Abstract
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is yet to be controlled worldwide, especially in India. The second wave of coronavirus disease 2019 (COVID-19) led to panic and confusion in India, owing to the overwhelming number of the population that fell prey to this highly infectious virus of recent times. In the second wave of COVID-19, the patients had to fight both the virus and opportunistic infections triggered by fungi and bacteria. Repeated use of steroids, antibiotics, and oxygen masks during the management of severely and critically ill COVID-19 patients nurtured opportunistic infections such as mucormycosis. Despite mucormycosis being a decades-old disease, it has gained notice of its widespread occurrence in COVID-19 patients throughout India. Instances of mucormycosis are usually unearthed in immunocompromised individuals and are caused by the inhalation of filamentous fungi, either from the natural environment or through supportive care units. In the recent outbreak during the second wave of COVID-19 in India, it has been seen to cause secondary infection as it grows along with the treatment of COVID-19. Furthermore, COVID-19 patients with comorbidities such as diabetes were more likely to have the mucormycosis co-infection because of their challenged immune systems’ inability to fight it.
Despite the hype, mucormycosis still remains neglected and least studied, which is predominantly due to all focus on diagnostics, vaccine, and therapeutic research. In this review, we emphasize mainly on the association of mucormycosis in COVID-19 patients. We also present the molecular mechanism of mucormycosis for a better understanding of the fungal infections in patients who have recently been infected with SARS-CoV-2. Better understanding of fungal pathogens, immediate diagnosis, and management of the infections are crucial in COVID-19 patients, as high mortalities have been recorded in co-infected patients despite recovery from COVID-19.
Abbreviations: ACE2, Angiotensin-converting enzyme 2; AmB, Amphotericin B; COVID-19, Coronavirus disease 2019; CAM, COVID-19 associated mucormycosis; EFGR, Epidermal growth factor receptor; GI, Gastrointestinal; IFN- γ, Interferon-γ; L-AmB, Lipid formulations of amphotericin B; ROCM, Rhino-orbital cerebral mucormycosis; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; T2D, Type II diabetes
Keywords: Mucormycosis, COVID-19, Fungal diagnostics, SARS-CoV-2, Co-infections
Introduction
The pandemic initiated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19), has now angled to be associated with a large number of opportunistic fungal and bacterial infections [1]. Within a few months, SARS-CoV-2 spread to almost 150 countries globally. Despite efforts to develop an effective vaccine and other therapeutics for SARS-CoV-2, COVID-19-associated mucormycosis (CAM) cases have increased dramatically in succeeding COVID-19 waves [2], [3]. In India’s deadly second wave of COVID-19, the exponential rise in CAM has also been recorded. The main reason for the spread of this opportunistic fungal infection is the ability of the fungal spores to germinate in the ideal environment set up by the respiratory system of COVID-19 patients [4]. COVID-19 patients typically possess low oxygen, an elevated glucose level, an acidic environment (metabolic acidosis), diabetic ketoacidosis (DKA), high iron concentration (augmented ferritins), and reduced phagocytic activity [5]. These factors combine to encourage opportunistic pathogens (fungi and bacteria) to grow in immunosuppressed patients.
Owing to the highly contagious nature of SARS-CoV-2, WHO (World Health Organization) declared the outbreak pandemic on Mar 11, 2020, and issued advisory guidelines for containing the virus. Coronavirus affected millions of lives in terms of morbidity and mortality. As of today (February 03, 2022), more than 386 million people are affected worldwide, and 5.7 million people have lost their lives [6], [7].
The genetic material of SARS CoV-2 is RNA (positive-sense single-strand RNA virus +SSRNA). It belongs to the genus beta coronavirus, and the corresponding family is coronaviridae [8]. The promiscuous biological nature of SARS-CoV-2 and its properties have been reviewed by our group [9]. Several groups have also addressed the problems associated with SARS-CoV-2 variants and COVID-19 vaccination to assist in the battle against pandemic and the challenges of post-COVID-19 illnesses [10]. Coronavirus utilizes human angiotensin-converting enzyme 2 (ACE2), a receptor on cells, tissue, and organs, to access the cells and start its replication using host machinery [11]. Coronavirus mainly attacks the respiratory system of the host. Infection by SARS-CoV-2 shows in various signs and symptoms such as fever, congestion, running nose, chill, coughing, fatigue, headache, body ache, sore throat, loss of taste and smell, difficulty breathing, and gastrointestinal symptoms, including nausea, vomiting, diarrhea, and abdominal pain [12]. Initially, COVID-19 were perceived as flu (influenza) due to their overlapping sign and symptoms. The distinction between these two highly contagious respiratory illnesses is based on proper testing to confirm a diagnosis.
COVID-19 has been linked with a gamut of opportunistic fungal and bacterial infections, which develop once the highly infectious virus destroys the patients' natural immunity [1]. In India, numerous cases of mucormycosis were reported during the recovery from COVID-19 of patients with a previous history of diabetes or exhibiting other comorbidities. In a recent report of 275 CAM cases, 233 of which were reported from India and 42 from the rest of the world, which indicate diabetes mellitus was the most frequent underlying risk factor for CAM in India compared to other nations [13]. In another investigation on 99 CAM patients, it was found that most incidences (72%) were reported in India, and most of the patients (78%) were males with diabetes mellitus (85%). A history of COVID-19 was found in 37% of individuals who had mucormycosis after an initial recovery [14]. Mucormycosis mortality was at 50% before the COVID-19 pandemic. However, during the current pandemic in India, it has risen to 85% [15]. The incidence of CAM was 50 times higher (seven instances per 1000 patients) than the highest known background of mucormycosis cases (0.14 cases per 1000 patients), according to a meta-analysis of 52,916 COVID-19 patients [16]. A case-control analysis of 352 patients (152 cases and 200 controls) found that diabetes, systemic steroids, extended use of cloth and surgical masks, and frequent nasopharyngeal swab testing were all linked to an elevated risk of CAM [17]. SARS-CoV-2 infected patients typically show a low level of oxygen, high blood glucose (diabetes), and immunocompromised system to fight the disease (SARS-CoV-2 mediated or steroid-induced) [18].
In this article, we detailed mucormycosis and summarized its association with SARS-CoV-2. During COVID-19, intermittent use of steroids, supportive oxygen from cylinders, and ventilators (in critically ill patients) have played a crucial role in patients' acquisition of a secondary opportunistic infection such as mucormycosis, also known as a black fungus. Mucormycosis is an angioinvasive severe fungal infection which spreads very fast to other parts of the body. If not diagnosed and managed within a stipulated time, mucormycosis can have serious repercussions and lethal to the affected patients.
Study selection
For this article, we searched and reviewed publications on COVID- 19 and mucormycosis. PubMed, ScienceDirect and Scopus were searched for research articles written on COVID-19 in English using the search words COVID-19/Pandemic, SARS-CoV-2, Mucormycosis, the second wave of coronavirus disease/mucormycosis, and coronavirus. The search was done in duplicate by two different individuals for the reproducibility with exclusion criteria for non-English articles and non-COVID-19 papers. Reports and updates from the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), and from other authentic sources were added. The results were grouped and systematically presented in this review.
Brief history of mucormycosis
Mucormycosis has a long history dating back to 1885, when Palatuf described this as phacomycosis or zygomycosis [19]. The term mucormycosis was later coined by Baker in 1957 [20]. Mucormycosis is a rare but possibly fatal fungal infection triggered by a group of molds collectively called mucormycetes. The mold fungi belong to Rhizopus, Mucor, Rhizomucor, Cunninghamella, and Absidia [21]. Mucormycosis is a severe and deadly fungal infection by causative organisms belonging to the subphylum mucoromycotina and the order in Mucorales [22]. Mucorales are widely available in nature, including in soil, decaying vegetable matter, bread, and dust. The infection of mucormycosis occurs due to the inhalation of spores containing Mucorales from the intake of contaminated food materials or through damaged skin and wounds [23]. In both developing and developed nations, mucormycosis is mainly found in the immunocompromised hosts. It is generally found in patients with uncontrolled diabetes, influenza, SARS-CoV-2, and poor hygiene conditions, and in persons.
who have suffered from severe trauma [24]. Mucormycosis is primarily caused by the invasion of blood vessels, which leads to thrombosis, necrosis infraction of tissue, and death. Generally, the global incidences of mucormycosis varies in the range of 0.003 – 0.005–1.7 million of the population; however, in 2019 – 2020, the incidence of prevalence became 80 times higher in India than any other nation [24], [25], [26], making India the country with the highest number of mucormycosis globally. Besides COVID-19, the other important reason for these elevated numbers is directly linked to India’s highest population suffering from type II diabetes (T2D) [27]. Many nations, including Austria, Brazil, Egypt, France, India, Iran, Italy, and the United States, have documented CAM as presented in Table 1, which could be directly linked to modern-day surgeries such as organ transplants and hematological diseases [28]. Table 1 is the simple and updated presentation of the previously published data by Singh AK et al., [29]. The increasing figures of mucormycosis cases associated with COVID-19 have become the primary cause of concern, with an elevated overall mortality rate of more than 45% [30].
Table 1.
Case representation of COVID-19 associated mucormycosis from India and other parts of the world.
| S. No. | Study type | Reported COVID-19 Associated mucormycosis cases (Number) | Reported Country | Type of mucormycosis | References |
|---|---|---|---|---|---|
| 1 | Case Report | 1 | India | Rhino-orbital cerebral | [31] |
| 2 | Case Report | 1 | India | Pulmonary | [32] |
| 3 | Case Report | 1 | India | Rhino-orbital cerebral | [33] |
| 4 | Case Report | 1 | India | Rhino-orbital cerebral | [34] |
| 5 | Case Report | 1 | India | Rhino-orbital cerebral | [35] |
| 6 | Retrospective, interventional study | 6 | India | Rhino-orbital cerebral | [36] |
| 7 | Comments | 10 | India | Rhino-orbital cerebral | [37] |
| 8 | Case series | 10 | India | Rhino-orbital cerebral | [38] |
| 9 | Case series | 11 | India | Rhino-orbital cerebral | [39] |
| 10 | Retrospective, Multicentric Study | 17 | India | Rhino-orbital cerebral | [40] |
| 11 | Observational Study | 23 | India | Rhino-orbital cerebral | [41] |
| 12 | Observational Study | 2826 | India | Rhino-orbital cerebral | [42] |
| 13 | Multicenter Study | 178 | India | Rhino-orbital cerebral | [43] |
| 14 | Case Report | 1 | India | Gastrointestinal | [44] |
| 15 | Case series | 10 | India | Rhino-orbital cerebral | [45] |
| 16 | Case series | 6 | India | Rhino-orbital cerebral | [46] |
| 17 | Case Report | 1 | India | Gastrointestinal | [47] |
| 18 | Case Report | 1 | India | Rhino-orbital cerebral | [48] |
| 19 | Case series | 84 | India | Rhino-orbital cerebral | [49] |
| 20 | Case Report | 1 | India | Rhino-orbital cerebral | [50] |
| 21 | Retrospective, institutional cohort, interventional study | 19 | India | Rhino-orbital cerebral | [51] |
| 22 | Case Report | 1 | UK | Pulmonary | [52] |
| 23 | Case Study | 2 | USA | Rhino-orbital cerebral | [53] |
| 24 | Case Report | 1 | USA | Rhino-orbital cerebral | [54] |
| 25 | Case Report | 1 | USA | Pulmonary | [55] |
| 26 | Case Report | 1 | USA | Rhino-orbital cerebral | [56] |
| 27 | Case Report | 1 | USA | Rhino-orbital cerebral | [57] |
| 28 | Case Report | 1 | USA | Pulmonary | [58] |
| 29 | Case Report | 1 | USA | Pulmonary | [59] |
| 30 | Case Report | 1 | USA | Cutaneous mucormycosis | [60] |
| 31 | Case Report | 1 | Brazil | Gastrointestinal | [61] |
| 32 | Case Report | 1 | Italy | Rhino-orbital cerebral, Pulmonary | [62] |
| 33 | Case Report | 1 | France | Pulmonary | [63] |
| 34 | Case Report | 1 | Iran | Rhino-orbital cerebral | [64] |
| 35 | Case Report | 2 | Iran | Rhino-orbital cerebral | [65] |
| 36 | Cross-sectional descriptive multicenter study | 15 | Iran | Rhino-orbital cerebral | [66] |
| 37 | Case Report | 1 | Turkey | Rhino-orbital cerebral | [67] |
| 38 | Case Report | 1 | Mexico | Rhino-orbital cerebral | [68] |
| 39 | Case Report | 1 | Austria | Pulmonary | [69] |
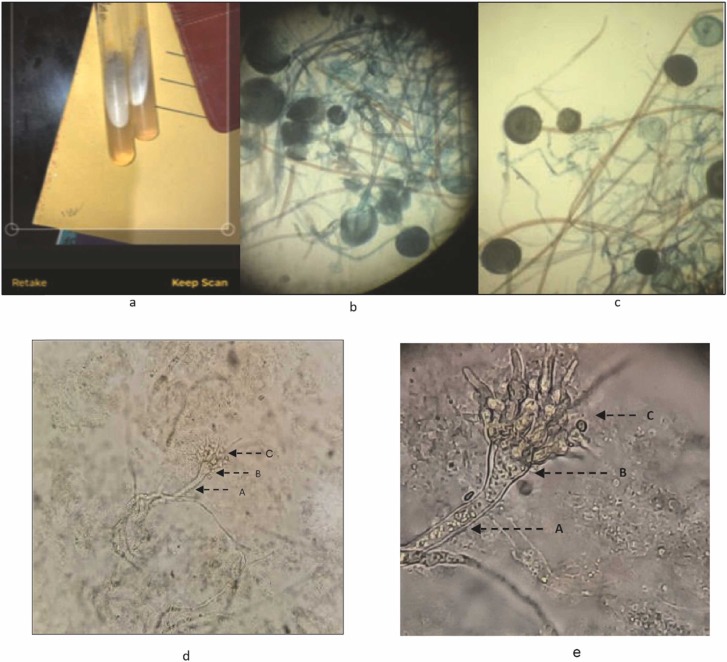
Mucormycosis, if diagnosed at a right time can be controlled; however, if not treated at the right time, mucormycosis can affect organs such as the eyes, upper part of the nasal cavity, and brain. In these cases, the patient needs immediate surgical intervention to stop other vital organs from dangerous infections. As mucormycosis is an opportunistic infection, it is often misidentified and therefore, resulting in poor prognosis of the disease [70]. In low- and middle-income countries including India, diagnoses of mucormycosis-causing pathogens are often identified by phenotypic characteristics such as growth rate, colony morphology, and reproductive structures [71]. In our laboratory, culturing and routine microscopy was used to identify the different forms of mucors from the biopsy, aspirate, and autopsy specimens ( Fig. 1).
Fig. 1.
Phenotypic identification of pathogen causing mucormycosis: (a) All biopsy, aspirate, and autopsy specimens were subjected to routine fungal cultures on agar media; (b), and (c) growth of fungi under microscopic examination of culture; (d) and (e) a high-resolution figure of the mucor (A: Sporangiophore, B: Columella, C: Sporangium).
Types of mucormycosis
The signs and symptoms of mucormycosis are swelling of the face, fever, headache, nasal or sinus congestion, black scratch on the nose or upper inside of the mouth. Mucormycosis affects different body parts such as the sinuses, brain, and lungs [72]. Based on an anatomical site, mucormycosis infection is categorized into six types, namely (i) rhino-orbital cerebral mucormycosis (ROCM), (ii) pulmonary (iii) cutaneous (iv) gastrointestinal (v) disseminated, and (vi) miscellaneous forms.
Rhino-orbital cerebral mucormycosis (ROCM)
ROCM is the most common type of mucormycosis seen in immunocompromised patients exhibiting uncontrolled diabetes mellitus (particularly with acidosis and ketoacidosis), hematologic dyscrasias, retroviral disease, steroid therapy, and malnourishment [69], [73]. Cases of ROCM are also reported in patients undergoing deferoxamine therapy, overload of iron, and in some cases of drug abuse.
The most common pathophysiology of ROCM is high blood sugar, where Rhizopus grows efficiently. In a retrospective study on six COVID-19 patients who had ROCM, all of the patients were men, with a mean blood glucose level of 222.5 ± 144.4 (86−404) mg/dL except for one patient who had mucormycosis at the same time as COVID-19 [29]. A study describes the instance of a patient with COVID-19 infection who developed rhino-orbital mucormycosis following therapy [24]. The fungus utilizes its ketone reductase system to survive in the acidic environment of DKA. Acidosis helps in the dissociation of iron, thereby sequestering proteins in the serum, which encourage virulence and survival of the fungus. Deferoxamine causes iron-richness, which acts as an iron-chelating agent, favoring fungal propagation by serving as siderophores to the fungus for its propagation [74].
The fungus exhibits the ability to damage the internal lamina of blood vessels, particularly the arteries, lymphatics, and veins by toxic and mechanical means. Once spores enter sinuses, the angioinvasive infection starts germinating into manifold hyphae in the immunocompromised patients. It is believed that the pterygopalatine fossa is the largest reservoir of the fungal hyphae [75]. In the next few hours, the fungus spreads and invaginates into vascular and neuronal regions. The invagination cause thrombosis and nerve dysfunction. The ensuing pathological manifestation involves blood vessels, cartilage, bone, neural and perineural areas, and often meninges. The necrosis at the palate region causes palatine eschars and damages the nasal turbinates. The infection from the sinuses erodes the bone, and successively encompasses the orbital structures. Thus, the infection spreads to the brain through the retro-orbital path or frontal lobes through the ethmoid sinuses. As the infection spreads along the regions of the sphenoid sinuses to the adjacent cavernous sinus, it may result in cranial nerve palsies. The extensive thrombosis in juglar veins, the cavernous sinus, and the carotid artery may further cause deterioration of the patient's condition. With the advent of new imaging techniques, the extent of tissue invasion can be fully delineated. Magnetic resonance imaging and computed tomography scans of the affected regions such as the paranasal sinus, the bones of the nasal, the septa, the orbit maxilla, and the mandible can assist detection. The radiodiagnosis techniques can examine any destruction of bones in nasal septa; osseous erosion, mucosal thickening, hypo/mild/hyper sinusitis, orbital cellulitis, soft tissue infiltration in the optical apex, optic neuritis, bone rarefaction and erosion of the skull base, infracts, and intracranial abscesses in the brain [76], [77].
Pulmonary mucormycosis
Many fungal infection cases are reported nowadays in COVID-19 hosts, including candidiasis and pulmonary aspergillosis. There are several mechanisms by which mucormycosis affects COVID-19 patients. In COVID-19 infection, there is a high impact on the immune system through inflammation, an increase in the number of neutrophil cells, a decrease in the number of lymphocyte cells, and mainly by its effect on CD4+ and CD8+ T cells. In SARS-CoV-2 infection, the number of neutrophils increases. CD4+ and CD8+ T cells play an essential role in mucormycosis infection in COVID-19 hosts through IL-4, IL-10, IL- 17, and interferon-γ (IFN- γ) [78].
Coronavirus infection with mucormycosis has a high mortality rate owing to complications such as cavernous sinus thrombosis, disseminated infection, and osteomyelitis. Diagnosis of mucormycosis is involved in routine blood check-ups, biopsy, and radio imaging. The standard treatment for mucormycosis is first to reduce risk factors, remove the damaged tissue or organ by surgical procedure, and use intravenous anti-fungal drugs such as liposomal Amphotericin B (L-AmB) [79].
Historically, the first case of pulmonary mucormycosis was explained in 1876 by Furbringer [80]. In 1971, Baker comprehensively illustrated most of the results of mucormycosis hitherto described [20]. Infection in the pulmonary regions starts owing to inhalation of spores either by the hematogenous or lymphatic spread in immunocompromised patients. Mucorales enter through the respiratory tract and quickly attack arteries, veins, and lymphatics, where the fungus cause thrombosis and infarction [81], [82], [83]. Depending upon the infection in the region of the lungs, pulmonary mucormycosis can be subdivided into unilateral (most common 62–75%), bilateral (16–25%), and mediastinal (3%). Pulmonary mucormycosis is a destructive and life-threatening infection if not diagnosed and managed at the right time [84]. Usual hosts possess armed mononuclear and polymorphonuclear phagocytes and destroy Mucorales by generating oxidative metabolites and the cationic defensins in the pulmonary system. Defense systems in normal individuals are facilitated by macrophages that prevent spores sprouting, while neutrophils kill elements of hyphae by oxidative burst [85]. The immunocompromised host does not have effective phagocytes and macrophages to fight the growing fungus in the pulmonary regions and succumbs to the coinfection induced by COVID-19. Recently, pulmonary mucormycosis in patients with COVID-19 has been associated with increased morbidity and mortality, where there are pre-existing comorbidities such as diabetes and the continuous use of corticosteroids during COVID-19 management [86], [87].
Cutaneous mucormycosis
Cutaneous mucormycosis is a rare fungal infection caused by an opportunistic fungus of the Glomeromycota phylum and Mucormycotina subphylum. Mucormycosis may occur in a variety of clinical manifestations and locations. Among all forms of mucormycosis, cutaneous mucormycosis is a relatively uncommon manifestation of mucor infection and represents a 15% mortality rate [88]. Histopathology and culture indicate the fungal infection in a cutaneous mucormycosis problem. Cutaneous mucormycosis is characterized by vascular invasion and irregularly formed wide, non-septate, twisted, ribbon-like, and thick-walled fungal hyphae [89], [90]. It is becoming increasingly common because of the growing number of patients who have been immunocompromised due to COVID-19 therapy. Wound damage, lacerations, surgical injuries, puncture sites of arterial lines or insulin injections, burns, abrasions, bug bites, tattoos, and contaminated adhesive tapes or dressings have all been linked to cutaneous mucormycosis [91]. Patients with allogeneic hematopoietic stem cell transplants and other hematologic malignancies often show mucormycosis in developed countries [73], [92]. Uncontrolled diabetes mellitus, on the other hand, is the most common related illness in developing countries [93], [94], [95]. Early detection allows for better infection management and increases patient survival. A strong index of suspicion is required for an accurate diagnosis. Presumptive diagnosis is critical, and physicians must examine these fungal infections in patients who have predisposing factors, negative bacterial cultures, and a fast progressing and severe clinical course that is resistant to standard therapy [96], [97]. The arms and legs are the most affected regions of the skin. The scalp, face, thorax, back, belly, perineum, breast, neck, and gluteal region are among the other places where it may be found. Mucormycosis requires a comprehensive approach to therapy. Uncontrolled diabetes, metabolic acidosis, excessive corticosteroid doses, extended neutropenia, organ transplantation, skin trauma (cuts, scrapes, punctures, or burns), and catheter infection are all known risk factors for developing mucormycosis [98].
It is recommended that a biopsy and molecular diagnostic testing be performed. A biopsy of the lesion, including subcutaneous fat, should be taken. In primary cutaneous mucormycosis, histology is more beneficial. Recently, novel molecular diagnostic techniques have been developed to identify the fungal species causing opportunistic coinfection. Even yet, most patients do not have access to this technology. These tests are specific for 18 S ribosomal DNA and have no cross-reactivity with other fungi [99]. Mucorales may be identified with excellent specificity in tissue samples and clinical isolates using real-time polymerase chain reaction (RT-PCR). A researcher created a single-tube multiplex RT-PCR to identify R. microsporus and Mucor, R. oryzae, from culture and clinical isolates. This technique is 100% specific and takes 2–3 h to get final results [100].
Underlying immunosuppression must be treated for appropriate fungal infection care and better survival chances, and a combination of intensive surgical therapy and antifungal medication should be explored.
Gastrointestinal tract mucormycosis
Patients with COVID-19 may be more prone to fungal infections. Mucormycosis of the gastrointestinal (GI) system is caused by ingestion of fungus spores. Although occurrences of GI mucormycosis in immunocompetent hosts are uncommon, the mortality rate from GI mucormycosis can reach up to 85% [73]. GI involvement is found in about 8% of the patients. The stomach and colon are the most frequently affected organs in the GI system. Fever, nausea, GI bleeding, perforation, abdominal pain are all signs of GI mucormycosis. Establishing a correct diagnosis is challenging because of the non-specific clinical signs/symptoms that may or may not lead to suspicion of mucormycosis.
Endoscopically, stomach mucormycosis appears as a big ulcer with necrosis, ultimately presenting with an adherent, thick, green exudate [101], [102]. Surgical debridement of affected tissues and antifungal medication are used in treatment. The prescription of choice for the first treatment is intravenous amphotericin B (AmB) [103], [104]. Despite early diagnosis and intensive combination surgical and medicinal treatment [73]. COVID-19 has been linked to immunological dysregulation [105]. COVID-19 patients may be at risk of acquiring invasive fungal infections such as invasive candidiasis, aspergillosis, and Pneumocystis jiroveci infection [79], [106]. GI mucormycosis is an uncommon illness that should be considered if a COVID-19 patient develops an unusual stomach ulcer. Clinical suspicion and early treatment are essential for disease eradication. As a result, in the presence of signs suggestive of mucormycosis, preemptive treatment should be explored. More research is needed to determine if these two diseases are linked.
Disseminated mucormycosis
Disseminated mucormycosis is an uncommon condition that often affects neutropenic individuals with hematologic malignancies, transplant recipients, immunocompromised individuals, and people using deferoxamine [107]. Invasive fungal infections (IFIs) in COVID-19 have a unique etiology. Due to changes in host signaling pathways, fungi may infect people in a high-inflammatory state just as they do in immunosuppressed individuals [108]. Disseminated mucormycosis accounts for 9% of mucormycosis cases, and it has a high mortality rate. Renal involvement is common in disseminated illness [109]. The disease manifests with nonspecific clinical signs and is typically identified at autopsy. In the post-mortem of a young man hospitalized with an acute anterior cerebral artery area infarction, an episode of disseminated mucormycosis in an immunocompetent host with COVID-19 infection was found. Following an autopsy, the cause of death was determined to be multi-organ failure due to disseminated mucormycosis caused by SARS-CoV-2 infection. Steatohepatitis, hypothyroidism, thrombo-embolic illness, and a high BMI were all contributing factors [110]. Clinicians dealing with COVID-19 patients have a unique challenge owing to the lack of sensitive non-invasive modalities and biomarkers for identifying mucormycosis and the high mortality rate in untreated cases. The main comorbid predisposing characteristics are uncontrolled diabetes mellitus and systemic steroid usage [111]. The reported death rate for disseminated mucormycosis is between 68% and 96%, depending on several conditions, including host immunosuppression, early diagnosis, AmB treatment, and surgical debridement [69], [77], [112], [113]. The clinical and radiological symptoms of pulmonary and disseminated mucormycosis are non-specific and may coincide with attributes associated with COVID-19, making CAM diagnosis difficult [112]. A systematic review and meta-analysis of 851 case reports published in 2018 found that 389/851 (46%) deaths occurred. Patients with disseminated mucormycosis had the greatest case fatality rate (68%) [69]. Because severe COVID-19 infection is associated with a high risk of death, detecting treatable co-infections, and intervening early may improve survival. There should be a high index of suspicion for invasive mycoses, including mucormycosis, in critically ill SARS-CoV-2 infected patients with systemic illness who are unresponsive or partially responsive to conventional antimicrobial agents. Furthermore, research in developing new strong diagnostic biomarkers that may identify mucormycosis early is critically required to avoid bad patient outcomes [110].
Many fungal infection cases are reported nowadays in COVID-19 hosts, including candidiasis and pulmonary aspergillosis. There are several mechanisms through which mucormycosis affects the COVID-19 patients. In COVID-19 infection, there is a high impact on the immune system through inflammation, an increase in the number of neutrophil cells, a decrease in the number of lymphocyte cells, and mainly in its effect on CD4+ and CD8+ T cells. In SARS-CoV-2 infection, the number of neutrophils increases. CD4+ and CD8+ T cells play an essential role in mucormycosis infection of COVID-19 hosts through IL-4, IL-10, IL-17, and IFN-γ [78].
Coronavirus infection with mucormycosis exhibits a high mortality rate due to complications such as cavernous sinus thrombosis, disseminated infection, and osteomyelitis. Diagnosis of mucormycosis is involved routine blood check-ups, biopsy, and radio imaging. The standard treatment for mucormycosis is first to reduce risk factors, remove the damaged tissue or organ by surgical procedure, and use intravenous antifungal drugs such as liposomal AmB [114], [115].
Miscellaneous forms
Nearly any position of the human body can have an infection by the Mucorales. Earlier reports have indicated mucormycosis in kidneys [116], bones [117], [118], trachea [119], [120], peritoneum [88], [121], external otitis [122], and superior vena cava syndrome [81].
Immunocompromised conditions in COVID-19 patients favoring mucormycosis
From the first COVID-19 case reported in December 2019 in Wuhan, China, there have been many ups and downs in the treatment, pathophysiology, diagnosis, and management of associated diseases. For example, the treatment of severe COVID-19 infections requires a critical care unit and mechanical ventilation, corticosteroids, and interleukin antagonists such as tocilizumab to reduce constriction in inflamed airways and cytokine burst. On the other hand, uncontrolled diabetes mellitus and imprudent use of immunosuppressive drugs such as corticosteroids and interleukins hamper the host's immune system and increase the chance of many opportunistic infections caused by mucormycosis.
Lymphopenia or lymphocytopenia is an illness with abnormally low counts of lymphocytes in the blood. It has recently been reported that severely ill patients infected with SARS-CoV-2 possess reduced lymphocytes levels [123], [124]. The patients who succumbed to COVID-19 exhibited lower lymphocytes than survivors [125]. Zheng et al. presented a study that revealed that NK cells and CTLs were dramatically diminished in COVID-19 patients [126]. Likewise, another study displayed by Diao et al. of older patients (aged more than 60 years) has shown sufficiently reduced levels of CD8+ and CD4+ T cells [127]. Since lymphocytes play a crucial role in maintaining immune homeostasis, SARS-CoV-2 infected patients are more likely to have co-infection [128]. Usually, SARS-CoV-2 attacks lung tissue and alveolar interstitial lesions. In these conditions, patients are prone to invasive fungal infections, particularly those that enter through the pulmonary system.
The fungus, ubiquitously found in the atmosphere, enters immunocompromised host cells, produces fungal-specific T cells, and generates IL-4, IL-10, IL-17, and IFN-γ. The pro-inflammatory cytokines stimulate CD4+ T cells and further impair the host cell [129], [130]. The hyphae of fungi interfere in the release of numerous immunomodulatory molecules such as RANTES (regulated upon activation, normal T cell expressed and secreted) and IFN-γ, secreted by NK cells and appear in the early phase of infection [131], [132].
Probable cause of lymphopenia
In severe cases of SARS-CoV-2 infection, the principal pathophysiology may include hypercytokinemia and associated traumas. The association of hypercytokinemia and lymphopenia in severe COVID-19 patients may have repercussions in the progress of uncontrolled opportunistic pathogens. Recently, Potenza et al. summarized a brief report on a group of hematological patients co-infected with mucormycosis termed Mucorales-specific CD4+ and CD8+ cells and were active against Mucorales generating cytokines IL-4, IL-10, IL-17, and IFN-γ [130]. Thus, there is a strong assertion that lymphopenia may increase the chance of developing mucormycosis.
Molecular mechanism of mucormycosis
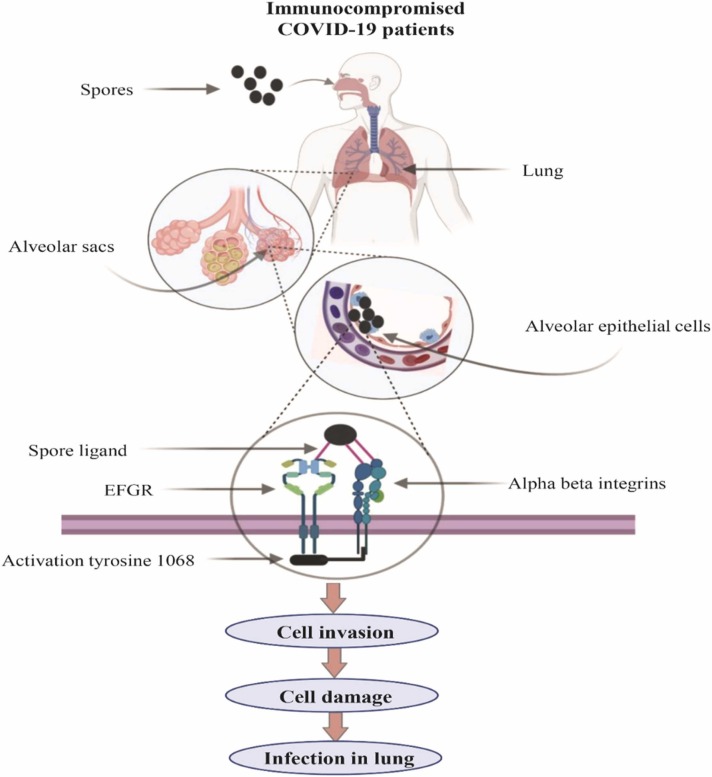
Raised iron, glucose, and ketone bodies in the COVID-19 patient significantly induce host cell receptor GRP78 (glucose-regulated protein, 78 kDa) and spore coat protein, CotH3 [133], [134]. The ligands of fungus identify host receptors distinctive to particular cell types, for example, alveolar, nasal, or endothelial cells. The interaction between fungal ligands and host receptors is augmented by other host factors, carrying the infection to the respective host niches ( Fig. 2). The fungal protein (CotH3) substantially comes in contact with GRP78 of nasal epithelial cells. On the other hand, Rhizopus interacts with β1 integrin at the time of alveolar epithelial cell invasion (Fig. 2). It is reported that the interaction with β1 integrin initiates the activation of the epidermal growth factor receptor (EFGR) [135]. Recently, Alqarihi et al. also presented a study showing antibodies against either CotH3 or GRP78 annul fungal invasion and nasal epithelial cells [136].
Fig. 2.
Pictorial representation of mucormycosis in immunocompromised COVID-19 patients. The molecular mechanism involves the inhalation of spores reaching the alveoli of the lungs. Spores interact with alpha-beta integrins through ligands. The following molecular events trigger activation of EFGR, cell invasion, and finally, infection in the lungs.
Mucorales not only damage endothelial cells by recognizing CotH3 and GRP78 but by other non-viable fungal factors which are killed by heat and chemicals such as glutaraldehyde and ethanol. The considerable amount of damage to endothelial cells by nonviable cells is not destroyed by strong chemicals. Secondary metabolites produced by Mucorales facilitate the interaction between the host and pathogen, and cause food poisoning sickness recently reported [137]. The genomic study supports the idea that Mucorales secrete secondary metabolites such as polyketide synthases, L-tryptophan dimethylallyl transferases, and non-ribosomal peptide synthetases and indirectly affect the signaling mechanism [138]. These secondary metabolites directly affect the signaling mechanism between the host and fungal pathogens and facilitate the spreading of mucormycosis in the COVID-19 patients.
Identification of fungal agents
As mentioned above, misidentification and misdiagnosis of mucormycosis are one of the leading causes related to the high mortality rates of this infection. Therefore, it is vital to identify the fungal agents causing the infections. The gold standard for clinically identifying mucormycosis in the lab is based on the principle of Smith and Krichner criteria [139]. The essential criteria for microbiological identification are (i) black necrotic turbinate’s easily, mistaken for dried crusted blood, (ii) blood-tinged nasal discharge with facial pain, (iii) soft peri-orbital, peri-nasal swelling plus discoloration and induration, (iv) ptosis of the eyelid, proptosis of the eyeball and complete ophthalmoplegia, and (v) numerous cranial nerve palsies.
Mycological identification of mucormycosis is based on the diameter of hyphae, the occurrence of septa, pigmentation, and the branching angle, so distinguishing this from other fungal infections.
The foundation for diagnosis of fungal infection relies on a few basic diagnostic techniques such as direct microscopy, fungal culture, histopathology, susceptibility testing, antigen testing, antibody testing, and molecular tests (DNA and RNA) of the specimen of the suspected specimen patient. In the next section, we briefly discuss the microbiological and pathological tests carried out regularly in labs to determine the fungal infection [140].
Potassium hydroxide test
This rapid test is commonly applied for superficial fungal infection and routinely performed in microbiological or pathological labs [141], [142].
Procedure
-
(i)
Samples (body fluids, swabs, tissues, etc.) are placed on a clean glass slide, and about 5–10 uL KOH solution is poured. The KOH solution completely dissolves non-fungal components while fungal hyphae and yeast cells are exposed to visualization under a microscope.
-
(ii)
Place the cover glass slip on the top of the slide and gently press to remove any air bubble. Wipe off excess solution with non-sticky tissue paper.
-
(iii)
Now microscopic slide is ready for examination under the microscope. Low power magnification (10X) is utilized to visualize epithelial cells.
-
(iv)
Fungal structure (hyphae or yeast) can also be examined at 10X magnification if any suspicious specimen is seen, then high magnification (40X) to ascertain other budding fungi.
Calcofluor white stain
The basic principle behind the calcofluor white stain test is that the stain (non-specific fluorochrome) binds with cellulose and chitin present in the cell walls of fungi and other microscopic organisms. The rapid white stain can detect yeast and pathogenic fungi, including Acanthamoeba, Balamuthia species, Naegleria, and Pneumocystis spp [143], [144], [145].
Procedure
-
(i)
Place the sample (swabs, body fluids, hair, nail and skin scrapings, sputum, etc.) on the clean glass slide.
-
(ii)
Pour carefully one drop of calcofluor white stain and one drop of potassium hydroxide solution (10%).
-
(iii)
Place the clear cover glass slip over the sample and leave it for 1 min to settle the solution and wipe off the remaining solution from the slide.
-
(iv)
Observe the slide under UV light at low magnification (100X) and then higher magnification (400X).
Fungal culture test
A fungal culture test is a primary technique to confirm and diagnose fungal infection. Growing culture in culture medium takes several weeks.
Procedure
-
(i)
Culture medium is prepared to grow specimen.
-
(ii)
Sample is placed onto Sabouraud’s dextrose agar containing cycloheximide and chloramphenicol.
-
(iii)
Samples containing nutrient media are incubated at a particular temperature over in an incubator and closely monitored during the experiment.
Fungal susceptibility test
Following the identification of fungal species in the fungal culture, a susceptibility test is performed to determine the most suitable antifungal agents of the pathogenic microorganisms. The standard disk-diffusion method is exploited to test non-dermatophyte filamentous fungi isolated from the fungal culture previously [146].
Procedure
-
(i)
Mueller-Hinton agar (pH, 7.2–7.4) placed onto agar plates for optimal growth of molds at 24–48 hr [147].
-
(ii)
Agar plates should be streaked in three different directions after drying.
-
(iii)
Disk (6 mm) containing AmB) (10 µg), itraconazole (10 µg), posaconazole (5 µg), caspofungin (5 µg), and voriconazole (1 µg) must be pressed down onto the inoculated agar plates so that the entire disk encounters the agar.
-
(iv)
Following incubation 16–24 hr, the zone of inhibition is noted down in mm. Zone of inhibition of A. fumigatus, A. flavus, and A. niger are visible after 24 hr of incubation.
Antibody testing
This is a non-culture-based immunodiagnostic test performed to establish the fungal infection of endemic mycoses. The most common tests under antibody testing are immunodiffusion, complement fixation, and enzyme immunoassay [140]. The immunodiffusion test identifies precipitating antibodies to Histoplasma M and H antigens. The qualitative immunodiffusion test reveals the diagnosis of coccidioidomycosis by displaying coccidioidal IgM or IgG. In the diagnosis of acute primary coccidioidomycosis, the presence of IgM is beneficial [148]. IgG antibodies are produced in chronic infection and are typically detected by complement fixation assay [140].
Molecular diagnostic test for fungi
In this method, biomarkers for DNA, RNA, or gene products of pathogenic fungal species are investigated [149].
The molecular diagnostic method exploits the use of PCR, restriction fragment length polymorphism analysis, DNA sequencing of internal transcribed spacer, and 18 S rRNA gene region for identification and detection of Mucorales [150], [151]. Voigt et al. reported a method for rapid and accurate identification of etiological agents mucormycosis where 18 S rDNA and the D1-D2 domains of the 28 S rDNA were identified for 42 isolates of Zygomycetes and 13 taxon-specific PCR primers [152]. Recently, bronchoalveolar lavage fluid was taken from the patient for rapid and exact detection of invasive pulmonary mucormycosis with the help of real-time quantitative PCR assays [153].
For precise and accurate identification of fungal species, molecular diagnostic tests are exploited where biochemical, microbiological, and phenotypic examination failed to recognize the pathogenic species.
Nanotechnology in fungal detection
Nanotechnology has been used in medicine since the 1980 s. However, nanotechnology has not been adequately utilized for fungal diagnostic purposes. Our group recently has reported the potential use of nanotechnology-based approaches to detect the SARS-CoV-2. The strategy employs graphene (G-FET), biosensor-based, as well as plasmonic photothermal-associated sensors to detect the virus (naked-eye detection on changing the color) [154], [155]. Lately, gold nanoparticles have recently been used for the detection of Aspergillus niger in human cutaneous fungal infections based on the difference in color from red to blue in a short time [156]. The nanoemulsion has been used as an effective treatment against human pathogenic fungi [157]. Myconanotechnology is an emerging science to tackle fungal infection with the aid of nanotechnology. The multidisciplinary approach of nanotechnology is the interface between fungal science and nanotechnology and has considerable potential to detect fungal species [158].
Treatment
The treatment approaches to counter mucormycosis are primarily based on novel methods. The introduction of statins into CAM therapy has recently been proposed due to the potential benefit 159]. The possible reason for the inclusion of statins in the CAM therapy may be due to (i) cytoprotective GRP78 expression, (ii) reduce infection risk, and (iii) synergize anti-CAM levels of the drug in plasma. Few drugs under preclinical investigation, such as VT-1161, SCH 42427, APX001A, colistin, and PC1244, possess potential against Mucorales [160], [161].
The successful management of mucormycosis is based on five basic steps, namely (i) immediate diagnosis, (ii) urgent start of antifungal medication, (iii) adjunct therapy, (iv) reversal of essential predisposing factors, and (v) surgical intervention, if needed [162]. Ascertaining an early diagnosis of mucormycosis is the mainstay for facilitating and initiating the start of antifungal therapy [163].
Immediate diagnosis
The diagnosis of mucormycosis relies upon the identification of fungal species grown on laboratory culture media for mucormycosis and subsequent confirmation from histopathology [85]. The major disadvantage associated with culture identification is that most of the time, culture yields no growth. In that scenario, clinicians must utilize appropriate invasive testing to establish an early diagnosis. Physical examination of the patient’s nostril airways and other signs and symptoms are necessary to diagnose mucormycosis.
With the advent of PCR, molecular diagnosis of mucormycosis has gained tremendously, exploiting 18 S rDNA [164]. Recently, Gholinejad-Ghadi et al. amplified 18 S rDNA regions using endpoint detection to identify mucormycetes [165]. Several articles report DNA sequencing of either internal transcribed spacer region of 18 S rDNA regions to establish mucormycosis diagnosis [166]. PCR-based assay effectively detects circulating DNA and assist in molecular detection of pulmonary mucormycosis.
In the patient with ROCM, computed tomography (CT) scans usually discover sinusitis and cannot scan deeper tissues. A CT scan is routinely performed for early detection of pulmonary mucormycosis. A CT scan can differentiate pulmonary mucormycosis from aspergillosis based on sinusitis and numerous nodules (more than 10) in the scan. A more powerful magnetic resonance imaging technique detects infection in the orbital and central nervous systems.
Urgent start of antifungal medication
The two main classes of antifungal medicines against mucormycosis are polyenes (amphotericin derivatives) and triazoles (isavuconazole and posaconazole). AmB and isavuconazole remain the only two anti-fungal agents approved by FDA for the management. Posaconazole is given as salvage treatment to the patient showing intolerance to AmB [167], [168]. Posaconazole's moderate strength and isavuconazole are also suggestive of first-line antifungal monotherapy [169].
To provide high doses of antifungal, clinicians begin with lipid formulations of L-AmB rather than AmB deoxycholate since L-AmB has less nephrotoxicity in immunocompromised patients. The recommended beginning dose of liposomal AmB or L-AmB complex is 5 mg/kg per day. The dose may increase up to 10 mg/kg daily to control the co-infection.
Recently, combinatorial therapy has been proposed. Combinations of L-AmB with posaconazole, L-AmB with echinocandins, and posaconazole with echinocandins have been used to treat mucormycosis in a retrospective cohort study [169]. Combinatorial therapy like (AmB+ atorvastatin/lovastatin) exhibited better efficacy against Rhizopus arrhizus as compared to AmB alone [170]. However, no convincing results in mortality between monotherapy and combinatorial therapy were reported. Posaconazole and isavuconazole are broad-spectrum azoles and are highly effective against mucormycosis in oral and parenteral formulations [171], [172]. Patients responding to L-Amb, posaconazole, and isavuconazole can be prescribed for step-down therapy. Another line of treatment for mucormycosis is salvage therapy, where those patients not responding to AmB are given posaconazole or isavuconazole intravenously with a dose of 300 mg/kg every 12 h, followed by 300 mg every 24 h afterward.
Itraconazole and terbinafine may be applied depending on the strains of Mucorales [71]. Combinatorial therapy utilizes polyene and caspofungin as a promising alternative and better therapy to treat the ROCM as compared to polyene monotherapy [173].
Adjunct therapy
Patients with elevated serum iron concentrations are more prone to mucormycosis infection. Iron metabolism plays a significant role in spreading infection with Mucorales, thus exploiting iron chelators as an adjunct therapy to control the mucormycosis [174], [175]. Some of the iron chelators approved by the FDA are deferiprone, injectable desferrioxamine, deferasirox [176]. Iron chelators (deferiprone and deferasirox) have been reported to possess in vitro as well as in vivo antifungal activity against Mucorales and thus exploited as effective iron chelators in clinical management [177]. Therefore, iron chelators may be exploited as an adjunct therapy to regulate the iron concentration in the body. As of now, iron chelators have not been utilized regularly to contain the CAM. Thus, combinatorial therapy of antifungal agents and non-xenosiderophore iron chelators could be engaged in the treatment management for an apparent synergistic effect [150].
Reversal of essential predisposing factors
The patient undergoing immunosuppressive medication, mainly steroids, and corticosteroids, should be carefully monitored and checked every 12 h. In the follow-up process, biochemical parameters are checked, and if no sign of improvement is recorded, then immediately all immunosuppressive medications are stopped. Restoring glucose levels is critical to balance normal acid-base status in a patient with a history of DKA.
The use of immunosuppressants, especially corticosteroids, should be minimized or stopped if possible. The immunosuppressant has been extensively used to reduce hospital stay and reduce the mortality of CAM patients [33]. Furthermore, to reduce the severity of infection caused by mucormycosis, the administration of iron, blood transfusion, and desferrioxamine should be carefully regulated.
Surgical intervention
In mucormycosis, debridement of necrotic tissue may cause blood vessel thrombosis, leading to poor penetration and absorption of antifungal drugs at the site of infection. Therefore, removal of necrotic tissue through surgical intervention is critical for the survival of the afflicted mucormycotic patient. Previously several case reports involving mucormycosis have presented that patients who did not opt for surgical intervention of the affected regions had a higher mortality rate as compared to the patients who underwent surgical debridement [178], [179], [180], [181]. The amount and timing of surgical intervention in the affected portion of fungal infection have not been fully delineated, and expert clinicians can choose when to operate. It also depends on the case-to-case basis. The surgical treatment depends on the site and extent of the infection, and it is the preferred line of treatment for ROCM and soft tissue mucormycosis.
In ROCM, surgical care involves drainage of sinuses and may require excision of orbital contents of the affected brain region. For complete control of pulmonary mucormycosis, excision of pulmonary lesions is performed if restricted to a single lobe. In cutaneous mucormycosis, entire cutaneous lesions are excised, while in the GI, necessary resection of GI masses is executed [182].
Recently, Hussain et al. conducted a study involving evidence-based mapping of CAM patients and observed a higher percentage (64.96%) of survival who underwent surgical procedures in adjunct to antifungal therapy [86]. Hence, the timing of surgical intervention is crucial to stop the spread of infection to nearby tissues and organs. The subsequent prognosis essentially depends on the infection site, etiology of fungus, spectrum, and comorbidities associated with the patient. Surgical management becomes challenging in cases of disseminated mucormycosis or the absence of removal of infected focus.
Conclusion
COVID-19 has put the entire world population in a precarious situation. Due to the unavailability of an exact cure for the deadly viral infection, and the advent of the second wave in India, conditions became out of hand control of the pandemic by the frontline health workers & paramedical staff with limited medical resources was impossible as the rate of infection was increasing day by day. During the handling of COVID-19, routine use of steroids, antibiotics, and supportive care (supportive oxygen and ventilators) to patients with reported comorbidities such as diabetes and cardiovascular diseases have worsened the management problem. Patients showing comorbidities are more likely to have secondary coinfection such as mucormycosis. Due to their immunocompromised system, individuals with a history of comorbidities may develop a life-threatening fungal infection, accompanied by high glucose levels, DKA, neutropenia, and amplified serum level (iron). Combining all these parameters may reduce levels of WBC, T cells, and other immune factors, leading to cytokine storm and impairment of cellular organs. Hence, frontline medical staff, including clinicians and health workers, may quickly control mucormycosis infection in the COVID-19 patients. A multidisciplinary approach in diagnosis is essential as well as treatment with antifungals within few hours, as the timing of mucormycosis management is critical to the subject. Sometimes surgical intervention is needed in the affected organs to control the spread of infection to other patient body parts. Further research and investigations are suggested to determine the molecular pathways involved in COVID-19 patients developing mucormycosis who have a history of comorbidities. Finally, a regular follow-up procedure is recommended to the COVID-19 patient with a prior history of comorbidities who has recently recovered from mucormycosis.
Funding
Dr. Ahmad reports receiving grant support from the South African National Research Foundation (NRF) Research Development Grant for Y-Rated Researchers (RDYR180418322304; Grant No: 116339).
Data Availability
Not applicable.
Acknowledgment
Authors are highly thankful to Virus Research and Diagnostic Lab (VRDL), Jawaharlal Nehru Medical College, and Hospital (JNMCH) laboratory staff for generating the different phenotypic pictures of mucormycosis for this review. Authors also acknowledge that all these pictures have been generated for this article only and have not been used for other clinical or research purposes.
Competing Interests
All authors declare no conflict of interest.
Consent for publication
Not applicable.
References
- 1.Kubin C.J., McConville T.H., Dietz D., Zucker J., May M., Nelson B., et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with health care-associated infections. Open Forum Infect Dis. 2021;8:ofab201. doi: 10.1093/ofid/ofab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soni S., Namdeo Pudake R., Jain U., Chauhan N. A systematic review on SARS-CoV-2-associated fungal coinfections. J Med Virol. 2021 doi: 10.1002/jmv.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan W.H., Hashmi Z., Goel A., Ahmad R., Gupta K., Khan N., et al. COVID-19 pandemic and vaccines update on challenges and resolutions. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.690621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahalaxmi I., Jayaramayya K., Venkatesan D., Subramaniam M.D., Renu K., Vijayakumar P., et al. Mucormycosis: an opportunistic pathogen during COVID-19. Environ Res. 2021;201 doi: 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020:105. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus (COVID-19) Dashboard n.d. 〈https://covid19.who.int〉 (Accessed 9 November, 2021).
- 7.〈https://coronavirus.jhu.edu/map.html〉 n.d.
- 8.Chauhan N., Jaggi M., Chauhan S.C., Yallapu M.M. Expert review of anti-infective therapy COVID-19: fighting the invisible enemy with microRNAs Abstract. Expert Rev Anti Infect Ther. 2021;19:137–146. doi: 10.1080/14787210.2020.1812385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azhar A., Al-hosaini K., Khan P.A., Oanz A.M., Zia Q., Banawas S., et al. Promiscuous biological features of newly emerged SARS-CoV-2 facilitate its unrestrained outbreak: an update. Coronaviruses. 2021:02. doi: 10.2174/2666796702666210202125638. [DOI] [Google Scholar]
- 10.Zeyaullah Md, AlShahrani A.M., Muzammil K., Ahmad I., Alam S., Khan W.H., et al. COVID-19 and SARS-CoV-2 variants: current challenges and health concern. Front Genet. 2021;12 doi: 10.3389/fgene.2021.693916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini A., Sm R., Mclellan A.D. Implications of SARS-CoV-2 mutations for genomic RNA structure and host microRNA targeting. Int J Mol Sci. 2020 doi: 10.3390/ijms21134807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthu V., Rudramurthy S.M., Chakrabarti A., Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: india versus the rest of the world. Mycopathologia. 2021;186:739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., et al. COVID‐19–associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64:1452–1459. doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aranjani J.M., Manuel A., Abdul Razack H.I., Mathew S.T. COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain S., Riad A., Singh A., Klugarová J., Antony B., Banna H., et al. Global prevalence of COVID-19-associated mucormycosis (CAM): living systematic review and meta-analysis. J Fungi. 2021;7:985. doi: 10.3390/jof7110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora U., Priyadarshi M., Katiyar V., Soneja M., Garg P., Gupta I., et al. Novel risk factors for coronavirus disease-associated mucormycosis (CAM): a case control study during the outbreak in India. Epidemiology. 2021 doi: 10.1101/2021.07.24.21261040. [DOI] [Google Scholar]
- 18.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paltauf A. Mycosis mucorina: Ein Beitrag zur Kenntniss der menschlichen Fadenpilzerkrankungen. Arch Für Pathol Anat Physiol Für Klin Med. 1885;102:543–564. doi: 10.1007/BF01932420. [DOI] [Google Scholar]
- 20.Baker R.D. Mucormycosis; a new disease? J Am Med Assoc. 1957;163:805–808. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- 21.Eucker J., Sezer O., Graf B., Possinger K. Mucormycoses. Mycoses. 2001;44:253–260. [PubMed] [Google Scholar]
- 22.Reid G., Lynch J.P., Fishbein M.C., Clark N.M. Mucormycosis. Semin Respir Crit Care Med. 2020;41:99–114. doi: 10.1055/s-0039-3401992. [DOI] [PubMed] [Google Scholar]
- 23.Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2009;15(Suppl 5):2–9. doi: 10.1111/j.1469-0691.2009.02972.x. [DOI] [PubMed] [Google Scholar]
- 24.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6 doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chander J., Kaur M., Singla N., Punia R.P.S., Singhal S.K., Attri A.K., et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi. 2018;4 doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5 doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.〈https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf〉 n.d.
- 28.Dilek A., Ozaras R., Ozkaya S., Sunbul M., Sen E.I., Leblebicioglu H. COVID-19-associated mucormycosis: case report and systematic review. Travel Med Infect Dis. 2021;44 doi: 10.1016/j.tmaid.2021.102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15 doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Mehta S., Pandey A. Rhino-orbital mucormycosis associated With COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saldanha M., Reddy R., Vincent M.J. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg Publ Assoc Otolaryngol India. 2021:1–4. doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revannavar S.M., P S S., Samaga L., V K V. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra N., Mutya V.S.S., Thomas A., Rai G., Reddy B., M A.A., et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867. doi: 10.18203/issn.2454-5929.ijohns20211583. [DOI] [Google Scholar]
- 39.Satish D., Joy D., Ross A., B. Mucormycosis coinfection associated with global COVID-19: a case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021;7:815. doi: 10.18203/issn.2454-5929.ijohns20211574. [DOI] [Google Scholar]
- 40.Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K.K., Kale P.G., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021 doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen M., Honavar S.G., Bansal R., Sengupta S., Rao R., Kim U., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R.P., Gupta N., Kaur T., Gupta A. Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arjun R., Felix V., Niyas V.K.M., Kumar M.A.S., Krishnan R.B., Mohan V., et al. COVID-19-associated rhino-orbital mucormycosis: a single-centre experience of 10 cases. QJM Int J Med. 2022;114:831–834. doi: 10.1093/qjmed/hcab176. [DOI] [PubMed] [Google Scholar]
- 46.Saidha P.K., Kapoor S., Das P., Gupta A., Kakkar V., Kumar A., et al. Mucormycosis of paranasal sinuses of odontogenic origin post COVID19 infection: a case series. Indian J Otolaryngol Head Neck Surg Publ Assoc Otolaryngol India. 2021:1–5. doi: 10.1007/s12070-021-02638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain M., Tyagi R., Tyagi R., Jain G. Post-COVID-19 gastrointestinal invasive mucormycosis. Indian J Surg. 2021:1–3. doi: 10.1007/s12262-021-03007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baskar H.C., Chandran A., Reddy C.S., Singh S. Rhino-orbital mucormycosis in a COVID-19 patient. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi A.R., Muthe M.M., Patankar S.H., Athawale A., Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: experience from a single center in India. AJR Am J Roentgenol. 2021;217:1431–1432. doi: 10.2214/AJR.21.26205. [DOI] [PubMed] [Google Scholar]
- 50.Rao R., Shetty A.P., Nagesh C.P. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: Clinico-radiological features. Indian J Ophthalmol. 2021;69:1627–1630. doi: 10.4103/ijo.IJO_1053_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.a Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2021:1–4. doi: 10.1080/01676830.2021.1903044. [DOI] [PubMed] [Google Scholar]
- 53.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radio Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12:85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of rhizopus azygosporus pneumonia following COVID-19. J Fungi. 2021;7:174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khatri A., Chang K.-M., Berlinrut I., Wallach F. Mucormycosis after coronavirus disease 2019 infection in a heart transplant recipient - case report and review of literature. J Mycol Med. 2021;31 doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monte Junior E.S. do, Santos M.E.L.D., Ribeiro I.B., Luz G. de O., Baba E.R., Hirsch B.S., et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53:746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020 doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellanger A.-P., Navellou J.-C., Lepiller Q., Brion A., Brunel A.-S., Millon L., et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021;S2666–9919(21) doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karimi‐Galougahi M., Arastou S., Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID‐19) Int Forum Allergy Rhinol. 2021;11:1029–1030. doi: 10.1002/alr.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pakdel F., Ahmadikia K., Salehi M., Tabari A., Jafari R., Mehrparvar G., et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64:1238–1252. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sargin F., Akbulut M., Karaduman S., Sungurtekin H. Severe rhinocerebral mucormycosis case developed after COVID 19. J Bacteriol Parasitol. 2021:12. [Google Scholar]
- 67.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R., et al. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically Ill COVID-19 patient with underlying hematological malignancy. J Fungi. 2021;7:88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Van Steenweghen S., Maertens J., Boogaerts M., Deneffe G., Verbeken E., Nackaerts K. Mucormycosis, a threatening opportunistic mycotic infection. Acta Clin Belg. 1999;54:99–102. doi: 10.1080/17843286.1999.11754215. [DOI] [PubMed] [Google Scholar]
- 71.Skiada A., Lass-Floerl C., Klimko N., Ibrahim A., Roilides E., Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56:93–101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babu A., Santosh R. Fungal infections of oral cavity: diagnosis. Manag, Assoc COVID. 2021;19 [Google Scholar]
- 73.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis Publ Infect Dis Soc Am. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 74.Ibrahim A.S., Spellberg B., Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hosseini S.M.S., Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Oto Rhino Laryngol J Eur Fed Oto Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto Rhino Laryngol Head Neck Surg. 2005;262:932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 76.Therakathu J., Prabhu S., Irodi A., Sudhakar S.V., Yadav V.K., Rupa V. Imaging features of rhinocerebral mucormycosis: a study of 43 patients. Egypt J Radio Nucl Med. 2018;49:447–452. doi: 10.1016/j.ejrnm.2018.01.001. [DOI] [Google Scholar]
- 77.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis Publ Infect Dis Soc Am. 2012;54(Suppl 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 78.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi. 2021 doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fürbringer P. Beobachtungen über lungenmycose beim menschen. Arch Für Pathol Anat Physiol Für Klin Med. 1876;66:330–365. doi: 10.1007/BF01878266. [DOI] [Google Scholar]
- 81.Helenglass G., Elliott J.A., Lucie N.P. An unusual presentation of opportunistic mucormycosis. Br Med J Clin Res Ed. 1981;282:108–109. doi: 10.1136/bmj.282.6258.108-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Green J.P., Karras D.J. Commentary. Ann Emerg Med. 2012;59:54–55. doi: 10.1016/j.annemergmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Yamin H.S., Alastal A.Y., Bakri I. Pulmonary mucormycosis over 130 years: a case report and literature review. Turk Thorac J. 2017;18:1–5. doi: 10.5152/TurkThoracJ.2017.16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iqbal N., Irfan M., Jabeen K., Kazmi M.M., Tariq M.U. Chronic pulmonary mucormycosis: an emerging fungal infection in diabetes mellitus. J Thorac Dis. 2017;9:E121–E125. doi: 10.21037/jtd.2017.02.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hussain S., Baxi H., Riad A., Klugarová J., Pokorná A., Slezáková S., et al. COVID-19-associated mucormycosis (CAM): an updated evidence mapping. Int J Environ Res Public Health. 2021;18:10340. doi: 10.3390/ijerph181910340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Tawfiq J.A., Alhumaid S., Alshukairi A.N., Temsah M.-H., Barry M., Al Mutair A., et al. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. 2021;49:833–853. doi: 10.1007/s15010-021-01670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adam R.D., Hunter G., DiTomasso J., Comerci G. Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis Publ Infect Dis Soc Am. 1994;19:67–76. doi: 10.1093/clinids/19.1.67. [DOI] [PubMed] [Google Scholar]
- 89.Umbert I.J., Su W.P. Cutaneous mucormycosis. J Am Acad Dermatol. 1989;21:1232–1234. doi: 10.1016/s0190-9622(89)70336-4. [DOI] [PubMed] [Google Scholar]
- 90.Mizutari K., Nishimoto K., Ono T. Cutaneous mucormycosis. J Dermatol. 1999;26:174–177. doi: 10.1111/j.1346-8138.1999.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 91.Horré R., Jovanić B., Herff S., Marklein G., Zhou H., Heinze I., et al. Wound infection due to Absidia corymbifera and Candida albicans with fatal outcome. Med Mycol. 2004;42:373–378. doi: 10.1080/1369378032000141426. [DOI] [PubMed] [Google Scholar]
- 92.Skiada A., Pagano L., Groll A., Zimmerli S., Dupont B., Lagrou K., et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2011;17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 93.Skiada A., Petrikkos G. Cutaneous zygomycosis. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2009;15(Suppl 5):41–45. doi: 10.1111/j.1469-0691.2009.02979.x. [DOI] [PubMed] [Google Scholar]
- 94.Tehmeena W., Hussain W., Zargar H.R., Sheikh A.R., Iqbal S. Primary cutaneous mucormycosis in an immunocompetent host. Mycopathologia. 2007;164:197–199. doi: 10.1007/s11046-007-9041-8. [DOI] [PubMed] [Google Scholar]
- 95.Simbli M., Hakim F., Koudieh M., Tleyjeh I.M. Nosocomial post-traumatic cutaneous mucormycosis: a systematic review. Scand J Infect Dis. 2008;40:577–582. doi: 10.1080/00365540701840096. [DOI] [PubMed] [Google Scholar]
- 96.Hata T.R., Johnson R.A., Barnhill R., Dover J.S. Ecthymalike lesions on the leg of an immunocompromised patient. Primary cutaneous mucormycosis. Arch Dermatol. 1995;131(833–4):836–837. doi: 10.1001/archderm.1995.01690190089018. [DOI] [PubMed] [Google Scholar]
- 97.Freifeld A.G., Iwen P.C. Zygomycosis. Semin Respir Crit Care Med. 2004;25:221–231. doi: 10.1055/s-2004-824905. [DOI] [PubMed] [Google Scholar]
- 98.Hocker T.L., Wada D.A., Bridges A., el-Azhary R. Disseminated zygomycosis heralded by a subtle cutaneous finding. Dermatol Online J. 2010;16:3. [PubMed] [Google Scholar]
- 99.Rickerts V., Just-Nübling G., Konrad F., Kern J., Lambrecht E., Böhme A., et al. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur J Clin Microbiol Infect Dis Publ Eur Soc Clin Microbiol. 2006;25:8–13. doi: 10.1007/s10096-005-0078-7. [DOI] [PubMed] [Google Scholar]
- 100.Bernal-Martínez L., Buitrago M.J., Castelli M.V., Rodriguez-Tudela J.L., Cuenca-Estrella M. Development of a single tube multiplex real-time PCR to detect the most clinically relevant mucormycetes species. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2013;19:E1–E7. doi: 10.1111/j.1469-0691.2012.03976.x. [DOI] [PubMed] [Google Scholar]
- 101.Cherney C.L., Chutuape A., Fikrig M.K. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: case report and literature review. Am J Gastroenterol. 1999;94:252–256. doi: 10.1111/j.1572-0241.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 102.Moura D.T.H., de, Proença I.M., McCarty T.R., Sagae V.M.T., Ribeiro I.B., Oliveira G.H.P. de, et al. Gastrointestinal manifestations and associated health outcomes of covid-19: a Brazilian experience from the largest South American Public Hospital. Clinics. 2020;75 doi: 10.6061/clinics/2020/e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spellberg B., Walsh T.J., Kontoyiannis D.P., Edwards J., Ibrahim A.S. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis Publ Infect Dis Soc Am. 2009;48:1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zia Q., Azhar A., Kamal M.A., Aliev G., Owais M., Ashraf G.M. Super aggregated form of Amphotericin B: a novel way to increase its therapeutic index. Curr Pharm Des. 2016;22:792–803. doi: 10.2174/1381612822666151209151719. [DOI] [PubMed] [Google Scholar]
- 105.Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuvelier I., Vogelaers D., Peleman R., Benoit D., Van Marck V., Offner F., et al. Two cases of disseminated mucormycosis in patients with hematological malignancies and literature review. Eur J Clin Microbiol Infect Dis Publ Eur Soc Clin Microbiol. 1998;17:859–863. doi: 10.1007/s100960050207. [DOI] [PubMed] [Google Scholar]
- 108.Cunha C., Carvalho A., Esposito A., Bistoni F., Romani L. DAMP signaling in fungal infections and diseases. Front Immunol. 2012;3:286. doi: 10.3389/fimmu.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dansky A.S., Lynne C.M., Politano V.A. Disseminated mucormycosis with renal involvement. J Urol. 1978;119:275–277. doi: 10.1016/s0022-5347(17)57455-8. [DOI] [PubMed] [Google Scholar]
- 110.Krishna V., Morjaria J., Jalandari R., Omar F., Kaul S. Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoenigl M., Seidel D., Carvalho A., Rudramurthy S.M., Arastehfar A., Gangneux J.P., et al. The emergence of COVID-19 associated mucormycosis: analysis of cases from 18 countries. SSRN Electron J. 2021 doi: 10.2139/ssrn.3844587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez-Morales A.J., Sah R., Millan-Oñate J., Gonzalez A., Montenegro-Idrogo J.J., Scherger S., et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021;8 doi: 10.1177/20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tilavberdiev S.A., Madaminov F.A. Associated FUngal Infections, Related to the Global Covid-19 pandemic (Literature review) Int J Multicult Multirelig. Under. 2021;8:619–627. doi: 10.18415/ijmmu.v8i5.2720. [DOI] [Google Scholar]
- 115.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Welk B., House A.A., Ralph E., Tweedy E., Luke P.P.W. Successful treatment of primary bilateral renal mucormycosis with bilateral nephrectomy. Urology. 2004;64:590. doi: 10.1016/j.urology.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 117.Maliwan N., Reyes C.V., Rippon J.W. Osteomyelitis secondary to cutaneous mucormycosis. Report of a case and a review of the literature. Am J Derm. 1984;6:479–481. doi: 10.1097/00000372-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 118.Pierce P.F., Wood M.B., Roberts G.D., Fitzgerald R.H., Robertson C., Edson R.S. Saksenaea vasiformis osteomyelitis. J Clin Microbiol. 1987;25:933–935. doi: 10.1128/jcm.25.5.933-935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews D.R., Allan A., Larbalestier R.I. Tracheal mucormycosis. Ann Thorac Surg. 1997;63:230–232. doi: 10.1016/s0003-4975(96)00817-x. [DOI] [PubMed] [Google Scholar]
- 120.Wolf O., Gil Z., Leider-Trejo L., Khafif A., Biderman P., Fliss D.M. Tracheal mucormycosis presented as an intraluminal soft tissue mass. Head Neck. 2004;26:541–543. doi: 10.1002/hed.20055. [DOI] [PubMed] [Google Scholar]
- 121.Serna J.H., Wanger A., Dosekun A.K. Successful treatment of mucormycosis peritonitis with liposomal amphotericin B in a patient on long-term peritoneal dialysis. Am J Kidney Dis J Natl Kidney Found. 2003;42:E14–E17. doi: 10.1016/s0272-6386(03)00797-2. [DOI] [PubMed] [Google Scholar]
- 122.Ogunlana E.O. Fungal air spora at Ibadan, Nigeria. Appl Microbiol. 1975;29:458–463. doi: 10.1128/am.29.4.458-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong N., Yang X., Ye L., Chen K., Chan E.W.-C., Yang M., et al. Genomic and protein structure modelling analysis depicts the origin and pathogenicity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. F1000 Res. 2020;9:121. doi: 10.12688/f1000research.22357.1. [DOI] [Google Scholar]
- 129.Castillo P., Wright K.E., Kontoyiannis D.P., Walsh T., Patel S., Chorvinsky E., et al. A new method for reactivating and expanding T cells specific for rhizopus oryzae. Mol Ther Methods Clin Dev. 2018;9:305–312. doi: 10.1016/j.omtm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Potenza L., Vallerini D., Barozzi P., Riva G., Forghieri F., Zanetti E., et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011;118:5416–5419. doi: 10.1182/blood-2011-07-366526. [DOI] [PubMed] [Google Scholar]
- 131.Schmidt S., Tramsen L., Perkhofer S., Lass-Flörl C., Hanisch M., Röger F., et al. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–944. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 132.Schmidt S., Schneider A., Demir A., Lass-Flörl C., Lehrnbecher T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses. 2016;59:34–38. doi: 10.1111/myc.12431. [DOI] [PubMed] [Google Scholar]
- 133.Liu M., Spellberg B., Phan Q.T., Fu Y., Fu Y., Lee A.S., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gebremariam T., Liu M., Luo G., Bruno V., Phan Q.T., Waring A.J., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124:237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watkins T.N., Gebremariam T., Swidergall M., Shetty A.C., Graf K.T., Alqarihi A., et al. Inhibition of EGFR signaling protects from mucormycosis. MBio. 2018;9:e01384–18. doi: 10.1128/mBio.01384-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alqarihi A., Gebremariam T., Gu Y., Swidergall M., Alkhazraji S., Soliman S.S.M., et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. MBio. 2020;11:e01087–20. doi: 10.1128/mBio.01087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee S.C., Billmyre R.B., Li A., Carson S., Sykes S.M., Huh E.Y., et al. Analysis of a food-borne fungal pathogen outbreak: virulence and genome of a Mucor circinelloides isolate from yogurt. MBio. 2014;5:e01390–01314. doi: 10.1128/mBio.01390-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Voigt K., Wolf T., Ochsenreiter K., Nagy G., Kaeger K., Shelest E. Genetic and Metabolic Aspects of Primary and Secondary Metabolism of the Zygomycetes. vol. The Mycota Vol III: Biochemistry and Molecular Biology. 3rd edition III ed: Springer Verlag, Berlin; 2016.
- 139.Smith H.W., Kirchner J.A. Cerebral mucormycosis; a report of three cases. AMA Arch Otolaryngol. 1958;68:715–726. doi: 10.1001/archotol.1958.00730020739010. [DOI] [PubMed] [Google Scholar]
- 140.Kozel T.R., Wickes B. Fungal diagnostics. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weinberg J.M., Koestenblatt E.K., Tutrone W.D., Tishler H.R., Najarian L. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193–197. doi: 10.1067/s0190-9622(03)01480-4. [DOI] [PubMed] [Google Scholar]
- 142.Ponka D., Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician Med Fam Can. 2014;60:57. [PMC free article] [PubMed] [Google Scholar]
- 143.Monheit J.E., Cowan D.F., Moore D.G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108:616–618. [PubMed] [Google Scholar]
- 144.Wilhelmus K.R., Osato M.S., Font R.L., Robinson N.M., Jones D.B. Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch Ophthalmol Chic Ill 1960. 1986;104:1309–1312. doi: 10.1001/archopht.1986.01050210063026. [DOI] [PubMed] [Google Scholar]
- 145.Harrington B.J., Hageage G.J. Calcofluor white: a review of its uses and applications in clinical mycology and parasitology. Lab Med. 2003;34:361–367. doi: 10.1309/EPH2TDT8335GH0R3. [DOI] [Google Scholar]
- 146.Alastruey-Izquierdo A., Melhem M.S.C., Bonfietti L.X., Rodriguez-Tudela J.L. Susceptibility test for fungi: clinical and laboratorial correlations in medical mycology. Rev Inst Med Trop Sao Paulo. 2015;57(Suppl 19):57–64. doi: 10.1590/S0036-46652015000700011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Espinel-Ingroff A., Cantón E., Pemán J. Antifungal susceptibility testing of filamentous fungi. Curr Fungal Infect Rep. 2012;6:41–50. doi: 10.1007/s12281-011-0079-1. [DOI] [Google Scholar]
- 148.Saubolle M.A., McKellar P.P., Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45:26–30. doi: 10.1128/JCM.02230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharm Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 150.Samson R., Dharne M. COVID-19 associated mucormycosis: evolving technologies for early and rapid diagnosis. 3 Biotech. 2022;12:6. doi: 10.1007/s13205-021-03080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lackner M., Caramalho R., Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9:683–695. doi: 10.2217/fmb.14.23. [DOI] [PubMed] [Google Scholar]
- 152.Voigt K., Cigelnik E., O’donnell K. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J Clin Microbiol. 1999;37:3957–3964. doi: 10.1128/JCM.37.12.3957-3964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scherer E., Iriart X., Bellanger A.P., Dupont D., Guitard J., Gabriel F., et al. Quantitative PCR (qPCR) detection of mucorales DNA in bronchoalveolar lavage fluid to diagnose pulmonary mucormycosis. J Clin Microbiol. 2018;56 doi: 10.1128/JCM.00289-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Azhar A., Hassan N., Singh M., Al-hosaini K., Kamal M.A. Synopsis on pharmotechnological approaches in diagnostic to management strategies in fighting against COVID-19. Curr Pharm Des. 2021:27. doi: 10.2174/1381612827666210715154004. [DOI] [PubMed] [Google Scholar]
- 155.Asdaq S.M.B., Rajan A., Damodaran A., Kamath S.R., Nair K.S., Zachariah S.M., et al. Identifying mucormycosis severity in Indian COVID-19 patients: a nano-based diagnosis and the necessity for critical therapeutic intervention. Antibiotics. 2021;10:1308. doi: 10.3390/antibiotics10111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sojinrin T., Conde J., Liu K., Curtin J., Byrne H.J., Cui D., et al. Plasmonic gold nanoparticles for detection of fungi and human cutaneous fungal infections. Anal Bioanal Chem. 2017;409:4647–4658. doi: 10.1007/s00216-017-0414-7. [DOI] [PubMed] [Google Scholar]
- 157.Garcia A., Fan Y.Y., Vellanki S., Huh E.Y., Vanegas D., Wang S.H., et al. Nanoemulsion as an effective treatment against human-pathogenic fungi. MSphere. 2019;4 doi: 10.1128/mSphere.00729-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mahendra Rai M.R., Alka Yadav A.Y., Bridge P., Aniket Gade A.G. In: Appl. Mycol. Rai M., Bridge P.D., editors. CABI; Wallingford: 2009. Myconanotechnology: A New and Emerging Science; pp. 258–267. [DOI] [Google Scholar]
- 159.Vuorio A., Kovanen P.T. Mucormycosis and glucose-regulated protein 78 in COVID-19: amenable to statin treatment? J Intern Med. 2021;290:931–933. doi: 10.1111/joim.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mycovia Pharmaceuticals Inc. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Oteseconazole (VT-1161) Oral Capsules in the Treatment of Subjects With Recurrent Vulvovaginal Candidiasis. clinicaltrials.gov; 2021.
- 161.Amplyx Pharmaceuticals. A Phase 2, Open-Label Study to Evaluate the Safety and Efficacy of APX001 in the Treatment of Patients With Invasive Mold Infections Caused by Aspergillus Species or Rare Molds. clinicaltrials.gov; 2021.
- 162.Spellberg B., Edwards J., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zubair S., Azhar A., Khan N., Ahmad E., Ajmal M., Owais M. In: Kalkum M., Semis M., editors. Vol. 1625. Springer New York; New York, NY: 2017. Nanoparticle-Based Mycosis Vaccine; pp. 169–211. (Vaccines Invasive Fungal Infect.). [DOI] [Google Scholar]
- 164.Millon L., Scherer E., Rocchi S., Bellanger A.-P. Molecular strategies to diagnose mucormycosis. J Fungi. 2019;5 doi: 10.3390/jof5010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gholinejad-Ghadi N., Shokohi T., Seifi Z., Aghili S.R., Roilides E., Nikkhah M., et al. Identification of Mucorales in patients with proven invasive mucormycosis by polymerase chain reaction in tissue samples. Mycoses. 2018;61:909–915. doi: 10.1111/myc.12837. [DOI] [PubMed] [Google Scholar]
- 166.Guinea J., Escribano P., Vena A., Muñoz P., Martínez-Jiménez M.D.C., Padilla B., et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PloS One. 2017;12 doi: 10.1371/journal.pone.0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ashkenazi-Hoffnung L., Bilavsky E., Avitzur Y., Amir J. Successful treatment of cutaneous zygomycosis with intravenous amphotericin B followed by oral posaconazole in a multivisceral transplant recipient. Transplantation. 2010;90:1133–1135. doi: 10.1097/TP.0b013e3181f86916. [DOI] [PubMed] [Google Scholar]
- 168.Yoon Y.K., Kim M.J., Chung Y.G., Shin I.Y. Successful treatment of a case with rhino-orbital-cerebral mucormycosis by the combination of neurosurgical intervention and the sequential use of amphotericin B and posaconazole. J Korean Neurosurg Soc. 2010;47:74–77. doi: 10.3340/jkns.2010.47.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kyvernitakis A., Torres H.A., Jiang Y., Chamilos G., Lewis R.E., Kontoyiannis D.P. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect Publ Eur Soc Clin Microbiol Infect Dis. 2016;22:811.e1–811.e8. doi: 10.1016/j.cmi.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Chatterjee S., Vardhan B., Singh D.K., Maitra A., Ojha U.K. Should statins be considered for the management of mucormycosis in COVID-19. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thompson G.R., Wiederhold N.P. Isavuconazole: a comprehensive review of spectrum of activity of a new triazole. Mycopathologia. 2010;170:291–313. doi: 10.1007/s11046-010-9324-3. [DOI] [PubMed] [Google Scholar]
- 172.Arendrup M.C., Jensen R.H., Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the mucorales order. Antimicrob Agents Chemother. 2015;59:7735–7742. doi: 10.1128/AAC.01919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reed C., Bryant R., Ibrahim A.S., Edwards J., Filler S.G., Goldberg R., et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis Publ Infect Dis Soc Am. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bullen J.J., Rogers H.J., Spalding P.B., Ward C.G. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55:251–258. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- 175.Roilides E., Lyman C.A., Panagopoulou P., Chanock S. Immunomodulation of invasive fungal infections. Infect Dis Clin North Am. 2003;17:193–219. doi: 10.1016/s0891-5520(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 176.Scott C., Arora G., Dickson K., Lehmann C. Iron chelation in local infection. Molecules. 2021;26 doi: 10.3390/molecules26010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahalmani V., Sarma P., Prakash A., Medhi B. Role of iron chelators in mucormycosis. Indian J Pharm. 2021;53:261–263. doi: 10.4103/ijp.ijp_604_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Khor B.-S., Lee M.-H., Leu H.-S., Liu J.-W. Rhinocerebral mucormycosis in Taiwan. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2003;36:266–269. [PubMed] [Google Scholar]
- 179.Petrikkos G., Skiada A., Sambatakou H., Toskas A., Vaiopoulos G., Giannopoulou M., et al. Mucormycosis: ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis Publ Eur Soc Clin Microbiol. 2003;22:753–756. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]
- 180.Pavie J., Lafaurie M., Lacroix C., Marie Zagdanski A., Debrosse D., Socié G., et al. Successful treatment of pulmonary mucormycosis in an allogenic bone-marrow transplant recipient with combined medical and surgical therapy. Scand J Infect Dis. 2004;36:767–769. doi: 10.1080/00365540410021081. [DOI] [PubMed] [Google Scholar]
- 181.Kontoyiannis D.P., Wessel V.C., Bodey G.P., Rolston K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis Publ Infect Dis Soc Am. 2000;30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 182.Huang H., Xie L., Zheng Z., Yu H., Tu L., Cui C., et al. Mucormycosis-induced upper gastrointestinal ulcer perforation in immunocompetent patients: a report of two cases. BMC Gastroenterol. 2021;21:311. doi: 10.1186/s12876-021-01881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.