Abstract
Background
Cysteine is a precursor of glutathione, an antioxidant that may reduce oxidation injury. The addition of cysteine to parenteral nutrition (PN) allows for the reduction of the amount of methionine in PN, thereby limiting hepatotoxicity and acidifies the solution, thereby increasing calcium and phosphate solubility and potentially improving bone mineralization.
Objectives
To determine the effects of supplementing PN with cysteine, cystine or its precursor N‐acetylcysteine on neonatal growth and short and long‐term outcomes.
Search methods
The standard search method of the Cochrane Neonatal Review Group was used. MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (The Cochrane Library) and recent abstracts from the Society for Pediatric Research/ American Pediatric Society, Eastern Society for Pediatric Research, and Society for Parenteral and Enteral Nutrition were originally searched in 2005. In August 2009 updated searches were done of The Cochrane Library, MEDLINE (search via PubMed), CINAHL and EMBASE from 2006 to 2009.
Selection criteria
All randomized (RCTs) and quasi‐randomized trials that examined the effects of cysteine, cystine or N‐acetylcysteine supplementation of neonatal PN were reviewed.
Data collection and analysis
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. Statistical analysis included relative risk, risk difference, and weighted mean difference (WMD).
Main results
Six trials fulfilled entry criteria. The majority of patients in these trials were preterm. Five small trials evaluated short‐term cysteine supplementation of cysteine‐free PN. One large multicenter RCT evaluated short‐term N‐acetylcysteine supplementation of cysteine‐containing PN in extremely low birth weight infants (≤ 1000 grams).
Growth was not significantly affected by cysteine supplementation (1 trial) or by N‐acetylcysteine supplementation (1 trial). Nitrogen retention was significantly increased by cysteine supplementation (4 trials) (WMD 31.8 mg/kg/day, 95% confidence interval +8.2, +55.4, n = 95, including 73 preterm infants).
Plasma levels of cysteine were significantly increased by cysteine supplementation but not by N‐acetylcysteine supplementation. N‐acetylcysteine supplementation did not significantly affect the risks of death by 36 postmenstrual weeks, bronchopulmonary dysplasia (BPD), death or BPD, retinopathy of prematurity (ROP), severe ROP, necrotizing enterocolitis requiring surgery, periventricular leukomalacia, intraventricular hemorrhage (IVH), or severe IVH.
Authors' conclusions
Available evidence from RCTs shows that routine short‐term cysteine chloride supplementation of cysteine‐free PN in preterm infants improves nitrogen balance. However, there is insufficient evidence to assess the risks of cysteine supplementation, especially regarding metabolic acidosis, which has been reported during the first two weeks of cysteine chloride administration. Available evidence from a large RCT trial does not support routine N‐acetylcysteine supplementation of cysteine‐containing PN in extremely low birth weight infants.
Plain language summary
Cysteine, cystine or N‐acetylcysteine supplementation in parenterally fed neonates
Sick or preterm newborn infants may require intravenous nutrition, including intravenous administration of solutions containing amino acids. Newborn infants need cysteine (an amino acid) for growth under certain conditions. Cysteine may decrease the chance of liver disease and brittle bones. This systemic review was done to analyze whether adding cysteine (or related compounds) to intravenous nutrition affects growth and other outcomes in newborn infants. Five trials studied the effects of adding cysteine to intravenous nutrition that did not contain cysteine. Addition of cysteine significantly improved the babies' ability to build body proteins (analyzed in four studies); however, it did not improve growth (analyzed in one study); no other outcomes were available. One large randomized trial studied the effect of adding another chemical, N‐acetyl‐cysteine, to intravenous nutrition that already contained cysteine. This study showed no benefit and no toxicity of this intervention. We conclude that present data are insufficient to justify routine addition of cysteine to the intravenous nutrition of newborn infants that does not contain cysteine. Available evidence does not support routine addition of N‐acetylcysteine to intravenous nutrition of newborn infants containing cysteine.
Background
Description of the condition
The inability of sick term and preterm infants to feed enterally frequently leads to the use of total parenteral nutrition (TPN) in order to provide adequate calories and nutrients to promote growth. Most commercial parenteral solutions contain a mixture of both essential and non‐essential amino acids. Low plasma levels of any essential amino acid indicate a relative deficiency state and this may be detrimental to nitrogen balance and growth (Rose 1955).
Description of the intervention
Several facts suggest that the addition of L‐cysteine, cystine or N‐acetylcysteine to TPN may be beneficial in neonates, especially very low birth weight (VLBW) infants (infants with birth weight < 1500 g) .
Several publications have suggested that L‐cysteine could be a conditionally essential (i.e., essential under certain conditions) amino acid for very preterm but not for borderline preterm or full‐term neonates (Uauy 1993; Brunton 2000; Heird 1998; Riedijk 2006). In contrast, one randomized trial comparing various intakes of cyst(e)ine in enterally fed very low birth weight infants did not support the hypothesis that cyst(e)ine is a conditionally essential amino acid in these infants. (Riedijk 2007; Riedijk 2008).
Cysteine is incorporated into proteins and is a precursor of taurine and glutathione. In adults, cysteine can be synthesized from ingested methionine via the trans‐sulfuration pathway and hence is considered one of the non‐essential amino acids (Rose 1955). In the fetus, hepatic cystathionase, the rate‐limiting enzyme that converts cystathionine to cysteine, is either absent or barely detectable, resulting in decreased synthesis of cysteine from methionine and high serum concentration of cystathionine (Sturman 1970; Pascal 1972; Gaull 1972; Heinonen 1974). Preterm infants have higher plasma cystathionine levels than term infants (Viña 1995). Hepatic cystathionase activity increases with gestational age (Sturman 1970; Heinonen 1974; Zlotkin 1982) and increases rapidly after birth (Zlotkin 1982) reaching mature levels at about three months in term infants. In vivo studies have shown that cysteine synthesized in preterm liver may be present in apolipoprotein B but not in plasma, suggesting that endogenous cysteine is used preferentially for hepatic protein synthesis (Miller 1996). In contrast, a recent study showed that cysteine synthesized in the liver was found in plasma of VLBW infants in amounts that increased with maturation (Shew 2005). Extrahepatic cystathionase, which matures earlier than the hepatic enzyme, might account for some conversion of methionine into cysteine even in preterm infants (Uauy 1993).
How the intervention might work
Parenterally fed infants or adults who do not receive cysteine supplements in TPN have low plasma levels of cystine (Zlotkin 1981; Uauy 1993), the oxidized, disulfide form of cysteine. Regression analysis suggests that an intravenous intake of cysteine of 500 micromoles per kg per day is required in preterm infants to reach plasma cysteine levels comparable to those in full‐term, breast‐fed infants (Van Goudoever 1994). Snyderman found that enteral supplementation of cysteine improved growth in preterm infants at two to four months of age (Snyderman 1971). These data support the possibility that cysteine may be a conditionally essential amino acid in preterm infants; supplementation of cysteine could improve growth and nitrogen retention under certain conditions. Liver toxicity may be induced by high methionine content of commercial parenteral nutritional solutions that do not contain cysteine (Moss 1999; Brunton 2000). Because of this, some manufacturers have added cysteine to commercial TPN and reduced methionine content accordingly. Supplementation of an essential amino acid (in this case cysteine) may not result in any improvement in growth if caloric intake or intake of other essential or conditionally essential nutrients (e.g., tyrosine) is limiting (Helms 1987; Heird 1993).
Studies have suggested that preterm infants have a poorly developed antioxidant system, making various organs more susceptible to oxidation injury, thereby increasing the risk for intracranial hemorrhage, periventricular leukomalacia, retinopathy of prematurity, chronic lung disease, and necrotizing enterocolitis (Thibeault 2000). Cysteine deficiency may be an etiological factor in the limited ability of preterm babies to produce glutathione, a natural antioxidant. The first step of synthesis of glutathione involves gammma‐glutamylcysteine synthetase, which mediates a peptide linkage between glutamate and cysteine. Studies have demonstrated a decrease in glutathione synthesis in erythrocytes of neonates when methionine was used as a cysteine precursor (Viña 1995). The effect was more pronounced in preterm infants less than 32 weeks of age. Supplementation of parenteral nutrition with cysteine increases endogenous synthesis of taurine and glutathione (Shew 2000a).
Taurine is present in all cells and is conjugated to biliary pigments, which are excreted into bile as soluble biliary salts. In adults, taurine can be synthesized from cysteine and methionine. However, in preterm infants, taurine is also considered a conditionally essential amino acid. Taurine needs to be included in parenteral solutions to prevent cholestasis (Howard 1992).
Adding cysteine‐hydrochloride (cysteine‐HCl) to TPN solutions increases calcium and phosphate solubility. Cysteine‐HCl reduces the pH of TPN significantly (Laine 1991; Parikh 2005), thereby increasing calcium and phosphate solubility and allowing increased mineral intake, a limiting factor in mineral accretion in preterm infants receiving TPN. Increased mineral accretion could reduce the incidence of osteopenia of prematurity and fractures in VLBW infants. However, acidification of the TPN by cysteine‐chloride in the smallest infants (especially those < 1250 g) may be associated with metabolic acidosis for the first two weeks (Uauy 1993; Laine 1991; Heird 1988). Such metabolic acidosis can be prevented by the administration of base (acetate or lactate) at a 2:1 molar (base:cysteine hydrochloride) ratio (Uauy 1993; Laine 1991).
Stability of cysteine in TPN The poor stability of cysteine in commercial amino acid solutions has led to the development of cysteine substitutes with greater stability. Cysteine is easily oxidized to cystine, which precipitates rapidly and is virtually insoluble in TPN solutions. In solutions containing both cysteine and glucose, the cysteine content of the fluid decreases by 40% within the first 10 hours from both oxidization and formation of D‐glucocysteine (Bjelton 1990). N‐acetyl‐cysteine, a soluble precursor, improves stability in solution, thereby allowing higher total concentrations of cysteine, cystine and its precursor in TPN. However, plasma cystine levels have been shown to be less than one third the lower limit of the reference range when cyst(e)ine intake consisted of small doses and no correlation was found between plasma cystine levels and N‐acetyl‐cysteine intake at these doses (Van Goudoever 1994).
Review Authors' note: In this review, international units (micromoles) were used. The molecular weights of cysteine‐hydrochloride and N‐acetyl‐cysteine are, respectively, 157.7 and 163.2. Therefore, to convert micromole to mg, multiply by 0.1577 and 0.1632, respectively.
Why it is important to do this review
This updates the review "Cysteine, cystine or N‐acetylcysteine supplementation in parenterally fed neonates (Soghier 2006a)".
Objectives
The primary aim of this review was to determine the effect of supplementation of parenterally fed neonates with cysteine, cystine or its precursor N‐acetylcysteine on growth assessed by weight gain, length, head circumference and nitrogen retention. The secondary aims were to determine the effect of cysteine, cystine or its precursor N‐acetylcysteine supplementation on mortality, morbidity secondary to oxidation injury (neurological, respiratory, ophthalmologic, gastrointestinal), bone accretion, acidosis or cholestasis.
Primary comparisons:
Cysteine or cystine vs. placebo supplementation to cysteine‐free PN
N‐acetylcysteine vs. placebo
Cysteine or cystine vs. N‐acetylcysteine
Planned subgroup analyses included the following:
Enteral versus parenteral (cysteine, cystine or N‐acetylcysteine) route
Gestational age: preterm (< 37 weeks of gestation) versus full‐term infants.
Adequacy of intake of calories and other amino acids, or lack of adequate ((Kleinman 2004; Roberts 2001) intake of at least one other element
TPN versus partial parenteral nutrition (PN)
RCTs versus quasi‐randomized trials
Methods
Criteria for considering studies for this review
Types of studies
All randomized and quasi‐randomized controlled trials in which cysteine, cystine or N‐acetyl cysteine was used for supplementation in comparison to placebo, other interventions or no supplementation in neonates. Cross‐over were eligible if they assessed short‐term outcomes. Cluster trials were eligible.
Types of participants
Infants 28 days postnatal age or less receiving no more than minimal enteral nutrition (maximum 15% of the daily calorie intake) were included.
Types of interventions
Intravenous or enteral supplementation of cysteine, cystine or N‐acetylcysteine versus no supplementation, placebo or other intervention. This included any dose, formulation, or duration, but excluded studies in which the intervention included not only supplementation of cysteine, cystine or N‐acetylcysteine but also supplementation of other nutrients. Starting age needed to be within 28 days of birth.
Types of outcome measures
Primary outcomes
Growth: weight gain (g), head circumference gain (cm) and length gain (cm), both short‐term (during the intervention period after one week and at the end of the intervention) and long‐term (after the intervention period at the time of discharge or at 36 weeks of postmenstrual age [obtained by adding gestational age and postnatal age]) and at one year of age
Nitrogen retention (mg/kg/day).
Secondary outcomes
Mortality before discharge
Chronic lung disease defined as continuous positive airway pressure or oxygen requirement (with or without characteristic radiographic findings) persisting beyond 36 weeks postmenstrual age
Retinopathy of prematurity of any stage or severe (stage 3 or more) (ICROP 2005)
Necrotizing enterocolitis defined as clinical evidence of abdominal distension and feeding intolerance plus radiological evidence of pneumatosis intestinalis with or without portal venous gas and pneumoperitoneum (greater than stage 1, using Bell's modified criteria) (Kanto 1994)
Cystic periventricular leukomalacia defined as cysts in the periventricular area on ultrasonogram, computerized tomography or magnetic resonance imaging scan
Intracranial hemorrhage of any grade or severe (grade 3 or 4) (Papile 1978)
Incidence of osteopenia of prematurity (defined as decrease bone mineral content and biochemical abnormalities), fractures
Incidence of metabolic acidosis (defined based on normal values [mean minus 2 standard deviations] of pH and base deficit for gestational age and postnatal age)
Incidence of cholestasis (serum level of direct bilirubin > 20% of total serum bilirubin, or serum level of direct bilirubin > 1 mg/dl if total level of bilirubin is < 5 mg/dl) (AAP 2004)
Calcium and phosphate retention (mmol/kg/day)
Plasma cyst(e)ine levels (µmol/100 ml), single measurement on the last day of the intervention period. This must be obtained before completion of the intervention because of rapid decrease in plasma level afterwards (Gomez 1993). Unless indicated, levels were assumed to correspond to free cyst(e)ine, because the bound fraction is not measured by conventional methods (Uauy 1993). Plasma levels of cyst(e)ine were calculated by adding the levels of cysteine and hemicystine.
In the protocol there was a typographical error. There was a mismatch between aim, objectives and outcomes: nitrogen retention was listed as primary in aim and objectives and as secondary in outcome measures. This mismatch was corrected in the review.
Search methods for identification of studies
Electronic searches
Searches were conducted using MEDLINE (1966 to December 2005), EMBASE (1974 to December 2005), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2006) and personal files. The MeSH headings used were cysteine, cysteine, cystine, N‐acetyl cysteine and parenteral nutrition, limited to infant, newborn. Reports in any language were examined.
In August 2009, the search was updated as follows: The Cochrane Library, MEDLINE (search via PubMed), CINAHL and EMBASE were searched from 2006 to 2009. Clinicaltrials.gov was searched with no date restriction. Search terms: cysteine OR cystine OR n acetyl cysteine AND parenteral nutrition. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searching other resources
The proceedings of Society for Pediatric Research/ American Pediatric Society, Eastern Society for Pediatric Research, and Society for Parenteral and Enteral Nutrition (1991 to December 2005) were hand searched for abstracts using the headings: cysteine and N‐acetylcysteine. For the 1992 and 1993 editions of the Society for Pediatric Research/ American Pediatric Society (no subject headings) the sections on developmental biology, developmental pharmacology, gastroenterology and nutrition, and neonatal nutrition and metabolism were searched.
Data collection and analysis
The criteria and standard methods of the Cochrane Collaboration and its Neonatal Review Group were used.
Selection of studies
Studies identified in the search were included if they met the inclusion criteria. All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. All investigators reviewed the results of the search and separately select the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
The review authors separately extracted, assessed and coded all data for each study using a form that was designed specifically for this review. Differences of opinion between review authors were resolved by discussion. Additional data were sought from the authors of all the trials included in the systematic review.
Where justified clinically, two or more subgroups of a published study, using standard formulae to calculate the weighted mean and to estimate the weighted standard deviation were merged (Altman 1991; Brion 1990; Zahr 1984).
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were employed. The methodological quality of the studies were assessed using the following key criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. For each criterion, assessment was yes, no, can't tell. Two review authors separately assessed each study. Any disagreement was resolved by discussion. This information was added to the Characteristics of Included Studies Table.
In addition, the following issues were evaluated and entered into the the Risk of Bias table:
Sequence generation: Was the allocation sequence adequately generated?
Allocation concealment: Was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
Incomplete outcome data: Were incomplete outcome data adequately addressed?
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
Statistical analysis was carried out using the standard method of the Cochrane Neonatal Review Group. Specifically, treatment effect was expressed as weighted mean difference (WMD) for continuous variables, and as risk difference (RD) and relative risk (RR) for dichotomous variables, using a fixed effect model for meta‐analysis and 95% confidence intervals (CI). For cross‐over trials, the inverse variance method, [assuming a correlation coefficient of either 0.4 (as presented in the RevMan Analysis figure), or 0.7 was used (sensitivity analysis)].
Assessment of heterogeneity
Meta‐analysis was performed for studies where supplementation was used for similar patients, design and dosage. Tests of between‐study heterogeneity (chi‐square analysis and I2 statistic) were used to determine if pooling of data was appropriate; if there was inconsistency in the direction of the effect, the data of the meta‐analysis was not presented.
Data synthesis
Statistical analysis was carried out using the standard method of the Cochrane Neonatal Review Group. Specifically, treatment effect was expressed as weighted mean difference (WMD) for continuous variables, and as risk difference (RD) and relative risk (RR) for dichotomous variables, using a fixed effect model for meta‐analysis and 95% confidence intervals (CI). For cross‐over trials, the inverse variance method, [assuming a correlation coefficient of either 0.4 (as presented in the RevMan Analysis figure), or 0.7 was used (sensitivity analysis)]. Meta‐analysis was performed for studies where supplementation was used for similar patients, design and dosage.
Subgroup analysis and investigation of heterogeneity
Enteral versus parenteral (cysteine, cystine or N‐acetylcysteine) route
Gestational age: preterm (< 37 weeks of gestation) versus full‐term infants.
Adequacy of intake of calories and other amino acids, or lack of adequate ((Kleinman 2004; Roberts 2001) intake of at least one other element
TPN versus partial parenteral nutrition (PN)
RCTs versus quasi‐randomized trials
Results
Description of studies
The MEDLINE search retrieved seven studies and the abstract search four additional studies. The EMBASE and the CCRT search did not retrieve any additional studies. Among these eleven studies, six fulfilled the predetermined criteria established for this review, and five were excluded.
Included studies: Specific information about these six studies is presented in the table entitled "Characteristics of Included Studies." All six trials allocated individual patients. Five trials used a parallel design, and one (Storm 2003) a cross‐over design. Five trials randomized cysteine and one (Ahola 2003) used N‐acetylcysteine supplementation. All trials used parenteral supplementation (of cysteine or N‐acetylcysteine) versus placebo or control. Five studies enrolled exclusively preterm infants, and (Zlotkin 1981) enrolled preterm infants and full‐term infants. Inadequate nitrogen intake was documented in a subset of one study (Malloy 1984). Two studies (Malloy 1984; Zlotkin 1981) used as baseline amino acid solution Aminosyn (Abbott Laboratories), which contains neither tyrosine, nor cysteine in any form. One study (Ahola 2003) used as baseline amino acid solution Vaminolac (Fresenius‐Kabi, Uppsala, Sweden), which contains cysteine, cystine and tyrosine. The other three studies (Kashyap 1992; Shew 1999; Storm 2003) presumably (Heird 1988, Uauy 1993; Miller 1996; Shew 2000a) used Trophamine, which contains N‐acetyltyrosine and no cysteine. Five studies exclusively enrolled patients on TPN, and one (Ahola 2003) enrolled patients with PN and some enteral feeding. Four trials were RCTs (Ahola 2003; Malloy 1984; Shew 1999; Storm 2003), and two were quasi‐randomized trials (Kashyap 1992; Zlotkin 1981).
When all trials were analyzed, it appeared that they belonged to two groups which differed in many characteristics: enrolled patients (large range of birth weight and gestational age versus exclusively extremely low birth weight [ELBW] infants), cysteine content of the baseline parenteral nutrition (PN) (cysteine‐free PN versus cysteine‐containing PN), intervention (cysteine versus N‐acetylcysteine), and PN (TPN versus partial PN). The first group included five small (36 patients or less) trials, involving patients with a wide range of birth weights and gestational ages, which analyzed the effects of short‐term cysteine (0.5 ‐ 1 millimoles/kg/day) supplementation of cysteine‐free PN (Kashyap 1992; Malloy 1984; Shew 1999; Storm 2003; Zlotkin 1981) on growth (Zlotkin 1981), nitrogen retention (Kashyap 1992; Malloy 1984; Shew 1999; Zlotkin 1981) and plasma levels of cysteine (Kashyap 1992; Malloy 1984; Storm 2003; Zlotkin 1981). The second group included a single large multicenter RCT involving 391 ELBW infants, which analyzed the effects of N‐acetylcysteine supplementation of cysteine‐containing PN (Ahola 2003) on growth, death, death or bronchopulmonary dysplasia by 36 weeks of postmenstrual age, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis (operated), periventricular leukomalacia, intraventricular hemorrhage and plasma cysteine levels. The only outcome variable for the cross‐over study that is reported in this systematic review is the plasma level of cysteine.
Cysteine supplementation of cysteine‐free PN:
Kashyap 1992: The total number of patients entered into the study was 20. They were allocated to one of three arms: treatment arm (1) n = 7, treatment arm (2) n = 6, and control group n = 7. Entry criteria included: VLBW infants receiving total parenteral nutrition. The mean birth weight was 1010 g and mean gestational age 27.8 weeks. Patients in treatment arm (1) received intravenous cysteine supplementation at a dose of 1 millimole/kg/day in the parenteral solution, those in treatment arm (2) received the same dose of cysteine infused separately, and controls received no supplementation. For the purpose of this systematic review, the two treatment arms were combined as described in the method section. The duration of supplementation was not provided. Outcome variables included nitrogen retention and plasma free cysteine concentration. Caloric and nitrogen intake were not provided.
Malloy 1984: The total number of patients entered into the study was 20, including ten in the treatment group and ten in the control group. Entry criteria included: preterm infants at least two days old, unable to take enteral feeds and unlikely to begin enteral feeding within five to seven days; or without enteral intake for 48 hours and unable to tolerate enteral feeds for at least five to seven days. Birth weight ranged between 900 g and 2500 g, and postnatal age between three and 53 days; gestational age is not provided. Patients were randomized to receive either cysteine‐hydrochloride (Cysteine‐HCl, Abbott Laboratories, Abbott Park, Chicago, IL) (72 mg/kg/day or 0.46 millimoles/kg/day) added to the parental solution for 6 days, or no cysteine supplementation. Randomization was stratified by the amount of nitrogen intake: those receiving low nitrogen intake (1.5 g/kg/day of amino acids) and those receiving higher nitrogen intake (2.5 g/kg/day of amino acids). In this systematic review, merged data (combining groups with different levels of nitrogen intake) and original data (separate groups by nitrogen intake) were both evaluated. Amino acids were provided by Aminosyn (Abbott Laboratories), which contains neither cysteine, nor tyrosine in any form. Non‐protein calories ranged between 63 and 73 kcal/kg/day. Outcome variables included weight gain (no SD provided), nitrogen retention, and plasma levels of total and free cysteine.
Shew 1999: The total number of patients entered into the study was 19, including nine in the treatment group and ten in the control group. Entry criteria included: neonates receiving parenteral nutrition. Patients were randomized to receive either cysteine hydrochloride supplementation (0.78 [SD 0.10] millimoles/kg/day) in parenteral nutrition for 5.9 (SD 1.5) days versus no supplementation for 6.8 (SD 1.6) days. The main outcome variable was the nitrogen balance during the final 48 hours of the study. Caloric intake was 92 (SD 12) kcal/kg/day in the treatment group and 99 (SD 19) kcal/kg/day in the control group, and amino acid intake was 2.9 (SD 0.3) and 2.7 (SD 0.3) g/kg/day, respectively.
Storm 2003: The total number of patients entered into the study was 18. The entry criteria included: low birth weight, gestational age 30 ‐ 37 weeks, postnatal age less than four weeks, requiring PN for at least two weeks. Exclusion criteria included: inborn genetic error or significant organ failure. Target PN dosage was 2.5 g/kg of amino acids with total caloric intake of 135 kcal/kg/day. After a three‐day PN without cysteine, infants were randomly allocated to a series of six three‐day dosing schedules of cysteine hydrochloride, ranging between 0 and 40 mg cysteine hydrochloride per g of amino acids. For this systematic review we compared the levels of cyst(e)ine on 40 mg of cysteine hydrochloride per g of amino acid (treatment) versus 0 mg/g (control). Outcome variables included plasma levels of taurine, cystine and methionine. Zlotkin 1981: The total number of patients entered into the study was 36, including 18 in the treatment group and 18 in the control group. Entry criteria included: Infants (age 2 ‐ 46 days) receiving total parenteral nutrition. The average birth weight in the treatment group and the control group was, respectively, 2231 g (SD 1659 g) and 2141 g (SD 1515 g); average gestational age was, respectively, 34.4 weeks (SD 8.5) and 34.2 weeks (SD 9.3); average postnatal age was 13.7 days (SD 23.3) and 15 days (SD 23.8). Patients were allocated to receive either cysteine hydrochloride supplementation (Abbott Laboratories) (77 mg/kg/day of cysteine or 0.64 millimoles/kg/day) for 6 days or no supplementation. Both groups received an average of 87 kcal/kg/day and 3.2 g/kg/day of protein. Amino acids were provided by Aminosyn (Abbott), which contains neither cysteine, nor tyrosine in any form. Outcome variables included weight gain, changes in length and head circumference, nitrogen balance and plasma levels of cysteine and hemicystine.
N‐acetylcysteine supplementation of cysteine‐containing PN:
Ahola 2003: The total number of patients entered into the study was 391, including 194 in the treatment group and 197 in the control group. Entry criteria included: Infants weighing 500 to 999 grams and on ventilator or nasal continuous positive airway pressure before the age of 36 hours. Patients were treated according to the routine procedures in each intensive care unit and received PN and some enteral nutrition. Average birth weights in the treatment group and the control group, were respectively, 0.776 kg (SD 0.128 kg) and 0.78 kg (SD 0.134 kg); average gestational ages 26.3 weeks (SD 1.7) and 26.5 weeks (SD 1.8). Patients were randomized to either intravenous N‐acetyl‐L‐cysteine (Parvolex, Evans, UK or Mucomyst, Draco Läkemedel AB, Lund, Sweden) at a constant rate of 16 ‐ 32 mg/kg/day (0.10‐0.20 millimole/kg/day, calculated on the basis of birth weight, with the smallest infants receiving the lowest dosages for six days versus placebo (0.9% sodium chloride or solvent of Mucomyst). The dosage was based on a pharmacokinetic study, aiming at a steady‐state plasma concentrations of N‐acetyl‐L‐cysteine of 100 ‐ 300 micromoles/L (Ahola 1999). Both groups received a commercial intravenous amino acid solution (Vaminolac, Fresenius‐Kabi, Uppsala, Sweden), which contains 1 mg/ml of cysteine + cystine, and 0.5 mg/ml tyrosine. Amino acid intake was started on day one or two, with the first dose 8 ml/kg/day, and increased daily to a maximum of 40 ‐ 60 ml/kg/day (0.33 ‐ 0.50 millimoles/kg/day of cysteine equivalent). The primary outcome was death or bronchopulmonary dysplasia by 36 postmenstrual weeks. Secondary outcomes included death, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis (operated), periventricular leukomalacia, intraventricular hemorrhage and plasma cysteine levels. Dr. Ahola provided us with the standard deviation of cysteine levels at the end of therapy and with unpublished individual data on weight.
Excluded studies: Specific information is presented in the table entitled "Characteristics of Excluded Studies." Reasons for study exclusion included (1) additional intervention (methionine, N‐acetyltyrosine or different amino acid solutions) besides cysteine, cystine or N‐acetylcysteine, (2) non contemporaneous groups (pre/post design), (3) lack of analysis of any of the outcome variables that had been predefined in this systematic review. Interventions and outcome variables are briefly described here. Helms 1995 analyzed the effect of sulfur amino acid supplementation on plasma levels of taurine and cyst(e)ine. Laine 1991 analyzed the effect of cysteine chloride supplementation on acidity of the PN solution, serum carbon dioxide content and intake of acetate to compensate metabolic acidosis. This study showed that cysteine supplementation significantly reduced serum carbon dioxide content despite an increased intake of acetate. Pointdexter 2000 analyzed the effect of N‐acetyltyrosine and cysteine supplementation on amino acid metabolism and protein balance. Shew 2000 analyzed the effect of cysteine supplementation on amino acid metabolism. Van Goudoever 1994 analyzed the effect of N‐acetylcysteine and N‐acetyltyrosine on plasma levels and urine excretion of amino acids.
Risk of bias in included studies
Cysteine supplementation of cysteine‐free PN: Among these five small trials, three were randomized, and two of them used blind allocation. Three trials were RCTs (Malloy 1984; Shew 1999; Storm 2003), one trial used alternate allocation (Zlotkin 1981) and for one trial the method of allocation is not provided (Kashyap 1992). Blinding of allocation: yes in two trials (Shew 1999; Storm 2003), unclear in one trial (Malloy 1984) and no in two trials (Kashyap 1992; Zlotkin 1981). Blinding of intervention: yes in two trials (Shew 1999; Storm 2003), unclear in one trial (Malloy 1984) and no in two trials (Kashyap 1992; Zlotkin 1981) Follow‐up complete in all studies. Blinding of outcome: yes in two trials (Shew 1999; Storm 2003), unclear in one trial (Malloy 1984) and no in two trials (Kashyap 1992; Zlotkin 1981). Three trials were published in abstract form only (Kashyap 1992; Shew 1999; Storm 2003), thereby limiting our ability to critically and fully assess design and methodology.
N‐acetylcysteine supplementation of cysteine‐containing PN in ELBW infants:
Ahola 2003: This large multicenter double‐blind randomized trial is of very good quality. Blinding of randomization: yes. Blinding of intervention: yes. Complete follow‐up: yes. Blinding of outcome measurement: yes.
Effects of interventions
Cysteine supplementation of cysteine‐free PN (Comparison 1): This group included five small trials (three RCTs and two quasi‐randomized trials) (Kashyap 1992; Malloy 1984; Shew 1999; Storm 2003; Zlotkin 1981), which compared short‐term cysteine (0.5 ‐ 1 millimole/kg/day) with placebo supplementation of cysteine‐free PN of cysteine‐free PN.
1.1. Primary outcomes:
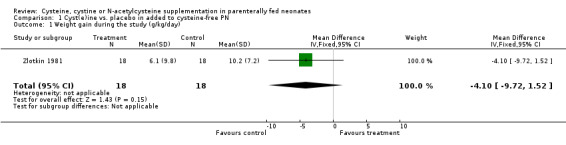
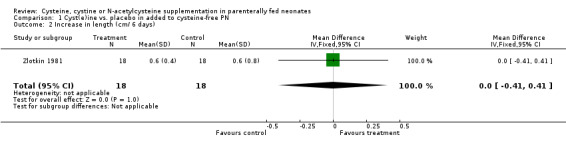
Growth (Outcomes 1.1 ‐ 1.3): Effects on growth (gains in weight, length or head circumference) were reported in only two studies (Zlotkin 1981; Malloy 1984). Zlotkin 1981 found no statistically significant effects on either weight gain (mean difference [MD] ‐4.1 g/kg/day, 95% CI ‐9.7, +1.5), length gain (MD 0 cm/6 days, 95% CI ‐0.41, +0.41), or head circumference (MD 0.3 cm/ 6 days, 95% CI ‐0.11, + 0.71). Malloy stated that there was no statistically significant effect of cysteine supplementation on weight gain, but reported only means and ranges. Thus, we could not combine these data in meta‐analysis.
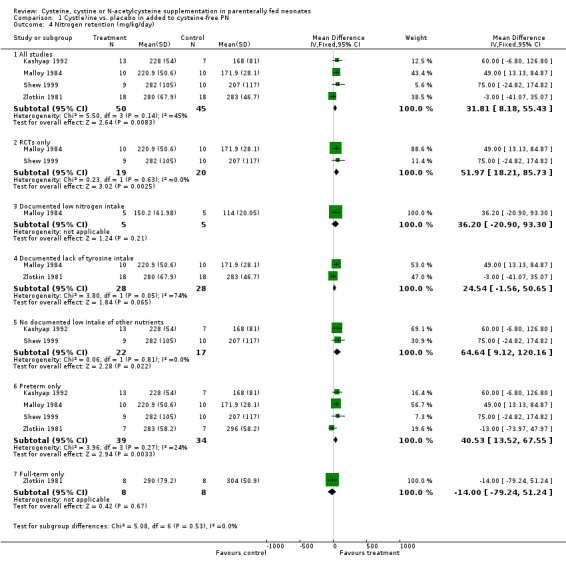
Nitrogen retention (Outcome 1.4): Effects on nitrogen retention was reported in four studies (Kashyap 1992; Malloy 1984; Shew 1999; Zlotkin 1981). Cysteine supplementation significantly increased nitrogen retention, WMD 31.8 mg/kg/day, 95% CI +8.2, +55.4. Sensitivity analysis did not significantly affect the results. Subgroup analyses showed significant improvement in nitrogen retention when analyzing infants without documented low intake of other nutrients, RCTs only, preterm infants only, but not in patients with documented low nitrogen intake (Malloy 1984), in those infants with documented lack of tyrosine intake (Malloy 1984; Zlotkin 1981) or in full‐term infants (Zlotkin 1981). However, one should note that the single study analyzing full‐term infants (Zlotkin 1981) used Aminosyn, which does not contain tyrosine.
1.2. Secondary outcomes:
Mortality and morbidity No data were reported on death, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, periventricular leukomalacia, intraventricular hemorrhage, or osteopenia of prematurity. No exact data were provided on metabolic acidosis (Kashyap 1992) and no data were reported on cholestasis, or calcium and phosphorus retention.
Plasma level of free cyst(e)ine (Outcome 1.5): The effect of cysteine supplementation on free plasma cyst(e)ine concentration at the end of therapy was reported in four trials (Malloy 1984; Storm 2003; Kashyap 1992; Zlotkin 1981). Cysteine supplementation significantly increased free plasma cyst(e)ine levels (MD 2.48 micromoles/100 ml, 95% CI 1.44,3.53). There was significant heterogeneity between the trials. Subgroup analyses also showed a significant increase in plasma free cyst(e)ine levels in RCTs only, in patients with demonstrated low nitrogen intake, those patients with lack of tyrosine intake, those patients with no documented low intake of or other nutrients, and in preterm infants, but not in full‐term infants. In Storm 2003, average cysteine levels were significantly correlated with the rate of cysteine administration (r = 0.97).
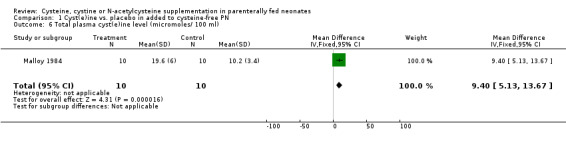
Total plasma cyst(e)ine level (Outcome 1.6): The effect of cysteine supplementation on total plasma cyst(e)ine concentration was reported in one trial (MD 9.4 micromoles/100 ml, 95% CI 5.1,13.7) (Malloy 1984).
N‐acetylcysteine supplementation of cysteine‐containing PN in ELBW infants (Comparison 2): This group included a single multicenter RCT (Ahola 2003), which compared N‐acetylcysteine with placebo supplementation of cysteine‐containing PN. Since only one trial analyzed this comparison no subgroup analyses could be conducted.
2.1. Primary outcomes:
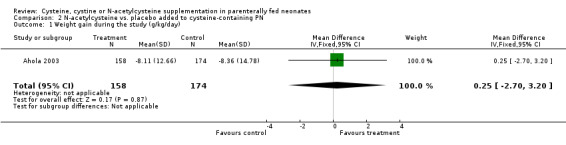
Growth (Outcomes 2.1 ‐ 2.2): Weight gain during the first week of life was available in 158 infants in the treatment group and 174 in the placebo group. N‐acetylcysteine supplementation did not significantly affect weight gain (MD 0.25 g/kg/day, 95% CI ‐2.70,+3.20). Weight at 36 weeks postmenstrual age was available in 157 infants in the treatment group and 166 in the placebo group. N‐acetylcysteine supplementation did not significantly affect the weight at 36 weeks (MD ‐4.8 g, 95% CI ‐90.7, +81.1).
2.2. Secondary outcomes:
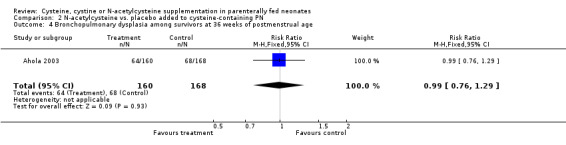
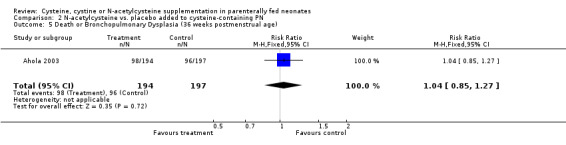
Death and bronchopulmonary dysplasia at 36 weeks corrected age (Outcomes 2.3 ‐ 2.5): The effect of N‐acetylcysteine supplementation on death and bronchopulmonary dysplasia at 36 weeks corrected age was available in 391 infants (194 in the treatment group and 197 in the placebo group). N‐acetylcysteine supplementation did not significantly affect the risk of death by 36 weeks of corrected age (RR 1.23, 95% CI 0.78,1.95; RD 0.03; 95% CI ‐0.04, +0.11). N‐acetylcysteine supplementation did not affect the risk of bronchopulmonary dysplasia among 328 survivors at 36 weeks corrected age (RR 0.99, 95% CI 0.76,1.29; RD 0.00, 95% CI ‐0.11, +0.10), or the risk of combined outcome death or bronchopulmonary dysplasia (RR 1.04, 95% CI 0.85,1.27; RD 0.02, 95% CI ‐0.08, +0.12).
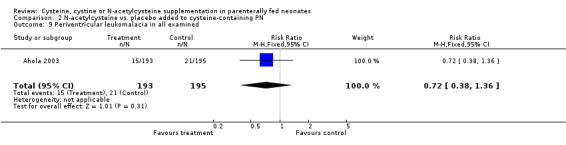
Other morbidity (Outcomes 2.6 ‐ 2.11): The effect of N‐acetylcysteine supplementation on retinopathy of prematurity, necrotizing enterocolitis requiring surgical intervention, periventricular leukomalacia and intraventricular hemorrhage was available in 391 infants, including 194 in the treatment group and 197 in the placebo group (Ahola 2003). N‐acetylcysteine supplementation did not significantly affect the risk of retinopathy of prematurity among infants examined (RR 0.89, 95% CI 0.71, 1.12; RD ‐0.06, 95% CI ‐0.16, +0.05); severe retinopathy of prematurity among infants examined (RR 1.06, 95% CI 0.59,1.90; RD 0.01, 95% CI ‐0.06, +0.08); necrotizing enterocolitis requiring surgical intervention (RR 1.08, 95% CI 0.56,2.07; RD 0.01; 95% CI ‐0.05, +0.06); periventricular leukomalacia among all infants examined (RR 0.72, 95% CI 0.38,1.36; RD ‐0.03, 95% CI ‐0.09, +0.03); intraventricular hemorrhage (RR 1.10, 95% CI 0.78, 1.55; RD 0.02, 95% CI ‐0.06, + 0.11); and severe intraventricular hemorrhage (RR 1.18, 95% CI 0.66,2.11; RD 0.02, 95% CI ‐0.04, +0.08). Data on metabolic acidosis and osteopenia were not available.
Plasma level of free cyst(e)ine (Outcome 2.12): The effect of N‐acetylcysteine supplementation on free plasma cysteine concentration at the end of therapy was available in 85 infants in the treatment group and 80 in the control group. Infants in the N‐acetylcysteine supplementation had a lower plasma cysteine concentration at the end of the study than those in the control group (MD ‐2.5 micromoles/100 ml, 95% CI ‐4.61, ‐0.39). However, the increase from baseline (third day) to the end of the study (day seven) was not significantly different between the two groups (Ahola 2003).
Subgroup analyses: not applicable
3. Cysteine or cystine vs. Nacetylcysteine supplementation of PN No studies found
Discussion
All trials analyzed the effects of short‐term cysteine supplementation on some of the predefined outcomes for this systematic review. All trials but one enrolled preterm infants only. The quality of the trials ranged from high‐quality multicenter RCT (Ahola 2003) to small quasi‐randomized trials published as abstracts only and providing only some of the important parameters required to assess quality.
As discussed previously, the six trials described and analyzed in this systematic review are best presented in two separate groups. Cysteine‐supplementation of cysteine‐free PN was analyzed in five small trials in which the authors were mainly interested in nitrogen retention and amino acid levels. Limitations of these trials include small size, lack of adequate or unclear evidence of allocation blinding (n = 3), and abstract publication only (n = 3). In contrast, N‐acetylcysteine supplementation of cysteine‐containing PN was analyzed in a large high‐quality multicenter trial in which the authors analyzed mortality and morbidity.
Cysteine supplementation of cysteine‐free PN: There was a discrepancy between the effects of cysteine supplementation on growth and those on nitrogen balance. One quasi‐randomized trial (Zlotkin 1981) showed no effects of cysteine supplementation on weight, length, head circumference or nitrogen retention in full‐term and preterm infants. Malloy 1984 showed that cysteine supplementation significantly increased nitrogen balance and stated it did not affect weight gain. In contrast, summary statistics showed that cysteine supplementation significantly increased nitrogen retention. These discrepancies in growth outcome may have resulted from differences in entry criteria, study duration, or nutritional intake other than cysteine. Cysteine is thought to be a conditionally essential amino acid, as shown by a study showing reduction in proteolysis and improvement in protein balance with simultaneous cysteine and tyrosine (or N‐acetyltyrosine) supplementation (Uauy 1993; Pointdexter 2000). The effect of cysteine or N‐acetyl‐cysteine supplementation on growth and nitrogen balance may depend on adequacy of tyrosine (or N‐acetyltyrosine) intake (Uauy 1993). Zlotkin 1981and Malloy 1984 used Aminosyn, which contained no tyrosine, as opposed to Aminosyn PF, which contains tyrosine (Kleinman 2004). More recent studies used either Vaminolac (Fresenius‐Kabi, Uppsala, Sweden), which contains tyrosine (Ahola 2003) or presumably Trophamine, which contains N‐acetyltyrosine (Kashyap 1992; Shew 1999; Storm 2003). The latter yields normal levels for virtually all amino acids except for low levels of cysteine (Heird 1987; Heird 1988). Subgroup analysis supports the hypothesis that the discrepancy between lack of improved nitrogen retention weight gain in two studies published in the 80's and significant improved nitrogen in two more recent studies may have resulted from lack of tyrosine in the first two studies.
Lack of data on mortality, toxicity (especially metabolic acidosis), or other short‐term and long‐term morbidity, and lack of any study using long‐term cysteine supplementation prevent us from giving a strong recommendation about the routine use of cysteine supplementation of cysteine‐free PN. Since data are insufficient to make a strong recommendation, we recommend a large randomized trial (see Conclusion).
N‐acetylcysteine supplementation of cysteine‐containing PN in ELBW infants: N‐acetylcysteine supplementation (6‐day course at a dose of 0.1 ‐ 0.2 millimoles/kg/day) of cysteine‐containing PN in EVLBW infants had no significant effect on primary and secondary outcomes in a large multicenter trial (Ahola 2003), except for a significant decrease in cysteine levels at the end of therapy. Possible reasons for these findings are multiple. First, glutathione deficiency is unlikely to be the single mechanism of oxidation injury. Second, glutathione levels were similar in both groups, suggesting that cysteine and cystine provided in PN prevented glutathione deficiency in both groups, thereby minimizing any effect of N‐acetylcysteine supplementation. Third, lack of efficacy of N‐acetylcysteine supplementation in VLBW infants may be due in part to urinary loss of acetylated amino acids (Van Goudoever 1994); however, the dosage used in this study led to adequate plasma levels of N‐acetylcysteine. Although plasma cysteine levels were lower in N‐acetylcysteine‐supplemented infants than in controls, the increase in plasma cysteine levels from day three to day seven, i.e., from baseline to the end of the study was similar in the two arms of the trial. N‐acetylcysteine may enter into the cells, where it can be deacetylated into cysteine (Ahola 2003). Fourth, lack of efficacy of N‐acetylcysteine supplementation may have resulted from too short an intervention. Finally, sample size was sufficient to detect changes in the primary outcome, but insufficient for secondary outcomes (e.g., periventricular leukomalacia). Lack of any significant effect of N‐acetylcysteine supplementation on mortality or any of the other outcomes in this large trial argues against the use of this supplementation in this setting.
Authors' conclusions
Implications for practice.
Available evidence from RCTs shows that routine short‐term cysteine chloride supplementation of cysteine‐free PN in preterm infants improves nitrogen balance. However, there is insufficient evidence to assess the risks of this supplementation, especially regarding metabolic acidosis, which has been reported during the first two weeks of cysteine chloride administration. Such acidosis may mostly affect the most immature infants during the first week of life.
Available evidence from one large RCT trial does not support routine N‐acetylcysteine supplementation of cysteine‐containing PN in extremely low birth weight infants.
Implications for research.
A large double‐blind RCT would be required to assess whether routine prolonged cysteine supplementation affects quantitative (weight, length, head circumference, nitrogen retention) and qualitative (body composition), and short‐ and long‐term neonatal outcomes in VLBW infants receiving cysteine‐free PN. Patients should receive a cysteine‐free amino acid solution that yields recommended amino acid concentrations (including tyrosine) except for cysteine, and adequate amounts of calories. To assess the effect of cysteine supplementation on bone mineral content, supplementation should be continued beyond two weeks, until cysteine‐induced metabolic acidosis is expected to resolve, thereby allowing investigators to stop alkalinization of the PN solution and thus increase its mineral content. The design of this double‐blind trial should allow the pharmacist to optimize mineral intake, based on solubility curves of calcium and phosphate in PN solutions (taking into account amino acids and pH) (Parikh 2005), without exceeding recommended osmolality.
Feedback
Comment from Jamie Kirkham, 7 August 2007
Summary
In the review you specify two primary outcomes: 1) Growth 2) Nitrogen Retention. However, in the review protocol, Growth is listed as the single most important primary outcome, while nitrogen retention was a secondary outcome.
Reply
In the protocol there was a typographical error: there was a mismatch between aim, objectives and outcomes: nitrogen retention was listed as primary in aim and objectives and as secondary in outcome measures. This mismatch was corrected in the review.
Contributors
Dr. Jamie Kirkham, MRC funded project ORBIT (Outcome Reporting Bias in Trials) (G0500952)
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2009 | New search has been performed | This updates the review "Cysteine, cystine or N‐acetylcysteine supplementation in parenterally fed neonates" published in the Cochrane Database of Systematic Reviews, Issue 4, 2006 (Soghier 2006a). Updated search conducted in August 2009 did not identify any new completed studies. One ongoing study was identified in Clinicaltrials.gov. The background in the Abstract and in the text was amended based on new information on cysteine in very low birth weight infants (Riedijk 2007; Riedijk 2008). Amendment made to text at the end of 'Types of outcome measures' section. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 12 March 2008 | Amended | Converted to new review format. |
| 10 July 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Dr. Ahola, who has generously provided us with additional data on weight and plasma cysteine levels.
We wish to thank Jamie Kirkham for pointing out an inconsistency in "Types of Outcome Measures". The published protocol listed "nitrogen retention" as primary in aim and objectives and as secondary in outcome measures. This inconsistency was corrected so that "nitrogen retention" appears under only Primary Outcomes in the completed review.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Cyst(e)ine vs. placebo in added to cysteine‐free PN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain during the study (g/kg/day) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐9.72, 1.52] |
| 2 Increase in length (cm/ 6 days) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.41, 0.41] |
| 3 Increase in head circumference (cm/ 6 days) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.11, 0.71] |
| 4 Nitrogen retention (mg/kg/day) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 All studies | 4 | 95 | Mean Difference (IV, Fixed, 95% CI) | 31.81 [8.18, 55.43] |
| 4.2 RCTs only | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 51.97 [18.21, 85.73] |
| 4.3 Documented low nitrogen intake | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 36.20 [‐20.90, 93.30] |
| 4.4 Documented lack of tyrosine intake | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 24.54 [‐1.56, 50.65] |
| 4.5 No documented low intake of other nutrients | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 64.64 [9.12, 120.16] |
| 4.6 Preterm only | 4 | 73 | Mean Difference (IV, Fixed, 95% CI) | 40.53 [13.52, 67.55] |
| 4.7 Full‐term only | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐79.24, 51.24] |
| 5 Plasma level of free cyst(e)ine (micromoles/100 ml) | 4 | WMD (Fixed, 95% CI) | Subtotals only | |
| 5.1 All studies | 4 | 112 | WMD (Fixed, 95% CI) | 2.48 [1.44, 3.53] |
| 5.2 RCTs only | 2 | 56 | WMD (Fixed, 95% CI) | 2.21 [0.86, 3.57] |
| 5.3 Document low nitrogen intake | 1 | 10 | WMD (Fixed, 95% CI) | 6.88 [1.58, 12.18] |
| 5.4 Documented lack of tyrosine intake | 2 | 56 | WMD (Fixed, 95% CI) | 3.19 [1.50, 4.88] |
| 5.5 No documented low intake of other nutrients | 2 | 56 | WMD (Fixed, 95% CI) | 2.05 [0.72, 3.38] |
| 5.6 Preterm only | 4 | 90 | WMD (Fixed, 95% CI) | 2.46 [1.28, 3.64] |
| 5.7 Full‐term only | 1 | 16 | WMD (Fixed, 95% CI) | 1.79 [‐1.76, 5.34] |
| 6 Total plasma cyst(e)ine level (micromoles/ 100 ml) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [5.13, 13.67] |
1.1. Analysis.
Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 1 Weight gain during the study (g/kg/day).
1.2. Analysis.
Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 2 Increase in length (cm/ 6 days).
1.3. Analysis.

Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 3 Increase in head circumference (cm/ 6 days).
1.4. Analysis.
Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 4 Nitrogen retention (mg/kg/day).
1.5. Analysis.

Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 5 Plasma level of free cyst(e)ine (micromoles/100 ml).
1.6. Analysis.
Comparison 1 Cyst(e)ine vs. placebo in added to cysteine‐free PN, Outcome 6 Total plasma cyst(e)ine level (micromoles/ 100 ml).
Comparison 2. N‐acetylcysteine vs. placebo added to cysteine‐containing PN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain during the study (g/kg/day) | 1 | 332 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐2.70, 3.20] |
| 2 Weight (g) at 36 weeks of postmenstrual age | 1 | 323 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐90.66, 81.06] |
| 3 Death by 36 weeks of postmenstrual age | 1 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.78, 1.95] |
| 4 Bronchopulmonary dysplasia among survivors at 36 weeks of postmenstrual age | 1 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.76, 1.29] |
| 5 Death or Bronchopulmonary Dysplasia (36 weeks postmenstrual age) | 1 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.85, 1.27] |
| 6 Retinopathy of prematurity (any stage) among those examined | 1 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 7 Severe (stage 3 or more) retinopathy of prematurity among those examined | 1 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 8 Necrotizing enterocolitis (operated) | 1 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.07] |
| 9 Periventricular leukomalacia in all examined | 1 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.36] |
| 10 Intraventricular hemorrhage (any grade) | 1 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.78, 1.55] |
| 11 Severe intraventricular hemorrhage (grade 3‐4) | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.66, 2.11] |
| 12 Plasma level of free cyst(e)ine (micromoles/ 100 ml) | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.61, ‐0.39] |
2.1. Analysis.
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 1 Weight gain during the study (g/kg/day).
2.2. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 2 Weight (g) at 36 weeks of postmenstrual age.
2.3. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 3 Death by 36 weeks of postmenstrual age.
2.4. Analysis.
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 4 Bronchopulmonary dysplasia among survivors at 36 weeks of postmenstrual age.
2.5. Analysis.
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 5 Death or Bronchopulmonary Dysplasia (36 weeks postmenstrual age).
2.6. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 6 Retinopathy of prematurity (any stage) among those examined.
2.7. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 7 Severe (stage 3 or more) retinopathy of prematurity among those examined.
2.8. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 8 Necrotizing enterocolitis (operated).
2.9. Analysis.
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 9 Periventricular leukomalacia in all examined.
2.10. Analysis.

Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 10 Intraventricular hemorrhage (any grade).
2.11. Analysis.
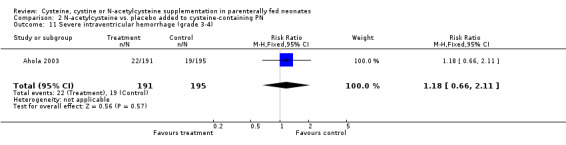
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 11 Severe intraventricular hemorrhage (grade 3‐4).
2.12. Analysis.
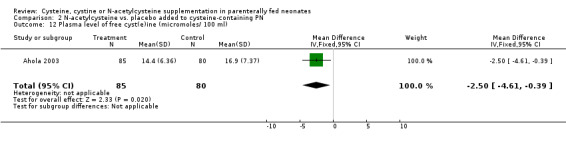
Comparison 2 N‐acetylcysteine vs. placebo added to cysteine‐containing PN, Outcome 12 Plasma level of free cyst(e)ine (micromoles/ 100 ml).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahola 2003.
| Methods | Multicenter double‐blind randomized trial. Blinding of randomization: yes. Blinding of intervention: yes. Complete follow‐up: yes. Blinding of outcome measurement: yes. | |
| Participants | Total number of patients entered into the study: 391. Entry criteria: Infants weighing 500 to 999 grams and on ventilator or nasal continuous positive airway pressure before the age of 36 hours. Average birth weight in the treatment group and the control group, respectively, 776 g (SD 128 g) and 780 g (SD 134 g) ; average gestational age 26.3 weeks (SD 1.7) and 26.5 weeks (SD 1.8); average postnatal age 24 hours (SD 9) and 23 hours (SD 9). | |
| Interventions | Intravenous N‐acetyl‐L‐cysteine (Parvolex, Evans, UK) or Mucomyst (Draco Läkemedel AB, Lund, Sweden) 16‐32 mg/kg/day (0.10‐0.20 millimole/kg/day) for 6 days (n = 194); versus placebo (0.9% sodium chloride or solvent of Mucomyst) (n = 197). | |
| Outcomes | Primary outcome of the main manuscript: death or bronchopulmonary dysplasia by 36 postmenstrual weeks. Secondary outcomes: death, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis (operated), periventricular leukomalacia, intraventricular hemorrhage and plasma cysteine levels on days 3 and 7. In addition to the original report, the authors also analyzed subgroups, one in which they found no effect of N‐acetyl‐L‐cysteine supplementation on pulmonary function (Sandberg 2004), and one in which they found evidence for lipid peroxidation (Ahola 2004). Dr. Ahola has generously provided us with data on weight and on plasma cysteine levels. Long‐term follow‐up data are not available yet. No data were available on bone accretion or level of acidosis. | |
| Notes | Both groups received an amino acid solution (Vaminolac, Fresenius‐Kabi, Uppsala, Sweden) containing 1 mg/ml of cysteine + cystine, and 0.5 mg/ml tyrosine, and providing a dose of cysteine starting at 8 mg/kg/day on day 1 or 2 and increasing daily up to 40‐60 mg/kg/day (0.33‐0.50 millimoles/kg/day of cysteine equivalent). Average nitrogen and caloric intake not provided. Setting: Finland, Sweden, Iceland, Denmark, Norway. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Multicenter double‐blind randomized trial. Randomization stratified for each center in blocks of 10 patients Blinding of randomization: yes |
| Allocation concealment? | Low risk | Multicenter double‐blind randomized trial Blinding of randomization: yes |
| Blinding? All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Kashyap 1992.
| Methods | Single‐site 3‐arm trial. Method of allocation: not provided. Blinding of allocation: no. Blinding of intervention: no. Complete follow‐up: yes. Blinding of outcome measurement: no. | |
| Participants | Total number of patients entered into the study: 20. Entry criteria: Very low birth weight infants receiving total parenteral nutrition. Mean birth weight: 1010 g, mean gestational age 27.8 weeks; postnatal age not provided. | |
| Interventions | Intravenous cysteine supplementation 1 millimole/kg/day (either in the parenteral solution [n=7] or separately [n=6]) versus no supplementation (n=7). Duration not provided. | |
| Outcomes | Nitrogen retention; plasma free cysteine concentration. | |
| Notes | Abstract only. Caloric and nitrogen intake not provided. Patients in treatment arm (1) required twice as much base to maintain normal acid‐base status as those in treatment arm (2). Controls required no base. For the purpose of this systematic review, the two treatment arms were combined as described in the method section. Nitrogen retention and plasma free cysteine concentration were not affected by the type of cysteine supplementation. Amino acid solution: contains N‐acetyltyrosine. Setting: United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single‐site 3‐arm trial Method of allocation: not provided |
| Allocation concealment? | High risk | Blinding of allocation: no |
| Blinding? All outcomes | High risk | Blinding of intervention: no. Blinding of outcome measurement: no |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
Malloy 1984.
| Methods | Single‐site randomized trial. Blinding of allocation: can't tell. Blinding of intervention: can't tell. Complete follow‐up : yes. Blinding of outcome measurement: can't tell. | |
| Participants | Total number of patients entered into the study: 20. Entry criteria: preterm infants at least 2 days old, unable to take enteral feeds and unlikely to begin enteral feeding within 5‐7 days; or without enteral intake for 48 hours and would be unable to tolerate enteral feeds for at least 5‐7 days. Birthweight ranged between 900 g and 2500 g, and postnatal age between 3 and 53 days; gestational age is not provided. | |
| Interventions | Cysteine‐chloride (Abbott Laboratories) (72 mg/kg/day or 0.46 millimoles/kg/day) added to the parental solution for 6 days (n=10) versus no cysteine supplementation (n=10). | |
| Outcomes | Weight gain (no SD provided), nitrogen retention, plasma levels of total and free cysteine. | |
| Notes | Randomization stratified by nitrogen intake: those receiving low nitrogen intake (1.5 g/kg/day of amino acids) and those receiving high nitrogen intake (2.5 g/kg/day of amino acids). Amino acids: provided by Aminosyn (Abbott Laboratories), which contains neither cysteine, nor tyrosine in any form. Non protein calories ranged between 63 and 73 kcal/kg/day. Setting: United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single‐site randomized trial. Blinding of allocation: can't tell |
| Allocation concealment? | Unclear risk | Single‐site randomized trial. Blinding of allocation: can't tell |
| Blinding? All outcomes | Unclear risk | Blinding of intervention: can't tell Blinding of outcome measurement: can't tell |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up : yes |
Shew 1999.
| Methods | Single site randomized trial. Blinding of randomization: yes. Blinding of intervention: yes. Complete follow‐up: yes. Blinding of outcome measurement: yes. | |
| Participants | Total number of patients entered into the study: 19. Entry criteria: neonates receiving parenteral nutrition. Average birth weight in the treatment group and the control group, respectively, 1470 g (SD 750 g) and 1540 g (SD 510 g); average gestational age 30.8 weeks (SD 3.0) and 29.9 weeks (SD 4.4); average postnatal age not provided. | |
| Interventions | Cysteine hydrochloride supplementation (0.78 [SD 0.90] millimoles/kg/day) in parenteral nutrition for 5.9 (SD 1.5) days (n = 9) versus no supplementation for 6.8 (SD 1.6) days (n =10). | |
| Outcomes | Nitrogen balance during the final 48 hours of the study. | |
| Notes | Abstract only. Caloric intake was 92 (SD 12) kcal/kg/day in the treatment group and 99 (SD 19) kcal/kg/day in the control group, and amino acid intake was 2.9 (SD 0.3) and 2.7 (SD 0.3) g/kg/day, respectively. There was no difference in glutathione concentration between the two groups. Setting: United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single site randomized trial. Blinding of randomization: yes |
| Allocation concealment? | Low risk | Single site randomized trial. Blinding of randomization: yes |
| Blinding? All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
Storm 2003.
| Methods | Single site cross‐over randomized trial. Blinding of randomization: yes. Blinding of intervention: yes. Complete follow‐up: yes. Blinding of outcome measurement: yes. | |
| Participants | Total number of patients entered into the study: 18. Entry criteria: low birth weight premature infants, gestational age ranged 30‐37 weeks, postnatal age less than 4 weeks, requiring PN for at least two weeks. Exclusion criteria: inborn genetic error or significant organ failure. | |
| Interventions | After a 3‐day PN without cysteine, infants were randomly allocated to one of six successive schedules dosing schedules of cysteine hydrochloride for 3 days, ranging between 0 and 40 mg/g amino acids. For this systematic review we compared the levels of cyst(e)ine at the end of the schedule with the highest amount of cysteine intake (40 mg of cysteine hydrochloride per g of amino acid) (treatment, n = 18) versus the levels at the end of the lowest, i.e., 0 mg/g (control, n = 18). | |
| Outcomes | Plasma levels of taurine, cystine and methionine at the end of each successive schedule. | |
| Notes | Abstract only. Target PN dosage was 2.5 g/kg of amino acids with total caloric intake of 135 kcal/kg/day. Setting: United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single site cross‐over randomized trial Blinding of randomization: yes |
| Allocation concealment? | Low risk | Blinding of randomization: yes |
| Blinding? All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
Zlotkin 1981.
| Methods | Single site trial. Alternate allocation to treatment versus control. Blinding of allocation: no. Blinding of intervention: no. Complete follow‐up: yes. Blinding of outcome: no. | |
| Participants | Total number of patients entered into the study: 36. Entry criteria: Infants (age 2‐46 days) receiving total parenteral nutrition. Average birth weight in the treatment group and the control group, respectively, 2231 g (SD 1659 g) and 2141 g (SD 1515 g); average gestational age 34.4 weeks (SD 8.5) and 34.2 weeks (SD 9.3); average postnatal age 13.7 days (SD 23.3) and 15 days (SD 23.8). | |
| Interventions | Cysteine hydrochloride (Abbott Laboratories) supplementation (77 mg/kg/day of cysteine or 0.64 millimoles/kg/day) for 6 days (n = 18) versus no supplementation (n = 18). | |
| Outcomes | Weight gain, changes in length and head circumference, nitrogen balance, plasma cysteine levels. | |
| Notes | Both groups received an average of 87 kcal/kg/day and 3.2 g/kg/day of amino acids, provided by Aminosyn (Abbott Laboratories), which contains neither cysteine, nor tyrosine in any form. Setting: Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Single site trial Alternate allocation to treatment versus control |
| Allocation concealment? | High risk | Alternate allocation to treatment versus control Blinding of allocation: no |
| Blinding? All outcomes | High risk | Blinding of intervention: no Blinding of outcome: no |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Helms 1995 | The intervention was either methionine supplementation, or methionine and cysteine supplementation. |
| Laine 1991 | The study did not include two simultaneous groups but had a before/after design. |
| Pointdexter 2000 | The intervention was N‐acetyltyrosine and cysteine hydrochloride supplementation |
| Shew 2000 | None of the predetermined outcome variables for this systematic review were analyzed. |
| Van Goudoever 1994 | The intervention was not limited to cysteine supplementation; it included different amounts of cysteine and tyrosine, or N‐acetyl‐L‐tyrosine and N‐acetyl‐L‐cysteine. |
Characteristics of ongoing studies [ordered by study ID]
NCT00254176.
| Trial name or title | Cysteine Supplementation in Critically Ill Neonates |
| Methods | Treatment, Randomized, Double Blind (Subject, Caregiver, Investigator), Placebo Control, Single Group Assignment, Bio‐availability Study |
| Participants | Mechanically ventilated neonates of all gestational ages and birth weights, less than 1 month of postnatal age admitted to the NICU, SNAP (Score of Neonatal Acute Physiology) > 10, projected requirement for continued parenteral nutrition of at least 1 week duration |
| Interventions | Random allocation to receive a cysteine or an isonitrogenous cysteine‐free supplement to their TPN (total parenteral nutrition) regimen |
| Outcomes | Primary Outcome Measures:Total RBC glutathione Secondary Outcome Measures: Tumor necrosis factor (TNF), l Interleukin‐6 (IL‐6), Oxygen dependency, Ventilation dependency, Erythrocyte oxidized: reduced glutathione ratio, In vivo erythrocyte glutathione synthetic rate, Plasma malondialdehyde concentration |
| Starting date | September 2006 |
| Contact information | sshew@mednet.ucla.edu |
| Notes |
Cysteine supplementation in critically ill neonates
Sponsor: University of California, Los Angeles NCT 00254176
Contributions of authors
Lamia Soghier wrote the first draft of the protocol and searched the databases. Luc Brion searched the abstracts and wrote the first draft of the review.
Luc Brion (LB) updated the review in 2006. The August 2009 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Roger Soll, Diane Haughton) and reviewed and approved by LB.
Sources of support
Internal sources
Albert Einstein College of Medicine, Children's Hospital at Montefiore, Division of Neonatology, USA.
External sources
No sources of support supplied
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahola 2003 {published data only}
- Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R. Plasma 8‐isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatric Research 2004;56:88–93. [DOI] [PubMed] [Google Scholar]
- Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, Jonsbo F, Esberg G, Stovring S, Kjartansson S, Stiris T, Lossius K, Virkola K, Fellman V. N‐acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. Journal of Pediatrics 2003;143:713‐9. [DOI] [PubMed] [Google Scholar]
- Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N‐acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biology of the Neonate 2004;86:275‐9. [DOI] [PubMed] [Google Scholar]
Kashyap 1992 {published and unpublished data}
- Kashyap S, Abildskov K, Heird WC. Cyst(e)ine supplementation of very low birth weight IVLBW) infants receiving parenteral nutrition (TPN). Pediatric Research 1992;31:290A. [Google Scholar]
Malloy 1984 {published data only}
- Malloy MH, Rassin DK, Richardson CJ. Cyst(e)ine measurements during total parenteral nutrition. Am J Clin Nutr 1983;37:188‐91. [DOI] [PubMed] [Google Scholar]
- Malloy MH, Rassin DK, Richardson CJ. Total parenteral nutrition in sick preterm infants: effects of cysteine supplementation with nitrogen intakes of 240 and 400 mg/kg/day. Journal of Pediatric Gastroenterology and Nutrition 1984;3:239‐44. [PubMed] [Google Scholar]
Shew 1999 {published and unpublished data}
- Shew SB, Jahoor F, Jaksic T, Heird WC. Improved protein metabolism in neonates receiving parenteral cystein supplementation. Pediatric Research 1999; Vol. 45:290A.
Storm 2003 {published and unpublished data}
- Storm MC, Helms RA. Cysteine supplementation normalizes plasma taurine concentrations in low birth weight premature infants requiring parenteral nutrition support. Journal of Parenteral and Enteral Nutrition 2003; Vol. 27:S4‐S5.
Zlotkin 1981 {published data only}
- Zlotkin SH, Bryan MH, Anderson GH. Cysteine supplementation to cysteine‐free intravenous feeding regimens in newborn infants. American Journal of Clinical Nutrition 1981;34:914‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Helms 1995 {published data only}
- Helms RA, Chesney RW, Storm MC. Sulfur amino acid metabolism in infants on parenteral nutrition. Clinical Nutrition 1995;14:381‐7. [DOI] [PubMed] [Google Scholar]
Laine 1991 {published data only}
- Laine L, Shulman RJ, Pitre D, Lifschitz CH, Adams J. Cysteine usage increases the need for acetate in neonates who receive total parenteral nutrition. American Journal of Clinical Nutrition 1991;54:565‐7. [DOI] [PubMed] [Google Scholar]
Pointdexter 2000 {published and unpublished data}
- Pointdexter BB, Wright‐Coltart S, Denne SC. The effect of N‐acetyltyrosine and cysteine in parenteral nutrition on protein metabolism in extremely low birth weight infants. Pediatric Research 2000; Vol. 47:294A.
Shew 2000 {published data only}
- Shew SB, Jaksic T, Jahoor F, Heird WC. Effects of Cysteine (CYS) on Endogenous Synthesis of Taurine (TAU) and Glutathione (GSH) by Premature Neonates Receiving Parenteral Nutrition. Pediatric Research 2000;47:296A. [Google Scholar]
Van Goudoever 1994 {published data only}
- Goudoever JB, Sulkers EJ, Timmerman M, Huijmans JGM, Langer K, Carnielli VP, Sauer PJJ. Amino acid solutions for premature neonates during the first week of life: the role of N‐acetyl‐L‐cysteine and N‐acetyl‐L‐tyrosine. Journal of Parenteral and Enteral Nutrition 1994;18:404‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00254176 {unpublished data only}
- Cysteine Supplementation in Critically Ill Neonates. Ongoing study September 2006.
Additional references
AAP 2004
- American Academy of Pediatrics. Clinical Practice Guideline. Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2004;114:297‐316. [DOI] [PubMed] [Google Scholar]
Ahola 1999
- Ahola T, Fellman V, Laaksonen R, Laitila J, Lapatto R, Neuvonen PJ, Raivio KO. Pharmacokinetics of intravenous N‐acetylcysteine in pre‐term new‐born infants. European Journal of Clinical Pharmacology 1999;55:645‐50. [DOI] [PubMed] [Google Scholar]
Altman 1991
- Altman DG. Comparing groups ‐ continuous data. In: Altman DG editor(s). Practical statistics for medical research. London: Chapman & Hall, 1991:192. [Google Scholar]
Bjelton 1990
- Bjelton L, Fransson GB. Availability of cysteine and of L‐2‐oxo‐thiazolidine‐4‐carboxylic acid as a source of cysteine in intravenous nutrition. Journal of Parenteral and Enteral Nutrition 1990;14:177‐82. [DOI] [PubMed] [Google Scholar]
Brion 1990
- Brion LP. Use of a Lotus 1‐2‐3 spreadsheet to plot the confidence and the prediction intervals in linear regression analysis. Computers in Biology and Medicine 1990;20:129‐34. [DOI] [PubMed] [Google Scholar]
Brunton 2000
- Brunton JA, Balla RO, Pencharzb PB. Current total parenteral nutrition solutions for the neonate are inadequate. Current Opinion in Clinical Nutrition and Metabolic Care 2000;3:299‐304. [DOI] [PubMed] [Google Scholar]
Gaull 1972
- Gaull G, Sturman JA, Raiha NCR. Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatric Research 1972;6:538‐47. [DOI] [PubMed] [Google Scholar]
Gomez 1993
- Gomez MR, Rogers LK, Smith CV, Heird WC. The fate of parenteral D‐glucose‐Lcysteine (DGC) in infants. Pediatric Research 1993; Vol. 33:303A.
Heinonen 1974
- Heinonen K, Raiha NCR. Induction of cystathionase in human foetal liver. The Biochemical Journal 1974;144:607‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heird 1987
- Heird WC, Dell RB, Helms RA, Greene HL, Ament ME, Karna P, Storm MC. Amino acid mixture designed to maintain normal plasma amino acid patterns in infants and children requiring parenteral nutrition. Pediatrics 1987;80:401‐8. [PubMed] [Google Scholar]
Heird 1988
- Heird WC, Hay W, Helms RA, Storm MC, Kashyap S, Dell RB. Pediatric parenteral amino acid mixture in low birth weight infants. Pediatrics 1988;81:41‐50. [PubMed] [Google Scholar]
Heird 1993
- Heird WC, Gomez MR. Parenteral nutrition. In: Tsang RC, Lucas A, Uauy R, Zlotkin S editor(s). Nutritional needs of the preterm infant. Scientific basis and practical guidelines. Baltimore: Williams & Wilkins, 1993:225‐242. [Google Scholar]
Heird 1998
- Heird WC. Amino acids in pediatric and neonatal nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 1998;1:73‐8. [DOI] [PubMed] [Google Scholar]
Helms 1987
- Helms RA, Christensen ML, Mauer EC, Strorm MC. Comparison of a pediatric versus standard amino acid formulation in preterm neonates requiring parenteral nutrition. Journal of Pediatrics 1987;110:466‐70. [DOI] [PubMed] [Google Scholar]
Howard 1992
- Howard D, Thompson DF. Taurine: an essential amino acid to prevent cholestasis in neonates?. Annals of Pharmacotherapy 1992;11:1390‐2. [DOI] [PubMed] [Google Scholar]
ICROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematury revisited. Archives of Ophthalmology 2005;123:991‐9. [DOI] [PubMed] [Google Scholar]
Kanto 1994
- Kanto WP, Hunter JE, Stoll BJ. Recognition and medical management of necrotizing enterocolitis. Clinics in Perinatology 1994;21:335‐46. [PubMed] [Google Scholar]
Kleinman 2004
- Ronald E Kleinman. Committee on Nutrition. Pediatric Nutrition Handbook. Fifth. Elk Grove Village, IL: American Academy of Pediatrics, 2004. [Google Scholar]
Miller 1996
- Miller RG, Keshen TH, Jahoor F, Shew SB, Jaksic T. Compartmentation of endogenously synthesized amino acids in neonates. Journal of Surgical Research 1996;63:199‐203. [DOI] [PubMed] [Google Scholar]
Moss 1999
- Moss RL, Haynes AL, Pastuszyn A, Glew RH. Methionine infusion reproduces liver injury of parenteral infusion cholestasis. Pediatric Research 1999;45:664‐8. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92:529‐34. [DOI] [PubMed] [Google Scholar]
Parikh 2005
- Parikh MJ, Dumas G, Silvestri A, Bistrian BR, Driscoll DF. Physical compatibility of neonatal total parenteral nutrient admixtures containing organic calcium and inorganic phosphate salts. American Journal of Health‐System Pharmacy 2005;62:1177‐83. [DOI] [PubMed] [Google Scholar]
Pascal 1972
- Pascal TA, Gillam BM, Gaull GE. Cystathionase: immunochemical evidence for absence from human fetal liver. Pediatric Research 1972;6:773‐8. [DOI] [PubMed] [Google Scholar]
Riedijk 2006
- Riedijk MA, Voortman G, Beek RHT, Wattimena DJ, Goudoever JB. Is Cyst(e)ine an essential amino acid in borderline premature infants?. Pediatric Academic Societies Annual Meeting 2006, 4680.6.
Riedijk 2007
- Riedijk MA, Beek RH, Voortman G, Bie HM, Dassel AC, Goudoever JB. Cysteine: a conditionally essential amino acid in low‐birth‐weight preterm infants?. Am J Clin Nutr 2007;86:1120‐5. [DOI] [PubMed] [Google Scholar]
Riedijk 2008
- Riedijk MA, Voortman G, Beek RH, Baartmans MG, Wafelman LS, Goudoever JB. Cyst(e)ine requirements in enterally fed very low birth weight preterm infants. Pediatrics 2008;121:e561‐7. [DOI] [PubMed] [Google Scholar]
Roberts 2001
- Roberts SA, Ball RO, Moore AM, Filler RM, Pencharz PB. The effect of graded intake of glycyl‐L‐tyrosine on phenylalanine and tyrosine metabolism in parenterally fed neonates with an estimation of tyrosine requirement. Pediatr Res 2001;49:111‐9. [DOI] [PubMed] [Google Scholar]
Rose 1955
- Rose WC, Wixom RL. The amino acid requirements of man. XIII. The sparing effect of cystine on the methionine requirement. The Journal of Biological Chemistry 1955;216:753‐73. [PubMed] [Google Scholar]
Shew 2000a
- Shew SB, Keshen TH, Glass NL, Jahoor F, Jaksic T. Ligation of a patent ductus arteriosus under fentanyl anesthesia improves protein metabolism in premature neonates. Journal of Pediatric Surgery 2000;35:1277‐81. [DOI] [PubMed] [Google Scholar]
Shew 2005
- Shew SB, Keshen TH, Jahoor F, Jaksic T. Assessment of cysteine synthesis in very low‐birth weight neonates using a [13C6]glucose tracer. Journal of Pediatric Surgery 2005;40:52‐6. [DOI] [PubMed] [Google Scholar]
Snyderman 1971
- Synderman SE. The protein and amino acid requirements of the premature infant. In: Visser HKA, Toreistra JA editor(s). Nutricia Symposium: metabolic processes in the foetus and newborn infant. Leiden, Netherlands: Stenfer Kroese, 1971. [Google Scholar]
Sturman 1970
- Sturman JA, Gaull G, Raiha NCR. Absence of cystathionase in human fetal liver: Is cystine essential?. Science 1970;169:74‐6. [DOI] [PubMed] [Google Scholar]
Thibeault 2000
- Thibeault DW. The precarious antioxidant defenses of the preterm infant. American Journal of Perinatology 2000;17:167‐81. [DOI] [PubMed] [Google Scholar]
Uauy 1993
- Uauy R, Greene HL, Heird WC. Conditionally essential nutrients: cysteine, taurine, tyrosine, arginine, glutamine, choline, inositol, and nucleotides. In: Tsang RC, Lucas A, Uauy R, Zlotkin S editor(s). Nutritional needs of the preterm infant. Scientific basis and practical guidelines. Baltimore: Williams & Wilkins, 1993:267‐280. [Google Scholar]
Viña 1995
- Viña J, Vento M, García‐Sala F, Puertes IR, Gascó E, Sastre J, Asensi M, Pallardó FV. L‐cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. American Journal of Clinical Nutrition 1995;61:1067‐9. [DOI] [PubMed] [Google Scholar]
Zahr 1984
- Zahr JH. Two‐sample hypotheses; Multiple comparisons. In: Zar JH editor(s). Biostatistical analysis. 2nd Edition. Englewood Cliffs, NJ: Prentice‐Hall, Inc, 1984:122‐149; 162‐184. [Google Scholar]
Zlotkin 1982
- Zlotkin SH, Anderson GH. The development of cystathionase activity during the first year of life. Pediatric Research 1982;16:65‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Soghier 2006
- Soghier LM, Brion LP. Cysteine supplementation in parenterally fed neonates: Systematic review. Pediatric Academic Societies Annual Meeting 2006; 5572.442. [DOI] [PMC free article] [PubMed]
Soghier 2006a
- Soghier LM, Brion LP. Cysteine, cystine or N‐acetylcysteine supplementation in parenterally fed neonatews. Cochrane Database of Systematic Reviews 4, Issue 2006. [DOI: 10.1002/14651858.CD004869.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


