Abstract
Background
Research protocols regarding the use of ActiGraph wGT3X+ accelerometers in care home residents are yet to be established. The purpose of this study was to identify the minimal wear time criteria required to achieve reliable estimates of physical activity (PA) and sedentary behaviour (SB) in older care home residents.
Methods
Ninety-four older adults from 14 care homes wore an ActiGraph wGT3X+ accelerometer on the right hip for 7 consecutive days. A pragmatic, staged approach was adopted in order to explore the effect of: monitoring day; minimum daily wear time and number of wear days on estimates of four outcomes derived from the accelerometer data: counts.day− 1, counts.minute− 1, PA time and SB time.
Results
Data from 91 participants (mean age: 84 ± 9 years, 34% male) was included in the analysis. No effect of monitoring day was observed. Lowering the daily wear time to ≥ 8 h (compared to ≥10 h) had no effect on the outcomes of interest. Four days of monitoring was sufficient to provide reliable estimates of all four outcomes.
Conclusion
In this study, a minimum wear time criterion of ≥ 8 h on any 4 days was required to derive reliable estimates of PA and SB from ActiGraph wGT3X+ accelerometer data in older care home residents.
Keywords: Physical activity, Sedentary behaviour, Older adults, Care home residents, Accelerometer, ActiGraph wGT3X + , Measurement, Methodology, Reliability
Background
Over the previous century there has been a shift in the demographics of the world’s population [1], with a particular expansion of the 85 years and above age group (i.e. the oldest old). As longevity in later life improves [2] this trend is likely to continue and in the United Kingdom (UK), the number of individuals aged over 85 years is expected to more than double between 2014 and 2034 to 3.2 million [3].
Whilst population ageing may be viewed positively; the fact that increases in life expectancy are typically mirrored by extended periods of morbidity and disability cannot be overlooked [4–6]. Many older adults will experience complex and interacting health needs and will ultimately require some form of support in their later years [1]. Recent estimates suggest there are over 400,000 older adults residing in care homes in the UK [7]. In the UK care homes provide overnight accommodation and communal living facilities for long-term care for individuals who may not be able to live independently. Care homes also provide personal care, and in some instances nursing care, for those people with illness, disability or dependence [8].
By definition residents of care homes are amongst the frailest individuals in the UK population, distinguishable from community-dwelling older adults of the same age because of their possible physical limitations, multi-morbidity, dependency on others and / or cognitive impairment [9]. Still, frailty may be considered a dynamic process and whilst it is likely that care home residents become frailer and are at higher risk of worsening disability, falls and admission to hospital, this deterioration is not immutable and there is scope to intervene [10].
Indeed, there is now ample evidence that engagement in physical activity (PA) has beneficial effects on a range of outcomes related to health in older adults, including those residing in care homes [8, 11–13]. In addition, there is mounting evidence regarding the negative impact sedentary behaviour (SB), independent of PA, may have on a number of health parameters [14–16]. Nevertheless, research suggests older adults residing in care homes spend the majority of their time sedentary [17–19]. This suggests a shift in the emphasis of interventions from simply targeting PA to whole-home initiatives which look to encourage residents to engage in more routine PA and reduce the amount of time they spend being sedentary for prolonged periods of time would be well placed. However, in order to develop such interventions a thorough understanding of the levels and patterns of PA and SB in this population is needed.
Recent advances in PA and SB monitoring afford researchers the opportunity to further increase and refine understanding of these behaviours. Accelerometers in particular are increasingly being used in studies involving care home residents due to their capability to provide information not only on the total volume of PA (which is typically low in this population) and SB; but also, on the intensity of PA and the patterns of both PA and SB [20, 21].
Still, it is important to acknowledge that the complexity of accelerometers means the use of these devices is not without challenges [22]. Several decisions pertaining to the data collection and processing methods need to be made [23]. Whilst best practice recommendations have been published regarding accelerometer use [24, 25], the paucity of high-quality measurement specific research involving older adults means much of the evidence informing these recommendations is derived from studies with younger adults. Thus, whilst these offer a valuable resource, some of these recommendations may not be applicable to older adults residing in care homes.
Participants in habitual PA studies are typically asked to wear an accelerometer during all waking hours over a 7-day period [24, 25]; yet compliance to this protocol, particularly in older adults, is variable. We recently reported that 19% of older care home residents approached to wear an accelerometer declined and cited not wishing to wear the accelerometer for so many days as their reason for doing so [18]. Furthermore, reports of individuals forgetting to put the accelerometer back on after removing it for reasons such as showering (many of the monitors are not water-proof); discomfort and sleeping are not uncommon [26, 27]. Accordingly, researchers typically apply a minimum wear time (i.e. the proportion of a day and the number of days) which is required to ensure estimates of PA and SB are representative of habitual PA behaviour.
In studies involving community dwelling older adults a threshold of ≥ 10 h of accelerometer wear is widely used to define a valid day [28, 29]. Nevertheless, this threshold is not universally accepted [30] and it has been acknowledged that what constitutes a ‘day’ is likely to vary considerably between individuals. This point is particularly pertinent when considering older adults as they are such a heterogeneous group, with those residing in a care home tending to be frailer than their counterparts living in a community setting [9]. At the same time, it is probable that the variability in PA and SB across days is likely reduced in care home residents given there is often more structure to their daily routines [31]. This is supported by recent studies involving older adults in both a retirement community (mean age: 83.5 y ± 6.5 y) [32] and a care home setting (mean age = 82.6 y ± 9.2 y) [18] which report no difference in PA across days of the week. Consequently, whilst 5 days wear has previously been deemed necessary to ensure reliable estimates of PA behaviour in samples of community-dwelling older adults [33, 34], it may be the number of days of wear could be reduced in care home residents without distorting data.
Further work is required to investigate the minimum wear criteria necessary to ensure estimates of PA and SB in older adults living in care homes are reliable. Such work is warranted as the application of a minimum wear time criterion derived from studies involving different populations may result in either insufficient data being collected or data being needlessly excluded from analysis which can ultimately effect conclusions made.
Thus, the aim of this study was to identify the minimal wear time criteria required to achieve reliable estimates of PA and SB in older care home residents.
Methods
Participants
This study is based on data collected from older care home residents recruited from ten care homes enrolled in the Research Exploring Physical Activity in Care Homes (REACH) programme between June 2013 and March 2015 and four care homes involved in the associated development work between September 2011 and January 2012. All of the care homes were located in West Yorkshire. Ethical approval was obtained from the Yorkshire and The Humber - Bradford and East of England-Essex NHS research ethics committees (REACH programme) and the University of Leeds research ethics committee (development work).
All residents within each of the care homes were screened for eligibility. The eligibility criteria were as follows: aged 65 years or over; a permanent resident; not bed bound or in receipt of palliative care. A permanent resident was defined as someone who was residing in the care home and not in receipt of respite, day-care, or short-term rehabilitation.
An initial assessment of the mental capacity of all eligible residents was undertaken by the care home manager or an (appropriate) nominated person. As per the Mental Capacity Act (MCA) guidance, all residents were assumed to have capacity unless it was established that they did not [35]. The following question was used to guide this process: “Does the individual have the capacity to consent (or refuse) to take part in the research study at this point in time?” [36]. Highlighting that an individual’s mental capacity is “decision-specific” was deemed particularly important as it is possible that whilst an individual may be deemed unable to make a decision about their finances (as an example) they may be capable of making a decision about taking part in a research study. Prospective participants were only deemed to lack capacity if there was evidence that:
they did not have a general understanding of the research project and what was expected of them (following the provision of information in an appropriate way, e.g. large print information sheets, verbal explanation of the research project);
they were unable to retain the information long enough to be able to consider it and make an informed decision;
they were unable to consider the potential benefits or risks of taking part in the research project;
they were unable to communicate their decision [37].
In cases where the manager / nominated individual did not feel able to make a judgement on a resident’s capacity the researcher conducted this assessment.
Those deemed to have capacity were approached and, if they wished to participate, written or verbal (witnessed) informed consent was gained. For those residents who were considered not to have capacity, assent from a personal consultee (identified by the care home manager / nominated individual) or an Advanced Directive relevant to research was sought. If neither could be identified; the personal consultee did not feel able to take on the role or did not respond within the pre-determined timeframes, an appropriate member of staff (again identified by the care home manager / nominated individual) was asked to act as a nominated consultee.
Procedures
An appropriate staff member within the care home (identified as someone who was familiar with the participants capabilities) was asked to complete the Barthel Index (BI) [38, 39]; the Functional Ambulation Classification (FAC) [40]; and report on the participants medical history (based on the Charlson Comorbidities Index (CCI) [41]. The following demographic information was also collected: gender, age, length of residence in the care home, height and weight.
Participants were asked to wear an ActiGraph wGT3X+ accelerometer (Actigraph, Pensacola, FL, USA) on the right hip, secured using an elasticated belt, during waking hours over the course of 7 days. Accelerometers were initialised to record raw data at a sampling rate of 30 Hz. No stop time was entered to allow for flexibility in wear time. Participants were advised that they were to remove the accelerometer if they were going to be engaging in any water-based activities (e.g. bathing) and were reassured that if they wished to remove the accelerometer for any reason (e.g. discomfort) they were permitted to do so. However, participants were encouraged to keep the accelerometer on during all waking hours for the duration of the monitoring period. Participants were also reassured that they did not need to “do anything” with the accelerometer other than wear it. A member of the research team visited the participant or spoke with a member of staff at the care home within one - two days of the accelerometer being fitted to ensure its proper use. Further contact with the participants and / or care home staff was undertaken only if needed.
For all participants, it was requested that a daily log of wear time (i.e. the time the monitor was put on and removed) was kept for the duration of the monitoring period. Participants capable of completing the log and putting the accelerometer on themselves were encouraged to do so; though the process of completing the activity log was also explained to staff and they were asked to offer support with this where appropriate.
Accelerometery data processing
Raw acceleration data were downloaded and initially processed using the normal filter option and aggregated over 60 s epochs using the proprietary ActiLife software (version 6.8.0, ActiGraph Pensacola, Florida, USA). Vector magnitude (VM) counts were used for analysis. In the absence of consensus on the best approach to correctly identify and remove non-wear time from an accelerometer data set and in an effort to reduce the risk of distorting data provided by the least active participants [42], all data were manually screened (guided by the original rules detailed in Table 1) alongside the activity logs and periods of non-wear time were removed.
Table 1.
Rules utilised to guide the manual screening of the accelerometer data to identified non-wear time
| Accelerometer administration was indicated if one of the following conditions were met: | The removal of an accelerometer was indicated if one of the following conditions were met: |
|---|---|
| a) If 60 min of consecutive 0’s (with the allowance of a 5-min interruption in the string of consecutive zero’s) in the VM axis precedes a VA count value of ≥760 cpm (i.e. light intensity PA) then this value is assumed to indicate the accelerometer being put on. Note, if another light count is identified in the following 5 min use the latter count as the on time. | a) If the 60 min following a VA count value of ≥760 cpm (i.e. light intensity PA) contains is a string of consecutive 0’s (with the allowance of a 5-min interruption in the string of consecutive zero’s) in the VM axis. |
| b) If 120 min of consecutive 0’s (with the allowance of a 5-min interruption in the string of consecutive zero’s) in the VM axis precedes a VA count value of ≥100 cpm (i.e. low intensity PA) then this value is assumed to indicate the accelerometer being put on. Note, if another count ≥100 cpm identified in the following 5 min use the latter count as the on time | b) If the 120 min following a VA count value of ≥100 cpm (i.e. low intensity PA) contains is a string of consecutive 0’s (with the allowance of a 5-min interruption in the string of consecutive zero’s) in the VM axis. |
Abbreviations: VM vector magnitude; VA vertical axis; cpm counts per minute
Daily wear time was determined by subtracting non-wear time from the total possible minutes in a day (1440 min). Data were then reviewed and the first monitoring day was removed if the monitor was administered after 1 pm. Partial days, defined as being < 4 h, were also removed as this amount of wear time was deemed insufficient to provide a reliable estimate of PA or SB outcomes.
In the current study the specific outcomes of interest were: total volume of PA (i.e. counts.day− 1 and counts.minute− 1), daily PA time and daily SB time. In the absence of cut-points developed specifically with care home residents, a published cut-point, developed in a sample of community-dwelling older adults (n = 37, mean age: 73.5 ± 7.3 y), was applied to the VM activity cpm to identify time spent engaging in PA (≥ 200 cpm) and SB (< 200 cpm) [43].
Statistical analysis
All analyses were completed using SPSS version 21 (IBM Corporation, Somers, NY, USA). Data were first assessed for normality of distribution visually (via histograms) and using the Shapiro-Wilk test. In cases where data were non-normally distributed (i.e. counts.day− 1, counts.minute− 1 and daily PA time), data were log (log10) transformed prior to statistical testing to satisfy normality assumptions and permit the use of parametric statistics. Data are presented as mean ± standard deviation (SD) unless otherwise specified and significance was defined as p < 0.05.
In order to explore the impact of different wear time criteria on the assessment of key accelerometer outcomes (i.e.counts.day− 1, counts.minute− 1, PA time and SB time) a pragmatic, staged approach was adopted as follows:
The effect of monitoring day on accelerometer outcomes
Given the hierarchical structure of the data (i.e. repeated measures within participants) and the fact that not all participants had an equal number of repeated measures (i.e. not all had data on 7 days), linear mixed effect models were used to explore the effect of monitoring day on the accelerometer outcomes. As wear time and sedentary time are related, all data were adjusted for daily wear time [44]. Each model included a random intercept for participants and care home to account for the nesting of observations within participants and the clustering of participants within care homes.
The impact of differing minimum daily wear time criteria on accelerometer outcomes
Wear times of 6 h, 7 h, 8 h, 9 h and 10 h were compared. Only data from participants who provided ≥ 1 day of data with a minimum daily wear time of ≥ 10 hours over the course of the measurement period were included in this section of analysis to ensure the sample was consistent. Linear mixed effect models were conducted for each accelerometer outcome as not all participants had seven days of data. The models included a random intercept for participants and care home to account for the nesting of observations within participants and the clustering of participants within care homes. Where the outcomes differed between the minimum daily wear time criteria, Bonferroni corrected pairwise comparisons were conducted. Recognising that it is not appropriate to conclude that the outcomes of interest are the same / equivalent based solely on the absence of a statistical difference, tests of equivalence using a confidence approach were also untaken to demonstrate the comparability between estimates of the outcomes of interest when different minimum daily wear time criteria were used [45]. The threshold of equivalency was set at ±10% of the mean of each of the PA outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) when minimum daily wear time was ≥10 h as this was chosen as the criterion. Given that accelerometry data presents as both direct and derived measures, a 10% threshold of PA time equated to ±14 min. We felt that for this population a difference of 14 min spent in PA over a day would be practically meaningful.
The impact of number of monitoring days on the reliability of accelerometer outcomes
Data from participants who provided 7 valid days were averaged over an increasing number of days of data collection (i.e. day one average, day one and two average, day one, two and three average etc.). Linear mixed effect models were then conducted to explore the effect of varying the number of monitoring days. The models included a random intercept for participants and care home to account for the nesting of observations within participants and the clustering of participants within care homes. Where the accelerometer outcomes differed according to the number of monitoring days, Bonferroni corrected pairwise comparisons were conducted. The magnitude of the difference in the accelerometer outcomes based on fewer days of monitoring was also determined using standardised effect size [46]. In addition, tests of equivalence using a confidence approach [45] were untaken to demonstrate the comparability between the estimates of the outcomes of interest based on a different number of monitoring days. The threshold of equivalency was set at ±10% of the mean of each of the PA outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) based on 7 days of monitoring as this was chosen as the criterion.
Results
Sample characteristics
A hip-worn accelerometer was administered to 94 participants. However, data from three participants were not considered further as it did not meet the initial screening criteria (i.e. 1 day with a minimum daily wear time of ≥ 4 hours). Thus, the analysis sample comprised 91 participants. Personal characteristics did not differ between those participants not included in the analysis sample and those who met the initial screening criteria (p > 0.05). The personal characteristics of those participants included in the analysis (n = 91) are presented in Table 2.
Table 2.
Participant characteristics (n = 91)
| n | N (%) or Mean ± SD | |
|---|---|---|
| Gender (male) | 91 | 31 (34%) |
| Age (y) | 85 | 84 ± 9 |
| Age group | 85 | |
| < 85 y | 39 (46%) | |
| ≥ 85 y | 46 (54%) | |
| Length of residence (months) | 85 | 35 ± 65 |
| Height (cm) | 72 | 161.9 ± 10.7 |
| Weight (kg) | 83 | 66.7 ± 15.4 |
| Capacity to consent (yes) | 91 | 68 (75%) |
| Number of comorbiditiesa: | 73 | |
| None | 4 (5%) | |
| 1–2 | 54 (74%) | |
| ≥ 3 | 15 (21%) | |
| BI Score (score on a 21-point scale; 0–20) | 86 | 12 ± 5 |
| BI score ≤ 11 (dependent) | 38 (44%) | |
| BI score > 11 (independent) | 48 (56%) | |
| FAC | 80 | |
| Level 0 (non-functional ambulation) | 11 (14%) | |
| Level 1 (ambulatory-dependent for physical assistance – level II) | 9 (11%) | |
| Level 2 (ambulatory-dependent for physical assistance – level I) | 4 (5%) | |
| Level 3 (ambulatory-dependent for supervision) | 3 (4%) | |
| Level 4 (ambulatory-independent on level surfaces) | 34 (42%) | |
| Level 5 (ambulatory-independent) | 19 (24%) |
Notes: Number of participants (n) is not equal to the total number of residents recruited due to missing data; a based on the Charlson Comorbidity Index
Abbreviations: BI Barthel Index; FAC Functional Ambulation Classification
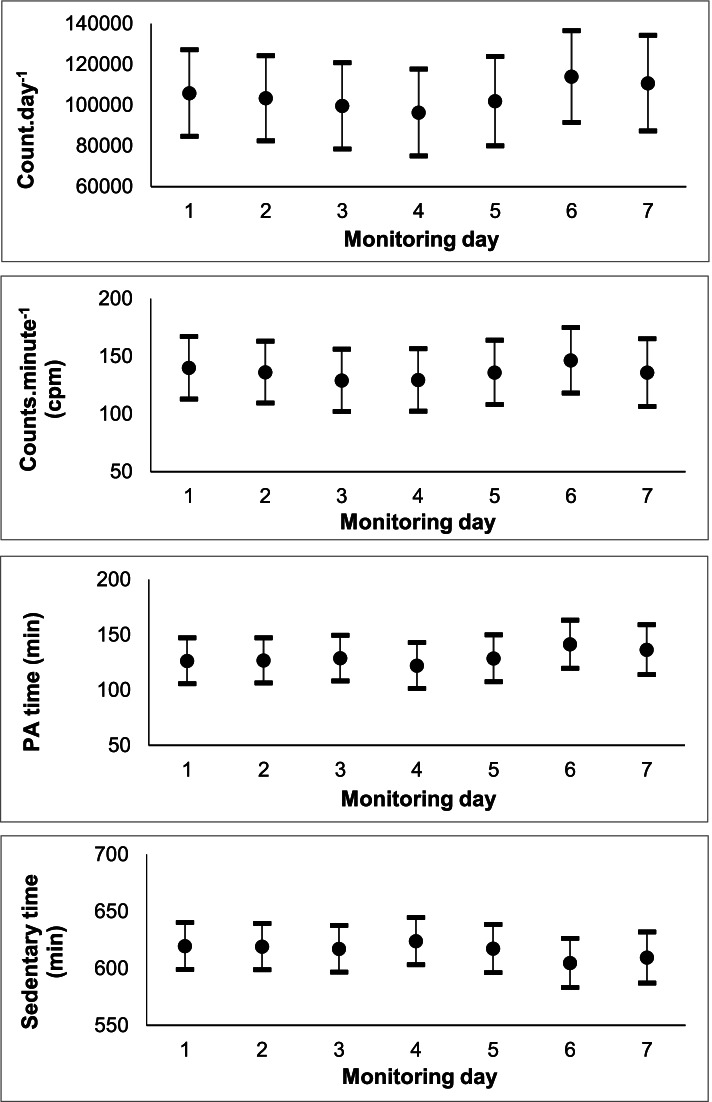
Effect of monitoring day on accelerometer outcomes
Estimates of the accelerometer outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) across monitoring days are displayed in Fig. 1. Recorded accelerometer outcomes did not differ with monitoring day (p > 0.05).
Fig. 1.
Mean and 95% confidence intervals of (reading top to bottom): counts.day− 1, counts.minute− 1, physical activity (PA) time and sedentary behaviour (SB) time across monitoring days (one-seven) (n = 91)
Impact of differing minimum daily wear time criteria on accelerometer outcomes
Data from participants (n = 85) who provided 1 day of data with a minimum daily wear time of ≥10 h were included in this section of analysis. The number of days available for analysis increased as the minimum number of hours used to define a valid day decreased. As can be seen in Table 3, population estimates of counts.day− 1, counts.minute− 1 and PA time were similar, irrespective of the minimum daily wear time criteria employed (p > 0.05). Conversely, estimates of SB time were significantly lower when valid days were defined as ≥7 h (Mean difference: − 21 min) or ≥ 6 h (Mean difference: − 26 min) compared to ≥ 10 h (p < 0.05) (Table 3).
Table 3.
Accelerometer outcomes calculated based on different definitions of a valid day and equivalence results
| Minimum daily wear time criteria (h) | Mean (95% CI) | Mean Difference | Standard Error | 90% CI around mean difference | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| counts.day− 1 | |||||
|
≥ 10 h (EI: ± 11,170) |
111,695 (90,870, 132,520) |
n/a | n/a | n/a | n/a |
| ≥ 9 h |
109,297 (88,545, 130,049) |
2398 | 3864 | − 7563 | 12,359 |
| ≥ 8 h |
107,976 (87,262, 128,690) |
3719 | 3821 | − 6132 | 13,569 |
| ≥ 7 h |
107,569 (86,870, 128,269) |
4126 | 3802 | − 5676 | 1392 |
| ≥ 6 h |
107,054 (86,364 127,744) |
4641 | 3789 | − 5127 | 14,409 |
| counts.minute−1 (cpm) | |||||
|
≥ 10 h (EI: ± 14) |
140 (114, 166) |
n/a | n/a | n/a | n/a |
| ≥ 9 h |
140 (113, 166) |
0 | 4 | −10 | 11b |
| ≥ 8 h |
139 (113, 165) |
1 | 4 | −10 | 11b |
| ≥ 7 h |
139 (113,166) |
1 | 4 | −10 | 11b |
| ≥ 6 h |
139 (113,165) |
1 | 4 | −10 | 11b |
| PA time (h and min) | |||||
|
≥ 10 h (EI: ± 14 min) |
2 h 20 min (1 h 58 min, 2 h 41 min) |
n/a | n/a | n/a | n/a |
| ≥ 9 h |
2 h 17 min (1 h 55 min, 2 h 38 min) |
3 min | 3 min | - 6 min | 12 minb |
| ≥ 8 h |
2 h 15 min (1 h 53 min, 2 h 36 min) |
5 min | 3 min | - 4 min | 14 minb |
| ≥ 7 h |
2 h 14 min (1 h 52 min, 2 h 36 min) |
6 min | 3 min | - 3 min | 14 minb |
| ≥ 6 h |
2 h 13 min (1 h 52 min, 2 h 35 min) |
6 min | 3 min | - 2 min | 15 min |
| SB time (h and min) | |||||
|
≥ 10 h (EI: ± 65 min) |
10 h 54 min (10 h 25 min, 11 h 20 min) |
n/a | n/a | n/a | n/a |
| ≥ 9 h |
10 h 41 min (10 h 14 min, 11 h 8 min) |
12 min | 7 min | - 6 min | 30 minb |
| ≥ 8 h |
10 h 35 min (10 h 8 min, 11 h 2 min) |
17 min | 7 min | - 1 min | 35 minb |
| ≥ 7 h |
10 h 31 min (10 h 4 min, 10 h 58 min) |
21 mina | 7 min | 4 min | 39 minb |
| ≥ 6 h |
10 h 29 min (9 h 59 min, 10 h 53 min) |
26 mina | 7 min | 8 min | 44 minb |
Notes: Accelerometer outcomes are: counts.day− 1, counts.minute− 1, PA time and SB time. Mean difference from the 10 h reference point. a denotes that the mean difference from the estimates based on 10 h wear time criteria is significant at the 0.05 level. Bonferroni correction applied. Equivalence interval = ± 10% of mean value when the minimum daily WT of ≥10 h was used. For counts.day− 1 = 11,170 count.day− 1; counts.minute− 1 = 14 count.minute− 1; PA time = 14 min; SB time = 65 min. b indicates equivalency
Abbreviations: EI equivalency interval; cpm counts per minute; PA physical activity; SB sedentary behaviour
Table 3 also presents the equivalency test results for each of the daily minimum wear time criteria. These results show that estimates of counts.minute− 1 and SB time were equivalent to estimates based on the reference minimum wear time criteria of ≥ 10 h irrespective of the minimum daily wear time employed. For PA time, estimates were equivalent to those based on the reference minimum wear time criteria of ≥ 10 h for the ≥ 9 h, ≥ 8 h and ≥ 7 h criterion. Conversely, none of the estimates of counts.day− 1 based on alternative daily minimum wear time criteria were equivalent to those based on the reference minimum wear time criteria of ≥ 10 h. Setting the equivalency interval at ±15% of the mean of the counts.day− 1 (i.e. ± 16,754 counts.day− 1) revealed all estimates to be equivalent to the reference.
The impact of number of monitoring days on the reliability of accelerometer outcomes
Data from participants (n = 35) who provided 7 days of data with a minimum daily wear time of ≥ 8 h were included in this section of analysis. Importantly these participants did not differ from those who did not meet this wear time criterion (Table 4, p > 0.05).
Table 4.
Characteristics of participants stratified according to whether they met the minimum wear time criteriaa
| Did participants provide 7 days with a minimum daily wear time of ≥ 8 h? | Yes (n = 35) | No (n = 59) | ||
|---|---|---|---|---|
| n | N (%) or Mean ± SD | n | N (%) or Mean ± SD | |
| Gender (male) | 35 | 12 (34%) | 59 | 20 (34%) |
| Age (y) | 31 | 83 ± 8 | 56 | 85 ± 9 |
| Length of residence (months) | 31 | 44 ± 97 | 56 | 30 ± 34 |
| Height (cm) | 28 | 161.3 ± 10.3 | 46 | 162.3 ± 10.9 |
| Weight (kg) | 30 | 67.8 ± 17.8 | 55 | 65.9 ± 13.9 |
| Capacity to consent (yes) | 35 | 24 (69%) | 59 | 46 (78%) |
| Number of comorbiditiesb: | 28 | 47 | ||
| None | 1 (4%) | 3 (6%) | ||
| 1–2 | 21 (75%) | 35 (75%) | ||
| ≥ 3 | 6 (21%) | 9 (19%) | ||
| BI score (score on a 21-point scale; 0–20) | 35 | 13 ± 5 | 54 | 12 ± 6 |
| BI score ≤ 11 (dependent) | 15 (43%) | 23 (43%) | ||
| BI score > 11 (independent) | 20 (57%) | 31 (57%) | ||
| FAC | 31 | 51 | ||
| Level 0 (non-functional ambulation) | 1 (3%) | 10 (20%) | ||
| Level 1 (ambulatory-dependent for physical assistance – level II) | 3 (10%) | 6 (12%) | ||
| Level 2 (ambulatory-dependent for physical assistance – level I) | 1 (3%) | 3 (6%) | ||
| Level 3 (ambulatory-dependent for supervision) | 1 (3%) | 2 (4%) | ||
| Level 4 (ambulatory-independent on level surfaces) | 16 (52%) | 19 (37%) | ||
| Level 5 (ambulatory-independent) | 9 (29%) | 11 (21%) | ||
Notes: a Wear time criteria defined as ≥ 8 h on 7 days. Number of participants (n) is not equal to the total number of residents recruited due to missing data. b based on the Charlson Comorbidity Index
Abbreviations: BI Barthel Index; FAC Functional Ambulation Classification
Estimates of the accelerometer outcomes derived by averaging over an increasing number of repeated days are presented in Table 5. Counts.minute− 1 was unaffected by the number of monitoring days included in analysis (p > 0.05). However, the number of monitoring days included in analysis did impact estimates of counts.day− 1 (F [6] = 2.713, p = 0.05); PA time (F [6] = 4.641, p < 0.01) and SB time (F [6] = 22.013, p < 0.01). For counts.day− 1 and PA time, only the estimate based on 1 monitoring day differed significantly from the 7-day average (p < 0.05). Estimates of SB time based on 1, 2 and 3 days of monitoring all significantly differed from the 7-day average (p < 0.05). Estimates based on a minimum of 4 days and above did not (p > 0.05).
Table 5.
Accelerometer outcomes calculated based on an increasing number of days of data collection and equivalence results
| Counts.day−1 | |||||||
| Day (n) | 1 | 2 | 3 | 4 | 5 | 6 | 7 (EI: ± 14,151) |
| Mean ± SD | 124,046 ± 85,406 | 140,581 ± 94,191 | 137,584 ± 97,130 | 136,500 ± 96,012 | 136,262 ± 93,744 | 139,427 ± 98,997 | 141,510 ± 104,549 |
| Mean differenceb | 17463a | 929 | 3926 | 5010 | 5248 | 2082 | n/a |
| Effect Size | 0.18 | 0.01 | 0.04 | 0.05 | 0.05 | 0.02 | n/a |
| 90% CI around mean difference | |||||||
| Lower | 3295 | −13,239 | −10,242 | − 9158 | − 8920 | − 12,085 | n/a |
| Upper | 31,631 | 15,097 | 18,093 | 19,177 | 19,415 | 16,250 | n/a |
| Counts.minute−1 (cpm) | |||||||
| Day (n) | 1 | 2 | 3 | 4 | 5 | 6 | 7 (EI: ± 18) |
| Mean ± SD | 181 ± 123 | 186 ± 129 | 174 ± 123 | 171 ± 124 | 170 ± 119 | 172 ± 123 | 172 ± 125 |
| Mean differenceb | 9 | 14 | 2 | 1 | 2 | 0 | n/a |
| Effect Size | 0.08 | 0.11 | 0.02 | 0.01 | 0.02 | 0.00 | n/a |
| 90% CI around mean difference | |||||||
| Lower | - 24 | - 28 | −17 | - 13 | - 12 | - 14 | n/a |
| Upper | 4b | 1b | 12b | 15b | 17b | 14b | n/a |
| PA time (h and min) | |||||||
| Day (n) | 1 | 2 | 3 | 4 | 5 | 6 | 7 (EI: ± 18 min) |
| Mean ± SD | 2 h 27 min ± 1 h 34 min | 2 h 44 min ± 1 h 40 min | 2 h 45 min ± 1 h 46 min | 2 h 45 min ± 1 h 46 min | 2 h 45 min ± 1 h 44 min | 2 h 48 min ± 1 h 45 min | 2 h 50 min ± 1 h 46 min |
| Mean difference† | 23 mina | 6 min | 5 min | 5 min | 5 min | 2 min | n/a |
| Effect Size | 0.23 | 0.06 | 0.05 | 0.04 | 0.04 | 0.02 | n/a |
| 90% CI around mean difference | |||||||
| Lower | 9 min | - 8 min | - 9 min | - 8 min | - 9 min | - 12 min | n/a |
| Upper | 37 min | 20 min | 19 min | 19 min | 19 min | 16 minb | n/a |
| SB time (h and min) | |||||||
| Day (n) | 1 | 2 | 3 | 4 | 5 | 6 | 7 (EI: ± 1 h 4 min) |
| Mean ± SD | 9 h 3 min ± 2 h 2 min | 9 h 50 min ± 2 h 2 min | 10 h 15 min ± 1 h 51 min | 10 h 28 min ± 1 h 51 min | 10 h 34 min ± 1 h 51 min | 10 h 35 min ± 1 h 49 min |
10 h 38 min ± 1 h 45 min |
| Mean differenceb | 1 h 35 mina | 48 mina | 23 min | 10 min | 5 min | 3 min | n/a |
| Effect Size | 0.83 | 0.44 | 0.21 | 0.09 | 0.04 | 0.03 | n/a |
| 90% CI around mean difference | |||||||
| Lower | 1 h 6 min | 19 min | - 6 min | - 19 min | - 25 min | - 27 min | n/a |
| Upper | 2 h 4 min | 1 h 17 min | 52 minb | 39 minb | 34 minb | 32 minb | n/a |
Notes. Accelerometer outcomes are: counts.day− 1, counts.minute− 1, PA time and SB time. A valid day being defined as a having a wear time of ≥ 8 h. Mean difference from the seven-day reference point. a denotes that the mean difference from the estimate based on 7 days is significant at the 0.10 level. Bonferroni correction applied. Equivalence interval = ± 10% of mean when the minimum daily WT of ≥10 h was used. For counts.day− 1 = 14,151 count.day− 1; cpm = 18 cpm; PA time = 18 min; SB time = 1 h 4 min. b indicates equivalency
Abbreviations: CI confidence interval; PA physical activity; SB sedentary behaviour; cpm counts per minute
Table 5 also presents the equivalency tests based on differing number of monitoring days. These results show that estimates of counts.minute− 1 were equivalent to estimates based on the reference of 7 monitoring days irrespective of the number of monitoring days included in the analysis. For SB time estimates based on 3, 4, 5 and 6 days of monitoring were equivalent to the criterion of 7 days. However, for PA time, only the estimate based on at least 6 days was equivalent to the estimate based on reference of 7 days. For counts.day− 1, none of the estimates based on fewer monitoring days were equivalent to those based on a minimum of 7 days of monitoring. When the equivalency interval was set at ±15% of the 7-day mean for both counts.day− 1 (i.e. ± 21,227 counts.day− 1) and PA time (i.e. ± 26 min), estimates based on 2, 3, 4, 5 and 6 days were equivalent to estimates based on the reference of 7 days of monitoring.
Discussion
Although accelerometers are increasingly being used with care home residents [17, 18, 47], to our knowledge the current study is the first to explore the minimal wear time criteria necessitated to achieve reliable estimates of PA and SB in this population.
In the absence of a consensus on how many hours of wear constitutes a valid day it seemed prudent to explore whether population estimates of PA and SB varied dependent on the criteria used to define a valid day in care home residents. In order to do this the current paper adopted two methods of demonstrating comparability to the chosen criterion (i.e. ≥ 10 h): significance testing (which is often used within the literature) and equivalency testing (the more appropriate approach). We provided both methods for discussion. For count.minute− 1 the results from both methods indicated that estimates were equivalent irrespective of the minimum daily wear time employed. For SB time estimates were significantly lower when the minimal wear time criteria was lowered from ≥10 h (reference) to ≥ 7 h or ≥ 6 h (p < 0.05). However, equivalency tests demonstrated that estimates were equivalent irrespective of the minimum daily wear time employed. For counts.day− 1 and PA time estimates did not differ significantly when the minimal wear time criteria was lowered; however, estimates based on different minimal wear time criteria were not always equivalent to the reference (i.e. ≥ 10 h). For PA time the estimate based on a minimal wear time criteria of ≥ 6 h was not equivalent to the reference. Conversely, for counts.day− 1, none of the estimates based on lower wear time criteria were deemed to be equivalent to the reference at a threshold of 10%. Still, it is interesting to note that when the equivalency interval was set at ±15% estimates were equivalent, irrespective of the minimum daily wear time employed.
It is important to note that there are no set standards for setting an equivalency interval, rather it is suggested that an equivalency interval is selected based on practical relevance and strong rationale [48]. Given accurate measurement of both PA and SB outcomes is imperative, measurement error must be a consideration. Whilst the exact measurement error for the PA outcomes reported on is unknown, reports suggest it may be upwards of 20–30% at an individual level [49]. In light of this it is important to acknowledge that whilst setting the equivalency interval to ±10% may be deemed appropriate for outcomes such as PA time and SB time it may not be appropriate for counts.day− 1.
Based on the results of the current study an argument can be made for reducing the minimum wear time criteria used to define a valid day. Whilst lower thresholds, most notably ≥ 8 h of accelerometer wear, have been used previously in studies with older adults (including those residing in care homes) [17, 47, 50] the current study is the first to provide empirical evidence to support this decision.
While it may be surmised that a 7-day monitoring protocol may be too burdensome for older care home residents [18] there is a concern that using fewer days would lead to inaccurate estimates of PA and SB if there is considerable variation between days. However, it may be inferred that the variability in PA across days is likely reduced in care home residents given the functional impairments characteristic of this population and structured routine typical of a care home setting [9, 31]. The results presented in the current study support this hypothesis, as key outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) were consistent across monitoring days. These findings are in accordance with recent studies conducted in both a retirement community and care home setting which reported no difference in outcomes across days of the week [18, 31]. Moreover, in the present study, estimates of accelerometer outcomes based on as few as 4 days of monitoring did not differ significantly from estimates based on 7 days (p > 0.05). Taken together these findings suggest it is not necessary to be prescriptive regarding the ‘type’ of day (i.e. weekend day or weekday) included in analysis and that the number of days wear could be reduced without distorting data.
Caution is warranted when considering whether to reduce the number of monitoring days. Based on the equivalency results the number of days of monitoring deemed necessary would differ dependent on the outcome of interest. For example, whilst estimates for SB time based on 3, 4, 5 and 6 days of monitoring were deemed equivalent to the estimate based on the reference of 7 days, for PA time, only the estimate based on at least 6 days was deemed equivalent. In light of this it is recommended that whenever possible the 7-day monitoring period should be implemented.
Limitations
In considering the findings presented it is important to acknowledge the current study is not without limitations. Firstly, the characteristics and PA and SB profile of the participants included will have had an impact on the findings therefore the results are only likely to be relevant to “similar” populations. However, participants were resident in a number of care homes in different social-economic areas and demographic data suggest they are similar to residents of care homes reported elsewhere [16, 51, 52]. In addition, there were no differences in the participant characteristics between those participants who were included in the analysis sample and those who were not.
Secondly, four specific accelerometer outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) were considered therefore the results presented are specific to these. It is not appropriate to assume that the minimal wear time criteria proposed would be sufficient to achieve reliable estimates of different outcomes. Previous research conducted in adults and older adults suggests the number of valid days needed to achieve reliable estimates of PA decreases as the intensity of the PA increases [34, 53, 54]. This may be attributable to the fact engagement in moderate-vigorous (MV) PA tends to be planned therefore is less variable. Although estimates of differing intensities of PA were not considered in the present study, it may be postulated that the reverse would be true for care home residents. Any engagement in higher intensity PA is likely to be sporadic thus more valid days would be required to ensure estimates were reliable. Nevertheless, given the profile of PA and SB in older care home residents [17, 18], the outcomes included in this study were deemed to be most relevant to this population. Furthermore, in the absence of a consensus on wear time criteria and lack of empirical evidence supporting the superiority of one criterion over another, the current study offers a considerable contribution to the existing literature. It is however important to recognise that the normal filter was used when processing the accelerometer count data which may resulted in an underestimation of PA time and overestimation of SB time given the cut point applied was derived using the low-frequency extension filter.
Finally, it is important to recognise that, as a consequence of the pragmatic, staged approach adopted, inferences made about the minimum accelerometer wear time criteria required to achieve reliable estimates of key accelerometer outcomes of interest in this population were made based on analysis conducted with smaller sub-groups of the larger analysis sample which could lead to concerns around bias. For example, when exploring whether the number of days of monitoring could be reduced, only participants who had 7 complete days of data were included in the analysis. In light of this, whilst there were no differences in the measured characteristics of participants included and those who were not in any of stage of the analysis, future studies may want to consider undertaking some statistical simulation work.
Implications
In the absence of best practice recommendations regarding the use of accelerometers in field-based research with older care home residents the empirical evidence provided in the current study can help researchers design accelerometer data collection protocols and facilitate decision making. For example, reducing participant burden is a particularly pertinent consideration in studies with older care home residents given the fragility of the population therefore a researcher may wish to use a shorter monitoring period. However, the results of the current study support the continued use of a 7-day monitoring period wherever possible in an effort to ensure sufficient data is collected to be confident that estimates of PA and SB are accurate. Having said this, undertaking research in a care home setting is particularly challenging therefore it is important to acknowledge that there may be cases where participants are not fully compliant with the data collection protocol. Hence, the application of a population specific minimum wear time criterion is beneficial as it maximises the sample size and subsequently the volume of useful accelerometer data retained in cases where a substantial proportion of participants do not present 7 days’ worth of data.
Conclusion
Determining the volume of data required to reliably estimate PA and SB whilst minimising participant burden and ensuring compliance is challenging. A balance between measurement reliability and sample size is needed to warrant confidence in the outcomes reported. In adopting a pragmatic approach, this study demonstrated that the minimum daily accelerometer wear time could be reduced in care home population without affecting the reliability of estimates of key PA and SB outcomes (i.e. counts.day− 1, counts.minute− 1, PA time and SB time) in a care home population. The results also suggest the number of days of wear necessary to ensure estimates are reliable could be reduced however this may be dependent on the outcome of interest. Accordingly a 7-day monitoring protocol should be utilised wherever possible to increase the likelihood of participants meeting the minimal wear time requirements and increase the chances of achieving reliable estimates of various outcomes simultaneously.
Acknowledgements
The authors would like to thank all of the residents, their relatives and the staff at each of the care homes who participated for all of their time and help.
In addition, as this work emerged from the larger REACH programme of work, the authors would like to acknowledge the help and support of the programme manager Nicola McMaster and also to our research colleagues Sally Barber, Arvin Prashar, Adelaide Lusambili, Alan Wright and Carolyn McCrorie who helped with data collection. The authors would also like to thank members of the REACH Programme Management Group for their important contributions throughout the REACH programme of research.
The REACH Programme Management Group comprises: Karen Birch, School of Biomedical Sciences, University of Leeds, UK; David Ellard, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick, UK; Amanda Farrin, Leeds Institute of Clinical Trials Research, University of Leeds; Joan Firth, personal and public representative; Anne Forster, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK and Leeds Institute of Health Sciences, University of Leeds, UK; Bev Gallagher, NHS Bradford Districts Clinical Commissioning Group, UK; Mary Godfrey (deceased), Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK and Leeds Institute of Health Sciences, University of Leeds, UK; Elizabeth Graham, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK; Claire Hulme, Health Economics Group, Institute of Health Research, University of Exeter, UK; Rebecca Lawton, School of Psychology, Faculty of Medicine and Health, University of Leeds, UK; Najma Siddiqi, Department of Health Sciences, Hull York Medical School, University of York, UK; and John Young, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK.
Abbreviations
- BI
Barthel Index
- CCI
Charlson Comorbidity Index
- CPM
counts per minute
- FAC
Functional Ambulation Category
- Hz
Hertz
- MCA
Mental Capacity Act
- MVPA
moderate-vigorous physical activity
- NIHR
National Institute for Health Research
- PA
physical activity
- PAG
physical activity guidelines
- REACH
Research Exploring physical Activity in Care Homes
- SB
sedentary behaviour
- SD
standard deviation
- UK
United Kingdom
- VA
vertical axis
- VM
vector magnitude
- WHO
World Health Organisation
Authors’ contributions
JA: study concept and design; data acquisition, analysis and interpretation; manuscript preparation. AF is the Chief Investigator of the REACH programme from which this study emerged, study concept and design, helped to draft and critique the output for important intellectual content. KMB: study concept and design; data analysis and interpretation; helped to draft and critique the output for important intellectual content. All authors read and approved the final manuscript.
Authors’ information
JA: Twitter handle: @JenAirlie.
Funding
This publication presents independent research funded by the UK NIHR under the following funding schemes: Programme Development Grants (RP-DG-0709-10141) and Programme Grants for Applied Research Programme (Development and preliminary testing of strategies to enhance routine physical activity in care homes (REACH); Grant Reference Number RP-PG-1210-12017). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Availability of data and materials
The dataset generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. Approval to conduct workstream 2 and 4 of the REACH programme was granted by the Yorkshire and The Humber - Bradford (Ref: 11/YH/0564) and East of England - Essex (Ref: 13/EE/1169) NHS research ethics committees respectively. Approval to conduct the associated development work was granted by the University of Leeds ethics committee (HSLTLM/10/007).
Written or verbal (witnessed) informed consent was provided by those with capacity. For those residents who were considered not to have capacity, a personal or nominated consultee provided assent via the completion of a declaration form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation (WHO). Global Health and Aging. 2011. http://www.who.int/ageing/publications/global_health.pdf. Accessed July 2018.
- 2.Age UK. Improving later life. Understanding the oldest old 2013. http://www.ageuk.org.uk/Documents/EN-GB/For-professionals/Research/Improving%20Later%20Life%202%20WEB.pdf?dtrk=true%20. Accessed November 2013.
- 3.Office for National Statistics (ONS). National Population Projections, 2014-based Statistical Bulletin. 2015. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2015-10-29#changing-age-structure. Accessed July 2018.
- 4.Prince MJ, Wu F, Guo Y, Robledo LMG, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 5.Kirchberger I, Meisinger C, Heier M, Zimmermann A-K, Thorand B, Autenrieth CS, et al. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLOS One. 2012;7(1):e30556. [DOI] [PMC free article] [PubMed]
- 6.Caughey GE, Ramsay EN, Vitry AI, Gilbert AL, Luszcz MA, Ryan P, et al. Comorbid chronic diseases, discordant impact on mortality in older people: a 14-year longitudinal population study. J Epidemiol Community Health. 2010;64(12):1036–1042. doi: 10.1136/jech.2009.088260. [DOI] [PubMed] [Google Scholar]
- 7.LaingBuisson Care homes for older people UK Market Report. July 2018.
- 8.Crocker T, Young J, Forster A, Brown L, Ozer S, Greenwood DC. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: systematic review with meta-analysis. Age Ageing. 2013;42(6):682–688. doi: 10.1093/ageing/aft133. [DOI] [PubMed] [Google Scholar]
- 9.Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JRF. Health status of UK care home residents: a cohort study. Age Ageing. 2014;43(1):97–103. doi: 10.1093/ageing/aft077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weening-Dijksterhuis E, de Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: a comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90(2):156–168. doi: 10.1097/PHM.0b013e3181f703ef. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health, Physical Activity, Health Improvement and Protection. Start active, stay active: a report on physical activity from the four home countries’ chief medical officers. London: Department of Health, Physical Activity, Health Improvement and Protection. 2011. https://www.gov.uk/government/publications/start-active-stay-active-a-report-on-physical-activity-from-the-four-home-countries-chief-medical-officers. Accessed 13th November 2013.
- 13.Physical Activity Guidelines (PAG) Advisory Committee. PAG Advisory Committee scientific report. Washington, DC: US Department of Health and Human Services. 2018. https://health.gov/paguidelines/second-edition/report.aspx. Accessed 14th March 2018.
- 14.de Rezende LFM, Rey-López JP, Matsudo VKR, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. 2014;14(1):333. [DOI] [PMC free article] [PubMed]
- 15.Sardinha LB, Santos DA, Silva AM, Baptista F, Owen N. Breaking-up sedentary time is associated with physical function in older adults. J Gerontol Ser A Biol Med Sci. 2015;70(1):119–124. doi: 10.1093/gerona/glu193. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill C, Dogra S. Different types of sedentary activities and their association with perceived health and wellness among middle-aged and older adults: a cross-sectional analysis. Am J Health Promot. 2016;30(5):314–322. doi: 10.1177/0890117116646334. [DOI] [PubMed] [Google Scholar]
- 17.Marmeleira J, Ferreira S, Raimundo A. Physical activity and physical fitness of nursing home residents with cognitive impairment: a pilot study. Exp Gerontol. 2017;100:63–69. doi: 10.1016/j.exger.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Barber SE, Forster A, Birch KM. Levels and patterns of daily physical activity and sedentary behaviour measured objectively in older care home residents in the UK. J Aging Phys Act. 2015;23(1):133–143. doi: 10.1123/JAPA.2013-0091. [DOI] [PubMed] [Google Scholar]
- 19.Sackley C, Hoppitt T, Levin S, Cardoso K. Observations of activity levels and social interaction in a residential care setting. Int J Ther Rehabil. 2006;13(8):370–373. doi: 10.12968/ijtr.2006.13.8.370. [DOI] [Google Scholar]
- 20.Shiroma EJ, Schrack JA, Harris TB. Accelerating accelerometer research in aging. J Gerontol Series A. 2018;73(5):619–621. doi: 10.1093/gerona/gly033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Cahalin L, Buman M, Ross R. The current state of physical activity assessment tools. Prog Cardiovasc Dis. 2015;57(4):387–395. doi: 10.1016/j.pcad.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the assessment of physical activity: clinical and research applications a scientific statement from the American Heart Association. Circulation. 2013;128(20):2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 23.Migueles JH, Cadenas-Sanchez C, Ekelund U, Nyström CD, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68–S76. doi: 10.1249/MSS.0b013e3182399e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37(11 Suppl):S582–S588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 26.Huberty J, Ehlers DK, Kurka J, Ainsworth B, Buman M. Feasibility of three wearable sensors for 24 hour monitoring in middle-aged women. BMC Womens Health. 2015;15(1):55. doi: 10.1186/s12905-015-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48(13):1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartini C, Wannamethee SG, Iliffe S, Morris RW, Ash S, Lennon L, et al. Diurnal patterns of objectively measured physical activity and sedentary behaviour in older men. BMC Public Health. 2015;15(1):609. doi: 10.1186/s12889-015-1976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferis BJ, Sartini C, Shiroma EJ, Whincup PH, Wannamethee SG, Lee I-M. Duration and breaks in sedentary behaviour: accelerometer data from 1566 community-dwelling older men (British regional heart study) Br J Sports Med. 2015;49(24):1591–1594. doi: 10.1136/bjsports-2014-093514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann SD, Barreira TV, Kang M, Ainsworth BE. Impact of accelerometer wear time on physical activity data: a NHANES semisimulation data approach. Br J Sports Med. 2014;48(3):278–282. doi: 10.1136/bjsports-2012-091410. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins RJ, Prashar A, Lusambili A, Ellard DR, Godfrey M. ‘If they don't use it, they lose it’: how organisational structures and practices shape residents’ physical movement in care home settings. Ageing Soc. 2017:1–26.
- 32.Marshall SJ, Kerr J, Carlson J, Cadmus-Bertram L, Patterson R, Wasilenko K, et al. Patterns of weekday and weekend sedentary behavior among older adults. J Aging Phys Act. 2015;23(4):534–541. doi: 10.1123/japa.2013-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki JE, Júnior JH, Meneguci J, Tribess S, Marocolo Júnior M, Stabelini Neto A, et al. Number of days required for reliably estimating physical activity and sedentary behaviour from accelerometer data in older adults. J Sports Sci. 2017:1–6. [DOI] [PubMed]
- 34.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? International Journal of Behavioral Nutrition and Physical Activity. 2011;8(62). [DOI] [PMC free article] [PubMed]
- 35.Mental Capacity Act. Regulations 2007. London: HM Government; 2005.
- 36.Dobson C. Conducting research with people not having the capacity to consent to their participation. A practical guide for researchers. Leicester: British Psychological Society; 2008.
- 37.Johnston C, Liddle J. The mental capacity act 2005: a new framework for healthcare decision making. J Med Ethics. 2007;33(2):94–97. doi: 10.1136/jme.2006.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Disabil Rehabil. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 39.Wade D, Collin C. The Barthel ADL index: a standard measure of physical disability? Disabil Rehabil. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 40.Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired reliability and meaningfulness. Phys Ther. 1984;64(1):35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.Hutto B, Howard VJ, Blair SN, Colabianchi N, Vena JE, Rhodes D, et al. Identifying accelerometer nonwear and wear time in older adults. International Journal of Behavioral Nutrition and Physical Activity. 2013;10(120). [DOI] [PMC free article] [PubMed]
- 43.Aguilar-Farias N, Brown W, Peeters G. ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. J Sci Med Sport. 2014;17(3):293–299. doi: 10.1016/j.jsams.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Aadland E, Ylvisåker E. Reliability of objectively measured sedentary time and physical activity in adults. PLoS One. 2015;10(7):e0133296. doi: 10.1371/journal.pone.0133296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusticus SA, Lovato CY. Applying tests of equivalence for multiple group comparisons: demonstration of the confidence interval approach. Pract Assessment Res Eval. 2011;16(1):7.
- 46.Coe R. It's the effect size, stupid: What effect size is and why it is important. Annual Conference of the British Educational Research Association2002.
- 47.Leung P-M, Ejupi A, van Schooten KS, Aziz O, Feldman F, Mackey DC, et al. Association between sedentary behaviour and physical, cognitive, and psychosocial status among older adults in assisted living. Biomed Res Int. 2017. 10.1155/2017/9160504. [DOI] [PMC free article] [PubMed]
- 48.Lewis I, Watson B, White KM. Internet versus paper-and-pencil survey methods in psychological experiments: equivalence testing of participant responses to health-related messages. Aust J Psychol. 2009;61(2):107–116. doi: 10.1080/00049530802105865. [DOI] [Google Scholar]
- 49.Arvidsson D, Fridolfsson J, Börjesson M. Measurement of physical activity in clinical practice using accelerometers. J Internal Medicine. 2019;286(2):137-. [DOI] [PubMed]
- 50.Orme M, Wijndaele K, Sharp SJ, Westgate K, Ekelund U, Brage S. Combined influence of epoch length, cut-point and bout duration on accelerometry-derived physical activity. Int J Behav Nutr Phys Act. 2014;11(1):34. doi: 10.1186/1479-5868-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham L, Ellwood A, Hull K, Fisher J, Cundill B, Holland M, et al. A posture and mobility training package for care home staff: results of a cluster randomised controlled feasibility trial (the PATCH trial) Age Ageing. 2020;49(5):821–828. doi: 10.1093/ageing/afaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurley MV, Wood J, Smith R, Grant R, Jordan J, Gage H, et al. The feasibility of increasing physical activity in care home residents: active residents in care homes (ARCH) programme. Physiotherapy. 2020;107:50–57. doi: 10.1016/j.physio.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Rowe DA, Kemble CD, Robinson TS, Mahar MT. Daily walking in older adults: day-to-day variability and criterion-referenced validity of total daily step counts. J Phys Act Health. 2007;4(4):434–446. doi: 10.1123/jpah.4.4.435. [DOI] [PubMed] [Google Scholar]
- 54.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analysed during the current study are available from the corresponding author on reasonable request.