Abstract
A synthetic peptide, (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was used to investigate the signal transduction mechanisms of bombesin receptor subtype-3. Using NCI-1299#5 human lung cancer cells stably transfected with bombesin receptor subtype-3, 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) elevated the cytosolic Ca2+ from 150 to 250 nM within 10 s. Addition of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused phosphorylation of mitogen activated protein kinase in a time- and concentration-dependent manner. The mitogen activated protein kinase phosphorylation caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited by 2′-amino-3′-methyoxyflavone (PD98059), a mitogen activated protein kinase kinase (MEK-1) inhibitor. Using a luciferase reporter gene construct, (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused Elk-1 activation after 10 min and the increase in Elk-1 activation caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited by PD98059 as well as a dominant-negative MEK-1. (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused increased c-fos as well as c-jun mRNAs 1 h after addition to NCI-H1299#5 cells. The 47-fold increase in c-fos mRNA caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited by PD98059, a dominant-negative MEK-1 and a substance P antagonist but not (3-phenylpropanoyl-d-Ala24, Pro26, Psi26,27, Phe27)GRP-(20–27) (BW2258U89), a GRP receptor antagonist. These results indicate that (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused increased nuclear oncogene expression and upstream events include mitogen activated protein kinase phosphorylation and Elk-1 activation.
Keywords: Lung cancer, Bombesin receptor subtype-3, Elk-1, Mitogen activated protein kinase, c-fos mRNA
1. Introduction
The bombesin family of peptides, including gastrin releasing peptide and neuromedin B, are biologically active in the central nervous system and periphery (Anastasi et al., 1973; Merali et al., 1983; Moody et al., 1981). Four types of bombesin receptors have been cloned. The gastrin releasing peptide receptor is a 384 amino acid protein which contains seven transmembrane domains and is coupled to guanine nucleotide binding protein (Battey et al.,1991; Spindel et al., 1990). The activated gastrin releasing peptide receptor and the neuromedin B receptor, which is a 390 amino acid protein, cause phosphatidylinositol turnover (Wada et al., 1991). The resulting inositol-1,4,5-trisphosphate causes release of Ca2+ from intracellular organelles (Moody et al., 1995b). The diacylglycerol released activates protein kinase C, which phosphorylates cytosolic protein substrates (Draoui et al., 1993). Mitogen activated protein kinase is activated, leading to increased nuclear oncogene expression (Moody et al., 1996). Both gastrin releasing peptide and neuromedin B receptor activation stimulate the proliferation of small cell lung cancer (SCLC) cells; however, the role of bombesin receptor subtype-3 is unknown.
The bombesin receptor subtype-3 receptor is a 399 amino acid protein that has approximately 50% amino acid sequence homology to the gastrin releasing peptide and neuromedin B receptor (Fathi et al., 1993). The bombesin receptor subtype-3 has been localized to spermatocytes, pregnant uterus, the central nervous system and breast (Gorbulev et al., 1992). In bombesin receptor subtype-3 deficient mice, however, obesity develops as do hypertension and diabetes (Ohki-Hamazaki et al., 1997). The endogenous ligand for bombesin receptor subtype-3 is unknown. Bombesin receptor subtype-3 binds gastrin releasing peptide, bombesin and neuromedin B with low affinity; however, the synthetic peptide (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) binds with high affinity to bombesin receptor subtype-3 as well as gastrin releasing peptide and neuromedin B receptors (Mantey et al., 1997). The second messengers induced by bombesin receptor subtype-3 activation are just beginning to be explored (Ryan et al., 1998a, b). Bombesin receptor subtype-4 has been cloned and binds bombesin with high affinity (Nagalla et al., 1995).
In the present study, the second messengers associated with bombesin receptor subtype-3 activation were investigated. (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) increased cytosolic Ca2+, mitogen activated protein kinase phosphorylation, Elk-1 activation and nuclear oncogene after addition to a human lung cancer cell line NCI-H1299 transfected with bombesin receptor subtype-3. The increase in c-fos gene mRNA caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited by a dominant-negative MEK-1 and the MEK-1 inhibitor 2′-amino-3′-methyoxyflavone (PD98059). Our results indicate that (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused increased nuclear oncogene expression in a MEK-1-dependent manner using lung cancer cells.
2. Materials and methods
2.1. Cell culture
NCI-H1299 cells were cultured in RPMI-1640 (Carney et al., 1985) containing 10% heat-inactivated fetal bovine serum (Life Technologies, Rockville, MD). The cells were split weekly with trypsin-ethylenediamino-tetraacetic acid (EDTA) (Life Technologies). The cells were mycoplasma free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2/95% air. Because native NCI-H1299 cells have low levels of bombesin receptor subtype-3, they were transfected with human bombesin receptor subtype-3 cDNA containing an amino-terminal flag epitope subcloned into the expression vectors pCD2 and pcDNA3 with lipofectamine (Mantey et al., 1997). The resulting stably transfected cells overexpressing bombesin receptor subtype-3 were cultured in RPMI-1640 containing 10% fetal bovine serum and 300 μg/ml G418 sulfate (Sigma, St. Louis, MO). Clone NCI-H1299#5 had a bombesin receptor subtype-3 density of 80,000 receptors/cell.
2.2. Cytosolic Ca2+
The ability of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to alter cytosolic Ca2+ was investigated. NCI-H1299#5 cells were harvested (2.5 × 106/ml) and loaded with 5 μM Fura 2-AM at 37°C for 30 min in SIT medium (RPMI-1640 containing 3 × 10−8 M sodium selenite, 5 μg/ml bovine insulin and 10 μg/ml transferrin (Sigma) as described previously (Moody et al., 1987). The fluorescence intensity was continuously monitored using a Perkin-Elmer LS2 spectrofluorometer equipped with a magnetic stirring mechanism and temperature (37°C)-regulated cuvette holder prior to and after the addition of peptide, and the Ca2+ concentrations were calculated.
2.3. Western blot
The ability of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to cause phosphorylation of mitogen activated protein kinase was investigated (Villalba et al., 1997). NCI-H1299#5 cells were cultured in 15-cm dishes. When a monolayer of cells was formed, they were placed in SIT media containing 0.5% fetal bovine serum overnight. Three hours before treatment cells were placed in fresh SIT media. Cells were treated with (d-Phe6, β-Ala11, Phe13, Nle14 )bombesin-(6–14) for 2 min, washed twice with PBS and lysed in buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM sodium chloride, 1% Triton X-100, 1% deoxycholate, 1% sodium azide, 1 mM ethyeneglycol bis (β-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA), 0.4 mM EDTA, 1.5 μg/ml aprotinin, 1.5 μg/ml leupeptin, 1 mM phenylmethylsulfonylfluoride and 0.2 mM sodium vanadate (Sigma). The lysate was sonicated for 5 s at 4°C and centrifuged at 10,000 × g for 15 min. Protein concentration was measured by using a kit (Pierce Chemical, Rockland, IL); 150 μg/ml of protein was incubated with 4 μg of anti-mitogen activated protein kinase (Upstate Biotechnologies, Lake Placid, NY) antibody, 4 μg of goat anti-rabbit immunoglobulin (Ig) G and 30 μl of protein A-agarose (Sigma) overnight at 4°C. The immunoprecipitates were washed three times with phosphate buffered saline (PBS) and analyzed by sodiumdodecylsulfate/polyacrylamide gel electrophoresis and Western blotting. Immunoprecipitates were fractionated using 10% polyacrylamide gels. Membranes were blocked overnight at 4°C using blotto (5% non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0), 2 mM calcium chloride, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide (Sigma). The membranes were treated with anti-phospho mitogen activated protein kinase antibody (Upstate Biotechnologies) and incubated for 2 h at 25°C with anti-mouse IgG-horseradish peroxidase conjugate. The membrane was washed for 10 min with blotto and twice for 10 min with washing solution (50 mM Tris–HCl (pH 8.0), 2 mM calcium chloride, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide) (Sigma). The blot was incubated with enhanced chemiluminescence detection reagent for 5 min and exposed to Hyperfilm ECL (Amersham, Chicago, IL). The density of bands was determined using a densitometer.
2.4. Elk-1
A reporter construct system was used to study Elk-1 activation (Westwick et al., 1994). NCI-H1299#5 cells were cotransfected with the Elk-1 transcription factor activation domain fused to a Gal-4 binding protein (0.5 μg) and the Gal-4 promoter coupled to the luciferase gene (1 μg) (Life Technologies) using lipofectamine. After 6 h in SIT medium, the cells were fed with RPMI-1640 containing 10% fetal bovine serum. After 24 h, the cells were placed in SIT medium and with (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) added. After 10 min, the old medium was removed and 300 μl of lysis buffer added. ATP and luciferin were added to the sample (20 μl) and relative intensity determined in a luminometer.
2.5. Northern blot
The ability of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to stimulate nuclear oncogene expression was investigated. For the c-fos experiments, NCI-H1299#5 cells were cultured with SIT medium containing 0.5% fetal bovine serum, treated with 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) in the presence or absence of competitor for 60 min, as described previously (Draoui et al., 1995).
3. Results
3.1. Cytosolic Ca2+
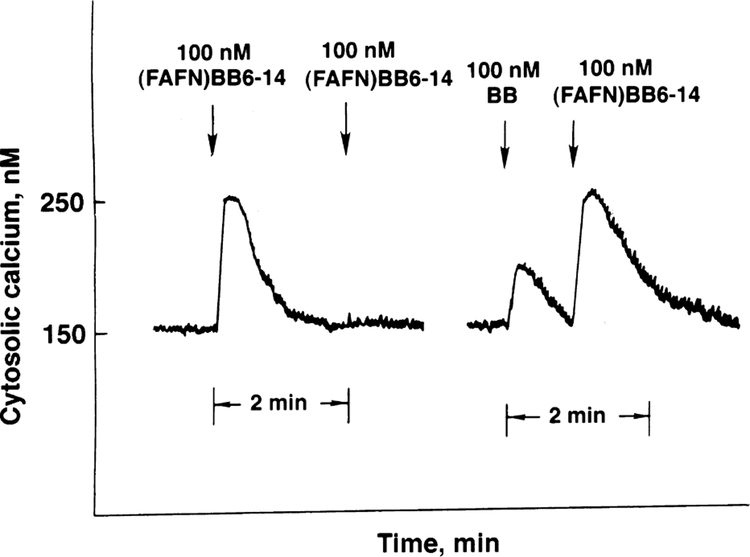
Fig. 1 shows that addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused cytosolic Ca2+ to increase from 150 to 250 nM within 10 s after addition to NCI-H1299#5 cells. The response was maximal after 10 s and the cytosolic Ca2+ returned to baseline after 60 s. If a second dose of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was added, no response was seen. Similarly, 10 nM bombesin caused cytosolic Ca2+ to increase in NCI-H1299#5 within 10 s from 150 to 190 nM. If 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was subsequently added, the cytosolic Ca2+ increased from 150 to 250 nM. In contrast, if a second dose of 10 nM bombesin was added, there was no increase in the cytosolic Ca2+ (data not shown). These results suggest that bombesin primarily activates gastrin releasing peptide receptors on NCI-H1299#5 cells whereas (d-Phe6, β-Ala11, Phe13, Nle14 )bombesin-(6–14) primarily activates bombesin receptor subtype-3. In this experiment and two others, the cytosolic Ca2+ response induced by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was larger than that of bombesin.
Fig. 1.
Cytosolic Ca2+. (Left) (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) ((fAFN)BB6–14), 100 nM, increased the cytosolic Ca2+ in NCI-H1299#5 cells after a first, but not second, addition. (Right) Bombesin (BB), 100 nM, increased the cytosolic Ca2+ as did subsequent addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14). This experiment is representative of two others.
3.2. Mitogen activated protein kinase
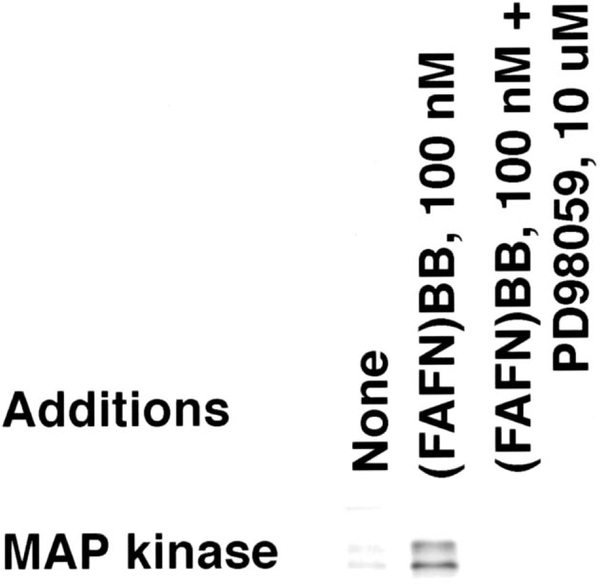
Fig. 2 shows that low levels of phosphorylated mitogen activated protein kinase were detected in NCI-H1299#5 cells. Both the 42 and 44 kDa form of mitogen activated protein kinase were maximally phosphorylated, 1–2 min after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14 )bombesin-(6–14), whereas 10 min after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) there was little increase in mitogen activated protein kinase phosphorylation. The MEK-1 inhibitor, PD98059, inhibited the increase in mitogen activated protein kinase phosphorylation caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14). In contrast, 100 nM bombesin weakly increased mitogen activated protein kinase phosphorylation (data not shown). These results suggest that mitogen activated protein kinase is phosphorylated by MEK-1 in NCI-H1299#5 cells after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14).
Fig. 2.
Mitogen activated protein kinase phosphorylation. By Western blot, the phosphorylation of mitogen activated protein kinase p42 and p44 was determined after no additions, addition of (FAFN)BB6–14), and 100 nM (FAFN)BB6–14 plus 10 μM PD98059. This experiment is representative of two others.
3.3. Elk-1
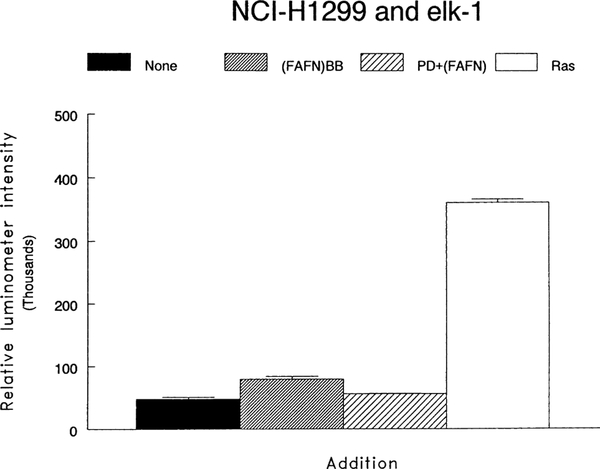
A reporter gene system was used to study the activation of Elk-1. Fig. 3 shows that the baseline was 47,914 relative luciferase units whereas activation by a ras construct increased the intensity 7-fold to 361,042. The intensity increased to 79,607, 10 min after the addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14). The increase in Elk-1 activation caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was reduced by 10 μM PD98059 to 55,956. Transfection with a dominant-negative MEK-1 reduced by 78% the stimulation caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) (data not shown). These results indicate that (d-Phe6, β-Ala11, Phe13, Nle14 )bombesin6–14 causes Elk-1 activation in a MEK-1 dependent manner in NCI-H1299#5 cells.
Fig. 3.
Elk-1 activation and PD98059. The ability of no additions (□), 100 nM (FAFN)BB6–14 ( ), 100 nM (FAFN)BB6–14 + 10 μM PD98059 (
), 100 nM (FAFN)BB6–14 + 10 μM PD98059 ( ) and ras (□) to activate elk-1 was determined using a reporter gene construct. The relative luminometer intensity was determined. The mean value ± S.D. of two determinations is indicated. This experiment is representative of two others.
) and ras (□) to activate elk-1 was determined using a reporter gene construct. The relative luminometer intensity was determined. The mean value ± S.D. of two determinations is indicated. This experiment is representative of two others.
3.4. Nuclear oncogene expression
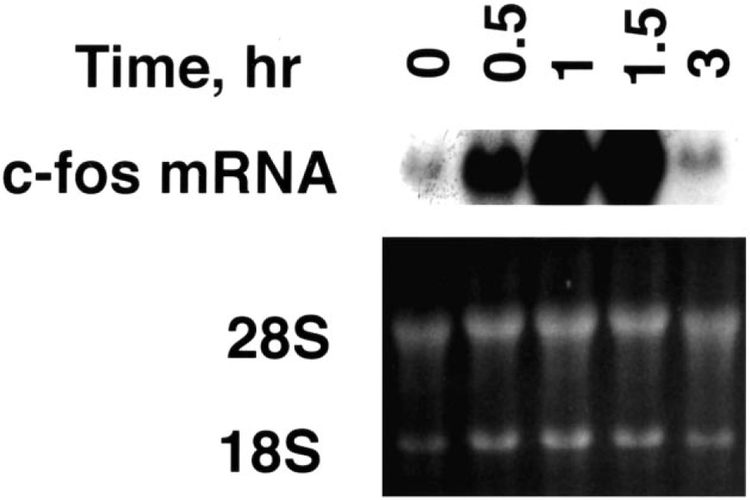
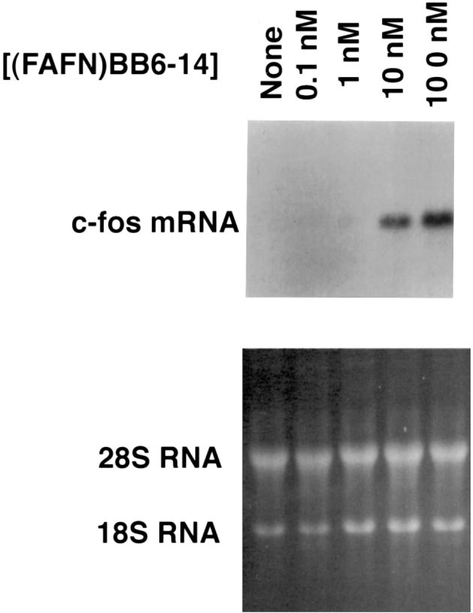
(d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused increased c-fos mRNA in a time-dependent manner. Fig. 4 shows that 1–1.5 h after the addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14), c-fos mRNA was maximal. c-fos mRNA was only slightly increased 3 h after addition of (d-Phe6, β-Ala11, Phe13, Nle14.bombesin-(6–14) to NCI-H1299#5 cells. c-fos mRNA increased strongly 1 h after addition of 10 and 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) but not 0.1 and 1 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) (Fig. 5). Thus, c-fos mRNA was increased in a time- and concentration-dependent manner after (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) addition.
Fig. 4.
c-fos mRNA. (Top) The c-fos mRNA was determined as a function of time after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells. (Bottom) Ethidium bromide staining. This experiment is representative of three others.
Fig. 5.
c-fos mRNA and (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) concentration. (Top) The c-fos mRNA was determined 1 h after the addition of varying concentrations of (FAFN)BB6–14 to NCI-H1299#5 cells. (Bottom) Ethidium bromide staining. This experiment is representative of three others.
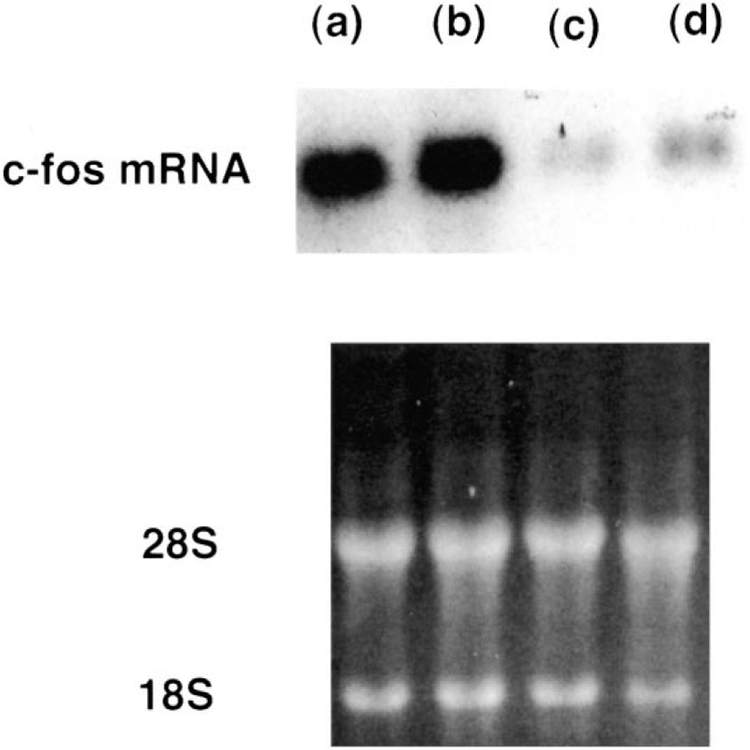
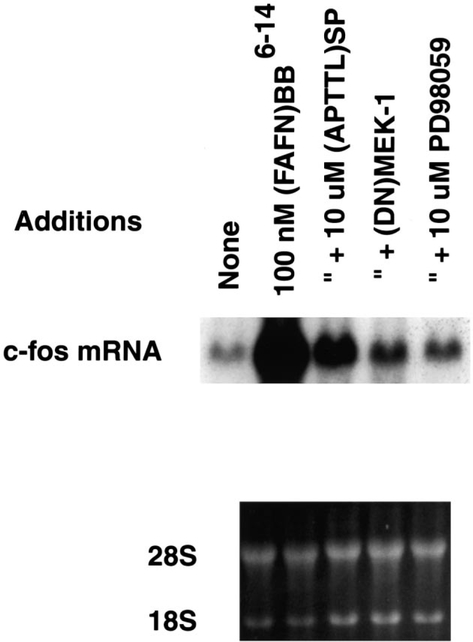
Fig. 6 shows that, 10 nM bombesin caused a slight increase in c-fos gene expression 1 h after addition to NCI-H1299#5 cells. The increase in c-fos mRNA caused by bombesin was blocked by 1 μM (3-phenylpropanoyl-d-Ala24, Pro26, Psi26,27, Phe27)GRP-(20–27) (BW225U89), a gastrin releasing peptide receptor antagonist. c-fos mRNA was strongly increased 1 h after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells and the increase in c-fos gene expression was minimally affected by 1 μM BW2258U89. These results indicate that BW2258U89 is a gastrin releasing peptide receptor but not bombesin receptor subtype-3 antagonist. Fig. 7 shows that 10 μM (d-Arg1, d-Pro2, d-Trp7,9, Leu11 )substance P and 10 μM PD98059 strongly inhibited the increase in c-fos mRNA caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) after 1 h. Similarly, if NCI-H1299#5 cells were transfected with a dominant-negative MEK-1 (0.5 μg), 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) had little effect on c-fos mRNA. (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) increased c-fos, c-jun and c-myc mRNA by 47-, 6- and 12-fold, respectively (data not shown). These results suggest that (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused increased nuclear oncogene expression in a MEK-1 dependent manner.
Fig. 6.
c-fos mRNA and gastrin releasling peptide receptor antagonists. (Top) The ability of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) (a,b) or 10 nM bombesin (c,d) to increase c-fos mRNA was determined in the absence (b,d) or presence (a,c) of 1 μM BW2258U89. (Bottom) Ethidium bromide staining. This experiment is representative of three others.
Fig. 7.
c-fos mRNA and MEK-1. The increase in c-fos mRNA caused by addition of 100 nM (FAFN)BB6–14 to NCI-H1299#5 cells was inhibited by 10 μM (d-Arg1, d-Pro2, d-Trp7,9, Leu11)substance P ((APTTL)SP) and 10 μM PD98059. Also, if NCI-H1299#5 cells were transfected with a dominant-negative (DN) MEK-1, 100 nM (FAFN)BB6–14 had little effect on c-fos mRNA. (Bottom) Ethidium bromide staining. This experiment is representative of two others.
4. Discussion
Bombesin receptor subtype-3 is an orphan receptor of the bombesin family. Previously, we found that gastrin releasing peptide and neuromedin B activated unique receptors causing phosphatidylinositol turnover (Mahmoud et al., 1991; Wada et al., 1991). The diacylglycerol released activated protein kinase C resulting in phosphorylation of protein substrates. Mitogen activated protein kinase was activated leading to increased expression of nuclear oncogenes. Here the signal transduction mechanisms of bombesin receptor subtype-3 were investigated using (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14).
Because few native cell lines that express high levels of bombesin receptor subtype-3 are known, NCI-H1299 cells were transfected with bombesin receptor subtype-3 (clone #5 has 80,000 receptors/cell and slightly lower receptor densities were observed in clones #12, #25 and #26 (Mantey et al., 1997)). Native NCI-H1299 cells have low levels of gastrin releasing peptide receptors (Moody et al., 1996). Bombesin inhibited 125I-gastrin releasing peptide binding to NCI-H1299 cells with an IC50 value of 2 nM. (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) bound with a Kd of 30 nM to NCI-H1299#5 cells (Mantey et al., 1997). Also, 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused phosphatidylinositol turnover and elevated cytosolic Ca2+ with an ED50 value of 50 nM (Ryan et al., 1998a,b). Using NCI-H1299#5 cells (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) primarily activated bombesin receptor subtype-3 and caused a strong biological response; however, bombesin primarily activated gastrin releasing peptide receptors and caused a weaker response. The signal transduction mechanisms, however, for bombesin receptor subtype-3 and gastrin releasing peptide receptors appear similar.
(d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) caused an increase in cytosolic Ca2+ within 10 s after addition to NCI-H1299#5 cells. In contrast, the increase in phosphorylation of both the 42 and 44 kDa forms of mitogen activated protein kinase was maximal 2 min after the addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells. The increase in mitogen activated protein kinase phosphorylation but not cytosolic Ca2+ caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited by PD98059; PD98059 inhibits the ability of MEK-1 to phosphorylate mitogen activated protein kinase on Thr183 and Tyr185 (Alessi et al., 1995; Dudley et al., 1995). Preliminary data (T. Moody, unpublished) indicate that mitogen activated protein kinase phosphorylation was inhibited by a dominant-negative MEK-1 and GF109203x, a protein kinase C inhibitor. These results suggest that addition of (d-Phe6, ß-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells causes mitogen activated protein kinase phosphorylation in a MEK-1 and protein kinase C dependent manner. Previously, we found that bombesin caused phosphorylation of the mitogen activated protein kinase substrate myelin basic protein in a MEK-1 dependent manner using NCI-H1299 cells (Koh et al., 1999).
A reporter system was used to assess Elk-1 activity. The number of relative luciferase units increased by approximately 50%, 20 min after the addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells. A dominant negative MEK-1, GF109203x and PD98059 inhibited most of the increase in Elk-1 activation caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14). As a positive control, ras transfection maximally increased Elk-1 expression 9-fold. It remains to be determined if ras and/or raf is activated after addition of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells (Whitmarsh and Davies, 1996). Previous studies indicate that protein kinase C inactivation reduces mitogen activated protein kinase activity (Hoshi et al., 1989). Further protein kinase C activation of mitogen activated protein kinase can be both ras-dependent and ras-independent (VanRenterghem et al., 1994). The Ras-independent activation of mitogen activated protein kinase may result when protein kinase C phosphorylates Raf (Sozeri et al., 1992; Kolch et al., 1993). In turn, MEK-1 and mitogen activated protein kinase are phosphorylated (Chen et al., 1992). Mitogen activated protein kinase may then be translocated into the nucleus where it activates Elk-1 (Kortenjann et al., 1994). Elk-1 phosphorylation leads to activation of serum response factor promoter elements, leading to increased immediate-early gene expression such as c-fos (Hipskind et al., 1994; Beno et al., 1995).
Addition of (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells increased c-fos and c-jun mRNAs. The increase in c-fos gene expression caused by (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was time- and concentration-dependent, being maximal 1 h after addition of 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) to NCI-H1299#5 cells. The increase in c-fos gene expression caused by 100 nM (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) was inhibited approximately 68% and 78% by a dominant-negative MEK-1 and PD98059, respectively. The c-fos and c-jun may form heterodimers and activate AP-1 sites on the 5′ upstream regulatory regions of lung cancer growth factor genes.
Gastrin releasing peptide receptor antagonists such as BW2258U89 blocked the increase in c-fos mRNA caused by bombesin using NCI-H1299#5 cells. Peptide antagonists for the gastrin releasing peptide receptor inhibit lung cancer proliferation in vitro and in vivo (Moody et al., 1995a; Yano et al., 1992). Gastrin releasing peptide receptor antagonists and neuromedin B receptor antagonists have little effect on bombesin receptor subtype-3 (Mantey et al., 1997, Eden et al., 1996). The broad spectrum substance P receptor antagonist (d-Arg1, d-Pro2, d-Trp7,9, Leu11 )substance P can block both gastrin releasing peptide and neuromedin B receptors with low affinity (Bepler et al., 1988; Woll and Rozengurt, 1988). Also, substance P receptor antagonists inhibit the growth of SCLC xenografts in nude mice (Sethi et al., 1992). Preliminary data (T. Moody, unpublished) indicate that (d-Arg1, d-Pro2, d-Trp7,9, Leu11 )substance P inhibited the increase in cytosolic Ca2+, mitogen activated protein kinase phosphorylation and elk-1 activation caused by (d-Phe6, β-Ala11, Phe13, Nle14 )bombesin-(6–14). These results suggest that (d-Arg1, d-Pro2, d-Trp7,9, Leu11 )substance P is also a bombesin receptor subtype-3 antagonist.
Previously we found that addition of gastrin releasing peptide or neuromedin B increased SCLC colony formation (Carney et al., 1985). Recently, we found that treatment of NCI-H1299#5 cells with (d-Phe6, β-Ala11, Phe13, Nle14)bombesin-(6–14) increases colony number (T. Moody unpublished). Thus, bombesin receptor subtype-3 activation may cause proliferation of lung cancer cells. Previously we found that PD98059 decreased lung cancer colony number (Koh et al., 1999). These results suggest that mitogen activated protein kinase activation is important for lung cancer proliferation in vitro.
In summary, bombesin receptor subtype-3 activation causes increased c-fos mRNA in lung cancer cells and upstream events include mitogen activated protein kinase phosphorylation and elk-1 activation.
Acknowledgements
The authors thank Dr. M. Koh for helpful discussions.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR, 1995. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Anastasi A, Erspamer V, Bucci M, 1973. Isolation and amino acid sequences of alytesin and bombesin: two analogous active tetradecapeptides from the skin of European discoglossid frogs. Arch. Biochem. Biophys 148, 443–446. [DOI] [PubMed] [Google Scholar]
- Battey JF, Way J, Corjay MH, Shapira H, Kusano K, Harkins R, Wu JM, Slattery T, Mann E, Feldman R, 1991. Molecular cloning of the bombesin/GRP receptor from Swiss 3T3 cells. Proc. Natl. Acad. Sci. U. S. A 88, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno DW, Brady LM, Bissonnette M, Davis BH, 1995. Protein kinase C and MAP kinase are required for 1,25-dihydroxy-vitamin D3 stimulation of EGR induction. J. Biol. Chem 270, 3642–3647. [DOI] [PubMed] [Google Scholar]
- Bepler G, Zeymer U, Mahmoud S, Fiskum G, Palaszynski E, Rotsch M, Willey J, Koros A, Cuttitta F, Moody TW, 1988. Substance P analogues function as bombesin receptor antagonists and inhibit small cell lung cancer clonal growth. Peptides 9, 1367–1372. [DOI] [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Bepler G, Guccion JG, Marangos PJ, Moody TW, Zweig MH, Minna JD, 1985. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 45, 2913–2923. [PubMed] [Google Scholar]
- Chen R, Sarnecki C, Blenis J, 1992. Inhibition of v-raf dependent c-fos expression and transformation by a kinase-defective mutant of the MAP kinase Erk2. Mol. Cell. Biol 12, 1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draoui M, Moody TW, Fathi Z, Battey J, 1993. GRP gene expression is stimulated in small cell lung cancer cells by phorbol ester. Cell Growth Differ. 4, 723–729. [PubMed] [Google Scholar]
- Draoui M, Chung P, Park M, Birrer M, Jakowlew S, Moody TW, 1995. Bombesin stimulates c-fos and c-jun mRNAs in small cell lung cancer cells. Peptides 16, 289–292. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR, 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U. S. A 92, 7686–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden JM, Hall MD, Higginbottom M, Horwell DC, Howson W, Hughes J, Jordan RE, Lewthwaite RA, Martin K, Mcknight AT, O’Tolle C, Pinnock RD, Pritchard MC, Suman-Chauhan N, Williams SC, 1996. PD165929 — The first high affinity non-peptide neuromedin B (NMB) receptor selective antagonist. Bioorg. Med. Chem. Lett 6, 2617–2623. [Google Scholar]
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Villet J, Sausville EA, Battey JF, 1993. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J. Biol. Chem 268, 5979–5984. [PubMed] [Google Scholar]
- Gorbulev V, Akhundova A, Buchner H, Fahrenholz F, 1992. Molecular cloning of a new bombesin receptor subtype expressed in uterus during pregnancy. Eur. J. Biochem 208, 405–410. [DOI] [PubMed] [Google Scholar]
- Hipskind RA, Baccarini M, Nordheim A, 1994. Transient activation of RAF-1, MEK and ERK2 coincides kinetically with ternary complex factor and immediate-early gene promoter activity in vivo. Mol. Cell Biol 14, 6219–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M, Nishida E, Sakai H, 1989. Characterization of a mitogen associated Ca2+ sensitive microtubule associate protein 2 kinase. Eur. J. Biochem 184, 477–486. [DOI] [PubMed] [Google Scholar]
- Kolch W, Heidecker G, Troppmair J, Yanagihara K, Bassin RH, Rapp UR, 1993. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Nature 364, 249–252.8321321 [Google Scholar]
- Kortenjann M, Thomae O, Shaw PE, 1994. Inhibition of v-raf dependent c-fos expression and transformation by a kinase defective mutant of the mitogen activated protein kinase ERK-2. Mol. Cell Biol 14, 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SWM, Leyton J, Moody TW, 1999. Bombesin activates MAP kinase in non-small cell lung cancer cells. Peptides 20, 121–126. [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Staley J, Taylor J, Bogden A, Moreau JP, Coy D, Avis I, Cuttitta F, Mulshine J, Moody TW, 1991. (Psi13,14)Bombesin analogues inhibit the growth of small cell lung cancer in vitro and in vivo. Cancer Res. 51, 1798–1802. [PubMed] [Google Scholar]
- Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, Searles RP, Spindel ER, Battey JF, Coy DH, Jensen RT, 1997. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates that it has a unique pharmacology compared with other mammalian bombesin receptors. J. Biol. Chem 272, 26062–26071. [DOI] [PubMed] [Google Scholar]
- Merali Z, Johnston S, Zakman S, 1983. Bombesin induced behavioral changes: antagonism by neuroleptics. Peptides 4, 693–699. [DOI] [PubMed] [Google Scholar]
- Moody TW, Pert CB, Gazdar AF, Carney DN, Minna JD, 1981. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science 214, 1246–1248. [DOI] [PubMed] [Google Scholar]
- Moody TW, Murphy A, Mahmoud S, Fiskum G, 1987. Bombesin-like peptides elevate cytosolic calcium in small cell lung cancer cells. Biochem. Biophys. Res. Commun 147, 189–195. [DOI] [PubMed] [Google Scholar]
- Moody TW, Venugopal R, Gozes Y, Hu V, McDermed J, Leban JJ, 1995a. BW1023U90: a new GRP receptor probe for small cell lung cancer cells. Peptides 17, 1337–1343. [DOI] [PubMed] [Google Scholar]
- Moody TW, Venugopal R, Zia F, Patierno S, Leban JJ, McDermed J, 1995b. BW2258: a GRP receptor antagonist which inhibits small cell lung cancer growth. Life Sci. 5, 521–529. [DOI] [PubMed] [Google Scholar]
- Moody TW, Zia F, Venugopal R, Fagarasan M, Oie H, Hu V, 1996. GRP receptors are present on non-small cell lung cancer cell lines. J. Cell Biochem 24s, 247–256. [DOI] [PubMed] [Google Scholar]
- Nagalla SR, Barry BJ, Creswick KC, Eden P, Taylor JT, Spindel ER, 1995. Cloning of a receptor for amphibian (Phe13)bombesin distinct from the receptor for GRP: Identification of a 4th bombesin receptor sybtype (BB4). Proc. Natl. Acad. Sci. U. S. A 92, 6205–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamamno M, Yamada K, Maeno H, Imaki J, Kikuyama S, Wada E, Wada K, 1997. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390, 165–169. [DOI] [PubMed] [Google Scholar]
- Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, Battey JF, Coy DH, Jensen RT, 1998a. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J. Biol. Chem 273, 13613–13624. [DOI] [PubMed] [Google Scholar]
- Ryan RR, Weber HC, Mantey SA, How W, Hilburger ME, Pradhan TK, Coy DH, Jensen RT, 1998b. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J. Pharmacol. Exp. Ther 187, 366–380. [PubMed] [Google Scholar]
- Sethi T, Langdon S, Smyth J, Rozengurt E, 1992. Growth of small cell lung cancer cells: Stimulation by multiple neuropeptides and inhibition by a broad spectrum antagonists in vitro and in vivo. Cancer Res. 52, 2737s–2742s. [PubMed] [Google Scholar]
- Sozeri O, Volllmer K, Liyange M, Frith D, Kour G, Mark GE, Stabel S, 1992. Protein kinase Cα activated raf-1 by direct phosphorylation. Oncogene 7, 2259–2262. [PubMed] [Google Scholar]
- Spindel ER, Giladi E, Brehm TP, Goodman RH, Segerson TP, 1990. Cloning and functional characterization of a cDNA encoding the murine fibroblast bombesin/GRP receptor. Mol. Endocrinol 4, 1956–1963. [DOI] [PubMed] [Google Scholar]
- VanRenterghem B, Browning MD, Maller JL, 1994. Activation of the c-raf protein kinase by protein kinase C. J. Biol. Chem 269, 24666–24672. [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L, 1997. Pituitary adenylate cyclase activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activation of the mitogen-activated protein kinase (MAP) kinase pathway. J. Neurosci 17, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battey J, 1991. cDNA cloning, characterization and brain region-specific expression of a neuromedin-B preferring bombesin receptor. Neuron 6, 421–430. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Cox AD, Der CJ, Cobb NH, Hibi M, Karin M, Brenner DA, 1994. Oncogenic ras activated c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc. Natl. Acad. Sci. U. S. A 91, 6030–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davies RJ, 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathwaysm. J. Mol. Med 74, 589–607. [DOI] [PubMed] [Google Scholar]
- Woll PJ, Rozengurt E, 1988. (d-Arg1, d-Phe5, d-Trp7,9, Leu11) substance P, a potent bombesin antagonist in murine Swiss 3T3 cells, inhibits the growth of human small cell lung cancer. Proc. Natl. Acad. Sci. U. S. A 85, 1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Pinski J, Groot K, Schally AV, 1992. Stimulation by bombesin and inhibition by BB-GRP antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 52, 4545–4547. [PubMed] [Google Scholar]