Abstract
Background
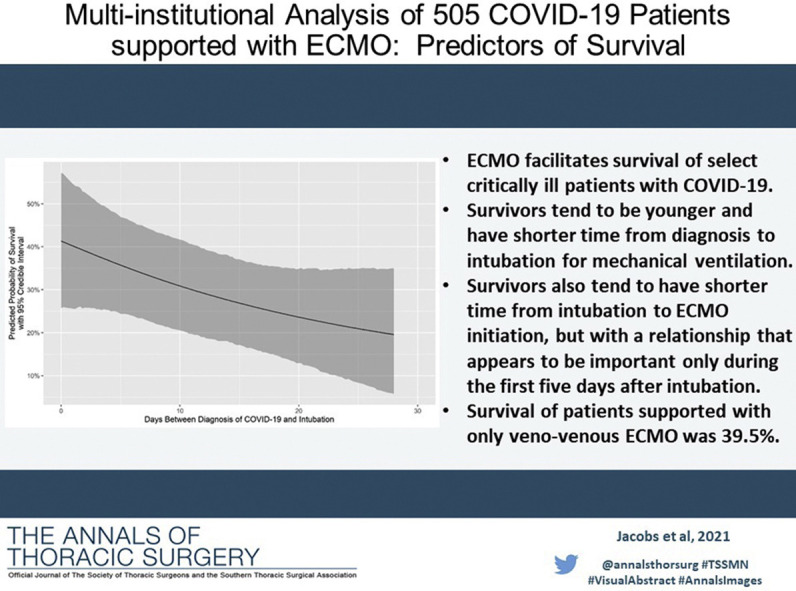
We reviewed our experience with 505 patients with confirmed coronavirus disease-2019 (COVID-19) supported with extracorporeal membrane oxygenation (ECMO) at 45 hospitals and estimated risk factors for mortality.
Methods
A multi-institutional database was created and used to assess all patients with COVID-19 who were supported with ECMO. A Bayesian mixed-effects logistic regression model was estimated to assess the effect on survival of multiple potential risk factors for mortality, including age at cannulation for ECMO as well as days between diagnosis of COVID-19 and intubation and days between intubation and cannulation for ECMO.
Results
Median time on ECMO was 18 days (interquartile range, 10-29 days). All 505 patients separated from ECMO: 194 patients (38.4%) survived and 311 patients (61.6%) died. Survival with venovenous ECMO was 184 of 466 patients (39.5%), and survival with venoarterial ECMO was 8 of 30 patients (26.7%). Survivors had lower median age (44 vs 51 years, P < .001) and shorter median time interval from diagnosis to intubation (7 vs 11 days, P = .001). Adjusting for several confounding factors, we estimated that an ECMO patient intubated on day 14 after the diagnosis of COVID-19 vs day 4 had a relative odds of survival of 0.65 (95% credible interval, 0.44-0.96; posterior probability of negative effect, 98.5%). Age was also negatively associated with survival: relative to a 38-year-old patient, we estimated that a 57-year-old patient had a relative odds of survival of 0.43 (95% credible interval, 0.30-0.61; posterior probability of negative effect, >99.99%).
Conclusions
ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors tend to be younger and have shorter time from diagnosis to intubation. Survival of patients supported with only venovenous ECMO was 39.5%.
Visual Abstract
Mr Coley, Dr Sestokas, and Mr Stammers disclose a financial relationship with SpecialtyCare.
As of September 29, 2021, 232 909 046 patients around the world have been diagnosed with coronavirus disease-2019 (COVID-19), with 4 768 139 associated deaths (2.05% mortality worldwide).1 Meanwhile, in the United States (US), as of September 29, 2021, 43 235 477 patients have been diagnosed with confirmed COVID-19, with 693 076 associated deaths to date (1.60% mortality in the US).1 The cause of death in most patients with COVID-19 is severe respiratory failure, with a small group succumbing to combined pulmonary and cardiac failure.2 , 3
Extracorporeal membrane oxygenation (ECMO) emerged as a vital therapeutic strategy for severely ill COVID-19 patients with inadequate oxygenation via conventional and ventilatory means.4, 5, 6, 7 As such, the role of ECMO in the management of severely ill patients with COVID-19 continues to be defined. We previously published analyses of our initial 32, 100, and then 200 patients with COVID-19 and severe pulmonary compromise supported with ECMO.8, 9, 10 These prior analyses documented the evolution of the use ECMO to support patients with COVID-19 and supported the concept that “ECMO facilitates survival of select critically ill patients with COVID-19.”8, 9, 10 Although substantial variation exists in drug treatment of patients with COVID-19, ECMO offers a reasonable rescue strategy.9 , 10
Several previously published analyses describe cohorts of patients with COVID-19 supported with ECMO.8, 9, 10, 11, 12, 13, 14, 15, 16 Early data from Wuhan, China, reported an alarmingly high rate of mortality of 83% (5 of 6) in patients with COVID-19 supported with ECMO.11 , 12 More recent data, however, reveal improved survival of patients with COVID-19 supported with ECMO.8, 9, 10 , 13, 14, 15, 16 Individual institutional reports13 and reports from multi-institutional registries14 both present detailed analyses with promising results. Our previous reports from our multi-institutional database8, 9, 10 corroborate these findings from individual institutions13 and multi-institutional registries,14 but additionally provide more granular, detailed information than large-scale registries14 and more generalizable information than can be garnered from analysis from a single institution.13
Increased knowledge about risk factors for mortality in patients with COVID-19 supported with ECMO can help guide physicians at the bedside with decisions about the use of ECMO in this complex population. This review details our clinical experience in 505 patients with confirmed COVID-19 supported with ECMO and estimates risk factors for mortality.
Material and Methods
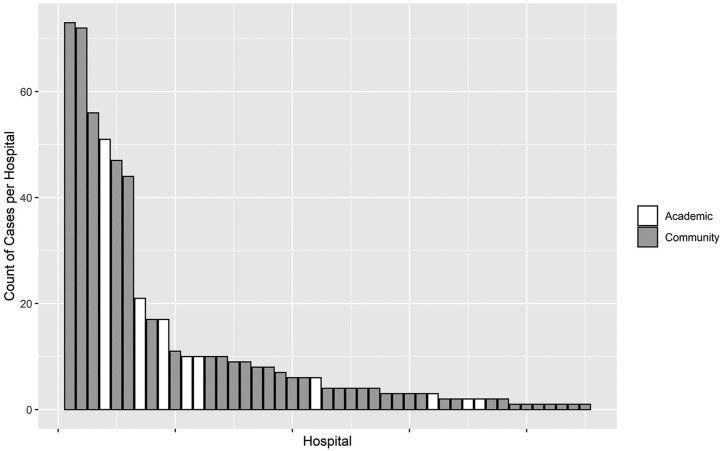
A multi-institutional database was created and used to assess all patients with COVID-19 who were supported with ECMO at 45 hospitals located in 21 US states. This database is prospectively maintained on all patients supported with ECMO and has been used for data collection and analysis. The database used is a component of the SpecialtyCare Operative Procedural REgistry (SCOPE Registry; https://specialtycareus.com/). SpecialtyCare is a US provider of Allied Health services, and the SCOPE Registry contains data from >1 million perfusion procedures in >40 states at >300 hospitals. Although the SCOPE Registry contains data from >300 hospitals, only 45 of these hospitals provided ECMO support to patients with COVID-19. This report therefore describes the ECMO experience at these 45 hospitals that have provided support with ECMO to patients with COVID-19. Of the 45 hospitals enrolling patients in this study, 36 were private hospitals and 9 were university/teaching hospitals. The mean number of COVID-19 ECMO cases at each of the 45 hospitals was 12.49 (range, 1-73; median, 4; interquartile range [IQR], 2-10).
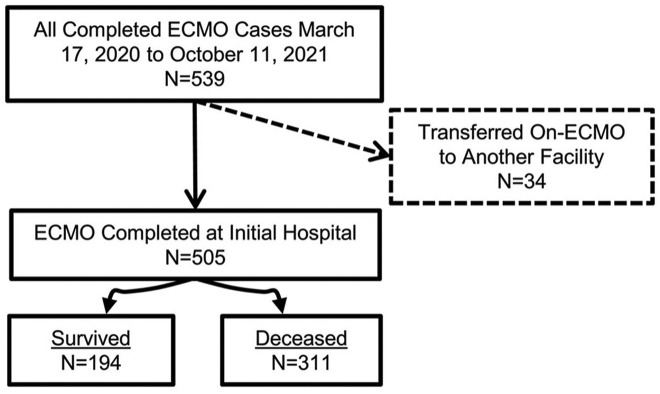
This analysis includes 505 patients with confirmed COVID-19 who were supported with and separated from ECMO between March 17, 2020, when our first COVID-19 patient was placed on ECMO, and October 11, 2021, when our last patient in this series was decannulated. The analysis does not include 34 patients who were cannulated but transferred to other hospitals on ECMO (Figure 1 ). Data analyzed included patient characteristics, pre–COVID-19 risk factors and comorbidities, confirmation of the diagnosis of COVID-19, features of ECMO support, specific medications used in an attempt to treat COVID-19, and short-term outcomes through hospital discharge.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram shows the distribution of all 505 patients by category of outcome. (ECMO, extracorporeal membrane oxygenation.)
Criteria for placement on ECMO were determined by the individual patient care team(s) at each of the contributing 45 hospitals. All patients who were placed on ECMO had the diagnosis of COVID-19 with severe respiratory failure deemed to be refractory to conventional management. The decision to initiate ECMO, the mode of therapy (ie, venovenous, venoarterial, etc), and the cannulation strategy were each determined by the individual ECMO teams in keeping with their respective individual institutional protocols and guidelines. This analysis includes all patients with COVID-19 placed on ECMO at the 45 hospitals participating in this study during the period of this analysis. None of these 505 patients were placed on ECMO during cardiopulmonary resuscitation. Extracorporeal cardiopulmonary resuscitation (eCPR) was not used for patients with COVID-19 at these 45 hospitals.
Institutional Review Board Approval
Institutional Review Board (IRB) approval and waiver of the need for consent were obtained. The human subjects research protocol for this study was reviewed and approved by an independent IRB. Institutional Ethics Review Board approval was obtained for the use of data from the SCOPE Registry (Protocol #012017; ADVARRA Center for IRB Intelligence, Columbia, MD). This study involved a retrospective review of data contained within the SCOPE Registry, which documented the individualized ECMO care provided at the direction of each patient’s medical team. Consent for ECMO treatment was managed according to local hospital protocols. ECMO care was not altered for purposes of this study. ECMO records were archived in the SCOPE Registry for quality review purposes. A full waiver of the need for patient consent for retrospective research through the SCOPE Registry was approved by the ADVARRA IRB (Protocol #012017).
Statistics
Descriptive summaries of the data were tabulated according to survival group using median and IQR (with Kruskal-Wallis rank sum test) for continuous variables, or count and percentage (with χ2 test) for categorical variables. The primary outcome of interest was mortality during the index hospitalization.
Missing data on covariates of interest was addressed by means of multiple imputation with chained equations as implemented by Harrell and colleagues.17 Less than 10% of cases had missing data for most covariates of interest. A total of 25 imputed data sets were created, modeled, and combined into a single set of regression results to properly account for uncertainty due to missing data.
To assess the effects of patient and care variables on survival, we estimated a Bayesian mixed-effects logistic regression model that included terms for age, sex, the presence of ≥1 key comorbidities (among asthma, cancer, chronic renal failure, diabetes, heart disease, hypertension, and obesity), days between the diagnosis of COVID-19 and intubation, days between intubation and the initiation of ECMO, and whether or not a patient was placed prone before ECMO, with a random effect term controlling for the hospital at which care was given. Effects for age, days between diagnosis and intubation, and days between intubation and initiation of ECMO were all modeled using restricted cubic splines with 3 knots placed at the 10th, 50th, and 90th percentiles of each variable’s distribution, respectively.
Prior distributions for each covariate were relatively uninformative: normal with a mean of zero and standard deviation (SD) of 100, allowing for a wide range of possible effects to be identified within the regression analysis. Individual model effects were summarized using the posterior predictive mean and 95% credible interval (CrI), with marginal effect contrasts between observations at the 25th and 75th percentile of each variable’s distribution, unless otherwise noted. Predictive ability of the overall model was assessed using the C-index and Somers’ Dxy. All analyses were conducted within R 4.0.3 software (The R Foundation for Statistical Computing)18 with the use of the “Hmisc”17 and “rmsb”19 packages.
Results
During the 19 months of this study, 505 consecutive patients with COVID-19 were supported with and separated from ECMO at 45 different hospitals: 194 patients survived (38.4%) and 311 patients died (61.6%). Table 1 provides detailed data about all 505 patients with COVID-19 supported with ECMO. Of note, of 505 patients, 317 (64.0%) were obese, 213 (46.1%) had hypertension, 174 (37.7%) had diabetes, 60 (13.0%) had asthma, 44 (9.5%) had heart disease, 35 (7.7%) had chronic renal failure, and 10 (2.2%) had cancer. The median time on ECMO was 18 days (IQR, 10-29 days). Survival with venovenous ECMO was 184 of 466 patients (39.5%), and survival with venoarterial ECMO was 8 of 30 patients (26.7%). The median per-hospital survival rate for venovenous ECMO was 33.3% (range, 0%-100%; IQR, 0%-51.3%).
Table 1.
Descriptive Summary Stratified by Survival
| Variable | All | Nonsurvivors | Survivors | P Value | No. |
|---|---|---|---|---|---|
| Total observations, n | 505 | 311 (61.6%) | 194 (38.4%) | ||
| Days from | |||||
| COVID-19 diagnosis to intubation | 10.0 (4.00; 14.0) | 11.0 (5.00; 15.0) | 7.00 (3.00; 12.8) | .001 | 405 |
| Intubation to cannulation | 4.00 (1.00; 6.00) | 4.00 (1.00; 7.00) | 3.50 (1.00; 5.75) | .523 | 443 |
| COVID-19 diagnosis to cannulation | 13.0 (7.00; 18.0) | 15.0 (9.00; 19.0) | 10.0 (5.25; 16.0) | <.001 | 428 |
| Days on ECMO | 18.0 (10.0; 29.0) | 20.0 (11.0; 29.5) | 15.0 (9.00; 25.8) | .009 | 505 |
| Hours on ECMO | 413 (223; 674) | 457 (246; 690) | 338 (212; 602) | .01 | 505 |
| Age | 48.0 (38.0; 57.0) | 51.0 (42.0; 59.0) | 44.0 (35.0; 52.8) | <.001 | 505 |
| Sex | .053 | 505 | |||
| Female | 158 (31.3) | 87 (28.0) | 71 (36.6) | ||
| Male | 347 (68.7) | 224 (72.0) | 123 (63.4) | ||
| Race | .091 | 480 | |||
| American Indian or Alaska Native | 11 (2.29) | 10 (3.44) | 1 (0.53) | ||
| Asian | 28 (5.83) | 15 (5.15) | 13 (6.88) | ||
| Black or African American | 77 (16.0) | 50 (17.2) | 27 (14.3) | ||
| Hawaiian or Pacific Islander | 10 (2.08) | 4 (1.37) | 6 (3.17) | ||
| Hispanic or Latino | 213 (44.4) | 134 (46.0) | 79 (41.8) | ||
| White | 141 (29.4) | 78 (26.8) | 63 (33.3) | ||
| Asthma | 60 (13.0) | 39 (13.8) | 21 (11.9) | .652 | 460 |
| Cancer | 10 (2.16) | 7 (2.46) | 3 (1.69) | .748 | 462 |
| Chronic renal failure | 35 (7.68) | 22 (7.86) | 13 (7.39) | .997 | 456 |
| Diabetes | 174 (37.7) | 120 (42.1) | 54 (30.7) | .018 | 461 |
| Heart disease | 44 (9.54) | 29 (10.2) | 15 (8.47) | .65 | 461 |
| Hypertension | 213 (46.1) | 138 (48.6) | 75 (42.1) | .208 | 462 |
| Obesity | 317 (64.0) | 194 (64.0) | 123 (64.1) | 1 | 495 |
| One or more comorbid conditions | 411 (83.0) | 256 (84.5) | 155 (80.7) | .336 | 495 |
| Placed prone before ECMO | 299 (68.0) | 179 (67.0) | 120 (69.4) | .685 | 440 |
| Tracheostomy performed | 208 (41.2) | 119 (38.3) | 89 (45.9) | .11 | 505 |
| One or more circuit changes | 176 (35.9) | 113 (37.7) | 63 (33.2) | .359 | 490 |
| CVVH or CRRT used | 127 (28.3) | 87 (31.6) | 40 (23.1) | .066 | 448 |
| ECMO type | .229 | 496 | |||
| Venoarterial | 30 (6.05) | 22 (7.24) | 8 (4.17) | ||
| Venovenous | 466 (94.0) | 282 (92.8) | 184 (95.8) | ||
| Anticoagulation type | .421 | 504 | |||
| Argatroban | 28 (5.56) | 17 (5.48) | 11 (5.67) | ||
| Bivalirudin | 128 (25.4) | 85 (27.4) | 43 (22.2) | ||
| Heparin | 345 (68.5) | 207 (66.8) | 138 (71.1) | ||
| None | 3 (0.60) | 1 (0.32) | 2 (1.03) | ||
| Antiviral medication | 326 (73.1) | 209 (76.3) | 117 (68.0) | .071 | 446 |
| Convalescent plasma | 212 (49.5) | 129 (48.7) | 83 (50.9) | .726 | 428 |
| Hydroxychloroquine | 56 (12.6) | 31 (11.3) | 25 (14.5) | .394 | 446 |
| Interleukin-6 blocker | 157 (35.3) | 87 (32.0) | 70 (40.5) | .085 | 445 |
| Prostaglandin | 152 (34.9) | 100 (37.5) | 52 (31.0) | .2 | 435 |
| Steroids | 374 (85.2) | 231 (85.9) | 143 (84.1) | .714 | 439 |
Data are presented as median (25th; 75th) or as n (%).
COVID-19, coronavirus disease-2019; CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; ECMO, extracorporeal membrane oxygenation.
Table 1 also provides detailed data comparing the characteristics of 194 survivors with 311 nonsurvivors. Survivors were generally younger, with a lower median age (44 vs 51 years, P < .001). Survivors also had a shorter median time interval from the diagnosis of COVID-19 to intubation (7 days vs 11 days, P = .001). Duration on ECMO was shorter in survivors than nonsurvivors: median time on ECMO was 15 days (IQR, 9-25.8 days) in survivors vs 20 days (IQR, 11-29.5) in nonsurvivors (P = .009).
In the 194 surviving patients, adjunctive therapies received while on ECMO were intravenous steroids in 143 (84.1%), antiviral medications in 117 (68.0%), convalescent plasma in 83 (50.9%), anti–interleukin-6 receptor monoclonal antibodies in 70 (40.5%), prostaglandin in 52 (31.0%), and hydroxychloroquine in 25 (14.5%).
Table 2 provides the results of our Bayesian mixed-effects logistic regression model. Overall model predictive performance was somewhat modest with a C-index of 0.65 (95% CrI, 0.63-0.66) and Somer’s Dxy of 0.30 (95% CrI, 0.26-0.33). Adjusting for several confounding factors, we estimated that an ECMO patient intubated on day 14 after the diagnosis of COVID-19 vs day 4 had a relative odds of survival of 0.65 (95% CrI, 0.44-0.96; posterior probability of negative effect, 98.5%). Age was also negatively associated with survival: relative to a 38-year-old patient, we estimated that a 57-year-old patient had a relative odds of survival of 0.43 (95% CrI, 0.30-0.61; posterior probability of negative effect, >99.99%). Female sex was positively associated with survival (odds ratio, 1.48; 95% CrI, 0.95-2.30; posterior probability of positive effect, 96.0%).
Table 2.
Bayesian Mixed-Effects Logistic Regression Results
| Variable | Contrast | Odds Ratio for Survival (95% Credible Interval) |
Posterior Probability That Effect on Survival Is (%) |
|---|---|---|---|
| Age, y | 57:38 | 0.43 (0.30-0.61) | Negative: >99.99% |
| Days between diagnosis and intubation | 14:4 | 0.65 (0.44-0.96) | Negative: 98.47% |
| Days between intubation and ECMO start | 6:1 | 0.82 (0.49-1.38) | Negative: 77.71% |
| Female sex | Yes:No | 1.48 (0.95-2.30) | Positive: 95.97% |
| Any comorbidity present | Yes:No | 0.78 (0.45-1.35) | Negative: 80.75% |
| Prone position pre-ECMO | Yes:No | 1.33 (0.83-2.17) | Positive: 87.85% |
ECMO, extracorporeal membrane oxygenation.
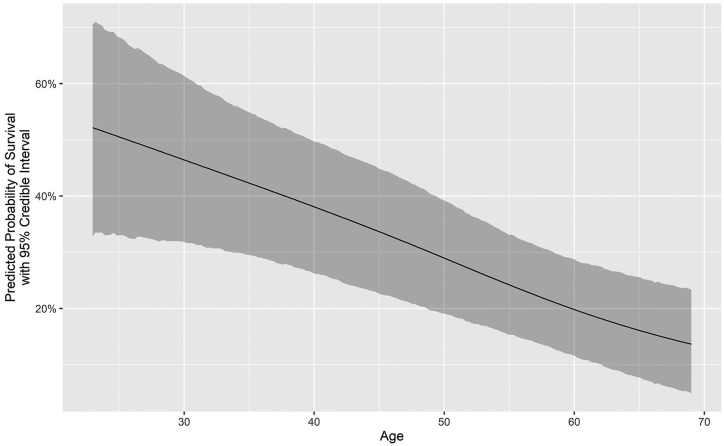
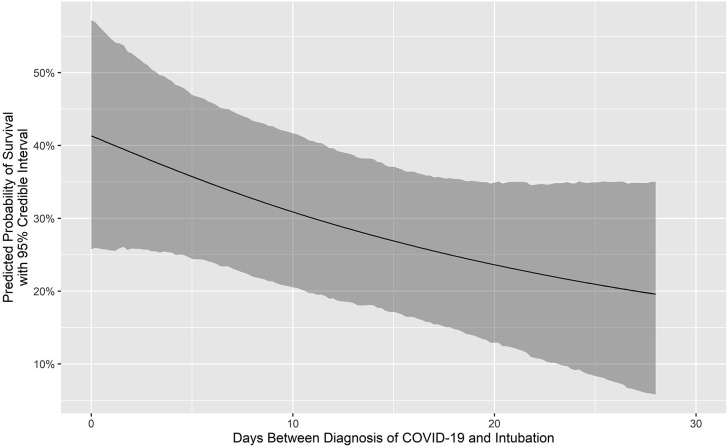
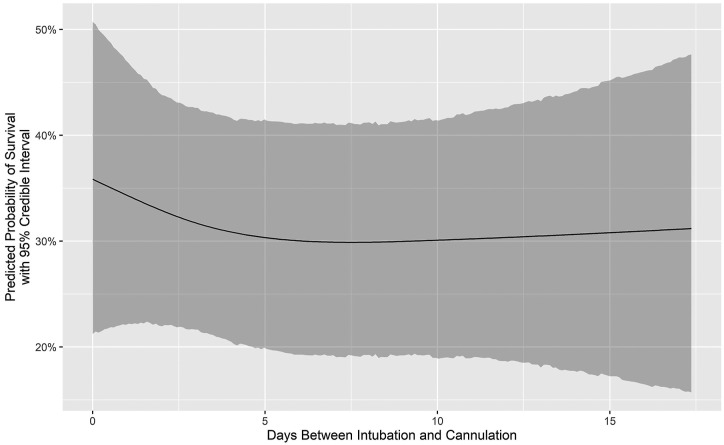
Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) flow diagram that depicts the distribution of all 505 patients by category of outcome. Figure 2 depicts the number of COVID-19 ECMO cases at each of the 45 hospitals (mean, 12.49 [range, 1-73], with a median of 4 [IQR, 2-10]). Figure 3 depicts the predicted probability of survival by age and shows improved survival with younger age. Figure 4 depicts the predicted probability of survival by days between the diagnosis of COVID-19 and intubation and shows improved survival with a shorter time interval between the diagnosis of COVID-19 and intubation. Figure 5 depicts the predicted probability of survival by days between intubation and ECMO initiation; this figure shows a somewhat improved survival with a shorter time interval between intubation and ECMO initiation, but with a less consistent relationship that appears to be important only during the first 5 days after intubation.
Figure 2.
The number of patients with coronavirus disease-2019 (COVID-19) supported with extracorporeal membrane oxygenation at each of the 45 hospitals.
Figure 3.
Predicted probability of survival by age.
Figure 4.
Predicted probability of survival by days between the diagnosis of coronavirus disease-2019 (COVID-19) and intubation.
Figure 5.
Predicted probability of survival by days between intubation and initiation of extracorporeal membrane oxygenation.
Comment
Our multi-institutional analysis of 505 consecutive patients with COVID-19 who were supported with ECMO and subsequently decannulated provides clear evidence that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors had lower median age (44 vs 51 years, P < .001) and shorter median time interval from diagnosis to intubation (7 days vs 11 days, P = .001). Female sex was positively associated with survival. Survival with venovenous ECMO was 184 of 466 patients (39.5%), and survival with venoarterial ECMO was 8 of 30 patients (26.7%). Substantial variation exists in the use of adjunctive drugs and therapies in the treatment of COVID-19, but these findings support the selective use of venovenous ECMO as a reasonable rescue strategy.
It is not surprising that we found that the time interval from COVID diagnosis to ECMO cannulation was inversely related to survival after ECMO for COVID-19. Indeed, as documented in Table 1, median “Days from COVID diagnosis to ECMO cannulation” was 10 days in survivors vs 15 days in nonsurvivors (P < .001). A plausible biological explanation for this finding is based on the concept that lungs have substantial intrinsic regenerative capacity.20, 21, 22, 23 Fulminant acute respiratory failure, occurring over a short time interval after infection and diagnosis, is a manifestation of extensive and rapid lung damage. When using ECMO as a bridge to recovery of the lungs, ECMO provides pulmonary support, during which time lung recovery is dependent upon regenerative functions and may occur in a setting of minimally traumatic mechanical ventilation24 or even in the absence of mechanical ventilation.25 Patients who have a longer time interval from the diagnosis of COVID-19 to respiratory failure severe enough to warrant support with ECMO by definition have (at least by physiological criteria) slowly progressive lung damage. Slowly progressive lung damage necessarily means that the pathogenic process exceeds lung generative function over a prolonged time period. If this is true, then it is entirely predictable that survival after ECMO cannulation is inversely related to the time interval from diagnosis of the disease to cannulation.
Although it is not surprising to some that “Days from COVID diagnosis to ECMO cannulation” is inversely related to survival after ECMO for COVID-19, it may be surprising to some that “Days from COVID diagnosis to intubation” is a more important predictor of outcome than “Days from intubation to ECMO cannulation.” As documented in Table 1, median “Days from COVID diagnosis to intubation” was 7 days in survivors vs 11 days in nonsurvivors (P = .001), while median “Days from intubation to ECMO cannulation” was 3.5 days in survivors vs 4 days in nonsurvivors (P = .523). In other words, as documented in Table 2, when comparing the 25th and 75th percentile of “Days from COVID diagnosis to cannulation” (4 days vs 14 days), we find that patients in the 75th percentile have a relative odds of survival of 0.65 (95% CrI, 0.44-0.96; posterior probability of negative effect, 98.5%); however, when comparing the 25th and 75th percentile of “Days from intubation to ECMO cannulation” (1 day vs 6 days), we find that patients in the 75th percentile have a relative odds of survival of 0.82 (95% CrI, 0.49-1.38; posterior probability of negative effect, 77.7%).
This observation might be explained by the theory that the “clock” on lung damage starts before the diagnosis of the disease, and it is the development of symptoms that typically triggers the test for the disease. The time from diagnosis of disease (mild lung damage typically, unless the patient presents with fulminant respiratory failure) to intubation is a period during which lung damage is ongoing,26 without sufficient net regeneration (because if there were sufficient regeneration, the patient’s pulmonary function would not deteriorate). However, during the time from intubation to cannulation for ECMO, the patient is going from poor lung function to extremely poor lung function, which may not be that different quantitatively. One might consider an analogy to perioperative kidney injury. The change from normal urine output and normal creatinine immediately after surgery to oliguria with a creatinine of 2.0 represents a much larger loss of renal function than the change from oliguria with a creatinine of 2.0 to anuria and dialysis dependence. (That stated, dialysis dependence is more predictive of mortality than is oliguria alone, but our current study does not include patients who required mechanical ventilation but not ECMO).
Another important observation is that the length of time supported with ECMO for COVID-19 is inversely related to survival after ECMO for COVID-19. As documented in Table 1, median “Days on ECMO” was 15 days in survivors vs 20 days in nonsurvivors (P = .009). Patients with COVID-19 who are supported with ECMO for extremely long periods of time without evidence of lung recovery have often developed de facto end-stage lung disease and should truly be considered for ongoing ECMO support only if they might be reasonably hypothesized to be or become candidates for lung transplantation.27 , 28
The Value of this Analysis
Our study adds to the body of knowledge and the literature by providing more granular multi-institutional data about our cohort of 505 patients with COVID-19 supported with ECMO at 45 hospitals. As previously described, several published analyses have studied the outcomes of ECMO in patients with COVID-19, and these outcomes have been quite heterogenous.8, 9, 10, 11, 12, 13, 14, 15, 16 Our analysis of the SCOPE Registry adds another data set of multi-institutional data to the growing body of literature about the use of ECMO in patients with COVID-19 and demonstrates that support with ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors had lower median age (44 vs 51 years, P < .001) and shorter median time interval from diagnosis to intubation (7 days vs 11 days, P = .001).
Future Directions
Much remains to be learned about the role of ECMO in these patients. From our analysis, no specific demographic, clinical, or laboratory data to date are predictive of outcome with ECMO in patients with COVID-19, with the exception of younger age and shorter time from diagnosis to intubation. Survivors tend to be younger and have a shorter duration from diagnosis to intubation. Meanwhile, the role of multiple medications in the treatment of COVID-19 remains unclear: none of the adjunct therapies appeared to be associated with survival. More information is needed to better determine which patients with COVID-19 will benefit from ECMO and which patients with COVID-19 will benefit from lung transplantation. Lessons learned from the use of ECMO to support patients with COVID-19 will inform the management of other patients with different forms of severe respiratory failure.
Limitations
This analysis is based on the available data in our database. Potential limitations include patient selection bias, institutional bias, confounding bias, and potentially underpowering of the analysis. Additional follow-up is required on all surviving patients. Further patient accrual will enhance continued analysis of outcomes. We plan to continue gathering data to provide additional insight about guideposts for patient selection and predictors of outcomes. We hope that by sharing our experience, other centers and patients may benefit.
Conclusion
Our experience and analysis of 505 consecutive patients at 45 hospitals reveal that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors tend to be younger. Survival of patients supported with only venovenous ECMO is 39.5% in our cohort. Survivors had a shorter median time interval from the diagnosis of COVID-19 to ECMO cannulation, driven mostly by the observation that survivors also had a shorter median time interval from the diagnosis of COVID-19 to intubation for mechanical ventilation. Substantial variation exists in drug treatment of COVID-19, but ECMO offers a reasonable rescue strategy.
Additional gathering and analysis of data will inform appropriate selection of patients and provide guidance on best use of ECMO in terms of timing, implementation, duration of support, and best criteria for discontinuation. Expansion of studies, such as the current analysis presented here, will provide a means to further define the role of ECMO in the management of severely compromised patients with COVID-19 and will serve to refine the optimal use of ECMO in these patients, with the goal of continuing to enhance survival.
References
- 1.Johns Hopkins University & Medicine Coronavirus Resource Center Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) https://coronavirus.jhu.edu/map.html
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopal K., Keller S.P., Akkanti B., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO–a “Living Working Document.”. ASAIO J. 2020;66:588–598. doi: 10.1097/MAT.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopal K., Keller S.P., Akkanti B., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO—a Living Working Document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett R.H., Ogino M.T., Brodie D., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. Published correction appears in ASAIO J. 2020;66:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badulak J., Antonini M.V., Stead C.M., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs J.P., Stammers A.H., St Louis, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs J.P., Stammers A.H., St Louis J., et al. Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J. 2021;67:496–502. doi: 10.1097/MAT.0000000000001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs J.P., Stammers A.H., St Louis J.D., et al. Multi-institutional analysis of 200 COVID-19 patients treated with extracorporeal membrane oxygenation: outcomes and trends. Ann Thorac Surg. 2022;113:1452–1460. doi: 10.1016/j.athoracsur.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry B.M. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kon Z.N., Smith D.E., Chang S.H., et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111:537–543. doi: 10.1016/j.athoracsur.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. Published correction appears in Lancet. 2020;396:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih E., DiMaio J.M., Squiers J.J., et al. Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): multicenter experience of referral hospitals in a large health care system. J Thorac Cardiovasc Surg. 2022;163:1071–1079.e3. doi: 10.1016/j.jtcvs.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih E., Squiers J.J., DiMaio J.M., et al. Outcomes of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome caused by COVID-19 versus influenza. Ann Thorac Surg. 2022;113:1445–1451. doi: 10.1016/j.athoracsur.2021.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrell F. Hmisc: Harrell Miscellaneous. R package version 4.4-2. https://CRAN.R-project.org/package=Hmisc
- 18.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 19.Harrell F. rmsb: Bayesian Regression Modeling Strategies. R package version 0.0.2. 2021. https://CRAN.R-project.org/package=rmsb
- 20.Kumar P.A., Hu Y., Yamamoto Y., et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotton D.N., Morrisey E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Liu K., Cui G., et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet. 2019;51:728–738. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- 23.Basil M.C., Katzen J., Engler A.E., et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann M., Bein T., Arlt M., et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loyalka P., Cheema F.H., Rao H., Rame J.E., Rajagopal K. Early usage of extracorporeal membrane oxygenation in the absence of invasive mechanical ventilation to treat COVID-19-related hypoxemic respiratory failure. ASAIO J. 2021;67:392–394. doi: 10.1097/MAT.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 26.Brochard L., Slutsky A., Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs J.P., Falasa M.P., Machuca T.N. Commentary: extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019: what do we know and what do we need to learn? J Thorac Cardiovasc Surg. 2022;163:1080–1082. doi: 10.1016/j.jtcvs.2020.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharat A., Machuca T.N., Querrey M., et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]