Abstract
Objective:
Past research suggests that certain sociodemographic factors may put youth with spina bifida (SB) at risk for poor outcomes. The aims of this study were to examine: (1) associations between ten sociodemographic factors and health-related, neuropsychological, and psychosocial functioning among youth with SB; (2) cumulative sociodemographic risk as a predictor of youth outcomes as moderated by age, and; (3) SB-related family stress as a mediator of longitudinal associations between cumulative sociodemographic risk and youth outcomes.
Methods:
Participants were youth with SB (N = 140 at Time 1; M age at Time 1 = 11.43, 53.6% female) recruited as part of a larger, longitudinal study. The study included questionnaire (parent-, teacher-, and youth-report), neuropsychological testing, and medical chart data across three time points, spaced two years apart.
Results:
A subset of the sociodemographic factors and their cumulative risk were associated with study outcomes. Specifically, youth characterized by sociodemographic risk had greater pain and lower academic achievement, but also fewer UTIs and fewer attention and executive function problems. Age did not moderate the association between cumulative risk and outcomes. Cumulative risk predicted lower SB-related family stress, which, in turn, predicted several outcomes.
Conclusions:
Examining a range of sociodemographic factors is warranted. Sociodemographic risk is linked to poorer outcomes for some risk indicators but similar or better outcomes for others. Results have implications for delivering evidence-based, diversity-sensitive clinical care to youth with SB.
Keywords: Family Stress, Health Disparities, Longitudinal Research, Sociodemographic Factors, Spina Bifida
Spina bifida (SB) is a congenital malformation caused by the failed closure of the embryonic neural tube during the early stages of pregnancy, resulting in malformations of the spinal cord and cerebral cortex (Copp et al., 2015). It is one of the most common congenital birth defects in the United States, occurring in roughly 3 of every 10,000 live births (Canfield et al., 2014). Individuals with SB face a host of health-related, neuropsychological, and psychosocial challenges throughout their lives. Health-related challenges may include neurological and gross motor impairments as well as neurogenic bowel and bladder (Copp et al., 2015). Impairments in neuropsychological functioning may include deficits in overall intellectual ability, executive functions, attention, memory, and visual-motor integration (Dennis, Landry, Barnes, & Fletcher, 2006). Lastly, youth with SB have poorer psychosocial outcomes compared to typically-developing youth, including being at risk for increased internalizing symptoms (Kabra, Feustal, & Kogan, 2015), social difficulties (Devine, Holmbeck, Gayes, & Purnell, 2012), and lower health-related quality of life (HRQOL; Murray et al., 2015).
Past research has examined factors that impact outcomes among youth with SB and their families, and there is growing evidence that sociodemographic factors may put youth at risk for poor health-related, neuropsychological, and psychosocial functioning. Studies on health-related outcomes have found, for example, that non-Hispanic African Americans with SB and those without private insurance were more likely to have bladder and bowel incontinence (Schechter et al., 2015). Similarly, studies examining neuropsychological outcomes have found that household income explained variance in overall cognitive functioning (Wohlfeiler, Macias, & Saylor, 2008) and lower SES was associated with poorer associative cognitive processes (Dennis et al., 2006) and vocabulary scores (Bier, Morales, Liebling, Geddes, & Kim, 1997). Finally, studies on psychosocial outcomes have found that youth with SB participated in less social activities if they were Hispanic (Liptak, Kennedy, & Dosa, 2010) or from families reporting lower income, lower parent education, and single-parent status (Law et al., 2006). Also, children with SB from low-SES homes had more social problems (Holmbeck et al., 2003) and fewer friends than typically-developing children (Zukerman, Devine, & Holmbeck, 2011).
A more comprehensive examination of how youth with SB are impacted by sociodemographic factors is needed, given the complex nature of the condition and the numerous challenges that these youth may face. This is especially true considering that the prevalence rates of SB differ based on SES (e.g., women with less than a high school education have a 1.7-fold increased risk of delivering infants with a neural tube defect; Grewal, Carmichael, Song, & Shaw, 2009) and ethnicity (Hispanics have the highest rates at 4.2 per 10,000 live births; Canfield et al., 2014). Moreover, families of youth with SB face a significant economic burden, with the lifetime cost of care for a person with SB estimated at $791, 900 (Grosse, Berry, Mick Tilford, Kucik, & Waitzman, 2016). Another reason why more research is needed to understand the impact of sociodemographic factors among youth with SB is the lack of clarity and consistency across the various conceptual and methodological approaches used in this area of research (Cheng et al., 2015). For example, multiple indicators (e.g., income, education, insurance status) are often used as proxies for “SES”, and such factors are frequently examined in isolation and not in comparison to each other, indicating that variance explained by related constructs is not taken into account (Cheng et al., 2015). This inconsistency across studies poses a significant challenge to understanding mechanisms through which these relations occur, thereby hindering the development of interventions and policies that can help reduce or eliminate the negative impact of such sociodemographic factors at both the individual and societal level (AAP, 2010; Cheng et al., 2015).
Because the impact of sociodemographic factors on child outcomes unfolds via complex processes, various theoretical models have been proposed to explain these pathways, many drawing on the bioecological model of human development (Bronfenbrenner, & Morris, 2006). One such model is the cumulative risk model, which has been proposed to explain how the accumulation of sociodemographic risks affects development (Rutter, 1993). It has also been suggested that assessing the timing and duration of risk across development is important. While some studies suggest that risk during early “sensitive” periods is a greater predictor of poor long-term outcomes (e.g., Atkinson et al., 2015), studies that have tested competing models have found that the persistence of cumulative risk overtime is more influential (e.g., Dunn et al., 2018; Schoon et al., 2002). While cumulative risk has not been examined in a sample of youth with SB, a study on youth with cancer found cumulative risk to be associated with child and parent outcomes (Bemis et al., 2015). Another theoretical model used to study the impact of sociodemographic factors is the family stress model, which posits that sociodemographic factors influence child development indirectly through parent and family functioning (Conger & Donnellan, 2007). This model has not been tested in youth with SB, but it has found support in other pediatric health condition populations (e.g., type 1 diabetes; Drew et al., 2011). Both the cumulative risk model and the family stress model offer valuable frameworks for investigating how sociodemographic factors may impact outcomes among youth with SB, which would allow for identification of targets for intervention (Cheng et al., 2015).
The current study sought to expand upon the limited understanding of how various sociodemographic risk factors are associated with health-related, neuropsychological, and psychosocial functioning in youth with SB, by drawing on established theoretical models that have not yet been tested in this population, and by addressing several methodological issues that exist in the current research. The first objective was to examine associations between ten sociodemographic factors and health-related, neuropsychological, and psychosocial functioning in youth with SB. It was hypothesized that, compared to youth not characterized by sociodemographic risk, those who are characterized by sociodemographic risk will demonstrate poorer health-related, neuropsychological, and psychosocial functioning (see Figure 1). The second objective was to examine the cumulative effect of sociodemographic risk as a predictor of youth health-related, neuropsychological, and psychosocial functioning, as moderated by age. It was hypothesized that greater cumulative risk would be associated with poorer health-related, neuropsychological, and psychosocial functioning concurrently, and these associations would be stronger for older youth. The third objective was to examine SB-related family stress as a mediator of the association between cumulative risk and youth health-related, neuropsychological, and psychosocial functioning over time. It was hypothesized that cumulative risk at Time 1 would predict greater SB-related family stress at Time 2, which would, in turn, predict poorer youth health-related, neuropsychological, and psychosocial functioning at Time 3.
Figure 1.
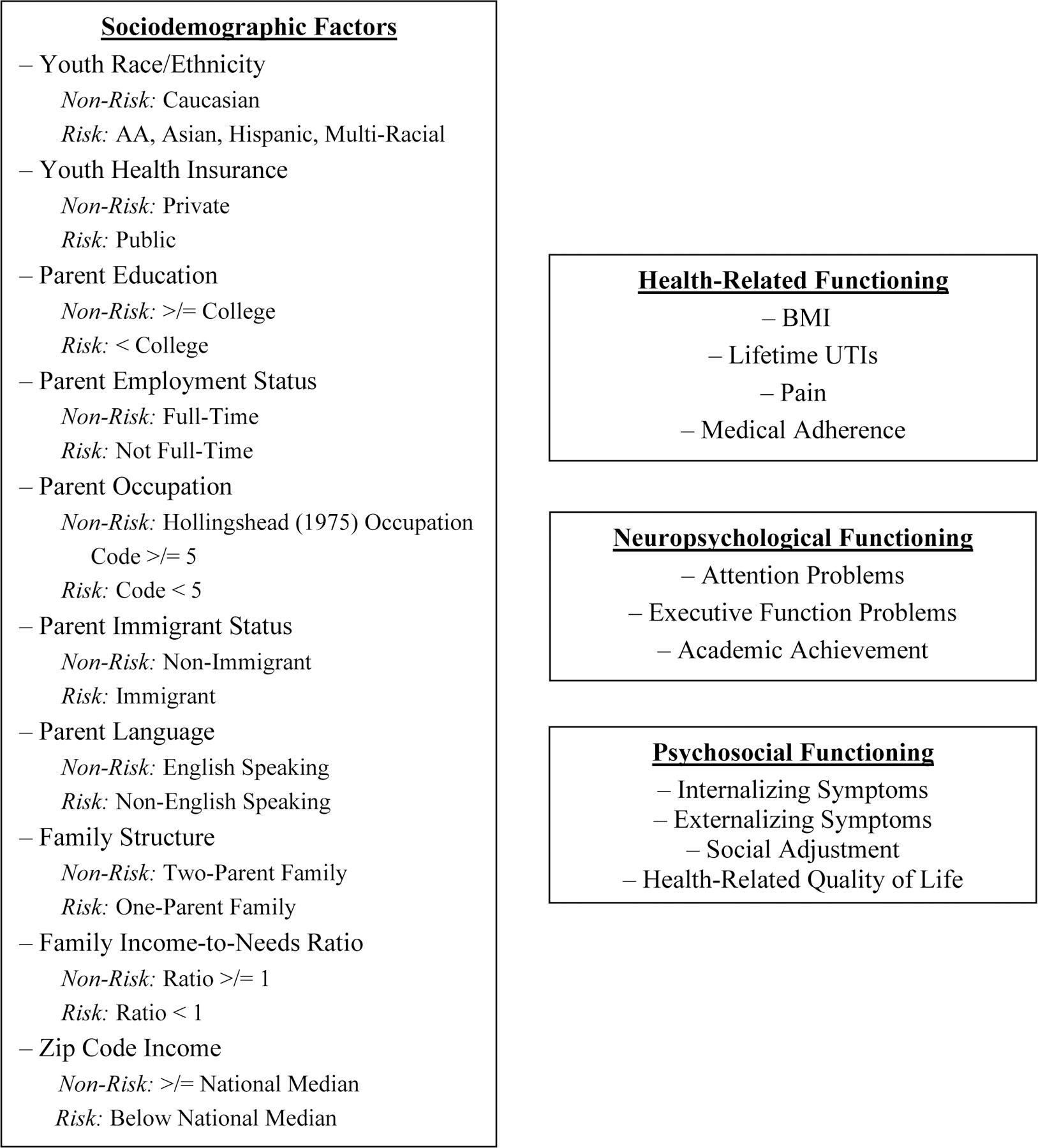
Study Variables
Methods
Data were drawn from a larger longitudinal study examining family, psychosocial, and neuropsychological functioning in youth with SB.
Participants
Families of youth with SB were recruited from four hospitals and a statewide SB association in the Midwest. Interested families were screened in-person during clinic visits or by phone by a member of the research team. Inclusion criteria for children with SB consisted of: (1) a diagnosis of SB (types included myelomeningocele, lipomeningocele, and myelocystocle); (2) age 8–15 years at Time 1; (3) ability to speak and read English and/or Spanish; (4) involvement of at least one primary caregiver; and (5) residence within 300 miles of laboratory (to allow for home visits for data collection).
A total of 246 families were approached during recruitment, of which 163 agreed to participate. However, of those 163 families, 21 families could not be contacted or later declined, and 2 families did not meet inclusion criteria. Thus, the final sample of participants included 140 families of youth with SB (53.6% female; M age = 11.43). Of these 140 children, 52.9% were Caucasian, 27.9% were Hispanic, 13.6% were African American, 1.4% were Asian, and 4.3% were multiracial. Hispanic families were oversampled to better study this population of youth with SB. Table 1 displays demographic and SB-related information for youth at Time 1. Youth of families who declined to participate did not differ from participants with respect to type of SB, shunt status, or occurrence of shunt infections (p’s > .05). Data were collected every two years, and the current study includes data from the first three time points. Participants were ages 8–15 at Time 1, ages 10–17 at Time 2, and ages 12–19 at Time 3. Data were collected at Time 2 for 110 (79%) of the original 140 participants, and at Time 3 for 103 (74%) of the original 140 participants.
Table 1.
Youth Demographic and Spina Bifida Information at Time 1
|
M (SD) or N (%) n = 140 |
|
|---|---|
| Age | 11.43 (2.46) |
| Gender: female | 75 (53.6%) |
| Race/ethnicity | |
| African American | 19 (13.6%) |
| Asian | 2 (1.4%) |
| Caucasian | 74 (52.9%) |
| Hispanic | 39 (27.9%) |
| Multiracial | 6 (4.3%) |
| Spina bifida type | |
| Myelomeningocele | 122 (87.1%) |
| Lipomeningocele | 10 (7.1%) |
| Other | 8 (5.7 %) |
| Lesion level | |
| Thoracic | 23 (16.4%) |
| Lumbar | 72 (51.4%) |
| Sacral | 43 (30.7%) |
| Unknown/not reported | 2 (1.4%) |
| Shunt present | 110 (78.6%) |
| Gross Motor Function | |
| Level I | 18 (12.9%) |
| Level II | 34 (24.3%) |
| Level III | 30 (21.4%) |
| Level IV | 53 (37.9%) |
| Unknown/not reported | 5 (3.5%) |
| IQ | 85.68 (19.67) |
Note. Gross Motor Function Level I = minimal limitations and Level IV = high degree of gross motor dysfunction. IQ = WASI estimated full-scale IQ.
Procedure
The current study was approved by university and hospital Institutional Review Boards. Data were collected by trained research assistants during two separate three-hour home visits at Time 1 and one three-hour home visit each at Times 2 and 3. For home visits with families who primarily spoke Spanish in the home, at least one research assistant was bilingual. Parental consent and child assent were obtained at the start of the first visit. Parents completed release forms to allow for data collection from medical charts, health professionals, and teachers. The current study included youth-, parent-, and teacher-reported questionnaire data, youth neuropsychological testing data, and medical chart data. Questionnaires that were only available in English were adapted for Spanish-speaking participants using forward and back translation by a translation team. At Time 3, 24 participants were 18 years or older (i.e., “young adults”), and therefore completed an abbreviated study protocol that did not include the participation of parents, peers, or teachers. At all time points, families received $150 and small gifts, teachers received $25, and health professionals received $10.
Measures: Condition-Related and Sociodemographic
Unless otherwise noted, all measures were collected at Times 1, 2, and 3. However, Objectives 1 and 2 only used data from Time 1 (see the Statistical Treatment section for details). Alphas reported in the text are for dependent variables at Times 1 and 3 and for mediating variables at Time 2.
Condition-related information.
Data regarding youth’s type of SB, lesion level, and shunt status were drawn from medical charts but, in cases where such data were missing, data were drawn from a medical history questionnaire completed by parents. Gross motor functioning was coded using the Gross Motor Function Classification System for SB (Wilson, Washington, Engel, Ciol, & Jensen, 2006). A condition severity composite score was computed for each participant, with scores ranging from 4 to 11 (higher scores indicate higher levels of severity). Scores were computed based on the following variables: myelomeningocele (no = 1, yes = 2), lesion level (sacral = 1, lumbar = 2, thoracic = 3), shunt status (no = 1, yes = 2), and gross motor function classification (Level I = 1, Level 2 = 2, Level 3 = 3, Level 4 = 4). Six participants did not have complete date for all 4 variables used to create the condition severity composite. Therefore, each participant’s sum score was divided by the highest possible sum based on their available data, to generate a condition severity percentage.
Sociodemographic information.
Parents reported on child age, gender, race/ethnicity, and health insurance, and reported on their own age, race/ethnicity, education, occupation, employment status, immigrant status, preferred language, family structure, family income, and number of family members living in the home. Data from mother-report was given preference. The ten sociodemographic variables used as independent variables included (see Figure 1 for non-risk and risk classification descriptors): (1) youth race/ethnicity; (2) youth health insurance; (3) parent education; (4) parent employment status; (5) parent occupation; (6) parent immigrant status; (7) parent language; (8) family structure; (9) income-to-needs ratio [calculated by dividing parent-reported annual family income by the 2009 standard of 150% of the federal poverty line, with a lower ratio indicating higher risk (USDHHS, 2009) for a family of the same size]; (10) zip code income (the median annual household income for participants’ residential area based on data from the American Community Survey (ACS); USCB, 2010; Franks, Tancredi, Winter, & Fiscella, 2010). These ten variables were chosen to allow for a comprehensive assessment of sociodemographic characteristics, as these variables are commonly used in existing research, although often in isolation (Cheng et al., 2015).
Cumulative risk.
Consistent with past research (Rutter, 1993), the ten sociodemographic factors were dichotomized and assigned a value of 0 (risk absent) or 1 (risk present) and then summed to calculate the cumulative risk index, with scores ranging from 0 to 10.
Measures: Health-Related Functioning
Body mass index (BMI).
Parents reported on youth height and weight on a health questionnaire adapted for this study from the CDC’s 1999 Youth Risk Behavior Survey (CDC, 1999). In cases where parent report was unavailable, data from medical charts were used. BMI percentile scores for each participant were computed using the CDC’s BMI Percentile Calculator for Children and Teenagers (CDC, 2015).
Urinary tract infections (UTIs).
Parents reported on the number of lifetime UTIs on a medical history questionnaire. In cases where such data were missing, data were drawn from the medical chart.
Pain.
Youth completed the Pain Questionnaire, (Klepper, 1999; Palermo, Zebracki, Newman, & Singer, 2004), which includes 14 items to assess a variety of pain characteristics. The current study included two items assessing pain frequency (over the past 3 months) and pain intensity (on a scale of 0 to 10), which were multiplied to compute an overall pain score, with higher scores indicating greater pain.
Medical adherence.
Youth adherence to their SB medical regimen (e.g., bowel and bladder regimen, medications) was measured by parent-report on the Spina Bifida Self-Management Profile (SBSMP; Wysocki & Gavin), a 14-item structured interview that was adapted to questionnaire format for the current study. The current study used the mean of all endorsed items, with higher scores indicating greater adherence. Owing to the number of participants who completed each item (i.e., parents could endorse “not applicable” for certain items), scale reliability could not be computed.
Measures: Neuropsychological Functioning
IQ.
Youth were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), to compute an estimated Full Scale IQ (FSIQ). The current study used standard scores.
Attention problems.
Attention problems were measured using parent- and teacher-report on the Swanson, Nolan, and Pelham Rating Scale-IV (SNAP-IV; Swanson et al., 2001). The current study used the mean of all 9 inattention items rated on a 4-point scale, with higher scores indicating greater attention problems (α’s = .93, .92, and .94 for mother-, father-, and teacher-report, respectively, at Time 1; α’s = .95, .92, and .96 for mother-, father-, and teacher-report, respectively, at Time 3). Attention problems were also measured using parent-report on the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) and teacher-report on the Teacher Report Form (TRF; Achenbach & Rescorla, 2001). The current study used T scores from the Attention Problems subscale, with higher scores indicating greater attention problems.
Executive function problems.
Executive functions problems were measured using parent- and teacher-report on the Behavior Rating Inventory of Executive Functions (BRIEF; Gioia, Isquith, Guy, Kentworthy, 2000). The parent-report version includes 85 items whereas the teacher-report version includes 86 items. Mean scores were used in the current study, with higher scores indicating more executive function problems (α’s = .97, .97, and .89 for mother-, father-, and teacher-report, respectively, at Time 1; α’s = .98, .98, and .98 for mother-, father-, and teacher-report, respectively, at Time 3).
Academic achievement.
Youth were administrated the reading, spelling, and arithmetic subtests of the Wide Range Achievement Test-3 (WRAT-3; Wilkinson, 1993) to assess basic academic ability at Times 1 and 3 only. Raw scores were converted to standard scores (α’s = .85 to .90; Wilkinson, 1993).
Measures: Psychosocial Functioning
Internalizing and externalizing symptoms.
Youth completed the Children’s Depression Inventory (CDI; Kovacs, 1992), a 27-item self-rated measure, with higher scores indicating higher depressive symptoms. The mean of all items was used in the current study (α’s = .80 and .77 at Times 1 and 3, respectively). Parents completed the CBCL and teachers completed the TRF (Achenbach & Rescorla, 2001). T-scores on the Internalizing Problems and Externalizing Problems subscales were used for this study.
Social adjustment.
Youth completed the Children’s Self Efficacy for Peer Interaction Scale (CSPI; Wheeler & Ladd, 1982) as a measure of perceived social competence, which consists of 22 items describing a social situation requiring the respondent to evaluate his/her ability to perform a verbal persuasive skill on a 4-point scale, with higher scores indicating greater self-efficacy. The current study used the mean across all 18 items (α’s = .82 and .91 at Times 1 and 3, respectively). Parents also completed the social competence subscale from the CBCL (Achenbach & Rescorla, 2001), which contains 9 items regarding: a) participation in organizations, clubs, teams, or groups, b) number of close friends, c) amount of time spent with friends outside of regular school hours, and d) behavior with others and when alone. The current study used T-scores on the Social Competence subscale.
Peer acceptance was assessed with youth, parent, and teacher report on the Social Acceptance subscale of Harter’s (1985) Self-Perception Profile for Children Scale (SPPC). The youth version consists of 6 items and the parent and teacher versions consist of 3 items, with higher scores indicating greater peer acceptance. The current study used mean scores (α’s = .67, .67, .76, and .60 for mother-, father-, teacher-, and child-report, respectively, at Time 1; α’s = .72, .59, .72, and .82 for mother-, father-, teacher-, and child-report, respectively, at Time 3).
Friendship quality was assessed with youth report on the Friendship Activity Questionnaire (FAQ; Bukowski, Hoza, & Boivin, 1994). The FAQ consists of 46 items across five scales of friendship qualities: companionship, conflict, help, security, and closeness. The current study used the mean score (α’s = .90 and .91 at Times 1 and 3, respectively). Youth also completed the Emotional Support Questionnaire Scale (ESQ; Slavin, 1991) to assess peer social support, which asks youth to identify and rate relationships based on 7 items measuring social support (e.g., how much they talk about personal concerns). The current study utilized data on how respondents rate their peer relationships by computing a mean score across all 7 items (α’s = .88 and .85 at Times 1 and 3, respectively).
Health-related quality of life.
Youths’ HRQOL was assessed using parent- (i.e., proxy) and youth-report on the psychosocial scale of the PedsQL Scale (PedsQL 4.0 Generic Core Scales; Varni, Seid, & Kurtin, 2001). The current study used mean scores, with higher scores indicating greater HRQOL (α’s = .79, .86, and .81 for mother-, father-, and child-report, respectively, at Time 1; α’s = .76, .84, and .81 for mother-, father-, and child-report, respectively, at Time 3).
Measures: Spina Bifida-Related Family Stress
Parents completed the Family Stress Scale (FSS; Quittner, Glueckauf, & Jackson, 1990), which consists of 19 items to assess common stressors in families of a child with SB; 13 items are non-disease specific (e.g., “mealtimes and bedtimes”) and 6 items are disease-specific (e.g., “medical care/appointments”). Higher scores indicate higher levels of stress. The current study used the mean of all 19 items (α’s = .92 and .90 for mother- and father-report, respectively, at Time 2).
Statistical Treatment
Missing data.
The present study had missing data due to item non-response, attrition, and an altered study protocol for youth 18 years and older at Time 3 (e.g., no inclusion of parent- or teacher-report). For all variables across all three time points, a non-significant Little’s missing completely at random (MCAR) test (Little, 1988) revealed that data were missing completely at random, 20.30% missing, χ2(2790) = 2889.60, p = .09. Listwise deletion was used to handle missing data, as this is considered a valid approach when data are found to be MCAR (Schafer & Graham, 2002).
Data reduction.
To reduce the number of analyses, and therefore reduce the chance of type I error, Pearson correlation coefficients (for two reporters/measures) or Cronbach’s alpha coefficients (for three or more reporters/measures) were computed to assess associations among multiple reporters of each measure and to assess associations among multiple measures for each construct. With the exception of BMI, UTIs, and pain (constructs which only had one reporter/measure), composite variables were created for all other outcomes given significant associations (p’s < .05) among reporters and/or measures.
Covariates.
Given that the range in participant age at each time point spans several developmental stages (ages 8–15 at Time 1, ages 10–17 at Time 2, and ages 12–19 at Time 3), age was included as a covariate in analyses for Objectives 1 and 3 to understand whether associations among study variables would emerge regardless of age. It was included as a moderator in analyses for Objective 2 to understand whether associations among variables varied as a function of age. In addition, t-tests revealed significant differences between risk and non-risk sociodemographic groups on IQ and condition severity (p’s < .05); thus, both IQ and condition severity were included as covariates in all analyses to understand whether associations among study variables existed regardless of one’s IQ or condition severity.
Objective 1.
Multivariate analyses of covariance (MANCOVAs) with univariate follow-up analyses were conducted to examine differences in health-related, neuropsychological, and psychosocial functioning between youth who are and are not characterized by sociodemographic risk at Time 1 (see Figure 1). Three MANCOVAs (one each for health-related, neuropsychological, and psychosocial functioning outcomes) were conducted for each sociodemographic factor.
Objective 2.
Cross-sectional hierarchical multiple regression analyses testing moderation effects (Holmbeck, 2002) were conducted to examine associations between the cumulative effect of sociodemographic risk (i.e., cumulative risk) at Time 1 and youth health-related, neuropsychological, and psychosocial functioning at Time 1, as moderated by age at Time 1. A separate regression analysis was conducted for each outcome. Variables were entered simultaneously within each of the following steps: (1) covariates, (2) cumulative risk index main effect and age main effects, (3) cumulative risk index X age interaction.
Objective 3.
Bootstrapping methods (Hayes, 2013) were used to examine SB-related family stress at Time 2 as a mediator of longitudinal associations between cumulative risk at Time 1 and youth health-related, neuropsychological, and psychosocial functioning at Time 3. The current study used Hayes’ PROCESS v2.16 statistical software to conduct bootstrapping analyses.
Statistical power.
A power of .80 and an alpha of .05 was assumed for all analyses. For objective 1, a sample size of 64 was required to detect medium effect sizes for analyses with 2 groups (ƞ2 = .10, .25, and .40 for small, medium, and large effects, respectively; Cohen, 1992). For objective 2, a sample size of 84 was required to detect medium effect sizes for analyses with 4 predictors (R2 = .02, .15, and .35 for small, medium, and large effects, respectively; Cohen, 1992). For objective 3, when using percentile bootstrapping methods, a sample size of 78 was required to detect medium effect sizes (abps = .14, .39, and .59 for small, medium, and large effects, respectively; Fritz & MacKinnon, 2007). Thus, the current study was sufficiently powered to detect medium effect sizes for all analyses.
Results
Preliminary Analyses
All variables were examined for skewness and, when significant skewness was present, the variable was corrected according to methods recommended by Tabachnick and Fidell (2013). Table 2 displays descriptive information across levels of the cumulative risk index at Time 1. Table 3 displays bivariate correlations among cumulative risk, outcome variables, and covariates (age, IQ, condition severity) at Time 1.
Table 2.
Descriptive Information on Cumulative Risk Index
|
M (SD) or N (%) n = 97 |
|
|---|---|
| Cumulative Risk Index | 3.26 (2.56) |
| 0 Risks | 14 (14.4%) |
| 1 Risk | 18 (18.6%) |
| 2 Risks | 16 (16.5%) |
| 3 Risks | 7 (7.2%) |
| 4 Risks | 10 (10.3%) |
| 5 Risks | 10 (10.3%) |
| 6 Risks | 5 (5.2%) |
| 7 Risks | 12 (12.4%) |
| 8 Risks | 4 (4.1%) |
| 9 Risks | 1 (1.0%) |
| 10 Risks | 0 (0.0%) |
Note. Sample was reduced from full sample of n = 140 because cases with missing data on any single sociodemographic factor used to create cumulative risk index were not included
Table 3.
Correlations among Cumulative Risk, Health-Related, Neuropsychological, and Psychosocial Functioning, and Covariates at Time 1
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CR | – | .21 | −.30** | .39** | .15 | −.16 | −.17 | −.34** | .18 | −.03 | −.25* | −.05 | .14 | −.43*** | .17 |
| Health | |||||||||||||||
| 2. BMI | – | .06 | .01 | .01 | −.18 | −.17 | −.06 | .08 | −.06 | −.18 | .06 | .22* | −.20* | .21* | |
| 3. UTIa | – | −.24 | .07 | −.18 | −.20 | .20 | −.10 | −.01 | .21* | .13 | −.05 | .27** | .22* | ||
| 4. Paina | – | .22 | .06 | .05 | −.28* | .32** | .18 | −.09 | −.26* | −.04 | −.30** | .15 | |||
| 5. Adherence | – | −.24** | −.29** | −.03 | −.14 | −.23* | .05 | .00 | .06 | −.13 | .33*** | ||||
| Neuropsychological | |||||||||||||||
| 6. Att Problems | – | .87*** | −.23** | .46*** | .48*** | −.52*** | −.36*** | −.07 | −.30** | .08 | |||||
| 7. EF Problems | – | −.19* | .44*** | .59*** | −.47*** | −.36*** | −.13 | −.24** | .07 | ||||||
| 8. Academics | – | −.23* | −.10 | .37*** | .18 | −.18* | .75*** | −.13 | |||||||
| Psychosocial | |||||||||||||||
| 9. Internalizing | – | .54*** | −.47*** | −.48*** | .05 | −.30** | .03 | ||||||||
| 10. Externalizing | – | −.21* | −.25** | −.15 | −.11 | −.13 | |||||||||
| 11. Social | – | .41*** | −.16 | .45*** | −.16 | ||||||||||
| 12. HRQOL | – | .03 | .23** | −.12 | |||||||||||
| Covariates | |||||||||||||||
| 13. Age | – | −.24** | .13 | ||||||||||||
| 14. IQ | – | −.31*** | |||||||||||||
| 15. Severity | – |
Note. n’s range from 81 to 140 across variables. CR = cumulative risk. BMI = body mass index percentile. UTI = lifetime number of urinary tract infections. Att Problems = attention problems. EF Problems = executive function problems. Internalizing = internalizing symptoms. Externalizing = externalizing symptoms. Social = (positive) social adjustment. HRQOL = health-related quality of life. IQ = WASI estimated full-scale IQ. Severity = condition severity.
This variable was log transformed to correct for skewness.
p < .05
p < .01
p <.001.
Objective 1
For Objective 1 results, adjusted means are presented in the text and in Table 4 and represent means after transformations and inclusion of covariates.
Table 4.
MANCOVAs and Significant ANCOVA Follow-Up Findings for Neuropsychological Functioning Outcomes
| MANCOVA | ANCOVA | Effect Size | Non-Risk M (SD) | Risk M (SD) | |
|---|---|---|---|---|---|
| Race/Ethnicity | F (3, 116) = 3.68* | .09 | |||
| Att: F (1, 124) = 8.36** | .06 | 0.04 (0.83) | −0.12 (0.66) | ||
| EF: F (1, 124) = 6.59* | .05 | 1.71 (0.32) | 1.64 (0.30) | ||
| Health Insurance | F (3, 112) = 2.84* | .07 | |||
| Att: F (1, 118) = 3.94* | .03 | −0.01 (0.78) | −0.10 (0.73) | ||
| Parent Education | F (3, 114) = 5.06** | .12 | |||
| Att: F (1, 120) = 5.64* | .05 | 0.05 (0.81) | −0.11 (0.71) | ||
| EF: F (1, 120) = 6.62* | .05 | 1.73 (0.30) | 1.64 (0.30) | ||
| AA: F (1, 120) = 8.67** | .07 | 100.70 (15.71) | 85.80 (19.69) | ||
| Parent Employment | F (3, 115) = 1.81 | .05 | |||
| AA: F (1, 117) = 4.71* A | .04 | 95.19 (18.48) | 81.48 (19.16) | ||
| Parent Occupation | F (3, 110) = 3.76* | .09 | |||
| Att: F (1, 116) = 9.32** | .07 | 0.01 (0.74) | −0.11 (0.76) | ||
| EF: F (1, 116) = 5.61* | .05 | 1.69 (0.26) | 1.65 (0.34) | ||
| Parent Immigrant Status | F (3, 94) = 1.54 | .05 | |||
| Att: F (1, 98) = 4.40* A | .04 | 0.02 (0.80) | −0.19 (0.51) | ||
| EF: F (1, 98) = 3.95* A | .04 | 1.70 (0.31) | 1.60 (0.22) | ||
| Parent Language | F (3, 116) = 1.49 | .04 | |||
| Att: F (1, 124) = 6.53* A | .05 | 0.02 (0.80) | −0.24 (0.54) | ||
| EF: F (1, 124) = 7.77** A | .06 | 1.71 (0.32) | 1.57 (0.28) | ||
| Family Structure | F (3, 112) = 0.62 | .02 | |||
| Family Income-to-Needs | F (3, 104) = 0.80 | .02 | |||
| Zip Code Income | F (3, 116) = 0.43 | .01 |
Note. Due to missing data and the use of listwise deletion, n’s range from 101 to 123 across MANCOVAs and from 103 to 129 across ANCOVAs. Each MANCOVA included attention problems (Att; z score), executive function problems (EF; mean score), and academic achievement (AA; standard score). See main document for explanation of Non-Risk and Risk categorization. Partial eta squared is reported as an effect size estimate (.10 = small effect size, .25 = medium effect size). Means are adjusted after transformations and inclusion of covariates. All analyses controlled for age, IQ, and condition severity.’
Univariate follow-up analysis is considered exploratory given the MANCOVA was not significant.
p < .05
p < .01
p <.001.
For health-related functioning, there was a significant multivariate effect for family income-to-needs [F (5, 41) = 2.71, p < .05, ES = .25]. Follow-up univariate analyses revealed a significant group difference effect for number of lifetime UTIs [F (1, 82), = 4.87, p < .05, ES = .06], such that youth who had a low family income-to-needs ratio had significantly less UTIs (M = 0.45) compared to youth who had a higher ratio (M = 0.69). Univariate analyses also revealed a significant effect for pain [F (1, 65) = 8.58, p < .01, ES .12], such that youth who had a low family income-to-needs ratio had significantly more pain (M = 1.30) compared to youth who had a higher ratio (M = 0.57). In addition, for health insurance, while the multivariate analysis was not significant (p > .05), exploratory univariate follow-up analyses revealed a significant finding for number of lifetime UTIs[F (1, 87) = 5.06, p < .05, ES = .06] in that youth without private health insurance had significantly less UTIs (M = 0.46) compared to youth with private health insurance (M = 0.69). No other significant results were found for health-related functioning (p’s > .05).
With respect to neuropsychological functioning, there were significant multivariate effects for race/ethnicity and parent occupation. Univariate follow-up analyses revealed effects for both attention problems and executive function problems for both of these sociodemographic factors such that non-Caucasian youth and youth with parents who have an occupation of lower status had significantly fewer attention and executive function problems compared to less at-risk youth. There was also a significant multivariate effect for health insurance, and univariate follow-up analyses revealed a significant association for attention problems, in that youth without private health insurance had significantly fewer attention problems compared to youth with private insurance. Lastly, a significant multivariate effect was found based on parent education. Univariate follow-up analyses revealed significant differences in attention problems, executive function problems, and academic achievement, in that compared to youth with a college-educated parent, youth with non-college educated parents had significantly fewer attention and executive function problems, but also lower academic achievement. In addition, while multivariate analyses were not significant for parent employment, parent immigrant status, and parent language, exploratory univariate follow-up analyses were significant (see Table 4).
There were no significant multivariate or univariate findings for the psychosocial functioning outcomes (p’s > .05).
Objective 2
The second objective was to examine the association between the cumulative effect of sociodemographic risk (i.e., cumulative risk) and youth health-related, neuropsychological, and psychosocial functioning, as moderated by age (all at Time 1). Results revealed that higher cumulative risk was found to be associated with more pain (b = .12, SE = .06, β = .33, t = 2.13, p < .05, ΔR2 = .07). However, higher cumulative risk was also found to be associated with fewer lifetime UTI’s (b = −.04, SE = .02, β = −.23, t = −2.06, p < .05, ΔR2 = .04), less attention problems (b = −.08, SE = .03, β = −.29, t = −2.73, p < .01, ΔR2 = .07), and less executive function problems (b = −.03, SE = .01, β = −.27, t = −2.42, p < .05, ΔR2 = .06. In addition, there were no significant interactions between cumulative risk and age (p’s > .05).
Objective 3
The third objective was to examine SB-related family stress at Time 2 as a mediator of the longitudinal association between cumulative risk at Time 1 and youth health-related, neuropsychological, and psychosocial functioning at Time 3. Refer to Table 5 for results of significant indirect mediation models. Results revealed significant indirect mediation models when predicting attention problems, internalizing symptoms, and HRQOL. Specifically, greater cumulative risk predicted less SB-related family stress, and less SB-related family stress predicted fewer attention problems, fewer internalizing symptoms, and greater HRQOL.
Table 5.
Significant Indirect Mediation Models of Cumulative Risk at Time 1, Predicting Outcomes at Time 3, as Mediated by Spina Bifida-Related Family Stress at Time 2
| Dependent Variable | Path A | Path B | Path C’ | Path C | Coeff. | SE | Indirect Effect | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effect | Total Effect | 95% CI | |||||||||||
| Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | Lower | Upper | ||||
| Attention Problems | −0.08* | 0.03 | 0.33* | 0.13 | −0.01 | 0.03 | −0.03 | 0.03 | −0.02 | 0.02 | −0.08 | −0.00 | −.05 |
| Internalizing Symptoms | −0.07* | 0.03 | 0.41 | 0.21 | 0.05 | 0.04 | 0.02 | 0.04 | −0.03 | 0.02 | −0.08 | −0.00 | −.04 |
| HRQOL | −0.07* | 0.03 | −0.27** | 0.10 | −0.06** | 0.02 | −0.04 | 0.02 | 0.02 | 0.01 | 0.00 | 0.05 | .05 |
Note. Due to missing data and the use of listwise deletion, n’s range from 59 to 62 across models. Coeff. = estimated effect coefficient. SE = standard error. Path A is the direct effect of the independent variable (cumulative risk) on the mediating variable (spina bifida-related family stress). Path B is the direct effect of the mediating variable on the outcome variable (neuropsychosocial or psychosocial functioning), while controlling for the independent variable. Patch C’ is the direct effect of the independent variable on the outcome variable, while controlling for the mediating variable. Path C is the total effect of the independent variable on the outcome variable. Partially standardized indirect effect coefficients are reported as estimates of effect size (.14 = small effect size). All analyses controlled for age, IQ, and condition severity.
p<.01
p<.05
Discussion
The purpose of this study was to examine how sociodemographic factors are associated with health-related, neuropsychological, and psychosocial functioning among youth with SB, and to understand the mechanisms and conditions through which these associations occur. Overall, results highlight the salience of several sociodemographic factors as being predictive of the outcomes included in this study. These associations seem to hold for youth at various ages and SB-related family stress appears to play an important role in how these associations unfold over time.
Differences between Sociodemographic Risk and Non-Risk Groups on Outcomes
Consistent with hypotheses for Objective 1, youth with SB who were characterized by sociodemographic risk (based on family income-to-needs) reported higher pain. For the most part, this has also been shown to be true for typically-developing youth and youth with chronic illnesses, but inconsistencies in the literature exist (King et al., 2011). Among youth with SB, pain has been described as more prevalent and pertinent to psychosocial health than what was previously believed, and it has been found to predict outcomes such as social activity involvement (Essner, Murray, & Holmbeck, 2014). Also consistent with hypotheses, as well as past studies on youth with SB and typically-developing youth (Davis-Kean, 2005; Holmbeck et al., 2003), youth with SB characterized by sociodemographic risk (based on parent education, as well as parent employment based on exploratory analyses) had lower academic achievement.
Contrary to hypotheses, youth with SB characterized by sociodemographic risk based on family income-to-needs (and health insurance based on exploratory analyses) reported fewer lifetime UTIs. One possibility is that parents at sociodemographic risk may be less vigilant in detecting UTIs due to the presence of other stressors in their lives, thus leading to an under-reporting of lifetime UTI numbers. Recent data on children with special health care needs suggests that, compared to children with private insurance, children with public insurance are more likely to have multiple chronic conditions and more health care needs, are less likely to access health care services, are more likely to be from low-income households, but are more likely to report their healthcare is affordable and meets their needs (Musumeci & Chidambaram, 2019). Together, this suggests that the relation between income, insurance, and urological function in youth with SB must be further examined, because urological issues can be a significant source of morbidity and mortality, and are implicated in at least 1/3 of all deaths of patients with SB (Oakeshott et al., 2010).
Also contrary to hypotheses, youth characterized by sociodemographic risk across several factors (race/ethnicity, health insurance, parent education, parent occupation, as well as parent immigrant status and parent language based on exploratory analyses) were reported to have fewer attention and executive function problems. In studies of typically-developing youth, some have found ADHD diagnoses as reported by parents to be higher among low-income youth and youth without private insurance, while other studies have found ADHD diagnoses as reported by parents and medical charts to be higher among high-income and Caucasian youth (Getahun et al., 2013; Pastor et al., 2015). Perhaps due to biases, it could be that the parents and teachers of youth at sociodemographic risk have lower expectations for attention and executive function skills, thus making them less likely to report such problems accurately.
Finally, no significant differences were found between risk and non-risk groups for BMI, medical adherence, or any psychosocial functioning outcomes. It is surprising that no differences were observed for BMI given that past research has found that, among typically-developing youth, those who are racial/ethnic minorities, from low-income families, or who have parents with less than high school educations are more likely to have higher BMIs and to be obese (Frederick, Snellman, & Putnam et al., 2014). It is important to recognize that there may be limitations to the BMI data collected in the present study, as these data were based on self-report. With respect to adherence, given the complex medical regimens that are required for those with SB, it is encouraging that youth who may be under-resourced (e.g., due to low income, low education) are not significantly less adherent than youth who are not under-resourced.
Associations between Cumulative Sociodemographic Risk and Outcomes
Consistent with the hypotheses for Objective 2, higher cumulative risk was cross-sectionally associated with more pain, but, interestingly, it was also associated with fewer lifetime UTI’s and less attention and executive function problems. These main effect results largely reflect the findings that were revealed in the Objective 1 analyses. The similar findings between Objectives 1 and 2 suggests that cumulative risk did not necessarily reveal itself to be a more explanatory variable compared to examining sociodemographic risk variables individually. Past research examining cumulative risk in families of youth with cancer found similar results (Bemis et al., 2015). However, both the study by Bemis and colleagues (2015) and the current study did not directly compare the influence of cumulative risk with that of individual sociodemographic factors. Past studies that have made direct comparisons have found that examining both individual and cumulative indices of risk was warranted because both indices uniquely predicted outcomes such as internalizing and externalizing problems in typically developing children (e.g., Jones, Forehand, Brody, & Armistad, 2002).
Further, age did not significantly moderate associations between cumulative risk and outcomes, thus indicating that relations between cumulative risk and pain, UTIs, attention problems, and executive function problems do not vary as a function of age. There is reason to suspect that these relations would have varied by age, based on what the developmental psychopathology literature tells us about the differential impact of cumulative risk occurring at different ages (Sameroff, 2000). The moderation analyses in the present study tested whether the timing of risks (e.g., risk at age 8 compared to risk at an older age) impacted how risk and outcomes are associated; these analyses did not test the chronicity (e.g., being at risk for 1 year compared to 10 years) or change in risk (e.g., increases in risk) over time, which have been shown to be strong predictors of outcomes in past research (Atkinson et al., 2015).
SB-Related Family Stress as a Mediator of Cumulative Risk and Outcomes
Finally, for Objective 3, SB-related family stress was found to mediate the relation between cumulative risk and attention problems, internalizing symptoms, and HRQOL. Importantly, and contrary to hypotheses, cumulative risk predicted lower SB-related family stress across all models. This is surprising given that previous studies applying the family stress model to pediatric populations have found lower SES to be linked with greater family stress (e.g., among youth with asthma; Chen, Fisher, Bacharier, & Strunk, 2003), and given that the literature on typically-developing youth has found that income, SES, and poverty are consistently linked to higher levels of stress (Chen & Miller, 2013). Past research on youth with SB has not examined whether SB-related family stress varies based on sociodemographic factors. It could be that families who are already experiencing significant sociodemographic stress may report lower levels of SB-related family stress because such condition-related stress may seem less impactful compared to other stressors in their lives. Indeed, research on pediatric cancer patients suggest that when examining the impact of sociodemographic variables, assessing both general and disease-specific stress can be fruitful (Bemis et al., 2015). There may also be other variables (e.g., coping strategies) that explain why families of youth with SB who are, presumably, less advantaged report less stress related to caring for a child with SB. It could be that these families possess strengths that mitigate or eliminate the adverse impact of risks.
Chen and Miller (2013) proposed that there are “shift-and-persist” characteristics that may benefit families who face socioeconomic adversity. Clearly, such families are confronted with repeated, unpredictable, and uncontrollable demands. Because such families may have limited options for problem-solving, they may “shift” by adjusting their response to stressors in a way that is consistent with what other scholars have termed “secondary control coping” (e.g., acceptance, cognitive restructuring, positive thinking, distraction; Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000). Secondary control coping has been found to be adaptive in the face of challenges that are unchangeable or cannot be problem-solved, such as those presented by sociodemographic adversity (Santiago & Wadsworth, 2009) or challenges presented by a chronic illness. Further, the “persist” aspect of Chen and Miller’s (2013) theory refers to the ability to endure adversity by finding meaning in difficult situations, having optimism about the future, and maintaining a focus on long-term goals. Interestingly, research has found that parents of youth with SB tend to be optimistic in their expectations for their children’s development (Holbein et al., 2017). Together, these speculations suggest that adaptive forms of coping, meaning-making, optimism, and hope, are strength-based areas that should be researched further in families of youth with SB, especially those at sociodemographic risk. While the finding that greater risk predicted less SB-related family stress was counter-intuitive, the associations between SB-related family stress and outcomes were in the expected directions, in that more SB-related family stress was associated with more attention problems and internalizing symptoms, as well as lower HRQOL.
Limitations and Future Research
This study had several strengths, including the comprehensive assessment of sociodemographic factors, the use of theoretical models to identify mechanisms through which sociodemographic factors might impact youth outcomes, and the collection of longitudinal, multi-method, and multi-source data. Still, there are limitations of the current study that should be addressed in future work. First, the present study had missing data owing to item nonresponse, attrition, and protocol changes for youth who were 18 years or older at Time 3, which limited the sample size across analyses, thus limiting the power to detect significant findings. Indeed, most effect sizes in this study were small to medium. Second, while the collection of data from multiple sources was a strength of the study, certain variables were only measured from one source, which impacts the interpretation of findings. For example, parents reported on number of lifetime UTIs and height and weight used to calculate BMI; if parent-report was unavailable, medical chart data were used. Past research has found variable evidence about the accuracy of parent-report of child health history (Schwarz, Monti, Savelli-Castillo, & Nelson, 2004). However, data reported in medical records is not always accurate either, as sometimes UTIs may be reported to either primary care physicians or specialty physicians but not both, and height and weight measurements are often not routinely assessed during clinic visits among youth with physical disabilities (McPherson, Swift, Yung, Lyons, & Church, 2013). Future research should continue efforts to collect data from multiple sources, giving consideration to the quality of the collected data, while making efforts to reconcile or examine differences between sources when appropriate. Third, while this study attempted to address methodological weaknesses of past health disparity research, we made conceptual and analytic decisions that may make generalizability of these findings to other samples difficult (e.g., dichotomization of sociodemographic factors into risk and non-risk categories). Fourth, without having a comparison sample, conclusions cannot be drawn about how findings from the present study are unique to the SB population.
Lastly, this study takes a “risk” approach when strengths-based or asset-focused approaches have been recommended, especially to prevent individuals with disabilities from being narrowly characterized as vulnerable (Alschuler, Kratz, & Ehde, 2016; Perrin, 2019; Sameroff, 2000). Risk factors are largely socially and contextually determined and may vary based on the region/country and over time (Cheng et al., 2015). For example, categorizing racial/ethnic minority status as a “risk” fails to acknowledge that this “risk” is due to social determinants that are active in American culture today rather than to one’s race in and of itself. Future research would benefit from assessing family perspectives about the influence of sociodemographic factors to better understand whether factors that we identify as “risks” are indeed perceived as such to families. The importance of such stakeholder engagement approaches has been increasingly recognized as away to ensure that research questions are of relevance and importance to the population being studied, especially among individuals with disabilities (e.g., Dunn, Ehde, Wegener, 2016). Moreover, health disparities are largely a result of unjust or discriminatory policies, provider and institutional biases, and other such social factors (Agency for Healthcare Research and Quality, 2016). Therefore, future research must be vigilant in examining the greater contextual factors that likely contribute to health disparities (e.g., discrimination experiences, access to care), as well as the intersectionality of identities, as individuals with a disability who are also a member of a vulnerable social group (e.g., low SES) may be even more likely to face disparities in health care (Levine & Breshears, 2019).
Clinical Implications and Conclusions
The results of the current study have implications for delivering evidence-based, diversity-sensitive clinical care to youth with SB. It appears that, despite the evidence that suggests certain sociodemographic characteristics put youth at risk for poor outcomes, youth with SB in the current study who were characterized by such risks (e.g., low income) were found, in some ways, to have similar or better outcomes than youth not characterized by risk. This finding highlights that youth with SB have areas of resiliency that, if identified, can be used to promote better adjustment outcomes. Indeed, the literature on families of youth with SB supports a resilience-disruption view of functioning; that is, while the presence of having a child with SB may disrupt normative family functioning in certain ways, these families are able to adapt and demonstrate considerable resilience (Lennon, Murray, Bechtel, & Holmbeck, 2015). In other words, results suggest that the resilience-disruption view of functioning which has been supported in the general literature on youth with SB may also be applied to the subset of youth with SB who are characterized by sociodemographic factors that may put them at risk.
Still, there are ways in which youth characterized by risk were more likely to have poorer outcomes, such as in the domains of pain and academic achievement. Youth would benefit from thoughtful and comprehensive clinical assessment of the sociodemographic factors that may put them at risk for adverse outcomes. Screening tools designed specifically to assess sociodemographic risk are lacking, often requiring such assessments to occur through interviews with families (Chung et al., 2016). An example of one useful screening tool is the Psychosocial Assessment Tool 2.0 _GEN (Kazak, 2011), which has been well-validated in pediatric populations (e.g., Crosby et al., 2006), and can be used to assess psychosocial risk in pediatric health by identifying a family’s areas of risk and resiliency across multiple domains (e.g., finances/resources, family problems, social support) and providing a total score for family risk. Youth who are identified as being at risk may then receive additional screening in other relevant domains to further inform clinical intervention (e.g., assessing pain using the Pediatric Pain Screening Tool; Simons, Smith, & Ibagon, 2015).
In conclusion, it is hoped that findings from the proposed study will improve the lives of youth with SB by informing future research questions, clinical care, and local and national policies aimed at improving outcomes among youth with SB. As previously stated, health disparities are largely a function of social conditions, policies, and institutions, and while these arenas may be challenging to reform, they can be improved upon (AAP, 2010; Cheng et al., 2015). Thus, using data from the present study to inform health care reform and social policies will benefit youth with spina bifida, as well as all children.
Impact.
The current study found that youth with spina bifida characterized by certain sociodemographic risk characteristics were more likely to have poorer outcomes in certain domains (e.g., pain, academic achievement). However, this was not a consistent finding, as many youth characterized by sociodemographic risk (e.g., low income) were found to have similar or better outcomes in other domains, compared to youth not characterized by risk.
These findings suggest youth with spina bifida would benefit from thoughtful and comprehensive clinical assessments of the sociodemographic factors that may put youth at risk for adverse outcomes.
These findings can also inform health care reform and social policies aimed at addressing healthcare disparities in youth with spina bifida.
Acknowledgements
The authors would like to thank the Illinois Spina Bifida Association as well as staff of the spina bifida clinics at Ann & Robert H. Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Medical Center. We also thank the numerous research assistants who helped with data collection and data entry. Finally, and most importantly, we would like to thank the parents, children, and teachers who participated in this study.
Funding
This work was supported by grants from the National Institute of Nursing Research and the Office of Behavioral and Social Sciences Research (R01 NR016235), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD048629), and the March of Dimes Birth Defects Foundation (12-FY13-271). This study is part of an ongoing, longitudinal study.
References
- Achenbach TM, & Rescorla LA (2001). ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA. [Google Scholar]
- Agency for Healthcare Research and Quality. (2016). National healthcare quality and disparities Report. Rockville, MD: Author. [Google Scholar]
- Alschuler KN, Kratz AL, & Ehde DM (2016). Resilience and vulnerability in individuals with chronic pain and physical disability. Rehabilitation Psychology, 61(1), 7–18. doi: 10.1037/rep0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics [AAP]. (2010). Health equity and children’s rights. Pediatrics, 125(4), 1018–1021. doi: 10.1542/peds.2010-0235 [DOI] [Google Scholar]
- Atkinson L, Beitchman J, Gonzalez A, Young A, Wilson B, Escobar M, … Villani V (2015). Cumulative risk, cumulative outcome: A 20-year longitudinal study. PLoS ONE 10(6), e0127650. Doi: 10.1371/journal.pone.0127650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis H, Yarboi J, Gerhardt CA, Vannatta K, Desjardins L, Murphy LK, … & Compas BE (2015). Childhood cancer in context: Sociodemographic factors, stress, and psychological distress among mothers and children. Journal of Pediatric Psychology, 40(8), 733–743. doi: 10.1093/jpepsy/jsv024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier JB, Morales Y, Liebling J, Geddes L, & Kim E (1997). Medical and social factors associated with cognitive outcome in individuals with myelomeningocele. Developmental Medicine & Child Neurology, 39, 263–266. doi: 10.1111/j.1469-8749.1997.tb07423.x [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Morris PA (2006). The bioecological model of human development. In Lerner RM (Ed.) Theoretical models of human development (6th ed., Vol. 1, pp. 793–828). Hoboken, NJ: Wiley. [Google Scholar]
- Bukowski WM, Hoza B, & Boivin M (1994). Measuring friendship quality during pre- and early adolescence: The development and psychometric properties of the friendship qualities scales. Journal of Social and Personal Relationships, 11(3), 471–484. doi: 10.1177/0265407594113011 [DOI] [Google Scholar]
- Canfield MA, Mai CT, Wang Y, O’Halloran A, Marengo LK, Olney RS … Kirby RS (2014). The association between race/ethnicity and major birth defects in the United States, 1999–2007. American Journal of Public Health, 104(9), e14 – 23. doi: 10.2105/AJPH.2014.302098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC]. (1999). Youth risk behavior surveillance system: 1999 youth risk behavior survey. Retrieved from http://www.cdc.gov/nccdphp/dash/yrbs/survey99.htm.
- Centers for Disease Control and Prevention [CDC]. (2015). BMI percentile calculator for child and teen. Retrieved from https://nccd.cdc.gov/dnpabmi/Calculator.aspx
- Chen E, Fisher EB, Bacharier LB, & Strunk RC (2003). Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine, 65(6), 984–992. doi: 10.1097/01.PSY.0000097340.54195.3C [DOI] [PubMed] [Google Scholar]
- Chen E, & Miller GE (2013). Socioeconomic status and health: Mediating and moderating factors. Annual Review of Clinical Psychology, 9, 723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Goodman E, & The Committee on Pediatric Research (2015). Race, ethnicity, and socioeconomic status in research on child health. Pediatrics, 135(1), e225–237, doi: 10.1542/peds.2014-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EK, Siegel BS, Garg A, Conroy K, Gross RS, Long DA, … Fierman AH (2016). Screening for Social determinants of health among children and families living in poverty: A guide for clinicians. Current Problems in Pediatric and Adolescent Health Care, 46(5), 135–153. doi: 10.1016/j.cppeds.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Quantitative Methods in Psychology, 112(1), 155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Conger RD, & Donnellan MB (2007). An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology, 58, 175–199. doi: 10.1146/annurev.psych.58.110405.085551 [DOI] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, & Saltzman H (2000). Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology, 68(6), 976–992. doi: 10.1037/0022-006X.68.6.976 [DOI] [PubMed] [Google Scholar]
- Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN & Shaw GM (2015). Spina bifida. Nature Review, Disease Primers, 1(15007), doi: 10.1038/nrdp.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby LE, Joffe NE, Reynolds N, Peugh JL, Manegold E, & Pai AL (2016). Psychometric properties of the Psychosocial Assessment Tool-General in adolescents and young adults with sickle cell disease. Journal of Pediatric Psychology, 41(4), 397–405. doi: 10.1093/jpepsy/jsv073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Kean PE (2005). The influence of parent education and family income on child achievement: The indirect role of parental expectations and the home environment. Journal of Family Psychology, 19(2), 294–304. doi: 10.1037/0893-3200.19.2.294 [DOI] [PubMed] [Google Scholar]
- Dennis M, Landry SH, Barnes M, & Fletcher JM (2006). A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society, 12(2), 285–296. doi: 10.1017/S1355617706060371 [DOI] [PubMed] [Google Scholar]
- Devine KA, Holmbeck GN, Gayes L, & Purnell JQ (2012). Friendships of children and adolescents with spina bifida: Social adjustment, social performance, and social skills. Journal of Pediatric Psychology, 37(2), 220–231. doi: 10.1093/jpepsy/jsr075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LM, Berg C, King P, Verdant C, Griffith K, Butler J, & Wiebe DJ (2011). Depleted parental psychological resources as mediators of the association of income with adherence and metabolic control. Journal of Family Psychology, 25(5), 751–758. doi: 10.1037/a0025259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DS, Ehde DM, & Wegener ST (2016). The foundational principles as psychological lodestars: Theoretical inspiration and empirical direction in rehabilitation psychology. Rehabilitation Psychology, 61(1), 1–6. doi: 10.1037/rep0000082 [DOI] [PubMed] [Google Scholar]
- Dunn EC, Soare TW, Raffeld MR, Busso DS, Crawford KM, Davis KA, … Susser ES (2018). What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: Recency, accumulation, or sensitive periods? Psychological Medicine, 48(15), 2562–2572. doi: 10.1017/S0033291718000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner BS, Murray CB, & Holmbeck GN (2014). The influence of condition parameters and internalizing symptoms on social outcomes in youth with spina bifida. Journal of Pediatric Psychology, 39(7), 718–734. doi: 10.1093/jpepsy/jsu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P, Tancredi DJ, Winter P, & Fiscella K (2010) Including socioeconomic status in coronary heart disease risk estimation. Annals of Family Medicine, 8(5), 447–453. doi: 10.1370/afm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick CB, Snellman K, & Putnam RD (2014). Increasing socioeconomic disparities in adolescent obesity. Proceedings of the National Academy of Sciences of the United States of America [PNAS], 11(4), 1339–1342. doi: 10.1073/pnas.1321355110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon DP (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahum D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, & Rhoads GG (2013). Recent trends in childhood Attention-Deficit/Hyperactivity Disorder. Journal of the American Medical Association Pediatrics, 167(3), 282–288. doi: 10.1001/2013.jamapediatrics.401 [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2000). BRIEF: Behavior Rating Inventory of Executive Function: Professional manual. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Grewal J, Carmichael SL, Song J, & Shaw GM, (2009). Neural tube defects: an analysis of neighbourhood- and individual-level socio-economic characteristics. Paediatric Perinatal Epidemiology, 23(2), 116–124. doi: 10.1111/j.1365-3016.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, Berry RJ, Mick Tilford J, Kucik JE, & Waitzman NJ (2016). Retrospective assessment of cost savings from prevention: Folic acid fortification and spina bifida in the U.S. American Journal of Preventive Medicine, 50(5 Suppl 1), S74–S80. doi: 10.1016/j.amepre.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S (1985). Manual for Self-Perception Profile for Children: Revision of the Perceived Competence Scale for Children. Denver, CO: University of Denver. [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press [Google Scholar]
- Holbein CE, Zebracki K, Bechtel CF, Papadakis JL, Bruno EF, & Holmbeck GN (2017). Milestone achievement in emerging adulthood in spina bifida: a longitudinal investigation of parental expectations. Developmental Medicine & Child Neurology, 59(3), 311–316. doi: 10.1111/dmcn.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. New Haven, CT: Yale University. [Google Scholar]
- Holmbeck GN (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27(1), 87–96. doi: 10.1093/jpepsy/27.1.87 [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Westhoven VC, Phillips WS, Bowers R, Gruse C, Nikolopoulos T, Tortura CM, & Davison K (2003). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology, 71(4), 782–796. doi: 10.1037/0022-006X.71.4.782 [DOI] [PubMed] [Google Scholar]
- Jones DJ, Forehand R, Brody G, & Armistad L (2002). Psychosocial adjustment of African American children in single-mother families: A test of three risk model. Journal of Marriage and Family, 64(1), 105–115. doi: 10.1111/j.1741-3737.2002.00105.x [DOI] [Google Scholar]
- Kabra AT, Feustal PJ, & Kogan BA (2015) Screening for depression and anxiety in childhood neurogenic bladder dysfunction. Journal of Pediatric Urology, 11(2), 75.e1–775.e7. doi: 10.1016/j.jpurol.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Kazak AE (2011). The Psychosocial Assessment Tool© (PAT) User Manual. Philadelphia, PA: Children’s Hospital of Philadelphia & Center for Pediatric Traumatic Stress. [Google Scholar]
- King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker LJ, & MacDonald AJ (2011). The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain, 152(12), 2729–2738. doi: 10.1016/j.pain.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Klepper S (1999). Effects of an eight-week physical conditioning program on disease signs and symptoms in children with chronic arthritis. Arthritis Care and Research, 12(1), 52–60. doi: [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). Children’s Depression Inventory—Manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Law M, King G, King S, Kertoy M, Hurley P, Rosenbaum P, … Hanna S (2006). Patterns of participation in recreational and leisure activities among children with complex physical disabilities. Developmental Medicine & Child Neurology, 48(5), 337–342. doi: 10.1017/S0012162206000740 [DOI] [PubMed] [Google Scholar]
- Lennon JM, Murray CB, Bechtel CF, & Holmbeck GN (2015). Resilience and disruption in observed family interactions in youth with and without spina bifida: An eight-year, five wave longitudinal study. Journal of Pediatric Psychology, 40(9), 943–955. doi: 10.1093/jpepsy/jsv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, & Breshears B (2019). Discrimination at every turn: An intersectional ecological lens for rehabilitation. Rehabilitation Psychology, 64(2), 146–153. doi: 10.1037/rep0000266 [DOI] [PubMed] [Google Scholar]
- Liptak GS, Kennedy JA, & Dosa NP (2010). Youth with spina bifida and transitions: Health and social participation in a nationally represented sample. The Journal of Pediatrics, 157(4), 584–588. doi: 10.1016/j.jpeds.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404). 1198–1202. doi: 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- McPherson AC, Swift JA, Yung E, & Church P (2013). The assessment of weight status in children and young people attending a spina bifida outpatient clinic: a retrospective medical record review. Disability and Rehabilitation, 35(25) 2123–2131. doi: 10.3109/09638288.2013.771705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CB, Holmbeck GN Ros AM, Flores DM, Mir SA, & Varni JW (2015). A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. Journal of Pediatric Psychology, 40(4), 419–430. doi: 10.1093/jpepsy/jsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci M, & Chidambaram P (2019). How do Medicaid/CHIP children with special health care needs differ from those with private insurance? Henry J. Kaiser Family Foundation. Retrieved from: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101754881-pdf [Google Scholar]
- Oakeshott P, Hunt GM, Poulton A & Reid F, (2010). Expectation of life and unexpected death in open spina bifida: A 40-year complete, non-selective, longitudinal cohort study. Developmental Medicine & Child Neurology, 52(8), 749–753. doi: 10.1111/j.1469-8749.2009.03543.x. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Zebracki K, Newman A, & Singer N (2004). Juvenile idiopathic arthritis: Parent-child discrepancy on reports of pain and disability. Journal of Rheumatology, 31(9), 1840–1846. doi: 10.1.1.852.6487 [PubMed] [Google Scholar]
- Pastor PN, Reuben CA, Duran CR, & Hawkins LD (2015). Association between diagnosed ADHD and selected characteristics among children aged 4–17 years: United States, 2011–2013. National Center for Health Statistics Data Brief, no. 201. Hyattsville, MD: National Center for Health Statistics. 2015. [Google Scholar]
- Perrin PB (2019). Diversity and social justice in disability: The heart and soul of rehabilitation psychology. Rehabilitation Psychology, 64(2), 105–110. doi: 10.1037/rep0000278 [DOI] [PubMed] [Google Scholar]
- Quittner AL, Glueckauf RL, & Jackson DN (1990). Chronic parenting stress: Moderating versus mediating effects of social support. Journal of Personality and Social Psychology, 59(6), 1266–1278. doi: 10.1037/0022-3514.59.6.1266 [DOI] [PubMed] [Google Scholar]
- Rutter M (1993). Stress, coping, and development. In Garmezy N & Rutter M (Eds.), Stress, coping, and development (pp. 1–41). New York, NY: McGraw-Hill. [Google Scholar]
- Sameroff AJ (2000). Developmental systems and psychopathology. Developmental Psychopathology, 12(3), 297–312. doi: 10.1017/S0954579400003035 [DOI] [PubMed] [Google Scholar]
- Santiago CD, & Wadsworth ME (2009). Coping with family conflict: What’s helpful and what’s not for low-income adolescents. Journal of Child and Family Studies, 18(2), 192–202. doi: 10.1007/s10826-008-9219-9 [DOI] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. doi: 10.1037//1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Schechter MS, Liu T, Soe M, Swanson M, Ward E, & Thibadeau J (2015). Sociodemographic attributes and spina bifida outcomes. Pediatrics, 135(4), 957–964. doi: 10.1542/peds.2014-2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JN, Monti A, Savelli-Castillo L, Nelson NP (2004). Accuracy of familial reporting of a child’s medical history in a dental clinic setting. Pediatric Dentistry, 26(5), 433–439. [PubMed] [Google Scholar]
- Schoon I, Bynner J, Joshi H, Parsons S, Wiggins RD, & Sacker A (2002). The influence of context, timing, and duration of risk experiences for the passage from childhood to midadulthood. Child Development, 73(5), 1486–1504. doi: 10.1111/1467-8624.00485 [DOI] [PubMed] [Google Scholar]
- Simons LE, Smith A, & Ibagon C (2015). Pediatric Pain Screening Tool: Rapid identification of risk in youth with pain complaints. Pain, 156(8), 1511–1518. doi: 10.1097/j.pain.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin LA (1991). Validation studies of the PEPSS, a measure of perceived emotional support for use with adolescents. Journal of Adolescent Research, 6(3), 316–335. doi: 10.1177/074355489163004 [DOI] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, … Wu M (2001). Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry, 40(2),168–179. doi: 10.1097/00004583-200102000-00011 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2013) Using multivariate statistics (6th ed.) Upper Saddle River, NJ: Pearson Education. [Google Scholar]
- U.S. Census Bureau [USCB]. (2010). American Community Survey, data release. Retrieved from https://www.census.gov/programs-surveys/acs/news/data-releases.
- U.S. Department of Health and Human Services [USDHHS] (2009). The 2009 HHS poverty guidelines. Retrieved from https://aspe.hhs.gov/2009-hhs-poverty-guidelines
- Varni JW, Seid M, & Kurtin PS (2001). PedsQL 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). WASI: Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, Texas: Harcourt Assessment, Inc. [Google Scholar]
- Wheeler VA & Ladd GW (1982). Assessment of children’s self-efficacy for social interactions with peers. Developmental Psychology, 18(6), 795–805. doi: 10.1037/0012-1649.18.6.795 [DOI] [Google Scholar]
- Wilkinson GS (1993). WRAT3: Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range, Inc. [Google Scholar]
- Wilson S, Washington LA, Engel JM, Ciol MA, & Jensen MP (2006). Perceived social support, psychological adjustment, and functional ability in youths with physical disabilities. Rehabilitation Psychology, 51(4), 322–330. doi: 10.1037/0090-5550.51.4.322 [DOI] [Google Scholar]
- Wohlfeiler MW, Macias MM, & Saylor CF (2008). Paternal correlates of cognitive and behavioral functioning in children with myelomeningocele. Developmental Medicine & Child Neurology, 50, 864–869. doi: 10.1111/j.1469-8749.2008.03070.x [DOI] [PubMed] [Google Scholar]
- Wysocki T, & Gavin L (2006). Paternal involvement in the management of pediatric chronic diseases: Associations with adherence, quality of life, and health status. Journal of Pediatric Psychology, 31(5), 501–511. doi: 10.1093/jpepsy/jsj042 [DOI] [PubMed] [Google Scholar]
- Zukerman JM, Devine KA, & Holmbeck GN (2011). Adolescent predictors of emerging adulthood milestones in youth with spina bifida. Journal of Pediatric Psychology, 36(3), 265–276. doi: 10.1093/jpepsy/jsq075 [DOI] [PMC free article] [PubMed] [Google Scholar]


