden (TMB) in the First-Line Management of Advanced Non-oncogene Addicted Non-small-cell Lung Cancer (NSCLC): a Systematic Review and Meta-Analysis of Randomized Controlled Trials. ESMO Open 6, 100124. 10.1016/j.esmoop.2021.100124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwing M., Herrmann K., Helfen A., Schliemann C., Berdel W. E., Eisenblätter M., et al. (2019). The Beginning of the End for Conventional RECIST - Novel Therapies Require Novel Imaging Approaches. Nat. Rev. Clin. Oncol. 16, 442–458. 10.1038/s41571-019-0169-5 [DOI] [PubMed] [Google Scholar]
- Gettinger S. N., Horn L., Gandhi L., Spigel D. R., Antonia S. J., Rizvi N. A., et al. (2015). Overall Survival and Long-Term Safety of Nivolumab (Anti-programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients with Previously Treated Advanced Non-small-cell Lung Cancer. Jco 33, 2004–2012. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson H. M., McKnight B. N., Malysa A., Dyson G., Wiesend W. N., McCarthy C. E., et al. (2018). IFNγ PET Imaging as a Predictive Tool for Monitoring Response to Tumor Immunotherapy. Cancer Res. 78, 5706–5717. 10.1158/0008-5472.CAN-18-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb L., Duchemann B., Chouahnia K., Zelek L., Soussan M. (2019). Monitoring Anti-PD-1-based Immunotherapy in Non-small Cell Lung Cancer with FDG PET: Introduction of iPERCIST. Ejnmmi Res. 9, 8. 10.1186/s13550-019-0473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg J. P., Li T., Yoneda K. Y. (2019). Molecular Testing Strategies in Non-small Cell Lung Cancer: Optimizing the Diagnostic Journey. Transl. Lung Cancer Res. 8, 286–301. 10.21037/tlcr.2019.04.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Baas P., Kim D.-W., Felip E., Pérez-Gracia J. L., Han J.-Y., et al. (2016). Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-small-cell Lung Cancer (KEYNOTE-010): a Randomised Controlled Trial. The Lancet 387, 1540–1550. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Hodi F. S., Oble D. A., Drappatz J., Velazquez E. F., Ramaiya N., Ramakrishna N., et al. (2008). CTLA-4 Blockade with Ipilimumab Induces Significant Clinical Benefit in a Female with Melanoma Metastases to the CNS. Nat. Rev. Clin. Oncol. 5, 557–561. 10.1038/ncponc1183 [DOI] [PubMed] [Google Scholar]
- Huisman M. C., Niemeijer A.-L. N., Windhorst A. D., Schuit R. C., Leung D., Hayes W., et al. et al (2020). Quantification of PD-L1 Expression with (18)F-BMS-986192 PET/CT in Patients with Advanced-Stage Non-small Cell Lung Cancer. J. Nucl. Med. 61, 1455–1460. 10.2967/jnumed.119.240895 [DOI] [PubMed] [Google Scholar]
- Iacovelli R., Massari F., Albiges L., Loriot Y., Massard C., Fizazi K., et al. (2015). Evidence and Clinical Relevance of Tumor Flare in Patients Who Discontinue Tyrosine Kinase Inhibitors for Treatment of Metastatic Renal Cell Carcinoma. Eur. Urol. 68, 154–160. 10.1016/j.eururo.2014.10.034 [DOI] [PubMed] [Google Scholar]
- Ito K., Teng R., Schöder H., Humm J. L., Ni A., Michaud L., et al. (2019). (18F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 60, 335–341. 10.2967/jnumed.118.213652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazu M., Edahiro R., Krebe H., Nishida K., Ishijima M., Uenami T., et al. (2018). Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Nivolumab: A Case Series. Thorac. Cancer. 9(12), 1782–1787. 10.1111/1759-7714.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K., Serizawa M., Koh Y., Takahashi T., Yamaguchi A., Hanaoka H., et al. et al (2014). Biological Significance of 18F-FDG Uptake on PET in Patients with Non-small-cell Lung Cancer. Lung Cancer 83, 197–204. 10.1016/j.lungcan.2013.11.025 [DOI] [PubMed] [Google Scholar]
- Kelly M. P., Makonnen S., Hickey C., Arnold T. C., Giurleo J. T., Tavaré R., et al. et al (2021). Preclinical PET Imaging with the Novel Human Antibody (89)Zr-DFO-Regn3504 Sensitively Detects PD-L1 Expression in Tumors and normal Tissues. J. Immunother. Cancer 9. 10.1136/jitc-2020-002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Baek S. W., Jeong Y., Yang Y., Kwon J., Han H. S., et al. (2019). Pseudoprogression Presenting as Intestinal Perforation in Non-Small Cell Lung Cancer Treated with Anti-PD-1: A Case Report. Mol. Clin. Oncol. 11(2), 132–134. 10.3892/mco.2019.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurra V., Sullivan R. J., Gainor J. F., Hodi F. S., Gandhi L., Sadow C. A., et al. (2016). Pseudoprogression in Cancer Immunotherapy: Rates, Time Course and Patient Outcomes. J. Clin. Oncol. 34, 6580. 10.1200/JCO.2016.34.15_suppl.6580 [DOI] [Google Scholar]
- Laffon E., Marthan R. (2021). Distribution Volume of (18)F-BMS-986192 in NSCLC Patients. J. Nucl. Med. 62, 144. 10.2967/jnumed.120.248484 [DOI] [PubMed] [Google Scholar]
- Larimer B. M., Wehrenberg-Klee E., Dubois F., Mehta A., Kalomeris T., Flaherty K., et al. (2017). Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 77, 2318–2327. 10.1158/0008-5472.CAN-16-3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang Y., Rubins D., Bennacef I., Holahan M., Haley H., et al. et al (2021). PET/CT Imaging of (89)Zr-N-sucDf-Pembrolizumab in Healthy Cynomolgus Monkeys. Mol. Imaging Biol. 23, 250–259. 10.1007/s11307-020-01558-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Cui Y., Chen X., Di L., Tong Z., Liu M., et al. (2020). Primary Metabolic Tumor Volume from 18F-FDG PET/CT Associated with Epidermal Growth Factor Receptor Mutation in Lung Adenocarcinoma Patients. Nucl. Med. Commun. 41, 1210–1217. 10.1097/MNM.0000000000001274 [DOI] [PubMed] [Google Scholar]
- Liu Q., Jiang L., Li K., Li H., Lv G., Lin J., et al. (2021). Immuno-PET Imaging of (68)Ga-labeled Nanobody Nb109 for Dynamic Monitoring the PD-L1 Expression in Cancers. Cancer Immunol. Immunother. 70, 1721–1733. 10.1007/s00262-020-02818-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J. M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J. F., et al. et al (2008). Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 359, 378–390. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- Lopci E., Toschi L., Grizzi F., Rahal D., Olivari L., Castino G. F., et al. et al (2016). Correlation of Metabolic Information on FDG-PET with Tissue Expression of Immune Markers in Patients with Non-small Cell Lung Cancer (NSCLC) Who Are Candidates for Upfront Surgery. Eur. J. Nucl. Med. Mol. Imaging 43, 1954–1961. 10.1007/s00259-016-3425-2 [DOI] [PubMed] [Google Scholar]
- Markovic S. N., Galli F., Suman V. J., Nevala W. K., Paulsen A. M., Hung J. C., et al. et al (2018). Non-invasive Visualization of Tumor Infiltrating Lymphocytes in Patients with Metastatic Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: a Pilot Study. Oncotarget 9, 30268–30278. 10.18632/oncotarget.25666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melian M., Lorente D., Aparici F., Juan O. (2018). Lung Brain Metastasis Pseudoprogression after Nivolumab and Ipilimumab Combination Treatment. Thorac. Cancer. 9(12), 1770–1773. 10.1111/1759-7714.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellema W. W., Burgers S. A., Smit E. F. (2015). Tumor Flare after Start of RAF Inhibition in KRAS Mutated NSCLC: a Case Report. Lung Cancer 87, 201–203. 10.1016/j.lungcan.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Missailidis S. (2008). Anticancer Therapeutics. Wiley. [Google Scholar]
- Monaco L., Gemelli M., Gotuzzo I., Bauckneht M., Crivellaro C., Genova C., et al. et al (2021). Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-small Cell Lung Cancer (NSCLC): A Real World Experience. Cancers (Basel) 13, 1634. 10.3390/cancers13071634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S. J., Oh J. K., Hyun S. H., Lee J. W., Hong I. K., Song B. I., et al. et al (2017). (18F-FDG PET/CT Can Predict Survival of Advanced Hepatocellular Carcinoma Patients: A Multicenter Retrospective Cohort Study. J. Nucl. Med. 58, 730–736. 10.2967/jnumed.116.182022 [DOI] [PubMed] [Google Scholar]
- Niemeijer A. N., Oprea L. D., Huisman M. C., Hoekstra O. S., Boellaard R., van de Veen B., et al. et al (2021). First-in-human Study of (89)Zr-Pembrolizumab PET/CT in Patients with Advanced Stage Non-small-cell Lung Cancer. J. Nucl. Med. 121, 261926. 10.2967/jnumed.121.261926 [DOI] [Google Scholar]
- Nishino M., Sholl L. M., Awad M. M., Hatabu H., Armand P., Hodi F. S. (2018). Sarcoid-Like Granulomatosis of the Lung Related to Immune-Checkpoint Inhibitors: Distinct Clinical and Imaging Features of a Unique Immune-Related Adverse Event. Cancer Immunol. Res. 6, 630–635. 10.1158/2326-6066.CIR-17-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passiglia F., Galvano A., Gristina V., Barraco N., Castiglia M., Perez A., et al. (2021). Is There Any Place for PD-1/CTLA-4 Inhibitors Combination in the First-Line Treatment of Advanced NSCLC?-a Trial-Level Meta-Analysis in PD-L1 Selected Subgroups. Transl Lung Cancer Res. 10, 3106–3119. 10.21037/tlcr-21-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverari G., Ceci F., Bertaglia V., Reale M. L., Rampado O., Gallio E., et al. et al (2020). (18)F-FDG Pet Parameters and Radiomics Features Analysis in Advanced Nsclc Treated with Immunotherapy as Predictors of Therapy Response and Survival. Cancers (Basel) 12, 1163. 10.3390/cancers12051163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A. G., Hui R., Csőszi T., Fülöp A., et al. et al (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-small-cell Lung Cancer. N. Engl. J. Med. 375, 1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Seban R. D., Assié J. B., Giroux-Leprieur E., Massiani M. A., Soussan M., Bonardel G., et al. et al (2020). Association of the Metabolic Score Using Baseline FDG-PET/CT and dNLR with Immunotherapy Outcomes in Advanced NSCLC Patients Treated with First-Line Pembrolizumab. Cancers (Basel) 12, 2234. 10.3390/cancers12082234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seban R. D., Assie J. B., Giroux-Leprieur E., Massiani M. A., Soussan M., Bonardel G., et al. et al (2020). FDG-PET Biomarkers Associated with Long-Term Benefit from First-Line Immunotherapy in Patients with Advanced Non-small Cell Lung Cancer. Ann. Nucl. Med. 34, 968–974. 10.1007/s12149-020-01539-7 [DOI] [PubMed] [Google Scholar]
- Seban R. D., Mezquita L., Berenbaum A., Dercle L., Botticella A., Le Pechoux C., et al. (2020). Adam Jet Al (2020) Baseline Metabolic Tumor burden on FDG PET/CT Scans Predicts Outcome in Advanced NSCLC Patients Treated with Immune Checkpoint Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 47, 1147–1157. 10.1007/s00259-019-04615-x [DOI] [PubMed] [Google Scholar]
- Shankar L. K., Hoffman J. M., Bacharach S., Graham M. M., Karp J., Lammertsma A. A., et al. et al (2006). Consensus Recommendations for the Use of 18F-FDG PET as an Indicator of Therapeutic Response in Patients in National Cancer Institute Trials. J. Nucl. Med. 47, 1059–1066. [PubMed] [Google Scholar]
- Solinas C., Porcu M., Hlavata Z., De Silva P., Puzzoni M., Willard-Gallo K., et al. (2017). Critical Features and Challenges Associated with Imaging in Patients Undergoing Cancer Immunotherapy. Crit. Rev. Oncol. Hematol. 120, 13–21. 10.1016/j.critrevonc.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Song P., Zhang J., Shang C., Zhang L. (2019). Curative Effect Assessment of Immunotherapy for Non-small Cell Lung Cancer: The “blind area” of Immune Response Evaluation Criteria in Solid Tumors (iRECIST). Thorac. Cancer. 10(4), 587–592. 10.1111/1759-7714.13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa A., Lopez C. E., Simon O. C., Karachaliou N., Rosell R. (2018). Clinical Assessment of Immune-Related Adverse Events. Ther. Adv. Med. Oncol. 10, 433587628. 10.1177/1758835918764628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara Y., Zasadny K. R., Neuhoff A. W., Wahl R. L. (1999). Reevaluation of the Standardized Uptake Value for FDG: Variations with Body Weight and Methods for Correction. Radiology 213, 521–525. 10.1148/radiology.213.2.r99nv37521 [DOI] [PubMed] [Google Scholar]
- Sznol M., Postow M. A., Davies M. J., Pavlick A. C., Plimack E. R., Shaheen M., et al. (2017). Endocrine-related Adverse Events Associated with Immune Checkpoint Blockade and Expert Insights on Their Management. Cancer Treat. Rev. 58, 70–76. 10.1016/j.ctrv.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Takada K., Toyokawa G., Tagawa T., Kohashi K., Akamine T., Takamori S., et al. et al (2017). Association between PD-L1 Expression and Metabolic Activity on (18)F-FDG PET/CT in Patients with Small-Sized Lung Cancer. Anticancer Res. 37, 7073–7082. 10.21873/anticanres.12180 [DOI] [PubMed] [Google Scholar]
- Tomita M., Suzuki M., Kono Y., Nakajima K., Matsuda T., Kuge Y., et al. (2020). Influence on [(18)F]FDG Uptake by Cancer Cells after Anti-PD-1 Therapy in an Enforced-Immune Activated Mouse Tumor. Ejnmmi Res. 10, 24. 10.1186/s13550-020-0608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Yasui H., Higashikawa K., Nakajima K., Takakura H., Shiga T., et al. (2018). Anti PD-1 Treatment Increases [(18)F]FDG Uptake by Cancer Cells in a Mouse B16F10 Melanoma Model. Ejnmmi Res. 8, 82. 10.1186/s13550-018-0433-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truillet C., Oh H., Yeo S. P., Lee C. Y., Huynh L. T., Wei J., Parker M., Blakely C., Sevillano N., Wang Y. Het. al. (2018). Imaging PD-L1 Expression with ImmunoPET. Bioconjug. Chem. 29, 96–103. 10.1021/acs.bioconjchem.7b00631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele A. D. (2008). The Lessons of GIST--PET and PET/CT: a New Paradigm for Imaging. Oncologist 13 (Suppl. 2), 8–13. 10.1634/theoncologist.13-S2-8 [DOI] [PubMed] [Google Scholar]
- Wahl R. L., Henry C. A., Ethier S. P. (1992). Serum Glucose: Effects on Tumor and normal Tissue Accumulation of 2-[f-18]-Fluoro-2-Deoxy-D-Glucose in Rodents with Mammary Carcinoma. Radiology 183, 643–647. 10.1148/radiology.183.3.1584912 [DOI] [PubMed] [Google Scholar]
- Wahl R. L., Jacene H., Kasamon Y., Lodge M. A. (2009). From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 50 (Suppl. 1), 122S–150S. 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W. A., Ziegler S. I., Thödtmann R., Hanauske A. R., Schwaiger M. (1999). Reproducibility of Metabolic Measurements in Malignant Tumors Using FDG PET. J. Nucl. Med. 40, 1771–1777. [PubMed] [Google Scholar]
- Xing Y., Chand G., Liu C., Cook G., O'Doherty J., Zhao L., et al. (2019). Early Phase I Study of a (99m)Tc-Labeled Anti-programmed Death Ligand-1 (PD-L1) Single-Domain Antibody in SPECT/CT Assessment of PD-L1 Expression in Non-small Cell Lung Cancer. J. Nucl. Med. 60, 1213–1220. 10.2967/jnumed.118.224170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Li L., Jiang X., Li Q. (2019). Mismatch Repair Deficiency/microsatellite Instability-High as a Predictor for Anti-PD-1/pd-L1 Immunotherapy Efficacy. J. Hematol. Oncol. 12, 54. 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Zhang M., Li R., Xue J., Lu Y. (2020). Pseudoprogression and Hyperprogression in Lung Cancer: a Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 146, 3269–3279. 10.1007/s00432-020-03360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]