Summary
Background:
Spirometric restriction - defined by reduced forced vital capacity (FVC) with preserved forced expiratory volume in 1s (FEV1)/FVC ratio - is associated with increased co-morbidities and mortality in adulthood. Little is known about the early origins of this condition. We sought to identify early-life risk factors for spirometric restriction in adult life using three population-based birth cohorts.
Methods:
In the Tucson Children’s Respiratory Study (TCRS), 652 participants completed spirometry at years 22, 26, 32, and/or 36. At each survey, three mutually exclusive spirometric patterns were defined: Normal (FEV1/FVC≥10th percentile, FVC≥10th percentile), Restrictive (FEV1/FVC≥10th percentile, FVC<10th percentile), and Obstructive (FEV1/FVC<10th percentile). Early-life factors were assessed for association with spirometric patterns using multivariate multinomial logistic regressions. Significant risk factors were tested for replication in the Swedish BAMSE (n=1,817, spirometry at year 24) and UK MAAS (n=411, spirometry at year 18) birth cohorts. Measurements of body composition and total lung capacity (TLC) were also available for a subset of participants.
Findings:
In TCRS, in multivariate models, maternal nutritional problems during pregnancy (adjRRR, 95%CI: 2·5, 1·3–4·8, p=0·006), being born small for gestational age (SGA) (3·3, 1·3–7·9, p=0·009), and being underweight in childhood (3·5, 1·4–9·3, p=0·010) were independent predictors of adult spirometric restriction. Associations with SGA and childhood underweight were confirmed in meta-analyses across the three cohorts (p=0·003 and <0·001, respectively). Having low lean body mass index (<10th percentile) in childhood predicted adult spirometric restriction (3·7, 1·5–9·0, p=0·005). These associations were confirmed in participants with spirometric restriction who had diminished TLC, indicating that these factors increase specifically the risk for lung restriction.
Interpretation:
Poor growth and nutritional deficits, in utero and throughout childhood, precede and predict the development of spirometric restriction in adult life. Strategies to improve prenatal through childhood growth trajectories may help prevent spirometric restriction and its morbidity-mortality burden.
INTRODUCTION
Over the past few decades, there has been growing interest in the restrictive spirometric pattern (characterized by a reduced forced vital capacity [FVC] with a preserved forced expiratory volume in one second [FEV1]/FVC ratio) as a major determinant of morbidity and mortality.1 Also referred to as preserved ratio impaired spirometry (PRISm), restrictive ventilatory pattern (RVP), Global Initiative for Chronic Obstructive Lung Disease (GOLD)-unclassified or nonspecific pattern, the restrictive spirometric pattern has been identified in a significant proportion of the general population.1 The reported prevalence varies remarkably by geographic area. The worldwide estimate is of approximately 14%, but much higher rates are observed in regions with sizeable impoverished populations where the effects of maternal and child undernutrition are most evident.1–3 For example, the Burden of Obstructive Lung Disease study identified spirometric restriction in the majority of participants from multiple sites in India and The Philippines.4
This spirometry-defined restriction does not necessarily indicate restrictive lung defects, which require direct measurements of total lung capacity via plethysmography or other techniques. However, such measurements are time consuming to perform and difficult to integrate into population-based studies and, therefore, to date epidemiological studies have not determined to what extent the burden and risk factors of spirometric restriction pertain to true lung restriction, as contrasted with other lung conditions (e.g., air trapping due to airway obstruction) that are also associated with low FVC.5,6
Indeed, spirometric restriction has been consistently reported to be associated with poor quality of life,7 increased non-respiratory co-morbidities (including cardiovascular disease,2,8 diabetes,2,8 metabolic syndrome1), and all-cause mortality risk in adult life,8,9 indicating that this condition may be a critical marker of poor general health and linked to developmental and functional impairments of multiple organs. Early identification and intervention in at-risk individuals may be crucial for preventing the onset and reducing the high burden of spirometric restriction.
Factors associated with the development of spirometric restriction in the general population remain poorly defined. Studies in adults have linked the restrictive spirometric pattern to older age,2 female sex,2,8 being underweight2,7 or obese,2,7,8,10 reduced physical activity,11 and cigarette smoking.2,7,8,10 An association between histories of childhood pneumonia and/or pleurisy and adult spirometric restriction has been also observed.12 However, little is known about the early origins of this condition. To date, there have been no studies investigating risk factors as early as from birth for association with spirometric restriction in adulthood and the possible impact of early deficits in growth and nutrition on this condition. Such studies could provide insights into opportunities for early interventions to prevent the adverse outcomes associated with this condition. In this study, we sought to identify early-life risk factors for spirometric restriction in adult life using data from three population-based birth cohorts.
METHODS
Study participants
For the present study, we used data from the Tucson Children’s Respiratory Study (TCRS) as the primary sample in which risk factors in early life and childhood were examined for association with adult spirometric patterns. Main results were tested for replication using data from two other birth cohorts: the Swedish Child (Barn), Allergy, Milieu, Stockholm, Epidemiological survey (BAMSE) and the UK Manchester Asthma and Allergy Study (MAAS). For each cohort, study protocols were approved by the institutional ethics committee and written informed consent/assent was obtained from study participants or their parents. The STROBE guidelines were used to ensure appropriate reporting of this study.
TCRS is a longitudinal population-based study that recruited 1,246 healthy infants at birth between 1980–1984.13 Questionnaires were answered by the primary caregiver at enrollment immediately after the child’s birth, and multiple follow-up questionnaires were completed through childhood and adulthood. At years 22, 26, 32, and 36, lung function was measured with spirometry. We used data from 652 participants who had lung function test results from at least one of these surveys.
BAMSE is a population-based birth cohort that recruited 4,089 Swedish children born between 1994–1996.14 Questionnaires were answered at recruitment and multiple follow-up visits, and spirometry was performed at year 24. For the present study, we included 1,817 participants who had available lung function data at year 24.14
MAAS is a population-based birth cohort that recruited 1,184 participants prenatally from 1995 to 1997 and followed them prospectively.15 Questionnaires were completed at recruitment and multiple follow-up visits, and spirometry was assessed in early adulthood at age ≥ 18 years (mean age 19·4, SD 0·71). We used data from 411 participants who had available lung function test results.
Lung function and spirometric patterns
Pre-bronchodilator spirometry data were used. In each cohort and at each adult survey, we defined the 10th percentiles for FVC and FEV1/FVC based on the residuals estimated from regressing FVC (or FEV1/FVC) on age, height, and race/ethnicity after stratification by sex. Participants were then divided into one of three mutually exclusive spirometric patterns: Normal (both FEV1/FVC and FVC ≥ 10th percentile), Restrictive (FEV1/FVC ≥ 10th percentile and FVC < 10th percentile), and Obstructive (FEV1/FVC < 10th percentile, independent of FVC values). Primary analyses used the 10th percentile as the threshold, based on the rationale that it provided reasonable prevalence estimates of spirometric restriction and that FVC deficits influence outcomes due to continuous rather than tail effects.
Main results were further tested in three sensitivity analyses as follows:
Excluding participants with significant bronchodilator response (BDR), defined as ≥ 12%- and 200 mL- increase in either FEV1 or FVC.5 These analyses were performed in TCRS and BAMSE in which post-bronchodilator spirometry data were available.
Using lower limit of normal (LLN) from the Global Lung Function Initiative (GLI) 2012 reference equations16 as cut-offs for both FVC and FEV1/FVC. Participants were classified into one of three mutually exclusive spirometric patterns (Normal, Restrictive, Obstructive), similar to those of the primary analyses.
Using the 10th percentile for FEV1 (based on the residuals estimated from regressing FEV1 on age, height, and race/ethnicity after stratification by sex) as the cut-off to classify participants into one of two mutually exclusive groups: Normal lung function (FEV1 ≥ 10th percentile) and Low lung function (FEV1 < 10th percentile).
Total lung capacity (TLC) was measured by body plethysmography in a random sample of 173 TCRS participants at year 32 and in 407 MAAS participants at year 18. We defined low TLC as below the 10th percentile based on the residuals estimated from regressing TLC on age, height, sex, and race/ethnicity. Normal TLC was defined as ≥ 10th percentile.
Details of spirometry and body plethysmography procedures are provided in appendix p 3.
Early-life and childhood determinants
TCRS
In TCRS, information about demographics, parental asthma, parental smoking, and parental education was collected by questionnaire at the time of enrollment shortly after birth. Pregnancy and perinatal data were obtained by study nurses while the mothers were still in the hospital following delivery. Information on nutritional problems during pregnancy and gestational age estimated by physicians and/or nurses was obtained from medical records. Anemia was included as a nutritional problem, given that iron deficiency resulting from inadequate iron intake is the most common cause of anemia during pregnancy.17 However, analyses testing separately anemia from other nutritional problems were also conducted. A newborn was considered small for gestational age (SGA), appropriate for gestational age (AGA), or large for gestational age (LGA) if the birthweight was below the 10th percentile, between the 10th and 90th percentiles, or greater than the 90th percentile, respectively,18 based on the US birthweight for gestational age reference.19 Ponderal index at birth was calculated as 100 x weight in grams/length in cm3 (g/cm3). Acute lower respiratory illnesses (LRI) during the first three years were ascertained by pediatricians.
At visit years 6, 11, and 16, weight and height were recorded by study nurses. Body mass index (BMI) was calculated as weight/height2 (kg/m2). At each survey, the child’s nutritional status was classified into normal weight, underweight, overweight, and obese, per the Centers for Disease Control and Prevention (CDC) definitions:20 underweight, overweight, and obesity were defined as BMI-for-age < 5th percentile, ≥ 85th and < 95th percentile, and ≥ 95th percentile, respectively. BMI-for-age percentiles were generated based on the 2000 CDC Growth Reference21 using the Stata zanthro package. A longitudinal nutritional status across years 6, 11, and 16 was also assessed and participants categorized into four mutually exclusive groups as follows: 1) Normal weight: participants who had normal weight at all available surveys, 2) Childhood underweight: participants who were underweight at any of the surveys, 3) Childhood overweight: participants who were overweight at any of the surveys and were never underweight or obese, and 4) Childhood obesity: participants who were obese at any of the surveys.
Asthma was defined as a physician-confirmed diagnosis of asthma with active symptoms (asthma attacks or wheeze) during the previous year. Childhood asthma was defined as a positive report of asthma in at least one of the surveys at years 6, 11, and 16. Current smoking was assessed by questionnaire from age 16 onwards. Pack-years were computed prospectively based on questionnaire information on usual number of cigarettes smoked per day and age at starting/quitting smoking.
BAMSE and MAAS
In BAMSE and MAAS, the same definitions of early-life and childhood growth parameters were applied; however, data for nutritional problems during pregnancy were not available in these cohorts. Newborn’s size for gestational age was classified into AGA, SGA, and LGA using the Swedish22 and the British23 reference values for BAMSE and MAAS, respectively. Child’s BMI-for-age was generated based on the WHO Child Growth Standards24 and WHO Reference 200725 in BAMSE, and on the British 1990 Growth Reference23 in MAAS, using the Stata zanthro package. Longitudinal childhood nutritional status was assessed across years 4, 8, 16 in BAMSE, and across years 5, 8, 11, 16 in MAAS.
Additional information on body composition, as evaluated by the hand-to-foot eight-electrode bioelectrical impedance analyze (Tanita BC-418, Tokyo, Japan [BIA8]), was available at year 11 for 365 MAAS participants.26 Lean body mass index (LBMI) and fat mass index (FMI) were calculated by dividing total lean body mass and total fat mass (kg) by height (m) squared, respectively. Low LBMI and low FMI were defined as below the 10th percentile based on the residuals estimated from regressing LBMI (or FMI) on age, sex, and race/ethnicity.
Statistical analyses
In TCRS, to assess the relation of early determinants to adult spirometric restriction and obstruction, we used multinomial logistic regression models with subject-clustered sandwich estimators of standard errors to control for the serial correlation of repeated intrasubject observations because spirometry data were available from up to 4 time points per subject. Main results were also confirmed using generalized estimating equations (GEE) that contrasted separately spirometric restriction and spirometric obstruction with the normal spirometric pattern while controlling for serial correlation using an unstructured correlation pattern for the variance estimates. In univariate analyses, the three-category spirometric pattern (normal pattern as the reference group) at years 22, 26, 32, and 36 was entered as the outcome, and early determinants as the independent predictors, with adjustment for survey year, sex, and race/ethnicity. In multivariate analyses, significant factors for spirometric restriction and/or obstruction from univariate analyses were all entered in the initial model. Survey year, sex, and race/ethnicity were included as a priori forced covariates. Other significant predictors were retained by backward selection at p<0·050 (see appendix p 3).
Significant early risk factors of the adult restrictive spirometric pattern were tested for replication in BAMSE and MAAS using multinomial logistic regression models adjusted for sex and race/ethnicity, with the three-category spirometric pattern at year 24 in BAMSE and year 18 in MAAS as the outcome. Fixed-effect meta-analyses were conducted to generate a pooled estimate of the effect across studies using the Stata metan package.
Sensitivity analyses among participants without significant BDR and sensitivity analyses using GLI LLN- cut-offs were conducted using the same statistical methods as primary analyses. For sensitivity analyses using FEV1- defined low lung function, the two-category outcome (Low versus Normal lung function) was used as the dependent variable in generalized estimating equations with unstructured correlation panel to control for serial correlation in TCRS. For BAMSE and MAAS, logistic regression was used.
Analyses were based on nonmissing data, and missing data were not imputed. All analyses were done with Stata SE version 15·0.
Role of the funding source
The funding source had no role in the study design, the writing of this paper or the decision to submit for publication. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication.
RESULTS
In TCRS, 652 participants had available spirometry data from at least one of the surveys at years 22, 26, 32, and 36. Compared with the 594 TCRS participants excluded from analyses due to lack of available spirometry data, those included were more likely to be non-Hispanic white, to have older, higher-educated parents, and were less likely to have smoking parents at birth (appendix p 4). No differences between included and excluded participants were found in relation to their childhood BMI or nutritional status. The main characteristics of TCRS, BAMSE, and MAAS participants included in this study are shown in appendix p 5. Compared to TCRS, fewer participants in BAMSE and MAAS were obese (5% at age 24 in BAMSE and 7% at age 18 in MAAS vs. 19% at age 22 in TCRS). Participants from BAMSE and MAAS had older mothers and were less likely to have smoking parents at birth. In BAMSE, there was also a smaller percentage of parents who completed more than 12 years of formal education. Participants from the three cohorts were comparable with respect to all other characteristics.
In all cohorts, by design, spirometric restriction was present in approximately 10% of participants at each survey year, and so was spirometric obstruction (Table 1). Cross-sectional associations between adult characteristics and spirometric patterns are shown for all cohorts in appendix p 6. Spirometric restriction was significantly associated with lower BMI in adult life in BAMSE and MAAS, but an opposite trend was observed in TCRS. Notably, neither smoking nor asthma was associated with an increased risk for spirometric restriction in any of the three cohorts. In fact, smoking was inversely associated with spirometric restriction in BAMSE (but not in the other two cohorts). A relation of pack-years to spirometric obstruction was observed in TCRS. Participants in the obstructive – but not restrictive – spirometric group were more likely to have respiratory symptoms than participants in the normal group (appendix pp 7-8).
Table 1:
Prevalence of each spirometric pattern in the study
| TCRS (Total N = 652) |
BAMSE | MAAS | ||||
|---|---|---|---|---|---|---|
| Year 22 | Year 26 | Year 32 | Year 36 | Year 24 | Year 18 | |
| N=456 | N=356 | N=430 | N=426 | N=1817 | N=411 | |
| Normal spirometric pattern, n (%) | 368 (81) | 287 (80) | 350 (81) | 345 (81) | 1462 (80) | 332 (81) |
| Restrictive spirometric pattern, n (%) | 43 (9) | 34 (10) | 38 (9) | 40 (9) | 174 (10) | 39 (9) |
| Obstructive spirometric pattern, n (%) | 45 (10) | 35 (10) | 42 (10) | 41 (10) | 181 (10) | 40 (10) |
Associations of mother’s pregnancy-related, parental, and early-life factors with adult spirometric restriction in TCRS
Among pregnancy-related factors, TCRS participants whose mothers had nutritional problems during pregnancy were over twice as likely to have spirometric restriction as adults than participants whose mothers did not have nutritional problems (Table 2, adjusted relative risk ratio [adjRRR] 2·36, 95% confidence interval [CI] 1·37–4·05, p=0·002). Anemia and excessive vomiting were the two main nutritional problems reported during pregnancy (20% and 3%, respectively); both were significantly related to adult spirometric restriction (Table 2). Among parental factors, having an older mother or a smoking father decreased the risk for the adult restrictive pattern.
Table 2:
Associations of pregnancy-related, parental, and early-life determinants with spirometric patterns across years 22–36 in TCRS
| N Subjects^ | Adult Restrictive Spirometric Pattern | Adult Obstructive Spirometric Pattern | |||||
|---|---|---|---|---|---|---|---|
| RRR (95% CI)* | p value | RRR (95% CI)* | p value | ||||
| Pregnancy-related factors | |||||||
| Nutritional problems during pregnancy | No | 398 | ref | ||||
| Yes | 139 | 2·36 (1·37, 4·05) | 0·002 | 1·55 (0·85, 2·83) | 0·15 | ||
| Specific nutritional problems during pregnancy | No | 398 | ref | ||||
| Anemia only | 108 | 2·10 (1·15, 3·84) | 0·016 | 1·66 (0·86, 3·20) | 0·13 | ||
| Excessive vomiting | 15 | 9·55 (3·38, 26·96) | <0·001 | 3·41 (1·16, 10·05) | 0·026 | ||
| Others | 16 | 0·84 (0·10, 6·89) | 0·87 | NA | NA | ||
| Maternal smoking during pregnancy | No | 542 | ref | ||||
| Yes | 94 | 0·93 (0·46, 1·89) | 0·84 | 1·51 (0·82, 2·80) | 0·19 | ||
| Antibiotic therapy during pregnancy | No | 556 | ref | ||||
| Yes | 94 | 0·58 (0·24, 1·39) | 0·22 | 1·12 (0·57, 2·20) | 0·74 | ||
| Problems during delivery | No | 376 | ref | ||||
| Yes | 271 | 1·31 (0·82, 2·11) | 0·26 | 1·30 (0·79, 2·14) | 0·31 | ||
| Delivery type | Normal delivery | 535 | ref | ||||
| C-section | 117 | 0·77 (0·41, 1·44) | 0·41 | 0·95 (0·51, 1·77) | 0·87 | ||
| Number of previous birth | 0–1 | 507 | ref | ||||
| 2–3 | 128 | 1·10 (0·62, 1·96) | 0·74 | 1·06 (0·56, 1·99) | 0·86 | ||
| > 3 | 17 | 0·22 (0·03, 1·65) | 0·14 | 0·59 (0·13, 2·72) | 0·50 | ||
| Parental factors | |||||||
| Maternal | Age at child’s birth, year | 652 | 0·95 (0·90, 0·99) | 0·046 | 0·97 (0·91, 1·03) | 0·37 | |
| Smoking at birth | No | 556 | ref | ||||
| Yes | 96 | 0·92 (0·46, 1·82) | 0·81 | 1·06 (0·55, 2·05) | 0·86 | ||
| Asthma | No | 577 | ref | ||||
| Yes | 63 | 0·66 (0·27, 1·62) | 0·37 | 2·69 (1·38, 5·28) | 0·004 | ||
| Education | > 12 years | 488 | ref | ||||
| ≤ 12 years | 163 | 1·19 (0·69, 2·06) | 0·53 | 1·34 (0·72, 2·50) | 0·36 | ||
| Paternal | Age at child’s birth, year | 646 | 0·98 (0·94, 1·02) | 0·41 | 1·01 (0·96, 1·07) | 0·61 | |
| No | 471 | ref | |||||
| Smoking at birth | Yes | 175 | 0·53 (0·29, 0·96) | 0·037 | 0·96 (0·55, 1·67) | 0·88 | |
| Asthma | No | 541 | ref | ||||
| Yes | 78 | 0·93 (0·42, 2·10) | 0·87 | 1·25 (0·61, 2·56) | 0·55 | ||
| Education | > 12 years | 483 | ref | ||||
| ≤ 12 years | 160 | 0·79 (0·46, 1·35) | 0·39 | 1·18 (0·61, 2·29) | 0·63 | ||
| Factors at birth and infancy | |||||||
| Birthweight, per 100 g | 652 | 0·94 (0·89, 0·99) | 0·028 | 0·99 (0·95, 1·04) | 0·75 | ||
| Ponderal index, per 1 unit | 482 | 0·22 (0·05, 0·88) | 0·033 | 1·47 (0·87, 2·50) | 0·15 | ||
| Size for gestational age | AGA | 408 | ref | ||||
| SGA | 40 | 2·70 (1·20, 6·08) | 0·017 | 1·03 (0·45, 2·39) | 0·94 | ||
| LGA | 51 | 0·49 (0·16, 1·53) | 0·22 | 1·41 (0·63, 3·16) | 0·40 | ||
| Birth length, cm | 482 | 0·99 (0·87, 1·12) | 0·84 | 0·93 (0·83, 1·05) | 0·24 | ||
| Head circumference, cm | 500 | 0·90 (0·71, 1·13) | 0·35 | 0·96 (0·80, 1·17) | 0·71 | ||
| Chest circumference, cm | 500 | 0·88 (0·75, 1·04) | 0·14 | 1·04 (0·91, 1·20) | 0·58 | ||
| Gestational age, week | 499 | 1·00 (0·75, 1·33) | 0·99 | 1·01 (0·73, 1·38) | 0·96 | ||
| LRI in the first 3 years | No | 228 | ref | ||||
| Yes | 323 | 1·66 (0·98, 2·81) | 0·058 | 1·18 (0·70, 2·01) | 0·53 | ||
Models included up to 1,668 lung function observations from years 22, 26, 32, and 36 (1,350 with normal, 155 with restrictive, and 163 with obstructive spirometric pattern).
Relative risk ratios (RRRs) are from multinomial logistic regression models adjusted for survey year, sex, and race/ethnicity. Normal spirometric pattern as the reference group. AGA=appropriate for gestational age. SGA=small for gestational age. LGA=large for gestational age. LRI=lower respiratory illnesses. ref=reference category. NA=not applicable. Significant associations are reported in bold font.
Among early-life factors, birthweight and ponderal index were both inversely related to the restrictive spirometric pattern in adult life. In line with these findings, participants born SGA were nearly three times more likely to develop spirometric restriction as adults, compared to those born with size appropriate for gestational age. In addition, we observed a trend towards an increased risk for the restrictive pattern in participants who experienced LRI in early life. There were no significant associations between adult spirometric restriction and maternal smoking during pregnancy, maternal asthma, gestational age, or birth length. Maternal asthma was, however, related to spirometric obstruction.
Associations of childhood underweight with adult spirometric restriction in TCRS
Because of the above associations with indicators of fetal nutrition and growth restriction, we sought to determine whether childhood BMI was a predictor of the development of spirometric restriction in adult life (Table 3). At each of the childhood surveys at ages 6, 11, and 16, being underweight increased the risk for adult spirometric restriction. When childhood nutritional status was evaluated across years 6–16, participants in the underweight group were three times more likely to develop the restrictive spirometric pattern as adults, compared to those in the normal weight group (3·03, 1·39–6·59, p=0·005). In contrast, we found no association of childhood obesity with spirometric restriction. Neither smoking at age 16, nor childhood asthma increased the risk for adult spirometric restriction, while childhood asthma was strongly related to spirometric obstruction.
Table 3:
Associations of childhood determinants with adult spirometric patterns across years 22–36 in TCRS
| N subjects^ | Adult Restrictive Spirometric Pattern | Adult Obstructive Spirometric Pattern | |||||
|---|---|---|---|---|---|---|---|
| RRR (95% CI)* | p value | RRR (95% CI)* | p value | ||||
| Nutritional status | Year 6 | Normal weight | 399 | ref | |||
| Underweight | 18 | 2·83 (0·98, 8·22) | 0·055 | 1·37 (0·37, 5·05) | 0·63 | ||
| Overweight | 59 | 0·64 (0·23, 1·75) | 0·38 | 0·72 (0·28, 1·90) | 0·51 | ||
| Obese | 36 | 0·58 (0·19, 1·75) | 0·34 | 0·70 (0·27, 1·81) | 0·47 | ||
| Year 11 | Normal weight | 372 | ref | ||||
| Underweight | 23 | 7·58 (3·08, 18·65) | <0·001 | 2·52 (0·76, 8·34) | 0·13 | ||
| Overweight | 81 | 1·23 (0·57, 2·66) | 0·59 | 1·40 (0·64, 3·06) | 0·41 | ||
| Obese | 72 | 0·87 (0·38, 2·03) | 0·75 | 1·04 (0·49, 2·24) | 0·91 | ||
| Year 16 | Normal weight | 334 | ref | ||||
| Underweight | 19 | 5·34 (2·01, 14·19) | 0·001 | 2·62 (0·86, 8·00) | 0·091 | ||
| Overweight | 52 | 0·61 (0·22, 1·66) | 0·34 | 1·46 (0·55, 3·88) | 0·45 | ||
| Obese | 66 | 1·60 (0·78, 3·31) | 0·20 | 1·08 (0·49, 2·39) | 0·85 | ||
| Childhood nutritional status across Years 6–16 | Normal weight | 344 | ref | ||||
| Underweight | 41 | 3·03 (1·39, 6·59) | 0·005 | 1·57 (0·67, 3·66) | 0·30 | ||
| Overweight | 98 | 0·86 (0·41, 1·81) | 0·70 | 1·28 (0·62, 2·64) | 0·51 | ||
| Obese | 109 | 1·07 (0·56, 2·08) | 0·83 | 0·83 (0·42, 1·65) | 0·60 | ||
| Childhood asthma across Years 6–16 † | No | 492 | ref | ||||
| Yes | 157 | 1·51 (0·89, 2·57) | 0·13 | 2·80 (1·67, 4·69) | <0·001 | ||
| Current smoking Year 16 | No | 394 | ref | ||||
| Yes | 48 | 0·66 (0·30, 1·45) | 0·30 | 1·54 (0·67, 3·51) | 0·31 | ||
| Smoking pack-years Year 16, per pack-year | 454 | 0·34 (0·09, 1·27) | 0·11 | 1·23 (0·85, 1·77) | 0·27 | ||
Models included up to 1,659 lung function observations from years 22, 26, 32, and 36 (1,342 with normal, 155 with restrictive, and 162 with obstructive spirometric pattern)
Relative risk ratios (RRRs) are from multinomial logistic regression models adjusted for survey year, sex, and race/ethnicity. Normal spirometric pattern as the reference group.
Physician-confirmed diagnosis of asthma with active symptoms (asthma attacks or wheeze) at year 6, 11, or 16. ref=reference category. Significant associations are reported in bold font.
Multivariate analyses in TCRS
When all the above risk factors that were significantly associated with spirometric restriction and/or obstruction in univariate analyses were tested in a multivariate model, maternal nutritional problems during pregnancy, being born SGA, and childhood underweight were the only independent predictors that remained significant for spirometric restriction, whereas childhood asthma was the only independent predictor for obstruction (Table 4). This model yielded effect estimates for these factors that were similar to those observed in univariate analyses, suggesting that indicators of nutrition and growth deficits both in utero and in childhood are independent risk factors associated with the development of adult spirometric restriction. These results were also confirmed in GEE models (appendix p 9). Of note, the association of childhood underweight with adult spirometric restriction was confirmed when the model was further adjusted for child’s physical activity assessed by metabolic equivalent hours per week (data not shown).
Table 4:
Final multivariate model obtained with backwards variable selection for significant, independent determinants in early life for adult spirometric patterns across years 22–36 in TCRS
| N subjects | Adult Restrictive Spirometric Pattern | Adult Obstructive Spirometric Pattern | ||||
|---|---|---|---|---|---|---|
| RRR (95% CI)* | p value | RRR (95% CI)* | p value | |||
| Nutritional problems during pregnancy | No | 282 | ref | |||
| Yes | 101 | 2·48 (1·30, 4·76) | 0·006 | 1·40 (0·70, 2·79) | 0·34 | |
| Size for gestational age | AGA | 319 | ref | |||
| SGA | 31 | 3·26 (1·34, 7·93) | 0·009 | 1·11 (0·44, 2·83) | 0·82 | |
| LGA | 33 | 0·68 (0·18, 2·55) | 0·56 | 1·73 (0·63, 4·74) | 0·28 | |
| Childhood nutritional status across Years 6–16 | Normal weight | 224 | ref | |||
| Underweight | 24 | 3·54 (1·35, 9·26) | 0·010 | 1·32 (0·41, 4·25) | 0·65 | |
| Overweight | 60 | 1·58 (0·69, 3·63) | 0·28 | 1·55 (0·62, 3·90) | 0·35 | |
| Obese | 75 | 1·08 (0·50, 2·32) | 0·84 | 0·79 (0·32, 1·94) | 0·60 | |
| Childhood asthma across Years 6–16 | No | 279 | ref | |||
| Yes | 104 | 1·54 (0·78, 3·04) | 0·21 | 2·70 (1·29, 5·63) | 0·008 | |
The final model included 1,023 lung function observations from years 22, 26, 32, and 36 (814 with normal, 106 with restrictive, and 103 with obstructive spirometric pattern) from 383 participants. Initial model included significant predictors of adult spirometric restriction and/or obstruction from univariate analyses: maternal nutritional problems during pregnancy, maternal age, maternal asthma, paternal smoking, birthweight, ponderal index, size for gestational age, childhood nutritional status, and childhood asthma. Significant predictors were retained by backward selection at p <0·050. Survey year, sex, and race/ethnicity were also included in the model as a priori forced covariates.
Relative risk ratios (RRRs) are from multinomial logistic regression models. Normal spirometric pattern as the reference group. AGA=appropriate for gestational age. SGA=small for gestational age. LGA=large for gestational age. ref=reference category. Significant associations are reported in bold font.
Replication studies in BAMSE and MAAS
The early-life indicators of nutritional status and growth identified as determinants of adult spirometric restriction in TCRS were tested for replication in BAMSE and MAAS (Figure 1), with the exception of maternal nutritional problems during pregnancy for which information was not available in either of the replication cohorts. Birthweight, ponderal index, and size for gestational age (Figures 1A–C) yielded significant effect estimates for association with adult spirometric restriction when meta-analyzed across the three cohorts, although being born SGA was the only risk factor showing consistent associations across cohorts.
Figure 1: Associations of early-life and childhood indicators of nutritional status and growth with adult spirometric restriction in the three birth cohorts of TCRS, BAMSE, and MAAS.
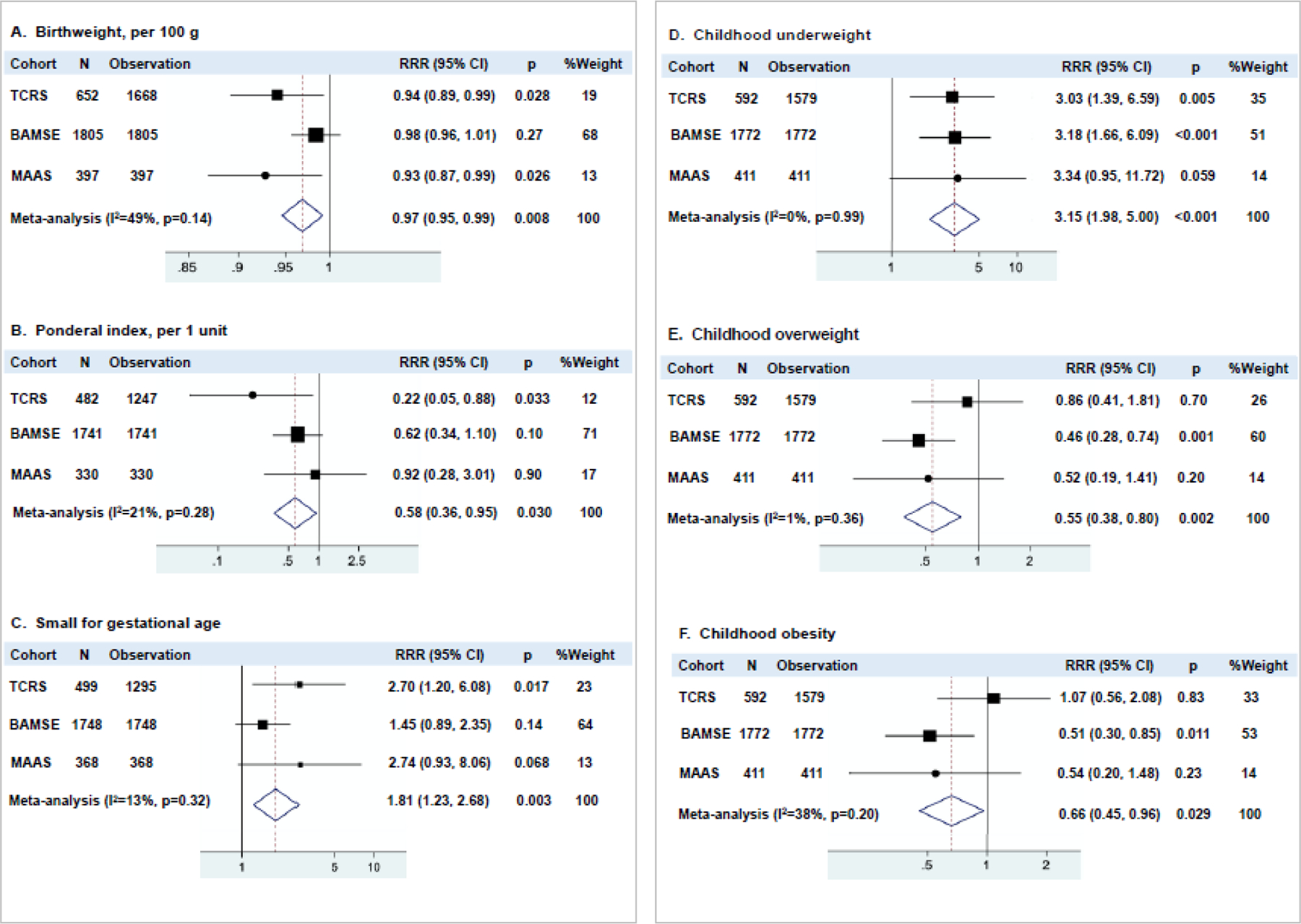
Results are from multinomial logistic regression models adjusted for sex, race/ethnicity (and survey year in TCRS). Spirometric patterns assessed at years 22–36 in TCRS, year 24 in BAMSE, and year 18 in MAAS. Normal spirometric pattern was used as the reference group. Childhood nutritional status was assessed across years 6, 11, 16 in TCRS, years 4, 8, 16 in BAMSE, and years 5, 8, 11, 16 in MAAS. RRR=relative risk ratio.
Associations between childhood underweight and adult spirometric restriction were remarkably consistent in all cohorts. In meta-analyses, children in the underweight group were three times more likely to develop the restrictive spirometric pattern as adults, compared to those in the normal weight group (p<0·001, Figure 1D). In contrast, children who were overweight (Figure 1E) or obese (Figure 1F) were protected from spirometric restriction in BAMSE and similar trends were observed in MAAS, although this association was not present in TCRS.
Consistent with these observations, as shown in Figure 2, participants with adult spirometric restriction had a lower BMI than participants with normal pattern, from early school ages through young adult life across all cohorts (meta-analyzed p<0·001).
Figure 2: Mean body mass index (BMI) at each age by adult spirometric patterns.
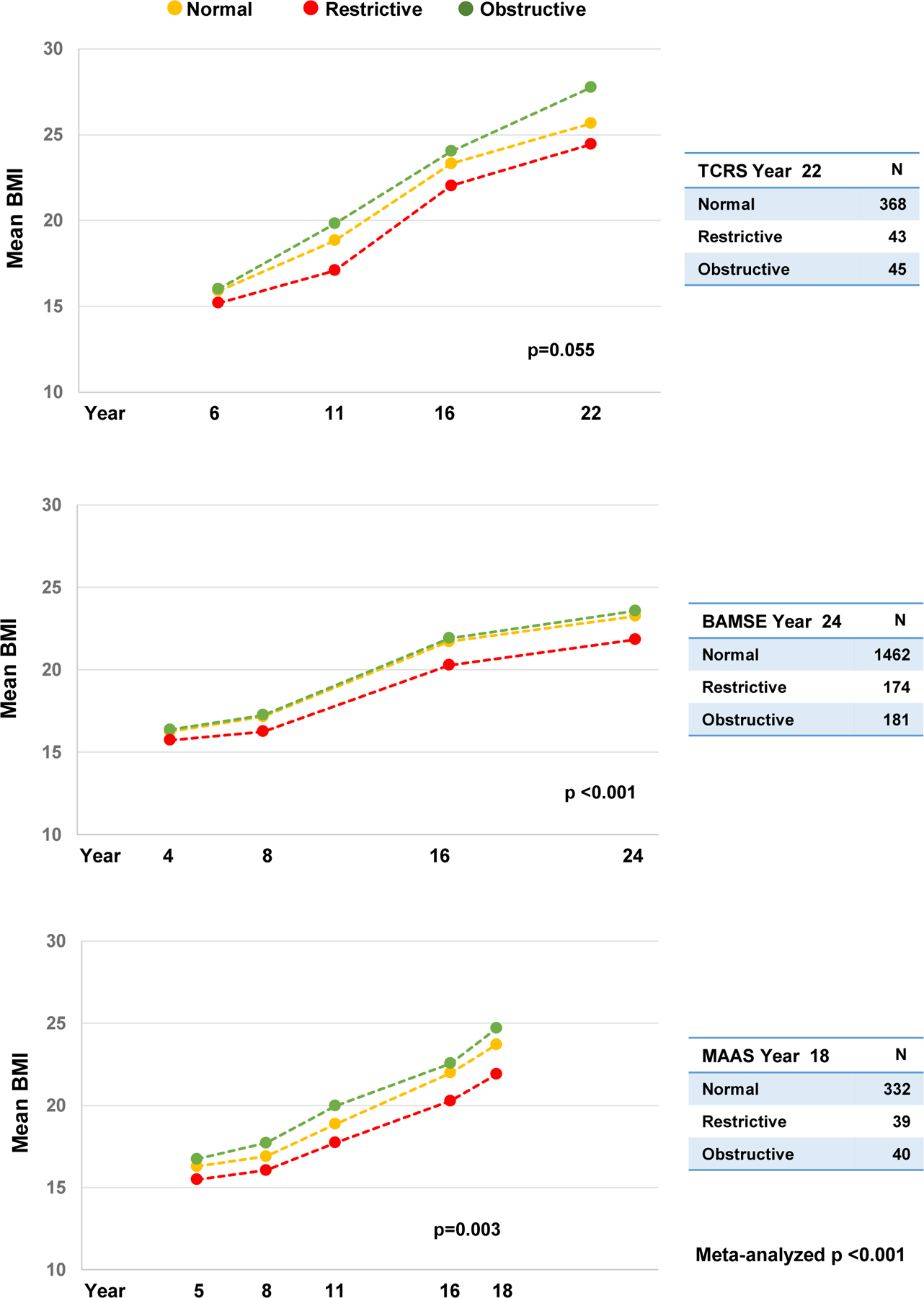
In TCRS, spirometry data from year 22 were used to closely parallel those of BAMSE (year 24) and MAAS (year 18). P values were obtained from random effect models assessing the relation between spirometric patterns (year 22 in TCRS, year 24 in BAMSE, year 18 in MAAS) and BMI across ages (years 6–22 in TCRS, years 4–24 in BAMSE, years 5–18 in MAAS), with adjustment for survey year, sex, and race/ethnicity. P-values shown for each cohort are for the comparison of the restrictive pattern versus normal pattern.
Child’s body composition and adult spirometric restriction in MAAS
The link between child’s body mass and adult spirometric restriction was further explored in MAAS, where body composition data were available at year 11. Interestingly, we found that the increased risk for adult spirometric restriction associated with low BMI was almost entirely due to deficits in lean body mass (Figure 3). A one-unit increase in LBMI at age 11 years was associated with a nearly 50% reduction in the risk of adult spirometric restriction (adjRRR 0·54, 95% CI 0·38–0·76, p<0·001), while there was no significant effect of FMI (0·86, 0·67–1·11, p=0·25). Consistent with these results, having low LBMI – but not low FMI – at age 11 increased significantly the risk for spirometric restriction in adulthood (3·66, 1·48–9·02, p=0·005; and 2·33, 0·93–5·85, p=0·072; respectively).
Figure 3: Child’s body composition at year 11 and adult spirometric patterns at year 18 in MAAS.
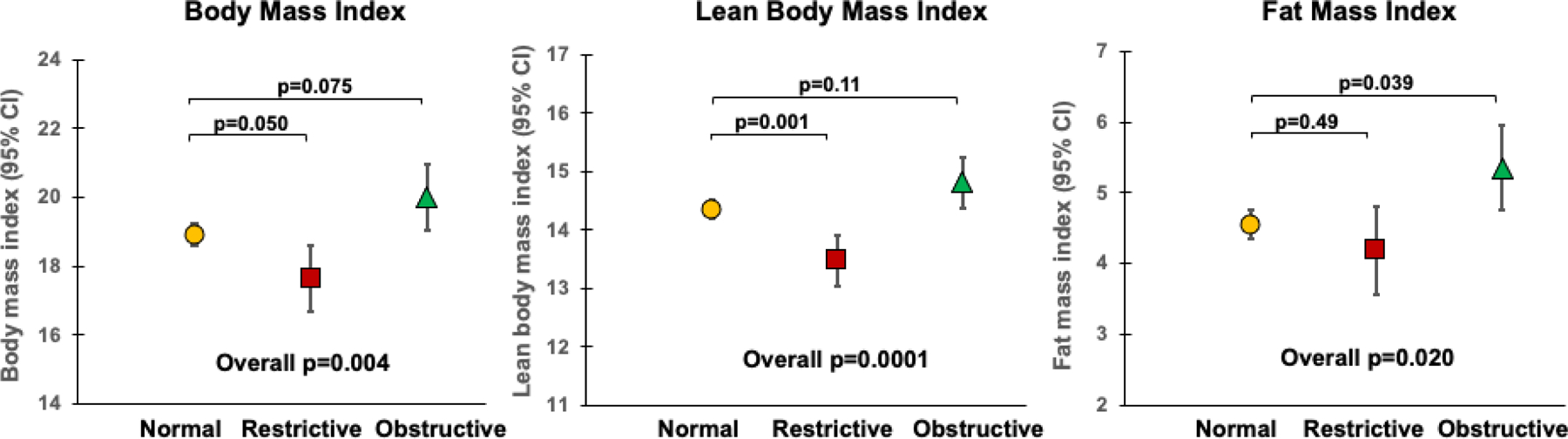
Normal spirometric pattern (n=297), restrictive pattern (n=33), obstructive pattern (n=35). Overall p value from ANOVA test. Pairwise comparisons were evaluated using Tukey’s post hoc test.
Early-life factors and adult spirometric restriction by TLC
TLC data were available for 173 TCRS participants at year 32 and 407 MAAS participants at year 18. In both TCRS and MAAS, the majority of participants with the restrictive spirometric pattern had low TLC (75% and 59%, respectively), as compared with only very small percentages of participants with normal or obstructive spirometric patterns (p=0·002 in TCRS and p<0·001 in MAAS, appendix p 16). These observations confirm that the restrictive spirometric pattern is associated with reduced static lung volumes in a notable proportion of cases. Importantly, in MAAS we found the associations of early-life risk factors, namely SGA, childhood underweight and childhood low lean body mass index, to be mainly present in participants with spirometric restriction and low TLC, rather than in those with spirometric restriction but normal TLC (Figure 4), suggesting that these factors increase specifically the risk for lung restriction. These relations could not be tested in TCRS due to the small number of participants with TLC information.
Figure 4: Associations of small for gestational age at birth, childhood underweight, and low lean body mass index at year 11 with spirometric restriction with and without low total lung capacity (TLC) at year 18 in MAAS.
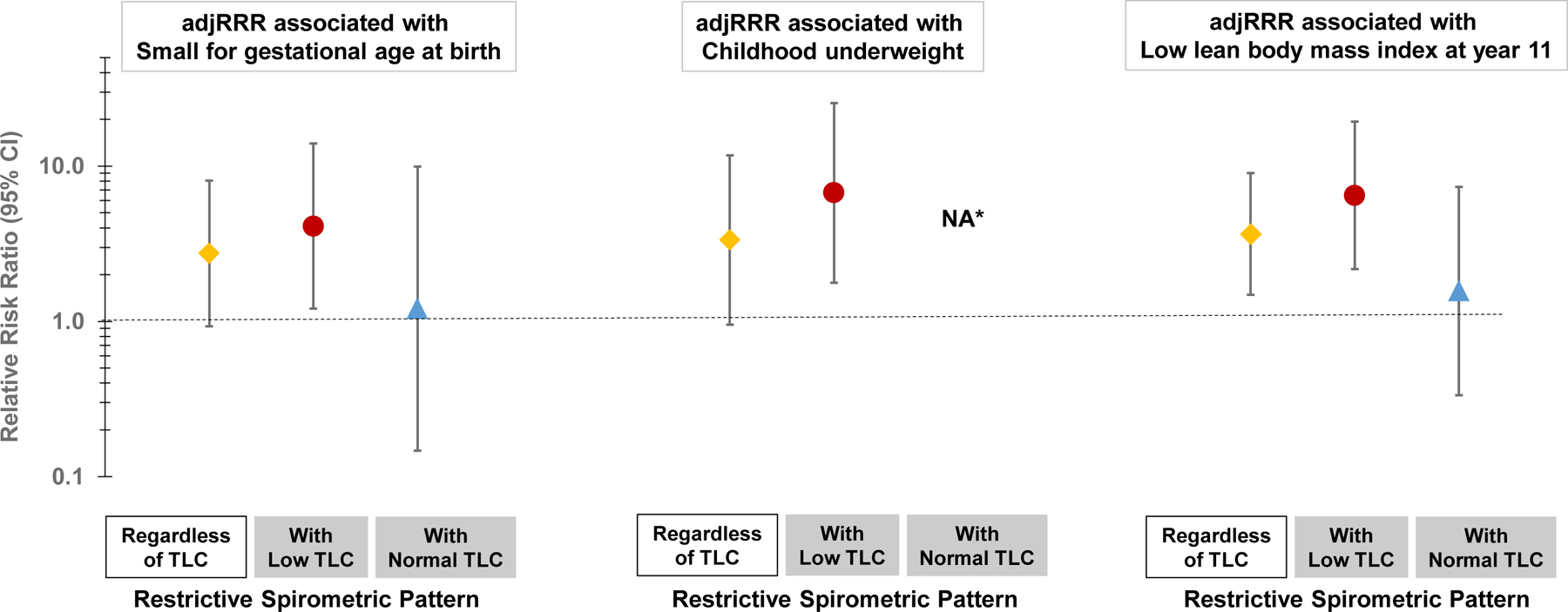
Results for associations with small for gestational age, childhood underweight, and low lean body mass index are from models that included 368, 411, and 365 MAAS participants, respectively. Relative risk ratios are from multinomial logistic regression models adjusted for sex and race/ethnicity. Normal spirometric pattern as the reference group. Low TLC defined as below the 10th percentile based on the residuals estimated from regressing TLC on age, height, sex, and race/ethnicity. Normal TLC defined as ≥ 10th percentile. Childhood nutritional status assessed across years 5, 8, 11, and 16. Low lean body mass index defined as below the 10th percentile based on the residuals estimated from regressing lean body mass index on age, sex, and race/ethnicity. *Estimate was not available because none of the participants in the restrictive spirometric pattern with normal TLC group was underweight during childhood.
Sensitivity analyses
Results from analyses performed among participants without significant BDR are provided in appendix p 10 and those from analyses using GLI LLN cut-offs are provided in appendix p 11. As expected, the prevalence of LLN-defined adult spirometric restriction was lower than that based on 10th percentiles used in primary analyses (1–3% in TCRS depending on the survey year, 2% in BAMSE, and 5% in MAAS). However, consistent with results from the main analyses, these sensitivity analyses confirmed significant relations of maternal nutritional problems during pregnancy, being born SGA, childhood underweight, and childhood low LBMI to the adult restrictive pattern.
When lung function groups were defined based only on FEV1 thresholds, univariate analyses in TCRS (appendix pp 12–13) showed nutritional problems during pregnancy, young maternal age, and childhood underweight to be associated with the FEV1-defined low lung function pattern, similar to what we found for spirometric restriction in primary analyses. However, the effects of indicators of growth in utero (i.e., SGA, birthweight, and ponderal index) became weaker and non-significant. Childhood asthma, which was associated with spirometric obstruction in primary analyses, was strongly related to the FEV1-defined low lung function pattern. In multivariate analyses (appendix p 14), maternal age, childhood underweight, and childhood asthma remained significant predictors for FEV1-defined low lung function. The effects of childhood underweight and childhood asthma were also confirmed in meta-analyses (appendix p 15).
DISCUSSION
In this study, we provide novel findings on early-life risk factors for adult spirometric restriction using three independent population-based birth cohorts. We found indicators of nutrition and growth impairment both in utero and in childhood to be associated with an increased risk for developing spirometric restriction in adult life. Specifically, we identified maternal nutritional problems during pregnancy, being born SGA, and childhood underweight as independent risk factors for adult spirometric restriction. Analyses on body composition showed that deficits in lean body mass were largely responsible for the association between childhood underweight and adult spirometric restriction. The effects of these risk factors were particularly strong among participants who had both a restrictive spirometric pattern and low total lung capacity, i.e., true lung restriction, in adulthood.
Our findings are consistent with mounting evidence that impairment of intrauterine growth may have long-term consequences on health and increase susceptibility to certain chronic conditions, including respiratory diseases, in later life.27,28 We found that being born SGA, or with lower birthweight, or lower ponderal index were all associated with an increased risk for spirometric restriction, but not obstruction, in adult life. These observations are in line with a recent meta-analysis29 that showed a reduction in FVC levels in adults born with a lower birthweight, while the association of birthweight with the FEV1/FVC ratio was weaker and inconsistent. Intrauterine growth is critically affected by maternal nutrition.30,31 Indeed, in our study not only we observed that mothers with nutritional problems during pregnancy tended to have more SGA babies (data not shown), but we also found a strong relation of a report of maternal nutritional problems during pregnancy, specifically anemia and excessive vomiting, with the development of spirometric restriction by the offspring in their adult life.
Besides these pregnancy and perinatal risk factors, our analysis demonstrated that being underweight throughout childhood was another independent predictor for spirometric restriction during adulthood, with strikingly consistent relative risk estimates in all three cohorts. These observations demonstrate that in utero and childhood growth and nutritional status have profound and independent influences on lung development and that the effects of childhood underweight on the risk of adult spirometric restriction cannot be simply ascribed to tracking of reduced intrauterine growth and low birthweight.
Although BMI is the most widely used measure for assessing nutritional status, it is unable to differentiate between fat and lean mass. Our analyses on body composition provided additional insight, indicating that deficits in child’s lean mass, rather than fat mass, were responsible for the inverse relation of childhood BMI with adult spirometric restriction. In accordance with our results, a previous report from the population-based UK ALSPAC birth cohort showed a positive dose-response relationship between LBMI in childhood and FVC levels during adolescence.32 Higher lean body mass in childhood may represent an indicator of developmental trajectories and/or increased respiratory muscle strength resulting in greater lung volumes. Our body composition data also suggest that the apparently protective effects observed in BAMSE and MAAS for having BMI over the 85th percentile during childhood against development of the adult restrictive pattern are likely to be explained by an increase in lean body mass rather than excessive fat mass.
Although inadequate nutrition is considered to be the prime determinant of deficits in fetal and child’s weight and growth, other factors (including genetic, socioeconomic, behavioral, metabolic) can contribute30,31,33 and our findings are open to different interpretations. Maternal and early-life nutritional status may have direct causal effects on lung development and lung function growth. In support of this scenario are the independent effects of both child’s and maternal nutritional indicators in our study and the remarkably high prevalence of spirometric restriction that has been reported from geographical areas where low birthweight and maternal and child malnutrition remain major public health problems.1,2,4 Of note, in this scenario, the results of our study, which are based on data from countries where poor growth and undernutrition in children are less common, are likely to underestimate considerably the true impact that growth and nutrition deficits have on lung health in children in low and middle-income countries. Reduced intrauterine growth, childhood BMI, and lean mass trajectories may be also markers of broader developmental alterations that impact simultaneously multiple organs, including the lungs, and result in functional deficits that are already evident by young adult life. Consistent with this hypothesis are our findings that spirometric restriction was not associated specifically with increased respiratory symptoms and recent observations that low levels of lung function in young adult life are associated with multiple non-respiratory comorbidities, including metabolic diseases, and with increased risk for early cardiovascular mortality.34–36 Taken together, our observations support that strategies intended to achieve optimal developmental trajectories throughout the prenatal period and into childhood may have critical roles in reducing risk for spirometric restriction and, in turn, its associated comorbidities and mortality.
Our study indicated that distinctive profiles of risk factors are at play for spirometric restriction (nutritional problems during pregnancy, SGA, childhood underweight) and obstruction (childhood asthma). In fact, in multivariate analyses none of the identified risk factors was shared by spirometric restriction and obstruction, supporting that these patterns have different contributing factors and, possibly, pathogenetic mechanisms. Consistent with these observations, in our sensitivity analyses, low FEV1 (a phenotype related to both spirometric patterns) was associated to some extent with risk factors for spirometric restriction as well as risk factors for spirometric obstruction, but the magnitude and consistency of these associations appeared weaker than those we observed when risk factors were tested directly against their specific spirometric patterns.
Varying definitions have been utilized for spirometric restriction, using fixed or LLN thresholds for FEV1/FVC and FVC.1 Notably, while sensitivity analyses using GLI-LLN cut-offs to define spirometric patterns yielded a lower prevalence of the restrictive pattern in our study, they confirmed all main results from primary analyses supporting the validity of our findings.
The gold standard for the diagnosis of restrictive defects requires measurement of TLC by body plethysmography or other techniques. However, the procedures are labor- and time-intensive, making them impractical in large population settings. Studies have indicated that spirometry-defined restriction has moderate sensitivity and high specificity for detecting TLC-based restriction.1,37 Consistent with these previous reports, we observed that a considerable proportion of individuals with spirometric restriction had diminished TLC, while those without spirometric restriction almost exclusively had normal TLC levels. Most importantly, the risk associated with growth and nutritional deficits early in life was more evident for participants who had spirometric restriction with reduced TLC (i.e., plethysmography-confirmed restriction) than for those who had spirometric restriction without TLC deficits. These findings indicate that spirometric restriction is associated with reduced static lung volumes in a notable proportion of cases and that early growth and nutrition risk factors affect specifically the risk for lung restriction. In line with these observations, in ours as in previous studies,7 well-known risk factors for obstructive lung diseases, including maternal asthma, childhood asthma, and cigarette smoke exposure, were not associated with the risk of the restrictive spirometric pattern.
Our study has certain limitations. Post-bronchodilator lung function was not performed in all cohorts; nonetheless, analyses from cohorts with available data confirmed the main results among participants without significant BDR. Furthermore, no relationship was observed between nutritional indices and obstruction. Our TLC data also indicated that the majority of individuals with spirometric restriction had valid lung restriction rather than a reduction in FVC due to gas trapping with an elevated residual volume to TLC ratio. Data on maternal nutritional problems during pregnancy were only available in the discovery TCRS cohort and no analyses could be completed on maternal weight gain during pregnancy to determine its potential effects on spirometric restriction. In addition, we did not explore genetic factors that have been associated with lung volume estimates (such as FVC) in adults,38 some of which are known to be involved in fetal lung development processes. Other environmental factors, such as air pollution,39 as well as socio-economic influences may also affect the risk for spirometric abnormalities in adult life, but they were beyond the scope of this study. This study’s major strength is the availability of three large, well-characterized population-based birth cohorts, with comprehensive prospective data collected shortly after birth and follow-up on lung function up to the fourth decade of life, and with remarkably consistent effect sizes in all three populations for the risk factors associated with spirometric restriction.
In conclusion, this study provides novel evidence that deficits in growth and nutritional status, as early as in utero and throughout childhood, increase the risk for restricted lung volume for body size in early adult life, indicating the congenital and developmental origins of this condition. Our findings support the long-term effects of early nutrition and developmental programming in mediating adult lung health and, possibly, protecting from the onset, morbidity, and mortality of adult spirometric restriction.
Supplementary Material
Research in Context.
Evidence before this study
Spirometric restriction has been identified as a significant determinant of morbidity and mortality in the adult general population. This spirometric pattern is remarkably prevalent in regions where maternal and child undernutrition are major public health concerns. However, whether early-life factors, particularly those related to growth and nutrition, contribute to the development of this condition remain unknown. We completed extensive PubMed searches with the search terms “spirometric restriction”, “preserved ratio impaired spirometry”, “PRISm”, “restrictive spirometric pattern”, “RSP”, “restrictive ventilatory pattern”, “RVP”, and “restrictive pattern”, without language restrictions. We identified several population-based cross-sectional and prospective studies reporting risk factors in adult life, and one study linking histories of childhood pneumonia and/or pleurisy to spirometric restriction during adulthood. However, no previous study assessed risk factors for adult spirometric restriction using prospective data collected as early as from birth. The date of our last search was April 15th, 2021.
Added value of this study
Using data from three-independent population-based birth cohorts, we found indicators of nutritional status and growth both in utero and in childhood to be the main determinants of spirometric restriction in adult life. In addition to maternal nutritional problems during pregnancy, we identified being born small for gestational age, childhood underweight, and childhood low lean body mass index as predictors of adult spirometric restriction. These associations were confirmed in participants with spirometric restriction who had diminished total lung capacity, indicating that these factors increase specifically the risk for lung restriction. Our study provides novel evidence that implicates deficits in growth and nutrition in utero through childhood as major risk factors for spirometric restriction in adult life.
Implications of all the available evidence
These findings support the long-term effects of early nutrition and developmental trajectories in mediating adult lung health and, possibly, protecting from the onset, morbidity, and mortality of adult spirometric restriction.
Acknowledgements
This study was supported by awards AI135108 from National Institute of Allergy and Infectious Diseases and HL132523 from National Heart, Lung, and Blood Institute, US National Institutes of Health. BAMSE was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, Region Stockholm, and the European Research Council TRIBAL, No 757919. MAAS was supported by the Asthma UK Grants No 301 (1995–1998), No 362 (1998–2001), No 01/012 (2001–2004), No 04/014 (2004–2007), BMA James Trust (2005) and The JP Moulton Charitable Foundation (2004-current), The North west Lung Centre Charity (1997-current) and the Medical Research Council (MRC) G0601361 (2007–2012), MR/K002449/1 (2013–2014) and MR/L012693/1 (2014–2018). UNICORN (Unified Cohorts Research Network): Disaggregating asthma MR/S025340/1.
We gratefully acknowledge the contributions of Lynn M. Taussig who started the Tucson Children’s Respiratory Study in 1980. We thank Darcie J. Revay for body plethysmography data and our study nurses for data collection and participant follow-up. We would like to thank the TCRS, BAMSE, and MAAS study participants and their parents for their continued support and enthusiasm.
Funding:
National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
WJM has received grant funding from the National Institutes of Health and Cystic Fibrosis Foundation and personal fees from the Cystic Fibrosis Foundation, the American Thoracic Society, and the American College of Chest Physicians. AC has received personal fees from Novartis, Thermo Fisher Scientific, Philips, Sanofi, Stallergenes Greer, and AstraZeneca. AS and CSM are supported by the NIHR Manchester Biomedical Research Centre. The other authors declare no competing interests.
Data sharing
De-identified data that were used for this study may be made available upon request within 1 year after publication. Requests for data will be reviewed on an individual basis by the principal investigators of the TCRS, BAMSE, and MAAS cohorts. Investigators must provide a methodologically sound approach to achieve scientific aims and proof of approval from the ethics committee of the investigator’s institution. Requests should be directed to the corresponding author.
REFERENCES
- 1.Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest 2016; 149: 238–51. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, McBurnie MA, Tan W, et al. Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis 2012; 16: 1405–11. [DOI] [PubMed] [Google Scholar]
- 3.Victora CG, Christian P, Vidaletti LP, Gatica-Dominguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet 2021; 397: 1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax 2014; 69: 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–68. [DOI] [PubMed] [Google Scholar]
- 6.Fortis S, Comellas A, Kim V, et al. Low FVC/TLC in Preserved Ratio Impaired Spirometry (PRISm) is associated with features of and progression to obstructive lung disease. Sci Rep 2020; 10: published online Mar 20. DOI: 10.1038/s41598-020-61932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra S, Carsin AE, Keidel D, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J 2017; 49: published online May 25. DOI: 10.1183/13993003.02096-2016. [DOI] [PubMed] [Google Scholar]
- 8.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 2010; 65: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58: 388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J 2020; 55: published online Jan 2. DOI: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 11.Carsin AE, Keidel D, Fuertes E, et al. Regular Physical Activity Levels and Incidence of Restrictive Spirometry Pattern: A Longitudinal Analysis of 2 Population–Based Cohorts. Am J Epidemiol 2020; 189: 1521–8. [DOI] [PubMed] [Google Scholar]
- 12.Perret JL, Lodge CJ, Lowe AJ, et al. Childhood pneumonia, pleurisy and lung function: a cohort study from the first to sixth decade of life. Thorax 2020; 75: 28–37. [DOI] [PubMed] [Google Scholar]
- 13.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol 1989; 129: 1219–31. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Hallberg J, Um Bergstrom P, et al. Assessment of chronic bronchitis and risk factors in young adults: results from BAMSE. Eur Respir J 2021; 57: published online Mar 4. DOI: 10.1183/13993003.02120-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Custovic A, Simpson BM, Murray CS, et al. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol 2002; 13: 32–7. [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sifakis S, Pharmakides G. Anemia in pregnancy. Ann N Y Acad Sci 2000; 900: 125–36. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995; 854: 1–452. [PubMed] [Google Scholar]
- 19.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 2014; 133: 844–53. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Defining childhood obesity https://www.cdc.gov/obesity/childhood/defining.html (accessed Feb 6, 2021).
- 21.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002: 1–190. [PubMed] [Google Scholar]
- 22.Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr 2008; 8: published online Feb 29. DOI: 10.1186/471-2431-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998; 17: 407–29. [PubMed] [Google Scholar]
- 24.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006; 450: 76–85. [DOI] [PubMed] [Google Scholar]
- 25.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007; 85: 660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Custovic A, Simpson A, Belgrave DC, Lowe LA, Murray CS. Differing associations of BMI and body fat with asthma and lung function in children. Pediatr Pulmonol 2014; 49: 1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker DJ. The foetal and infant origins of inequalities in health in Britain. J Public Health Med 1991; 13: 64–8. [PubMed] [Google Scholar]
- 28.Rasmussen KM. The “fetal origins” hypothesis: challenges and opportunities for maternal and child nutrition. Annu Rev Nutr 2001; 21: 73–95. [DOI] [PubMed] [Google Scholar]
- 29.Saad NJ, Patel J, Burney P, Minelli C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2017; 14: 994–1004. [DOI] [PubMed] [Google Scholar]
- 30.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008; 371: 243–60. [DOI] [PubMed] [Google Scholar]
- 31.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427–51. [DOI] [PubMed] [Google Scholar]
- 32.Peralta GP, Fuertes E, Granell R, et al. Childhood Body Composition Trajectories and Adolescent Lung Function. Findings from the ALSPAC study. Am J Respir Crit Care Med 2019; 200: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homan GJ. Failure to Thrive: A Practical Guide. Am Fam Physician 2016; 94: 295–9. [PubMed] [Google Scholar]
- 34.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low Lung Function in Young Adult Life Is Associated with Early Mortality. Am J Respir Crit Care Med 2017; 195: 1399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bush A Low Lung Function in Young Adult Life Is Associated with Early Mortality. Am J Respir Crit Care Med 2018; 197: 538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agusti A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017; 5: 935–45. [DOI] [PubMed] [Google Scholar]
- 37.Aaron SD, Dales RE, Cardinal P. How accurate is spirometry at predicting restrictive pulmonary impairment? Chest 1999; 115: 869–73. [DOI] [PubMed] [Google Scholar]
- 38.Portas L, Pereira M, Shaheen SO, et al. Lung Development Genes and Adult Lung Function. Am J Respir Crit Care Med 2020; 202: 853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz ES, Litonjua AA, Melen E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr Allergy Asthma Rep 2017; 17: published online June 17. DOI: 10.1007/s11882-017-0709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


