Abstract
Stroke is a major problem worldwide that impacts over 100 million adults and children annually. Rehabilitation therapy is the current standard of care to restore functional impairments post-stroke, however its effects are limited and many patients suffer persisting functional impairments and life-long disability. Noninvasive Brain Stimulation (NIBS) has emerged as a potential rehabilitation treatment option in both adults and children with brain injury. In the last decade, Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (tDCS) and Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) have been investigated to improve motor recovery in adults post-stroke. These promising adult findings using NIBS, however, have yet to be widely translated to the area of pediatrics. The limited studies exploring NIBS in children have demonstrated safety, feasibility, and utility of stimulation-augmented rehabilitation. This chapter will describe the mechanism of NIBS therapy (cortical excitability, neuroplasticity) that underlies its use in stroke and motor function and how TMS, tDCS, and taVNS are applied in adult stroke treatment paradigms. We will then discuss the current state of NIBS in early pediatric brain injury and will provide insight regarding practical considerations and future applications of NIBS in pediatrics to make this promising treatment option a viable therapy in children.
Keywords: Brain stimulation, VNS, TMS, tDCS, Pediatrics, taVNS, tVNS, NIBS, Brain injury, Stroke, Cerebral palsy
1. Introduction
Globally, the prevalence of stroke was 104.2 million people in 2017 (Virani et al., 2020). In the United States, approximately 795,000 people each year have a new or recurrent stroke. On average, that is one stroke every 40s with one death every 4min (Centers for Disease Control and Prevention, 2018). The majority (87%) of strokes are ischemic, which result from reductions in blood flow (ischemia) to the brain that disrupts and reorganizes neuronal connections leading to both sensorimotor and cognitive impairments (Centers for Disease Control and Prevention, 2018; Virani et al., 2020). The minority of strokes are caused by intracerebral or subarachnoid hemorrhage (Virani et al., 2020). Both forms of stroke are devastating neurological conditions and a primary cause of long-term disability in adults.
After a stroke, the brain undergoes major changes in synaptic function including changes in cortical excitability, deregulated plasticity and altered interhemispheric interactions (Murphy and Corbett, 2009). Stroke can lead to a variety of functional disabilities, including: hemiparesis, gait instability, blurred or loss in vision, speech difficulty, and dementia (Virani et al., 2020). These impairments significantly diminish a person’s quality of life and have limited treatment options. The main form of treatment is rehabilitation therapy—such as constraint induced movement therapy (CIMT) (Taub et al., 1998), robot-assisted therapy (Aisen et al., 1997), task-specific training (Michaelsen et al., 2006), and high-intensity resistance training (Langhorne et al., 2011; Ouellette et al., 2004). While these therapies have benefits for stroke, there is room for novel interventions that enhance their efficacy.
One of the main areas of research in effective stroke recovery is determining optimal therapies to modify and reorganize neuronal connections (Krakauer et al., 2012; Murphy and Corbett, 2009). Noninvasive brain stimulation (NIBS) has emerged as a method to modulate cortical excitability and enhance motor learning, leading to its investigation as a promising therapeutic tool in the adult stroke (Bolognini et al., 2009; Reis et al., 2008). While there are many forms of NIBS, the most actively studied methods are transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and vagus nerve stimulation (VNS). Administering tDCS and TMS to the motor cortex in adult stroke has shown promise in treating motor function impairments based on two general hypothesized mechanisms: (1) increasing excitability in the affected hemisphere or (2) suppressing excitability in the unaffected hemisphere (Bastani and Jaberzadeh, 2012; de Moura et al., 2019; Marquez et al., 2015; McDonnell and Stinear, 2017; Richards et al., 2008). These approaches, while grounded in clinical (Brunoni et al., 2012; Huang et al., 2017) and animal neurophysiology (Jackson et al., 2016) are not without conceptional limits. Firstly, the effects of tDCS/TMS may not be conceived of as simply “winding brain activity up and down,” rather the nature of modulation can be intensity (e.g., increasing current at cathode from −1 to −2 mA can produce excitatory changes) (Batsikadze et al., 2013; Samani et al., 2019), montage (Leite et al., 2018), neuronal pathways (Rahman et al., 2017; Rawji et al., 2018), and brain state dependent. Secondly, how the brain responds to injury and how these processes should be engaged by neuromodulation (e.g., if these is benefit in activating the healthy hemisphere) remains complex (Buch et al., 2017; Ziemann et al., 2008).
Across these and other neuromodulation approaches one can broadly conceive of two therapeutic schemes. The first scheme, and most common, depends directly on neuroplasticity activated by training and as such any effectiveness and specificity (Bikson and Rahman, 2013; Kronberg et al., 2019) depends on the paired training. In this scheme, NIBS is paired with rehabilitation to accelerate the functional improvements gained by rehabilitation training alone. In the second scheme, which is less common for NIBS, stimulation of the brain directly activates repair (restorative) mechanisms or otherwise increase brain capacity (Bahr Hosseini et al., 2019). In this scheme, NIBS can be applied and be effective independent of rehabilitation training.
While advancements have been made in developing rehabilitation interventions for adults post-stroke, few have been translated to a pediatric population. Perinatally acquired brain injury, including ischemic or neuroinflammatory insults, or hypoxic ischemic encephalopathy (HIE), (Adami et al., 2016; Virani et al., 2020) can lead to serious, debilitating conditions, including neurodevelopmental impairments such as cerebral palsy (CP), a non-progressive movement disorder (Rosenbaum et al., 2007). The overall prevalence of CP is 2.11 per 1000 live births (Oskoui et al., 2013). Treatment for CP and pediatric motor disorders is limited, and more effective options are needed. Due to the vulnerable population and unknown parametric and safety considerations, NIBS research in pediatrics is still in its early stages. There is some early evidence that the NIBS methods (TMS, tDCS, VNS) used to treat adult stroke may be clinically beneficial in children with brain injury (Badran et al., 2020; Chung and Lo, 2015; Elbanna et al., 2019; Hameed et al., 2017; Palm et al., 2016). The research into adult stroke rehabilitation had yielded important concepts and key paradigms that might be translated to the pediatric population.
2. NIBS applications in adult stroke
Noninvasive brain stimulation can be administered using a variety of different neuromodulation techniques that affect the central nervous system either directly (top-down), or indirectly (bottom-up). The three most common forms of neuromodulation are (1) dynamic electromagnetic fields applied using transcranial magnetic stimulation (TMS), (2) direct electricity applied using transcranial direct current stimulation (tDCS), and (3) electrical stimulation of cranial nerve X, the vagus nerve, via vagus nerve stimulation (VNS). TMS, tDCS, and VNS have all shown promise in restoring motor function and repairing aberrant neural functioning, however their underlying mechanisms are different. In general, TMS and tDCS are top-down forms of brain stimulation, which act directly on cortical tissue, whereas VNS is a bottom-up form of stimulation (Adair et al., 2020), which activates the vagus nerve and its effects are projected up to the brain stem (Fig. 1). In this section we will review the fundamental principles behind NIBS application in brain injury and stroke.
FIG. 1.
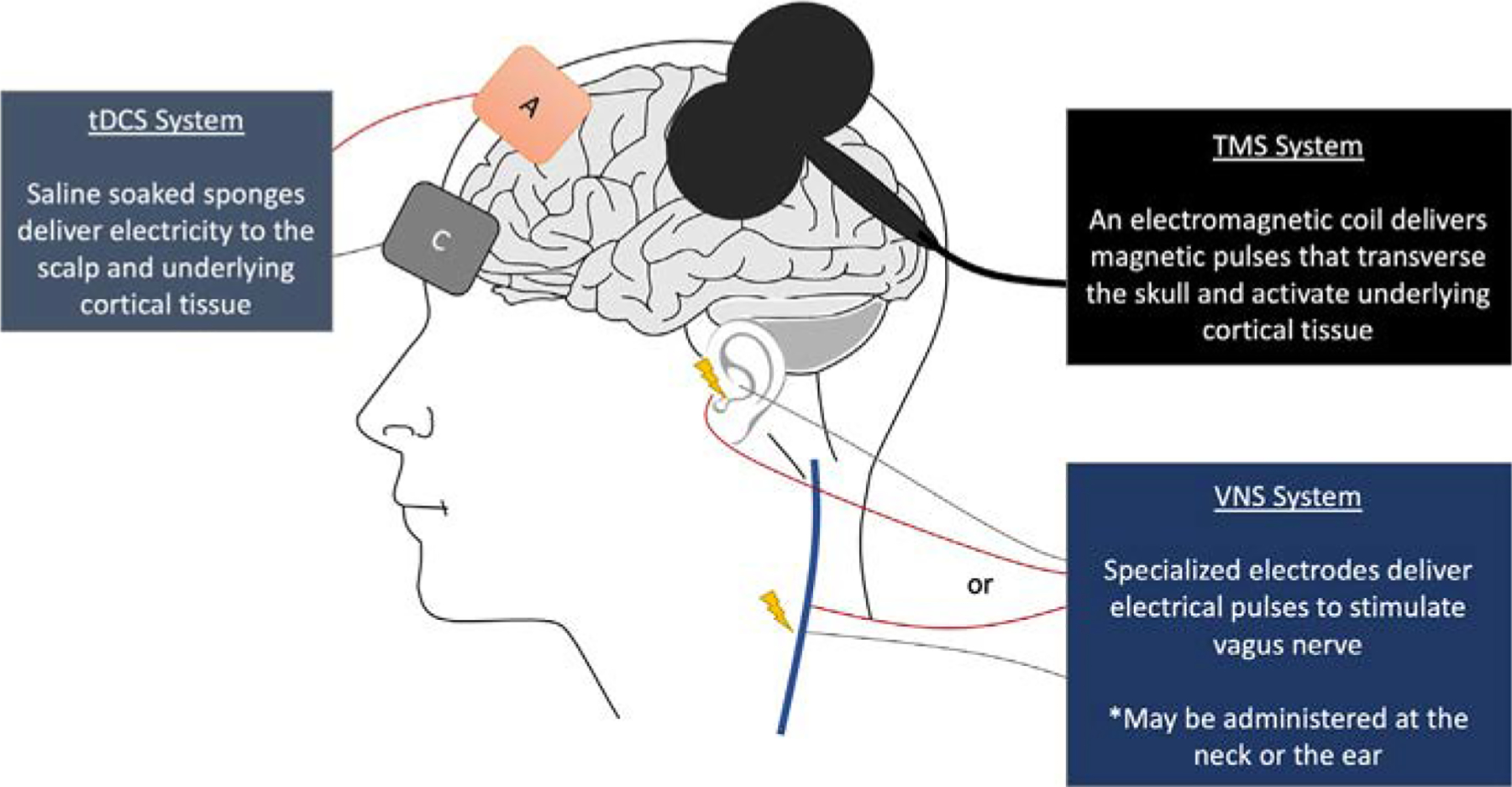
Noninvasive brain stimulation (NIBS) techniques and their varying activation targets. TMS and tDCS (top-down) act directly on cortical tissue, and VNS (bottom-up) activates the vagus nerve (cervical or auricular) and its effects are projected to the brain stem.
2.1. Transcranial magnetic stimulation (TMS) in adult stroke
Transcranial magnetic stimulation (TMS) is administered by placing an electromagnetic coil on the scalp of a patient and delivering brief, powerful (1–3T) magnetic pulses that traverse the scalp and skull, and depolarize neurons in the underlying cortical tissue. This magnetic field has a shallow, focal penetration depth (2–4cm) and can be pulsed at varying frequencies to directly modulate cortical excitability. TMS delivered at high-frequencies (>5Hz) can cause transient elevations in neuronal excitability, whereas at low-frequency (<2Hz), TMS suppresses excitability (Bolognini et al., 2009). Daily administration of TMS can enhance and prolong these behavioral effects, making TMS a powerful neuromodulatory tool and treatment intervention for neuropsychiatric disorders (Burt et al., 2002). TMS is a very safe form of brain stimulation when administered by trained staff, with less than 2% incidence of precipitating a seizure (Rossi et al., 2009). However, in adults with pre-existing epilepsy, stroke and multiple sclerosis, there is an increased risk of having a seizure induced by TMS (Chung and Lo, 2015; Garvey and Gilbert, 2004; Gilbert et al., 2004).
The neurophysiological underpinnings of stroke are complex, however it has been demonstrated that cortical excitability surrounding the stroke lesion as well as in the contralateral hemisphere is altered (Buchkremer-Ratzmann et al., 1996; Cicinelli et al., 2003). Both GABAergic and glutamatergic systems are involved in modulating these effects (Bütefisch et al., 2003), which result in a post-stroke cortical reorganization. A shift in cortical balance takes place following a stroke, where activity in the affected hemisphere decreases and the unaffected hemisphere increases (Murase et al., 2004). This is referred to as the interhemispheric inhibition model (Cicinelli et al., 2003; Murase et al., 2004), however, versions of this model have proposed a time and severity interaction in the effect. NIBS treatments have been developed that address these alterations in dynamic excitability states post-stroke. TMS has been used predominately to either enhance excitability of the lesioned hemisphere (high-frequency TMS) or suppress activity in the non-lesioned hemisphere in order to increase excitability in the lesioned cortex (low-frequency TMS).
In clinical trials, low-frequency repetitive TMS (LF-rTMS) has been investigated as an adjunctive therapy to the contralesional hemisphere, and has been shown to improve motor function by increasing cortical excitability in the lesioned motor cortex. A meta-analysis by Zhang and colleagues reviewed 22 randomized clinical trials (RCT) and 3 crossover studies in a total of 619 stroke participants investigating the effects of 1Hz rTMS over the contralesional M1 on upper limb motor recovery and cortical plasticity. Results demonstrate a positive effect on upper limb motor recovery, specifically in hand function, and researchers postulate that this most likely relates to the rebalancing of excitability in the two hemispheres by TMS (Zhang et al., 2017). High frequency rTMS (HF-rTMS) delivered to the lesioned hemisphere has also been investigated to treat motor deficits in adult participants with stroke, with modest results. In one pilot RCT, participants in the experimental group received 5Hz rTMS over the lesioned hemisphere before treadmill training for nine sessions. The rTMS group showed significant improvements in walking speed, gait, and motor function of lower extremities with improvements maintained at 1 month postintervention (Wang et al., 2019).
It is still unclear whether the LF-rTMS approach is superior to the HF-rTMS approach, however, current research suggests that LF-rTMS may be safer and may be more beneficial to stroke. Hsu and colleagues compared 18 RCTs (N = 370) in which adult stroke participants either received LF-rTMS, HF-rTMS or a combination of LF-rTMS and HF-rTMS. The results indicate that overall rTMS has positive effects on motor function, and that contralesional LF-rTMS may be more beneficial than ipsilesional HF-rTMS (Hsu et al., 2012). Furthermore, a study assessing HF-rTMS in participants with chronic stroke found that HF-rTMS at 20–25Hz may increase the risk of seizure occurrence in this participant group, indicating that LF-rTMS might be the safer option for this population (Lomarev et al., 2007). It is also very possible that different participants will be more responsive to one or the other of the two approaches, with many researchers noting a need for personalized or precision rehabilitation (Plow et al., 2016).
In order to begin exploring optimal parameters for rTMS to improve motor function in stroke, Xiang and colleagues broadly reviewed 43 RCTs using TMS in adults with motor function impairments (Xiang et al., 2019). 1168 participants were included in the meta-analysis and it was found that rTMS had a positive effect on limb motor function, specifically in improving daily living activities. They found no significant differences between stimulus frequencies in rTMS parameters, but a subgroup analysis of rTMS frequencies delivered between 1 and 10Hz revealed that 1Hz in particular had a positive effect on motor function in participants. The meta-analysis also showed that MEP changes in the stimulated hemisphere were more prevalent in HF-rTMS participants compared to those treated with LF-rTMS (Xiang et al., 2019).
A critical question is the optimal timing and duration of TMS, delivered either before or during rehabilitation therapy. Several studies report the use of concomitant rehabilitation therapy alongside rTMS and this may be more effective than rTMS alone (Smith and Stinear, 2016). Kim and colleagues applied HF-rTMS (10Hz) over the affected hemisphere as a form of priming before completion of a complex motor task in stroke participants. The results demonstrate that HF-rTMS priming induced a significant increase in MEP amplitude compared to sham and this was positively associated with increased motor performance (Kim et al., 2006). In a meta-analysis by Zhang and colleagues, specifically exploring the use of LF-rTMS, the majority of studies utilize 1Hz contralesional LF-rTMS and other training (Zhang et al., 2017). The question of whether LF-rTMS alone or in combination with other therapies has positive effects on upper motor function, has yet to be addressed by researchers (Fisicaro et al., 2019; Zhang et al., 2017). It is also unclear if TMS facilitates motor recovery based on the number of stimulation sessions a participant receives. Prior research has shown that a single session of rTMS may not provide as many benefits as multiple sessions, but more research is needed to draw definitive conclusions (Kandel et al., 2012). In summary, rTMS research in adult stroke has significantly increased over the past decade and is progressing toward becoming a part of clinical treatment options. In addition, further parametric optimization of TMS effects and perhaps new parameters such as theta-burst stimulation (Chen et al., 2019) may provide further improvements in functional outcomes for stroke.
2.2. Transcranial direct current stimulation (tDCS) in adult stroke
Transcranial direct current stimulation (tDCS) is an electrical form of NIBS that delivers direct electrical stimulation to the cortex by using a variety of different conductive electrodes (saline-soaked sponge electrodes, ring electrodes with conductive gel, or hydrogels) placed directly onto the scalp delivering low levels of electricity (DaSilva et al., 2011; Woods et al., 2016). tDCS has become widely used in many neuropsychiatric disorders due to its low cost and ease of administration (Bikson et al., 2018; Lefaucheur et al., 2017). tDCS also has the potential of being safely used at home which makes it particularly appealing (Bikson et al., 2020; Charvet et al., 2015; Dobbs et al., 2018; Kasschau et al., 2016). The main safety consideration for tDCS is transient and mild skin irritation (e.g., tingling) (Antal et al., 2017; Bikson et al., 2016; Krishnan et al., 2015) and transient erythema (Ezquerro et al., 2017). Lasting skin irritation (e.g., burns) is not an expected adverse event of tDCS when standard protocols and appropriate equipment is used (Woods et al., 2016). The consensus on the safety and tolerability of tDCS has been well demonstrated in subjects with brain injury both in adults (Russo et al., 2017) and children (Bikson et al., 2016; Zewdie et al., 2020).
The specific polarity of the tDCS electrodes attached to the scalp (either anode or cathode) is an important consideration for outcomes. At a basic level brain excitation is presumed to occur under the anode electrode (anodal tDCS) or brain inhibition is presumed to occur under the cathode electrode (cathodal tDCS) (Nitsche and Paulus, 2001). However, tDCS always requires an anode and cathode, so “anodal tDCS” and “cathodal tDCS” as used in the literature, and in this review, reflects statement of hypothesis (Bikson et al., 2019)—namely what the brain target is and if its closer to the anode and cathode. In general, anodal tDCS is presumed to excite underlying cortex and cathodal tDCS is assumed to inhibit underlying cortex. This derives from early clinical neurophysiology (Nitsche and Paulus, 2000) and animal studies (Bindman et al., 1964; Giordano et al., 2017; Jackson et al., 2016). However, ongoing studies have shown that either polarity of tDCS can produce excitatory or inhibitory effects depending on the stimulation dose and what brain processes are probed (Agboada et al., 2019; Batsikadze et al., 2013; Bikson et al., 2004).
Despite the above caveats, the directionality of current is an important consideration when administering tDCS, as the approaches requires the design of where the anode and cathode are placed. Disregarding convolutions (folding) of the cortical surface (Rahman et al., 2017), current under the anode enters into the brain, while current under the cathode exits the brain (Datta et al., 2008; Faria et al., 2011). This polarizing current can increase (anodal/inward) and decrease (cathodal/outward) neuronal excitability (Lafon et al., 2017; Liu et al., 2019; Radman et al., 2007). The polarization produced by tDCS is weak (~1mV) and sustained in duration to modulate ongoing plasticity (Fritsch et al., 2010; Kronberg et al., 2017; Sun et al., 2016). This serves as the mechanistic substrate for tDCS making rehabilitation training more effective: as the cellular level tDCS “boosts” the specific neuroplasticity activated by training (Kronberg et al., 2020).
Researchers have compared cathodal versus anodal tDCS, and both have been shown effective in adult stroke participants (Khedr et al., 2013). Butler and colleagues conducted a meta-analysis of anodal tDCS effects on upper limb motor recovery in eight randomized placebo-controlled trials (Butler et al., 2013). The results show when comparing participants functional outcome scores pre and post NIBS intervention, anodal tDCS over the affected M1 has a small to moderate effect on improving motor function. In one of the studies (Kim et al., 2009), 10 subacute stroke participants received either active anodal tDCS or sham over the ipsilesional hemisphere for 20min and a motor performance tasks (finger acceleration and Box and Block Test (BBT)) of the paretic hand was assessed before, during and after stimulation. Participants who received active anodal tDCS had significantly improved performance on the motor task and improvements were maintained after stimulation (30min for finger acceleration and 60min for BBT) (Kim et al., 2009).
Cathodal tDCS over the contralesional hemisphere has also been investigated in adult stroke. One study assessed the effect of cathodal tDCS on motor skill acquisition in 12 participants with subcortical stroke. Results demonstrated that two cathodal tDCS sessions (1.0mA for 20min) enhanced learning the new motor skill in comparison to sham and that there was a significant correlation between tDCS facilitated motor improvement and changes in tDCS-induced intracortical inhibition (Zimerman et al., 2012).
Researchers have found that combining tDCS with motor rehabilitation training programs promotes improved motor function compared to motor training alone (Chang et al., 2015; Lefebvre et al., 2013). Furthermore, bihemispheric or dual tDCS has emerged which combines the two forms, and may promote even more functional recovery. Dual tDCS consists of an anode positioned over the ipsilesional hemisphere and a cathode on the contralesional hemisphere. Lindenberg and colleagues investigated the effects dual tDCS in combination with physical and occupational therapy on motor function post-stroke. Participants received either active or sham bihemispheric tDCS (1.5mA, 30min) in conjunction with physical/occupational therapy (60min) for five consecutive sessions. The active tDCS group had significantly greater upper extremity motor function improvement (based on Fugyl-Meyer and Wolf Motor Function Test scores) in comparison to sham (Lindenberg et al., 2010). Currently, the majority of the studies investigating tDCS in adult stroke involve upper motor function, with only a small number assessing lower limb function and gait. Klomjai and colleagues sought to study the effects of a single dual tDCS session on lower limb motor function. Participants received active dual tDCS (2mA, 20min) before conventional physical therapy treatment. The results demonstrate that dual tDCS for a single session prior to physical therapy improved lower limb function but did not increase strength performance (Klomjai et al., 2018). Overall, tDCS has shown promise in the adult population but more research is needed to investigate the long-term effects of this intervention.
2.3. Vagus nerve stimulation (VNS) for adult stroke
Vagus nerve stimulation is a form of cranial nerve stimulation using electrodes either implanted on the left cervical bundle of the vagus nerve in the neck (conventional VNS) (George et al., 2000) or administered transcutaneously to the auricular branch of the vagus nerve that innervates the ears (taVNS) (Badran et al., 2017, 2018a, 2019). VNS indirectly activates the central nervous system, via afferent signals transmitted to the brain stem, which subsequently activate nucleus tractus solitarius (NTS). The NTS projects to the locus coeruleus (LC), another key brainstem structure which is responsible for the production of norepinephrine (NE) in the brain (Berridge and Waterhouse, 2003). This vagal-mediated release of NE projects diffusely to many key cortical (motor, sensory, prefrontal) and subcortical (thalamus, hippocampus) structures to facilitate neuroplasticity. Neuromodulators acetylcholine (Orsetti et al., 1996) and norepinephrine are released during taVNS as well as with attentional processing of a motor task to boost neuroplasticity (McIntyre et al., 2002). This increase in neuroplasticity facilitates cortical reorganization and the repair of aberrant neurological processes post-brain injury.
Animal models have demonstrated that pairing VNS with a motor or sensory experience can result in cortical reorganization that can provide beneficial effects in chronic tinnitus, stroke and posttraumatic stress disorder by driving specific forms of cortical plasticity (Hays et al., 2013). In stroke, researchers sought to determine if VNS paired with physical rehabilitation post-stroke would enhance plasticity in the motor cortex. Khodaparast and colleagues administered rehabilitative training either with or without VNS to adult rats after ischemic stroke. The VNS paired group showed full recovery of a previously learned motor task, as well as improved performance compared to rehabilitative training without VNS (Khodaparast et al., 2013, 2014). In another study by the same group, rodents who received VNS in conjunction with rehabilitation therapy post-stroke, showed greater forelimb strength on the isometric forelimb task compared to those with rehabilitation alone (Khodaparast et al., 2013, 2014). These results demonstrate that pairing VNS with rehabilitation training can restore motor function following brain injury in animals.
These animal model findings are currently being translated into the human stroke population, pairing rehabilitation training with a neuroplasticity-enhancing intervention such as VNS or taVNS to restore motor function (Baig et al., 2019; Capone et al., 2017; Redgrave et al., 2018). Twenty-one adult participants with ischemic stroke were enrolled and were randomized to receive cervically implanted VNS paired with rehabilitation therapy or rehabilitation therapy alone (without VNS). 0.5s VNS was delivered during movement with a current intensity of 0.8mA, frequency 30Hz, 100-μs pulse width. This study demonstrated that VNS is safe and feasible in adults with chronic stroke and there were no significant differences between groups in regard to upper limb motor function assessments (Dawson et al., 2016). Although there were no serious adverse device events in this study, VNS did not boost the effects of rehabilitation training and is a surgically form of stimulation making it less appealing.
A noninvasive alternative to VNS, taVNS, has been developed and is currently being investigated in the adult stroke population. In 2017, Capone and colleagues were the first researchers to investigate the safety and feasibility of taVNS in 14 adult participants with ischemic or hemorrhagic chronic stroke. Participants were randomized to robot-assisted therapy with active or sham taVNS delivered for 10 days. Participants received taVNS for 60min with a current intensity below pain threshold (1.1–9.0mA, 30s trains, 20Hz, pulse width: 300-μs). This study demonstrated that taVNS is feasible in stroke participants and may produce minor clinical improvement in arm motor function when combined with robotic assisted therapy (Capone et al., 2017). Another study also assessed taVNS (18, 60min sessions over 6 weeks) in conjunction with upper limb repetitive task practice in 13 adult stroke participants and concluded that taVNS is feasible and well tolerated with motor improvements, justifying a phase 2 clinical trial (Redgrave et al., 2018). taVNS is a promising, emerging facilitator of neuroplasticity and novel application of NIBS in stroke.
3. NIBS applications in pediatrics
NIBS has demonstrated early efficacy in the adult stroke population, but it is still novel in the field of pediatrics. Translation of stimulation techniques to a pediatric population may provide life-long benefits to this vulnerable group who otherwise have limited treatment options. Two specific rehabilitation protocols, pediatric Constraint-Induced Movement Therapy (P-CIMT) and Hand Arm Bimanual Intensive Therapy (HABIT), have demonstrated clinically meaningful and sustained benefits across multiple investigations with high levels of scientific evidence for children with CP (DeLuca et al., 2017; Gordon et al., 2011; Novak et al., 2013; Ramey et al., 2013) and emerging evidence in infants (PMID: 29175749). While protocols vary, the most efficacious forms of both P-CIMT and HABIT are usually reported at dosage levels involving between 60 and 120 treatment hours. These high-dosage therapy hours are then provided within a condensed time period, usually 4 weeks or less. There is substantial evidence that intensive therapeutic bursts (e.g., many hours each day on multiple consecutive days across multiple weeks) (Gordon et al., 2011; Ramey et al., 2013) create optimal opportunity for the development of increased motor and functional skills in children with hemiparesis (Novak et al., 2013). However, these intensive therapy models are difficult to implement in today’s rehabilitation environment since the families and children must participate in hours of intensive therapy daily over several weeks and the interventions are costly. These types of intensive therapies may interrupt normal family routines due to parents having other responsibilities during the day and may impact families financially.
Early brain injury, such as that incurred by a brain bleed in utero, may lead to the development of cerebral palsy (CP) which is characterized by a reduction in subcortical activity leading to diminished corticospinal and somatosensory circuit activity (Kurz and Wilson, 2011; Rose et al., 2011). Reductions in motor cortex excitability in children cause a variety of impairments in motor development and organization (Pitcher et al., 2012), and depending on the location of the brain lesion, different motor skills may be impacted causing life-long disability. Changes in muscle tone, increased spasticity, compromised gait, poor balance responses, altered tactile, proprioceptive and kinesthetic awareness are among the most common motor deficits in children with CP (Kurz and Wilson, 2011; Rosenbaum et al., 2007). Currently, the treatment options for CP are limited with the main forms of therapy for being intensive physical rehabilitation in the form of pediatric CIMT and bimanual therapy such as HABIT (Deluca et al., 2017; Gordon et al., 2011; Novak et al., 2013; Ramey et al., 2013).
While this is no cure for CP, emerging evidence indicates that brain stimulation could potentially help minimize motor impairments in children with CP by promoting the activation of the primary motor cortex (Dinomais et al., 2013) leading to positive effects on both immediate and long-term motor function. There is a higher propensity for neuroplasticity in the developing brain (Cioni et al., 2011; Hadders-Algra, 2001; MÜller et al., 1997) allowing for NIBS to accelerate rehabilitation in children. However, there is limited literature on administering NIBS in the developing brain (Davis, 2014; Gillick et al., 2014; Kowalski et al., 2019; Nemanich et al., 2019) and modulating cortical excitability in pediatric participants may require stimulation protocols that are distinct from adult protocols (Froemke, 2015; Gillick et al., 2014; Hameed et al., 2017; Kowalski et al., 2019; Nemanich et al., 2019; Takesian and Hensch, 2013).
Safety is paramount in using NIBS in pediatric populations. Prior research has indicated that NIBS modalities are generally safe and well tolerated in the pediatric population with minimal side effects. Elbanna and colleagues reviewed 14 RCTs using NIBS (10 tDCS, 4 rTMS) in pediatrics and report no cases where participants stopped stimulation sessions due to adverse events in 306 children (Elbanna et al., 2019). Furthermore, there were no participants with hemiparesis who had deterioration on the less-affected extremities after receiving NIBS therapy.
This portion of the chapter will discuss the safety, feasibility and potential efficacy of using NIBS to treat motor dysfunction following early pediatric brain injury, specifically children with CP. NIBS is a rapidly evolving field and currently TMS and tDCS are most actively being studied in children with brain injury. The majority of NIBS applications in pediatrics utilize neuromodulation to improve functional deficits resultant from such pediatric brain injury—such as upper and lower limb function, spasticity, gait and balance. Often NIBS is applied in conjunction with rehabilitative therapy such as P-CIMT, virtual reality mobility training and treadmill training. These NIBS-augmented therapies vary in length, ranging from a single treatment session up to 10 treatment session. Sessions last approximately 20min and utilize either TMS, tDCS, or VNS.
Table 1 highlights NIBS studies in early pediatric brain injury over the past decade.
Table 1.
Summary of study methods and outcomes for NIBS intervention in early pediatric brain injury.
| Authors, Year (References) | Title | Study design | Participant population | Age range | Subjects | NIBS intervention | Parameters | Functional outcomes |
|---|---|---|---|---|---|---|---|---|
| TMS | ||||||||
| Valle et al. (2007) | Low- and high-frequency repetitive transcranial magnetic stimulation for the treatment of spasticity | Randomized, double-blind, sham-controlled, parallel design clinical trial | Cerebral palsy and spastic quadriplegia | 5–18 years old | N = 17 | Active rTMS at 1Hz or 5Hz | Frequency: 1Hz or 5Hz, intensity: 90% of the motor threshold; duration: 5 consecutive days | Significant reduction of spasticity after 5Hz rTMS only, not sham or 1Hz rTMS as indexed by the degree of passive movement; this was not evident when using the Ashworth scale, although a trend for improvement was seen for elbow movement |
| Kirton et al. (2008a) | Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical pediatric stroke: a randomized trial | Randomized Clinical Trial | Arterial Ischemic stroke (AIS) | 8–20 years old | N = 10 | Low-frequency rTMS over contralesional motor cortex or sham treatment | Frequency: 1Hz, intensity: 100% resting motor threshold duration: one 20min session per day for 8 days | Safe and feasible for participants with AIS, and grip strength improved in rTMS group and persisted until day 17. MAUEF scores improved by more in rTMS group than in sham but did not persist at day 17 |
| Gillick et al. (2015) | Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis | Randomized, double-blinded, placebo-controlled pretest/posttest trial | Congenital hemiparesis caused by ischemic stroke or periventricular leukomalacia | 8–17years old | N = 19 | Low-frequency rTMS in contralesional hemisphere + mCIMT or sham rTMS + mCIMT | Frequency: Priming 6Hz intensity: 90% RMT for 10min followed by 1Hz at 90% RMT for 10min, duration: 5 treatments of real rTMS and 5 treatments of mCIMT on alternate weekdays over 2 weeks | Demonstrated rTMS can be used safely in participants with congenital hemiparesis in combination with mCIMT. No differences between groups in secondary cognitive and unaffected hand motor measures |
| Kirton et al. (2016) | Brain stimulation and constraint for perinatal stroke hemiparesis The PLASTIC CHAMPS Trial | Factorial-design, blinded, randomized controlled trial | Hemiparesis with MRI-confirmed perinatal stroke | 6–19 years old | N = 45 | Randomized to daily rTMS, CIMT, both, or neither along with 2-week, goal-directed, peer-supported motor learning camp | Frequency: 1Hz, intensity: 90% motor threshold duration: 20min daily for 2 weeks | Class II evidence that combined rTMS and CIMT enhance therapy-induced functional motor gains in children with stroke-induced hemiparetic cerebral palsy |
| Nemanich et al. (2019) | Safety and feasibility of transcranial magnetic stimulation as an exploratory assessment of corticospinal connectivity in infants after perinatal brain injury: an observational study | Pilot, cross sectional study | Infants with perinatal stroke or intracranial hemorrhage | 3–12 months old (corrected age) | N = 6 | Single-pulse TMS applied to each hemisphere | 14–37 single TMS pulses and MEP amplitudes ranged from 50 to 200 μV | Demonstrated safety and feasibility of TMS in infants with perinatal brain injury. Contralateral motor evoked responses observed in 4/6 infants and both contralateral and ipsilateral responses observed in 2/6 infants |
| Kowalski et al. (2019) | Motor evoked potentials as potential biomarkers of early atypical corticospinal tract development in infants with perinatal stroke | Pilot, cross sectional study | Infants with perinatal stroke | 3–12 months old | N = 10 | Single-pulse TMS applied to each hemisphere | Intensity: 50–90% maximum stimulator output (MSO) | Younger infants were more likely to have an MEP from the more affected hemisphere (MAH) compared to older infants, and absence of MEPs from MAH was associated with atypical movement. Suggests an influence of age on MEP presentation from the MAH |
| Aree-Uea et al. (2014) | Reduction of spasticity in cerebral palsy by anodal transcranial direct current stimulation | RCT | Spastic cerebral palsy | 8–18 years old | N = 46 | Anodal tDCS left primary motor cortex. Both groups received routine physical therapy | Current intensity: 1.0mA, duration: 20min daily sessions for 5 consecutive days | Reduction in CP-related spasticity (but not passive range of motion) in the short term. |
| Duarte et al. (2014) | Effect of transcranial direct current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial | Double-blind RCT | Cerebral palsy | 5–12 years old | N = 24 | Anodal tDCS primary motor cortex + treadmill training (experimental group) placebo tDCS + treadmill training (control group) | Current intensity: 1.0mA, duration: 5 weekly 20min sessions for 2 weeks | Experimental group had improvements in anteroposterior sway, mediolateral sway, and the Pediatric Balance Scale compared to control group both 1 week and 1 month after the completion of the protocol |
| Grecco et al. (2014) | Effect of a single session of transcranial direct current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: A randomized sham-controlled study | Randomized Sham-controlled study | Hemiparetic or diparetic cerebral palsy | 6–10 years old | N = 20 | Anodal tDCS primary motor cortex (experimental group) or placebo transcranial stimulation (control group) | Current intensity: 1.0mA, duration: Single 20min session | tDCS group had significant reductions in sway during standing in the anteroposterior and mediolateral directions with eyes open and eyes closed compared to the control group. Researchers conclude there are positive changes in static balance and gait velocity after single session of tDCS. Increases in gait velocity were not maintained for more than 20min after the end of stimulation |
| Grecco et al. (2014) | Transcranial direct current stimulation during treadmill training in children with cerebral palsy: A randomized controlled double-blind clinical trial | Double-blind RCT | Spastic diparetic cerebral palsy | 5–12 years old | N = 24 | Anodal tDCS over primary motor cortex in dominant hemisphere+treadmill training (experimental group) control group: placebo tDCS + treadmill training | Current intensity: 1.0mA, duration: 5 weekly 20min sessions for 2 weeks | Experimental group exhibited improvements in temporal functional mobility, gait variables (spatiotemporal and kinematics variables) with results maintaining 1 month after intervention. Cortical excitability was also measured in participants using TMS and improvements were found in MEP in experimental group |
| Lazzari et al. (2015) | Effect of a single session of transcranial direct current stimulation combined with virtual reality training on the balance of children with cerebral palsy: a randomized, controlled, double-blind trial | Double-blind RCT | Cerebral palsy | 4–12 years old | N = 12 | Anodal tDCS over primary motor cortex combined with mobility virtual reality training (experimental group) or placebo tDCS with mobility virtual reality training | Current intensity: 1.0mA, duration: Single 20min session | Only significant difference found-an increase in body sway velocity was found in children after a single session of tDCS |
| Collange Grecco et al. (2015) | Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: a pilot, randomized, controlled, double-blind, clinical trial | Pilot controlled RCT | Diparetic cerebral palsy | 5–10 years old | N = 20 | Anodal tDCS + virtual reality for gait training (experimental group) control group: placebo tDCS + virtual reality for gait training | Current intensity: 1.0mA, duration: 5 weekly 20min sessions for 2 weeks | tDCS led to significant increase in motor evoked potential. Experimental group had better performance in gait velocity, cadence, gross motor function, and independent mobility |
| Kirton et al. (2017) | Transcranial direct current stimulation for children with perinatal stroke and hemiparesis | Double-blind RCT | MRI-classified unilateral perinatal stroke and disabling hemiparesis | 6–18 years old | N = 24 | Contralesional M1 Cathodal tDCS during initial 20min of motor learning therapy (experimental) or sham tDCS during initial 20min of motor learning therapy (control group). Motor learning therapy lasted 2 weeks. | Current intensity: 1.0mA, duration: 20min daily for 2 weeks | tDCS is safe and feasible in hemiparetic children. No significant effects of tDCS on objective motor function but gains in subjective function. May reflect underdosing |
| Auvichayapat et al. (2017) | Transient Changes in Brain Metabolites after Transcranial Direct Current Stimulation in Spastic Cerebral Palsy: A Pilot Study | Pilot study | Spastic hemiparetic or diparetic cerebral palsy | 8–12 years old | N = 10 | Anodal tDCS left primary motor cortex | Current intensity:1.0mA, duration: 5 20min sessions | tDCS appeared to reduce the degree of spasticity and improve hand function. An increase N-acetyl aspartate (NAA), choline (Cho), myoinositol (mL), and glutamine-glutamate (Glx) was found |
| Grecco et al. (2017) | Cerebellar transcranial direct current stimulation in children with ataxic cerebral palsy: A sham-controlled, crossover, pilot study | Single blind-sham controlled, crossover, pilot study | Ataxic cerebral palsy with balance deficit | 5–11 years old | N = 6 | Anodal tDCS of cerebellar region + treadmill training (experimental group) sham tDCS + treadmill training (control group) | Current intensity: 1.0mA, duration: 5 weekly 20min sessions for 2 weeks | Reductions in oscillations of center of pressure in the anteroposterior and mediolateral directions with eyes closed 1 week and 1 month after the cessation of the intervention in active anodal tDCS only. Functional balance and functional performance in mobility from treadmill training were only maintained on month after in active tDCS group only. |
| Lazzari et al. (2017) | Effect of transcranial direct current stimulation combined with virtual reality training on balance in children with cerebral palsy: a randomized, controlled, double-blind, clinical trial | Double-blind RCT | Cerebral palsy | 4–12 years old | N = 20 | Anodal tDCS + virtual reality mobility training (experimental group) control group: placebo tDCS + virtual reality mobility training | Current intensity:1.0mA, duration: 5 weekly 20min sessions for 2 weeks | Significant postintervention and follow-up effects favoring experimental group over control group in regard to static and functional balance |
| Moura et al. (2017) | Effects of a single session of transcranial direct current stimulation on upper limb movements in children with cerebral palsy: A randomized, sham-controlled study | Randomized Sham-controlled study | Cerebral palsy | 6–12 years old | N = 20 | Anodal tDCS of primary motor cortex of the hemisphere ipsilateral to the brain lesion + functional training of paretic upper limb (experimental group) or sham tDCS functional training of paretic upper limb (control group) | Current intensity: 1.0mA, duration: Single 20min session | Reductions in total movement duration and returning movement duration were found in both the paretic and non-paretic limbs in active tDCS group only |
| Gillick et al. (2018) | Transcranial direct current stimulation and constraint-induced therapy in cerebral palsy: a randomized, blinded, sham-controlled clinical trial | Randomized, blinded, sham-controlled clinical trial | Unilateral cerebral palsy | 7–21 years old | N = 20 | Cathodal tDCS applied to contralesional M1 combined with constraint-induced movement therapy (CIMT) (experimental) or sham tDCS +CIMT (control) | Current intensity: 0.7mA, duration: 10 consecutive weekday 20min sessions | Study shows safety and feasibility of serial sessions of tDCS. Both active and sham groups improved in the Assisting Hand Assessment (AHA) with no significant effect of tDCS |
| Nemanich et al. (2019) | Influence of combined transcranial direct current stimulation and motor training on corticospinal excitability in children with unilateral cerebral palsy | Part of Gillick et al. (2018) RCT | Unilateral cerebral palsy | 7–21 years old | N = 20 | Active tDCS + CIMT or sham + CIMT. tDCS cathode positioned from TMS-derived motor hot spot. Cortical excitability was also assessed using single-pulse TMS. | Current intensity: 0.7mA, duration: 10 consecutive weekday 20min sessions | Reductions in contralesional M1 MEP amplitude in the Active + CIMT compared to Sham + CIMT group-no significant MEP amplitude differences between groups. Changes in corticospinal excitability were not statistically associated with improvements in hand function after the intervention |
| taVNS | ||||||||
| Badran et al. (2020) | Transcutaneous auricular vagus nerve stimulation-paired rehabilitation for oromotor feeding problems in newborns: an open-label pilot study | Open label pilot | Premature/Hypoxic-ischemic encephalopathy (HIE) infants | premature infants at ≤33 weeks gestational age and term infants suffering from HIE | N = 14 | taVNS paired with bottle feeding rehabilitation | Frequency: 25Hz, pulse width: 500 μs, and current intensity: 0.1mA below perceptual threshold (PT), duration: 30min per day for 2 weeks | Demonstrated safety and feasibility of taVNS in infants. Discharge without G-tube, adequate oral feeding volumes and weight gain. 8/14 infants attained full oral feeds and were discharged without a G-tube |
3.1. TMS safety and clinical utility in pediatrics
For TMS interventions, researchers have demonstrated safety and good tolerability in children, and the associated risks are minimal (Garvey and Gilbert, 2004; Gilbert et al., 2004; Krishnan et al., 2015; Quintana, 2005) (Rajapakse and Kirton, 2013). In a study of repetitive TMS (rTMS) in children with arterial ischemic stroke (AIS), two participants reported mild headaches that were self-resolving, one participant reported mild nausea and neck stiffness, and two participants experience neurocardiogenic syncope. The participants mean tolerability scores did not differ between sham and rTMS (Kirton et al., 2008a). Hearing impairment resulting from TMS has also been a concern for the pediatric population, but researchers studied auditory effects in children ages 2 months to 16 years of age before and after TMS with no hearing protection and did not find any abnormalities (Miguel Angel et al., 2001). Neurocardiogenic syncope has also been identified as a preventable adverse event from rTMS, and strategies to mitigate this effect include screening for predisposition to neurocardiogenic syncope, implementing precautions for hydration, food intake and blood pressure, and disclosing the risk of neurocardiogenic syncope during the consent process (Kirton et al., 2008b).
Gillick and colleagues also assessed the safety and feasibility of primed rTMS and Modified Constraint-Induced Movement Therapy (mCIMT) in participants with congenital hemiparesis. The stimulation was well tolerated and there was no worsening of motor function. Minor adverse events reported included headaches and cast irritation (Gillick et al., 2015). As far as TMS dosage for pediatric participants, it has been found that children, especially those under age 6, have higher motor thresholds compared to adolescents and adults (Garvey and Gilbert, 2004). rTMS parameters in the pediatric stroke population have ranged from 1 to 6Hz, 90–100% motor threshold for 5–20min (Table 1). In addition, noninvasive neuromodulation has been used on infants successfully and safely in recent studies. Reseachers have demonstrated that single-pulse TMS is safe and feasible in infants between ages 3–12months (Kowalski et al., 2019; Nemanich et al., 2019). No adverse events were reported during stimulation and all sessions were well tolerated. Furthermore, in a recent review on the use of noninvasive neuromodulation in improving motor skills for perinatal stroke, the authors recommended using a combination of manual therapy with neurostimulation in early infancy in order to implement this intervention during a critical period of development (Hilderley et al., 2019).
The ability of rTMS to modulate neural networks in the adult stroke population to promote motor function (Jin et al., 2002; Mansur et al., 2005; Takeuchi et al., 2005), are now being translated into a pediatric population. In one of the earliest rTMS studies in pediatrics, Valle studied low and high-frequency rTMS in a randomized, double-blind, sham-controlled, parallel design clinical trial in 17 children ages 5–18 with CP. For the active rTMS groups, stimulation was delivered in five 1-min trains at 1 or 5Hz with an intensity of 90% of the motor threshold for 5 consecutive days. The results demonstrate that there was a significant reduction of spasticity after 5Hz rTMS only, as measured by the degree of passive movement, but not by the Ashworth scale, although a trend for improvement was seen for elbow movement which was not seen with 1Hz rTMS or in sham rTMS (Valle et al., 2007). In a subsequent randomized clinical trial, Kirton and colleagues investigated the use of low-frequency rTMS (LF-rTMS) in children and adolescents ages 8–20 with subcortical arterial ischemic stroke (AIS) and hemiparesis. rTMS was applied at 1Hz with an intensity of 100% motor threshold for 20min per daily session over the contralesional primary motor cortex for 8 days. This study included 10 children and demonstrated safety and feasibility of rTMS in children with AIS, and suggested functional improvements (Kirton et al., 2008a). The rTMS group demonstrated improved grip strength (persisting to day 17) and Melbourne assessment scores (not persisting at day 17) in comparison to sham. Though preliminary, hand function appeared to have improved after rTMS intervention (Kirton et al., 2008a). Later, Gillick and colleagues reported significant improvements in hand function in a randomized controlled combined rTMS/CIMT study using LF-rTMS to the contralesional hemisphere in 19 children with perinatal stroke and resultant hemiparetic CP (Gillick et al., 2015).
In 2016, Kirton studied LF-rTMS in children ages 6–19 with hemiparesis due to MRI-confirmed perinatal stroke. The 45 children were randomized to daily rTMS, CIMT, both, or neither in a 2-week goal-directed, peer-supported motor learning camp. The rTMS group received 1Hz with an intensity of 90% motor threshold 20min daily for 2 weeks. The results demonstrate that children who received rTMS, CIMT or the combination of the two had twice the chance of significant clinical improvement, with children receiving both having the most gains on the Assisting Hand Assessment at 6 months. This study provides Class II evidence that rTMS and CIMT in combination promote therapy-induced functional motor gains in children with hemiparesis (Kirton et al., 2016). In summary, rTMS has been proven to be safe and well tolerated, with some hints of efficacy in the pediatric population, but more large-scale clinical trials are needed.
3.2. tDCS safety and clinical utility in pediatrics
tDCS is a safe and approachable form of NIBS for the pediatric population due to its simple and portable administration (Bikson et al., 2016) (Palm et al., 2016). However, infants and children have smaller skull thickness and corticospinal fluid volume, which needs to be taken into account when calculating optimized dosage (Beauchamp et al., 2011; Brain Development Cooperative Group, 2012). Early trials in children used an intensity comparable to adults, with no safety concerns reported. However, subsequent work using computational models based on anatomical MRI that incorporate subject-specific anatomy indicate that on average the current density in children brain is higher than average adults. This in turn suggests applying lower current in children (~1mA) may produce similar voltage changes in the cortex as an adult receiving a dose of 2mA (Kessler et al., 2013; Minhas et al., 2012). For example, one study used computer modeling to assess the induced electric field (e-field) of 0.7mA tDCS in the cortex of a 10-year old stroke participant. To induce a similar e-field in an adult would require 43% more current, or 1.0mA (Gillick et al., 2014).
This variance in dosimetry is consistent with neurophysiological studies in children. In adults, increasing cathodal intensity from −1 to −2mA, changes the resulting neuromodulation from inhibitory to excitatory. In children, this reversal happened from −0.5 to −1mA cathodal (Moliadze et al., 2015), which is precisely consistent with the same current producing more brain current density in children (Kessler et al., 2013). This biphasic response was not evident in anodal tDCS, where 0.5mA was not sufficient to produce the expected increase in MEP. 1.0mA current for anodal tDCS remains consistent with the adult tDCS parameters (Moliadze et al., 2015). Generally, this reinforces the value of considering tDCS dosing specifically for children.
Researchers have studied the use of both cathodal (inhibitory) and anodal (excitatory) in children with acquired brain injury, specifically children with resultant CP (Fleming et al., 2018). In 2018, Fleming and colleagues reviewed studies investigating the use of tDCS in children and adolescents with CP, and found that studies involving anodal tDCS in regard to lower limb function are promising, but upper limb function studies have mixed results (Fleming et al., 2018). Grecco and colleagues investigated the effect of a single 20min session of anodal tDCS (1mA) on lower limb function (Grecco et al., 2014). In the active group, tDCS was applied over the dominant hemisphere in children ages 6–10years old with hemiparetic or diparetic CP and researchers assessed the effects on balance and gait. Significant reductions in sway were found in the active tDCS group compared to sham; increases in gait velocity were also found but were not maintained for more than 20min after the end of stimulation (Grecco et al., 2014). Lazzari et al. (2015) also investigated the effects of a single session (20min) of anodal tDCS (1mA) in conjunction with virtual reality mobility training in children with CP. The outcomes of this study were not as encouraging, with an increase in sway velocity being found in the active tDCS group which could possibility be due to fatigue causing a deterioration in balance (Fleming et al., 2018; Lazzari et al., 2015).
Combining tDCS with motor training has also emerged as a pediatric therapy approach. Three studies assessed the effect of multiple tDCS sessions combined with either treadmill training or virtual reality training on lower limb function in children with CP (Collange Grecco et al., 2015; Duarte et al., 2014; Lazzari et al., 2017). Results demonstrate through within-group comparisons by Fleming’s 2018 review that participants in the active tDCS had improvements in balance based on the Pediatric Balance Scale (PBS) and Pediatric Evaluation Disability Inventory (PEDI-subjective measure) (Fleming et al., 2018). In addition, at the 1 month follow-up the active tDCS group had improvements in the PBS and lower sway based on between-group comparisons (Fleming et al., 2018). Furthermore, Collange Grecco’s studied explored motor evoked potential (MEP) induced by TMS and reported an increase in amplitude after 10 days of anodal tDCS (Collange Grecco et al., 2015).
Moura and colleagues investigated the effects of a single 20min anodal tDCS session (1mA) over the primary motor cortex of the hemisphere ipsilateral to the brain lesion in conjunction with functional training of the paretic upper limb. Results demonstrate reductions in total movement duration and returning movement duration during reaching in both paretic and non-paretic limbs in the active tDCS group only (Moura et al., 2017). Aree-Uea assessed the effect of anodal tDCS over the left primary motor cortex on spasticity in children aged 8–18years old with Spastic CP. Children also received routine physical therapy in both the active tDCS and sham group. tDCS was delivered at 1mA for 5 consecutive days and the results demonstrate that tDCS appeared to reduce CP-related spasticity in the short term in the shoulder, elbow, wrist and fingers (Aree-Uea et al., 2014).
Cathodal tDCS has also been investigated in two randomized controlled trials in combination with motor learning therapy and CIMT in children with CP. In one study, Gillick and colleagues applied cathodal tDCS (0.7mA) for 20min over the contralesional M1 in combination with CIMT for 10 sessions. Both active and sham groups improved in the Assisting Hand Assessment (AHA) with no significant effect of tDCS (Gillick et al., 2018). However, uniquely those children with retained crossed-corticospinal tract excitability or ‘contralateral circuitry’ performed better than those without “ipsilateral circuitry” (Gillick et al., 2018). The improvements were demonstrated independent of stimulation condition. Another study by Kirton and colleagues also investigated the effect of 10 sessions of cathodal tDCS over contralesional M1 in combination with motor learning therapy (Kirton et al., 2016). The results of this study found that children improved on the subjective self-report measure (Canadian Occupational Performance Measure) for the active tDCS group, but no significant effects on objective motor function were found (Kirton et al., 2017). These results of cathodal tDCS studies may reflect a dosage issue as discussed in previous section.
In summary, anodal tDCS appears to improve spasticity in children with CP, and researchers need to investigate tDCS effects on motor function further because the current data does not indicate definite improvements from tDCS.
3.3. taVNS safety and clinical unitility in pediatrics
Recently transcutaneous auricular vagus nerve stimulation (taVNS) has shown promise as a safe form of NIBS with limited data on taVNS in infants. taVNS was tested in over 600 sessions in 31 different babies for feeding dysfunction with no serious adverse events due to stimulation (Badran et al., 2020). Auricular neurostimulation is also being tested in neonates with opioid withdrawal. Adverse events are minimal, and transient skin redness has not been observed. Rapid decrease in heart rate accompanies the onset of stimulation with equally rapid rebound (Badran et al., 2018b). In addition, discomfort levels are monitored pre and post stimulation using the Neonatal Infant Pain Scale (NIPS) and were not significantly increased with stimulation.
Given its novelty, taVNS has the least amount of evidence in pediatrics, however it is rapidly being explored in newborn infants using the targeted-plasticity paradigm which pairs stimulation with motor rehabilitation to improve motor function. Researchers at the Medical University of South Carolina have begun investigating whether taVNS may help improve bottle suck behavior in newborns slated to receive a gastrostomy tube. Aree-Uea et al. (2014) and Jenkins paired taVNS with bottle feeding in 14 premature infants and term infants suffering from HIE with feeding dysfunction. taVNS-paired feeding was delivered for 30min per day for 2 weeks (frequency: 25Hz, pulse width: 500μs, current intensity: 0.1mA below perceptual threshold) in combination with bottle feeding rehabilitation (Badran et al., 2020). Eight out of the 14 infants enrolled in this study attained full oral feed volumes and were discharged from the hospital without a gastrostomy tube (Badran et al., 2020). These findings reveal preliminary evidence suggests that taVNS has positive benefits on improving motor coordination with feeding, as well as demonstrates safety and feasibility in utilizing taVNS in this population.
4. Practical considerations of NIBS in pediatrics
There is limited but growing data supporting the use of NIBS in pediatrics across ages, with methods that need further optimization and refinement. Furthermore, the majority of the studies utilized NIBS to treat motor and sensory impairments in children with cerebral palsy (CP). Further work is indicated before these methods can be fully translated to children and infants. Differences in size, connectivity, maturity, and organization of the developing brain must be taken into account when developing NIBS for pediatrics.
These differences also raise some practical considerations when applying NIBS in a pediatric population. Regarding TMS, researchers have expressed concerns about using an adult size head coil in children due to their smaller head circumference. This may only be important for children under 6 years of age though because after that brain volume remains similar (Rajapakse and Kirton, 2013). The magnetic field induced by the adult figure-8 coils is approximately the size of a silver dollar, and this field may be too broad when administering TMS in pediatrics. This may cause off-target effects in nearby cortical structures. In addition, TMS dosage needs to be established as discussed previously. Currently adults receive a titration of stimulation intensity as a scalar multiplier of motor threshold. This dosing titration is yet to be optimized for the pediatric population.
Stimulation systems for tDCS are generally inexpensive and offer flexibility in treatment, as they may be used at home, which is particularly appealing in pediatrics.
taVNS involves electrical stimulation of the ear, which in pediatrics is a small anatomical target that requires custom ear electrodes fabricated for this application. Electrodes often are less than 5mm in diameter and require considerations for adhesion and fit that make this technique particularly difficult to administer in untrained individuals. Ensuring electrodes maintain proper contact with the ear throughout treatment is also a monitoring consideration as it is difficult to maintain positioning in the newborn population that is moving or irritated. The electrode-skin interface in this population may also pose irritation risk that should be actively monitored.
As with any medical procedure, all NIBS administration in pediatrics require the proper training and clinical team to supervise and ensure safety of all participants. In-person hands-on courses for NIBS training are offered at several major academic institutions throughout the United States. With proper training and equipment, TMS, tDCS and taVNS have very promising therapeutic potential and practicality for infants and children with acquired brain injury. Ultimately larger scale multi-site trials are needed to further warrant the use of NIBS in pediatric populations.
5. Conclusions and future directions
As demonstrated in this chapter and in the studies listed in Table 1, NIBS appear to be safe and feasible in children who have incurred early injury to the brain with resultant motor impairments such as those with CP. Furthermore, NIBS may improve functional disabilities, modulate motor cortical excitability, and improve quality of life in these patients. The current research has demonstrated that NIBS in combination with other rehabilitative interventions is likely more effective than either alone in adults. There is clear need for large-scale longitudinal clinical trials investigating the use of NIBS in the pediatric population. In conclusion, NIBS appears to be effective and could become a promising future treatment option for infants and children with brain injury in the future.
References
- Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, Bremner JD, Badran BW, Napadow V, Clark VP, Bikson M, 2020. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul. 13, 717–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami RR, Grundy ME, Poretti A, Felling RJ, Lemmon M, Graham EM, 2016. Distinguishing arterial ischemic stroke from hypoxic–ischemic encephalopathy in the neonate at birth. Obstet. Gynecol 128, 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agboada D, Samani MM, Jamil A, Kuo M-F, Nitsche MA, 2019. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci. Rep 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT, 1997. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch. Neurol 54, 443–446. [DOI] [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, Cohen L, Dowthwaite G, Ellrich J, Flöel A, 2017. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol 128, 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aree-Uea B, Auvichayapat N, Janyacharoen T, Siritaratiwat W, Amatachaya A, Prasertnoo J, Tunkamnerdthai O, Thinkhamrop B, Jensen MP, Auvichayapat P, 2014. Reduction of spasticity in cerebral palsy by anodal transcranial direct current stimulation. J. Med. Assoc. Thail 97, 954–962. [PubMed] [Google Scholar]
- Auvichayapat P, Aree-Uea B, Auvichayapat N, Phuttharak W, Janyacharoen T, Tunkamnerdthai O, Boonphongsathian W, Ngernyam N, Keeratitanont K, 2017. Transient changes in brain metabolites after transcranial direct current stimulation in spastic cerebral palsy: a pilot study. Front. Neurol 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran B, Glusman C, Badran A, Austelle C, Devries W, Borckhardt J, George M, 2017. The physiological and neurobiological effects of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul. 10, 378. [Google Scholar]
- Badran BW, Brown JC, Dowdle LT, Mithoefer OJ, Labate NT, Coatsworth J, Devries WH, Austelle CW, Mcteague LM, Yu A, 2018a. Tragus or cymba conchae? Investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul. 11, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Mithoefer OJ, Summer CE, Labate NT, Glusman CE, Badran AW, Devries WH, Summers PM, Austelle CW, Mcteague LM, 2018b. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 11, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Alfred BY, Adair D, Mappin G, Devries WH, Jenkins DD, George MS, Bikson M, 2019. Laboratory administration of transcutaneous auricular vagus nerve stimulation (taVNS): technique, targeting, and considerations. J. Vis. Exp 143, e58984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Jenkins DD, Cook D, Thompson S, Dancy M, Devries WH, Mappin G, Summers P, Bikson M, George MS, 2020. Transcutaneous auricular vagus nerve stimulation-paired rehabilitation for oromotor feeding problems in newborns: an open-label pilot study. Front. Hum. Neurosci 14, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr Hosseini M, Hou J, Bikson M, Iacoboni M, Gornbein J, Saver JL, 2019. Central nervous system electrical stimulation for neuroprotection in acute cerebral ischemia: meta-analysis of preclinical studies. Stroke 50, 2892–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig SS, Falidas K, Laud PJ, Snowdon N, Farooq MU, Ali A, Majid A, Redgrave JN, 2019. Transcutaneous auricular vagus nerve stimulation with upper limb repetitive task practice may improve sensory recovery in chronic stroke. J. Stroke Cerebrovasc. Dis 28, 104348. [DOI] [PubMed] [Google Scholar]
- Bastani A, Jaberzadeh S, 2012. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin. Neurophysiol 123, 644–657. [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche M, 2013. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol 591, 1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Beurlot MR, Fava E, Nath AR, Parikh NA, Saad ZS, Bortfeld H, Oghalai JS, 2011. The developmental trajectory of brain-scalp distance from birth through childhood: implications for functional neuroimaging. PLoS One 6, e24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD, 2003. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev 42, 33–84. [DOI] [PubMed] [Google Scholar]
- Bikson M, Rahman A, 2013. Origins of specificity during TDCS: anatomical, activity-selective, and input-bias mechanisms. Front. Hum. Neurosci 7, 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG, 2004. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol 557, 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, 2016. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9, 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, Adair D, Datta A, Fang XH, Wingeier B, 2018. Limited output transcranial electrical stimulation (Lotes-2017): engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul. 11, 134–157. [DOI] [PubMed] [Google Scholar]
- Bikson M, Esmaeilpour Z, Adair D, Kronberg G, Tyler WJ, Antal A, Datta A, Sabel BA, Nitsche MA, Loo C, 2019. Transcranial electrical stimulation nomenclature. Brain Stimul. 12, 1349–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Hanlon CA, Woods AJ, Gillick BT, Charvet L, Lamm C, Madeo G, Holczer A, Almeida J, Antal A, 2020. Guidelines for TMS/tES clinical services and research through the COVID-19 pandemic. Brain Stimul. 13, 1124–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold O, Redfearn J, 1964. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol 172, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F, 2009. Using non-invasive brain stimulation to augment motor training-induced plasticity. J. Neuroeng. Rehabil 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Development Cooperative Group, 2012. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex 22, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, 2012. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 5, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, Gerloff C, Hallett M, Hummel FC, Nitsche MA, 2017. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin. Neurophysiol 128, 589–603. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW, 1996. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke 27, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Burt T, Lisanby SH, Sackeim HA, 2002. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int. J. Neuropsychopharmacol 5, 73–103. [DOI] [PubMed] [Google Scholar]
- BÜtefisch CM, Netz J, Wesslin M, Seitz RJ, Hömberg V, 2003. Remote changes in cortical excitability after stroke. Brain 126, 470–481. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Shuster M, O’hara E, Hurley K, Middlebrooks D, Guilkey K, 2013. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J. Hand Ther 26, 162–171. [DOI] [PubMed] [Google Scholar]
- Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, Florio L, Ranieri F, Falato E, Di Santo A, 2017. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast. 2017, 7876507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Underlying Cause of Death, 1999–2018. CDC WONDER Online Database. Centers for Disease Control and Prevention, Atlanta, GA. (Accessed March 12, 2020). [Google Scholar]
- Chang MC, Kim DY, Park DH, 2015. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 8, 561–566. [DOI] [PubMed] [Google Scholar]
- Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, Loo C, Krull KR, Bikson M, 2015. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front. Syst. Neurosci 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-J, Huang Y-Z, Chen C-Y, Chen C-L, Chen H-C, Wu C-Y, Lin K-C, Chang T-L, 2019. Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: a pilot randomized controlled trial. BMC Neurol. 19, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MG, Lo WD, 2015. Noninvasive brain stimulation: the potential for use in the rehabilitation of pediatric acquired brain injury. Arch. Phys. Med. Rehabil 96, S129–S137. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM, 2003. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke 34, 2653–2658. [DOI] [PubMed] [Google Scholar]
- Cioni G, D’acunto G, Guzzetta A, 2011. Perinatal Brain Damage in Children: Neuroplasticity, Early Intervention, and Molecular Mechanisms of Recovery. Progress in Brain Research, Elsevier. [DOI] [PubMed] [Google Scholar]
- Collange Grecco LA, De Almeida Carvalho Duarte N, Mendonça ME, Galli M, Fregni F, Oliveira CS, 2015. Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: a pilot, randomized, controlled, double-blind, clinical trial. Clin. Rehabil 29, 1212–1223. [DOI] [PubMed] [Google Scholar]
- Dasilva AF, Volz MS, Bikson M, Fregni F, 2011. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp 51, e2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Elwassif M, Battaglia F, Bikson M, 2008. Transcranial current stimulation focality using disc and ring electrode configurations: fem analysis. J. Neural Eng 5, 163. [DOI] [PubMed] [Google Scholar]
- Davis NJ, 2014. Transcranial stimulation of the developing brain: a plea for extreme caution. Front. Hum. Neurosci 8, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, Mclean J, Forbes K, Kilgard MP, 2016. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper limb rehabilitation after ischemic stroke. Stroke 47, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moura MCDS, Hazime FA, Marotti Aparicio LV, Grecco LA, Brunoni AR, Hasue RH, 2019. Effects of transcranial direct current stimulation (tDCS) on balance improvement: a systematic review and meta-analysis. Somatosens. Mot. Res 36, 122–135. [DOI] [PubMed] [Google Scholar]
- Deluca SC, Trucks MR, Wallace DA, Ramey SL, 2017. Practice-based evidence from a clinical cohort that received pediatric constraint-induced movement therapy. J. Pediatr. Rehabil. Med 10, 37–46. [DOI] [PubMed] [Google Scholar]
- Dinomais M, Lignon G, Chinier E, Richard I, Ter Minassian A, Tich SNGT, 2013. Effect of observation of simple hand movement on brain activations in patients with unilateral cerebral palsy: an fMRI study. Res. Dev. Disabil 34, 1928–1937. [DOI] [PubMed] [Google Scholar]
- Dobbs B, Pawlak N, Biagioni M, Agarwal S, Shaw M, Pilloni G, Bikson M, Datta A, Charvet L, 2018. Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson’s disease. J. Neuroeng. Rehabil 15, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte NDAC, Grecco LAC, Galli M, Fregni F, Oliveira CS, 2014. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PLoS One 9, e105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbanna ST, Elshennawy S, Ayad M, 2019. Noninvasive brain stimulation for rehabilitation of pediatric motor disorders following brain injury: systematic review of randomized controlled trials. Arch. Phys. Med. Rehabil 100, 1945–1963. [DOI] [PubMed] [Google Scholar]
- Ezquerro F, Moffa AH, Bikson M, Khadka N, Aparicio LV, De Sampaio-Junior B, Fregni F, Bensenor IM, Lotufo PA, Pereira AC, 2017. The influence of skin redness on blinding in transcranial direct current stimulation studies: a crossover trial. Neuromodulation 20, 248–255. [DOI] [PubMed] [Google Scholar]
- Faria P, Hallett M, Miranda PC, 2011. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J. Neural Eng 8, 066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M, 2019. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord 12, 1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MK, Theologis T, Buckingham R, Johansen-Berg H, 2018. Transcranial direct current stimulation for promoting motor function in cerebral palsy: a review. J. Neuroeng. Rehabil 15, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B, 2010. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, 2015. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci 38, 195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Gilbert DL, 2004. Transcranial magnetic stimulation in children. Eur. J. Paediatr. Neurol 8, 7–19. [DOI] [PubMed] [Google Scholar]
- George MS, Nahas Z, Bohning DE, Lomarev M, Denslow S, Osenbach R, Ballenger JC, 2000. Vagus nerve stimulation: a new form of therapeutic brain stimulation. CNS Spectr. 5, 43–52. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM, 2004. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin. Neurophysiol 115, 1730–1739. [DOI] [PubMed] [Google Scholar]
- Gillick BT, Kirton A, Carmel JB, Minhas P, Bikson M, 2014. Pediatric stroke and transcranial direct current stimulation: methods for rational individualized dose optimization. Front. Hum. Neurosci 8, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, Cassidy J, Kimberley T, Carey JR, 2015. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Arch. Phys. Med. Rehabil 96, S104–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillick B, Rich T, Nemanich S, Chen C-Y, Menk J, Mueller B, Chen M, Ward M, Meekins G, Feyma T, 2018. Transcranial direct current stimulation and constraint-induced therapy in cerebral palsy: a randomized, blinded, sham-controlled clinical trial. Eur. J. Paediatr. Neurol 22, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano J, Bikson M, Kappenman ES, Clark VP, Coslett HB, Hamblin MR, Hamilton R, Jankord R, Kozumbo WJ, McKinley RA, 2017. Mechanisms and effects of transcranial direct current stimulation. Dose-Response 15, 1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Hung Y-C, Brandao M, Ferre CL, Kuo H-C, Friel K, Petra E, Chinnan A, Charles JR, 2011. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil. Neural Repair 25, 692–702. [DOI] [PubMed] [Google Scholar]
- Grecco LA, Duarte NA, Zanon N, Galli M, Fregni F, Oliveira CS, 2014. Effect of a single session of transcranial direct-current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: a randomized sham-controlled study. Braz. J. Phys. Ther 18, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecco LAC, Oliveira CS, Duarte NDAC, Lima VLC, Zanon N, Fregni F, 2017. Cerebellar transcranial direct current stimulation in children with ataxic cerebral palsy: a sham-controlled, crossover, pilot study. Dev. Neurorehabil 20, 142–148. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, 2001. Early brain damage and the development of motor behavior in children: clues for therapeutic intervention? Neural Plast. 8, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed MQ, Dhamne SC, Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, Rotenberg A, 2017. Transcranial magnetic and direct current stimulation in children. Curr. Neurol. Neurosci. Rep 17, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, Kilgard MP, 2013. Targeting Plasticity With Vagus Nerve Stimulation to Treat Neurological Disease. Progress in Brain Research, Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderley AJ, Metzler MJ, Kirton A, 2019. Noninvasive neuromodulation to promote motor skill gains after perinatal stroke. Stroke 50, 233–239. [DOI] [PubMed] [Google Scholar]
- Hsu W-Y, Cheng C-H, Liao K-K, Lee I-H, Lin Y-Y, 2012. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke 43, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M, Ugawa Y, Jaberzadeh S, 2017. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin. Neurophysiol 128, 2318–2329. [DOI] [PubMed] [Google Scholar]
- Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, Bikson M, 2016. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin. Neurophysiol 127, 3425–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Wu X, Wang J, Huang B, Wang Q, Zhang T, Niu Z, Zhang X, 2002. Effect of transcranial magnetic stimulation on rehabilitation of motor function in patients with cerebral infarction. Zhonghua Yi Xue Za Zhi 82, 534–537. [PubMed] [Google Scholar]
- Kandel M, Beis J-M, Le Chapelain L, Guesdon H, Paysant J, 2012. Non-invasive cerebral stimulation for the upper limb rehabilitation after stroke: a review. Ann. Phys. Rehabil. Med 55, 657–680. [DOI] [PubMed] [Google Scholar]
- Kasschau M, Reisner J, Sherman K, Bikson M, Datta A, Charvet LE, 2016. Transcranial direct current stimulation is feasible for remotely supervised home delivery in multiple sclerosis. Neuromodulation 19, 824–831. [DOI] [PubMed] [Google Scholar]
- Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M, 2013. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One 8, e76112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Shawky OA, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM, 2013. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil. Neural Repair 27, 592–601. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker Ii RL, Kilgard MP, 2013. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis 60, 80–88. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, Kilgard MP, 2014. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil. Neural Repair 28, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-H, You SH, Ko M-H, Park J-W, Lee KH, Jang SH, Yoo W-K, Hallett M, 2006. Repetitive transcranial magnetic stimulation–induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 37, 1471–1476. [DOI] [PubMed] [Google Scholar]
- Kim DY, Ohn SH, Yang EJ, Park C-I, Jung KJ, 2009. Enhancing motor performance by anodal transcranial direct current stimulation in subacute stroke patients. Am. J. Phys. Med. Rehabil 88, 829–836. [DOI] [PubMed] [Google Scholar]
- Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon A-M, Deveber G, 2008a. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 7, 507–513. [DOI] [PubMed] [Google Scholar]
- Kirton A, Deveber G, Gunraj C, Chen R, 2008b. Neurocardiogenic syncope complicating pediatric transcranial magnetic stimulation. Pediatr. Neurol 39, 196–197. [DOI] [PubMed] [Google Scholar]
- Kirton A, Andersen J, Herrero M, Nettel-Aguirre A, Carsolio L, Damji O, Keess J, Mineyko A, Hodge J, Hill MD, 2016. Brain stimulation and constraint for perinatal stroke hemiparesis: the plastic champs trial. Neurology 86, 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton A, Ciechanski P, Zewdie E, Andersen J, Nettel-Aguirre A, Carlson H, Carsolio L, Herrero M, Quigley J, Mineyko A, 2017. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology 88, 259–267. [DOI] [PubMed] [Google Scholar]
- Klomjai W, Aneksan B, Pheungphrarattanatrai A, Chantanachai T, Choowong N, Bunleukhet S, Auvichayapat P, Nilanon Y, Hiengkaew V, 2018. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: a randomized sham-controlled crossover study. Ann. Phys. Rehabil. Med 61, 286–291. [DOI] [PubMed] [Google Scholar]
- Kowalski JL, Nemanich ST, Nawshin T, Chen M, Peyton C, Zorn E, Hickey M, Rao R, Georgieff M, Rudser K, Gillick BT, 2019. Motor evoked potentials as potential biomarkers of early atypical corticospinal tract development in infants with perinatal stroke. J. Clin. Med 8, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF, 2012. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil. Neural Repair 26, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Santos L, Peterson MD, Ehinger M, 2015. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 8, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronberg G, Bridi M, Abel T, Bikson M, Parra LC, 2017. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 10, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronberg G, Rahman A, Parra LC, Bikson M, 2019. Direct current stimulation boosts Hebbian plasticity in vitro. BioRxiv, 562322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronberg G, Rahman A, Sharma M, Bikson M, Parra LC, 2020. Direct current stimulation boosts hebbian plasticity in vitro. Brain Stimul. 13, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, 2011. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci. Lett 490, 1–5. [DOI] [PubMed] [Google Scholar]
- Lafon B, Rahman A, Bikson M, Parra LC, 2017. Direct current stimulation alters neuronal input/output function. Brain Stimul. 10, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J, Kwakkel G, 2011. Stroke rehabilitation. Lancet 377, 1693–1702. [DOI] [PubMed] [Google Scholar]
- Lazzari RD, Politti F, Santos CA, Dumont AJL, Rezende FL, Grecco LAC, Ferreira LAB, Oliveira CS, 2015. Effect of a single session of transcranial direct-current stimulation combined with virtual reality training on the balance of children with cerebral palsy: a randomized, controlled, double-blind trial. J. Phys. Ther. Sci 27, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari RD, Politti F, Belina SF, Collange Grecco LA, Santos CA, Dumont AJL, Lopes JBP, Cimolin V, Galli M, Santos Oliveira C, 2017. Effect of transcranial direct current stimulation combined with virtual reality training on balance in children with cerebral palsy: a randomized, controlled, double-blind, clinical trial. J. Mot. Behav 49, 329–336. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, 2017. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol 128, 56–92. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y, 2013. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front. Hum. Neurosci 6, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J, Gonçalves ÓF, Pereira P, Khadka N, Bikson M, Fregni F, Carvalho S, 2018. The differential effects of unihemispheric and bihemispheric tDCS over the inferior frontal gyrus on proactive control. Neurosci. Res 130, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu L, Nair D, Schlaug G, 2010. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 75, 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-H, He X-K, Chen H-Y, Peng C-W, Rotenberg A, Juan C-H, Pei Y-C, Liu H-L, Chiang Y-H, Wang J-Y, 2019. Neuromodulatory effects of transcranial direct current stimulation on motor excitability in rats. Neural Plast. 2019, 4252943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomarev M, Kim D, Richardson SP, Voller B, Hallett M, 2007. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin. Neurophysiol 118, 2072–2075. [DOI] [PubMed] [Google Scholar]
- Mansur C, Fregni F, Boggio P, Riberto M, Gallucci-Neto J, Santos C, Wagner T, Rigonatti S, Marcolin M, Pascual-Leone A, 2005. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64, 1802–1804. [DOI] [PubMed] [Google Scholar]
- Marquez J, Van Vliet P, Mcelduff P, Lagopoulos J, Parsons M, 2015. Transcranial direct current stimulation (tDCS): does it have merit in stroke rehabilitation? A systematic review. Int. J. Stroke 10, 306–316. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Stinear CM, 2017. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul. 10, 721–734. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, Mcgaugh JL, 2002. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci 16, 1223–1226. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Dannenbaum R, Levin MF, 2006. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke 37, 186–192. [DOI] [PubMed] [Google Scholar]
- Miguel Angel C-C, Ignacio M-M, Cordero G, René T-M, Mario S-Z, Matilde R-G, Adalberto G-A, 2001. Transcranial magnetic stimulation and acoustic trauma or hearing loss in children. Neurol. Res 23, 343–346. [DOI] [PubMed] [Google Scholar]
- Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK, 2012. Transcranial direct current stimulation in pediatric brain: a computational modeling study. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp. 859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze V, Schmanke T, Andreas S, Lyzhko E, Freitag CM, Siniatchkin M, 2015. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin. Neurophysiol 126, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Moura RCF, Santos C, Collange Grecco L, Albertini G, Cimolin V, Galli M, Oliveira C, 2017. Effects of a single session of transcranial direct current stimulation on upper limb movements in children with cerebral palsy: a randomized, sham-controlled study. Dev. Neurorehabil 20, 368–375. [DOI] [PubMed] [Google Scholar]
- MÜller R-A, Rothermel RD, Behen ME, Muzik O, Chakraborty PK, Chugani HT, 1997. Plasticity of motor organization in children and adults. Neuroreport 8, 3103–3108. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG, 2004. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol 55, 400–409. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D, 2009. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci 10, 861–872. [DOI] [PubMed] [Google Scholar]
- Nemanich ST, Chen CY, Chen M, Zorn E, Mueller B, Peyton C, Elison JT, Stinear J, Rao R, Georgieff M, Menk J, Rudser K, Gillick B, 2019. Safety and feasibility of transcranial magnetic stimulation as an exploratory assessment of corticospinal connectivity in infants after perinatal brain injury: an observational study. Phys. Ther 99, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol 527, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2001. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901. [DOI] [PubMed] [Google Scholar]
- Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson SA, Goldsmith S, 2013. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev. Med. Child Neurol 55, 885–910. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G, 1996. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 724, 89–96. [DOI] [PubMed] [Google Scholar]
- Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T, 2013. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev. Med. Child Neurol 55, 509–519. [DOI] [PubMed] [Google Scholar]
- Ouellette MM, Lebrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, Fielding RA, 2004. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke 35, 1404–1409. [DOI] [PubMed] [Google Scholar]
- Palm U, Segmiller FM, Epple AN, Freisleder F-J, Koutsouleris N, Schulte-Körne G, Padberg F, 2016. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J. Neural Transm 123, 1219–1234. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Riley AM, Doeltgen SH, Kurylowicz L, Rothwell JC, McAllister SM, Smith AE, Clow A, Kennaway DJ, Ridding MC, 2012. Physiological evidence consistent with reduced neuroplasticity in human adolescents born preterm. J. Neurosci 32, 16410–16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E, Sankarasubramanian V, Cunningham D, Potter-Baker K, Varnerin N, Cohen L, Sterr A, Conforto A, Machado A, 2016. Models to tailor brain stimulation therapies in stroke. Neural Plast. 2016, 4071620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana H, 2005. Transcranial magnetic stimulation in persons younger than the age of 18. J. ECT 21, 88–95. [DOI] [PubMed] [Google Scholar]
- Radman T, Su Y, An JH, Parra LC, Bikson M, 2007. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J. Neurosci 27, 3030–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Lafon B, Parra LC, Bikson M, 2017. Direct current stimulation boosts synaptic gain and cooperativity in vitro. J. Physiol 595, 3535–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse T, Kirton A, 2013. Non-invasive brain stimulation in children: applications and future directions. Transl. Neurosci 4, 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey SL, Coker-Bolt P, Deluca SC, 2013. Operationalizing pediatric constraint-induced movement therapy (P-CIMT): the core principles to inform training P-CIMT therapists, measuring fidelity of implementation, and developing post-treatment plans. In: Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT): A Guide for Occupational Therapy and Health Care Clinicians, Researchers, and Educators. The American Occupational Therapy Association, Bethesda, MD. [Google Scholar]
- Rawji V, Ciocca M, Zacharia A, Soares D, Truong D, Bikson M, Rothwell J, Bestmann S, 2018. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave JN, Moore L, Oyekunle T, Ebrahim M, Falidas K, Snowdon N, Ali A, Majid A, 2018. Transcutaneous auricular vagus nerve stimulation with concurrent upper limb repetitive task practice for poststroke motor recovery: a pilot study. J. Stroke Cerebrovasc. Dis 27, 1998–2005. [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik PA, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, Cohen LG, 2008. Consensus: “can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 1, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH, 2008. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia 46, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Guzzetta A, Pannek K, Boyd R, 2011. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect. 1, 309–316. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B, 2007. A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl 109, 8–14. [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120, 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo C, Souza Carneiro MI, Bolognini N, Fregni F, 2017. Safety review of transcranial direct current stimulation in stroke. Neuromodulation 20, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani MM, Agboada D, Jamil A, Kuo M-F, Nitsche MA, 2019. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119, 350–361. [DOI] [PubMed] [Google Scholar]
- Smith M-C, Stinear CM, 2016. Transcranial magnetic stimulation (TMS) in stroke: ready for clinical practice? J. Clin. Neurosci 31, 10–14. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lipton JO, Boyle LM, Madsen JR, Goldenberg MC, Pascual-Leone A, Sahin M, Rotenberg A, 2016. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann. Neurol 80, 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK, 2013. Balancing Plasticity/Stability Across Brain Development. Progress in Brain Research, Elsevier. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K, 2005. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36, 2681–2686. [DOI] [PubMed] [Google Scholar]
- Taub E, Crago JE, Uswatte G, 1998. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil. Psychol 43, 152. [Google Scholar]
- Valle AC, Dionisio K, Pitskel NB, Pascual-Leone A, Orsati F, Ferreira MJ, Boggio PS, Lima MC, Rigonatti SP, Fregni F, 2007. Low and high frequency repetitive transcranial magnetic stimulation for the treatment of spasticity. Dev. Med. Child Neurol 49, 534–538. [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, 2020. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141, e139–e596. [DOI] [PubMed] [Google Scholar]
- Wang R-Y, Wang F-Y, Huang S-F, Yang Y-R, 2019. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture 68, 382–387. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, 2016. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol 127, 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Sun J, Tang X, Zeng K, Wu X, 2019. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil 33, 847–864. [DOI] [PubMed] [Google Scholar]
- Zewdie E, Ciechanski P, Kuo H, Giuffre A, Kahl C, King R, Cole L, Godfrey H, Seeger T, Swansburg R, 2020. Safety and tolerability of transcranial magnetic and direct current stimulation in children: prospective single center evidence from 3.5 million stimulations. Brain Stimul. 13, 565–575. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing G, Shuai S, Guo Z, Chen H, McClure MA, Chen X, Mu Q, 2017. Low-frequency repetitive transcranial magnetic stimulation for stroke-induced upper limb motor deficit: a meta-analysis. Neural Plast. 2017, 2758097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC, 2008. Consensus: motor cortex plasticity protocols. Brain Stimul. 1, 164–182. [DOI] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC, 2012. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke 43, 2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]


