Abstract
Radial glial cells (RGC) are at the center of brain development in vertebrates, acting as progenitors for neurons and macroglia (oligodendrocytes and astrocytes) and as guides for migration of neurons from the ventricular surface to their final positions in the brain. These cells originate from neuroepithelial cells (NEC) from which they inherit their epithelial features and polarized morphology, with processes extending from the ventricular to the pial surface of the embryonic cerebrum. We have learnt a great deal since the first descriptions of these cells at the end of the nineteenth century. However, there are still questions regarding how and when NEC transform into RGC or about the function of intermediate filaments such as glial fibrillary acidic protein (GFAP) in RGCs and their dynamics during neurogenesis. For example, it is not clear why RGCs in primates, including humans, express GFAP at the onset of cortical neurogenesis while in rodents it is expressed when it is essentially complete. Based on an ultrastructural analysis of GFAP expression and cell morphology of dividing progenitors in the developing neocortex of the macaque monkey, we show that RGCs become the main progenitor in the developing cerebrum by the start of neurogenesis, as all dividing cells show glial features such as GFAP expression and lack of tight junctions. Also, our data suggest that RGCs retract their apical process during mitosis. We discuss our findings in the context of the role and molecular characteristics of RGCs in the vertebrate brain, their differences with NECs and their dynamic behavior during the process of neurogenesis.
Keywords: Radial glia, GFAP, Neurogenesis, Cortical development, Ventricular zone, Neural stem cells
Introduction
Radial glia has a central role in the development of the nervous system of vertebrates, generating the neurons and glia that will populate the brain, and guiding the migration of neurons from their birthplace around the ventricle to their final positions in the cortical plate [1]. It is well established that neuroepithelial cells (NEC) proliferate symmetrically in the neural tube to establish the brain neuroepithelium. Around the time of neurogenesis NECs lose some epithelial features gaining glial characteristics and transform into radial glial cells (RGCs) [2]. RGCs will then either self-renew expanding their pool or generate committed neuronal or glial progenitors through asymmetric division [3, 4], defined by uneven distribution of subcellular structures or molecules between daughter cells that influence cell fate [5, 6]. Although RGCs will produce the vast majority of neurons and glia [1, 2, 7], it is not clear what is the relative contribution of NECs and RGCs to neurogenesis during those early stages of development in the primate, and how long NECs remain in the primate brain.
Radial glial cells were initially described in the late nineteenth century as cells with long radial fibers covering the developing human neuroepithelium. The general morphology of the cells, revealed by the Golgi impregnation method, especially the long radial fibers with varicosities and the intermediate forms transforming into astrocytes during late development suggested a glial identity, and they were referred to as “Epithelial cells”, “Radial cells”, “Fetal ependymal cells”, “Spongioblasts”, “Tanycites” and “Faserglia” [8–13]. Rakic proposed the unifying term Radial Glia Cell (RGC) that over time became universally adopted [14]. Subsequent electron microscopy studies showed their mixed nature, as they exhibit epithelial features such as adherens junctions and primary cilium in the ventricular endfeet and also glial characteristics like pale cytoplasm, intermediate filaments, gap junctions and abundant glycogen granules [15–18]. And further molecular characterization have shown that RGCs share expression of several markers with NECs, such as nestin [19, 20], RC1 [21] or RC2 [22] that is a transcriptionally modified form of nestin [2, 23]. However, RGCs are characterized by the expression of a number of molecules that are typical of astrocytes, conferring them their glial phenotype: Brain lipid-binding protein (BLBP) [24]; astrocyte-specific glutamate transporter GLAST [25]; S100β, glutamine synthase (GS); [26]; vimentin [27]; tenascin-C (TN-C) [28], and GFAP [17].
Radial glial cells are characterized by a long basal process that extends from the ventricular zone (VZ) to the pial surface. While this distance is relatively short in small mammals such as rodents, it becomes increasingly long in species with large cerebrum such as primates [29]. In humans, it extends several centimeters from the VZ, across the transient subventricular zone (SVZ), intermediate zone (IZ), subplate and cortical plate to reach the pial surface [30]. This feature has posed an interesting question still not completely resolved: what happens with the radial process during cell division. Early ultrastructural studies suggested that the radial process was reabsorbed during mitosis and regrown afterwards [31, 32]. But data from ex-vivo studies of neurogenesis showed that the radial process remained in place during the division of the RGC [33, 34]. Recent data suggest that this apparent controversy might be related to the developmental timing, with early RGCs reabsorbing their processes and late RGCs maintaining it [35].
GFAP was the first marker associated to astrocytes [36] and soon after was described in radial glia since the onset of neurogenesis in primates and humans [15, 17, 37], in contrast to rodents [38] where it is only detected later in development when gliogenesis advances [39]. We therefore used GFAP staining and electron microscopic (EM) 3D reconstruction of the developing cortex of macaques to analyze the differentiation of RGCs from NECs and their relative contribution to neurogenesis at early and middle fetal stages namely at embryonic day (E) 45 and 65. We focused on dividing cells in the ventricular zone (VZ) to target progenitors, trying to identify NECs and RGCs based on their ultrastructural features and expression of GFAP. Our findings shed light on the questions posed above. Regarding the relative population of NECs to RGCs at early stages, we found a predominant radial glial phenotype both at E45 and E61/65. In addition, we obtained evidence that the basal process of radial glia might be reabsorbed during mitosis at early neurogenesis, giving support to the idea that reabsorption might be time-dependent. We discuss our findings in the context of three specific aspects of the biology of RGCs: GFAP expression across development and across species, the transition of NECs into RGCs and the dynamics of the basal process of RGCs during neurogenesis.
Materials and Methods
Six macaque monkey embryos were used for the immunohistochemistry (IHC) and EM study at E37, E42, E45 (n = 2), E61 and E65. Immunoperoxidase labeling of GFAP was performed with rat anti-GFAP (Invitrogen, Eugene, OR, USA; dilution 1:5000), or rabbit anti-GFAP (Dako Cytomation, Denmark; dilution 1:1000) polyclonal antibodies. Controls in which the primary antibody was replaced with normal serum showed absence of staining. Electron microscopy and 3D reconstruction were performed as we previously described [40–42]. We reconstructed 46 dividing cells and 5 interphase cells from GFAP stained tissue, and 2 dividing cells from negative control tissue. For extended material and methods see supplementary material.
Results
Variation of GFAP Content in Dividing Cortical VZ Progenitors
In our samples, GFAP was detected as early as E37 in the dorsal region of the developing macaque pallium, and by E42 it was expressed all over the cortical neuroepithelium (Fig. 1), suggesting the transformation of NECs into radial glia. Although the expression of GFAP looked homogeneous through neocortical VZ cells by light microscopy observation, electron microscopy (EM) showed its local accumulation in certain cells, while others were immunonegative in single ultrathin sections (Fig. 2). To investigate the expression of GFAP in a larger population of dividing cells of the VZ, we used 3D reconstruction from EM images of uninterrupted serial sections. In total, we analyzed 46 dividing RGCs from different regions of the dorsal telencephalon at E45 and E61/65 (Fig. 3 and Table S1). For all cells, we quantified the GFAP immunoreaction end-product depositions per total cell volume as a percentage. At both ages analyzed, we found that all dividing cells contained variable amount of GFAP: almost half of the cells had less than 1% GFAP, while others had up to 20% GFAP per volume (Fig. 3). The average GFAP immunolabeling in dividing progenitors increased from E45 to E61/65 (Fig. 4a), as expected since transitioning from early to mature radial glia. We arbitrarily divided all 3D reconstructed cells in two groups according to the normalized volume of anti-GFAP depositions, distinguishing 23 cells with lower GFAP expression defined as “GFAP-low” and the 23 cells with higher GFAP as the “GFAP-high” group. Cells from negative controls reconstructed showed absence of staining, confirming the specificity of GFAP in our sample (Fig. S1). Also, we assessed the effect of antibody penetration in the labeling (see methodological considerations and Fig. S2 in supplementary material).
Fig. 1.
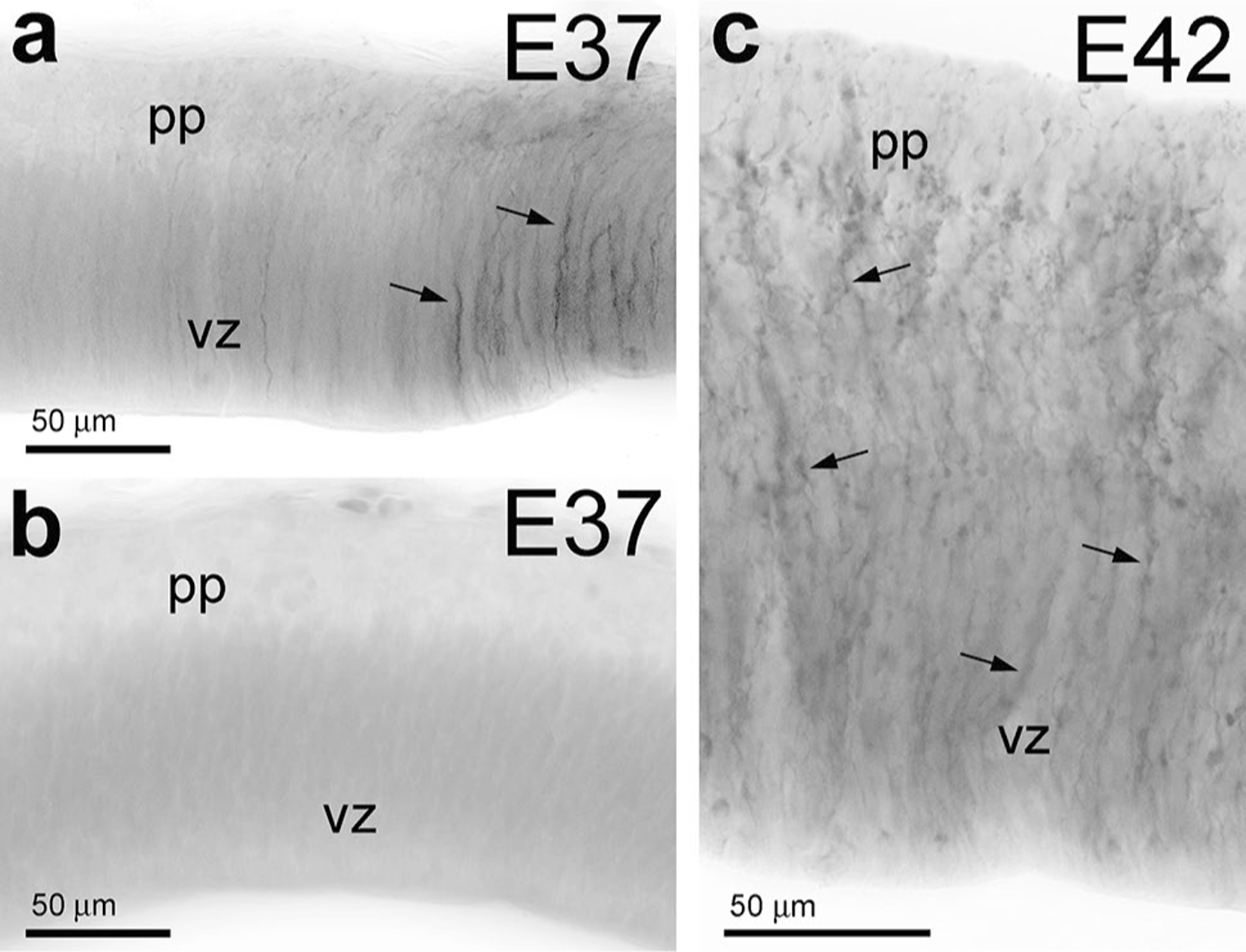
GFAP immunolabeling in the early embryonic rhesus macaque brain. At E37, the neocortical neuroepithelium of the macaque monkey starts expressing GFAP (arrows) in the dorsomedial region (a), while the latero-ventral developing cortex remains GFAP negative (b). c At E42, the entire cerebral cortex contains numerous GFAP-positive radial processes (arrows). pp preplate, vz ventricular zone
Fig. 2.
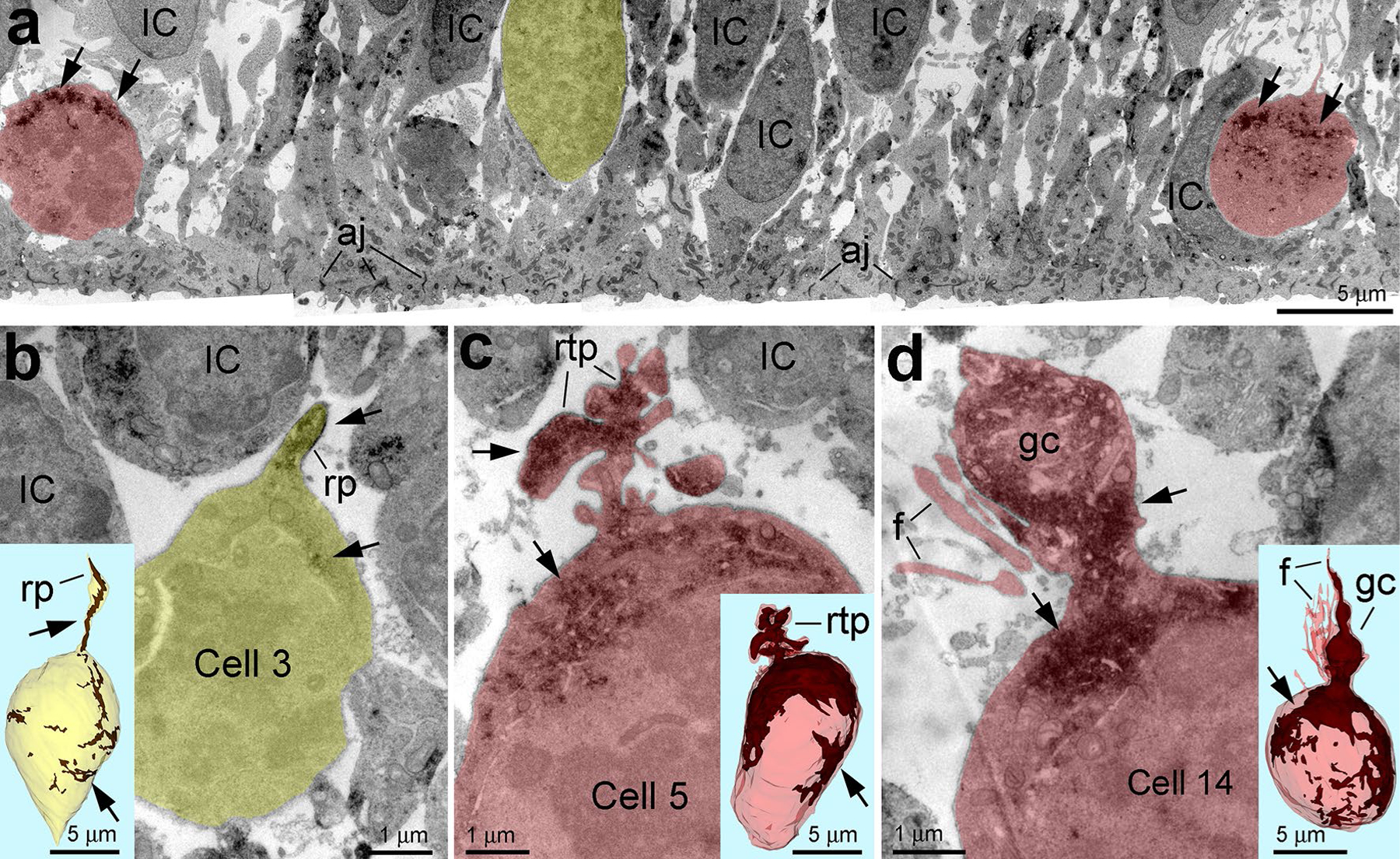
GFAP content is very variable in dividing VZ progenitors. a Differential expression of GFAP (arrows) in dividing cells in the VZ of E45 rhesus monkey embryo. Mitotic cells with low and high GFAP content are depicted semitransparent yellow and red, respectively. b–d Estimation of GFAP content in distinct cells using 3D reconstruction from uninterrupted serial sections. b Example of low GFAP expressing cell emitting potentially long radial process (rp) truncated in the serial sections. c, d High GFAP expressing cells emit short retracting-like process (rtp in c) or growth cone-like process (gc) and numerous filopodia (f in d). Insertions show 3D reconstructions of the cell bodies with GFAP immunoreaction end-product depositions (arrows; brown in the 3D images). Analyzed cells are numbered according to Table S1 and represented with basal aspect upwards. aj adherens junctions at the ventricular surface, IC interphase cells
Fig. 3.
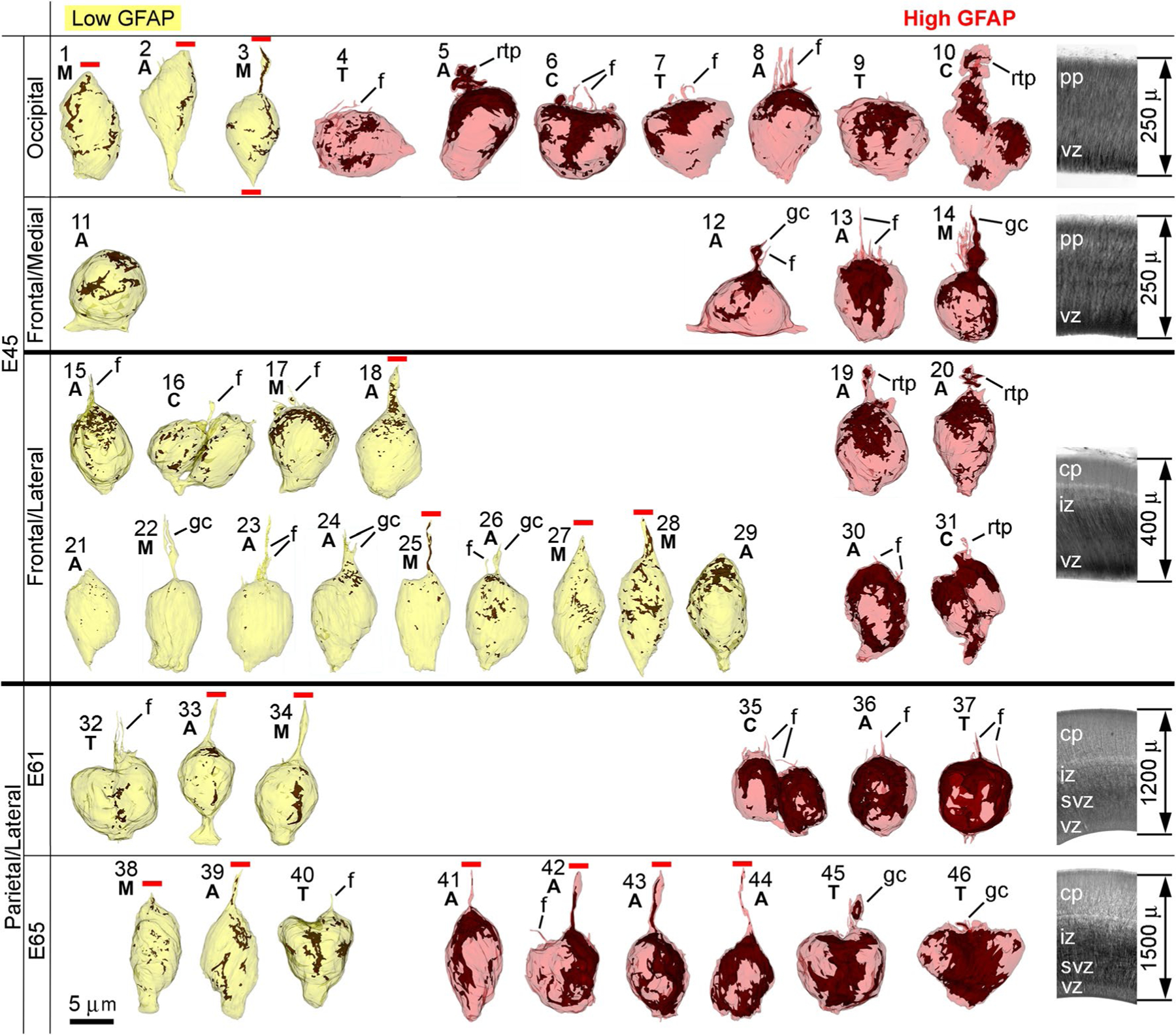
Montage of 3D reconstructions of the 46 cells analyzed. Age and cortical regions are indicated in the left side, and images of the cortical layering and thickness in the right side. GFAP immunolabeling is depicted in brown and cells are oriented with the basal segments upwards. Mitotic phases (M metaphase, A anaphase, T telophase, C cytokinesis) are indicated for each cell. Cells are ordered in ascending order of relative volume of GFAP immunolabeling (Table S1). Note the variability in the amount of immunolabeling and the morphology of the basal processes. Cells number 21, 22 and 23 show minute content of GFAP (albeit above background labeling; see Supplementary data for details). Truncated radial processes are indicated with horizontal red lines. Cells may emit growth cone-like processes (gc), retracting-like processes (rtp) or thin filopodia (f) terminated within the analyzed tissue segments. cp cortical plate, iz intermediate zone, pp preplate, svz subventricular zone, vz ventricular zone
Fig. 4.
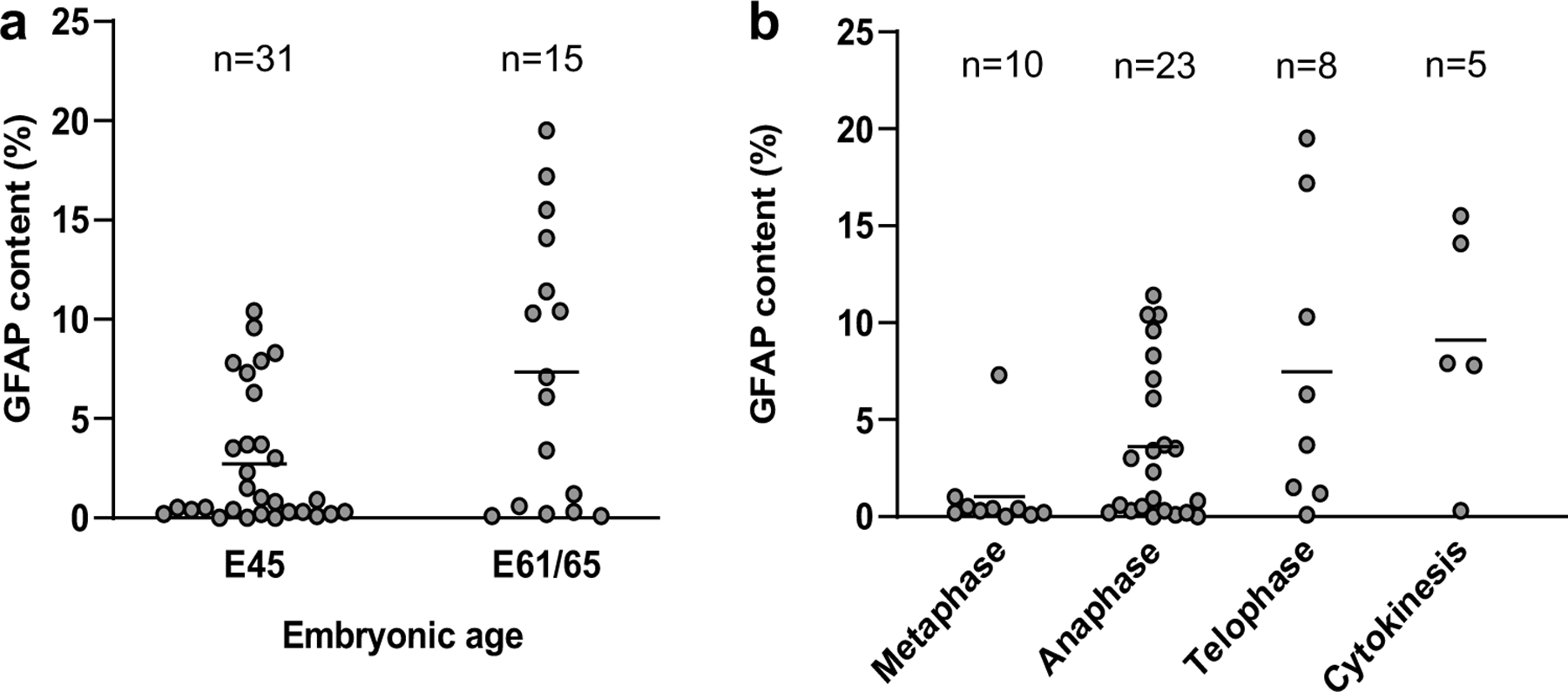
Changes of GFAP content per volume (%) with age and mitotic phase. a Average of GFAP content (horizontal line) almost doubles from E45 to E61/65 (p = 0.17). b Average GFAP content (horizontal line) increases as cell division progresses (Spearman rho = 0.48, p (2-tailed) = 0.00076). This trend was still present when considering ages separately (see main text)
Electron microscopy allows to evaluate the morphology and distribution of the chromatin and centrioles in dividing cells to assess their mitotic phase (Fig. S3). Hence, we found 10 cells in metaphase, 23 cells in anaphase, 8 cells in telophase and 5 cells in cytokinesis (Fig. 3). Interestingly, when considering GFAP content as mitosis progresses, a moderate correlation emerged: cells in metaphase showed on average low levels of GFAP that increased in the subsequent phases as mitosis progressed (Spearman rho = 0.48, p (2-tailed) = 0.00076; Fig. 4b). Experimental conditions (Fig. S2) or age did not seem to be a factor, as a trend was still observable when considering both ages independently (E45 rho = 0.43, p (2-tailed) = 0.016, n = 31; E61/65 rho = 0.51, p (2-tailed) = 0.055, n = 15; Fig. 4a). Therefore, this finding suggests that GFAP accumulates in the soma as mitosis progresses, probably related to the retraction of the basal process (see below).
We also looked at a possible asymmetric distribution of GFAP in the dividing cells. As neurogenesis is ongoing at E45 and E61/65, one can assume that most of the divisions in the VZ surface will be asymmetrical to produce either neurons directly, or more likely intermediate progenitors, that do not express GFAP [43, 44]. We found asymmetric distribution of GFAP in 3 cells in anaphase (out of 23 anaphase cells studied), when the morphology of the daughter cells starts to be defined (Fig. S3), but we did not observe asymmetry of GFAP content in the 13 cells in telophase and cytokinesis, where the daughter cells can be easily identified (Fig. 3). These findings suggest that segregation of GFAP is not common in progenitors, and the disappearance of GFAP in differentiating daughter cells might be consequence of quick depolymerization and degradation of GFAP after division is concluded.
Retraction of the Basal Process in Dividing Cortical VZ Progenitors
The apical endfeet of the cells analyzed presented adherens junctions and a cilium protruding to the ventricle during interphase (Fig. 5). We could not detect tight junctions, but instead we observed close junctions [31], located apical to the adherens junctions, consisting in a narrow apposition of the neighbor cell membranes separated by an intercellular space of 5–8 nm. (Fig. 5).
Fig. 5.
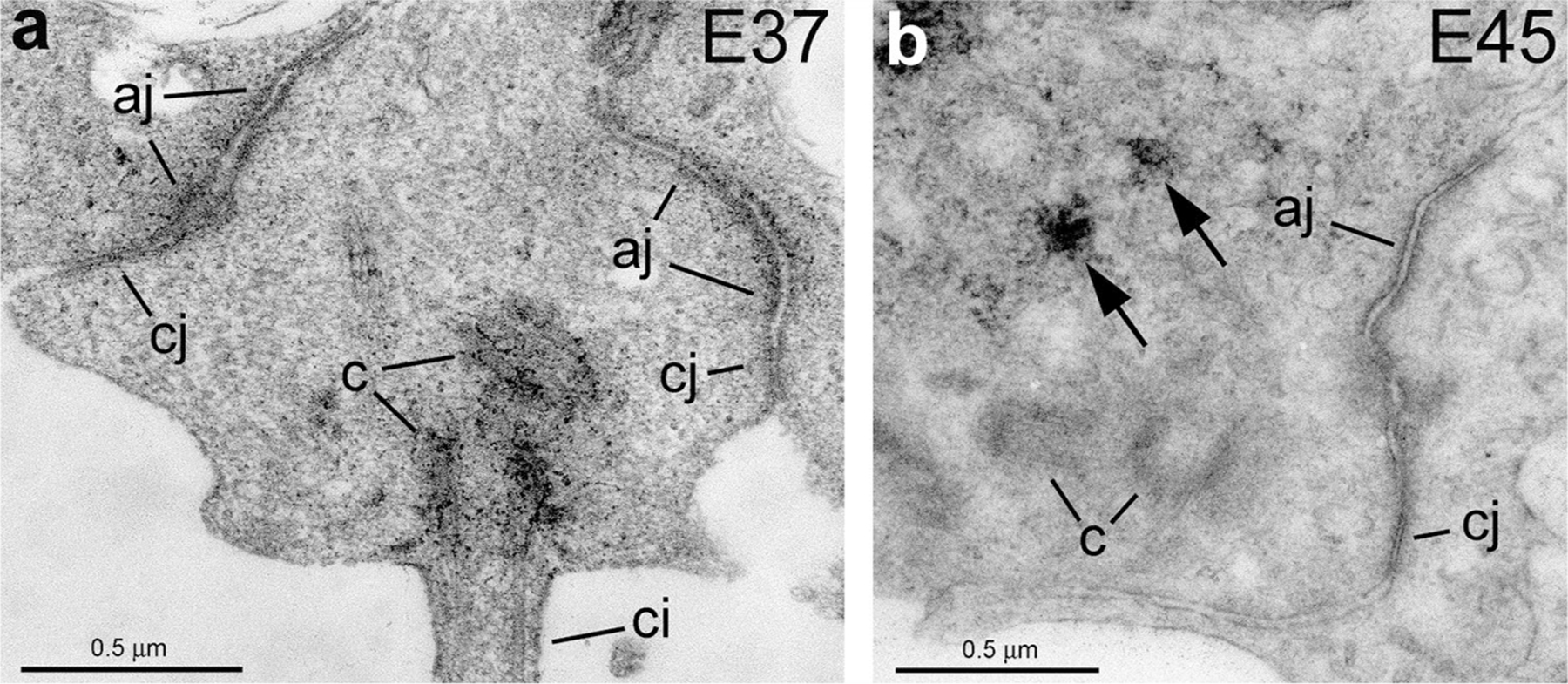
Close junctions in radial glial cells at E37 (a) and E45 (b). Close junctions with an intermembrane space of 5–8 nm were found in the most apical region of the ventricular endfoot at all ages analyzed. Arrows point to GFAP labeling. aj adherens junction, c centrioles, ci cilium, cj close junction
The basal aspect of the studied cells were heterogeneous and four different morphological types could be established based on the presence of: (i) smooth basal surface without protrusions or with thin filopodia (0.1–0.2 um in diameter; 19 cells); (ii) radial process ending in a growth cone-like structure usually accompanied by filopodia (8 cells); (iii) potentially long radial process (0.5–1.0 um in diameter; 16 cells) truncated in the serial sections that might reach the pial surface); or (iv) retracting-like radial process (5 cells) (Table S1). Thin filopodia may appear single or numerous in certain cells and do not contain detectable GFAP. Radial processes were always single and GFAP-positive (Figs. 2, 3). Retracting-like processes had a characteristic ultrastructure with several rounded-up lobes that differ from the sharp tips associated with filopodia in growth cone-like processes (see labels and compare cells 5 and 14 in Figs. 2 and 3). The presence of retracting-like basal processes in some cells (e.g. cells 19 and 20 in Fig. 3) and processes ending in growth cone-like structures (e.g. cells 12 and 14 in Fig. 3) suggests that the basal process in dividing progenitors may be a dynamic structure at the ages analyzed. We assessed chromatin morphology and centrosome positions to establish the mitotic phase of the studied cells and we found that all 16 cells that maintain potentially long radial process were in the initial stages of mitosis, meta- or anaphase, while none of the 13 cells in telophase or cytokinesis had a long process (see labels in Fig. 3). This suggests that radial processes might exist at the beginning of mitosis but they are retracted and no longer present at the time of cell division, and would regrow in the daughter cells. However, growth cone-like structures were detected in cells at meta-, ana- and telophase, when the process is supposed to be retracting, suggesting that the reabsorption of the process might not be a smooth process and can be accompanied by episodes of growth.
When correlating with GFAP levels at E45, cells emitting potentially long radial processes had the lower expression of GFAP, while cells showing either short or no processes had higher expression of GFAP. As illustrated in Fig. 3, most of the cells exhibiting a potentially long radial process (e.g. cells 3 and 18 in Fig. 3) are labeled in yellow indicating low GFAP content. Only cells #41–44 at E65 exhibit a putative long radial process in the cells labeled in red with high GFAP content. This appears counterintuitive to the idea that higher expression of GFAP would contribute to a more robust cytoskeletal scaffolding in RGCs with a long process. However, since technical constrains only allow us to study the cell body and proximal segment of the basal process, this might reflect the accumulation of depolymerized GFAP in the soma during retraction of the basal process. Nevertheless, some cells lacking a potential long radial process show low GFAP levels (e.g. cells 16 and 32 in Fig. 3), suggesting there might be a regulation of GFAP expression and/or degradation independent of the mitotic/morphologic stage of the cell.
Thus, the combined findings that dividing cells in the VZ surface contain some degree of GFAP and lack tight junctions in their endfeet, substituted by close junctions, strongly suggests that most if not all dividing cells in the VZ surface are RGCs, from early neurogenic stages. In addition, our data support a dynamic behavior (retraction/regrowth) of the radial processes during progenitor division at early neurogenic stages, and suggest that the retraction of the basal process contributes to the large differences in GFAP levels between VZ progenitors.
Variable GFAP Content in Interphase Progenitors
In the course of our analysis, we found interphase progenitors, identifiable by their apical endfoot bearing adherens junctions and a primary cilium, which exhibited variable amounts of GFAP immuno-precipitate, including cells that lacked presence of GFAP in single ultrathin sections. To assess the possibility of some interphase progenitors not expressing GFAP in the VZ, we performed 3D reconstruction of 5 arbitrarily chosen interphase cells showing immuno-negativity in single ultrathin sections of the cell body. We found relatively low GFAP content in all these cells: between 0.1 and 0.9% of anti-GFAP deposition by volume (Fig. S4 and Table S1). Thus, similar to mitotic cells, interphase cells exhibit large differences in GFAP content, and we could not find GFAP-negative cells.
Discussion
Radial glial cells (RGC) are the main neurogenic and gliogenic progenitor in the vertebrate brain. They originate from neuroepithelial cells (NEC) that populate and proliferate to expand the embryonic neural tube. After the closing of the neural tube in the prospective telencephalon, NECs transition to radial glial cells that will proliferate mostly asymmetrically, generating one radial glia cell and either one neuron or one intermediate progenitor cell that will self-renew and ultimately divide symmetrically to produce two neurons [1, 43].
The transition from NECs to RGCs implies that RGCs will retain some epithelial features such as their polarized morphology with an apical endfoot in the ventricle bearing adherens junctions and a primary cilium, and a longer basal process reaching to the pial surface. They will also maintain the expression of the intermediate filament nestin [19, 20], However, RGCs lose other epithelial features like the presence of tight junctions in the endfoot [45], while they gain typical astroglial characteristics like specific intermediate filaments vimentin and GFAP [17, 27], gap junctions or the presence of abundant glycogen granules [15–18].
Radial glia is therefore a peculiar cell type, with a mix of epithelial and glial features that is however ubiquitous in the developing vertebrate brain and plays a fundamental role as mother of both neurons and macroglia, astrocytes and oligodendrocytes. In addition, their radial processes serve as a guide for neurons to migrate from their birthplace in the ventricular surface to their final positions in the cortical plate [14]. This feature generates developmental radial units in the brain that serve to transfer the protomap of progenitors in the ventricular surface to the map of their progeny in the final destinations in the brain [46].
Expression of GFAP in Radial Glial Cells in the Vertebrate Brain
Glial fibrillary acidic protein is a type III intermediate filament similar to vimentin and desmin that is pretty conserved in the vertebrate lineage with the exception of Lampreys and Anurans [47, 48]. In non-mammalian vertebrates, several studies have shown that developmental radial glia express some degree of GFAP from early stages in fish [49–52], reptiles [53–55] and avians [56]. Amphibians offer a more complex picture: we lack data about caudata (newts and salamanders) regarding GFAP expression in developing radial glia (although is present in adult RGCs that remain as the main form of glia in this group [57]. However, anurans (frogs and toads) and some caecilians suffered a deletion of GFAP at the time of their differentiation from caudata, around 290 million years ago. Although they lack the GFAP gene, there is no observable phenotype, and they show the expected glial response after lesions, only lacking upregulation of GFAP [48]. This explains the lack of immunostaining in some studies [58], while the positive results in others [47, 59] have been proven to be caused by unspecific staining with GFAP antibodies that label other intermediate filaments such as vimentin, desmin, peripherin or α-internexin [48]. It is interesting to mention that in fish, amphibians and reptiles, which continue neurogenesis in certain regions during their entire life span [60], RGCs persists in the adult brain. Astrocytes start to appear throughout the brain in reptiles and they will become the dominant form of glia in avians and mammals [55, 61].
Overall, although GFAP is present in developing RGCsthroughout non-mammalian vertebrates, it has been consistently reported that its expression typically increases postnatally when RGCs and astrocytes establish themselves as the glial complement in these species [55, 62]. In primates, including humans, glial cells are more numerous than neurons and play crucial roles in brain homeostasis and defense against pathological insults [63].
In mammals, early studies on GFAP revealed it was present in RGCs in the developing cortex of humans and macaque monkeys [15–17, 37]. However, subsequent studies in other species like mice, rat, guinea pig or ferret showed radial glia lacking expression of GFAP that only appears at the end of neurogenesis when radial glia starts to transform into astrocytes [38, 64–68]. And this pattern seems to be the typical in mammals, where GFAP is selectively expressed in mature astrocytes, typically at low levels [36, 69], but it is highly upregulated in case of injury [70, 71]. Radial glia in mammals almost completely disappears after gliogenesis, however, specialized forms of radial glia subsist in certain brain regions such as tanycytes in the hypothalamus, [72], Bergmann cells in the cerebellum [73], Schwann cells [74], and reactive Muller cells in the retina [75].
Overall, the pattern that arises in vertebrates is that developmental RGCs express vimentin as the major intermediate filament. After neurogenesis, as developmental RGCs transition to adult RGCs or astrocytes, vimentin is progressively replaced by GFAP as the major intermediate filament in adult astrocytes/RGCs [55, 62, 68, 71]. The lower expression of GFAP in developmental RGCs in non-mammalian vertebrates seems to disappear in the mammalian line, except in primates, where GFAP is highly upregulated during the transformation of NECs into RGCs [16, 17]. The expression of GFAP in RGCs in the primate line is linked to changes in the GFAP promoter. Specifically, the human GFAP promoter has been used to induce strong GFAP expression in RGCs in mice [76, 77].
The specific expression of GFAP in astrocytes (and primate radial glia) suggests an important role in those cells. However, so far the function of GFAP has been elusive. In the 90 s, four different groups created KOs of GFAP in mice [78–81], and all had similar results: animals grew, behaved and reproduced normally, without major deficits, although a decrease in long term depression in the cerebellum was reported [82]. Those models were based on regular KO technology that imply lack of GFAP since early development, allowing compensation to mitigate the effects of the deletion. A similar scenario is present in anurans, where the lack of the GFAP gene might be compensated early on by other intermediate filaments such as vimentin. Inducible mouse KOs would be a better approach, since deletion of GFAP would be sudden during development or at adult stages, and would not allow for a quick compensatory response. However, inducible models are not available, maybe because the lack of phenotype in traditional models tempered the interest for further research. In this note, it is interesting to mention that KO mice for Vimentin and even the double KO for both Vimentin and GFAP showed a similar absence of clear phenotype, although some alterations of the glial response to injury were recorded [83, 84].
Overall, it seems that GFAP is not required for the function of RGCs or astrocytes, likely because it might be replaced by other intermediate filaments such as vimentin or desmin without consequence. However, if present, there is little flexibility for changes in the GFAP molecule, and variations of a single amino acid can result in severe or lethal forms of Alexander disease [71]. This rare disease is associated in 90% of cases with changes of one or several aminoacids of the GFAP protein, that at tissue level are manifested as Rosenthal fibers: accumulations of GFAP and other intermediate filaments such as vimentin and synemin occurring in astrocytes that can be detected both with light and electron microscopy. Alexander disease has homogeneous prevalence by age, sex or ethnicity, and produces a variety of symptoms that depends on the age and region of onset and includes developmental delay, seizures and intellectual disability (reviewed in [71]).
In this context, it is not clear if the upregulated expression of GFAP in primate RGCs is functionally relevant. However, it seems reasonable to speculate that the change in the regulatory elements of the GFAP gene could be beneficial for the enlarging primate brain, as radial glia in large brains extend several centimeters during cortical development, and the radial processes would benefit from a stronger cytoskeleton supported by GFAP [43]. Comparison with other species with large brains could give clues to support this hypothesis. Unfortunately, embryonic studies of large mammals are not common for obvious technical and logistical reasons, and as far as we know, only sheep and horse have been analyzed. In the sheep, three studies showed divergent results: one indicated that GFAP is present in radial glia at E50, although it is not illustrated [85] while two other reports showed expression in what looks like astrocytes, but only scarce and short putative radial fibers could be identified at different time from 8 to 20 weeks of gestation [86, 87]. Regarding horse, GFAP + cells were described in the developing pallium, but the figures in the paper suggests that radial glia does not express GFAP [88]. This limited sample of large brain mammals is not conclusive, and data from other groups such as cetaceans would be informative to validate this hypothesis. However, with all the available evidence, it seems that the early and strong expression of GFAP in primate radial glia might be an eccentricity in the peculiar distribution of GFAP in the vertebrate brain.
Transition of Neuroepithelial Cells to Radial Glial Cells
Our data show that GFAP is detected as soon as neurogenesis starts in the dorsal developing macaque cortex [89, 90] in agreement with previous data from our lab [17, 91]. The fact that all the dividing cells we found in the ventricular zone (VZ) were positive for GFAP, even if at low levels, suggests that the NEC to RGC transition occurs quickly, and therefore RGCs would become the main progenitors already during the early steps of neurogenesis. The presence of cells with low expression of GFAP undetectable in single ultrathin sections likely represents the population of cells lacking GFAP expression in the VZ of the macaque monkey previously described by our lab [16, 91].
One of the features of the transition from NECs to RGCs is the loss of functional tight junctions [45, 92, 93]. Early morphological studies in amphibians and chick showed that by the closing of the neural tube, tight junctions disappear while large gap junctions become smaller in the apical aspect of NECs [93, 94]. Later studies on mice confirmed these results, showing also the disappearance of occludin expression, a protein specific of tight junctions [45]. Several ultrastructural studies have reported the lack of tight junctions in RGCs in mice that seem to be replaced by close junctions [31], with an intermembrane space between 3–4 nm to 20 nm that could correspond to gap junctions [31, 95–97]. Other report described tight junctions in the early human embryo between 8 and 15 gestational weeks, but the data is not definitive, as the images provided suggest that the junctions are cut at oblique angle, and therefore do not show clear membrane appositions [98]. Our data shows close junctions with an intermembrane space of 5–8 nm located apical to the adherens junctions at all ages studied, E42, E61/65 (Fig. 5) and also at E80 (data not shown). They resemble the junctions described in amphibians by Decker and Friend [93] and by Hinds in similar positions in the ventricular surface of the mouse at E13 and E15 that he described as potential gap junctions [31, 99]. However, neither their study nor our data allows identification of any specific feature of gap junctions.
Dynamics of the RGC Basal Process During Cell Division
The earliest description of radial cells in the developing neural tube by His in 1889 already made a difference between the elongated radial cells that he called spongioblasts and the rounded dividing cells located in the ventricular surface he called germinal cells. However, soon afterwards scientists concluded that both were the same cell type at different cell cycle phases [13, 100]. Sauer proposed the interkinetic nuclear movement to explain how the transition occurs [101], and the idea of radial cells rounding up and losing their radial process during mitosis became associated to the dynamics of NECs and RGCs.
Sauer described mitotic cells in the VZ as round up, assuming the radial process would be reabsorbed before mitosis [101]. With few exceptions (see [102]), subsequent ultrastructural studies in several species including chick, rabbit and mouse confirmed Sauer observations, showing round mitotic cells without traceable radial processes, and intermediate stages of process retraction and growth at early mitotic phases and early interphase cells respectively [31, 32, 99, 103–105]. Specifically, in the cortical ventricular surface of the mouse at E13, Hinds and Ruffet described that dividing cells in interphase and early prophase had a long basal process, cells in metaphase had short or no processes while cells in anaphase and early telophase were all rounded [31].
Subsequent ex-vivo studies using fluorescent markers to visualize the dynamics of RGC proliferation in the developing cortex, showed a different scenario, in which radial progenitors would divide without losing the radial process [33, 34, 106, 107]. However, these studies described different events, particularly after division. Noctor and coworkers described that the basal process was inherited by the progenitor remaining in the proliferative zone [33, 107], while Miyata and coworkers reported the process is inherited by the cell migrating basally to the cortex [34]. Also, Das and collaborators, studying the retina of zebrafish, described that all clear analyzed progenitor divisions showed a basal process, and it was inherited by one daughter cell, while the other daughter cell grew a process quickly after division [106]. However, other studies analyzing developing retina in newborn rats indicated that in about 80% of the cells they could not detect a basal process during mitosis [108]. Interestingly, Das and collaborators reported one case in which the cell that did not inherited the basal process divided again after 18 h. [106], and Cayouette and coworkers indicated that when they could follow the daughter cells in cases in which the basal process was present, the process was consistently inherited by the cell that occupied a more basal position [108], suggesting that, when the process is present during division, is typically inherited by the differentiating daughter cell that will take a basal position, similar to the findings of Miyata and collaborators [34].
Our data strongly suggests that the process is retracted during mitosis. The fact that all cells in telophase or cytokinesis lack long processes and exhibit mostly round morphologies or short retracting processes and filopodia clearly agrees with previous EM studies described above. The only caveat to this hypothesis is the presence of short processes with growth cones in cells in meta-, ana- and telophase that suggests growth at a time when the process is expected to be retracting. Cells growing a basal process during cell division have not been observed and it is unlikely since is metabolically expensive and during mitosis the cell machinery is focused on the complex dynamic of cell division, the condensed chromosomes do not facilitate gene expression and protein synthesis is highly reduced [109]. One possibility to explain this observation is that the retraction of the process is not a smooth phenomenon, and can alternate with brief, spurious bouts of growth. This alternation between growth and retraction is characteristic of growing processes [110] and might be also present in retracting ones.
The reason for the different results between EM studies like ours and ex-vivo imaging data is not totally clear, although it might be related to different methodology, species and systems analyzed. Recently, Subramanian and collaborators proposed that timing is the key factor, as they show that NECs normally retract their processes when analyzed in early human telencephalic VZ at 8–10 gestational weeks (GW), while RGCs, showing longer radial processes, tend to maintain their basal process when dividing later on, between 9 and 10 GW [35]. Similar results were found in brain organoids after 5 or 10 weeks in culture: NECs in the early organoids retracted their process while later RGCs in the late organoids preserved their basal process during division [35]. Although this might be correct, still it is not clear why ultrastructural studies carried out during equivalent or even later stages of neurogenic RGC proliferation, including ours, consistently show rounding mitotic RGCs with evidence of basal process retraction and regrowth after cytokinesis. Live imaging of the basal process during mitosis is difficult, as it becomes almost undetectable, thin and varicose [33, 34, 107]. This suggest that during mitosis it might become extremely thin, like a long filopodium, that is either broken during the processing of the tissue for EM study, or is missed in the analysis. However, as described above, we and other authors [31, 32] were able to observe and reconstruct such thin filopodia, but failed to find a long process in late mitotic cells. Therefore, we believe that there might be a more complex picture in which most radial glial cells retract their processes, while some maintain them during the whole division process.
GFAP Content Changes Along the Cell Cycle
We observed a correlation between the content of GFAP and the mitotic phase of the dividing cells. Cells entering mitoses showed in average low content of GFAP in the soma that progressively increased as they advance in the mitotic phases, reaching average highest levels at telophase and cytokinesis (Fig. 4c). In fact, glioma cells in vitro show an inverse relation between GFAP expression and proliferation [111–113] and primary cultures of astrocytes from mice lacking GFAP show increased proliferation [114]. In addition, since the chromosomes duplicate and condensate for division, de novo GFAP synthesis does not seem a likely possibility. One possible explanation is that the increase in GFAP in the soma originates from the accumulation of depolymerized GFAP from the retraction of the basal process during the mitotic cycle. The relative accumulation of GFAP in the basal region of the dividing cell, close to the process when it is present, supports this possibility. Our observations in dividing cells only revealed 3 cell in anaphase with asymmetric distribution of GFAP between daughter cells, but we could not detect asymmetry in the distribution of GFAP in cells in telophase and cytokinesis, when daughter cells are more clearly identifiable and closer to scission. Since most cell division at these times is supposed to be asymmetric, it suggests that in most cases, GFAP may be inherited by daughter RGCs but also initially by differentiating daughter cells, either intermediate progenitors or neurons. It is likely though that the degradation of GFAP occurs quickly during the last steps of cytokinesis or early after division, since intermediate progenitors and neurons by definition do not express or contain GFAP. Nevertheless, some cells lacking a radial process show low GFAP levels (Fig. 3), suggesting there might be more processes at play regulating the content of GFAP, independent of the mitotic/morphologic stage of the cell. Studies in astrocytes in culture has described disassembly of GFAP by phosphorylation during mitosis, especially around the cleavage furrow [115, 116], and organized distribution of GFAP structures during cell division, that suggested a participation in the mitotic process [117, 118]. Our data do not show a particular organization of GFAP in dividing cells, and we found large heterogeneity in the amount and distribution of GFAP, precluding any possible functional conclusion.
We also observed large differences in the amount of GFAP content in cells in interphase, suggesting a possible role in progenitors during quiescence. One possibility is different timing after cell division. According to our data, at cytokinesis, progenitors exhibit relatively large amounts of GFAP in the soma but lack a basal process. After mitosis, GFAP content in the soma might change as GFAP is trafficked to the growing basal process, explaining the differences we observe. It is possible that the interphase cells with low content of GFAP observed here represent quiescent progenitors with a grown process that may exhibit relatively high content of GFAP in the process but low GFAP levels in the soma, as has been consistently observed in cells of the astroglial lineage [71].
In summary, in this study we revisit important questions regarding the biology and dynamics of cortical progenitors in vertebrates. We started with data obtained with a very specific technique: EM analysis of GFAP stained VZ cells of the developing macaque neocortex. However, combining 3D cell reconstruction and ultrastructural analysis with GFAP expression, we produced a limited but unique data set that served as a primer to discuss some interesting questions about early neocortical progenitors in the vertebrate brain, such as the relationship between NECs and RGCs, the differential expression of GFAP in progenitors between species or the dynamics of the basal process in RGCs.
Further studies are required to conclusively answer these questions, but our current data and review, although limited in its scope, allows shed some light on specific questions and hopefully will inspire more studies on these topics. We are currently using longitudinal transcriptomic analysis during macaque neurogenesis that will help pursuing that aim and more generally, shed light on how differential gene expression in the RGC population configures the VZ/SVZ protomap that will instruct the neurons aimed for different regions and layers in the cortex, and the subsequent production of specific types of glial cells.
Supplementary Material
Acknowledgements
This work supported in part by the National Institutes of Health NIDA grant DA023999. We thank Dr. Alvaro Duque, Yale Department of Neuroscience and MacBrainResource which is supported by MH113257 to Alvaro Duque at Yale Medical School.
Footnotes
Special Issue: In Honor of Prof. Vladimir Parpura.
Supplementary Information The online version containssupplementary material available at (https://doi.org/10.1007/s11064–021-03296-z.
Declarations
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval Experiments were performed with approval from the ethics committee and IACUC at Yale University.
References
- 1.Rakic P (2009) Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci 10(10):724–735. 10.1038/nrn2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori T, Buffo A, Götz M (2005) The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol 69:67–99. 10.1016/s0070-2153(05)69004-7 [DOI] [PubMed] [Google Scholar]
- 3.Breunig JJ, Haydar TF, Rakic P (2011) Neural stem cells: historical perspective and future prospects. Neuron 70(4):614–625. 10.1016/j.neuron.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haydar TF, Wang F, Schwartz ML, Rakic P (2000) Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci 20(15):5764–5774. 10.1523/jneurosci.20-15-05764.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huttner WB, Kosodo Y (2005) Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol 17(6):648–657. 10.1016/j.ceb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Goderie SK, Temple S (2005) Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron 45(6):873–886. 10.1016/j.neuron.2005.01.045 [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Buylla A, Kriegstein A (2013) Neural stem cells among glia. Patterning and cell type specification in the developing CNS and PNS Academic Press, Cambridge, pp 685–705. 10.1016/b978-0-12-397265-1.00079-4 [DOI] [Google Scholar]
- 8.His W (1889) Die Neuroblasten und deren Entstehung im embrionalen Mark. Abh Kgl Sachs Ges Wissensch Math Phys Kl 15:311–372 [Google Scholar]
- 9.Koelliker A (1879) Entwicklungsgeschichte des Menschen und der hoeheren Thiere W. Engelmann, Leipzig [Google Scholar]
- 10.Magini G (1888) Nevroglia e cellule nervose cerebrali nei feti, vol 1. Atti del Dodicesimo Congresso della Associazione Medica Italiana Tipografia Fratelli Fusi, Pavia [Google Scholar]
- 11.Golgi C (1885) Sulla fina anatomia degli organi centrali del sistema nervoso. Tipografia di Stefano Calderini e Figlio, Reggio Emilia
- 12.Lenhossek M (1893) Der feinere bau des Nervensystems in Lichte neuester Forschung. In: Kornfeld H (ed) Fischer’s Medicinische Buchhandlung. Fischer, Berlin [Google Scholar]
- 13.Ramón y Cajal S (1909) Histologie du système nerveux de l’homme et des vertébrés A. Maloine, Paris [Google Scholar]
- 14.Rakic P (1972) Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145(1):61–83. 10.1002/cne.901450105 [DOI] [PubMed] [Google Scholar]
- 15.Choi BH, Lapham LW (1978) Radial glia in the human fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res 148(2):295–311. 10.1016/0006-8993(78)90721-7 [DOI] [PubMed] [Google Scholar]
- 16.Levitt P, Cooper ML, Rakic P (1983) Early divergence and changing proportions of neuronal and glial precursor cells in the primate cerebral ventricular zone. Dev Biol 96(2):472–484. 10.1016/0012-1606(83)90184-7 [DOI] [PubMed] [Google Scholar]
- 17.Levitt P, Rakic P (1980) Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol 193(3):815–840. 10.1002/cne.901930316 [DOI] [PubMed] [Google Scholar]
- 18.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ (1997) Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci 17(18):7037–7044. 10.1523/jneurosci.17-18-07037.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hockfield S, McKay RD (1985) Identification of major cell classes in the developing mammalian nervous system. J Neurosci 5(12):3310–3328. 10.1523/jneurosci.05-12-03310.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60(4):585–595. 10.1016/0092-8674(90)90662-x [DOI] [PubMed] [Google Scholar]
- 21.Edwards MA, Yamamoto M, Caviness VS Jr (1990) Organization of radial glia and related cells in the developing murine CNS. An analysis based upon a new monoclonal antibody marker. Neuroscience 36(1):121–144. 10.1016/0306-4522(90)90356-9 [DOI] [PubMed] [Google Scholar]
- 22.Misson JP, Edwards MA, Yamamoto M, Caviness VS Jr (1988) Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Brain Res Dev Brain Res 44(1):95–108. 10.1016/0165-3806(88)90121-6 [DOI] [PubMed] [Google Scholar]
- 23.Park D, Xiang AP, Zhang L, Mao FF, Walton NM, Choi SS, Lahn BT (2009) The radial glia antibody RC2 recognizes a protein encoded by Nestin. Biochem Biophys Res Commun 382(3):588–592. 10.1016/j.bbrc.2009.03.074 [DOI] [PubMed] [Google Scholar]
- 24.Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12(4):895–908. 10.1016/0896-6273(94)90341-7 [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y (1997) Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci 17(23):9212–9219. 10.1523/jneurosci.17-23-09212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akimoto J, Itoh H, Miwa T, Ikeda K (1993) Immunohistochemical study of glutamine synthetase expression in early glial development. Brain Res Dev Brain Res 72(1):9–14. 10.1016/0165-3806(93)90154-3 [DOI] [PubMed] [Google Scholar]
- 27.Schnitzer J, Franke WW, Schachner M (1981) Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol 90(2):435–447. 10.1083/jcb.90.2.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker RP, Brunso-Bechtold JK, Jenrath DA, Khan NA, Poss PM, Sweatt AJ, Xu Y (1994) Cellular origins of tenascin in the developing nervous system. Perspect Dev Neurobiol 2(1):89–99. 10.1080/0907676x.1994.9961226 [DOI] [PubMed] [Google Scholar]
- 29.Schmechel DE, Rakic P (1979) A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 156(2):115–152. 10.1007/bf00300010 [DOI] [PubMed] [Google Scholar]
- 30.Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: boulder committee revisited. Nat Rev Neurosci 9(2):110–122. 10.1038/nrn2252 [DOI] [PubMed] [Google Scholar]
- 31.Hinds JW, Ruffett TL (1971) Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat 115(2):226–264. 10.1007/bf00391127 [DOI] [PubMed] [Google Scholar]
- 32.Seymour RM, Berry M (1975) Scanning and transmission electron microscope studies of interkinetic nuclear migration in the cerebral vesicles of the rat. J Comp Neurol 160(1):105–125. 10.1002/cne.901600107 [DOI] [PubMed] [Google Scholar]
- 33.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409(6821):714–720. 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- 34.Miyata T, Kawaguchi A, Okano H, Ogawa M (2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31(5):727–741. 10.1016/s0896-6273(01)00420-2 [DOI] [PubMed] [Google Scholar]
- 35.Subramanian L, Bershteyn M, Paredes MF, Kriegstein AR (2017) Dynamic behaviour of human neuroepithelial cells in the developing forebrain. Nat Commun 8:14167. 10.1038/ncomms14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43(2):429–435. 10.1016/0006-8993(72)90398-8 [DOI] [PubMed] [Google Scholar]
- 37.Antanitus DS, Choi BH, Lapham LW (1976) The demonstration of glial fibrillary acidic protein in the cerebrum of the human fetus by indirect immunofluorescence. Brain Res 103(3):613–616. 10.1016/0006-8993(76)90464-9 [DOI] [PubMed] [Google Scholar]
- 38.Sancho-Tello M, Vallés S, Montoliu C, Renau-Piqueras J, Guerri C (1995) Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia 15(2):157–166. 10.1002/glia.440150208 [DOI] [PubMed] [Google Scholar]
- 39.Bignami A, Dahl D (1989) Vimentin-GFAP transition in primary dissociated cultures of rat embryo spinal cord. Int J Dev Neurosci 7(4):343–357. 10.1016/0736-5748(89)90056-7 [DOI] [PubMed] [Google Scholar]
- 40.Morozov YM, Ayoub AE, Rakic P (2006) Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci 26(19):5017–5027. 10.1523/jneurosci.0272-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morozov YM, Koch M, Rakic P, Horvath TL (2017) Cannabinoid type 1 receptor-containing axons innervate NPY/AgRP neurons in the mouse arcuate nucleus. Mol Metab 6(4):374–381. 10.1016/j.molmet.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morozov YM, Sun YY, Kuan CY, Rakic P (2016) Alteration of SLP2-like immunolabeling in mitochondria signifies early cellular damage in developing and adult mouse brain. Eur J Neurosci 43(2):245–257. 10.1111/ejn.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molnár Z, Clowry GJ, Šestan N, Alzu’bi A, Bakken T, Hevner RF, Hüppi PS, Kostović I, Rakic P, Anton ES, Edwards D, Garcez P, Hoerder-Suabedissen A, Kriegstein A (2019) New insights into the development of the human cerebral cortex. J Anat 235(3):432–451. 10.1111/joa.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF (2005) Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci 25(1):247–251. 10.1523/jneurosci.2899-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aaku-Saraste E, Hellwig A, Huttner WB (1996) Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure–remodeling of the neuroepithelium prior to neurogenesis. Dev Biol 180(2):664–679. 10.1006/dbio.1996.0336 [DOI] [PubMed] [Google Scholar]
- 46.Rakic P (1988) Specification of cerebral cortical areas. Science 241(4862):170–176. 10.1126/science.3291116 [DOI] [PubMed] [Google Scholar]
- 47.Dahl D, Bignami A (1973) Immunochemical and immunofluorescence studies of the glial fibrillary acidic protein in vertebrates. Brain Res 61:279–293. 10.1016/0006-8993(73)90533-7 [DOI] [PubMed] [Google Scholar]
- 48.Martinez-De Luna RI, Ku RY, Aruck AM, Santiago F, Viczian AS, San Mauro D, Zuber ME (2017) Muller glia reactivity follows retinal injury despite the absence of the glial fibrillary acidic protein gene in Xenopus. Dev Biol 426(2):219–235. 10.1016/j.ydbio.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Docampo-Seara A, Santos-Duran GN, Candal E, Rodriguez Diaz MA (2019) Expression of radial glial markers (GFAP, BLBP and GS) during telencephalic development in the catshark (Scyliorhinus canicula). Brain Struct Funct 224(1):33–56. 10.1007/s00429-018-1758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arochena M, Anadón R, Díaz-Regueira SM (2004) Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J Comp Neurol 469(3):413–436. 10.1002/cne.11021 [DOI] [PubMed] [Google Scholar]
- 51.Marcus RC, Easter SS Jr (1995) Expression of glial fibrillary acidic protein and its relation to tract formation in embryonic zebrafish (Danio rerio). J Comp Neurol 359(3):365–381. 10.1002/cne.903590302 [DOI] [PubMed] [Google Scholar]
- 52.Johnson K, Barragan J, Bashiruddin S, Smith CJ, Tyrrell C, Parsons MJ, Doris R, Kucenas S, Downes GB, Velez CM, Schneider C, Sakai C, Pathak N, Anderson K, Stein R, Devoto SH, Mumm JS, Barresi MJ (2016) Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord. Glia 64(7):1170–1189. 10.1002/glia.22990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monzon-Mayor M, Yanes C, Ghandour MS, de Barry J, Gombos G (1990) Glial fibrillary acidic protein and vimentin immunohistochemistry in the developing and adult midbrain of the lizard Gallotia galloti. J Comp Neurol 295(4):569–579. 10.1002/cne.902950406 [DOI] [PubMed] [Google Scholar]
- 54.Yanes C, Monzon-Mayor M, Ghandour MS, de Barry J, Gombos G (1990) Radial glia and astrocytes in developing and adult telencephalon of the lizard Gallotia galloti as revealed by immunohistochemistry with anti-GFAP and anti-vimentin antibodies. J Comp Neurol 295(4):559–568. 10.1002/cne.902950405 [DOI] [PubMed] [Google Scholar]
- 55.Kálmán M, Pritz MB (2001) Glial fibrillary acidic protein-immunopositive structures in the brain of a Crocodilian, Caiman crocodilus, and its bearing on the evolution of astroglia. J Comp Neurol 431(4):460–480. [DOI] [PubMed] [Google Scholar]
- 56.Tapscott SJ, Bennett GS, Toyama Y, Kleinbart F, Holtzer H (1981) Intermediate filament proteins in the developing chick spinal cord. Dev Biol 86(1):40–54. 10.1016/0012-606(81)90313-4 [DOI] [PubMed] [Google Scholar]
- 57.Naujoks-Manteuffel C, Roth G (1989) Astroglial cells in a salamander brain (Salamandra salamandra) as compared to mammals: a glial fibrillary acidic protein immunohistochemistry study. Brain Res 487(2):397–401. 10.1016/0006-8993(89)90849-4 [DOI] [PubMed] [Google Scholar]
- 58.Onteniente B, Kimura H, Maeda T (1983) Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol 215(4):427–436. 10.1002/cne.902150407 [DOI] [PubMed] [Google Scholar]
- 59.Messenger NJ, Warner AE (1989) The appearance of neural and glial cell markers during early development of the nervous system in the amphibian embryo. Development 107(1):43–54 [DOI] [PubMed] [Google Scholar]
- 60.Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR (2003) Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex 13(6):550–559. 10.1093/cercor/13.6.550 [DOI] [PubMed] [Google Scholar]
- 61.Verkhratsky A, Ho MS, Parpura V (2019) Evolution of neuroglia. Adv Exp Med Biol 1175:15–44. 10.1007/978-981-13-9913-8_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bettini S, Lazzari M, Franceschini V (2019) Molecular markers in the study of non-model vertebrates: their significant contributions to the current knowledge of tetrapod glial cells and fish olfactory neurons. Results Probl Cell Differ 68:355–377. 10.1007/978-3-030-23459-1_15 [DOI] [PubMed] [Google Scholar]
- 63.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121(1):4–27. 10.1111/j.1471-4159.2012.07664.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bovolenta P, Liem RK, Mason CA (1987) Glial filament protein expression in astroglia in the mouse visual pathway. Brain Res 430(1):113–126. 10.1016/0165-3806(87)90181-7 [DOI] [PubMed] [Google Scholar]
- 65.Bovolenta P, Liem RK, Mason CA (1984) Development of cerebellar astroglia: transitions in form and cytoskeletal content. Dev Biol 102(1):248–259. 10.1016/0012-1606(84)90189-1 [DOI] [PubMed] [Google Scholar]
- 66.Landry CF, Ivy GO, Brown IR (1990) Developmental expression of glial fibrillary acidic protein mRNA in the rat brain analyzed by in situ hybridization. J Neurosci Res 25(2):194–203. 10.1002/jnr.490250207 [DOI] [PubMed] [Google Scholar]
- 67.Nitsos I, Rees S (1990) The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int J Dev Neurosci 8(3):233–244. 10.1016/0736-5748(90)90029-2 [DOI] [PubMed] [Google Scholar]
- 68.Voigt T (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289(1):74–88. 10.1002/cne.902890106 [DOI] [PubMed] [Google Scholar]
- 69.Raff MC, Fields KL, Hakomori SI, Mirsky R, Pruss RM, Winter J (1979) Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res 174(2):283–308. 10.1016/0006-8993(79)90851-5 [DOI] [PubMed] [Google Scholar]
- 70.Clarke SR, Shetty AK, Bradley JL, Turner DA (1994) Reactive astrocytes express the embryonic intermediate neurofilament nestin. NeuroReport 5(15):1885–1888. 10.1097/00001756-199410000-00011 [DOI] [PubMed] [Google Scholar]
- 71.Messing A, Brenner M (2020) GFAP at 50. ASN Neuro 12:1759091420949680. 10.1177/1759091420949680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Vitry F, Picart R, Jacque C, Tixier-Vidal A (1981) Glial fibrillary acidic protein. A cellular marker of tanycytes in the mouse hypothalamus. Dev Neurosci 4(6):457–460. 10.1159/000112813 [DOI] [PubMed] [Google Scholar]
- 73.Lazarides E (1982) Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem 51:219–250. 10.1146/annurev.bi.51.070182.001251 [DOI] [PubMed] [Google Scholar]
- 74.Yen SH, Fields KL (1981) Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol 88(1):115–126. 10.1083/jcb.88.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bignami A (1984) Glial fibrillary acidic (GFA) protein in Müller glia. Immunofluorescence study of the goldfish retina. Brain Res 300(1):175–178. 10.1016/0006-8993(84)91355-6 [DOI] [PubMed] [Google Scholar]
- 76.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A (1994) GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 14(3 Pt 1):1030–1037. 10.1523/jneurosci.14-03-01030.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A (2001) hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31(2):85–94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]
- 78.Gomi H, Yokoyama T, Fujimoto K, Ikeda T, Katoh A, Itoh T, Itohara S (1995) Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14(1):29–41. 10.1016/0896-6273(95)90238-4 [DOI] [PubMed] [Google Scholar]
- 79.Pekny M, Levéen P, Pekna M, Eliasson C, Berthold CH, Westermark B, Betsholtz C (1995) Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. Embo J 14(8):1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS (1996) GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17(4):607–615. 10.1016/s0896-6273(00)80194-4 [DOI] [PubMed] [Google Scholar]
- 81.McCall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, Zhang CL, Pearce RA, Chiu SY, Messing A (1996) Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci USA 93(13):6361–6366. 10.1073/pnas.93.13.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, Chen C, Thompson RF, Itohara S (1996) Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron 16(3):587–599. 10.1016/s0896-6273(00)80078-1 [DOI] [PubMed] [Google Scholar]
- 83.Galou M, Colucci-Guyon E, Ensergueix D, Ridet JL, Gimenez y Ribotta M, Privat A, Babinet C, Dupouey P (1996) Disrupted glial fibrillary acidic protein network in astrocytes from vimentin knockout mice. J Cell Biol 133(4):853–863. 10.1083/jcb.133.4.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallén A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisén J (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145(3):503–514. 10.1083/jcb.145.3.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dieriks BV, Dean JM, Aronica E, Waldvogel HJ, Faull RLM, Curtis MA (2018) Differential fatty acid-binding protein expression in persistent radial glia in the human and sheep subventricular zone. Dev Neurosci 40(2):145–161. 10.1159/000487633 [DOI] [PubMed] [Google Scholar]
- 86.Salouci M, Antoine N, Shikh Al Sook MK, Piret J, Mignon Y, Kirschvink N, Gabriel A (2014) Developmental profiles of GFAP-positive astrocytes in sheep cerebellum. Vet Res Commun 38(4):279–285. 10.1007/s11259-014-9614-1 [DOI] [PubMed] [Google Scholar]
- 87.Rana S (2019) Structural changes during fetal development of the gyrified brain: clues on determinants of cortical folding. School of Clinical Sciences, Monash University, Clayton [Google Scholar]
- 88.Rigoglio NN, Barreto RS, Favaron PO, Jacob JC, Smith LC, Gastal MO, Gastal EL, Miglino MA (2017) Central nervous system and vertebrae development in horses: a chronological study with differential temporal expression of nestin and GFAP. J Mol Neurosci 61(1):61–78. 10.1007/s12031-016-0805-9 [DOI] [PubMed] [Google Scholar]
- 89.Granger B, Tekaia F, Le Sourd AM, Rakic P, Bourgeois JP (1995) Tempo of neurogenesis and synaptogenesis in the primate cingulate mesocortex: comparison with the neocortex. J Comp Neurol 360(2):363–376. 10.1002/cne.903600212 [DOI] [PubMed] [Google Scholar]
- 90.Rakic P (1974) Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183(4123):425–427. 10.1126/science.183.4123.425 [DOI] [PubMed] [Google Scholar]
- 91.Levitt P, Cooper ML, Rakic P (1981) Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci 1(1):27–39. 10.1523/jneurosci.01-01-00027.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farquhar MG, Palade GE (1963) Junctional complexes in various epithelia. J Cell Biol 17(2):375–412. 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Decker RS, Friend DS (1974) Assembly of gap junctions during amphibian neurulation. J Cell Biol 62(1):32–47. 10.1083/jcb.62.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Revel JP, Brown SS (1976) Cell junctions in development, with particular reference to the neural tube. Cold Spring Harb Symp Quant Biol 40:443–455. 10.1101/sqb.1976.040.01.042 [DOI] [PubMed] [Google Scholar]
- 95.Brightman MW, Reese TS (1969) Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40(3):648–677. 10.1083/jcb.40.3.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Revel JP, Karnovsky MJ (1967) Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol 33(3):C7–c12. 10.1083/jcb.33.3.c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nadarajah B, Jones AM, Evans WH, Parnavelas JG (1997) Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci 17(9):3096–3111. 10.1523/jneurosci.17-09-03096.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duckett S (1968) The germinal layer of the growing human brain during early fetal life. Anat Rec 161(2):231–245. 10.1002/ar.1091610208 [DOI] [PubMed] [Google Scholar]
- 99.Shoukimas GM, Hinds JW (1978) The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol 179(4):795–830. 10.1002/cne.901790407 [DOI] [PubMed] [Google Scholar]
- 100.Koelliker A (1896) Handbuch der Gewebelehre des Menschen, 6th edn. W. Engelmann, Leipzig [Google Scholar]
- 101.Sauer FC (1935) Mitosis in the neural tube. J Comp Neurol 62:377–405 [Google Scholar]
- 102.Berry M, Rogers AW (1965) The migration of neuroblasts in the developing cerebral cortex. J Anat 99(Pt 4):691–709 [PMC free article] [PubMed] [Google Scholar]
- 103.Fujita H, Fujita S (1963) Electron microscopic studies on neuroblast differentiation in the central nervous system of domestic fowl. Z Zellforsch Mikrosk Anat 60:463–478. 10.1007/bf00336619 [DOI] [PubMed] [Google Scholar]
- 104.Stensaas LJ, Stensaas SS (1968) An electron microscope study of cells in the matrix and intermediate laminae of the cerebral hemisphere of the 45 mm rabbit embryo. Z Zellforsch Mikrosk Anat 91(3):341–365. 10.1007/bf00440763 [DOI] [PubMed] [Google Scholar]
- 105.Hinds JW, Hinds PL (1974) Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol 37(2):381–416. 10.1016/0012-1606(74)90156-0 [DOI] [PubMed] [Google Scholar]
- 106.Das T, Payer B, Cayouette M, Harris WA (2003) In vivo timelapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron 37(4):597–609. 10.1016/s0896-6273(03)00066-7 [DOI] [PubMed] [Google Scholar]
- 107.Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR (2002) Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 22(8):3161–3173. 10.1523/jneurosci.22-08-03161.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cayouette M, Raff M (2003) The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130(11):2329–2339. 10.1242/dev.00446 [DOI] [PubMed] [Google Scholar]
- 109.Tanenbaum ME, Stern-Ginossar N, Weissman JS, Vale RD (2015) Regulation of mRNA translation during mitosis. eLife 4:e07957. 10.7554/eLife.07957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rauch P, Heine P, Goettgens B, Käs JA (2013) Different modes of growth cone collapse in NG 108–15 cells. Eur Biophys J 42(8):591–605. 10.1007/s00249-013-0907-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rutka JT, Smith SL (1993) Transfection of human astrocytoma cells with glial fibrillary acidic protein complementary DNA: analysis of expression, proliferation, and tumorigenicity. Cancer Res 53(15):3624–3631 [PubMed] [Google Scholar]
- 112.Toda M, Miura M, Asou H, Sugiyama I, Kawase T, Uyemura K (1999) Suppression of glial tumor growth by expression of glial fibrillary acidic protein. Neurochem Res 24(2):339–343. 10.1023/a:1022538810581 [DOI] [PubMed] [Google Scholar]
- 113.Toda M, Miura M, Asou H, Toya S, Uyemura K (1994) Cell growth suppression of astrocytoma C6 cells by glial fibrillary acidic protein cDNA transfection. J Neurochem 63(5):1975–1978. 10.1046/j.1471-4159.1994.63051975.x [DOI] [PubMed] [Google Scholar]
- 114.Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, Betsholtz C (1998) GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp Cell Res 239(2):332–343. 10.1006/excr.1997.3922 [DOI] [PubMed] [Google Scholar]
- 115.Inagaki M, Nakamura Y, Takeda M, Nishimura T, Inagaki N (1994) Glial fibrillary acidic protein: dynamic property and regulation by phosphorylation. Brain Pathol 4(3):239–243. 10.1111/j.1750-3639.1994.tb00839.x [DOI] [PubMed] [Google Scholar]
- 116.Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M (1998) Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol 143(5):1249–1258. 10.1083/jcb.143.5.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoshida T, Tomozawa Y, Arisato T, Okamoto Y, Hirano H, Nakagawa M (2007) The functional alteration of mutant GFAP depends on the location of the domain: morphological and functional studies using astrocytoma-derived cells. J Hum Genet 52(4):362–369. 10.1007/s10038-007-0124-7 [DOI] [PubMed] [Google Scholar]
- 118.Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93(3):421–443. 10.1016/j.pneurobio.2011.01.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


