Abstract
Background:
The pulmonary artery pulsatility index (PAPi), calculated from the ratio of the pulmonary artery pulse pressure to right atrial pressure, is a predictor of right ventricular failure after inferior myocardial infarction and left ventricular assist device implantation. Whether PAPi is associated with adverse outcomes across a heterogenous population is unknown.
Methods:
We examined consecutive patients undergoing right heart catheterization between 2005–2016 in a hospital-based cohort. Multivariable Cox models were utilized to examine the association between PAPi and all-cause mortality, major adverse cardiac events (MACE), and heart failure (HF) hospitalizations.
Results:
We studied 8285 individuals (mean age 63 years, 39% women) with median PAPi across quartiles 1.7, 2.8, 4.2, and 8.7, who were followed over a mean follow-up of 6.7 ± 3.3 years. Patients in the lowest PAPi quartile had a 60% greater risk of death compared to the highest quartile (multivariable adjusted HR 1.60, 95% CI 1.36–1.88, p<0.001) and a higher risk of MACE and HF hospitalizations (HR 1.80, 95% CI 1.56–2.07, p<0.001 and HR 2.08, 95% CI 1.76–2.47, p<0.001, respectively). Of note, patients in quartiles 2 and 3 also had increased risk of cardiovascular events compared to quartile 4 (multivariable p<0.05 for all).
Conclusions:
Compared to the highest PAPi quartile, patients in PAPi quartiles 1–3 had a greater risk of all-cause mortality, MACE, and HF hospitalizations, with greatest risk observed in the lowest quartile. A low PAPi, even at values higher than previously reported, may serve an important role in identifying high-risk individuals across a broad spectrum of cardiovascular disease.
Keywords: Heart failure, right ventricular function, hemodynamic assessment, outcomes, Hemodynamics, Mortality/Survival, Diagnostic Testing
INTRODUCTION:
Right ventricular systolic dysfunction is independently associated with poor outcomes across a spectrum of cardiovascular and pulmonary disorders1–10. However, despite the prognostic importance of right ventricular function, accurate standard echocardiographic assessment is challenged by the complex geometry of the right ventricle11, 12, and cardiac magnetic resonance imaging has practical limitations to widespread use. The limitations of imaging assessment of the right ventricle have led to utilization of invasive hemodynamic indices of right ventricular performance such as right ventricular stroke work index (RVSWI) and the ratio of the right atrial pressure to pulmonary capillary wedge pressure (RA:PCWP).
More recently, the pulmonary artery pulsatility index (PAPi), defined as the ratio of pulmonary artery pulse pressure to right atrial pressure (), has emerged as a novel hemodynamic index of right ventricular performance. PAPi was initially reported as a strong predictor of right ventricular failure in patients with acute inferior myocardial infarction13 and in those undergoing left ventricular assist device placement for end-stage heart failure, with superior performance to RVSWI and RA:PCWP14, 15. Subsequent to these initial findings, PAPi has been shown to be independently associated with survival in pulmonary arterial hypertension16, progressive renal dysfunction in heart failure with preserved ejection fraction17, post-cardiopulmonary bypass right ventricular dysfunction18, and acute kidney injury after heart transplantation19. Among New York Heart Association class IV heart failure patients from the 2005 Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, PAPi was an independent predictor of death or hospitalization at 6 months (whereas RVSWI and RA:PCWP were not)20. More recently, PAPi has been shown to be a reliable indicator of adverse cardiac events in ambulatory heart failure patients21, 22.
While prior studies have focused on specific advanced disease samples, the clinical implications of PAPi across a large diverse sample of individuals with a range of cardiopulmonary diseases remains unclear. Utilizing a large database of consecutive patients undergoing right heart catheterization (RHC), we sought to 1) characterize the distribution of PAPi across a broad patient population, 2) identify clinical and echocardiographic correlates of PAPi, and 3) determine the association of PAPi with all-cause mortality and adverse cardiovascular events. We hypothesized that lower PAPi would be associated with all-cause mortality, major adverse cardiovascular events (MACE), and heart failure (HF) hospitalizations across a spectrum of cardiovascular disease and right ventricular function.
METHODS:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Sample:
We examined consecutive ambulatory and hospitalized patients undergoing right heart catheterization (RHC) between 2005 and 2016 at Massachusetts General Hospital. In cases where patients had multiple RHC procedures during this time period, only the initial RHC was included for analysis, leaving a total of 10,306 cases. Exclusion criteria included the following: acute myocardial infarction occurring on the same day as catheterization, cardiac arrest or shock within 24 hours, presence of mechanical ventilation, presence of intra-aortic balloon pump, history of heart or lung transplant, complicated adult congenital heart disease, history of valvular replacement, or those on dialysis (n=887 excluded). Cases were also excluded if there were missing key clinical covariates (n=484), patient identifier variables (n=398) or hemodynamic parameters (n=252), leaving a final study sample of 8285 for analysis. This study was approved by the Mass General Brigham Institutional Review Board.
Clinical, echocardiographic, and hemodynamic variables:
Clinical characteristics were ascertained from the medical records at the time of RHC and included age, sex, body mass index (BMI), smoking status, and presence of comorbidities (including diabetes mellitus, hypertension, history of myocardial infarction, history of heart failure, prior lung disease, and chronic kidney disease). Obstructive sleep apnea was identified by the electronic medical record utilizing appropriate International Classification of Diseases Ninth Revision (ICD-9) or Tenth Revision (ICD-10), codes.
Echocardiographic data within one year of RHC were reviewed with abstraction of quantitative and qualitative parameters regarding ventricular size and function and valvular pathology. Valvular pathology was recorded if moderate or greater stenosis or regurgitation were present. Abnormal RV function was recorded if there was any degree of RV dysfunction. For patients with more than one available echocardiogram, the nearest study to the RHC date was utilized. Echocardiographic data were available for 6750 out of 8285 patients (81.5%). Of note, 49% of patients had an echocardiogram within one week of RHC, an additional 17% within one month of RHC, and only 5% of patients had the closest echocardiogram longer than 6 months but less than one year within RHC. Quantitative parameters of right ventricular size and function, including right ventricular basal diameter, tricuspid annular plane systolic excursion (TAPSE), pulse tissue doppler S’ wave (tricuspid annulus systolic velocity), and right ventricular fractional area change (FAC), were available for a small subset of patients from manual review of patients who underwent same-day RHC and transthoracic echocardiogram.
Key hemodynamic measures recorded at the time of RHC included resting blood pressure, heart rate, mean right atrial (RA) pressure, pulmonary artery (PA) systolic and diastolic pressure, and mean pulmonary capillary wedge pressure (PCWP). Nonphysiologic parameters were set to missing. For example, if the PA systolic or diastolic pressure were listed as zero, these data were set to missing. PAPi was calculated as . For instances in which the RA pressure was recorded as zero, a physiologic value was confirmed by evaluation of the “a” and “v” wave measurements as well as the measured right ventricular diastolic pressure. If deemed physiologic, the RA pressure value was set to one for the purposes of PAPi calculation, else the RA pressure was set to missing. Cardiac output and cardiac index were derived via thermodilution methods utilizing the Mosteller formula for body surface area23. RVSWI was derived utilizing the thermodilution cardiac index measurement.
Clinical Outcomes:
Our primary outcomes were all-cause mortality and heart failure hospitalization, and occurrence of a major adverse cardiac event was our secondary outcome. All-cause mortality was ascertained using the National Social Security Death Master Index and hospital records, abstracted on 06/10/2020. The precise dates of deaths that occurred between 06/10/2017 and the date of abstraction (a three-year period) are protected nationally due to confidentiality purposes. Thus, these death events were imputed as occurring midway through this period, 12/10/2018, for standardization (n=242 individuals out of 3006 total death events).
Occurrence of a major adverse cardiac event (MACE) was defined as a composite of heart failure admission, cerebrovascular accident (CVA) or transient ischemic attack (TIA) or acute myocardial infarction (MI) by a corresponding ICD-9 or ICD-10 code as primary discharge diagnosis. A heart failure hospitalization was defined by an ICD-9 or ICD-10 code for heart failure as primary discharge diagnosis or a current procedural terminology (CPT) code for heart transplantation (OHT) or durable ventricular assist device (VAD). The follow-up period for each participant was defined as time from RHC to death date or date of final encounter in the electronic health record. Patients were censored based on time of last encounter.
Statistical Analysis:
The distribution of PAPi was winsorized to limit leverage of outliers on subsequent analyses, setting the minimum PAPi as 0.3 and maximum PAPi as 30 (2 patients had PAPi < 0.3, and 54 patients had PAPi > 30 prior to winsorizing to 0.3 to and 30, respectively). PAPi was further natural log-transformed to obtain a normal distribution and divided into quartiles for analysis. Baseline characteristics were summarized across the total sample and according to PAPi quartile. Continuous variables were reported using means and standard deviations or medians and interquartile ranges (25th – 75th percentile) as appropriate, whereas categorical variables were denoted using frequency and percentages. PAPi subgroups were compared using ANOVA, Chi-square or Kruskal-Wallis tests as appropriate. Correlations between PAPi and other continuous parameters of right ventricular function were performed via Spearman correlation due to skewed distribution. Clinical and echocardiographic correlates of log PAPi were determined using a multivariable adjusted linear regression model. A stepwise regression model was utilized to examine multivariable-adjusted determinants of PAPi with entry at p <0.1 and retention in the final model at p < 0.05.
We examined the association of log PAPi as a continuous variable and quartiles with clinical outcomes, including all-cause mortality (primary) and MACE or HF hospitalization (secondary). While the linear model was our primary analysis, we secondarily explored outcomes by PAPi quartile to provide clinically-relevant analyses. We used the Kaplan-Meier method to examine the association of PAPi with outcomes and log rank tests to examine differences between groups. Multivariable Cox models were then constructed, adjusting for age, sex, BMI, previous MI, previous HF, hypertension, diabetes, and chronic kidney disease. The proportional hazards assumption was tested, and minor violations were found using cumulative Martingale residuals24. We therefore included time-varying predictors to account for model fitness, including log-transformed PAPi, age, BMI, previous HF, and hypertension treatment. We investigated the relative performance of various hemodynamic parameters of RV function (log-transformed PAPi, RVSI, and RA:PCWP ratio) to predict adverse outcomes by evaluating C-statistics of each hemodynamic parameter when added to the multivariable model containing clinical variables outlined above. A two-sided p value <0.05 was deemed as statistically significant. Analyses were conducted using SAS software, Version 9.4 (Cary, North Carolina, USA).
RESULTS:
We studied 8285 individuals with mean age of 63 ± 13 years and 39% women. Comorbid conditions were common including 59% with hypertension, 23% diabetes mellitus, 16% chronic lung disease, 14% obstructive sleep apnea, 19% previous MI, and 32% previous HF. Among this sample, the median PAPi was 3.4 with interquartile range of 2.2 to 5.5 (Figure 1). Baseline characteristics stratified by log-transformed PAPi quartile are shown in Table 1, with median physiologic (non-log-transformed for clinical interpretation) PAPi values within each quartile as follows: Q1, 1.7 [1.3–2]; Q2, 2.8 [2.5–3]; Q3, 4.2 [3.8–4.8]; and Q4, 8.7 [6.8–14]. Patients in the lowest vs highest PAPi quartile were younger (60 vs 64 years), with a greater proportion of men (65% vs 54%), higher BMI (31.6 vs 26.5 kg/m2), and more frequent obstructive sleep apnea (20% vs 7%). NT-proBNP levels were clinically similar across quartiles (median NT-proBNP 1736 pg/mL across total sample).
Figure 1:
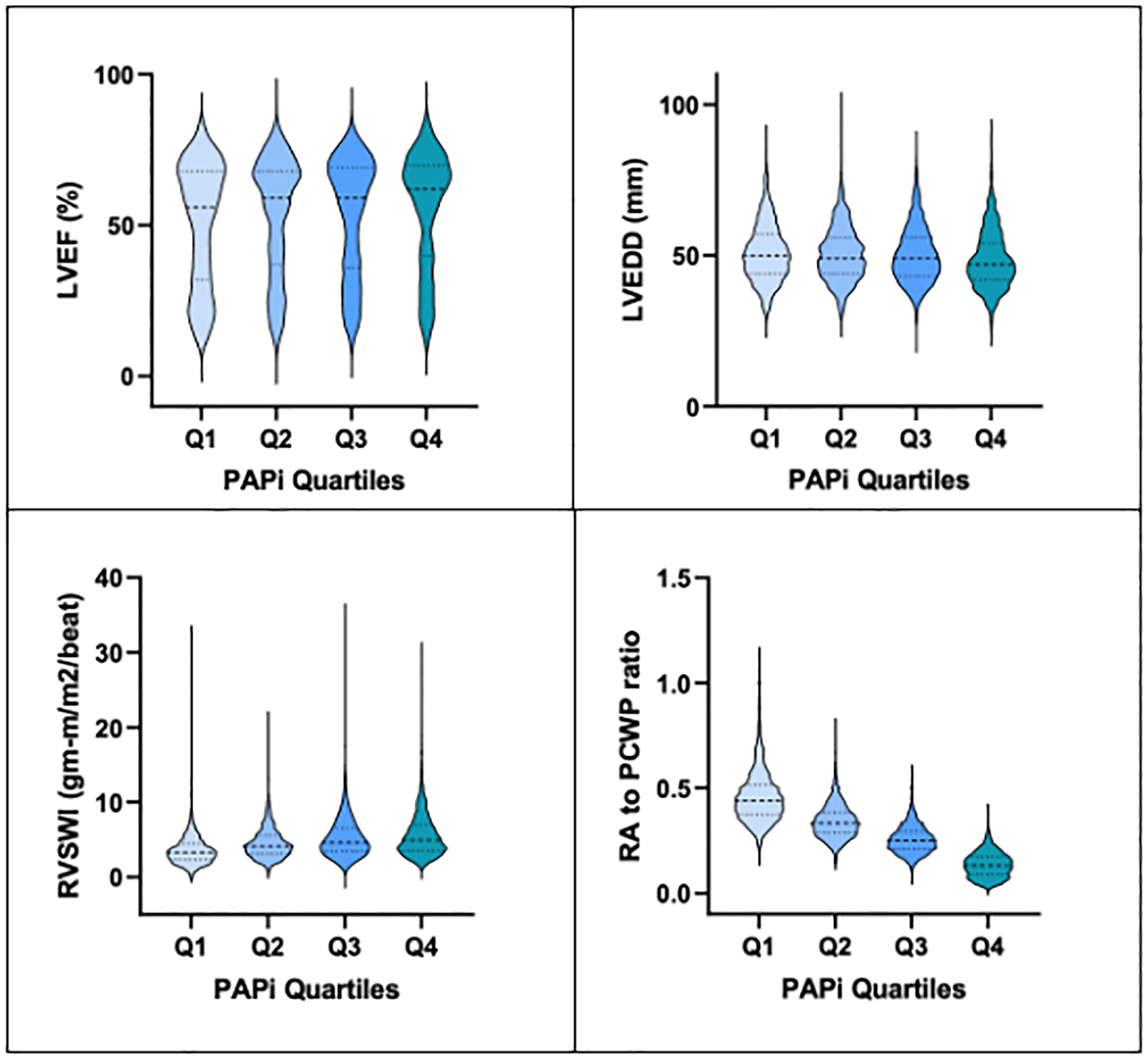
Distribution of echocardiographic and hemodynamic parameters across PAPi quartiles.
Abbreviations: PAPi, pulmonary artery pulsatility index; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; RVSWI, right ventricular stroke work index (calculated using thermodilution cardiac output); RA, right atrial; PCWP, pulmonary capillary wedge pressure.
Table 1:
Clinical and hemodynamic characteristics by PAPi quartile
| PAPi Quartile 1 (n=2090) |
PAPi Quartile 2 (n=2050) |
PAPi Quartile 3 (n=2099) |
PAPi Quartile 4 (n=2046) |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Clinical characteristics | |||||
| Age, years | 60.2 ± 13 | 63.2 ± 12.3 | 64.3 ± 12 | 64 ± 12.9 | <0.001 |
| Men, n (%) | 1365 (65.3) | 1268 (61.9) | 1288 (61.4) | 1101 (53.8) | <0.001 |
| Race/ethnicity, n (%) | 0.91 | ||||
| White | 1774 (84.9) | 1735 (84.6) | 1796 (85.6) | 1724 (84.3) | |
| Black | 78 (3.7) | 83 (4.1) | 71 (3.4) | 80 (3.9) | |
| Hispanic | 58 (2.8) | 66 (3.2) | 71 (3.4) | 68 (3.3) | |
| Asian | 38 (1.8) | 40 (2) | 41 (2) | 45 (2.2) | |
| Unknown | 142 (6.8) | 127 (6.2) | 120 (5.7) | 129 (6.3) | |
| Current/recent smoker, n (%) | 168 (11.8) | 121 (9.9) | 83 (7.6) | 88 (9.2) | 0.004 |
| Hypertension, n (%) | 1201 (57.4) | 1256 (61.3) | 1306 (62.2) | 1110 (54.3) | <0.001 |
| Diabetes Mellitus, n (%) | 508 (24.3) | 563 (27.5) | 468 (22.3) | 384 (18.8) | <0.001 |
| Chronic lung disease, n (%) | 304 (14.6) | 318 (15.5) | 350 (16.7) | 377 (18.4) | 0.006 |
| Chronic kidney disease, n (%) | 68 (3.3) | 72 (3.5) | 52 (2.5) | 49 (2.4) | 0.08 |
| Previous MI, n (%) | 354 (16.9) | 409 (20) | 450 (21.4) | 338 (16.5) | <0.001 |
| Previous HF, n (%) | 699 (33.4) | 665 (32.4) | 705 (33.6) | 587 (28.7) | 0.002 |
| Obstructive sleep apnea, n (%) | 425 (20.3) | 341 (16.6) | 236 (11.2) | 152 (7.4) | <0.001 |
| BMI (kg/m2) | 31.6 ± 7.4 | 30.5 ± 7 | 28.6 ± 6 | 26.5 ± 5.7 | <0.001 |
| NT-proBNP (pg/ml)* | 1682 [506–4693] | 1621 [562–4219] | 1979 [617–5011] | 1666 [419–4175] | 0.03 |
| Hemoglobin (g/dl) | 13 ± 2.4 | 13.1 ± 2.3 | 12.9 ± 2 | 13.1 ± 1.9 | 0.61 |
|
| |||||
| Hemodynamic parameter | |||||
| PAPi*† | 1.7 [1.3–2] | 2.8 [2.5–3] | 4.2 [3.8–4.8] | 8.7 [6.8–14] | <0.001 |
| PA systolic pressure, mmHg* | 37 [30–48] | 37 [29–50] | 38 [29–50] | 33 [26–46] | <0.001 |
| PA diastolic pressure, mmHg* | 19 [14–25] | 15 [11–21] | 13 [9–19] | 10 [6–15] | <0.001 |
| Mean RA pressure, mmHg* | 11 [9–15] | 8 [6–11] | 6 [4–8] | 3 [1–4] | <0.001 |
| PCWP, mmHg* | 18 [13–24] | 15 [11–21] | 13 [9–20] | 9 [6–14] | <0.001 |
| PVR, Wood units* | 1.6 [1.1–2.4] | 1.7 [1.1–2.6] | 1.8 [1.2–2.8] | 1.9 [1.3–3.1] | <0.001 |
| TPG, mmHg* | 8 [5–11] | 9 [6–12] | 9 [6–13] | 9 [7–14] | <0.001 |
| RVSWI, g/m/beat/m2*‡ | 6.6 [4.7–8.8] | 8 [6.1–10.8] | 8.9 [6.6–12.3] | 9 [6.6–12.6] | <0.001 |
| RA to PCWP ratio* | 0.4 [0.4–0.5] | 0.3 [0.3–0.4] | 0.3 [0.2–0.3] | 0.1 [0.1–0.2] | <0.001 |
| Td cardiac output, L/min*‡ | 5.1 [4–6.2] | 5.0 [4.2–6.2] | 5.0 [4.2–6] | 5.0 [4.2–6] | 0.79 |
| Td cardiac index, L/min/m2*‡ | 2.4 [2–2.9] | 2.5 [2.1–3] | 2.6 [2.2–3] | 2.7 [2.3–3.2] | <0.001 |
| Heart rate, bpm | 74.9 ± 17 | 71.3 ± 15.3 | 71.2 ± 14.3 | 72.9 ± 14.6 | <0.001 |
| Systolic blood pressure, mmHg | 121.9 ± 23.8 | 127.3 ± 23.2 | 127.7 ± 24.8 | 128.9 ± 25.5 | <0.001 |
| Mean arterial pressure, mmHg | 86.3 ± 14.1 | 86.1 ± 13.5 | 85 ± 14 | 84 ± 14 | <0.001 |
Table displays mean ± standard deviation.
denotes variables displayed as median [interquartile range].
Absolute range for PAPi quartiles: Quartile 1 0.3–2.2, Quartile 2 2.21–3.3, Quartile 3 3.4–5.5, Quartile 4 5.6–30.0.
854 subjects have missing cardiac output calculations and are not included in denoted variables.
Abbreviations: PAPi, pulmonary artery pulsatility index; MI, myocardial infarction; HF, heart failure; BMI, body mass index; NT-proBNP, N-terminal pro-B type natriuretic peptide; PA, pulmonary artery; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; RVSWI, right ventricular stroke work index (calculated using thermodilution cardiac output), Td, thermodilution method. PVR and RVSWI are calculated by thermodilution cardiac output measurement.
Echocardiographic parameters differed across PAPi quartiles (Supplemental Table 1). Patients in the lowest PAPi quartile had higher left ventricular end-diastolic dimension (LVEDD, 51 ± 10 mm) and lower LVEF (51 ± 20%) compared to other quartiles (Figure 1). In addition, factors suggestive of poorer right ventricular function were worse amongst the lowest PAPi quartile, including more frequent dilated right ventricular cavity size (26.3% of patients, compared to ≤ 20.6% amongst other quartiles), abnormal right ventricular function (27.2% of patients, compared to ≤ 20.1% amongst other quartiles), lower right ventricular systolic pressure (43 ± 12.1 mmHg, compared to mean > 47 mmHg amongst other quartiles) and more frequent moderate-or-greater tricuspid regurgitation (28.4% of patients, compared to ≤ 21.2% amongst other quartiles). Among the smaller subset of patients with quantitative echocardiographic parameters of right ventricular function, there was no significant difference in right ventricular basal diameter or fractional area change between PAPi quartiles, and there were modest interquartile differences in tricuspid annular plane systolic excursion, TAPSE (lowest vs highest PAPi quartile: 14.9 ± 5.0 mm vs 17.2 ± 4.7 mm, respectively), and pulsed tissue doppler tricuspid annular systolic velocity, S’ (lowest vs highest PAPi quartile: 8.8 ± 2.7 cm/s vs 10.7 ± 3.1 cm/s, respectively, Supplemental Table 2). Utilizing Spearman correlations, PAPi correlated positively with both TAPSE and S’ (TAPSE, correlation coefficient, rs = 0.21, p=0.01; S’, correlation coefficient, rs = 0.33, P<0.001).
In general, patients in the lowest PAPi quartile had worse hemodynamics than other quartiles with higher PCWP (18 mmHg, compared to 9 mmHg for the highest quartile) and lower thermodilution cardiac index (2.4 L/min/m2, compared to a cardiac index of 2.7 L/min/m2 in patients in the highest quartile). Lower PAPi values aligned with worse indices of other right ventricular hemodynamic parameters. Patients in the lowest PAPi quartile had lower RVSWI (3.3 g/m/beat/m2, compared to 4.9 g/m/beat/m2 in PAPi quartile 4) and a higher RA-to-PCWP ratio (0.4 compared to 0.1 for PAPi quartile 4). Utilizing Spearman correlations due to skewed values, PAPi as a continuous variable correlated positively with RVSWI (correlation coefficient, rs = 0.26, p < 0.001). In addition, PAPi demonstrated a strong negative correlation with RA-to-PCWP ratio (rs = −0.88, p< 0.001).
Clinical and echocardiographic correlates of log PAPi
We utilized comprehensive and stepwise regression models to examine clinical and echocardiographic associations with PAPi (Table 2, Figure 2). Younger age, male sex, higher BMI, and the presence of obstructive sleep apnea were all associated with lower PAPi in multivariable-adjusted analyses (P<0.001 for all). Specifically, in the stepwise regression model, a 1-SD higher BMI was associated with a 0.23 log-unit lower PAPi (standard error (s.e.) 0.01, p<0.001). For echocardiographic measures, lower LVEF, higher LVEDD, and the presence of tricuspid regurgitation were associated with lower PAPi (P<0.001 for all). Specifically, in the stepwise regression model, a one-standard deviation lower LVEF was associated with a 0.1 log-unit lower PAPi (s.e. 0.01), whereas the presence of moderate-or-greater tricuspid regurgitation was associated with a 0.24 unit lower PAPi (s.e. 0.023). The presence of abnormal right ventricular function or a dilated right ventricle were associated with PAPi in age- and sex-adjusted analyses (p<0.001 for both), but were not selected in the stepwise model in favor of LVEF and LVEDD. Overall, utilizing a stepwise model of only clinical predictors explained 10.4% of the variance of PAPi, which increased to 12.7% of the variance of PAPi after incorporating basic echocardiographic variables into the regression model.
Table 2:
Models of clinical and echocardiographic predictors of log PAPi
| Predictors | Age/sex adjusted model |
Stepwise model 1 (n=8285 Adj R2 = 10.4%) |
Stepwise model 2 (n=5466 Adj R2 = 12.73%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β estimate | Standard error | p value | β estimate | Standard error | p value | β estimate | Standard error | p value | |
| Clinical variables | |||||||||
| Age | 0.080 | 0.01 | <0.001 | 0.08 | 0.01 | <0.001 | 0.07 | 0.01 | <0.001 |
| Male sex | −0.13 | 0.02 | <0.001 | −0.11 | 0.02 | <0.001 | −0.15 | 0.02 | <0.001 |
| BMI | −0.21 | 0.01 | <0.001 | −0.20 | 0.01 | <0.001 | −0.23 | 0.01 | <0.001 |
| Diabetes | −0.12 | 0.02 | <0.001 | – | – | – | – | – | – |
| Hypertension | −0.09 | 0.02 | <0.001 | – | – | – | – | – | – |
| Previous MI | −0.02 | 0.02 | 0.274 | – | – | – | – | – | – |
| Previous HF | −0.09 | 0.02 | <0.001 | −0.08 | 0.02 | <0.001 | – | – | – |
| Previous Lung Disease | 0.06 | 0.02 | 0.009 | 0.09 | 0.02 | <0.001 | 0.08 | 0.03 | <0.001 |
| Obstructive sleep apnea | −0.30 | 0.02 | <0.001 | −0.12 | 0.02 | <0.001 | −0.10 | 0.03 | <0.001 |
| Chronic kidney disease | −0.11 | 0.05 | 0.029 | – | – | – | – | – | – |
| Echocardiographic variables | |||||||||
| LVEF | 0.06 | 0.01 | <0.001 | – | – | – | 0.10 | 0.01 | <0.001 |
| LVEDD | −0.05 | 0.01 | <0.001 | – | – | – | 0.06 | 0.01 | <0.001 |
| Abnormal RV function | −0.14 | 0.02 | <0.001 | – | – | – | – | – | – |
| Dilated RV | −0.15 | 0.02 | <0.001 | – | – | – | – | – | – |
| Tricuspid regurgitation | −0.22 | 0.02 | <0.001 | – | – | – | −0.24 | 0.02 | <0.001 |
Beta estimates display change in log-transformed PAPi per one standard deviation increment for continuous predictors or between-none for categorical predictors. Standard deviations (SD) for continuous predictors: Age = 12.6; BMI = 6.8; LVEF = 19.7; LVEDD = 9.5. Stepwise model 1 included all clinical variables eligible for entry; stepwise model 2 included all listed predictors as eligible for entry.
Abbreviations: S.E., standard error; BMI, body mass index; MI, myocardial infarction; HF, heart failure; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; RV, right ventricle/ventricular.
Figure 2:
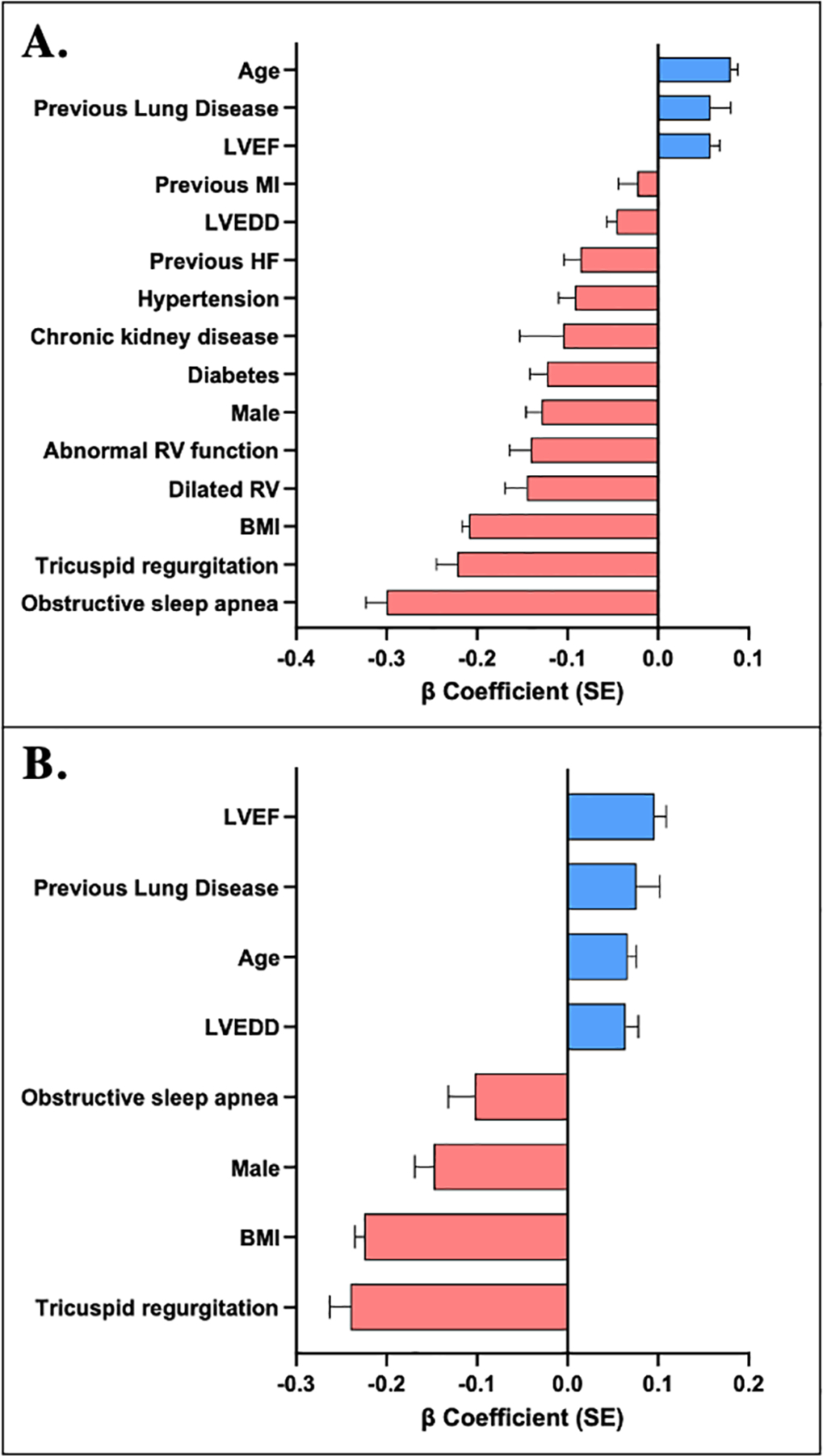
Clinical and echocardiographic correlates of log PAPi. A, Age- and sex-adjusted individual correlates with PAPi. B, Stepwise regression model identifying significant clinical and echocardiographic correlates with PAPi. Abbreviations: PAPi, pulmonary artery pulsatility index; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; MI, myocardial infarction; BMI, body mass index; RV, right ventricular.
Association of PAPi with mortality and adverse cardiovascular events:
Over a mean (SD) follow-up of 6.7 (3.3) years, there were 3006 deaths, 2668 MACE and 2004 HF hospitalizations. The Kaplan-Meier curves for probability of MACE and HF hospitalizations by PAPi quartile are displayed in Figure 3 and demonstrate greatest risk of adverse outcomes among individuals in the lowest PAPi quartile (adjusted log-rank p < 0.001 for Q1 vs Q4), with intermediate risk of outcomes among quartiles 2 and 3 when compared with the highest quartile (Q2 vs Q4, adjusted log-rank p=0.03 for MACE and p=0.02 for HF hospitalizations; Q3 vs Q4, adjusted log-rank p=0.02 for MACE and p=0.002 for HF hospitalizations).
Figure 3:
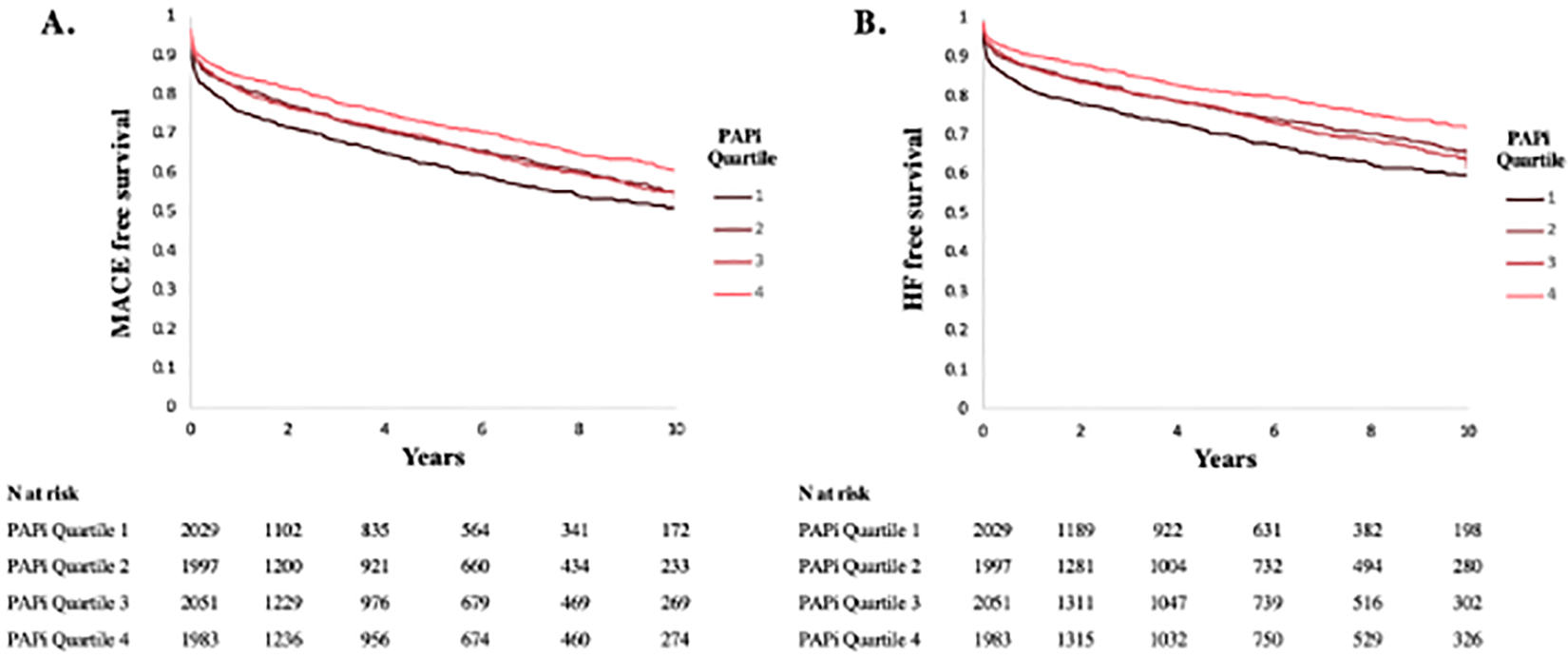
Event-free survival. Kaplan Meier curves displaying percent freedom of MACE (A) and HF hospitalization (B). Numbers of patients at risk per PAPi quartile are displayed below the figures. Abbreviations: PAPi, pulmonary artery pulsatility index; MACE, major adverse cardiovascular events; HF, heart failure.
A lower PAPi was associated with all-cause mortality (HR 0.90 per 1-standard deviation higher in log-transformed PAPi, 95% CI 0.85–0.96, p<0.001), MACE (HR 0.81, 95% CI 0.77–0.85, p<0.001), and HF hospitalization (HR 0.73, 95% CI 0.69–0.78, p<0.001) (Table 3). To understand the differences across PAPi quartiles, we then examined the association of PAPi quartile with all-cause mortality in multivariable analyses, adjusting for age, sex, BMI, previous myocardial infarction, previous HF, hypertension, diabetes mellitus, and chronic kidney disease (Table 4). With the highest PAPi quartile serving as the referent group, we found the risk of death was higher among individuals in the lower PAPi quartile groups, including a 15% higher risk of death among Q3, 20% among Q2, and 60% among Q1 (Figure 4).
Table 3:
Association between log PAPi and Death/Incident Cardiovascular Hospitalization
| Outcome | Age- and sex-adjusted | Multivariable adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| All-cause mortality n=3006/8285 |
0.90 (0.85, 0.96) | <0.001 | 0.85 (0.80, 0.90) | <0.001 |
| MACE n=2668/8060 |
0.81 (0.77, 0.85) | <0.001 | 0.79 (0.75, 0.84) | <0.001 |
| HF hospitalization n=2004/8060 |
0.73 (0.69, 0.78) | <0.001 | 0.73 (0.68, 0.78) | <0.001 |
HR per 1-standard deviation increment in log-transformed PAPi. Multivariable model adjusted for age, sex, BMI, diabetes, hypertension, chronic kidney disease, previous MI, previous HF.
Abbreviations: PAPi, pulmonary artery pulsatility index; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiovascular events; HF, heart failure.
Table 4:
Association between PAPi Quartile and Death/Incident Cardiovascular Hospitalization
| Outcome | PAPi quartile | Age- and sex-adjusted |
Multivariable adjusted |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | p-trend | HR (95% CI) | p-value | p trend | ||
| All-cause mortality | Q4 | referent | referent | <0.001 | referent | referent | <0.001 |
| n=3006/8285 | Q3 | 1.08 (0.97, 1.21) | 0.15 | 1.15 (1.03, 1.28) | 0.01 | ||
| Q2 | 1.08 (0.95, 1.24) | 0.24 | 1.20 (1.04, 1.37) | 0.01 | |||
| Q1 | 1.35 (1.15, 1.58) | <0.001 | 1.60 (1.36, 1.88) | <0.001 | |||
| MACE | Q4 | referent | referent | <0.001 | referent | referent | <0.001 |
| n=2668/8060 | Q3 | 1.22 (1.09, 1.37) | <0.001 | 1.23 (1.09, 1.38) | <0.001 | ||
| Q2 | 1.26 (1.11, 1.44) | <0.001 | 1.32 (1.15, 1.50) | <0.001 | |||
| Q1 | 1.66 (1.44, 1.90) | <0.001 | 1.80 (1.56, 2.07) | <0.001 | |||
| HF hospitalization | Q4 | referent | referent | <0.001 | referent | referent | <0.001 |
| n=2004/8060 | Q3 | 1.34 (1.17, 1.53) | <0.001 | 1.32 (1.15, 1.51) | <0.001 | ||
| Q2 | 1.37 (1.18, 1.60) | <0.001 | 1.39 (1.19, 1.63) | <0.001 | |||
| Q1 | 2.00 (1.69, 2.35) | <0.001 | 2.08 (1.76, 2.47) | <0.001 | |||
Abbreviations: PAPi, pulmonary artery pulsatility index; HR, hazard ratio; Q, quartile; MACE, major adverse cardiovascular events; HF, heart failure.
Figure 4:
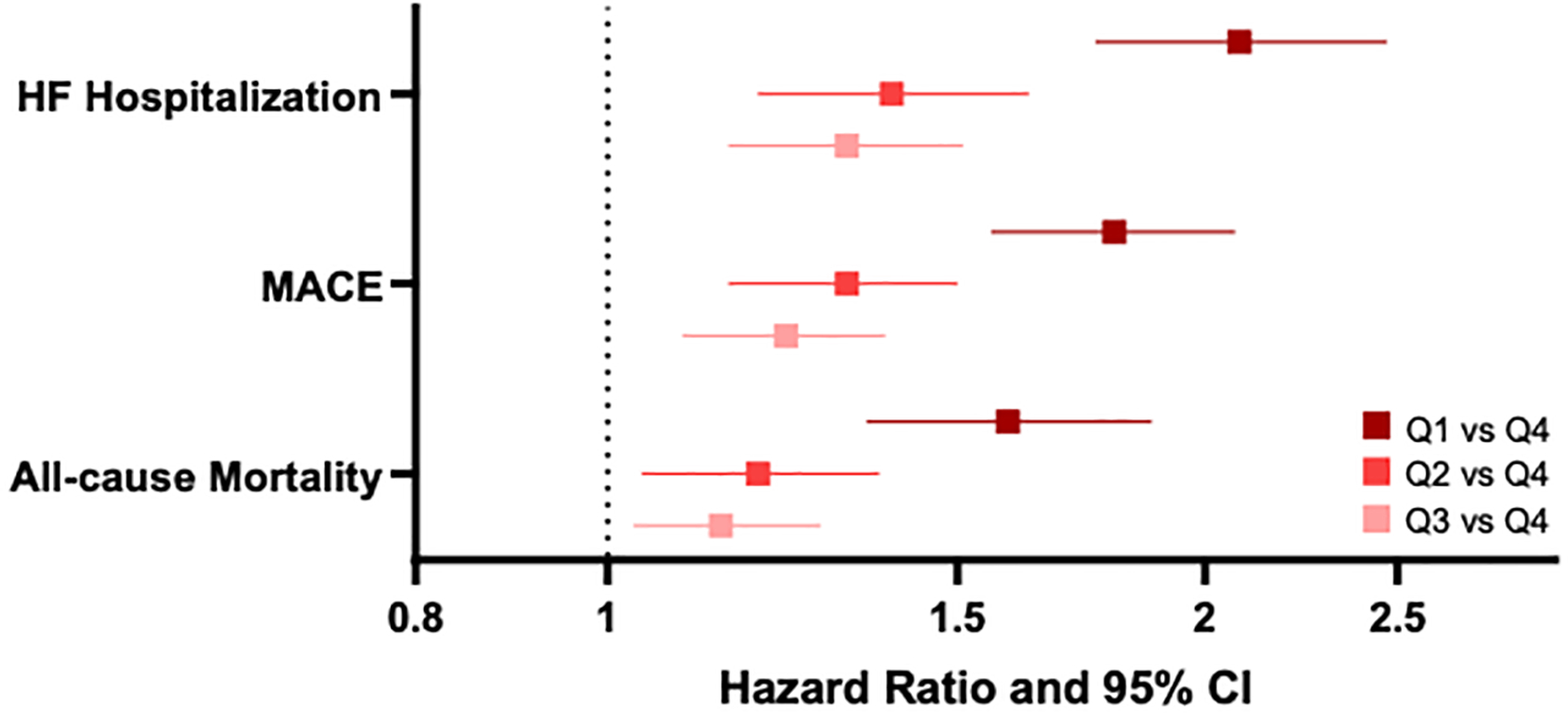
Association between PAPi quartiles and adverse outcomes. Model is adjusted for age, sex, BMI, previous MI, previous HF, hypertension, diabetes, and chronic kidney disease.
We found similar associations of PAPi quartiles with MACE and HF hospitalizations Specifically, patients in the lowest vs highest PAPi quartile were at increased risk of future MACE (multivariable adjusted HR 1.80, 95% CI 1.56–2.07, p < 0.001) and HF hospitalization (HR 2.08, 95% CI 1.76–2.47, p < 0.001). In addition, individuals in PAPi quartiles 2 and 3 appeared to be intermediate risk for both outcomes. As RV dysfunction is often linked to elevated afterload through increased pulmonary vascular resistance, we performed a stratified analysis by high vs low PVR (≥ vs < 3 Wood units, Supplemental Table 3). We confirmed that a higher PAPi was associated with a lower hazard for adverse outcomes irrespective of an elevated PVR.
Predictive ability of PAPi compared to other hemodynamic parameters of RV function
We next examined the association of other hemodynamic parameters of RV function with adverse clinical outcomes (Supplemental Table 4). Similar to PAPi, the log-transformed RA:PCWP ratio was associated with MACE (HR 1.15, 95% CI 1.09–1.22, p<0.001) and HF hospitalization (HR 1.16, 95% CI 1.09–1.24, p<0.001). By contrast, log-transformed RVSWI was not associated with MACE or HF hospitalization. We utilized C-statistics to examine incremental information of each metric of RV function (PAPi, RA:PCWP, and RVSWI) when added to the clinical model (Supplemental Table 5). PAPi improved the C-statistic for HF hospitalizations (p=0.008) and MACE (p=0.03), whereas RA:PCWP ratio did not. Amongst the sample of patients with available cardiac output measurements, PAPi improved the C-statistic for HF hospitalization to a marginally greater degree than RVSWI.
DISCUSSION:
In a hospital-based cohort of patients across a broad spectrum of cardiopulmonary disease referred for RHC, we described the clinical, hemodynamic, and echocardiographic profiles of patients with low PAPi and evaluated the association of PAPi with all-cause mortality and adverse cardiovascular outcomes over 10 years of follow-up. We found the following: 1) patients in the lowest PAPi quartile tended to be younger, greater percent men, have a higher BMI, and had worse hemodynamic parameters of right ventricular function with accompanying lower cardiac index; 2) the presence of a higher BMI or moderate-or-greater degree of tricuspid regurgitation were strongly associated with a worse PAPi; and 3) patients in the lowest PAPi quartile had a 60% greater risk of mortality and approximately twice the risk of MACE and HF hospitalization compared to patients in the highest PAPi quartile, though individuals with intermediate PAPi in the range of 2.2 to 5.5 were also at greater risk of cardiovascular events.
PAPi, the ratio of pulmonary artery pulse pressure to the RA pressure, is a relatively new hemodynamic parameter of right ventricular function, though it has become increasingly utilized for assessment of right ventricular failure, particularly as it relates to mechanical circulatory support device implantation25. PAPi is influenced by the degree of right ventricular afterload (influenced by pulmonary vascular resistance and PCWP) and contractile function (through the PA pulse pressure measurement) as well as the presence of congestion (through incorporation of the RA pressure). A low PAPi has been previously demonstrated in both the stable and acutely ill HF patients to predict mortality and adverse renal and cardiovascular outcomes14, 15, 17, 19–22. In the HF population, the decrease in PAPi reflective of progressive right ventricular dysfunction can be mediated through an increase in RA pressure or through a decrease in PA pulse pressure due to decreased right ventricular stroke volume. Our findings interestingly demonstrated that PAPi carries an important prognostic value even when RA-to-PCWP ratio is considered “normal.” In fact, a minority of patients, even amongst the lowest PAPi quartile, had a RA-to-PCWP ratio of > 0.6, a value traditionally thought to represent right ventricular dysfunction out-of-proportion to left ventricular dysfunction in the heart failure population. We also demonstrate that while PAPi is correlated negatively with RA:PCWP and positively with RVSWI, PAPi is associated with MACE and HF hospitalizations, whereas RVSWI is not. While both PAPi and RA:PCWP ratio are associated with adverse clinical outcomes, incremental improvement in the c-statistic is greatest when PAPi is added to a clinical model.
Prior studies have demonstrated the importance of PAPi in predicting severe right ventricular failure necessitating additional hemodynamic support in specific patient populations with advanced cardiac disease 13–15, 18. In addition to right ventricular failure, low PAPi has also previously been shown to be associated with higher mortality in the post-myocardial infarction, pulmonary arterial hypertension, and advanced heart failure populations 13, 16, 20. We now extend these findings to a broad hospital-based cohort of patients across a much greater spectrum of cardiopulmonary disease, and we demonstrate that lower PAPi is associated with increased risk of adverse events including all-cause mortality, MACE, and HF hospitalizations.
Stratification of the overall cohort of patients into PAPi quartile revealed unique phenotypic differences in the patients in the lowest PAPi quartile. In baseline demographic characteristics, male sex and a younger age were associated with a lower PAPi. In ambulatory studies of patients with heart failure and pulmonary hypertension and in the advanced heart failure population, there were no sex differences observed16, 20, 21, and only the ambulatory heart failure population mirrored our observations that a younger age was associated with a lower PAPi21. Further sex-and age-specific research is warranted. We also observed a higher BMI and higher prevalence of obstructive sleep apnea in the lowest PAPi quartile. There are growing data supporting the increase in right ventricular dysfunction among obese patients26, 27 and in patients with obstructive sleep apnea, though initiation of CPAP has been demonstrated to improve right ventricular structure and function in the latter28–30. In addition, we observed that the presence of moderate-or-greater tricuspid regurgitation was associated with a lower PAPi. Significant tricuspid regurgitation leads to right ventricular dilation through increased diastolic volume, and progressive right ventricular dilation/dysfunction and a lower cardiac output in these patients have been shown to be associated with mortality31. It has yet to be studied whether PAPi can help risk-stratify patients with significant tricuspid regurgitation to identify those who may benefit from intervention.
To date, there has been no determination of a “normal” PAPi range. In prior studies, receiver operating characteristic curves have demonstrated an optimal PAPi cutoff of < 1.85–2 for right ventricular failure after left ventricular assist device implantation14, 15, < 1.9 for right ventricular dysfunction after cardiac surgery18, < 3.65 for mortality or hospitalization in the advanced heart failure population20, and < 1 for in-hospital mortality and/or requirement for percutaneous right ventricular support device after inferior myocardial infarction13. In pulmonary arterial hypertension and ambulatory heart failure patients, only the lowest PAPi quartile patients (PAPi less than 3.7 and 1.4, respectively) were found to have adverse outcomes16, 21. However, outside of the aforementioned specific populations, a setpoint of PAPi below which all-cause mortality is increased has not been evaluated in a diverse population. In our study, the lowest PAPi quartile, which had the highest adjusted risk for mortality, MACE, and HF hospitalization, had a median PAPi of 1.7, aligning with the majority of above studies. Importantly, we observed that intermediate PAPi quartiles 2 and 3 (corresponding to a range of PAPi between 2.2 and 5.5) remained associated with worse cardiovascular outcomes compared with the highest PAPi quartile, extending prior thresholds studied across a broader sample.
While our study expands PAPi as a predictor of mortality and adverse events across a more inclusive and heterogeneous population, it is possible that clinical PAPi thresholds differ amongst selective populations 32. As above, our findings demonstrate the association of a higher BMI with a lower PAPi, though there is a known entity referred to as the “obesity paradox” where obese patients with cardiovascular disease have a better prognosis compared to non-obese patients26, 33. Whether obesity modifies the increased hazard for mortality, MACE, and HF hospitalizations observed in the lowest PAPi quartile has yet to be determined. We also acknowledge that pre-capillary pulmonary arterial hypertension was infrequent in our sample, as noted by the normal values for pulmonary vascular resistance and transpulmonary gradient across PAPi quartiles. Therefore, we cannot comment on the potential divergent effects of PAPi in pulmonary arterial hypertension, where a high PAPi may reflect patients with the highest degree of adverse pulmonary vascular remodeling, and a lower PAPi could represent either lower pulmonary pressures or progressive right ventricular dysfunction with elevated RA pressures. Further research to elucidate accurate interpretation of PAPi and its prognostic role in these specific populations is merited.
Several limitations deserve mention in our study. As our study was an observational sample of patients undergoing clinically-indicated RHC, we could not control for referral bias and confounding by indication for RHC. In addition, there are likely unmeasured clinical confounders that modify the association between PAPi and adverse outcomes. Given the observational nature of the study design, causal inferences cannot be drawn. For example, the mechanisms underlying our observation of greater BMI with lower PAPi remain incompletely understood. Another limitation of our study was the reliance on code-based diagnoses of MACE and HF endpoints, as well as follow-up that was restricted to patients who had longitudinal care in our health-care system. Our adjudication methods may have underestimated the number of non-fatal events, although we suspect this underestimation would not result in differential bias by PAPi quartile. We also lacked the precise dates of death of 242 individuals within three years of abstraction date due to confidentiality protections and imputed the midpoint between last disclosed date and pull date of data to approximate dates of death. We believe this likely had minimal influence on results, representing only 8% of all total death events. In addition, the majority of echocardiographic data were not extracted on the day of RHC, so it is possible that interventions could have been performed between the time of echocardiography and RHC. Our study excluded patients with recent shock, mechanical circulatory support, or cardiac arrest, and thus our findings cannot be extrapolated to the acute setting of a critically ill patient. Last, though we demonstrate that PAPi is an important prognostic marker across a spectrum of cardiovascular disease, these data can only be applied to patients who undergo invasive hemodynamic assessment. Within the small subset of patients with available same-day quantitative right ventricular echocardiographic data, the data overall demonstrate poor correlation with PAPi. Future studies may further elucidate the comparison of invasive versus more advanced noninvasive diagnostic assessment of right ventricular function with relation to adverse outcomes.
In sum, we provide growing evidence of the prognostic capability of PAPi across diverse patient populations. Patients in the lowest PAPi quartile are at greatest hazard for all-cause mortality and adverse cardiovascular outcomes, though we extend findings of greater cardiovascular event risk into the intermediate PAPi range beyond previous studies. Our findings suggest that a low PAPi can be utilized to serve as a risk factor for adverse events across a spectrum of disease. Future studies are needed to determine whether change in PAPi modifies risk of mortality or cardiovascular hospitalizations in specific disease populations and if identification of this at-risk population may allow for targeted intervention to change disease trajectory.
Supplementary Material
Commentary:
The pulmonary artery pulsatility index (PAPi), defined as the ratio of pulmonary artery pulse pressure to right atrial pressure (), is a hemodynamic parameter of right ventricular function.
Building on prior studies of PAPi in selected samples, we demonstrate that a lower PAPi is associated with all-cause mortality, major adverse cardiac events, and heart failure hospitalizations across a large heterogeneous patient sample. Lower PAPi was associated with adverse outcomes even at much higher ranges than previously reported. For example, patients in the third quartile of PAPi (PAPi range 3.4 – 5.5) had a 15% higher risk of all-cause mortality and > 20% higher risk of MACE and HF hospitalizations compared to patients in the fourth quartile of PAPi (PAPi ≥ 5.6).
A low PAPi, even at values higher than previously reported, may serve an important role in identifying high-risk individuals across a broad spectrum of cardiovascular disease. Future studies examining the utility of PAPi across higher ranges for identification of at-risk individuals and subsequent clinical implications are warranted.
Funding:
Dr. Ho was supported by grants from NIH (R01-HL134893, R01-HL140224, and K24-HL153669).
Disclosures:
Dr. Ho has received research grants from Gilead Sciences and Bayer AG, and research supplies from EcoNugenics. The remaining authors have nothing to disclose.
Non-standard Abbreviations and Acronyms
- BMI
body mass index
- HF
heart failure
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- NT-proBNP
N-terminal pro-B type natriuretic peptide
- PAPi
pulmonary artery pulsatility index
- RA:PCWP
ratio of right atrial to pulmonary capillary wedge pressure
- RHC
right heart catheterization
- RV
right ventricular
- RVSWI
right ventricular stroke work index
Footnotes
Supplemental Material: Supplemental Tables S1–S5.
Contributor Information
Emily K. Zern, Corrigan Minehan Heart Center, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Dongyu Wang, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA.
Paula Rambarat, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Samuel Bernard, Leon H. Charney Division of Cardiology, New York University Grossman School of Medicine.
Samantha M. Paniagua, Corrigan Minehan Heart Center, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Elizabeth E. Liu, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA.
Jenna McNeill, Pulmonary and Critical Care Division, Massachusetts General Hospital, Boston, MA.
Jessica K. Wang, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA.
Carl T. Andrews, Corrigan Minehan Heart Center, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Eugene V. Pomerantsev, Corrigan Minehan Heart Center, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Michael H. Picard, Corrigan Minehan Heart Center, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Jennifer E. Ho, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA.
REFERENCES:
- 1.Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV and Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F and Ling LH. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. [DOI] [PubMed] [Google Scholar]
- 3.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL and all i. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–879. [DOI] [PubMed] [Google Scholar]
- 4.Nochioka K, Querejeta Roca G, Claggett B, Biering-Sorensen T, Matsushita K, Hung CL, Solomon SD, Kitzman D and Shah AM. Right Ventricular Function, Right Ventricular-Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018;3:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prins KW, Rose L, Archer SL, Pritzker M, Weir EK, Olson MD and Thenappan T. Clinical Determinants and Prognostic Implications of Right Ventricular Dysfunction in Pulmonary Hypertension Caused by Chronic Lung Disease. J Am Heart Assoc. 2019;8:e011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah JP, Yang Y, Chen S, Hagar A, Pu XB, Xia T, Ou Y, Chen M and Chen Y. Prevalence and Prognostic Significance of Right Ventricular Dysfunction in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122:1932–1938. [DOI] [PubMed] [Google Scholar]
- 7.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD and Investigators S. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–5. [DOI] [PubMed] [Google Scholar]
- 8.Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja J, Brown TD, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi TC, Sharma R, Carpenter JP, Cook SA, Cowie MR, Assomull RG, Pennell DJ and Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013;128:1623–33. [DOI] [PubMed] [Google Scholar]
- 9.Merlo M, Gobbo M, Stolfo D, Losurdo P, Ramani F, Barbati G, Pivetta A, Di Lenarda A, Anzini M, Gigli M, Pinamonti B and Sinagra G. The Prognostic Impact of the Evolution of RV Function in Idiopathic DCM. JACC Cardiovasc Imaging. 2016;9:1034–1042. [DOI] [PubMed] [Google Scholar]
- 10.Purmah Y, Lei LY, Dykstra S, Mikami Y, Cornhill A, Satriano A, Flewitt J, Rivest S, Sandonato R, Seib M, Lydell CP, Howarth AG, Heydari B, Merchant N, Bristow M, Fine N, Gaztanaga J and White JA. Right Ventricular Ejection Fraction for the Prediction of Major Adverse Cardiovascular and Heart Failure-Related Events: A Cardiac MRI Based Study of 7131 Patients With Known or Suspected Cardiovascular Disease. Circ Cardiovasc Imaging. 2021;14:e011337. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Prakasa K, Bomma C, Tandri H, Dalal D, James C, Tichnell C, Corretti M, Bluemke D, Calkins H and Abraham TP. Comparison of novel echocardiographic parameters of right ventricular function with ejection fraction by cardiac magnetic resonance. J Am Soc Echocardiogr. 2007;20:1058–64. [DOI] [PubMed] [Google Scholar]
- 13.Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelstiel CD and Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. [DOI] [PubMed] [Google Scholar]
- 14.Kang G, Ha R and Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016;35:67–73. [DOI] [PubMed] [Google Scholar]
- 15.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D and Kapur NK. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After Left Ventricular Assist Device Surgery. J Card Fail. 2016;22:110–6. [DOI] [PubMed] [Google Scholar]
- 16.Mazimba S, Welch TS, Mwansa H, Breathett KK, Kennedy JLW, Mihalek AD, Harding WC, Mysore MM, Zhuo DX and Bilchick KC. Haemodynamically Derived Pulmonary Artery Pulsatility Index Predicts Mortality in Pulmonary Arterial Hypertension. Heart Lung Circ. 2019;28:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo KB, Mezue K, Ram P, Goyal A, Shah M, Krishnamoorthy P, Gupta S, Pressman GS and Rangaswami J. Echocardiographic and Hemodynamic Parameters Associated with Diminishing Renal Filtration among Patients with Heart Failure with Preserved Ejection Fraction. Cardiorenal Med. 2019;9:83–91. [DOI] [PubMed] [Google Scholar]
- 18.Rong LQ, Rahouma M, Neuburger PJ, Arguelles G, Emerson J, Mauer E, Tam C, Shore-Lesserson L, Pryor KO and Gaudino M. Use of Pulmonary Artery Pulsatility Index in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2020;34:1220–1225. [DOI] [PubMed] [Google Scholar]
- 19.Guven G, Brankovic M, Constantinescu AA, Brugts JJ, Hesselink DA, Akin S, Struijs A, Birim O, Ince C, Manintveld OC and Caliskan K. Preoperative right heart hemodynamics predict postoperative acute kidney injury after heart transplantation. Intensive Care Med. 2018;44:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochav SM, Flores RJ, Truby LK and Topkara VK. Prognostic Impact of Pulmonary Artery Pulsatility Index (PAPi) in Patients With Advanced Heart Failure: Insights From the ESCAPE Trial. J Card Fail. 2018;24:453–459. [DOI] [PubMed] [Google Scholar]
- 21.Cesini S, Bhagra S and Pettit SJ. Low Pulmonary Artery Pulsatility Index Is Associated With Adverse Outcomes in Ambulatory Patients With Advanced Heart Failure. J Card Fail. 2020;26:352–359. [DOI] [PubMed] [Google Scholar]
- 22.Kuwayama T, Morimoto R, Oishi H, Kato H, Kimura Y, Kazama S, Shibata N, Arao Y, Yamaguchi S, Hiraiwa H, Kondo T, Furusawa K, Okumura T and Murohara T. Efficacy of Pulmonary Artery Pulsatility Index as a Measure of Right Ventricular Dysfunction in Stable Phase of Dilated Cardiomyopathy. Circ J. 2020;84:1536–1543. [DOI] [PubMed] [Google Scholar]
- 23.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 24.Lin DW LJ; Ying Z Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 25.Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT and Burkhoff D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation. 2017;136:314–326. [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV and Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 27.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanner BM, Konermann M, Sturm A, Muller HJ and Zidek W. Right ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 1997;10:2079–83. [DOI] [PubMed] [Google Scholar]
- 29.Chu AA, Yu HM, Yang H, Tian LM, Hu ZY, Jiang N, Xie WX and Huang Y. Evaluation of right ventricular performance and impact of continuous positive airway pressure therapy in patients with obstructive sleep apnea living at high altitude. Sci Rep. 2020;10:20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Shim CY, Cho YJ, Park S, Lee CJ, Park JH, Cho HJ, Ha JW and Hong GR. Continuous Positive Airway Pressure Therapy Restores Cardiac Mechanical Function in Patients With Severe Obstructive Sleep Apnea: A Randomized, Sham-Controlled Study. J Am Soc Echocardiogr. 2019;32:826–835. [DOI] [PubMed] [Google Scholar]
- 31.Chen E, L’Official G, Guerin A, Dreyfus J, Lavie-Badie Y, Sportouch C, Eicher JC, Marechaux S, Le Tourneau T, Oger E and Donal E. Natural history of functional tricuspid regurgitation: impact of cardiac output. Eur Heart J Cardiovasc Imaging. 2021;22:878–885. [DOI] [PubMed] [Google Scholar]
- 32.Lim HS and Gustafsson F. Pulmonary artery pulsatility index: physiological basis and clinical application. Eur J Heart Fail. 2020;22:32–38. [DOI] [PubMed] [Google Scholar]
- 33.Lavie CJ, Milani RV and Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


