Abstract
Cytomegalovirus (CMV) increases tuberculosis (TB) risk, but its relationship with latent TB infection (LTBI) is unknown. Using US nationally representative data, we report that CMV was independently associated with LTBI (odds ratio, 2.94; 95% CI, 1.19–7.28; P=.02). CMV and LTBI were associated with higher C-reactive protein, suggesting chronic inflammation.
Keywords: cytomegalovirus, immune dysregulation, inflammation, latent tuberculosis infection
Latent tuberculosis infection (LTBI) and cytomegalovirus (CMV) have exceptionally high global prevalence worldwide. An estimated one-fourth of the world’s population has LTBI [1]. CMV has an even higher global prevalence. The epidemiology of the 2 infections is similar with regard to age and sex distribution, variation in regional prevalence, and overlapping risk factors [2, 3]. Both CMV and LTBI have been implicated in chronic inflammation and increased risk of noncommunicable diseases, including cardiovascular disease [4].
TB disease remains a top global cause of death from an infectious disease [5]. Chronic viral infections such as HIV, hepatitis C, and human T-lymphotropic virus type 1 increase TB disease risk [6–8]. Similarly, recent studies indicate that CMV infection increases risk of progression to TB disease, likely through enhanced CMV-driven T-cell activation and immune dysregulation [9]. However, whether CMV infection is associated with increased rates of LTBI has not been investigated. We used US nationally representative data to determine whether CMV seropositivity was associated with LTBI. We also explored whether LTBI and CMV infections were associated with enhanced systemic inflammation using C-reactive protein (CRP) levels.
METHODS
We used data from the National Health and Nutritional Examination Survey (NHANES) from 1999 through 2000 for cross-sectional analyses. NHANES is conducted by the National Center for Health Statistics, Centers for Diseases Control and Prevention, and comprises a series of cross-sectional surveys with a multistage probability cluster sampling design. For 1999–2000, NHANES examinations included tuberculin skin testing (TST), tuberculosis questionnaires, and cytomegalovirus (CMV) antibody testing.
Tuberculosis assessments were conducted for persons age ≥1 year. We defined LTBI as NHANES Tuberculin Skin Test skin induration ≥10mm. We excluded individuals who reported prior history of TB disease or prior LTBI treatment in the questionnaire.
CMV antibody testing of stored sera was conducted in NHANES participants age 6–49 years. CMV-specific immunoglobulin G (IgG) was measured with enzyme-linked immunosorbent assay (ELISA; Quest International, Inc., Miami, FL, USA). CMV IgG optical density (OD) was used as a quantitative measurement of CMV antibody responses [9, 10]. We also extracted available antibody testing results for Helicobacter pylori, Toxoplasma gondii, and hepatitis C virus.
CRP levels were quantified by latex-enhanced nephelometry. High CRP was defined as CRP concentration ≥0.3mg/dL [11]. History of diabetes mellitus and current tobacco use were defined as previously described [12]. We retrieved sociodemographic variables and available anthropometric and cardio-metabolic parameters including body mass index (BMI), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides.
Statistical Analyses
Statistical weights were used in analyses to account for complex survey design, survey nonresponse, and poststratification adjustment to match total population counts from the Census Bureau. Continuous variables were presented with weighted medians and interquartile ranges (IQRs), and categorical variables with frequencies and percentages. Categorical variables were compared using the Pearson χ2 test with a Rao and Scott second-order correction using an F statistic with noninteger degrees of freedom, accounting for survey design. Logistic regression models were used to assess the association between LTBI and CMV and between LTBI and high CRP adjusted for potential confounders. Model selection was based on Akaike information criterion (AIC). Analyses were performed using SAS, version 9.4. P values <.05 were considered statistically significant.
RESULTS
Of 7363 participants, 403 (5.5%) had LTBI. Participants with LTBI were older (median age [IQR], 44 [33–56] years) than those without LTBI (median age [IQR], 34 [18–50] years) and more likely to be male (61% LTBI vs 49% no LTBI), to be foreign born (61% LTBI vs 17% no LTBI), and to have a Bacille Calmette-Guérin (BCG) vaccination scar (20% LTBI vs 7% no LTBI) (Supplementary Table 1). Of 4215 tested for CMV, 90% of participants with LTBI were CMV positive, compared with 58% of those without LTBI. Similarly, a higher proportion of participants with LTBI had Helicobacter pylori, Toxoplasma gondii, and hepatitis C virus than those without LTBI among those tested. A higher proportion of participants with LTBI had high CRP than those without LTBI (45% vs 31%).
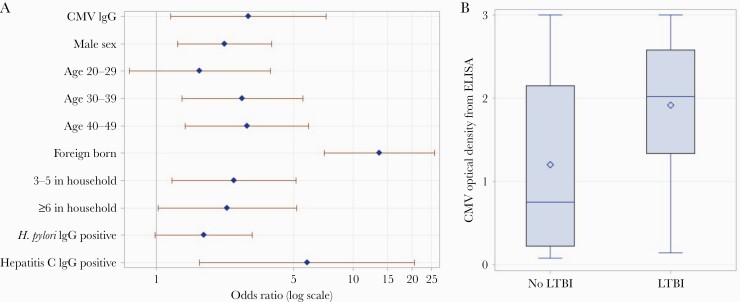
CMV IgG–positive status was associated with LTBI (unadjusted odds ratio [OR], 8.37; 95% CI, 3.67–19.05). Older age (age 30–39 years and age 40–49 years compared with age 6–19 years), male sex, foreign birth vs US birth, and large household size were also significantly associated with LTBI (Figure 1A). Positive CMV IgG remained associated with LTBI (adjusted OR, 2.94; 95% CI, 1.19–7.28) (Supplementary Table 2) in a multivariable regression model adjusted for age group, sex, US birth vs foreign birth, household size, H. pylori antibody status, and hepatitis C virus antibody status. Both LTBI (OR, 1.49; 95% CI, 1.06–2.07) and CMV (OR, 1.32; 95% CI, 1.08–1.61) were associated with high CRP in weighted univariate logistic regressions. LTBI (OR, 1.5; 95% CI, 1.03–2.2) was significantly associated with high CRP in weighted multivariable models adjusted for BMI, monocyte-to-lymphocyte ratio, current smoker, and sex (Table 1).
Figure 1.
A, Multivariable logistic regression model assessing the relationship between CMV and LTBI, adjusting for age group, sex, US birth vs foreign birth, household size, H. pylori antibody status, and hepatitis C virus antibody status. Adjusted odds ratios were determined by adjusting for each of the other factors. The marker on the horizontal lines denotes the odds ratio, and horizontal line denotes the 95% CI. The vertical line shows an odds ratio of 1.0. B, Box plot of CMV optical density by LTBI status. The top and bottom box edges denote quartiles, and the line in the box denotes the median. Minimum and maximum values are denoted by ends of lines. Abbreviations: CMV, cytomegalovirus; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; LTBI, latent tuberculosis infection.
Table 1.
Univariate Logistic Regression and Multivariable Logistic Regression Models Evaluating the Association Between High CRP and the Following Factors: LTBI, Body Mass Index, Monocyte-to-Lymphocyte Ratio, Smoking Status, and Sex
| Covariates | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| LTBI | 1.49 (1.06–2.07) | .02 | 1.5 (1.03–2.2) | .04 |
| Body mass index | 1.17 (1.15–1.18) | <.0001 | 1.17 (1.15–1.19) | <.0001 |
| Monocyte-to-lymphocyte ratio | 8.28 (4.61–14.9) | <.0001 | 26.81 (11.94–60.21) | <.0001 |
| Current smoker | 1.01 (0.82–1.23) | .90 | 1.47 (1.16–1.87) | .002 |
| Male sex | 0.49 (0.42–0.56) | <.0001 | 0.4 (0.33–0.49) | <.0001 |
Adjusted odds ratios determined by adjusting for each of the other factors
Abbreviations: CRP, C-reactive protein; LTBI, latent tuberculosis infection; OR, odds ratio.
Weighted median CMV optical density (IQR) was significantly higher in individuals with LTBI than those without LTBI (2.02 [1.36–2.58] vs 0.75 [0.22–2.15]; P<.001) (Figure 1B). CMV optical density tertiles also corresponded to increasing LTBI prevalence. In the first tertile of CMV optical density, LTBI prevalence was 1.3%. In the second tertile of CMV optical density, LTBI prevalence was 4.3%, and in the third tertile 7.5%.
DISCUSSION
Our cross-sectional study demonstrated a significant association between CMV seropositivity and LTBI in a nationally representative survey of the US population. Male sex, older age, birth outside of the United States, larger household size, and hepatitis C seropositivity were also independently associated with LTBI. Median CMV optical density was associated with LTBI, and tertiles of CMV optical density corresponded to increased prevalence of LTBI. Both LTBI and CMV were associated with high CRP levels.
TB prevention efforts focus on identification and treatment of those with LTBI who are most likely to progress to active TB disease. Although previous studies indicate that CMV may increase risk of progression to active TB disease [3, 9], no prior studies have investigated the occurrence of CMV and LTBI coinfection. Our results are important as they demonstrate that LTBI and CMV coinfection is common, which may have implications for TB progression and outcomes. Our findings also raise an important question of whether CMV may contribute to increased host susceptibility to establishing TB infection, particularly due to its broad impact on human immunity [13, 14]. There is increasing evidence on the role of coinfections in TB pathogenesis, as chronic viral infections have been demonstrated to promote Mycobacterium tuberculosis (Mtb) burden, delay Mtb-specific T-cell priming, and contribute to TB morbidity in experimental conditions [15]. Although our focus was on LTBI and CMV coinfection, we also found that hepatitis C virus was independently associated with LTBI. This suggests that LTBI clusters with other chronic viral infections besides CMV, which may also affect Mtb infection outcomes [8, 16].
Both LTBI and CMV infection have been associated with immune activation. Studies suggest that LTBI is associated with increased levels of T-cell activation markers and cytokines including interferon-gamma [12]. High tumor necrosis factor–alpha and interleukin-6 levels are associated with high CMV IgG responses and may mediate CMV-driven cardiovascular disease mortality [10]. The implications of chronic immune activation, inflammation, and associations with cardiovascular disease are particularly relevant given increasing attention to the high prevalence of cardiovascular disease and other noncommunicable diseases in low- and middle-income countries, many of which have high co-prevalence of LTBI and CMV infection. In this context, our study findings of associations between CMV, LTBI, and higher CRP levels highlight the need for further understanding of infection-related immunologic mechanisms for cardiovascular disease pathogenesis and associated targets for intervention, including whether upstream prevention or treatment of infection could impact outcomes. We analyzed CRP as it was the only inflammatory marker available in NHANES, but future studies could include a broader profiling of systemic inflammation and immune activation parameters in the setting of LTBI and CMV coinfection.
The strengths of our study include the large sample size of the NHANES database and associated testing including CMV IgG optical density measurement. The limitations of our study include the cross-sectional study design, which does not allow determination of causation, nor determination of whether CMV infection may increase risk of LTBI or the reverse. Furthermore, since acquisition of LTBI and CMV share similar risk factors, clustering of these infections may be a manifestation of common individual, socioeconomic, and environmental factors, which we could not fully evaluate. We used the most recent available NHANES data when both CMV and LTBI testing were performed; future studies with updated data using interferon gamma release assay results could be informative.
The association between CMV seropositivity and LTBI in our study raises important questions about immunologic mechanisms that may modulate host susceptibility to different infections and how exposure to multiple pathogens may contribute to the pathogenesis of noncommunicable diseases. Further studies on the interplay between TB and CMV in high-burden settings may lay additional groundwork for understanding important underlying mechanisms and subsequent targets for intervention.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number K08 AI106420 to Y.F.v.d.H.) and the National Center for Advancing Translational Science (grant number KL2 TR001426 to M.A.H.) at the National Institutes of Health, Bethesda, Maryland, USA.
Potential conflicts of interest. Yuri F. van der Heijden: no conflict. Bin Zhang: no conflict. Claire A. Chougnet: no conflict. Moises A. Huaman: no conflict. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This research was determined not human subjects research by the University of Cincinnati Institutional Review Board.
Contributor Information
Yuri F van der Heijden, Division of Infectious Diseases, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA; Vanderbilt Tuberculosis Center, Vanderbilt University School of Medicine, Nashville, Tennessee, USA; The Aurum Institute, Johannesburg, South Africa.
Bin Zhang, Division of Biostatistics and Epidemiology, Cincinnati Children’s Medical Center, Cincinnati, Ohio, USA.
Claire A Chougnet, Division of Immunobiology, Cincinnati Children’s Medical Center, Cincinnati, Ohio, USA.
Moises A Huaman, Vanderbilt Tuberculosis Center, Vanderbilt University School of Medicine, Nashville, Tennessee, USA; Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
References
- 1. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 2019; 29:e2034. [DOI] [PubMed] [Google Scholar]
- 3. Cobelens F, Nagelkerke N, Fletcher H. The convergent epidemiology of tuberculosis and human cytomegalovirus infection. F1000Res 2018; 7:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huaman MA, Henson D, Ticona E, et al. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines 2015; 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Global Tuberculosis Report 2019. World Health Organization; 2019. [Google Scholar]
- 6. Esmail H, Riou C, Bruyn ED, et al. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annu Rev Immunol 2018; 36:603–38. [DOI] [PubMed] [Google Scholar]
- 7. Grassi MF, Dos Santos NP, Lírio M, et al. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis 2016; 16:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu PH, Lin YT, Hsieh KP, et al. Hepatitis C virus infection is associated with an increased risk of active tuberculosis disease: a nationwide population-based study. Medicine (Baltimore) 2015; 94:e1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stockdale L, Nash S, Farmer R, et al. Cytomegalovirus antibody responses associated with increased risk of tuberculosis disease in Ugandan adults. J Infect Dis 2020; 221:1127–34. [DOI] [PubMed] [Google Scholar]
- 10. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simanek AM, Dowd JB, Pawelec G, et al. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 2011; 6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huaman MA, Deepe GS Jr, Fichtenbaum CJ. Elevated circulating concentrations of interferon-gamma in latent tuberculosis infection. Pathog Immun 2016; 1:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol 2018; 18:575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015; 160:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu W, Snell LM, Guo M, et al. Early innate and adaptive immune perturbations determine long-term severity of chronic virus and Mycobacterium tuberculosis coinfection. Immunity 2021; 54:526–41 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Serag HB, Anand B, Richardson P, Rabeneck L. Association between hepatitis C infection and other infectious diseases: a case for targeted screening? Am J Gastroenterol 2003; 98:167–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.