Abstract
The cessation of firing of serotonergic dorsal raphe neurons is a key controlling event of rapid eye movement (REM) sleep. We tested the hypothesis that this cessation of activity is due to γ-aminobutyric acid (GABA) release using the in vivo microdialysis technique. We found that REM sleep is accompanied by a selective increase in GABA release, but not by a change in glutamate or glycine release in the dorsal raphe nucleus. Microinjection of the GABA agonist muscimol into the dorsal raphe increased REM sleep, although microperfusion of the GABA antagonist picrotoxin blocked REM sleep. These results implicate GABA release as a critical element in the production of the REM sleep state and in the control of discharge in serotonergic neurons across the sleep/wake cycle.
Keywords: microdialysis, muscimol, picrotoxin, serotonin
The serotonergic neurons of the brain stem dorsal raphe (DR) nucleus innervate multiple neocortical, hippocampal, diencephalic, and brain stem regions of the mammalian brain (3, 33, 37, 43). The activity of serotonergic neurons has been implicated in the control of shifts between states of sleep, nociception, cortical desynchronization, and normal and abnormal emotional states (38, 42). The greatest change in the discharge rate of DR cells occurs across the sleep/wake cycle. In the freely behaving cat, serotonergic neurons fire tonically at a rate of 3–6 Hz throughout waking and at 1–2 Hz during slow-wave sleep (SWS). During rapid eye movement (REM) sleep, serotonergic neurons exhibit a near-complete cessation of activity (23, 25).
The decreased activity of serotonergic neurons during REM sleep is accompanied by a decrease in serotonin release in several brain areas implicated in the production of sleep including the hypothalamus and pontine reticular formation (PRF) (15, 28, 44). Evidence from several laboratories suggests that the inactivity of DR serotonergic neurons disinhibits neuronal mechanisms responsible for the production of REM sleep. Thus blockade of DR neuronal activity by local cooling has been shown to induce REM sleep (6), and electrical stimulation of the DR blocks REM sleep (5). An increase in REM sleep time occurs with continuous perfusion of the DR with 8-hydroxy-2(di-n-propylamino)-tetralin, a compound that inhibits the activity of DR serotonergic cells by activation of 5-hydroxytryptamine la (5-HT1a) autoreceptors (29). The cessation of activity of serotonergic neurons is required for the generation of ponto-geniculo-occipital (PGO) spikes, a key phasic event of REM sleep (6, 18, 36). Recent evidence suggests that serotonin modulates the production of PGO spikes through actions in the amygdala (32) as well as through inhibition of brain stem cholinergic neurons, which are known to fire in conjunction with PGO waves (20, 22, 39).
The neurochemical mechanism underlying the cessation of DR serotonergic unit activity during REM sleep is unknown. It is unlikely that serotonergic inhibition of DR serotonergic cells through an autoreceptor mechanism is responsible, because the concentration of serotonin in the DR is lowest during REM sleep (28). In addition, data suggest that autoinhibitory mechanisms of serotonergic DR cells are significantly activated only during waking behaviors (8). Disfacilitation by removal of an excitatory noradrenergic or glutamatergic input could contribute to reduced discharge of DR units during sleep. However, serotonergic DR neurons exhibit pacemaker potentials in the anesthetized rat (1) and are unresponsive to excitatory input during REM sleep (23). Therefore, we hypothesized that the cessation of activity in serotonergic DR neurons during REM sleep is mediated by active inhibition.
Both γ-aminobutyric acid (GABA) and glycine potently inhibit serotonergic DR neurons (10, 11, 26). GABAergic and glycinergic neurons densely innervate the serotonergic neurons of the DR (9,12). A glycinergic mechanism mediates the characteristic muscle atonia of REM sleep through active inhibition of spinal motoneurons (7). We hypothesized that GABA and/or glycine are selectively released on serotonergic neurons during REM sleep. We tested this hypothesis by developing techniques for the measurement of GABA and glycine release in the DR across sleep/wake states with in vivo microdialysis and high-performance liquid chromatography (HPLC).
METHODS
Adult mongrel cats served as subjects. Animals were anesthetized with pentobarbitol sodium (35 mg/kg ip). Screw electrodes were placed in the posterior orbit and sensorimotor cortex for the recording of eye movements and electroencephalogram. Flexible stainless steel wires were inserted into the neck musculature for the recording of electromyographic activity. Stainless steel guide cannulas (21 gauge thin wall) were implanted at a 36° angle to vertical, to a position 1 mm dorsal to the DR. Guide cannulas and electrodes were secured to the skull with dental cement.
Dialysis collection.
At least 2 wk after surgery, a concentric-style microdialysis probe with a 2-mm semipermeable membrane 270 μm in diameter was inserted such that the tip extended 3 mm beyond the tip of the guide. The probe was perfused at a rate of 2 μl/min with artificial cerebrospinal fluid (aCSF) of the following composition (in mM): 145 Na+, 2.7 K+ 1.0 Mg2+, 1.2 Ca2+, 152 Cl−, and 2 Na2HPO4, pH 7.2. Sample collection was timed precisely by the use of a fraction collector (Eicom), incorporating a 10-min delay for the perfusate to travel from the tip of the probe to the outlet of the tubing. At least 17 h elapsed between probe insertion and the start of sample collection to permit stabilization of the surrounding tissue. Microdialysis samples were collected from immediately adjacent 5- to 10-min periods of SWS, REM sleep, and wake. Thus comparisons of amino-acid neurotransmitter content as a function of sleep/wake state were made between samples collected over a total period of <30 min. This design minimizes potential confounds created by comparing transmitter release in samples from sleep/wake states collected at different time points subsequent to insertion of the microdialysis probe. Potential confounds caused by circadian rhythmicity of neurotransmitter release are also minimized by this procedure.
HPLC analysis.
Samples were analyzed for GABA content as well as glutamate and glycine content by HPLC coupled with electrochemical detection after precolumn derivitization with o-pthaldialdehyde.
HPLC data analysis.
Data from 19 sleep/wake cycles in four cats were analyzed by one-way analysis of variance (ANOVA) using the Newman-Keuls test for post hoc comparisons. Each of the 19 sleep/wake cycles was represented by values for SWS, REM sleep, and wake for each of the amino acids measured. Samples from two, four, five, and eight sleep/wake cycles were collected from each of the four animals, respectively.
Microinjection and microperfusion.
Microinjections of the GABAA receptor agonist muscimol (0.25 mg in 0.5 μl aCSF) were made over a period of 2 min in four DR sites in three cats at 9 AM. Vehicle control (0.5 μl aCSF) microinjections were also made in each case at the same time of day. The order of control and drug injections was randomized. Subsequent to microinjections, polysomnographic recordings were made for a period of 6 h. Microperfusions of the GABAA receptor antagonist picrotoxin (100 μM in aCSF at 2 ml/min) via microdialysis probes were made in three DR sites in three cats beginning at 10 AM. In each case, the microdialysis probe used for perfusion had been inserted 17 h before testing to allow for stabilization of the surrounding tissue. Microperfusions of the same areas with aCSF at the same time of day served as control. Microperfusions and polysomnographic recordings were made simultaneously for 4 h.
Microinjection / microperfusion data analysis.
Amount of time spent in SWS, REM sleep, and wake after drug or control microinjections/microperfusions was determined using standard criteria for the analysis of cat polysomnographs (41). Data from microinjection and microperfusion studies were analyzed by one-way ANOVA and Newman-Keuls post hoc t-tests.
Histology.
Brain stem coronal slices (50 μm width) from each animal were stained with neutral red. Microdialysis, microperfusion, and microinjection sites were identified. In two animals, alternate slices were immunohistochemically stained for serotonin by the following method: 1) 1-h incubation in blocking buffer of 0.1% Triton X-100, 0.1% bovine serum albumin, and 2% normal goat serum in 0.1 M phosphate-buffered saline; 2) 48-h incubation in 1:100,000 rabbit 5-HT antibody at 4°C (Incstar); 3) 2-h incubation in 1:500 biotinylated goat anti-rabbit immunoglobulin G; 4) 2-h incubation in 1:100 avidin-biotin-peroxidase complex with visualization by Vector 3,3′-diaminobenzidine kit.
RESULTS
Figure 1 indicates the locations of microdialysis, microinjection, and microperfusion sites. We found that the extracellular concentration of GABA in the DR rose selectively during REM sleep compared with both waking and SWS (ANOVA, degrees of freedom 2,51, P < 0.001). GABA levels were similar during wake and SWS sleep. Overall, GABA concentration in REM sleep samples was found to be 47 and 71% greater than in SWS and wake samples, respectively. No significant differences in release of glutamate or glycine as a function of state were found. Table 1 lists the concentrations of the three compounds as a function of sleep/wake state. Figure 2 depicts GABA content in microdialysis samples collected from an individual DR site over the course of several sleep/wake cycles. Mean values for GABA concentration as a function of sleep/wake state were also calculated for each animal/microdialysis site. The means for each state for each animal were then subjected to an additional ANOVA to ensure that results regarding state-dependent amino acid concentration were not confounded by overrepresentation of data from a single microdialysis site or animal. Only results significant under both statistical tests are reported; both statistical procedures yielded the same results for every comparison.
Fig. 1.

A: histologically identified microdialysis probe and microinjection placements from brain stem coronal slices stained with neutral red. Black rectangles, placement of microdialysis membranes used in microdialysis collection. Open rectangles, placement of microdialysis membranes used in microperfusion experiments. +, Placement of muscimol microinjections. IC, inferior colliculus; CP, cerebral pedunclc; LDT, laterodorsal tegmental nucleus: BC, brachium conjunctivum: PAG. periaqueductal gray, MLF, medial longitudinal fasciculus; BP. brachium pontis; FTC, central tegmental field; PI, AP0, anterior-posterior stereotaxic planes. B: photomicrograph of a microinjection site (dark lesioned area) on midline of the ventral PAG. C: photomicrograph of area outlined in B, demonstrating placement of microinjection within a population of neurons immunopositive for 5-hydroxytryptamine.
Table 1.
GABA, glutamate, and glycine concentration as a function of sleep / wake state
| GABA | Glutamate | Glycine | |
|---|---|---|---|
| Wake | 0.042 ± 0.005 | 35.84 ± 2.55 | 32.30 ± 4.29 |
| SWS | 0.049 ± 0.007 | 36.42 ± 4.57 | 34.05 ± 4.80 |
| REM | 0.072 ± 0.003* | 33.98 ± 2.03 | 33.89 ± 4.11 |
Mean γ-aminobutyric acid (GABA), glutamate, and glycine concentration (in pmol/μl) ± SE for slow-wave sleep (SWS), rapid eye movement (REM) sleep, and wake samples. Each mean is based on 19 samples.
P < 0.001, GABA concentration in REM sleep samples is significantly greater than GABA concentration in SWS and wake samples (analysis of variance with Newman-Keuls post hoc t-tests, degrees of freedom = 2,54).
Fig. 2.
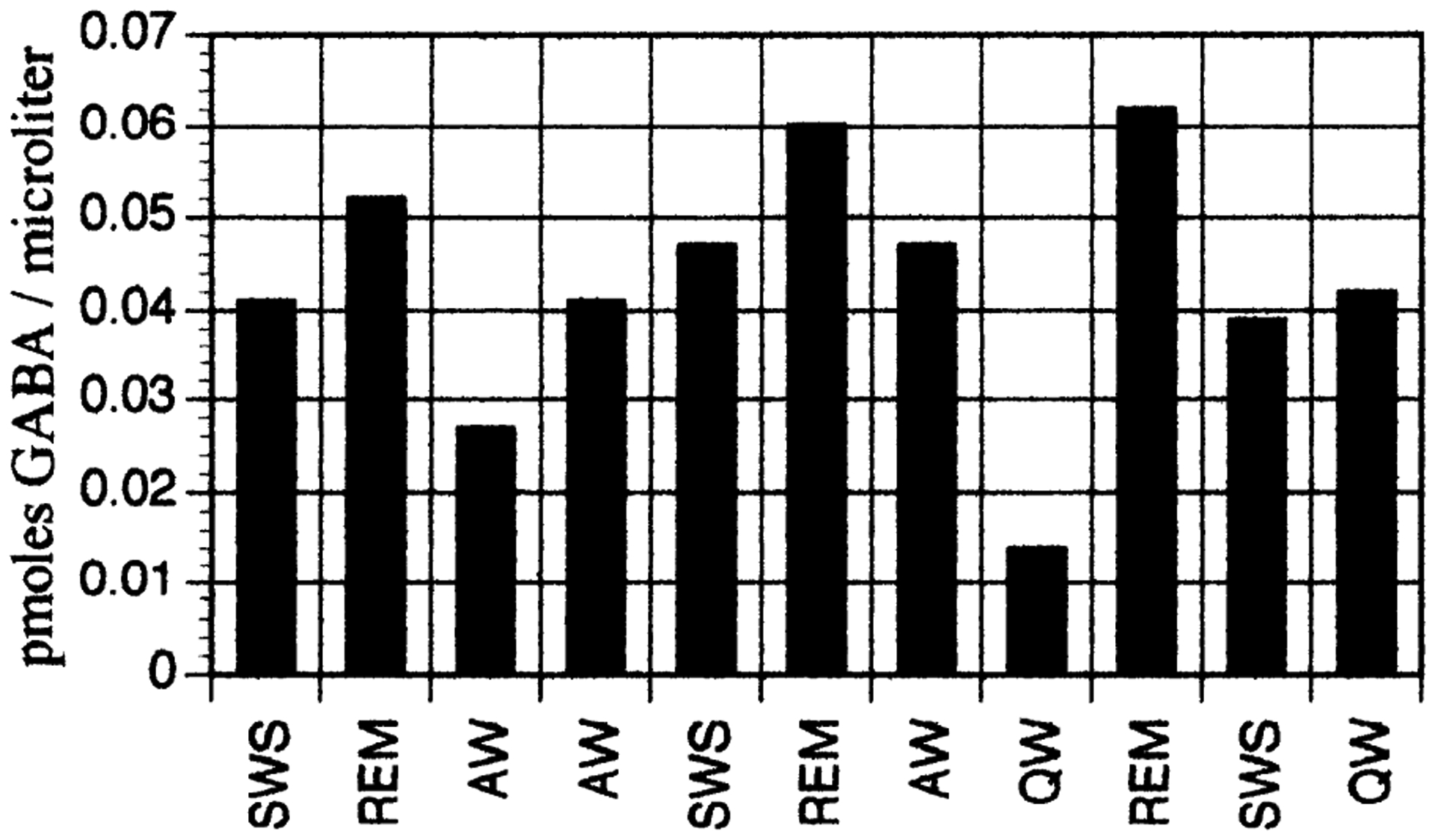
γ-Aminobutyric acid (GABA) content in microdialysis samples collected in an individual dorsal raphe (DR) site over the course of several sleep/wake cycles. Note consistent increase in GABA release in rapid eye movement (REM) sleep. SWS, slow-wave sleep; AW, active wake; QW, quiet wake.
Microinjection of the GABAA receptor agonist muscimol into the DR during waking produced a 67% increase in REM sleep time over vehicle control values over a 2-h period (t-test, n = 4, P < 0.03) (Fig. 3). Increases in REM sleep time were offset by decreases in SWS and wake time that were not statistically significant. REM sleep amounts returned to control levels during hours 3–6 of the recording period.
Fig. 3.

REM sleep time as a percentage of control (vehicle microinjection) values after microinjection of GABAA receptor agonist muscimol in the DR. Muscimol increased REM sleep time for a period of 2 h after injection. *P < 0.03. Open bars, SWS; solid bars, REM sleep; hatched bars, wake; n = 4. Error bars indicate SE.
Microperfusion of the DR area with the GABAA receptor Cl− channel blocker picrotoxin (0.1 mM) via a microdialysis probe completely blocked the production of REM sleep over a 4-h period in two animals and decreased REM sleep time by 83% in a third (t-test, n = 3, P < 0.05) (Fig. 4). In each case, the animals were able to initiate and maintain normal periods of SWS and wake. No significant differences in SWS or wake amounts were observed.
Fig. 4.

Time (as a percentage of vehicle control) spent in SWS, REM sleep, and wake during perfusion of the DR with 100 μm picrotoxin. Picrotoxin significantly decreased REM sleep time. *P < 0.05. Open bars, aCSF; black bars, picrotoxin; n = 3. Error bars indicate SE.
DISCUSSION
The finding of increased GABA in the DR during REM sleep is consistent with the hypothesis that GABA mediates active inhibition of DR serotonergic neurons during REM sleep. We also examined the efficacy and necessity of GABAergic input to the DR for REM sleep control. The large reduction in REM sleep amounts during picrotoxin perfusion in the DR indicates that the inhibition of DR serotonergic neurons by GABAA receptor activation is required for the production of REM sleep. Increases in REM sleep time after the microinjection of the GABAA receptor agonist muscimol are consistent with our hypothesis that the inhibition of DR serotonergic cells by a GABAergic mechanism is permissive to and facilitates REM sleep production.
Our microdialysis data suggest that GABAergic neurons, within or projecting to the DR, are selectively active during REM sleep. The location of neurons mediating the increased GABA release in the DR during REM sleep remains undetermined. Both intrinsic and extrinsic sources of GABA innervation in the DR nucleus are possible origins of state-dependent GABA release. Fos immunolabeling indicates that small, nonserotonergic, possibly GABAergic, neurons of the DR are activated after the induction of a REM sleep-like state with microinjection of carbachol in the PRF (34, 45). Aghajanian et al. (2) identified a population of nonserotonergic cells in the dorsal raphe that exhibited changes in firing opposite that of serotonergic cells in response to peripheral nerve stimulation. However, unit recording studies of the DR have not revealed units whose activity across the sleep/wake cycle parallel the changes we observed in GABA release. Thus GABA release selective to REM sleep could arise from small DR neurons not easily recorded with conventional extracellular recording techniques or from neurons external to the DR. There are GABAergic projections to the DR from the preoptic and posterior hypothalamus, the periaqueductal gray, the midbrain and PRF, the peribrachial region, and the prepositus hypoglossal nucleus (9,12). It is likely that the source of state-dependent GABA release in the DR is caudal to the midbrain because DR units have been demonstrated to retain their REM-off pattern of firing in decerebrate cats (14).
Neurons discharging selectively during REM sleep have been identified in the dorsolateral PRF (35). A population of GABAergic cells has been identified in this same region (17). The dorsolateral PRF sends projections to the DR, some of which are GABAergic (4, 27). Together with the GABA release data presented herein, these findings suggest that some PRF REM-on neurons may be GABAergic.
REM sleep behavior disorder in humans is characterized by fragmented REM sleep as well as a disruption of muscle atonia within REM sleep (24). It resembles a syndrome seen after lesions of the dorsolateral pons in the cat (13, 18). In contrast to normal REM sleep, serotonergic neurons are not completely silent during REM sleep in the REM sleep without atonia syndrome (40). REM sleep behavior disorder is successfully treated with clonazepam, a benzodiazepine that potentiates GABAA receptors and has been shown to decrease the use of serotonin in the mouse brain (30). This suggests that a disruption of the output of GABAergic PRF neurons projecting to the raphe nuclei could underlie this disorder of REM sleep.
Microiontophoretic application of the GABAA receptor antagonist bicuculline (Bic) reduces the decrease in DR unit discharge in SWS but not in REM sleep (21). We found no significant increase in GABA release in SWS. However, a small increase in the release of GABA, possibly beyond the resolution of the microdialysis technique, might be sufficient to reduce DR unit discharge in SWS. This interpretation is consistent with the report by Lydie et al. (23) indicating greatly decreased response of DR cells to excitatory input in REM sleep compared with SWS, despite a relatively small decrease in discharge rate from SWS to REM sleep. Greatly increased levels of GABA release would not be reflected in DR unit discharge because DR discharge in REM sleep falls to zero from the already minimal SWS rates. Our microdialysis and microperfusion data provide strong evidence that GABA mediates the suppression of serotonergic unit discharge during REM sleep. Indeed, as suggested by Levine and Jacobs (21), the inability of iontophoresed Bic to reverse REM sleep cessation of DR unit discharge could be due to incomplete antagonism of DR GABAA receptors as a result of increased GABA release. The results of the present study are compatible with their hypothesis. This interpretation is also consistent with the blockade of REM sleep production that we observed with picrotoxin perfusion. The antagonism of GABAergic activity in the DR by picrotoxin would not be blocked by increased GABA concentration because picrotoxin acts at the GABAA receptor Cl− channel rather than competing with GABA at the GABAA receptor binding site, as is the case for Bic (19).
In summary, we have demonstrated that GABA release in the DR is increased during REM sleep. Alterations in the production of REM sleep after the microinjection of a GABAA receptor agonist and antagonist into the DR are consistent with the hypothesis that inhibition of serotonergic DR neurons by a GABAergic mechanism is a key controlling event of REM sleep. Our findings suggest that a group of REM-on neurons responsible for initiating REM sleep is GABAergic.
Acknowledgments
This research was supported by the Medical Research Service of the Department of Veteran’s Affairs and National Institutes of Health Grants NS-14610, NS-23724, and HL-41370.
Footnotes
These results have been reported in preliminary form (D. A. Nitz and J. M. Siegel. World Fed. Sleep Res. Soc. Abs. 24A: 49, 1995).
REFERENCES
- 1.Aghajanian GK, and Vandermaelen CP. Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res. 238: 463–469,1982. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Wang RY, and Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 153:169–175,1978. [DOI] [PubMed] [Google Scholar]
- 3.Azmitia EC, and Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and medial raphe nuclei in the rat. J. Comp. Neurol 179: 641–668,1978. [DOI] [PubMed] [Google Scholar]
- 4.Belin MF, Aguera M, Tappaz M, McRae-Degueurce A, Bobillier P, and Pujol JF. GABA-accumulating neurons in the nucleus raphe dorsalis and periaqueductal gray in the rat: a biochemical and radioautgraphic study. Brain Res. 170: 279–297, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Benington JH, Woudenberg MC, and Heller HC. REM-sleep propensity accumulates during 2-h REM-sleep deprivation in the rest period in rats. Neurosci. Lett 180: 76–80,1994. [DOI] [PubMed] [Google Scholar]
- 6.Cespuglio R, Gomez ME, Walker E, and Jouvet M. Effet du refroidissement et de la stimulation des noyaux du systemes du raphe sur les etats de vigilance chez le chat. Electroencephalogr. Clin. Neurophysiol 3: 221–227,1979. [DOI] [PubMed] [Google Scholar]
- 7.Chase MH, and Morales FR. Principles and Practice of Sleep Medicine, edited by Kryger M, Roth T, and Dement WC. New York: Saunders, 1989, p. 74–85. [Google Scholar]
- 8.Fornal CA, Litto WJ, Metzler CW, and Marrosu F. Single unit responses of dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. J. Pharmacol. Exp. Ther 270:1345–1358,1994. [PubMed] [Google Scholar]
- 9.Fort P, Luppi PH, and Jouvet M. Glycine-immunoreactive neurones in the cat brainstem reticular formation. Neuroreport 4:1123–1126,1993. [PubMed] [Google Scholar]
- 10.Gallager DW, and Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur. J. Pharmacol 39: 357–364,1976. [DOI] [PubMed] [Google Scholar]
- 11.Gallager DW, Lakoski JM, Gonsalves SF, and Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature 308: 74–77,1984. [DOI] [PubMed] [Google Scholar]
- 12.Harandi M, Aguera M, Gamrani H, Didier M, Maitre M, Calas A, and Belin MF. Gamma-amino-butyric acid and 5-hydroxytryptamine interrelationship in the rat nucleus raphe dorsalis: combination of radioautographic and immunocytochemical techniques at light and electron microscopy levels. Neuroscience 21: 237–251,1987. [DOI] [PubMed] [Google Scholar]
- 13.Henley K, and Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol. Exp. (Warsz.) 34: 215–232, 1974. [PubMed] [Google Scholar]
- 14.Hoshino K, and Pompeiano O. Tonic inhibition of dorsal pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat. Arch. Ital. Biol 114: 310–340,1976. [PubMed] [Google Scholar]
- 15.Iwakiri H, Matsuyama K, and Mori S. Extracellular levels of serotonin in the medial pontine reticular formation in relation to sleep-wake cycle in cats: a microdialysis study. Neurosci. Res 18:157–170,1993. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs BL, Wilkinson LO, and Fornal CA. The role of brain serotonin. A neurophysiologic perspective. Neuropsychopharmacology 3: 473–479,1990. [PubMed] [Google Scholar]
- 17.Jones BE Noradrenergic locus coeruleus neurons: their distant connections and their relationship to neighboring (including cholinergic and GABAergic) neurons of the central grey and reticular formation. Prog. Brain Res 88:15–30,1991. [DOI] [PubMed] [Google Scholar]
- 18.Jouvet M, and Delorme JF. Supression du sommeil par le parachlorometamphetamine et la parachlorophenylalanine. C. R. Soc. Biol 160: 2347–2350, 1966. [PubMed] [Google Scholar]
- 19.Kardos J The GABAA receptor channel mediated chloride ion translocation through the plasma membrane: new insights for 36Cl− ion flux measurements. Synapse 13: 74–93,1993. [DOI] [PubMed] [Google Scholar]
- 20.Leonard CS, and Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience 59: 309–330,1994. [DOI] [PubMed] [Google Scholar]
- 21.Levine ES, and Jacobs BL. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. Neuroscience 12: 4037–4044,1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, and Reiner PB. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc. Natl. Acad. Sei. USA 89: 743–747,1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lydie R, McCarley RW, and Hobson JA. Serotonin neurons and sleep. II. Time course of dorsal raphe discharge. Neurosci. Lett 38: 35–40, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Mahowald M, and Schenck CH. Principles and Practice of Sleep Medicine, edited by Kryger M, Roth T, and Dement WC. New York: Saunders, 1989, p. 389–401. [Google Scholar]
- 25.McGinty DJ, and Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 101: 569–575, 1976. [DOI] [PubMed] [Google Scholar]
- 26.Pan ZZ, and Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol 61: 719–726,1989. [DOI] [PubMed] [Google Scholar]
- 27.Peyron C, and Luppi PH. GABAergic afferents to the rat dorsal raphe nucleus. Soc. Neurosci. Abstr 20:128.16,1994. [Google Scholar]
- 28.Portas CM, and McCarley RW. Behavioral state related changes of extracellular serotonin concentrations in the dorsal raphe nucleus: a microdialysis study in the freely behaving cat. Brain Res. 648: 306–312,1994. [DOI] [PubMed] [Google Scholar]
- 29.Portas CM, Thakkar M, Rainnie D, and McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OHDPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J. Neurosci 16: 2820–2828, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt J, Jenner P, Reynolds EH, and Marsden CD. Clonazepam induces decreased serotoninergic activity in the mouse brain. Neuropharmacology 18: 791–799,1979. [DOI] [PubMed] [Google Scholar]
- 31.Sanford LD, Ross RJ, Seggos AE, Morrison AR, and Ball WA. Central administration of two 5-HT receptor agonists: effect on REM sleep initiation and PGO waves. Pharmacol. Biochem. Behav 49: 93–100,1994. [DOI] [PubMed] [Google Scholar]
- 32.Sanford LD, Tejani-Butt SM, Ross RJ, and Morrison AR. Amygdaloid control of alerting and behavioral arousal in rats: involvement of serotonergic mechanisms. Arch. Ital. Biol 134: 81–99,1995. [PubMed] [Google Scholar]
- 33.Semba K Aminergic and cholinergic afferents to REM sleep induction regions of the pontine reticular formation in the rat. J. Comp. Neurol 330: 543–556,1993. [DOI] [PubMed] [Google Scholar]
- 34.Shiromani PJ, Malik M, Winston S, and McCarley RW. Time course of Fos-like immunoreactivity associated with cholinergically induced REM sleep. J. Neurosci 15: 3500–3508,1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel J Principles and Practice of Sleep Medicine (2nd ed.), edited by Kryger M, Roth T, and Dement WC. New York: Saunders, 1994, p. 125–144. [Google Scholar]
- 36.Simon RP, Gershon MD, and Brooks DC. The role of the raphe nuclei in the regulation of ponto-geniculo-occipital wave activity. Brain Res. 58: 313–330,1973. [DOI] [PubMed] [Google Scholar]
- 37.Steininger TL, Rye DB, and Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J. Comp. Neurol 321: 515–543, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Steriade M Basic mechanisms of sleep generation. Neurology 42: 9–17,1992. [PubMed] [Google Scholar]
- 39.Steriade M, Pare D, Datta S, Oakson G, and Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci 10: 2560–2579,1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trulson ME, Jacobs BL, and Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 226: 75–91,1981. [DOI] [PubMed] [Google Scholar]
- 41.Ursin R, and Sterman B. A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat. Los Angeles, CA: Brain Information Service, Brain Research Institute, UCLA, 1981. [Google Scholar]
- 42.Vanderwolf C, Baker G, and Dickson C. Serotonergic control of cerebral activity and behavior: models of dementia. Ann. NY Acad. Sei 600: 366–382,1990. [DOI] [PubMed] [Google Scholar]
- 43.Vertes RP, and Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J. Comp. Neurol 340: 11–26, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson LO, Auerbach SB, and Jacobs BL. Extracellular serotonin levels change with behavioral state but not with pyrogen-induced hyperthermia. J. Neurosci 11: 2732–2741, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamuy J, Sampogna S, Lopez-Rodriguez F, Luppi PH, Morales FR, and Chase MH. Fos and serotonin immunoreactivity in the raphe nuclei of the cat during carbachol-induced active sleep: a double-labelling study. Neuroscience 67: 211–223, 1995. [DOI] [PubMed] [Google Scholar]


