Abstract
Background
Parasite evolution has been conceptualized as a process of genetic loss and simplification. Contrary to this model, there is evidence of expansion and conservation of gene families related to essential functions of parasitism in some parasite genomes, reminiscent of widespread mosaic evolution—where subregions of a genome have different rates of evolutionary change. We found evidence of mosaic genome evolution in the cnidarian Myxobolus honghuensis, a myxozoan parasite of fish, with extremely simple morphology.
Results
We compared M. honghuensis with other myxozoans and free-living cnidarians, and determined that it has a relatively larger myxozoan genome (206 Mb), which is less reduced and less compact due to gene retention, large introns, transposon insertion, but not polyploidy. Relative to other metazoans, the M. honghuensis genome is depleted of neural genes and has only the simplest animal immune components. Conversely, it has relatively more genes involved in stress resistance, tissue invasion, energy metabolism, and cellular processes compared to other myxozoans and free-living cnidarians. We postulate that the expansion of these gene families is the result of evolutionary adaptations to endoparasitism. M. honghuensis retains genes found in free-living Cnidaria, including a reduced nervous system, myogenic components, ANTP class Homeobox genes, and components of the Wnt and Hedgehog pathways.
Conclusions
Our analyses suggest that the M. honghuensis genome evolved as a mosaic of conservative, divergent, depleted, and enhanced genes and pathways. These findings illustrate that myxozoans are not as genetically simple as previously regarded, and the evolution of some myxozoans is driven by both genomic streamlining and expansion.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-022-01249-8.
Keywords: Evolutionary genomics, Parasite evolution, Genome streamlining, Cnidaria, Myxozoa, Myxobolus honghuensis
Background
Parasite evolution is a focal issue in evolutionary biology and ecology [1, 2]. Understanding the evolutionary processes of the transition of ancestral free-living organisms to parasitism is important for human health and agriculture and offers a new testing ground for evolutionary and ecological theory [3, 4]. Relative to free-living species, parasite genomes are typically smaller and more tightly packed with protein-coding genes [5, 6]. Parasite evolution is usually considered to be accompanied by loss of genetic complexity, as exploitation of host metabolic pathways releases selective constraints on parts of the parasite genome, resulting in loss of now-redundant functions (and associated genes) [7]. Accordingly, an appealing reductive theory of evolution has been developed to describe this loss of function and subsequent streamlining that governs parasite genome evolution [8, 9]. However, there is evidence for conservation and even expansion of gene families associated with infection and survival in some parasite genomes [6, 10]. The opposing characters of genomic streamlining and expansion are components of a pervasive process known as mosaic evolution, which is the tendency for a genome to evolve as a set of discrete units, each with its own evolutionary mode, rather than being dominated by a uniform trend [11, 12]. Mosaic evolution is widely used to frame observed genomic changes in free-living taxa including mice, humans, and birds [12–15]. There is much less evidence of selective genetic expansion in parasites [6, 10, 16–18]; thus, mosaic evolution has rarely been emphasized in describing parasite evolutionary patterns. We propose that a more complete picture of parasite genome evolution should incorporate the opposing features of streamlining and novel complexity.
Cnidarians are early-diverging metazoans and ideally suited for investigating the genomic changes that underlie parasitism, as the group includes both free-living (e.g., Medusozoa, Anthozoa) and parasitic taxa (Myxozoa and Polypodiozoa) [19]. Myxozoa are microscopic, oligocellular endoparasites with simple body organization, but multiple morphologies in their complex life cycles [20, 21]. The few sequenced myxozoan genomes are more compact and smaller than those of free-living cnidarians [22], with some species having reduced metabolic capacity [23], or having lost core animal features such as cytosine methylation [24] or their mitochondrial genome [25]. These findings support the view that evolutionary loss and simplification have played a major role in shaping the evolution of myxozoans [22, 25, 26].
Here we characterize the genome of Myxobolus honghuensis, a parasite of economically important gibel carp [27]. We compare its genome with other myxozoans and free-living Cnidaria to reveal unique and conserved genomic features that characterize this cryptically complex parasite group.
Results
Genome assembly and annotation
We sequenced the M. honghuensis genome with a PacBio RSII, yielding ~136× coverage and a 16-kilobase (kb) average read length (Additional file 1: Table S1). The final long-read data contained 2,030,357 sequences with a mean length of 10.4 kb (Additional file 1: Table S2). The genome size was estimated by k-mer analysis using Jellyfish software (Additional file 2: Fig. S1) to be 206 megabases (Mb). The FALCON-assembled genome contained 1118 contigs (161 Mb, N50 1.3 Mb; Table 1). M. honghuensis has a relatively larger myxozoan genome (Additional file 1: Table S3). The assembly showed high integrity and quality, with >98.5% of Illumina genome survey reads mapped to the PacBio assembly (Additional file 1: Table S4), and successful reconstruction of the nuclear rRNAs. Core Eukaryotic Genes Mapping Approach (CEGMA) [28] identified only 42.7% of CEGs; a low percentage also seen in another myxozoan [25] and possibly due to fast-evolutionary rates rendering even common eukaryotic genes difficult to recognize.
Table 1.
Comparison of myxozoan genome assembly quality
| Species name | Myxobolus honghuensis | Thelohanellus kitauei | Myxobolus squamalis | Henneguya salminicola | Kudoa iwatai | Enteromyxum leei | Sphaeromyxa zaharoni |
|---|---|---|---|---|---|---|---|
| Assembly size (kb) | 161,092,274 | 150,348,159 | 43,671,844 | 61,443,780 | 31,197,353 | 68,163,509 | 173,585,031 |
| Number of scaffolds | 1118 | 5757 | 37,921 | 18,330 | 1639 | 69,053 | 70,914 |
| Number of contigs | 1118 | 15,632 | 37,919 | 18,330 | 1646 | 69,178 | 70,935 |
| Contig N50 | 1,273,483 | 13,033 | 1286 | 7570 | 39,532 | 998 | 4474 |
| Scaffold N50 | 1,273,483 | 149,756 | 1286 | 7570 | 40,195 | 998 | 4474 |
| CEGs (complete) | 42.7% | 46.8% | 37.5% | 53.6% | 73.0% | 25.8% | 39.1% |
| %GC | 22.8 | 37.5 | 27.3 | 29.0 | 23.6 | 33.5 | 28.0 |
| References | Present study | Yang et al., 2014 [23] | Yahalomi et al., 2020 [25] | Yahalomi et al., 2020 [25] | Chang et al., 2015 [22] | Chang et al., 2015 [22] | Chang et al., 2015 [22] |
The M. honghuensis genome contains 15,433 inferred protein-coding genes, based on combined ab initio gene prediction, homology searching, and transcript mapping (Table 2, Additional file 1: Table S5). We assigned functions to 39.3% (6072) of the gene models (Additional file 1: Table S6).
Table 2.
Summary of genome annotation
| Genome annotation | |
|---|---|
| Transposable elements | |
| LTR | 2,838,408 bp |
| LINE | 459,926 bp |
| SINE | 2,326,269 bp |
| DIRS | 339,533 bp |
| DNA | 20,248,630 bp |
| Total | 58,174,337 bp |
| Protein-coding genes | |
| Total number | 15,433 |
| Mean CDS length | 756 bp |
| Mean exon length | 132 bp |
| Mean intron length | 507 bp |
| Functional annotation | |
| GO | 1371 (8.9%) |
| KEGG | 2918 (18.9%) |
| KOG | 3946 (25.6%) |
| TrEMBL | 4935 (32.0%) |
| NR | 5847 (37.9%) |
| NT | 1826 (11.8%) |
| Total | 6072 (39.3%) |
Transposable element and whole-genome duplication analyses
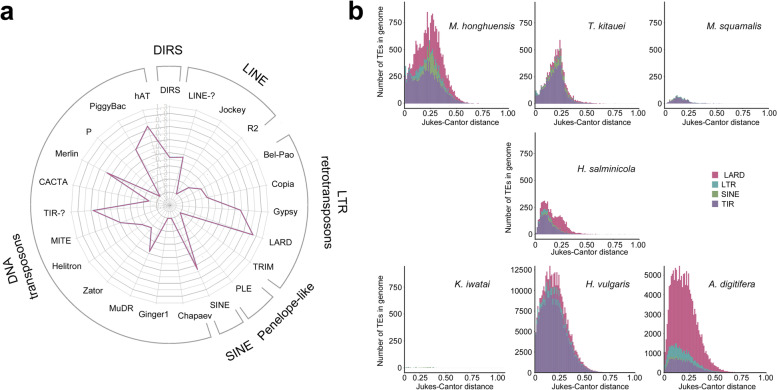
To identify factors that contribute to the relatively large genome of M. honghuensis, we analyzed the transposable element (TE) content and whole-genome duplications (WGDs) in M. honghuensis and compared it with other cnidarians [29, 30]. The M. honghuensis genome contained 36.1% (55.5 Mb) repetitive sequences, most of which are TEs (23.7% of the total genome; Table 2 and Additional file 1: Table S7). Long terminal repeats (LTRs, 9.2%) and terminal inverted repeats (TIRs, 11.9%) are the major contributors of retrotransposons (11.1%) and DNA transposons (12.6%) respectively (Fig. 1a). We calculated the relative age of transposable element copies using Kimura distance analyses and comparisons with other cnidarians, and revealed that M. honghuensis has had at least two transposon bursts (Fig. 1b).
Fig. 1.
Evolutionary history and expression of transposable elements (TEs). A Radar chart showing the proportion of TE superfamilies in the Myxobolus honghuensis genome. B Copy divergence analysis of TE classes in seven cnidarian genomes, based on Kimura 2 parameter distances. The percentage of TEs in genomes (y-axis) are clustered based on their Kimura values. Younger copies are located on the left side while older copies are located on the right side
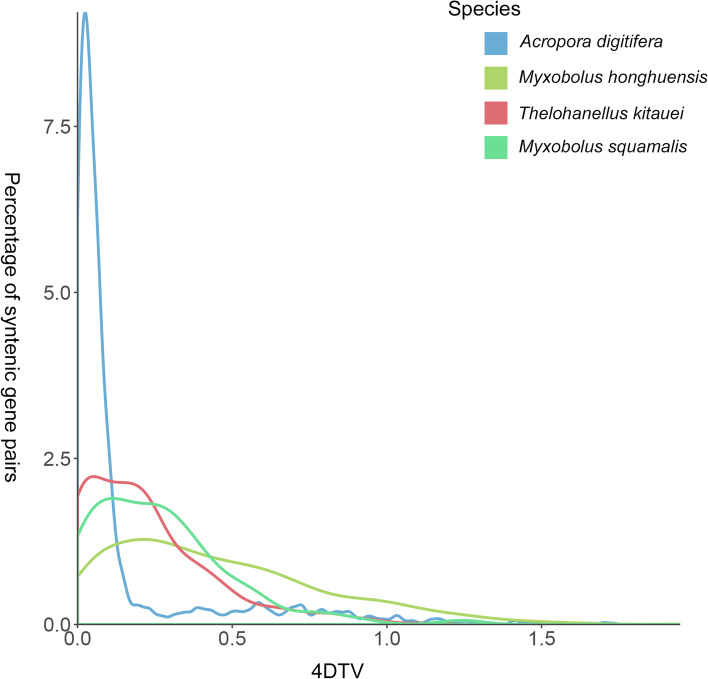
To identify potential WGD events in M. honghuensis, we determined the number of syntenic gene pairs in five myxozoans and two free-living cnidarian genomes (Additional file 1: Table S8). We found few syntenic blocks and syntenic gene pairs in the M. honghuensis genome, suggesting that it has not undergone WGD. We then calculated fourfold synonymous third-codon transversion (4DTv) values (a neutral genetic distance used to estimate the relative timing of evolutionary events [31]) for paralogous gene pairs in the free-living Acropora digitifera, and myxozoans M. honghuensis, Thelohanellus kitauei, and Myxobolus squamalis. We observed no sharp peaks in the 4DTv plots of the myxozoans, which supported a hypothesis that WGD has not occurred in these species (Fig. 2).
Fig. 2.
Distribution of the rate of transversions on fourfold degenerate synonymous sites (4DTv) among paralogs in genomes of four cnidarians
Mitochondrial (mt) genome sequences and nuclear-encoded proteins that are targeted to mitochondria
To determine whether there is mt genome in M. honghuensis, or it has lost its mt genome, as shown in the case of myxozoan Henneguya salminicola [25], we constructed a myxozoan seed database consisted of the complete mt genome of M. squamalis (MK087050) and the mt genome-containing sequence (JWZT01002463) of the closely related myxozoan T. kitauei [23, 25]. Then, BLASTN and TBLASTX searches were conducted against the PacBio and Illumina genome assemblies using seed sequences (MK087050 + JWZT01002463) as queries. We successfully identified three contigs (scaffold120386, scaffold85385, scaffold38991) in the Illumina genome assembly, which were considered to contain mt genome sequences of M. honghuensis. However, there were no positive hits in the PacBio genome assembly (e-value ≤ 1e−25). Then, the three Illumina contigs were added to the original seed to form new seed sequences. BLASTN and TBLASTX searches were further performed using the new seed sequences as queries against the PacBio long-read raw data (e-value ≤ 1e−25). In this way, 298 positive hits were found in the PacBio long-read raw data. These long-read PacBio raw reads were also considered to contain mt genome sequences. Our results showed that while M. honghuensis has a mt genome, it could not be recovered successfully using the PacBio assembly tools.
To explore nuclear-encoded protein target to mitochondria in M. honghuensis, we built a database of 198 nuclear-encoded proteins that function in mitochondria (e.g., cristae organization, mtDNA replication and translation, electron-transport chains) in fruit fly, human, and Hydra, and identified 97 homologs in M. honghuensis (Additional file 1: Table S9), 82 in H. salminicola, 75 in Myxobolus cerebralis, 63 in M. squamalis, and 22 in Kudoa iwatai [25]. We detected 7/11 cristae organization proteins in M. honghuensis, compared with 5/11 in H. salminicola and 4–6/11 for the other myxozoans. We identified 33/64 mt electron-transport chain complex proteins in M. honghuensis, compared with 10/64 in H. salminicola and 18–20/64 in the other published myxozoans. We detected 57/123 genes involved in mt genome replication and translation in M. honghuensis, 7/123 in H. salminicola, and 41–58/123 in the other myxozoans.
Myxozoans have diverse mitochondrial genome architectures and at least one—H. salminicola—has lost its mt genome [25, 32, 33]. We showed that M. honghuensis has a fast-evolving mt genome and retains many genes related to aerobic respiration, mitochondrial genome translation and replication, and cristae organization. However, it should be noted that we did not recover any specific complete mt genes of M. honghuensis in this study. We assumed that most of our blast hits of mt genome were located in the non-coding areas, which might reside in the large conserved region [25]. In the future work, assembling a complete M. honghuensis mt genome from improved long-read sequencing will help us to understand its structure and evolution. Furthermore, DAPI-staining and ultrastructural methods are needed to provide microscopic evidence of the M. honghuensis mt.
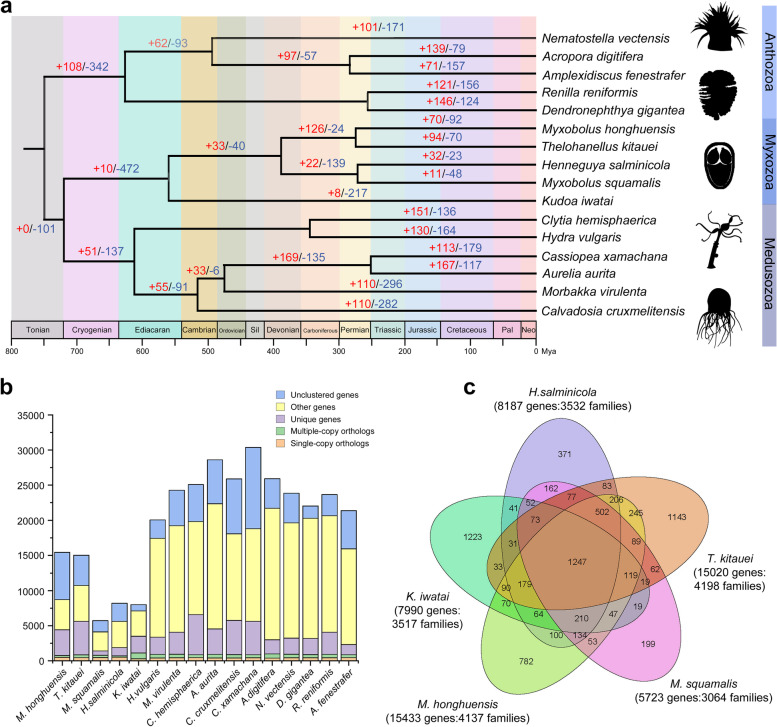
Evolution of gene families
A comparison of genome sequences by OrthoMCL showed that M. honghuensis has 4137 of the 12,300 orthologous gene families identified in 16 cnidarians (Fig. 3b and Additional file 1: Table S10). Compared with other cnidarians, the M. honghuensis genome has 10,362 species-specific genes (>67% of the entire gene repertoire) (Additional file 2: Fig. S2, Fig. S3), which were enriched (p < 0.05) in single-organism catabolic processes (GO: 0044712), cellular catabolic processes (GO: 0044248), and peptidase activity (GO:0070011) (Additional file 1: Table S11 and Additional file 2: Figs. S4-S6). We identified a core set of 1247 gene families shared among 5 myxozoans (M. honghuensis, T. kitauei, H. salminicola, M. squamalis, and K. iwatai), which accounted for 29.7–40.7% of their gene families (Fig. 3c). The M. honghuensis genome has 70 significantly expanded and 92 significantly contracted gene families based on the z-score of gene count differences among 16 cnidarians (Fig. 3a and Additional file 1: Table S12). The expanded families included 2068 genes (Additional file 1: Table S13), which over-represent energy metabolism, intra- and intercellular communication, and invasion (Additional file 2: Fig. S7, Fig. S8). GO enrichment analysis revealed that the expanded families were enriched in meiotic cell cycle processes (GO:1903046), meiotic nuclear division (GO:0007126), and macromolecular complexes (GO:0032991) (Additional file 2: Figs. S9-S11). The 92 contracted families (Additional file 1: Table S14) were related to respiratory, neural, and immunity functions, e.g., cytochrome c biogenesis protein, immunoglobulin v-set domain, and clustered mitochondria (Additional file 2: Fig. S12, Fig. S13). GO enrichment analysis showed that contracted families were enriched in macromolecular complexes, and s-adenosylmethionine-dependent methyltransferase activity (GO:0008757) (Additional file 2: Figs. S14-S16).
Fig. 3.
Gene family and phylogenetic analyses of Myxobolus honghuensis and other representative cnidarian genomes. Phylogenetic tree was generated from a matrix of 51 single-copy orthologs (11,323 amino acid positions) using maximum likelihood under the GTRGAMMA model. A Number of expanded (red) and contracted (blue) gene families in each evolutionary branch. The expanded families are related to energy metabolism, intra- and intercellular communication, and invasion. The contracted families are related to respiratory, neural, and immunity functions, e.g., cytochrome c biogenesis protein, immunoglobulin v-set domain, and clustered mitochondria. B Clusters of orthologous and paralogous gene families in M. honghuensis and 15 other cnidarians. C Overlap of gene families in M. honghuensis and four other myxozoans
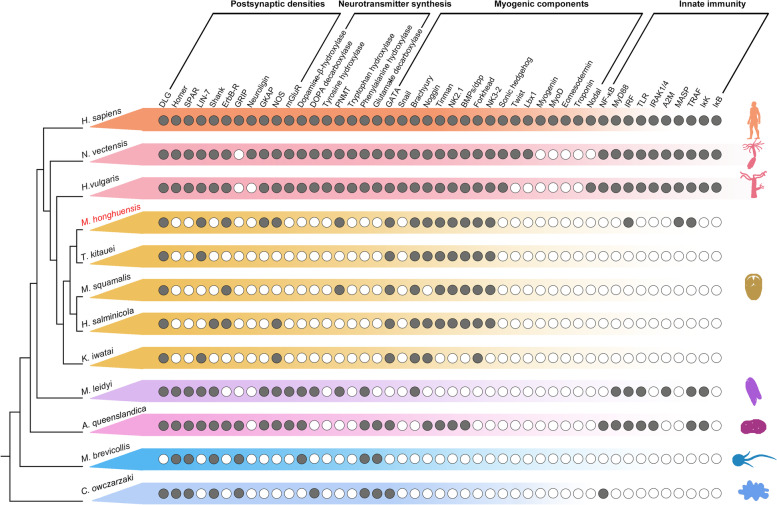
Streamlined neural molecules
To test if myxozoans retain any components of a nervous system, we searched for genes associated with postsynaptic densities (PSDs) and neurotransmitters (Fig. 4). We found 5/11 PSDs genes (DLG, LIN-7, ErbB-R, GKAP, and NOS) and 1/8 neurotransmitter genes (PNMT), but only in M. honghuensis and M. squamalis (Fig. 4). Other neural genes found in free-living Cnidaria (Homer, SPAR, Shank, neuroligin, and mGluR) were not detected [34, 35]. We consider the apparent loss of these in M. honghuensis to be secondary, except GRIP, which is not present in all cnidarians, and might be lost primarily in myxozoans.
Fig. 4.
Presence/absence of genes associated with postsynaptic densities (PSD), neurotransmitter synthesis, myogenic components, and innate immunity in myxozoans and other representative eukaryotes
Loss of innate immune genes
Innate immune components are found in nearly all bilaterians and cnidarians, and recently detected in placozoans, ctenophores, and sponges [36–38]. We found that all 10 major gene families involved in immunity [39] were present in free-living cnidarians, but reduced in the 5 analyzed myxozoans (Fig. 4). The reduced complement of immune system components implies that canonical immune system pathways are not functional in myxozoans.
Genes that promote the M. honghuensis life cycle
Myxobolus myxospores can resist drying, freezing, and animal digestion [40]. We identified 235 M. honghuensis genes involved in stress resistance (Additional file 1: Table S6), including the universal stress protein Sll1388 and DNA repair and recombination protein RadA, and determined these are under positive selection (Additional file 1: Table S15).
We detected the expansion of genes related to recognition, rapid proliferation, and migration. C-type lectins (CLEC), which recognize complex carbohydrates on cells and tissues [41], are significantly expanded in the M. honghuensis genome (Additional file 1: Table S13), as are gene families related to meiotic cell cycle processes and meiotic nuclear division (Additional file 1: Table S16; Additional file 2: Fig. S9). KEGG analysis revealed M. honghuensis is enriched in lineage-specific regulators of the actin cytoskeleton (Additional file 1: Table S17), which is involved with cell migration and adhesion [42]. In addition, gene families involved in tumor metastasis and hyperplasia, such as tenascin and extracellular matrix (ECM)-receptor interaction, are significantly expanded (Additional file 1: Table S13), which is in line with the enrichment of M. honghuensis lineage-specific genes of cancer pathways (Additional file 1: Table S17). A previous study indicated that the metastasis of cancer cells is partly analogous to the expansion mechanism of protozoan parasites [43]. Since miniaturization has enabled myxozoans to converge on patterns of host exploitation similar to those of protists [19], we suggest that this tumor-related gene expansion, together with enhanced meiotic and actin cytoskeleton activity, played an important role in the migration and rapid proliferation of M. honghuensis in host tissues.
Endoparasites live in a nutrition-restricted environment within the host and thus may have unique energy metabolism strategies [44]. We showed that the lineage-specific genes of M. honghuensis were enriched in GO terms associated with single-organism and cellular catabolic processes (Additional file 2: Fig. S4). By calculating Ka/Ks ratios, we detected positive selection in genes related to enzymatic catalysis in carbohydrate metabolism (Additional file 1: Table S15). Compared to other cnidarians, we observed marked expansion of gene families involved in fatty acid biosynthesis, elongation, and degradation, (Additional file 1: Table S13), but contraction of low-density lipoprotein receptors (LDLRs) (genes, which reduce cholesterol [45] Additional file 1: Table S14). Reduction of LDLRs might promote accumulation of cholesterol, which could improve success of M. honghuensis within its hosts, as has been shown for other parasites including amoeba and flagellates [46, 47].
We observed expansion of gene families related to the cellular processes of nutrient uptake and waste excretion, such as the ATP-binding cassette (ABC)-2 family transporter protein (involved in extra- and intracellular transmembrane nutrition and the extrusion of noxious substances [48]) and the major facilitator superfamily (MFS) facilitates the movement of small solutes across cell membranes and is responsible for drug metabolism and metabolite transport in other parasites [49, 50]) (Additional file 1: Table S13). Endocytosis pathways were also expanded (Additional file 1: Table S13). We observed genes linked with probable enhancement of intra- and intercellular communications in the M. honghuensis genome; e.g., the glycosphingolipid biosynthesis pathway, which mediates and modulates intercellular coordination [51] (Additional file 1: Table S18). Also, we found significant expansion of genes encoding Notch ligands Delta and Serrate and the pathway involved with gap junction, which play key roles in intercellular communication [52, 53] (Additional file 1: Table S13). Relative to other cnidarians, M. honghuensis has more genes of the focal adhesion pathway, which regulates communication between the cell and the surrounding extracellular matrix [54] (Additional file 1: Table S13).
Mesoderm and myogenic components
The mesoderm is a germ layer present in triploblastic animals that gives rise to skeletal structures, circulatory organs, and muscle tissue. However, both genes associated with mesoderm and muscle formation, and mesoderm-like musculature have been found in cnidarians [55]. In the Myxozoa, muscles and mesodermal musculature are only known from Malacosporea with worm-like life stages, not Myxosporea (which includes M. honghuensis) [56]. Here, as expected from prior work [57, 58], we did not detect myogenin, MyoD, eomesodermin, and troponin in any myxozoan or free-living species (Fig. 4), genes that typically play a role in muscle differentiation, specification, contraction, and atrophy [59]. We did however identify GATA, Brachyury, and Forkhead in the free-living cnidarians and myxozoans. These conservative transcription factors are involved in specification and differentiation of multiple cell types during embryogenesis and development [60, 61]. We did not detect either of two developmental regulator genes, sonic hedgehog and SNAIL, in the myxozoans, but found them in the free-living species as expected [62, 63] (Fig. 4).
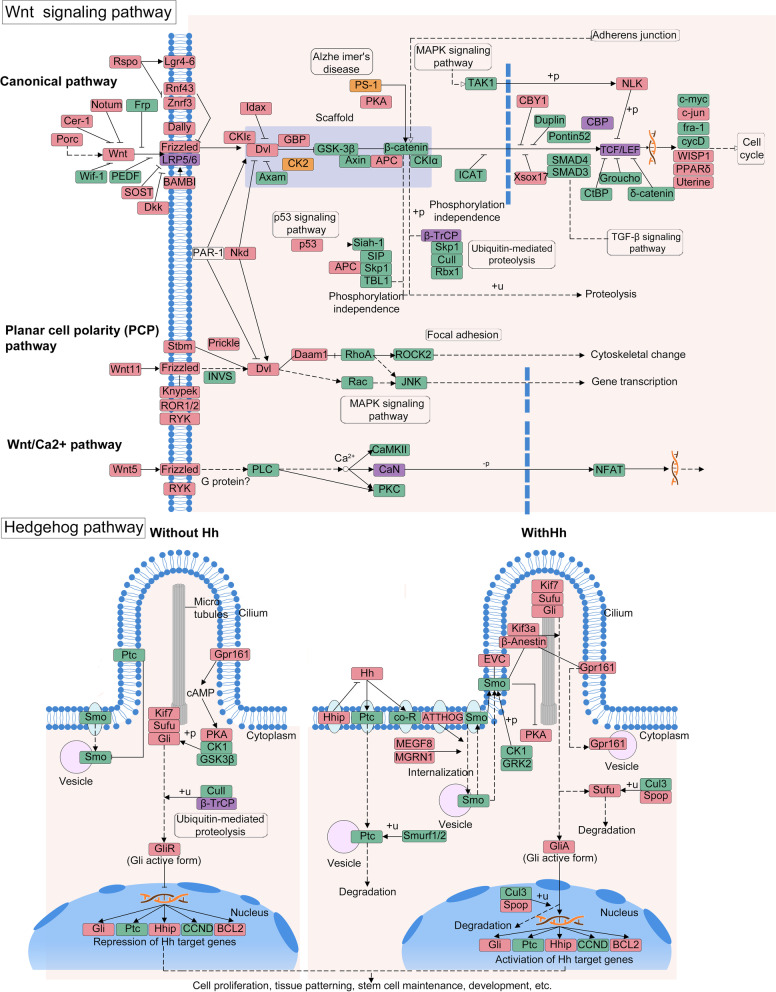
Wnt, Hedgehog, and Homeobox
The diverse life cycle development and body forms of cnidarians are regulated in part by homeobox genes, and the Wnt and Hedgehog signaling pathways, which determine body polarity, tissue identity, and polyp-to-jellyfish transition [64, 65]. In M. honghuensis, we detected 43/83 Wnt signaling pathway genes, including 9 key genes (Fig. 5) that could control neuronal development, left-right axis establishment, and mesoderm segmentation. Ten genes in the Wnt pathway are expanded (Additional file 1: Table S13), including LRP5 and beta-TrCP, which trigger beta-catenin signaling and mediate ubiquitination, respectively [66]. Two genes that stabilize beta-catenin and regulate many cellular processes, PS1 and csnk2b [67], are contracted (Additional file 1: Table S14). We detected 21/52 components of the Hedgehog pathway, including growth arrest-specific 1 (Fig. 5). More Wnt and Hedgehog signaling pathway genes were found in the M. honghuensis genome (43/83 = 51.8%, 21/52 = 40.4%) than in the other myxozoans K. iwatai (22/72 = 30.6%, 4/19 = 21.1%) and M. cerebralis (23/72 = 31.9%, 5/19 = 26.3%). We found 7/24 of ANTP class Homeobox genes in M. honghuensis (1 Hox-like and 6 NK-like), compared to 18-24/24 in free-living Cnidaria (Additional file 1: Table S19).
Fig. 5.
Myxobolus honghuensis genes involved in the Wnt and Hedgehog pathways. Components present in the M. honghuensis genome are in green, undetected components are in red. The purple and orange boxes indicate expanded and contracted genes respectively. Abbreviations, annotations, and connections are adapted from KEGG pathway (04310 and 04340) and follow KEGG standards
Discussion
While it seems intuitive that the evolutionary history of obligate parasites is characterized by reductions, including a massive loss of genetic and functional diversity [7], herein we show that the genome of the myxozoan M. honghuensis illustrates both reduction and expansion of different components—a pattern described as mosaic evolution. Comparison of this parasite with other myxozoans and free-living Cnidaria revealed aspects of genome size variation, adaptive mechanisms, and genome organization, which expand our understanding of parasite genome complexity and evolution.
Genome size and transposable elements
The genome size of M. honghuensis is 206 Mb, with a smaller final assembly size of 161 Mb likely due to abundant repetitive sequences. These estimates place M. honghuensis as having a slightly larger genome than closely related T. kitauei (188.5 Mb) [23], ninefold larger than the smallest (K. iwatai, 22.5 Mb) [22]. The M. honghuensis genome is equivalent in size to some free-living cnidarians, including Nemopilema nomurai (213 Mb) [68] and S. malayensis (185 Mb) (Additional file 1: Table S3) [69], despite having a body size at the micron scale, compared with vastly larger N. nomurai (up to 2 m in diameter) [70] and S. malayensis (up to 13.5 cm in diameter) [71]. Concomitant with its larger genome size, M. honghuensis has 2.8 times the number of protein-coding genes than the distantly related myxozoan K. iwatai (5533 genes, 22.5 Mb) [22], but is comparable to that of the more closely related T. kitauei (16,638 genes, 188.5 Mb) [23] (Additional file 2: Fig. S17), and similar to free-living cnidarians Hydra vulgaris (16,839 genes, 1,005 Mb) [72], and Nematostella vectensis (18,000 genes, 450 Mb) [73] (Additional file 1: Table S3). The M. honghuensis genome is less compact (mean intron size 507 bp) compared with myxozoans K. iwatai (82 bp), T. kitauei (240 bp), and the non-myxozoan Sanderia malayensis (381bp; Additional file 1: Table S3). M. honghuensis has a mean exon size of 132 bp, compared with 102 bp in K. iwatai, 235 bp in T. kitauei, 218 bp in H. vulgaris, and 208 bp in N. vectensis (Additional file 1: Table S3).
Transposable element (TE) and whole-genome duplications (WGD) contribute to genome size variation [29, 30]. We found that the M. honghuensis genome differs from that of most animals by possessing more DNA transposons than retrotransposons, and no evidence of WGD. We did not detect evidence for WGD in other myxozoans, but confirmed a likely ancient genome duplication in the free-living cnidarian A. digitifera, previously reported by Mao and Satoh, 2019 [74]. TE abundances in the myxozoans we analyzed were proportional to their genome sizes, which suggested that differences in myxozoan genome sizes are caused partly by different TE insertion/expansion rates. Massive mobilization of TEs has been implicated in many cases of adaptive evolution, such as adaptation to novel environments, stressors, or environmental change [75], and is thus a likely feature of many myxozoans given their extensive colonization of aquatic and even terrestrial hosts and habitats [76]. A further genetic signature of a dynamic evolutionary history related to parasitism was the finding that M. honghuensis transposons were acquired in two bursts, which we propose is an artifact of the host-acquisition or host-switch events that likely occurred several times in evolution of the Myxozoa [77]. The TE results, together with our findings of gene number and intron size, suggest that gene retention, large intron, transposon insertion, but not polyploidy, are the major factors contributing to the relatively large genome size of M. honghuensis.
Species-specific adaptations
M. honghuensis has 10,362 species-specific genes (>67% of its gene complement), compared with 1.4 to 63.6% for other animals [78–80]. This high proportion could be integral to adaptations for parasitism in alternate fish and worm hosts. We also found that although M. honghuensis has about the same number of expanded (70) versus contracted (92) gene families, many more genes are represented in the expanded families (2068) than contracted (only 90). This suggests that there have been large-scale duplication events leading to expansion of genic content in some families. Myxozoans must succeed in diverse environments during their 2-host life cycles, including invasion and development within multiple host tissues, and persistence in the external environment as spores. We found expansion and positive selection of a series of genes conceivably related to this parasite life cycle, including temperature and salinity stress resistance (Sll1388), and DNA repair (RadA). The strong positive selection of these stress resistance proteins may help spores survive in both the water column, and within the digestive system of their target hosts.
Host invasion by a myxozoan involves molecular and physical evasion of immune responses. We found genes coding for C-type lectins (CLEC) were expanded in M. honghuensis, and thus may be an important tool for mediating immunological recognition within its hosts, much as other parasites release CLEC homologous to key host receptors to possibly interfere with immune response or effector function [81]. Myxozoans require motile stages both to avoid host immune responses [82] and to reach target tissues. In Ceratonova shasta and Ceratomyxa puntazzi, actin has an important role in stage motility [83, 84], and our finding that here M. honghuensis is enriched in lineage-specific regulators of the actin cytoskeleton (Additional file 1: Table S17), suggests that actin may be important for success of diverse myxozoan taxa.
Primitive nervous system?
While free-living cnidarians have simple nerve nets [85], there is no evidence that similar nerves are present in myxozoans even though they can sense proximity of hosts and can have motile, multi-cellular stages [56]. In M. honghuensis, we detected 5 genes associated with postsynaptic densities, which raises the possibility that M. honghuensis have a miniaturized nervous system, perhaps similar to those typical of insects, especially parasitoids [86]. Both parasitoids and myxozoans have undergone extreme reduction in size during their evolutionary history, with insects retaining a nervous system [87, 88]. Retention of a genetic signature of nervous systems supports the idea that general evolutionary simplification of morphology should not be assumed to have corresponding losses in the genome [34]. In the future work, we can investigate the nerve-like structures in different myxozoans by immunogold labeling of synaptic proteins [89] or immunohistochemical labeling of FMRF-amide and α-tubulin [90].
Wnt, Hedgehog, and Homeobox
Myxozoans have diverse morphologies in their life cycles, which involve bilateral myxospores, triradial actinospores, and amoeboid multi-nucleate/-cellular sporoplasms. Little is known about the genetic basis of myxozoan development and body patternation, but at least two species have lost the ligands, receptors, and most downstream elements of the Wnt and Hedgehog signaling pathways [22], and no pathway-specific components were detected in a recent in-depth analysis of Tetracapsuloides bryosalmonae from both of its hosts [91]. In contrast, we identified Wnt and hedgehog components in M. honghuensis, demonstrating that at least M. honghuensis retain these signaling components (e.g., LRP5 and beta-TrCP) while losing others (e.g., PS1 and csnk2b). However, it should be noted that most of the ligand and/or receptor are still missing in the Wnt and Hedgehog pathways, and it is likely that the other elements are non-functional or operating in other pathways. For the homeobox, M. honghuensis is only the second myxozoan (after T. bryosalmonae [91]) in which unambiguous Homeobox-family genes/proteins have been observed. The identification of homeobox will facilitate future functional studies and spatial expression mapping to better understand whether and how these genes are involved in the radical morphological shifts that occur between myxozoan life stages (spores versus amoeboid sporoplasm versus plasmodia). Particularly informative will be discovery of how homeobox genes control the bilateral/triradial alternation between actinospore and myxospore stages, which should be tractable in the few species whose complex life cycles are both known and maintainable in the laboratory (C. shasta [92], Parvicapsula minibicornis [93], M. cerebralis [94]).
High genomic variation across Myxozoa
We revealed high genomic variation within myxozoans, including a wide range of genome sizes across myxozoans and a high number of non-overlapping gene families between species (Fig. 3c). Even between two closely related species, M. honghuensis and T. kitauei, the species-specific components account for a large proportion of the genome and several gene families, such as PSDs, neurotransmitter synthesis, and innate immunity, exhibit clear different pattern of presence and absence. This phenomenon raises the questions of why there is so much variation across myxozoa and why some species can survive without these gene pathways being retained and expanded. In fact, the variation in genome size and content has also been reported in other parasites, such as microsporidians [95], mesozoans [9, 16], and parasitic nematodes [10]. It is widely appreciated that parasites are prone to rapid evolution, and because of their often short generation times, large population sizes, and host-parasite arms races, parasite genome may show evidence of adaptive “plasticity” [4, 96]. Therefore, a possible answer for the above questions is that myxozoans exhibit highly species-specific adaptations to variable environments (e.g., different habitats, hosts, organs, and tissues), which are shown to be related to substantial genome size variation and increased proportion of non-overlapping gene families [80, 97, 98]. However, the causes of myxozoan genomic variation are still widely open for discussion and more works needs to be done to investigate the genomes from a diverse range of myxozoans.
Conclusions
In summary, we show that M. honghuensis has a relatively larger myxozoan genome, which is both less reduced and less compact, due to gene retention, larger introns, and transposon insertions, but not polyploidy. As evolutionary adaptations to endoparasitism, the M. honghuensis genome evolved in a mosaic fashion of conserved, divergent, depleted, and enhanced genes and pathways. It has the simplest animal immune components and has retained a reduced set of neurological and signaling genes, including components of the Wnt and Hedgehog pathways, and Homeobox domains. M. honghuensis has increased the number of genes associated with stress resistance, tissue invasion, energy metabolism, and cellular processes. These results illustrate that M. honghuensis genome evolution is not governed solely by streamlining, but rather embodies trade-offs between genomic simplification and complexification necessary for success of a multi-host parasite life cycle. Altogether, this study changes our view of parasite evolution, and furthermore provides an exciting new system and genomic resources to investigate the evolutionary plasticity and function of core cellular mechanisms in animals.
Methods
Sampling, DNA/RNA extraction and sequencing
M. honghuensis was collected from infected allogynogenetic gibel carp Carassius auratus gibelio in Zoumaling Farm, Hubei Province, China on July 29, 2015. Fish (66) were held on ice before being killed with an overdose of MS-222 (Sigma-Aldrich, Co., Ltd., St. Louis, MO, USA). From each fish, tissue containing one large cyst was homogenized by a manual glass tissue grinder and suspended in 0.1M phosphate-buffered saline (PBS), pH 7.2, then filtered through cotton gauze. Myxospores were separated from the filtrate by sucrose gradient centrifugation and Percoll gradient centrifugation in turn, washed several times with distilled water, and then examined microscopically to verify purity and identify. Purified myxospores were placed into RNAlater (Sigma) or 95% ethanol, frozen in liquid nitrogen and finally stored at – 80 °C. Samples with highest myxospore number and purity were selected for downstream processing: 15 for DNA, and 3 samples for RNA sequencing. All of these 18 samples were identified as M. honghuensis based on morphology and 18S sequencing [99, 100] (Additional file 1: Table S20). The maintenance and care of experimental animals complied with the National Institutes of Health Guide for the care and use of laboratory animals [101] and was approved by the animal care and use committee of Huazhong Agricultural University, China.
From the 15 DNA samples (from 15 different fishes), 3 of them were sent for Illumina genome survey. Genomic DNA was isolated and extracted using the CTAB method [102]. Libraries were built by TruSeq DNA library kit (Illumina). The ~500 bp insert size libraries were sequenced on Illumina HiSeq 2500 (2 × 125 PE). The 3 DNA candidates were also sent for PacBio library construction. To do this, 10 μg gDNA was sheared to an ~20-kb targeted size using a Covaris g-Tube, then used for size selection using Blue Pippin (Sage Science, Beverly, MA, USA). Then, 20-kb PacBio libraries were prepared following the standard PacBio protocol. After some trial and error (considering the DNA amount, contamination, library quality), only one sample (No. 51, it is unmixed) was sent for final PacBio sequencing. It was sequenced on a PacBio RSII platform (PacBio Sequel sequencer at Biomarker Technologies Corporation) with P6-C4 chemistry.
We have 3 RNA candidates (from three different fishes) for Illumina transcriptome sequencing. Before RNA extraction, we checked the three samples and found immature plasmodia that contained spores of different stages. Then we extracted the RNA separately, tested the RNA quality, and combined the RNA. RNA was isolated with TRIzol 550 (Invitrogen). The purity and integrity of RNA were assessed using NanoPhotometer® spectrophotometer (IMPLEN, CA, USA) and RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA) respectively. Only one library was constructed from the combined RNA and sent for RNA sequencing. Libraries were prepared using NEBNext® Ultra RNA kit (New England Biolab (NEB), Ipswich, MA) following the manufacturer’s recommendations and sequenced as 2 × 125 paired-end (PE) runs with Illumina HiSeq 2500. Raw sequence data from both the Illumina genome and transcriptome sequencing were cleaned and trimming of low-quality reads and adaptors using Trimmomatic v0.33 [103]
17-mer analysis and evaluation of genome size
All the filtered Illumina paired-end genomic reads were used for 17-mer frequency analysis. Jellyfish v2.1.3 [104] was used to count K-mer occurrences with a setting of K = 17, and a histogram of K-mer distributions was generated. Peak coverage was taken to be the average K-mer coverage, and genome size estimated by the formula: G = K-mer_num/Peak_depth, where the K-mer_num and Peak_depth are the total number and the average depth of 17-mer, respectively.
Genome assembly and decontamination
We assembled PacBio long-read data of M. honghuensis using FALCON v0.7 [105], then used arrow v2.2.2 [106] to polish this long-read-based genome through PacBio reads themselves. Next, we used two rounds of Pilon-based v1.22 [107] polishing to correct single-base variants, indels and to fill the gaps in the final assembly using Illumina genome survey raw data. To evaluate and remove contamination from genome, we first mapped the raw Illumina genome sequences to the common carp genome (NCBI: GCF_000951615.1, [108]) using SOAP software v2.21 [109]. This resulted a very low total mapped rate of 0.02% (Additional file 1: Table S21). This means that there is very few host contamination in our DNA. The Illumina sample is the same as that was used to make DNA for Pacbio. So for the Pacbio assembly, instead of searching against a host genome directly, we applied a customized decontamination protocol in our recent CCPRD framework [110], which consider both host and bacterial contamination. Briefly, Blobtools v1.0 [111] was used to construct a BlobDB, which contained the genome sequences, coverage, and species information. The results were processed by the Blobtools function “create” to annotate each scaffold. A TAGC was drawn at the rank of the phylum and under the taxrule “bestsum.” Using the Blobtools function “view,” taxonomically annotated non-cnidarian scaffolds with a bit-score of ≥200 were inspected manually and compared against the NCBI nucleotide database (BLASTN, -e value 1 × 10 652 −5, -max_target_seqs 20, -outfmt 6). Sequences strongly matched to Chordata and Proteobacteria were excluded. The bacterial sequences were extracted and independently assembled using wtdbg (https://github.com/ruanjue/wtdbg) (Additional file 1: Table S22). CEGMA v2.5 [28] was used to assess the completeness of the genome by mapping against genes that are highly conserved in eukaryotes.
Annotation of repeats
We used both homology-based and de novo approaches for repeat annotation. Using default settings, LTR_FINDER v1.0.5 [112], MITE-Hunter v1 [113], RepeatScout v1.0.5 [114], PILER-DF v1 [115] were used to build a de novo repeat library from our assembly. The predicted repeats were then classified using PASTEClassifier v1 [116] and merged with Repbase v19.06 [117]. Then, consensus sequences from the repeat library were used as queries for RepeatMasker v4.0.5 [118] to determine the repeat content of the M. honghuensis genome. The relative age of the different transposable element (TE) families was estimated through Copy Divergence Analysis (CDA) using Jukes-Cantor distances [119] between individual copies and their consensus sequence. The percentage differences between identified TE copies in the genome and the consensus sequences in the TE library were extracted from the RepeatMasker file (.out file), and converted to Jukes-Cantor distance by the formula d = −(3/4)loge(1−(4/3)p).
Gene prediction
We used ab initio prediction, homology search, and transcriptome-assisted prediction, for gene prediction in the repeat-masked genome. For the ab initio prediction, we used Genscan v2.1 [120], Augustus v3.2.3 [121], GlimmerHMM v3.0.1 [122], GeneID v1.4.4 [123], SNAP v2013-02-16 [124], with default parameters. For homology prediction, we used GeMoMa v1.3.2 [125] with the protein sequences from T. kitauei (NCBI: GCA_000827895.1, [23]), A. digitifera (NCBI: GCF_000222465.1, [126]), Exaiptasia pallida (NCBI: GCF_001417965.1, [127]), H. vulgaris (NCBI: GCF_000004095.1, [72]), N. vectensis (NCBI: GCF_000209225.1, [73]), Orbicella faveolata (NCBI: GCF_002042975.1, [128]), Caenorhabditis elegans (NCBI: GCA_000002985.3, [129]). For transcriptome-assisted gene prediction, we used Hisat2 v2.1.0 and Stringtie v1.3.3 [130] for the transcriptome assembly, then TransDecoder v5.3.0 [131] and GeneMarkS-T v5.1 [132] for gene prediction, and PASA v2.3.3 [133] to predict unigenes from de novo transcriptome assembly. To integrate data derived from these three methods into an EVM-derived gene set, we used EvidenceModeler (EVM) v1.1.1 [134]. Sequences of predicted genes were searched against GO, KEGG, KOG, Swissprot, TrEMBL, NR, and NT databases for annotation.
To annotate non-coding RNAs, we used tRNAscan-SE v2.0 [135] software to predict the tRNAs with eukaryotic parameters. Non-coding RNAs (miRNAs and rRNAs) were detected using BLASTN to search the Rfam [136] database.
Identification of mt genome sequences and nuclear-encoded proteins that are targeted to mitochondria
To detect whether there is mt genome in M. honghuensis, we searched the mt genome sequences in M. honghuensis Pacbio contigs, Illumina contigs (from genome survey), and PacBio long-read raw data respectively. First, we constructed myxozoan seed sequences containing mt genome sequences of the closely related myxozoan T. kitauei (JWZT01002463) and complete mt genome of M. squamalis (MK087050). Then, BLASTN and TBLASTX searches were conducted against the Pacbio and Illumina genomes using seed sequences (MK087050 + JWZT01002463) as queries. The positive hits (e-value ≤ 1e−25) were obtained and added to the seed sequences. Second, BLASTN and TBLASTX searches were performed using the new seed sequences as queries against the Pacbio long-read raw data (e-value were−25).
To search for presence and absence of nuclear-encoded proteins target to mitochondria in M. honghuensis, we first built a database based on Yahalomi et al [25], which contains 198 selected nuclear-encoded proteins that function in mitochondria from fruit fly, human, and/or Hydra proteome. These proteins include (i) proteins involved in cristae organization; (ii) genes involved in mitochondrial respiratory chain complexes (I-V); (iii) nuclear genes involved in the replication and translation of the mt genome. Using reciprocal BLASTP, the presence/absence of these proteins were determined in M. honghuensis proteome. The M. honghuensis proteome includes PacBio gene models and CCPRD from our previous work [110]. The 198 nuclear-encoded proteins target to mitochondria were used as seeds to search candidate homologous proteins in the M. honghuensis proteome (e-value to s−3). The candidate proteins were BLASTed against the UniProtKB/Swiss-Prot. Only those proteins with the best match to the same term of seed sequences were considered as present.
Gene family and phylogenetic analysis
The protein data of representative cnidarian species, including T. kitauei (NCBI: GCA_000827895.1, [23]), K. iwatai (NCBI: GCA_001407235.2, [22]), H. salminicola (NCBI: GCA_009887335.1, [25]), M. squamalis (NCBI: GCA_010108815.1, [25]), H. vulgaris (NCBI: GCF_000004095.1, [72]), Morbakka virulenta (NCBI: GCA_003991215.1, [65]), Clytia hemisphaerica (NCBI: GCA_902728285.1, [137]), Aurelia aurita (NCBI: GCA_004194415.1, [65]), Calvadosia cruxmelitensis (NCBI: GCA_900245855.1, [138]), Cassiopea xamachana (NCBI: GCA_900291935.1, [138]), A. digitifera (NCBI: GCF_000222465.1, [126]), N. vectensis (NCBI: GCF_000209225.1, [73]), Dendronephthya gigantea (NCBI: GCA_004324835.1, [139]), Renilla reniformis (NCBI: GCA_900177555.1, [140]), and Amplexidiscus fenestrafer (NCBI: PRJNA354436, [141]), were used for gene family clustering (Additional file 1: Table S23). The 16 cnidarians were chosen because they have relatively good genome quality and gene models. So, we can get more accurate estimation of gene family expansion and contraction. The gene family, phylogenetic and molecular clock analyses need to be conducted on the same tree. So the latter two analyses also used these 16 species. We used OrthoMCL v2.0.5 [142] to cluster orthologous groups among selected species, with low-quality gene model sequences removed based on the sequence length of OrthoMCL criteria. Sequences were used for all-versus-all BLAST searches with e-value cutoff of 1e−5. Homologous gene families of various species were determined using OrthoMCL with the Markov model. The Venn diagram of shared orthologous groups between myxozoans M. honghuensis, T. kitauei, K. iwatai, H. salminicola, and M. squamalis was plotted using R.
Fifty-one single-copy orthologous gene clusters of these 16 Cnidaria species were extracted from OrthoMCL results, and aligned using MAFFT v7.205 [143], poorly aligned regions were eliminated by GBlocks v0.91b with default parameters and trimmed sequences were concatenated to form a supermatrix of 16 taxa and 11,323 amino acid positions for phylogenetic analyses. We constructed a phylogenetic tree using RAxML v8.2.12 [144] with the GTRGAMMA model and 100 rapid bootstrap replicates.
Molecular clock and CAFÉ analysis
We estimated species divergence times through the Bayesian relaxed molecular clock approach using MCMCtree in PAML v4.8 [145]. Fossil records were downloaded from the TIMETREE [146] website for calibration. Three calibration points were used: the maximum age of Medusozoa + Myxozoa were set to and 680 million years ago (Mya), the node of crown cnidarians was constrained with a minimum age of 677 Mya and maximum age of 805 Mya, the minimum and maximum divergence age of sea anemones and stony corals were set to 437 Mya and 600 Mya. Expansion and contraction of gene clusters were determined using CAFÉ v4.1 [147]. The phylogenetic tree and the divergence times from the previous step were used in CAFÉ to infer changes in gene family size using a probabilistic model.
Detection of positively selected genes
To detect the positively selected genes in M. honghuensis, the single-copy genes of M. honghuensis and from the closely related species K. iwatai were aligned using MAFFT. The estimation of dN and dS values were obtained using yn00 software (part of the PAML program package). Genes with a dN/dS ratio >1 were considered as indicative of gene parts displaying a signal of positive selection. The positively selected genes were annotated by GO, KEGG, KOG, Pfam, Swissprot, and NR analyses as for the entire genome.
Gene gain and loss
Using reciprocal BLASTP, the presence/absence of the conserved developmental signaling pathways (Wnt and Hedgehog), neuronal signaling, myogenic components, and innate immune regulation were determined in five myxozoans (M. honghuensis, T. kitauei, K. iwatai, H. salminicola, M. squamalis). Briefly, Homo sapiens proteins (NCBI: GCA_000001405.28, [148]) were used as seeds to perform reciprocal BLAST searches against the proteome of the two free-living cnidarians, H. vulgaris and N. vectensis. Then the H. sapiens, H. vulgaris, and N. vectensis proteins were used to identify candidate homologous proteins in myxozoan predicted proteomes (e-value ≤ 1e−3). The candidate proteins were BLASTed against the UniProtKB/Swiss-Prot. Only those proteins with the best match to the same term of seed sequences were considered as present. As a control of our approach, we confirmed presence of these genes in ctenophore Mnemiopsis leidyi (NCBI: GCA_000226015.1, [149]), sponge Amphimedon queenslandica (NCBI: GCA_000090795.1, [39]), and two unicellular eukaryote species, Monosiga brevicollis (NCBI: GCA_000002865.1, [150]) and Capsaspora owczarzaki (NCBI: GCA_000151315.2, [151]). Additionally, using the same reciprocal BLAST searches and human and fruit fly proteins as seeds, we searched the 20 meiosis-related genes that were conserved among human, fruit fly, budding yeast, and dinoflagellates [152] in the genome and transcriptome of M. honghuensis.
4DTv analysis
To detect any whole genome duplication events in myxozoan genomes, we searched for putative paralogs within genomes using MCscanX v2.49 [153] and calculated transversion rates of fourfold generation sites (4DTv) between gene pairs located in synteny blocks using an in-house Perl script (https://github.com/qingxiangguo/scripts). For comparison and as a control, we analyzed protein sequences of A. digitifera, a cnidarian known to have experienced genome duplication [74]. For the initial 4DTv analysis, we have done syntenic gene analysis for 7 species in Additional file 1: Table S8, but among them, H. salminicola and H. vulgaris have too few gene pairs and cannot be used to do 4DTv analyses. For K. iwatai, the genome is very unusual and cannot be used to calculate 4DTv. Because there are a lot of nearly identical gene pairs in K. iwatai, which might be due to redundancy instead of WGDs, so we only included 4 species in 4DTv analyses.
Supplementary Information
Additional file 1: Table S1. Statistics of the Pacbio sequencing and assembly of the Myxobolus honghuensis genome. Table S2. Length distribution of the Pacbio subreads. Table S3. Estimated genome characteristics. Table S4. Clean reads mapping result. Table S5. Details of the gene prediction results. Table S6. Integration annotation file of Myxobolus honghuensis genes. Table S7. Composition of repetitive sequences in the Myxobolus honghuensis genome. Table S8. Syntenic analysis of Myxobolus honghuensis and 6 other cnidarian genomes. Table S9. Presence and absence of nuclear-encoded proteins that are targeted to mitochondria in the Myxobolus honghuensis proteome. Table S10. Summary of orthologous and paralogous gene families in Myxobolus honghuensis and 15 other sequenced cnidarian genomes. Table S11. Annotation of the unique genes in Myxobolus honghuensis genome. Table S12. Distribution of expanded and contracted gene families among the 16 cnidarian genomes. Table S13. Annotation of the expanded gene families in Myxobolus honghuensis genome. Table S14. Annotation of the contracted gene families in Myxobolus honghuensis genome. Table S15. Positively selected genes identified in Myxobolus honghuensis genome. Table S16. Presence and absence of 20 meiosis-related genes in the genome and transcriptome of Myxobolus honghuensis. Table S17. KEGG pathway enrichment analysis of unique genes. Table S18. KEGG pathway enrichment analysis of expanded and contracted genes. Table S19. Presence and absence of ANTP class Homeobox genes in M. honghuensis and other free-living cnidarians. Table S20. Details of sampling and sequencing. Table S21. Results for mapping Illumina genome sequences to the common carp genome (NCBI: GCF_000951615.1) using SOAP. Table S22. Statistics of the bacterial genome assembly. Table S23. Information of the species used in comparative genomics.
Additional file 2: Figure S1. K-mer distribution of survey genome sequencing reads of Myxobolus honghuensis. (A) 17-mer frequency percentage distribution curve of sequencing reads. (B) The product of 17-mer frequency and corresponding depth percentage distribution curve of sequencing reads. K-mers (K=17) were extracted from the paired-end library with an insert size of 500 bp. The total 17-mer count is 17,472,358,830. The peak 17-mer depth was 85, and the genome size was calculated as is 17,472,358,830/85 = 205.6 Mb. Figure S2. GO classification of unique genes in Myxobolus honghuensis. Figure S3. KEGG pathway analysis of unique genes in Myxobolus honghuensis. Figure S4. The biological process GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S5. The cellular component GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S6. The molecular function GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S7. GO classification of expanded genes in Myxobolus honghuensis. Figure S8. KEGG pathway analysis of expanded genes in Myxobolus honghuensis. Figure S9. The biological process GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S10. The cellular component GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S11. The molecular function GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S12. GO classification of contracted genes in Myxobolus honghuensis. Figure S13. KEGG pathway analysis of contracted genes in Myxobolus honghuensis. Figure S14. The biological process GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S15. The cellular component GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S16. The molecular function GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S17. Phylogenetic tree of Myxobolus honghuensis (in bold) and 15 other species based on maximum likelihood analysis of a concatenated alignment of widespread single-copy protein sequences (51 genes including 11,323 amino acids)
Acknowledgements
We thank Dr. Jinshui Zheng (HZAU, China) for discussions. We thank Biomarker Technologies Corporation, Beijing, China, for generating the sequencing data.
Abbreviations
- ABC
ATP-binding cassette
- ANTP
Antennapedia
- CLEC
C-type lectins
- ECM
Extracellular matrix
- kb
kilobase
- LDLRs
Low-density lipoprotein receptors
- LTRs
Long terminal repeats
- Mb
Megabase
- MFS
Major facilitator superfamily
- Mt
Mitochondrial
- Mya
Million years ago
- PSDs
Postsynaptic densities
- rRNA
Ribosomal RNA
- TE
Transposable element
- TIRs
Terminal inverted repeats
- WGDs
Whole-genome duplications
- 4DTv
Fourfold synonymous third-codon transversion
Authors’ contributions
QG, SDA, and ZG conceived the project and designed the research. QG, BX, and YZ collected the samples. QG, SDA, and BX analyzed the data. QG wrote the manuscript with contributions from SDA, JLB, YZ, and ZG. ZG and YZ acquired the funding. The authors read and approved the final manuscript.
Funding
This work was supported by Nature Science Foundation of China (grant nos. 32070431), China Agriculture Research System of MOF and MARA (CARS-46), the Hubei Agricultural Science and Technology Innovation Center (grant no. 2016620000001046), Featuring Talents Cultivation Project (grant no. 4611300108).
Availability of data and materials
Data generated and analyzed during this study are included in the published article, its additional files, and publicly available repositories. The raw reads of the M. honghuensis transcriptome sequencing, Illumina genome sequencing, and PacBio genome sequencing have been deposited at the NCBI Short Read Archive with the project accession numbers PRJNA779260 [154], PRJNA778632 [155], and PRJNA779846 [156]. The transcriptome assembly has been deposited at DDBJ/EMBL/GenBank under the accession GJPJ00000000 [157]. The Illumina genome assembly have been deposited in the Genome Warehouse (GWH) in National Genomics Data Center (NGDC) under the accession GWHBFXM00000000 [158]. The PacBio genome assembly and related annotation information have been deposited in the GWH in NGDC under the accession GWHBFXL00000000 [159] and Harvard Dataverse: 10.7910/DVN/INLEPM [160]. Alignment and maximum likelihood tree have been deposited in the TreeBASE repository: http://purl.org/phylo/treebase/phylows/study/TB2:S28997 [161].
Declarations
Ethics approval and consent to participate
The maintenance and care of experimental animals complied with the National Institutes of Health Guide for the care and use of laboratory animals and was approved by the animal care and use committee of Huazhong Agricultural University, China (HZAUFI-2015-011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingxiang Guo and Stephen D. Atkinson contributed equally to this work.
References
- 1.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Taenia solium Genome Consortium. Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consortium IHG Comparative genomics of the major parasitic worms. Nat Genet. 2019;51:163. doi: 10.1038/s41588-018-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert D, Fields PD. Host–parasite co-evolution and its genomic signature. Nat Rev Genet. 2020;21:754–768. doi: 10.1038/s41576-020-0269-1. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich C, Sommer RJ. How to become a parasite–lessons from the genomes of nematodes. Trends Genet. 2009;25:203–209. doi: 10.1016/j.tig.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AP. Genome evolution in trypanosomatid parasites. Parasitology. 2015;142:S40–S56. doi: 10.1017/S0031182014000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AP. The evolution of parasite genomes and the origins of parasitism. Parasitology. 2015;142:S1–S5. doi: 10.1017/S0031182014001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanifuji G, Takabayashi S, Kume K, Takagi M, Nakayama T, Kamikawa R, et al. The draft genome of Kipferlia bialata reveals reductive genome evolution in fornicate parasites. PLoS One. 2018;13:e0194487. doi: 10.1371/journal.pone.0194487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slyusarev GS, Starunov VV, Bondarenko AS, Zorina NA, Bondarenko NI. Extreme genome and nervous system streamlining in the invertebrate parasite Intoshia variabili. Curr Biol. 2020;30:1292–1298.e3. doi: 10.1016/j.cub.2020.01.061. [DOI] [PubMed] [Google Scholar]
- 10.Stevens L, Rooke S, Falzon LC, Machuka EM, Momanyi K, Murungi MK, et al. The genome of Caenorhabditis bovis. Curr Biol. 2020;30:1023–1031.e4. doi: 10.1016/j.cub.2020.01.074. [DOI] [PubMed] [Google Scholar]
- 11.Dunn KA, Bielawski JP, Ward TJ, Urquhart C, Gu H. Reconciling ecological and genomic divergence among lineages of Listeria under an “extended mosaic genome concept”. Mol Biol Evol. 2009;26:2605–2615. doi: 10.1093/molbev/msp176. [DOI] [PubMed] [Google Scholar]
- 12.Stryjewski KF, Sorenson MD. Mosaic genome evolution in a recent and rapid avian radiation. Nat Ecol Evol. 2017;1:1912–1922. doi: 10.1038/s41559-017-0364-7. [DOI] [PubMed] [Google Scholar]
- 13.Pääbo S. The mosaic that is our genome. Nature. 2003;421:409–412. doi: 10.1038/nature01400. [DOI] [PubMed] [Google Scholar]
- 14.Richter DJ, Fozouni P, Eisen MB, King N. Gene family innovation, conservation and loss on the animal stem lineage. Elife. 2018;7:e34226. doi: 10.7554/eLife.34226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koop BF. Human and rodent DNA sequence comparisons: a mosaic model of genomic evolution. Trends Genet. 1995;11:367–371. doi: 10.1016/S0168-9525(00)89108-8. [DOI] [PubMed] [Google Scholar]
- 16.Lu T-M, Kanda M, Furuya H, Satoh N. Dicyemid mesozoans: a unique parasitic lifestyle and a reduced genome. Genome Biol Evol. 2019;11:2232–2243. doi: 10.1093/gbe/evz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinz E, Williams TA, Nakjang S, Noël CJ, Swan DC, Goldberg AV, et al. The genome of the obligate intracellular parasite Trachipleistophora hominis: new insights into microsporidian genome dynamics and reductive evolution. PLoS Pathog. 2012;8:e1002979. doi: 10.1371/journal.ppat.1002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spanu PD, Abbott JC, Amselem J, Burgis TA, Soanes DM, Stüber K, et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330:1543–1546. doi: 10.1126/science.1194573. [DOI] [PubMed] [Google Scholar]
- 19.Okamura B, Gruhl A. Evolution, origins and diversification of parasitic cnidarians. EcoEvoRxiv. 2020; 10.32942/osf.io/qdpje.
- 20.Lom J, Dyková I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol (Praha). 2013;53:1–36. doi: 10.14411/fp.2006.001. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson SD, Bartholomew JL, Lotan T. Myxozoans: ancient metazoan parasites find a home in phylum Cnidaria. Zoology. 2018;129:66–68. doi: 10.1016/j.zool.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Chang ES, Neuhof M, Rubinstein ND, Diamant A, Philippe H, Huchon D, et al. Genomic insights into the evolutionary origin of Myxozoa within Cnidaria. Proc Natl Acad Sci. 2015;112:14912–14917. doi: 10.1073/pnas.1511468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Xiong J, Zhou Z, Huo F, Miao W, Ran C, et al. The genome of the myxosporean Thelohanellus kitauei shows adaptations to nutrient acquisition within its fish host. Genome Biol Evol. 2014;6:3182–3198. doi: 10.1093/gbe/evu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyger R, Luzuriaga-Neira A, Layman T, Milkewitz Sandberg TO, Singh D, Huchon D, et al. Myxosporea (Myxozoa, Cnidaria) lack DNA cytosine methylation. Mol Biol Evol. 2021;38:393–404. doi: 10.1093/molbev/msaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yahalomi D, Atkinson SD, Neuhof M, Chang ES, Philippe H, Cartwright P, et al. A cnidarian parasite of salmon (Myxozoa: Henneguya) lacks a mitochondrial genome. Proc Natl Acad Sci. 2020;117:5358–5363. doi: 10.1073/pnas.1909907117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alama-Bermejo G, Holzer AS. Advances and discoveries in myxozoan genomics. Trends Parasitol. 2021;37:552–568. doi: 10.1016/j.pt.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Whipps CM, Gu Z, Zeng C, Huang M. Myxobolus honghuensis n. sp. (Myxosporea: Bivalvulida) parasitizing the pharynx of allogynogenetic gibel carp Carassius auratus gibelio (Bloch) from Honghu Lake. China. Parasitol Res. 2012;110:1331–1336. doi: 10.1007/s00436-011-2629-4. [DOI] [PubMed] [Google Scholar]
- 28.Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- 29.Xia W, Li H, Cheng W, Li H, Mi Y, Gou X, et al. High-quality genome assembly of Chrysaora quinquecirrha provides insights into the adaptive evolution of jellyfish. Front Genet. 2020;11:535. doi: 10.3389/fgene.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu GT, Schmutzer T, Bull F, Cao HX, Fuchs J, Tran TD, et al. Comparative genome analysis reveals divergent genome size evolution in a carnivorous plant genus. Plant Genome. 2015;8:1–14. doi: 10.3835/plantgenome2015.04.0021. [DOI] [PubMed] [Google Scholar]
- 31.Tang H, Wang X, Bowers JE, Ming R, Alam M, Paterson AH. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008;18:1944–1954. doi: 10.1101/gr.080978.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi F, Sekizuka T, Ogasawara Y, Yokoyama H, Kamikawa R, Inagaki Y, et al. The mitochondrial genomes of a myxozoan genus Kudoa are extremely divergent in Metazoa. PLoS One. 2015;10:e0132030. doi: 10.1371/journal.pone.0132030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahalomi D, Haddas-Sasson M, Rubinstein ND, Feldstein T, Diamant A, Huchon D. The multipartite mitochondrial genome of Enteromyxum leei (Myxozoa): eight fast-evolving megacircles. Mol Biol Evol. 2017;34:1551–1556. doi: 10.1093/molbev/msx072. [DOI] [PubMed] [Google Scholar]
- 34.Poulin R, Randhawa HS. Evolution of parasitism along convergent lines: from ecology to genomics. Parasitology. 2015;142:S6–15. doi: 10.1017/S0031182013001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Roch KG, Johnson JR, Florens L, Zhou Y, Santrosyan A, Grainger M, et al. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 2004;14:2308–2318. doi: 10.1101/gr.2523904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riesgo A, Farrar N, Windsor PJ, Giribet G, Leys SP. The analysis of eight transcriptomes from all poriferan classes reveals surprising genetic complexity in sponges. Mol Biol Evol. 2014;31:1102–1120. doi: 10.1093/molbev/msu057. [DOI] [PubMed] [Google Scholar]
- 37.Kamm K, Schierwater B, DeSalle R. Innate immunity in the simplest animals – placozoans. BMC Genomics. 2019;20:5. doi: 10.1186/s12864-018-5377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traylor-Knowles N, Vandepas LE, Browne WE. Still enigmatic: innate immunity in the ctenophore Mnemiopsis leidyi. Integr Comp Biol. 2019;59:811–818. doi: 10.1093/icb/icz116. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Matbouhi M, Sobottka I, Schumacher U, Schottelius J. Effect of passage through the gastrointestinal tract of mice on the viability of Myxobolus cerebralis (Myxozoa) spores. Bull Eur Assoc Fish Pathol. 2005;25:276–279. [Google Scholar]
- 41.Takeuchi T, Sennari R, Sugiura K, Tateno H, Hirabayashi J, Kasai K. A C-type lectin of Caenorhabditis elegans: its sugar-binding property revealed by glycoconjugate microarray analysis. Biochem Biophys Res Commun. 2008;377:303–306. doi: 10.1016/j.bbrc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18:54. doi: 10.1186/s12931-017-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lun Z-R, Lai D-H, Wen Y-Z, Zheng L-L, Shen J-L, Yang T-B, et al. Cancer in the parasitic protozoans Trypanosoma brucei and Toxoplasma gondii. Proc Natl Acad Sci. 2015;112:8835–8842. doi: 10.1073/pnas.1502599112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kita K. Energy metabolism of parasite: their strategy for adaptation. Jpn J Vet Res. 1997;44:212–213. [Google Scholar]
- 45.Go G, Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med. 2012;85:19–28. [PMC free article] [PubMed] [Google Scholar]
- 46.Lujan HD, Mowatt MR, Byrd LG, Nash TE. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc Natl Acad Sci. 1996;93:7628–7633. doi: 10.1073/pnas.93.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal D, Bhatti HS, Sehgal R. Role of cholesterol in parasitic infections. Lipids Health Dis. 2005;4:10. doi: 10.1186/1476-511X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 49.Laranjeira-Silva MF, Wang W, Samuel TK, Maeda FY, Michailowsky V, Hamza I, et al. A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog. 2018;14:e1007140. doi: 10.1371/journal.ppat.1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin F, Dube F, Karlsson Lindsjö O, Eydal M, Höglund J, Bergström TF, et al. Transcriptional responses in Parascaris univalens after in vitro exposure to ivermectin, pyrantel citrate and thiabendazole. Parasit Vectors. 2020;13:342. doi: 10.1186/s13071-020-04212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- 52.Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 53.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 54.Horzum U, Ozdil B, Pesen-Okvur D. Step-by-step quantitative analysis of focal adhesions. MethodsX. 2014;1:56–59. doi: 10.1016/j.mex.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seipel K, Schmid V. Mesodermal anatomies in cnidarian polyps and medusae. Int J Dev Biol. 2006;50:589. doi: 10.1387/ijdb.062150ks. [DOI] [PubMed] [Google Scholar]
- 56.Okamura B, Gruhl A, Bartholomew JL. An introduction to Myxozoan evolution, ecology and development. In: Okamura B, Gruhl A, Bartholomew JL, editors. Myxozoan Evolution, Ecology and Development. Cham: Springer International Publishing; 2015. pp. 1–20. [Google Scholar]
- 57.Leclere L, Röttinger E. Diversity of cnidarian muscles: function, anatomy, development and regeneration. Front Cell Dev Biol. 2017;4:157. doi: 10.3389/fcell.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinmetz PRH, Kraus JEM, Larroux C, Hammel JU, Amon-Hassenzahl A, Houliston E, et al. Independent evolution of striated muscles in cnidarians and bilaterians. Nature. 2012;487:231–234. doi: 10.1038/nature11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang M, Lee E-J, Chung M-J, Park S, Jeong K-S. Five transcriptional factors reprogram fibroblast into myogenic lineage cells via paraxial mesoderm stage. Cell Cycle. 2020;19:1804–1816. doi: 10.1080/15384101.2020.1780384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuoka Y, Shinzato C, Satoh N. The mesoderm-forming gene brachyury regulates ectoderm-endoderm demarcation in the coral Acropora digitifera. Curr Biol. 2016;26:2885–2892. doi: 10.1016/j.cub.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, et al. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dattoli AA, Hink MA, DuBuc TQ, Teunisse BJ, Goedhart J, Röttinger E, et al. Domain analysis of the Nematostella vectensis SNAIL ortholog reveals unique nucleolar localization that depends on the zinc-finger domains. Sci Rep. 2015;5:12147. doi: 10.1038/srep12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matus DQ, Magie C, Pang K, Martindale MQ, Thomsen GH. The Hedgehog gene family of the cnidarian, Nematostella vectensis, and implications for understanding metazoan Hedgehog pathway evolution. Dev Biol. 2008;313:501–518. doi: 10.1016/j.ydbio.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C-Y, McKinney SA, Ellington LR, Gibson MC. Hedgehog signaling is required for endomesodermal patterning and germ cell development in the sea anemone Nematostella vectensis. eLife. 2020;9:e54573. doi: 10.7554/eLife.54573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khalturin K, Shinzato C, Khalturina M, Hamada M, Fujie M, Koyanagi R, et al. Medusozoan genomes inform the evolution of the jellyfish body plan. Nat Ecol Evol. 2019;3:811–822. doi: 10.1038/s41559-019-0853-y. [DOI] [PubMed] [Google Scholar]
- 66.Park H-B, Kim J-W, Baek K-H. Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int J Mol Sci. 2020;21:3904. doi: 10.3390/ijms21113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magold AI, Cacquevel M, Fraering PC. Gene expression profiling in cells with enhanced γ-secretase activity. PLoS One. 2009;4:e6952. doi: 10.1371/journal.pone.0006952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H-M, Weber JA, Lee N, Park SG, Cho YS, Bhak Y, et al. The genome of the giant Nomura’s jellyfish sheds light on the early evolution of active predation. BMC Biol. 2019;17:1–12. doi: 10.1186/s12915-019-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nong W, Cao J, Li Y, Qu Z, Sun J, Swale T, et al. Jellyfish genomes reveal distinct homeobox gene clusters and conservation of small RNA processing. Nat Commun. 2020;11:1–11. doi: 10.1038/s41467-020-16801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirose M, Mukai T, Hwang D, Iida K. The acoustic characteristics of three jellyfish species: Nemopilema nomurai, Cyanea nozakii, and Aurelia aurita. ICES J Mar Sci. 2009;66:1233–1237. doi: 10.1093/icesjms/fsp126. [DOI] [Google Scholar]
- 71.Morandini AC, Gul S. Rediscovery of Sanderia malayensis and remarks on Rhopilema nomadica record in Pakistan (Cnidaria: Scyphozoa) Papéis Avulsos Zool. 2016;56:171–175. doi: 10.1590/0031-1049.2016.56.15. [DOI] [Google Scholar]
- 72.Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, et al. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 74.Mao Y, Satoh N. A likely ancient genome duplication in the speciose reef-building coral genus, Acropora. iScience. 2019;13:20–32. doi: 10.1016/j.isci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marburger S, Alexandrou MA, Taggart JB, Creer S, Carvalho G, Oliveira C, et al. Whole genome duplication and transposable element proliferation drive genome expansion in Corydoradinae catfishes. Proc R Soc B Biol Sci. 2018;285:20172732. doi: 10.1098/rspb.2017.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallett SL, Hartigan A, Atkinson SD. Myxozoans on the move: dispersal modes, exotic species and emerging diseases. In: Okamura B, Gruhl A, Bartholomew JL, editors. Myxozoan Evolution, Ecology and Development. Cham: Springer International Publishing; 2015. pp. 343–362. [Google Scholar]
- 77.Holzer AS, Bartošová-Sojková P, Born-Torrijos A, Lövy A, Hartigan A, Fiala I. The joint evolution of the Myxozoa and their alternate hosts: a cnidarian recipe for success and vast biodiversity. Mol Ecol. 2018;27:1651–1666. doi: 10.1111/mec.14558. [DOI] [PubMed] [Google Scholar]
- 78.Gong L, Fan G, Ren Y, Chen Y, Qiu Q, Liu L, et al. Chromosomal level reference genome of Tachypleus tridentatus provides insights into evolution and adaptation of horseshoe crabs. Mol Ecol Resour. 2019;19:744–756. doi: 10.1111/1755-0998.12988. [DOI] [PubMed] [Google Scholar]
- 79.Lehmann R, Lightfoot DJ, Schunter C, Michell CT, Ohyanagi H, Mineta K, et al. Finding Nemo’s genes: a chromosome-scale reference assembly of the genome of the orange clownfish Amphiprion percula. Mol Ecol Resour. 2019;19:570–585. doi: 10.1111/1755-0998.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Yuan J, Sun Y, Li S, Gao Y, Yu Y, et al. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-018-08197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vázquez-Mendoza A, Carrero JC, Rodriguez-Sosa M. Parasitic infections: a role for C-type lectins receptors. BioMed Res Int. 2013;2013:e456352. doi: 10.1155/2013/456352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartigan A, Estensoro I, Vancová M, Bílý T, Patra S, Eszterbauer E, et al. New cell motility model observed in parasitic cnidarian Sphaerospora molnari (Myxozoa: Myxosporea) blood stages in fish. Sci Rep. 2016;6:39093. doi: 10.1038/srep39093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alama-Bermejo G, Bron JE, Raga JA, Holzer AS. 3D Morphology, ultrastructure and development of Ceratomyxa puntazzi stages: first insights into the mechanisms of motility and budding in the Myxozoa. PLoS One. 2012;7:e32679. doi: 10.1371/journal.pone.0032679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brekhman V, Ofek-Lalzar M, Atkinson SD, Alama-Bermejo G, Maor-Landaw K, Malik A, et al. Proteomic analysis of the parasitic cnidarian Ceratonova shasta (Cnidaria: Myxozoa) reveals diverse roles of actin in motility and spore formation. Front Mar Sci. 2021;8:632700. doi: 10.3389/fmars.2021.632700. [DOI] [Google Scholar]
- 85.Watanabe H, Fujisawa T, Holstein TW. Cnidarians and the evolutionary origin of the nervous system. Dev Growth Differ. 2009;51:167–183. doi: 10.1111/j.1440-169X.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 86.Niven JE, Farris SM. Miniaturization of nervous systems and neurons. Curr Biol. 2012;22:R323–R329. doi: 10.1016/j.cub.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Okamura B, Curry A, Wood TS, Canning EU. Ultrastructure of Buddenbrockia identifies it as a myxozoan and verifies the bilaterian origin of the Myxozoa. Parasitology. 2002;124:215–223. doi: 10.1017/s0031182001001184. [DOI] [PubMed] [Google Scholar]
- 88.El-Matbouli M, Hoffmann RW. Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the actinosporean stage in Tubifex tubifex. Int J Parasitol. 1998;28:195–217. doi: 10.1016/S0020-7519(97)00176-8. [DOI] [PubMed] [Google Scholar]
- 89.Zhong L, Brown JC, Wells C, Gerges NZ. Post-embedding immunogold labeling of synaptic proteins in hippocampal slice cultures. JoVE J Vis Exp. 2013:e50273. 10.3791/50273. [DOI] [PMC free article] [PubMed]
- 90.Raikova EV, Raikova OI. Nervous system immunohistochemistry of the parasitic cnidarian Polypodium hydriforme at its free-living stage. Zoology. 2016;119:143–152. doi: 10.1016/j.zool.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Faber M, Shaw S, Yoon S, de Paiva AE, Wang B, Qi Z, et al. Comparative transcriptomics and host-specific parasite gene expression profiles inform on drivers of proliferative kidney disease. Sci Rep. 2021;11:2149. doi: 10.1038/s41598-020-77881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartholomew JL, Whipple MJ, Stevens DG, Fryer JL. The life cycle of Ceratomyxa shasta, a myxosporean parasite of salmonids, requires a freshwater polychaete as an alternate host. J Parasitol. 1997;83:859–868. doi: 10.2307/3284281. [DOI] [PubMed] [Google Scholar]
- 93.Bartholomew JL, Atkinson SD, Hallett SL. Involvement of Manayunkia speciosa (Annelida: Polychaeta: Sabellidae) in the life cycle of Parvicapsula minibicornis, a myxozoan parasite of pacific salmon. J Parasitol. 2006;92:742–748. doi: 10.1645/GE-781R.1. [DOI] [PubMed] [Google Scholar]
- 94.Wolf K, Markiw ME. Biology contravenes taxonomy in the Myxozoa: new discoveries show alternation of invertebrate and vertebrate hosts. Science. 1984;225:1449–1452. doi: 10.1126/science.225.4669.1449. [DOI] [PubMed] [Google Scholar]
- 95.Wadi L, Reinke AW. Evolution of microsporidia: an extremely successful group of eukaryotic intracellular parasites. PLOS Pathog. 2020;16:e1008276. doi: 10.1371/journal.ppat.1008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Van Kerckhoven L, Ebert D, et al. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- 97.Kapusta A, Suh A, Feschotte C. Dynamics of genome size evolution in birds and mammals. Proc Natl Acad Sci. 2017;114:E1460–E1469. doi: 10.1073/pnas.1616702114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hahn MW, Han MV, Han S-G. Gene Family Evolution across 12 Drosophila Genomes. PLoS Genet. 2007;3:e197. doi: 10.1371/journal.pgen.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naldoni J, Zatti SA, da Silva MRM, Maia AAM, Adriano EA. Morphological, ultrastructural, and phylogenetic analysis of two novel Myxobolus species (Cnidaria: Myxosporea) parasitizing bryconid fish from São Francisco River, Brazil. Parasitol Int. 2019;71:27–36. doi: 10.1016/j.parint.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 100.Lom J, Arthur JR. A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis. 1989;12:151–156. doi: 10.1111/j.1365-2761.1989.tb00287.x. [DOI] [Google Scholar]
- 101.Council NR. Guide for the care and use of laboratory animals. National Academies Press; 2010. [Google Scholar]
- 102.Cota-Sánchez JH, Remarchuk K, Ubayasena K. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol Biol Report. 2006;24:161. doi: 10.1007/BF02914055. [DOI] [Google Scholar]
- 103.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. https://doi.org/10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed]
- 105.Chin C-S, Peluso P, Sedlazeck FJ, Nattestad M, Concepcion GT, Clum A, et al. Phased diploid genome assembly with single-molecule real-time sequencing. Nat Methods. 2016;13:1050–1054. doi: 10.1038/nmeth.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 107.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PloS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu P, Zhang X, Wang X, Li J, Liu G, Kuang Y, et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat Genet. 2014. 10.1038/ng.3098. [DOI] [PubMed]
- 109.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 110.Guo Q, Li D, Zhai Y, Gu Z. CCPRD: a novel analytical framework for the comprehensive proteomic reference database construction of nonmodel organisms. ACS Omega. 2020;5:15370–15384. doi: 10.1021/acsomega.0c01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laetsch DR, Blaxter ML. BlobTools: Interrogation of genome assemblies. F1000Research. 2017;6:1287. doi: 10.12688/f1000research.12232.1. [DOI] [Google Scholar]
- 112.Xu Z, Wang H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007;35(suppl_2):W265–W268. doi: 10.1093/nar/gkm286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han Y, Wessler SR. MITE-Hunter: a program for discovering miniature inverted-repeat transposable elements from genomic sequences. Nucleic Acids Res. 2010;38:e199. doi: 10.1093/nar/gkq862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(suppl_1):i351–i358. doi: 10.1093/bioinformatics/bti1018. [DOI] [PubMed] [Google Scholar]
- 115.Edgar RC, Myers EW. PILER: identification and classification of genomic repeats. Bioinformatics. 2005;21(suppl_1):i152–i158. doi: 10.1093/bioinformatics/bti1003. [DOI] [PubMed] [Google Scholar]
- 116.Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165-c3. [DOI] [PubMed] [Google Scholar]
- 117.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–467. doi: 10.1186/s13100-015-0041-9. [DOI] [PubMed] [Google Scholar]
- 118.Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinforma. 2009;25:4–10. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 119.Jukes TH, Cantor CR, Munro HN. Evolution of protein molecules. Mamm Protein Metab Acad Press N Y; 1969. pp. 21–123. [Google Scholar]
- 120.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 121.Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33(suppl_2):W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majoros WH, Pertea M, Salzberg SL. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics. 2004;20:2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- 123.Alioto T, Blanco E, Parra G, Guigó R. Using geneid to identify genes. Curr Protoc Bioinforma. 2018;64:e56. doi: 10.1002/0471250953.bi0403s18. [DOI] [PubMed] [Google Scholar]
- 124.Korf I. Gene finding in novel genomes. BMC Bioinformatics. 2004;5:59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Keilwagen J, Wenk M, Erickson JL, Schattat MH, Grau J, Hartung F. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 2016;44:e89. doi: 10.1093/nar/gkw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476:320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- 127.Baumgarten S, Simakov O, Esherick LY, Liew YJ, Lehnert EM, Michell CT, et al. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc Natl Acad Sci. 2015;112:11893–11898. doi: 10.1073/pnas.1513318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prada C, Hanna B, Budd AF, Woodley CM, Schmutz J, Grimwood J, et al. Empty niches after extinctions increase population sizes of modern corals. Curr Biol. 2016;26:3190–3194. doi: 10.1016/j.cub.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 129.The C. elegans sequencing consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–8. 10.1126/science.282.5396.2012. [DOI] [PubMed]
- 130.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8:1494. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Besemer J, Lomsadze A, Borodovsky M. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001;29:2607–2618. doi: 10.1093/nar/29.12.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genomics. 2006;7:327. doi: 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33(suppl_1):D121–D124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leclère L, Horin C, Chevalier S, Lapébie P, Dru P, Peron S, et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat Ecol Evol. 2019;3:801–810. doi: 10.1038/s41559-019-0833-2. [DOI] [PubMed] [Google Scholar]
- 138.Ohdera A, Ames CL, Dikow RB, Kayal E, Chiodin M, Busby B, et al. Box, stalked, and upside-down? Draft genomes from diverse jellyfish (Cnidaria, Acraspeda) lineages: Alatina alata (Cubozoa), Calvadosia cruxmelitensis (Staurozoa), and Cassiopea xamachana (Scyphozoa) GigaScience. 2019;8:giz069. doi: 10.1093/gigascience/giz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeon Y, Park SG, Lee N, Weber JA, Kim H-S, Hwang S-J, et al. The draft genome of an octocoral, Dendronephthya gigantea. Genome Biol Evol. 2019;11:949–953. doi: 10.1093/gbe/evz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang JB, Quattrini AM, Francis WR, Ryan JF, Rodríguez E, McFadden CS. A hybrid de novo assembly of the sea pansy (Renilla muelleri) genome. GigaScience. 2019;8. 10.1093/gigascience/giz026. [DOI] [PMC free article] [PubMed]
- 141.Wang X, Liew YJ, Li Y, Zoccola D, Tambutte S, Aranda M. Draft genomes of the corallimorpharians Amplexidiscus fenestrafer and Discosoma sp. Mol Ecol Resour. 2017;17:e187–e195. doi: 10.1111/1755-0998.12680. [DOI] [PubMed] [Google Scholar]
- 142.Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 146.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 2017;34:1812–1819. doi: 10.1093/molbev/msx116. [DOI] [PubMed] [Google Scholar]
- 147.De Bie T, Cristianini N, Demuth JP, Hahn MW. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- 148.Mohajeri K, Cantsilieris S, Huddleston J, Nelson BJ, Coe BP, Campbell CD, et al. Interchromosomal core duplicons drive both evolutionary instability and disease susceptibility of the Chromosome 8p23.1 region. Genome Res. 2016;26:1453–1467. doi: 10.1101/gr.211284.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryan JF, Pang K, Schnitzler CE, Nguyen A-D, Moreland RT, Simmons DK, et al. The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342:1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suga H, Chen Z, De Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, et al. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat Commun. 2013;4:1–9. doi: 10.1038/ncomms3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morse D. A transcriptome-based perspective of meiosis in dinoflagellates. Protist. 2019;170:397–403. doi: 10.1016/j.protis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. Illumina sequencing of Myxobolus honghuensis spores RNA. NCBI Sequence Read Archive. 2022. https://identifiers.org/ncbi/insdc.sra:SRX13090597.
- 155.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. Illumina sequencing of Myxobolus honghuensis spores DNA. NCBI Sequence Read Archive. 2022. https://identifiers.org/ncbi/insdc.sra:SRX13090520.
- 156.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. PacBio sequencing of Myxobolus honghuensis spores DNA. NCBI Sequence Read Archive. 2022. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA779846/.
- 157.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. Myxobolus honghuensis transcriptome sequencing for genomic analysis. NCBI Transcriptome Shotgun Assembly Sequence Database. 2022. https://identifiers.org/ncbi/insdc:GJPJ00000000.
- 158.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. Myxobolus honghuensis Illumina genome assembly. NGDC Genome Warehouse. 2022. https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA007205.
- 159.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. Myxobolus honghuensis PacBio genome sequencing and assembly. NGDC Genome Warehouse. 2022. https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA007199.
- 160.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. A myxozoan genome reveals mosaic evolution in a parasitic cnidarian. Harvard Dataverse. 2022. https://doi.org/10.7910/DVN/INLEPM. [DOI] [PMC free article] [PubMed]
- 161.Guo Q, Atkinson SD, Xiao B, Zhai Y, Bartholomew JL, Gu Z. A myxozoan genome reveals mosaic evolution in a parasitic cnidarian. TreeBASE. 2022. http://purl.org/phylo/treebase/phylows/study/TB2:S28997. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Statistics of the Pacbio sequencing and assembly of the Myxobolus honghuensis genome. Table S2. Length distribution of the Pacbio subreads. Table S3. Estimated genome characteristics. Table S4. Clean reads mapping result. Table S5. Details of the gene prediction results. Table S6. Integration annotation file of Myxobolus honghuensis genes. Table S7. Composition of repetitive sequences in the Myxobolus honghuensis genome. Table S8. Syntenic analysis of Myxobolus honghuensis and 6 other cnidarian genomes. Table S9. Presence and absence of nuclear-encoded proteins that are targeted to mitochondria in the Myxobolus honghuensis proteome. Table S10. Summary of orthologous and paralogous gene families in Myxobolus honghuensis and 15 other sequenced cnidarian genomes. Table S11. Annotation of the unique genes in Myxobolus honghuensis genome. Table S12. Distribution of expanded and contracted gene families among the 16 cnidarian genomes. Table S13. Annotation of the expanded gene families in Myxobolus honghuensis genome. Table S14. Annotation of the contracted gene families in Myxobolus honghuensis genome. Table S15. Positively selected genes identified in Myxobolus honghuensis genome. Table S16. Presence and absence of 20 meiosis-related genes in the genome and transcriptome of Myxobolus honghuensis. Table S17. KEGG pathway enrichment analysis of unique genes. Table S18. KEGG pathway enrichment analysis of expanded and contracted genes. Table S19. Presence and absence of ANTP class Homeobox genes in M. honghuensis and other free-living cnidarians. Table S20. Details of sampling and sequencing. Table S21. Results for mapping Illumina genome sequences to the common carp genome (NCBI: GCF_000951615.1) using SOAP. Table S22. Statistics of the bacterial genome assembly. Table S23. Information of the species used in comparative genomics.
Additional file 2: Figure S1. K-mer distribution of survey genome sequencing reads of Myxobolus honghuensis. (A) 17-mer frequency percentage distribution curve of sequencing reads. (B) The product of 17-mer frequency and corresponding depth percentage distribution curve of sequencing reads. K-mers (K=17) were extracted from the paired-end library with an insert size of 500 bp. The total 17-mer count is 17,472,358,830. The peak 17-mer depth was 85, and the genome size was calculated as is 17,472,358,830/85 = 205.6 Mb. Figure S2. GO classification of unique genes in Myxobolus honghuensis. Figure S3. KEGG pathway analysis of unique genes in Myxobolus honghuensis. Figure S4. The biological process GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S5. The cellular component GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S6. The molecular function GO enrichment graph of unique genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S7. GO classification of expanded genes in Myxobolus honghuensis. Figure S8. KEGG pathway analysis of expanded genes in Myxobolus honghuensis. Figure S9. The biological process GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S10. The cellular component GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S11. The molecular function GO enrichment graph of expanded genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S12. GO classification of contracted genes in Myxobolus honghuensis. Figure S13. KEGG pathway analysis of contracted genes in Myxobolus honghuensis. Figure S14. The biological process GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S15. The cellular component GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S16. The molecular function GO enrichment graph of contracted genes in Myxobolus honghuensis. The redder the rectangle, the higher the degree of enrichment. Figure S17. Phylogenetic tree of Myxobolus honghuensis (in bold) and 15 other species based on maximum likelihood analysis of a concatenated alignment of widespread single-copy protein sequences (51 genes including 11,323 amino acids)
Data Availability Statement
Data generated and analyzed during this study are included in the published article, its additional files, and publicly available repositories. The raw reads of the M. honghuensis transcriptome sequencing, Illumina genome sequencing, and PacBio genome sequencing have been deposited at the NCBI Short Read Archive with the project accession numbers PRJNA779260 [154], PRJNA778632 [155], and PRJNA779846 [156]. The transcriptome assembly has been deposited at DDBJ/EMBL/GenBank under the accession GJPJ00000000 [157]. The Illumina genome assembly have been deposited in the Genome Warehouse (GWH) in National Genomics Data Center (NGDC) under the accession GWHBFXM00000000 [158]. The PacBio genome assembly and related annotation information have been deposited in the GWH in NGDC under the accession GWHBFXL00000000 [159] and Harvard Dataverse: 10.7910/DVN/INLEPM [160]. Alignment and maximum likelihood tree have been deposited in the TreeBASE repository: http://purl.org/phylo/treebase/phylows/study/TB2:S28997 [161].