Abstract
Purpose
To report a case of a branch retinal vein occlusion (BRVO) following mRNA COVID-19 vaccination.
Observations
A 34-year-old healthy male presented with blurriness in the inferior visual field, intermittent photopsia, multiple retinal hemorrhages, dilated and tortuous retinal vessels, and cotton wools spots in the right eye. The clinical examination and ancillary tests confirmed the diagnosis of a right eye BRVO. The visual symptoms started 2 days following first dose COVID-19 vaccination with the BNT162b2 (Pfizer-BioNTech) mRNA vaccine.
Conclusions and importance
This is a rare case of BRVO in an otherwise healthy young man, presenting after vaccination for COVID-19 in the absence of other coagulable risk factors. As the literature on venous thrombosis after COVID-19 vaccinations remains sparse, it is critical to raise awareness that BRVO could be a vaccine-related thrombotic adverse event. We highlight that as more of the population is vaccinated, an increased incidence of BRVO may confirm the link to COVID-19 vaccination.
Keywords: COVID-19, BRVO, Vaccination, Branch retinal vein occlusion, Retina, SARS-CoV-2
1. Introduction
Ophthalmic complications, including retinal vein occlusion (RVO) and other retinal findings,1,2 have been reported in the setting of coronavirus disease 2019 (COVID-19) infection. These events are postulated to occur due to hypercoagulability, inflammation, and microvascular alterations. Cerebral venous thrombosis has been reported to occur after both infection (39.0 per million) and vaccination (4.1–5 per million, depending on vaccine type).3 We report a rare case of branch retinal vein occlusion (BRVO), following COVID-19 vaccination with the BNT162b2 (Pfizer-BioNTech) mRNA vaccine.
2. Case report
A previously healthy 34-year-old man presented with visual symptoms that started 2 days following the first dose of BNT162b2 vaccination. He did not seek immediate medical attention as his visual symptoms were mild. When his symptoms did not resolve after 2 weeks, he was referred to ophthalmology and assessed on day 21 following vaccination. He reported blurriness in the inferior visual field in his right eye as well as intermittent photopsia. He had mild injection site soreness following vaccination, and no other systemic symptoms. Upon examination, visual acuity was 20/20 in both eyes with normal colour vision and no relative afferent pupillary defect. Dilated fundus examination showed a superior nasal BRVO in the right eye, with intraretinal hemorrhages in the superior retina extending to the periphery (Fig. 1). Fluorescein angiography clearly demonstrated delayed venous filling with late staining of the affected vessels (Fig. 2).
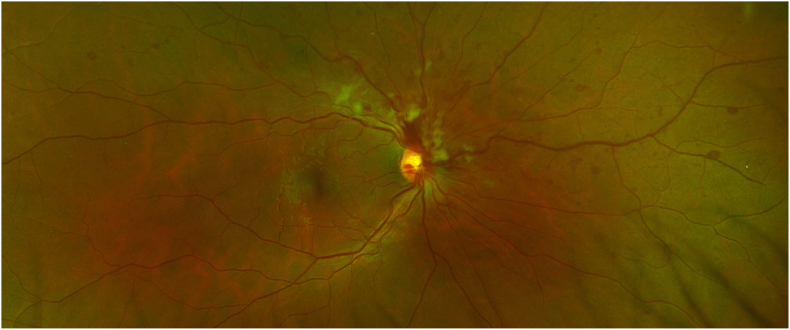
Fig. 1.
Colour photo of right eye superior nasal branch retinal vein occlusion demonstrating venous dilation and tortuosity, multiple retinal hemorrhages and cotton wool spots. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
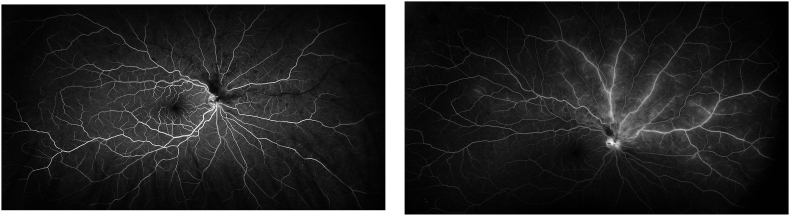
Fig. 2.
Fluorescein angiogram A) at 26 seconds showing delayed venous filling and B) at 6 minutes showing staining and leakage of the affected veins. The superior temporal branch remains uninvolved with normal filling and no leakage.
Laboratory tests including complete blood count, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, international normalized ratio/partial thromboplastin time (INR/PTT), homocysteine, anti-cardiolipin, lupus anticoagulant, factor V Leiden were all normal. The patient had no medical comorbidities, and was not taking any medication. Hematology consultation did not uncover any additional risk factors. Prior ocular history was significant only for laser vision correction 2 years prior for mild myopia.
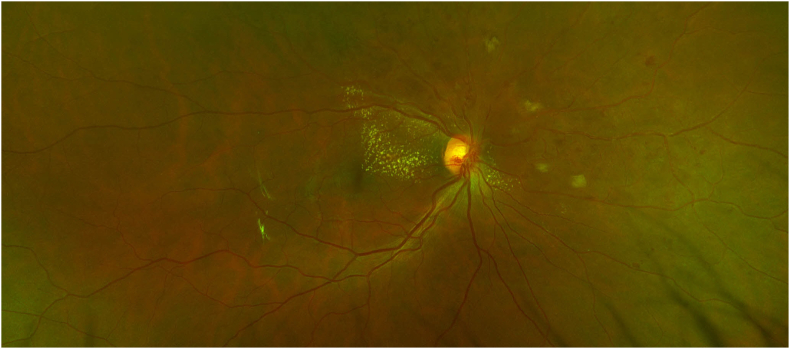
At follow up after 4, 7 and 10 months, visual acuity remained 20/20 with a persistent inferior visual field defect. Fundus examination showed gross resolution of intraretinal hemorrhages, but new macular exudates (Fig. 3). No macular edema was seen on OCT imaging at 4 and 7 months.
Fig. 3.
Colour photo of the right eye at 10 month follow up, showing resolution of intraretinal hemorrhages, but new macular exudates. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
We report a rare case of BRVO presenting after vaccination with BNT162b2 for COVID-19. BRVO1 and central retinal vein occlusion (CRVO)4 following COVID-19 have previously been reported in otherwise healthy patients. Although significantly lower than that associated with infection, the thrombotic risk of COVID-19 vaccination has been recognized, and occurs following vaccination with multiple different vaccines.3 BRVO was recently reported in three cases of vaccination with ChAdOx1 (AstraZeneca),3,4 and two cases with BNT162b2.4 These patients were older than the current case, mean age was 63.6, and three had comorbid hypertension.3,4 Other case reports demonstrated CRVO,4, 5, 6, 7 and combined central retinal artery occlusion (CRAO) and CRVO with ischemic optic neuropathy,8 occurring following vaccination with BNT162b2, CRVO after ChAdOx1,9 unilateral hemispheric RVO,10 and combined CRAO and CRVO11 with mRNA-1237 (Moderna), and superior hemi-retinal vein occlusion (HRVO) with severe cystoid macular edema following vaccination with adenoviral-based Gam-COVID-Vac/Sputnik V (Gamaleya Institute).12
We postulate that an immunological response was evoked by the vaccine, triggering venous thrombosis in this previously healthy patient. The pathogenesis may involve microvascular alternations at the level of the retina, as previously reported in the setting of COVID-19 infection.2 The hypothesis of abnormal coagulation is supported by findings of exacerbation of existing BRVO following vaccination with BNT162b2 in two older individuals (74 and 71 years old).13
BRVO typically occurs at arteriovenous crossings mainly due to microvascular disease. In younger adults, aged less than 50 years, hypertension, hyperlipidemia, and increased BMI were found to be predictors of BRVO. In the current case, thrombotic factors were not identified.6 Our patient did not have any risk factors and thrombophilia workup was found to be unremarkable.
4. Conclusions
In summary, we report a case of an otherwise healthy young man, presenting with BRVO following a first dose of COVID-19 vaccine in the absence of other coagulable risk factors. Although a direct causal relationship cannot be established, considering the reports of venous thrombosis after COVID-19 vaccination, this finding is suspicious for a vaccine-related thrombotic adverse event. The incidence of RVO either after infection or vaccination appears to be very low, but should prompt ophthalmic examination in patients with even mild visual complaints in these settings. As more of the population is vaccinated, an increased incidence of RVO may confirm the link to COVID-19 vaccination.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Intellectual property
FORMCHECKBOX We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
FORMCHECKBOX We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
FORMCHECKBOX IRB approval was obtained (required for studies and series of 3 or more cases).
FORMCHECKBOX Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: DRP, LLCDB, YI.
Acknowledgements
None.
References
- 1.Duff S.M., Wilde M., Khurshid G. Branch retinal vein occlusion in a COVID-19 positive patient. Cureus. 2021;13(2):2–5. doi: 10.7759/cureus.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raval N., Djougarian A., Lin J. Central retinal vein occlusion in the setting of COVID-19 infection. J Ophthal Inflamm Infect. 2021;11(1):10–12. doi: 10.1186/s12348-021-00241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H.S., Byun Y., Byeon S.H., Kim S.S., Kim Y.J., Lee C.S. Retinal hemorrhage after sars-cov-2 vaccination. J Clin Med. 2021;10(23) doi: 10.3390/jcm10235705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters M.C., Cheng S.S.H., Sharma A., Moloney T.P. Retinal vein occlusion following COVID‐19 vaccination. Clin Exp Ophthalmol. Publ online Feb. 2022;3 doi: 10.1111/ceo.14056. [DOI] [PubMed] [Google Scholar]
- 5.Endo B., Bahamon S., Martínez-Pulgarín D. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Indian J Ophthalmol. 2021;69(10):2865–2866. doi: 10.4103/ijo.IJO_1477_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bialasiewicz A.A., Farah-Diab M.S., Mebarki H.T. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol. 2021;41(12):3889–3892. doi: 10.1007/s10792-021-01971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PP, Gelnick S, Jonisch J, Verma R, Zucker B. Central Retinal Vein Occlusion Following BNT162b2 (Pfizer-BioNTech) COVID-19 Messenger RNA Vaccine. doi:10.1097/ICB.0000000000001214. [DOI] [PubMed]
- 8.Lee S., Sankhala K.K., Bose S., Gallemore R.P. Combined central retinal artery and vein occlusion with ischemic optic neuropathy after COVID-19 vaccination. Int Med Case Rep J. 2022;15:7–14. doi: 10.2147/imcrj.s328931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonawane N., Yadav D., Kota A., Singh H. Central retinal vein occlusion post-COVID-19 vaccination. Indian J Ophthalmol. 2022;70(1):308–309. doi: 10.4103/ijo.IJO_1757_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacconi R., Simona F., Forte P., Querques G. Retinal vein occlusion following two doses of mRNA-1237 (moderna) immunization for SARS-cov-2: a case report. Ophthalmol Ther. 2022;11(1):453–458. doi: 10.1007/s40123-021-00441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikegami Y., Numaga J., Okano N., Fukuda S., Yamamoto H., Terada Y. Combined central retinal artery and vein occlusion shortly after mRNA-SARS-CoV-2 vaccination. QJM: Int J Med. 2022;114(12):884–885. doi: 10.1093/qjmed/hcab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal M., Murthy S., Srinivas Y. Unilateral retinal vein occlusion in a young, healthy male following Sputnik v vaccination. Indian J Ophthalmol. 2021;69(12) doi: 10.4103/ijo.IJO_2412_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka H., Nagasato D., Nakakura S., et al. Exacerbation of branch retinal vein occlusion post SARS-CoV2 vaccination Case reports. Medicine (United States) 2021;100(50):E28236. doi: 10.1097/MD.0000000000028236. [DOI] [PMC free article] [PubMed] [Google Scholar]