OBJECTIVES:
Little is known about the epidemiology of ventilator-acquired pneumonia among coronavirus disease 2019 patients such as incidence or etiological agents. Some studies suggest a higher risk of ventilator-associated pneumonia in this specific population.
DESIGN:
Cohort exposed/nonexposed study among the REA-REZO surveillance network.
SETTING:
Multicentric; ICUs in France.
PATIENTS:
The coronavirus disease 2019 patients at admission were matched on the age, sex, center of inclusion, presence of antimicrobial therapy at admission, patient provenance, time from ICU admission to mechanical ventilation, and Simplified Acute Physiology Score II at admission to the patients included between 2016 and 2019 within the same surveillance network (1:1).
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The overall incidence of ventilator-associated pneumonia, the cumulative incidence, and hazard rate of the first and the second ventilator-associated pneumonia were estimated. In addition, the ventilator-associated pneumonia microbiological ecology and specific resistant pattern in coronavirus disease 2019 exposed and nonexposed patients were compared. Medication data were not collected. A total of 1,879 patients were included in each group. The overall incidence of ventilator-associated pneumonia was higher among coronavirus disease 2019 exposed patients (25.5; 95% CI [23.7–27.45] vs 15.4; 95% CI [13.7–17.3] ventilator-associated pneumonia per 1,000 ventilation days). The cumulative incidence was higher for the first and the second ventilator-associated pneumonia among the coronavirus disease 2019 exposed patients (respective Gray test p < 0.0001 and 0.0167). The microbiological ecology and resistance were comparable between groups with a predominance of Enterobacterales and nonfermenting Gram-negative bacteria. The documented resistance pattern was similar between groups, except for a lower rate of methicillin-resistant Staphylococcus aureus in the coronavirus disease 2019 exposed patient (6% vs 23%; p = 0.013).
CONCLUSIONS:
There was a higher incidence of ventilator-associated pneumonia occurring among coronavirus disease 2019 patient compared with the general ICU population, with a similar microbiological ecology and resistance pattern.
Keywords: coronavirus disease 2019, incidence, microbial ecology, ventilator-associated pneumonia
With the recent outbreak of the coronavirus disease 2019 (COVID-19) pandemic, the world has faced a massive flow of patients with viral pneumonia. Among hospitalized patients with COVID-19 in France, a proportion of 17% of them will require admission to an ICU and more than half of these will require mechanical ventilation (1–4). For the patients admitted to ICU, the 28-day mortality is around 40%, and the duration of mechanical ventilation is frequently prolonged (5).
During mechanical ventilation, ventilator-associated pneumonia (VAP) is common and is associated with increased duration of mechanical ventilation and length of ICU stay (6), but its association with mortality remains controversial (7, 8); in addition, there is an increased risk of VAP among patients with viral pneumonia (9–11). Several factors influence the occurrence of VAP during acute respiratory distress syndrome (ARDS), such as the dysregulation of lung immune defenses (12), or gastric and oropharyngeal colonization (13). In addition, certain treatments for COVID-19 infection are also known to increase the risk of VAP: for example, corticotherapy is currently one of the main treatments recommended during COVID-19 ARDS, leading to a decrease in mortality (14) and an increase in ventilation-free days (15).
Little is known about the epidemiology of VAP in COVID-19 patients. An European multicenter study including 568 cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection reported that the VAP incidence and ventilator-acquired tracheobronchitis were higher compared with the general population and influenza patients undergoing mechanical ventilation and reported that the majority of bacteria responsible for VAP were Gram-negative (Pseudomonas aeruginosa, Enterobacter species, and Klebsiella species) (16); similar results were found in the study reported by Maes et al (17) or Blonz et al (18). These concordant studies indicate that the incidence of VAP among COVID-19 patients is higher than that would be expected from other viral pneumonia. Furthermore, the second episode of VAP was never studied in detail, probably because of the lack of information (small studies), and the information currently available on the pathogens responsible for VAP does not allow us to clearly identify whether there is a difference between the COVID-19 and classically described VAP pathogens, both in terms of species or resistance, that could be of interest to physicians for adaptation of antibiotherapy.
We therefore conducted a study using the REA-REZO surveillance network, which is dedicated to the surveillance of ICU-acquired infections related to invasive devices. We matched COVID-19 patients with non–COVID-19 patients in order to estimate the incidence of VAP, study specifically the first and second episodes of VAP, and determine whether the local ecology among COVID-19 patients was similar to that of the general ICU population.
MATERIAL AND METHODS
Study Setting
We conducted a study nested in a cohort resulting from a prospective continuous multicenter surveillance of ICU-acquired infections and their risk factors since 2004. This cohort collects patient-level data from more than 100F adult ICUs participating in the REA-REZO surveillance network on a voluntary basis. For this study, in addition to the usual data collection, the COVID-19 status was collected.
The detailed protocol for data collection and monitoring are available at: http://rearezo.chu-lyon.fr/. All patients received information about the use of their personal data for research purposes and were given the opportunity to refuse it (during or after their intensive care stay, and in case the patient was unable refuse participation, family or close relatives could refuse the use of data on behalf of the patient). According to French law, written informed consent was not required. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The database was approved by the “Commission nationale de l’informatique et des libertés” (Number 919149) and by the Institutional Review Board (IRB) (CPP SUD EST—IRB 00009118).
Participants and Variables
The cohort included patients admitted for at least 2 days to an adult ICU. Patients with a diagnosis of COVID-19 (either strongly suspected on the basis of clinical condition and suggestive CT-scan or confirmed by a specific polymerase chain reaction [PCR]) during ICU stay until December 31, 2020, were selected (COVID-19 exposed patients). Patients from units participating in the surveillance network included between January 01, 2016, and December 31, 2019, before the French COVID-19 pandemic, were selected (COVID-19 nonexposed patients). It should be noted that the COVID-19 pandemic in France started at the end of January 2020. Patients undergoing extracorporeal membrane oxygenation (ECMO), with immunodepression according to Acute Physiology and Chronic Health Evaluation II definition (19), with a traumatic or surgical admission category were excluded from both groups, as were those who did not receive mechanical ventilation.
The following data were extracted: demographic characteristics: age, sex, presence of an antimicrobial treatment (excluding prophylaxis) 2 days before or after admission date, Simplified Acute Physiology Score (SAPS) II, type of admission (medical or surgical), patient provenance (from direct admission [community, nursing home] or secondary to hospital admission [acute care, rehabilitation, ICU…]), interval between admission and intubation, total duration of intubation, presence of immunosuppression, length of ICU stay, diagnosis of VAP episode, and ICU mortality. The medication data (including corticosteroid data) were not collected.
Information on VAP included the date of pulmonary infection, the diagnostic method of pneumonia (see below), up to two identified microorganisms per infection episode, and antimicrobial resistance status by key resistance patterns for selected bacteria. Susceptibility testing in all the units was conducted according to the French recommendations (Comité de l’antibiogramme—Société Francaise de Microbiologie) (20) compatible with the European Committee on Antimicrobial Susceptibility Testing recommendations.
Definition of ICU-Acquired Pneumonia
Pneumonia was defined according to the European Centers for Disease Control and Prevention, which includes clinical, radiological, and bacteriological criteria (21). Only the VAP with positive microbiological criteria was included. The bacteriological diagnosis was based on positive quantitative culture from minimally contaminated lower respiratory tract (LRT) specimen such as distal protected aspirate, positive quantitative culture from possibly contaminated LRT specimen such as endotracheal aspiration, or alternative microbiology methods such as positive blood culture not related to another source of infection. A pneumonia is considered ICU acquired if it occurs after more than 48 hours from admission, and VAP is defined by a pneumonia occurring after 48 hours of intubation.
The time from mechanical ventilation to the first VAP was the difference between the date of intubation and the date of the first documented VAP. For the hazard of the second VAP, the beginning time was the date of onset of the first VAP.
To consider VAP as a new episode, the combination of new signs and symptoms and radiographic evidence or other diagnostic testing was required, with a minimum of 2 days of clinical resolution after the first VAP. If the germ species was the same as the one identified in the first episode, a delay of 14 days after the time of resolution of the first VAP was required to consider VAP as a new episode.
COVID-19 Nonexposed Group
The COVID-19 nonexposed group was constructed by exact matching on inclusion center, sex, presence of antimicrobial therapy at admission, patient provenance (community/nursing home vs hospital), time to mechanical ventilation, and by nearest neighbor for SAPS II and age. A 1:1 ratio between COVID-19 exposed and nonexposed patient was applied (22).
Outcome
The primary outcome was to estimate the difference in cumulative incidence of the first and the second VAP between the COVID-19 and nonexposed patients.
The secondary outcomes were the hazard rate of VAP, in order to estimate the period with the highest risk of VAP for the patient, and to describe the bacteriological epidemiology of the COVID-19 VAP considering types of bacteria and resistance patterns compared with the COVID-19 nonexposed patients.
Statistical Methods
Descriptive statistics are expressed by the median and interquartile range (interquartile range) for quantitative variables and by the number of patients and proportion (%) for qualitative variables. For comparison between COVID-19 exposed and nonexposed patients, the McNemar, McNemar-Bowker, or Wilcoxon signed rank tests for the comparison of the matched group, and the chi-square or Fisher test for the comparison of the microbial ecology and resistance patterns. The adequate balancing of matching variables was evaluated using the standardized mean difference, with a threshold at 0.10.
The overall incidence rate was calculated using the number of VAP (all VAP episodes during the ICU stay) and the sum of ventilation exposure days among all patients. In order to estimate the cumulative incidence of the first VAP episode among groups, a model considering extubation and death as competitive risks was fitted using the Aalen-Johansen estimator. Then, specific 7-, 14-, and 28-day cumulative incidence was estimated for each group, and we compared the curves using the Gray test. Using Cox proportional hazards model, subdistribution hazard ratio (sHR) for COVID-19 exposed patients was estimated, both crude and adjusted on the age, sex, SAPS II, presence of antimicrobial therapy at admission, patient provenance, time to mechanical ventilation. We also estimated the hazard rate as a function of time with multidimensional penalized splines and estimated the day of the highest risk of VAP and its corresponding 95% CI using 1,000 bootstrap iteration.
Statistical significance was arbitrarily set at p value of less than 0.05. Analyses were performed using R software v3.4.3 (23) using the Survival, Matchit and survPen packages (24).
RESULTS
Population
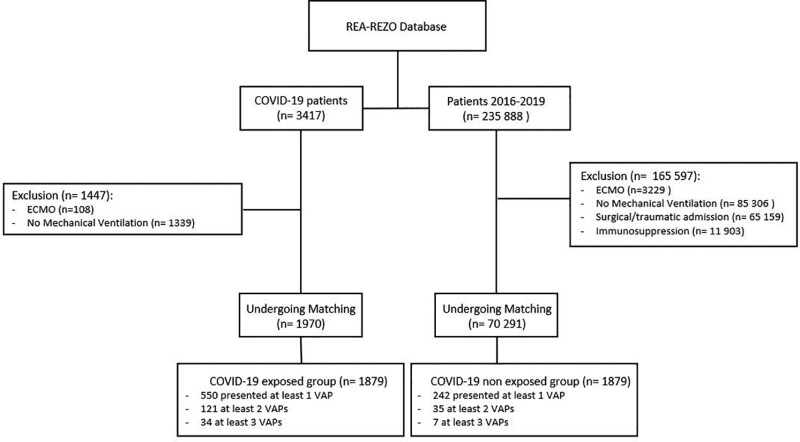
A total of 3,417 ICU patients with COVID-19 diagnosis in 2020 were identified in 94 centers (Supplementary Material App A, http://links.lww.com/CCM/G813). Among these, 108 patients who underwent ECMO and 1,339 who did not receive mechanical ventilation were excluded; 1,970 underwent matching. Between 2016 and 2019, there were 235,888 patients included in the cohort. Among these, 165,597 did not meet inclusion criteria; 70,291 patients underwent matching. In total, 1,879 COVID-19 patients were matched to 1,879 nonexposed COVID patients (Fig. 1). The COVID-19 diagnosis based on PCR for 1,605 of 1,879 COVID-19 patients (85%) or on clinical signs only for 274 of 1,879 (15%).
Figure 1.
Study flow chart. COVID-19 = coronavirus disease 2019, ECMO = extracorporeal membrane oxygenation, VAP = ventilator-associated pneumonia.
The majority of patients were men (72%), had a median age of 68 years, and the median SAPS II was 43. They were most frequently placed under mechanical ventilation during the first 24 hours after admission (65%). Compared with the COVID-19 nonexposed patients, COVID-19 exposed patients had a longer duration of exposure to mechanical ventilation (11 d [5–20 d] vs 6 d [2–12 d]) and longer length of ICU stay (15 d [8–25 d] vs 9 d [5–18 d]) (Table 1), and the crude ICU fatality rate was higher among COVID-19 exposed patients (Table 1); a greater proportion of the COVID-19 exposed patients presented a VAP episode (29% [n = 550] vs 13% [n = 242]; p < 0.001).
Table 1.
Patient Characteristics
| Variable | COVID-19 Exposed Patients (N = 1,879) | COVID-19 Nonexposed Patients (N = 1,879) | p/Standardized Mean Difference |
|---|---|---|---|
| Matching criteria | |||
| Age, yr, median (interquartile range) | 68 (59–74) | 68 (57–77) | 0.006 |
| Sex, male, n (%) | 1,356 (72) | 1,356 (72) | |
| Simplified Acute Physiology Score II, median (interquartile range) | 42 (34–54) | 44 (35–56) | 0.098 |
| Admission from community/nursing home, n (%) | 853 (45) | 853 (45) | |
| Antibiotherapy at admission, n (%) | 1,394 (74) | 1,394 (74) | |
| Time from admission to mechanical ventilation, hr, n (%) | |||
| < 24 | 1,216 (65) | 1,216 (65) | |
| 24–72 | 439 (23) | 439 (23) | |
| > 72 | 224 (12) | 224 (12) | |
| Patient outcomes | |||
| Total duration of mechanical ventilation, d, median (interquartile range) | 11 (5–20) | 6 (2–12) | < 0.001 |
| Length of ICU stay, d, median (interquartile range) | 15 (8–25) | 9 (5–18) | < 0.001 |
| Number of VAP episodes, n (%) | < 0.001 | ||
| None | 1,329 (71) | 1,637 (87) | |
| 1 | 429 (23) | 207 (11) | |
| 2 | 87 (4) | 28 (2) | |
| > 2 | 34 (2) | 7 (0) | |
| Time between admission and first VAP, d, median (interquartile range) | 8 (5–12) | 9 (6–13) | 0.032 |
| Time between first and second VAP, d, median (interquartile range) | 11 (8–15) | 14 (7–20) | 0.892 |
| ICU case fatality, n (%) | 575 (31) | 483 (26) | < 0.001 |
COVID-19 = coronavirus disease 2019, VAP = ventilator-associated pneumonia.
p values for the comparison between groups, except for the matching variables. Standardized mean difference is in italic.
Incidence of VAP
The overall incidence rate of VAP episodes was 25.5 (23.7–27.4) per 1,000 ventilation days in COVID-19 (718 VAP episodes for 28,154 days of mechanical ventilation), and 15.4 (13.7–17.3) VAP episodes per 1,000 ventilation days in the COVID-19 nonexposed patients (289 VAP for 18,765 d of mechanical ventilation).
First VAP Episode.
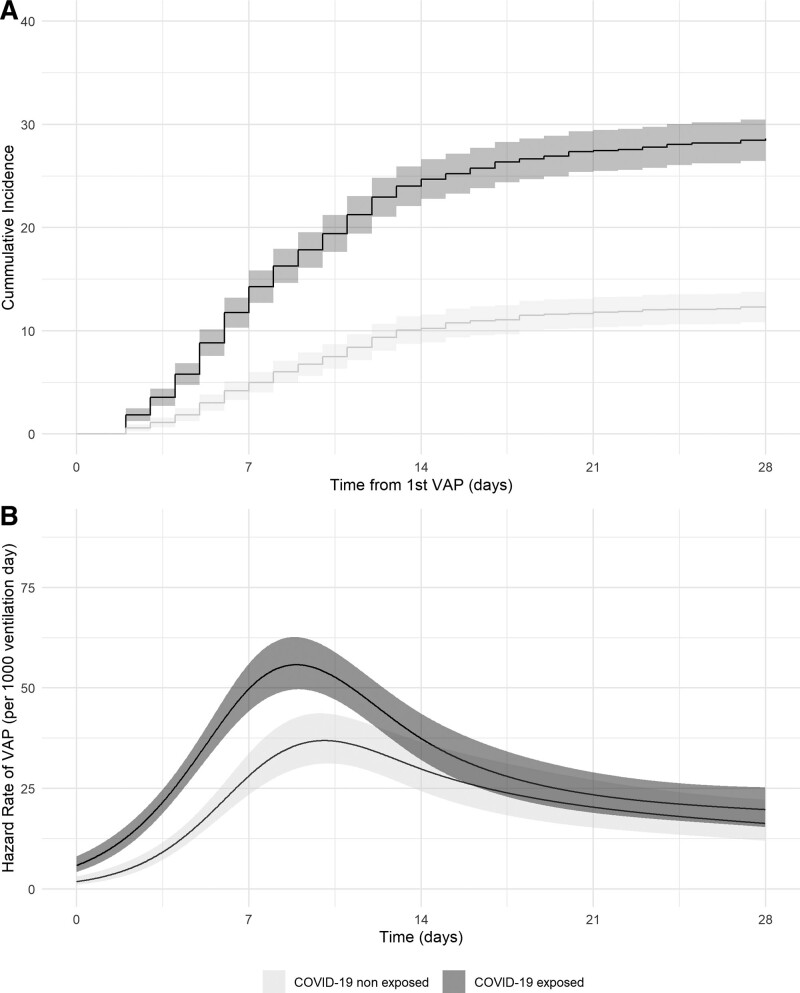
Compared with the COVID-19 nonexposed patients, the cumulative incidence for the first episode of VAP was higher in COVID-19 exposed patients at 7 days (14.3% [12.7–15.8%] vs 5.0% [4.0–6.0%]), at 14 days (24.7% [22.8–26.6%] vs 10.2% [8.9–11.6%]), and at 28 days (28.6% [26.6–30.6%] vs 12.3% [10.9–13.8%]) (Fig. 2) (p < 0.001).
Figure 2.
Cumulative incidence and time-dependent hazard rate of the first ventilator-associated pneumonia (VAP) episode. A, Cumulative incidence curve for the first VAP episode among coronavirus disease 2019 (COVID-19) exposed and nonexposed patients and their 95% CIs. B, Time-dependent hazard rate for the first VAP episode among COVID-19 exposed and nonexposed patients associated and their 95% CIs.
The highest hazard rate for the COVID-19 exposed patients was 56 (50–63) VAP per 1,000 ventilation days and 37 (31–44) for the COVID-19 nonexposed patients (Fig. 2B). The peak occurred at 8.9 days (8.3–9.4 d) for the COVID-19 exposed patients and at 10.0 days [8.8–11.0 d) for COVID-19 nonexposed patients.
The COVID-19 exposed patients had a higher risk of a first VAP episode than the COVID-19 nonexposed patients (sHR 1.70 [1.46–1.97]; p < 0.001 and adjusted sHR 1.68 [1.45–1.96]; p < 0.001).
Second VAP Episode.
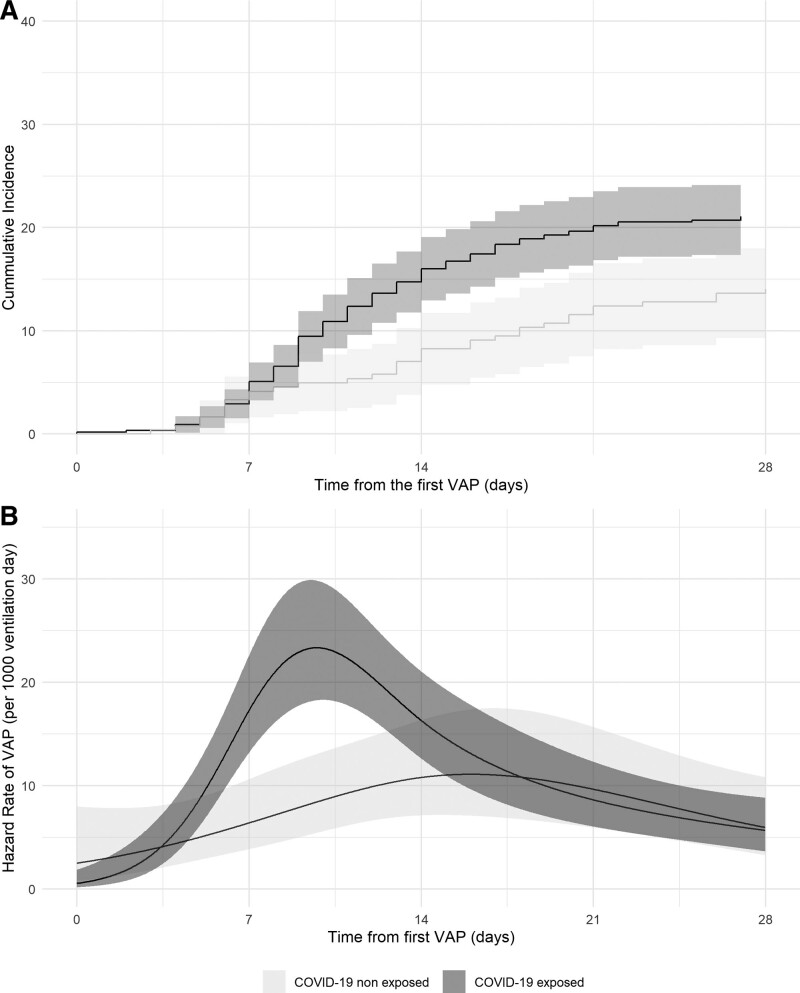
Concerning the second VAP episode, the cumulative incidence COVID-19 exposed patients was closer to that of the COVID-19 nonexposed patients than in the first episode (5.1% [3.3–6.9%] vs 4.1% [1.6–6.6%] at 7 d, 16.0% [12.9–19.1%] vs 8.3% [4.8–11.7%] at 14 d, and 21.1% [17.8–24.5%] vs 14.1% [9.7–18.4%] at 28 d, respectively for the COVID-19 and the COVID-19 nonexposed patients; p = 0.011) (Fig. 4A). This difference is related to a peak hazard rate of 23.3 (18.2–29.8) VAP per 1,000 ventilation days at 9.7 days (8.4–10.4 d) after the first VAP (Fig. 4B). The COVID-19 exposed patients had a higher risk of a VAP episode than the COVID-19 nonexposed patients (sHR 1.58 [1.09–2.31]; p = 0.016 and adjusted sHR 1.50 [1.03–2.19]; p = 0.036).
Microbiology.
Distribution of the bacterial species was homogeneous between the COVID-19 and COVID-19 nonexposed patients (p = 0.780). Among all VAP episodes, 319 of 1,007 (31%) were plurimicrobial: 223 of 718 (31%) in COVID-19 exposed patients and 96 of 289 (33%) in the COVID-19 nonexposed patients (p = 0.760).
Distribution of bacterial species was homogeneous between groups for the first episode (p = 0.915) and the second and more episode (p = 0.483) (Supplementary Tables 2 and 3, http://links.lww.com/CCM/G814). The majority of identified pathogens were Gram-negative bacteria (n = 986; 77%), with a large proportion of Enterobacterales (n = 602; 61%) followed by nonfermenting Gram-negative bacilli (n = 348; 35%). Gram-positive pathogens were less frequently isolated; Staphylococcus aureus was the most frequent. The frequency of resistance was homogeneous between COVID-19 exposed and nonexposed patients, with the exception of a lower rate of methicillin-resistant S. aureus in the COVID-19 exposed patients (n = 5; 6%) than in the COVID-19 nonexposed patients (n = 9; 23%; p = 0.013) (Table 2). The distribution of pathogen species between the first and the second VAP was comparable between groups (Cochran-Mantel-Haenszel chi-square test p = 0.668) (Supplementary Tables 2, 3, and 4, http://links.lww.com/CCM/G814).
Table 2.
Bacterial Ecology
| Variable | COVID-19 Exposed Patients (N = 925) | COVID-19 Nonexposed Patients (N = 359) | p |
|---|---|---|---|
| Gram-negative bacteria, n (%) | |||
| Enterobacterales | 444 (48) | 158 (44) | |
| Amoxicillin-clavulanic acid | 111 (70) | 299 (68) | 0.609 |
| Third-generation cephalosporins | 132 (30) | 52 (33) | 0.546 |
| Carbapenems | 5 (1) | 4 (2) | 0.256 |
| Escherichia coli | 85 (19) | 24 (15) | |
| Citrobacter species | 39 (9) | 5 (3) | |
| Enterobacter species | 125 (28) | 41 (26) | |
| Hafnia species | 26 (6) | 6 (4) | |
| Klebsiella species | 92 (21) | 53 (34) | |
| Morganella species | 13 (3) | 3 (2) | |
| Proteus species | 30 (7) | 8 (5) | |
| Serratia | 33 (7) | 18 (11) | |
| Other | 1 (0) | 0 (0) | |
| Nonfermenting | 244 (26) | 104 (29) | |
| Pseudomonas aeruginosa | 195 (80) | 75 (72) | |
| Piperacillin-tazobactam | 55 (28) | 23 (31) | 0.763 |
| Ceftazidime | 31 (16) | 18 (24) | 0.147 |
| Carbapenems | 42 (22) | 19 (25) | 0.526 |
| Stenotrophomonas maltophilia | 31 (13) | 19 (18) | |
| Acinetobacter baumannii | 7 (3) | 4 (4) | |
| Other | 11 (4) | 6 (6) | |
| Other | 25 (3) | 11 (3) | |
| Haemophilus species | 19 (76) | 10 (91) | |
| Other | 6 (24) | 1 (9) | |
| Gram-positive bacteria, n (%) | 168 (18) | 68 (19) | |
| Enterococcus speciesa | 47 (28) | 12 (18) | |
| Staphylococcus aureus | 87 (52) | 40 (59) | |
| Methicillin | 5 (6) | 9 (23) | 0.013 |
| Staphylococcus species | 10 (6) | 9 (13) | |
| Streptococcus species | 23 (13) | 7 (10) | |
| Other | 1 (1) | 0 (0) | |
| Other, n (%) | 44 (5) | 18 (5) | |
| Other bacteria | 11 (25) | 10 (55) | |
| Candida speciesa | 15 (34) | 4 (22) | |
| Aspergillus fumigatus | 11 (25) | 1 (6) | |
| Virus (cytomegalovirus, herpes simplex virus)a | 7 (16) | 3 (17) |
COVID-19 = coronavirus disease 2019.
aNonpathogenic pathogen.
Resistance to amoxicillin-clavulanic acid, third-generation cephalosporins, carbapenems, piperacillin-tazobactam, ceftazidime, and methicillin.
DISCUSSION
The results of the present study indicate that, although the incidence of VAP was higher among COVID-19 exposed patients, the occurrence of the peak of risk and the local ecology were comparable to that found in non–COVID-19 exposed patients, both in the first and second episode of VAP.
The incidence of VAP among COVID-19 nonexposed patients in the present study is consistent with that reported in the literature (from 12 to 20 VAP episodes per 1,000 ventilation days [25]). Similarly, the rate herein among COVID-19 patients is also close to that reported elsewhere; Maes et al (17) reported an incidence of 28 VAP episodes per 1,000 ventilation days, and an increased risk of VAP was also found in the study reported by Rouzé et al (16) (compared with a group without viral infection: sHR at 1.73 [1.38–2.20]). Several reasons might explain this high incidence of VAP among COVID-19 patients. Although the association between corticotherapy and risk of VAP remains debated, many of the other drugs used experimentally also have immunosuppressive properties, such as tocilizumab, anti-interleukin 1 inhibitors (anakinra), or inhibitors of Janus kinases such as baricitinib (26, 27). Furthermore, immunomodulation is also documented during COVID-19 infection: an initial immunostimulation (cytokine storm) followed by a deep immunosuppression occurring around the 11 and 14 days after first symptoms, with a “V curve” reduction of HLA-DR expression, lymphopenia, and monocytopenia (28).
There is also a frequent association between COVID-19 and ARDS, which increases the risk of VAP (12). COVID-19 is also associated with poor lung perfusion and pulmonary emboli, increasing the risk of pulmonary infarction (29). Another potential determinant for the increase of VAP incidence is the decrease in the level experience/organization related to non-ICU healthcare workers solicited during the pandemic, who are not familiar with infection prevention and control procedures in ICU associated with an increase in workload (30).
The time-dependent hazard rate of VAP allowed us to estimate the moment of the highest risk of VAP, which was similar in both COVID-19 exposed and nonexposed patients and around the 10th day. For the first VAP episode, the increased risk of VAP was persistent during time. However, we observed a lower impact of COVID-19 on the occurrence of the second episode of VAP; this difference was explained mainly by a peak occurring at the ninth day after the first VAP.
We observed a similar distribution in the infecting organisms among COVID-19 exposed and nonexposed patients, with a predominance of P. aeruginosa and Enterobacterales. This is consistent with several studies that did not find any difference between COVID-19 and non–COVID-19 patients (17). Furthermore, we did not find any difference in the distribution of microorganisms between the first and the second VAP episode. Regarding the antimicrobial resistance patterns studied, the only difference was the proportion of methicillin-resistant S. aureus. This might be because of the decline in the proportion of MRSA in Europe that has been observed recently over several years (31), or because of the increased use of the personal protective equipment during the COVID-19 pandemic. The lack of variation in bacterial epidemiology and resistance pattern should encourage physicians to maintain the usual antibiotic management for these patients.
The present study used an extensive (20-yr-old) surveillance network, familiar with the monitoring of the ICU-acquired infection; it confirms the conclusions of other prospective cohort studies and further explores this risk over time, with a high quality of data collected by intensivists and infection control practitioners and with standardized definition of ICU-acquired infection according to that proposed by the European Centers for Disease Control and Prevention (21). By implementing an early analysis of the collected data, it might have been possible to highlight this association between COVID-19 and VAP and to propose prophylactic interventions in the COVID-19 population, but in the context of the initial stages of the COVID-19 pandemic, data collection was not the priority. We included patients whose COVID-19 diagnosis was solely based on clinical and radiological signs because at the beginning of the pandemic, the SARS-CoV-2 specific PCR was not easily accessible and because of possible false-negative results due to PCR testing (32). However, due to the nature of the surveillance network, we did not document some variables, such as antibiotic treatment during ICU stay, specific COVID-19 treatments (including corticosteroid), and the follow-up was limited to ICU stay. Indeed, the corticosteroid widely used in ICU for the treatment of COVID-19 could be related to the incidence of VAP. As the COVID-19 patients often presented an ARDS, VAP may have been overdiagnosed, but only VAP with microbiological confirmation was included in our study. Furthermore, the patients undergoing ECMO were excluded because of the specific characteristics of this population, with a high deleterious inflammatory response, and immune modulation.
CONCLUSIONS
In conclusion, using prospectively collected data in an ongoing multicenter ICU surveillance network, we found that the risk of VAP was higher for COVID-19 patients, in particular for the first VAP episode. Onset is later than in non–COVID-19 ICU patients. However, there was no specific bacterial or resistance pattern, which suggests that the management of antibiotic treatment should therefore follow the usual recommendations.
Figure 3.
Cumulative incidence and time-dependent hazard rate of the second ventilator-associated pneumonia (VAP) episode. A, Cumulative incidence curve for the second VAP episode among coronavirus disease 2019 (COVID-19) exposed and nonexposed patients and their 95% CIs. B, Time-dependent hazard rate for the second VAP episode for COVID-19 exposed and nonexposed patients and their 95% CIs. T0 = time from the first VAP episode.
ACKNOWLEDGMENTS
We thank all the physicians and nurses of the REA-REZO network Helene Boyer and Philip Robinson (Hospices Civils de Lyon) for help in article preparation.
Supplementary Material
Footnotes
*See also p. 522.
The REA-REZO Study Group members can be found in Supplementary Table 1 (http://links.lww.com/CCM/G814).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Vacheron helped in writing the first draft of the article, reviewing the article, and performing the statistical analysis. Drs. Lepape, Savey, Machut, and Friggeri helped in data collection, conception, interpretation of the data, and revising the article substantially. Dr. Maucort-Boulch helped in statistical analysis, conception, interpretation of the data, and revising the article substantially. Other authors helped in the interpretation of the data, data collection, and revising the article substantially.
Dr. Vanhems’ institution received funding from Anios; he received funding from Astellas and Sanofi. Drs. Vanhem and Friggeri received funding from Pfizer. Dr. Friggeri received funding from MSD. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators: Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaeuffer C, Hyaric CL, Fabacher T, et al. : Clinical characteristics and risk factors associated with severe COVID-19: Prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Eurosurveillance. 2020; 25:2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhems P, Gustin MP, Elias C, et al. ; COVID-Outcomes-HCL Consortium: Factors associated with admission to intensive care units in COVID-19 patients in Lyon-France. PLoS One. 2021; 16:e0243709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators: Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021; 47:60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamberini L, Tonetti T, Spadaro S, et al. : Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: Multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Loeches I, Povoa P, Rodríguez A, et al. ; TAVeM study: Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): A multicentre, prospective, observational study. Lancet Respir Med. 2015; 3:859–868 [DOI] [PubMed] [Google Scholar]
- 7.Melsen WG, Rovers MM, Groenwold RHH, et al. : Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013; 13:665–71 [DOI] [PubMed] [Google Scholar]
- 8.Bekaert M, Timsit JF, Vansteelandt S, et al. ; Outcomerea Study Group: Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am J Respir Crit Care Med. 2011; 184:1133–1139 [DOI] [PubMed] [Google Scholar]
- 9.Curcio D, Ferreira Cabrera L, Duarte A, et al. : Ventilator-associated pneumonia in patients with 2009 pandemic influenza A (H1N1) infection: An observational study. J Chemother Florence Italy. 2010; 22:428–30 [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Benoit SR, Skarbinski J, et al. ; 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team: Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus--United States, 2009. Clin Infect Dis. 2012; 54:1221–1229 [DOI] [PubMed] [Google Scholar]
- 11.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. ; Dutch-Belgian Mycosis Study Group: Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir Med. 2018; 6:782–792 [DOI] [PubMed] [Google Scholar]
- 12.Luyt CE, Bouadma L, Morris AC, et al. : Pulmonary infections complicating ARDS. Intensive Care Med. 2020; 46:2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdar N, Crnich CJ, Maki DG: The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care. 2005; 50:725–739 [PubMed] [Google Scholar]
- 14.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N Engl J Med. 2020; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomazini BM, Maia IS, Cavalcanti AB, et al. ; COALITION COVID-19 Brazil III Investigators: Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020; 324:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouzé A, Martin-Loeches I, Povoa P, et al. : Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021; 47:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M, Higginson E, Pereira-Dias J, et al. : Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021; 25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blonz G, Kouatchet A, Chudeau N, et al. : Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit Care. 2021; 25:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: A severity of disease classification system. Crit Care Med. 1985; 13:818–829 [PubMed] [Google Scholar]
- 20.EUCAST: Comité de l’antibiogramme de la Société Francaise de Microbiologie. 2020 [Google Scholar]
- 21.Plachouras D, Lepape A, Suetens C: ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018; 44:2216–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho DE, Imai K, King G, et al. : Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007; 15:199–236 [Google Scholar]
- 23.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2017 [Google Scholar]
- 24.Fauvernier M, Roche L, Uhry Z, et al. : Multi-dimensional penalized hazard model with continuous covariates: Applications for studying trends and social inequalities in cancer survival. J R Stat Soc Ser C Appl Stat. 2019; 68:1233–1257 [Google Scholar]
- 25.Papazian L, Klompas M, Luyt CE: Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020; 46:888–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stasi C, Fallani S, Voller F, et al. : Treatment for COVID-19: An overview. Eur J Pharmacol. 2020; 889:173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health: COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed September 9, 2021 [PubMed]
- 28.Payen D, Cravat M, Maadadi H, et al. : A longitudinal study of immune cells in severe COVID-19 patients. Front Immunol. 2020; 11:580250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis): High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020; 46:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucchini A, Iozzo P, Bambi S: Nursing workload in the COVID-19 era. Intensive Crit Care Nurs. 2020; 61:102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EARS: Antimicrobial Resistance in the EU/EEA (EARS-Net): Annual Epidemiological Report for 2019 [Google Scholar]
- 32.Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. : False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS One. 2020; 15:e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.