OBJECTIVES:
To explore candidate prognostic and predictive biomarkers identified in retrospective observational studies (interleukin-6, C-reactive protein, lactate dehydrogenase, ferritin, lymphocytes, monocytes, neutrophils, d-dimer, and platelets) in patients with coronavirus disease 2019 pneumonia after treatment with tocilizumab, an anti–interleukin-6 receptor antibody, using data from the COVACTA trial in patients hospitalized with severe coronavirus disease 2019 pneumonia.
DESIGN:
Exploratory analysis from a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial.
SETTING:
Hospitals in North America and Europe.
PATIENTS:
Adults hospitalized with severe coronavirus disease 2019 pneumonia receiving standard care.
INTERVENTION:
Randomly assigned 2:1 to IV tocilizumab 8 mg/kg or placebo.
MEASUREMENTS AND MAIN RESULTS:
Candidate biomarkers were measured in 295 patients in the tocilizumab arm and 142 patients in the placebo arm. Efficacy outcomes assessed were clinical status on a seven-category ordinal scale (1, discharge; 7, death), mortality, time to hospital discharge, and mechanical ventilation (if not receiving it at randomization) through day 28. Prognostic and predictive biomarkers were evaluated continuously with proportional odds, binomial or Fine-Gray models, and additional sensitivity analyses. Modeling in the placebo arm showed all candidate biomarkers except lactate dehydrogenase and d-dimer were strongly prognostic for day 28 clinical outcomes of mortality, mechanical ventilation, clinical status, and time to hospital discharge. Modeling in the tocilizumab arm showed a predictive value of ferritin for day 28 clinical outcomes of mortality (predictive interaction, p = 0.03), mechanical ventilation (predictive interaction, p = 0.01), and clinical status (predictive interaction, p = 0.02) compared with placebo.
CONCLUSIONS:
Multiple biomarkers prognostic for clinical outcomes were confirmed in COVACTA. Ferritin was identified as a predictive biomarker for the effects of tocilizumab in the COVACTA patient population; high ferritin levels were associated with better clinical outcomes for tocilizumab compared with placebo at day 28.
Keywords: biomarkers, coronavirus disease 2019, ferritin, interleukin-6, severe acute respiratory syndrome coronavirus 2, tocilizumab
Proinflammatory cytokines, such as interleukin (IL)–6, may be involved in the immune dysregulation reported in coronavirus disease 2019 (COVID-19), possibly resulting in more severe clinical manifestations (1–3). Elevated IL-6 levels are associated with severe COVID-19 (4), and preliminary findings from case-control and retrospective cohort studies (5, 6) supported investigation of tocilizumab, an anti–IL-6 receptor α antibody, in severe COVID-19 pneumonia.
COVACTA was a global, double-blind, randomized, placebo-controlled, phase 3 trial of tocilizumab in patients hospitalized with severe COVID-19 pneumonia. The primary endpoint of improved clinical status on a seven-category ordinal scale at day 28 was not met. No difference was observed in mortality at day 28; however, time to hospital discharge was shorter in the tocilizumab group. Subgroup analysis suggested reduced risk for ICU transfer among patients not in the ICU at randomization and potential reduction in clinical failure (death, mechanical ventilation, ICU transfer, or study withdrawal) among patients not receiving mechanical ventilation at randomization. Additional studies are investigating these findings further (7).
Heterogeneity in clinical presentation and pathologic manifestations of COVID-19 (8) present challenges in investigating effective treatments and identifying biomarkers to prognosticate or predict treatment response. Laboratory measures of hyperinflammation (IL-6, C-reactive protein [CRP]), macrophage activation (ferritin), dysregulated immune cells (lymphocytes, monocytes, neutrophils), tissue damage (lactate dehydrogenase [LDH]), and coagulopathy (d-dimer, platelets) have been reported as risk factors for poor prognosis in COVID-19 (4, 9–13), and most of them are mechanistically linked to IL-6 activity (14–16). These biomarkers have been investigated only in retrospective observational studies of COVID-19.
We aimed to determine the prognostic and predictive value of previously identified candidate biomarkers regarding clinical outcomes in a prespecified exploratory analysis of the COVACTA trial.
METHODS
Patients and Study Design
COVACTA (ClinicalTrials.gov; NCT04320615) was a randomized, placebo-controlled, double-blind, global, multicenter, phase 3 trial investigating the efficacy and safety of tocilizumab versus placebo in patients hospitalized with severe COVID-19 pneumonia receiving standard care according to local practice (7). Tocilizumab is investigational for the treatment of severe COVID-19 pneumonia and is not currently indicated for use in this patient population. Eligible adult patients—stratified by geographic region (North America, Europe) and mechanical ventilation (yes, no)—received IV tocilizumab or placebo (2:1). Key inclusion criteria were hospitalization with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection confirmed by polymerase chain reaction of any specimen and blood oxygen saturation less than or equal to 93% or Pao2/Fio2 less than 300 mm Hg despite local standard care (could include antivirals and low-dose steroids). Key exclusion criteria were active or suspected infection (other than SARS-CoV-2), alanine aminotransferase or aspartate aminotransferase greater than 10× upper limit of normal, absolute neutrophil count less than 1,000/mL, and platelet count less than 50,000/mL. The primary endpoint was clinical status assessed using a seven-category ordinal scale. Secondary endpoints included mortality at day 28, incidence of mechanical ventilation (in patients not receiving it at randomization), and time to hospital discharge. Informed consent was obtained for all enrolled patients. The study was conducted in accordance with the International Council for Harmonization E6 guideline for good clinical practice and the Declaration of Helsinki or local regulations, whichever afforded greater patient protection. The protocol was reviewed and approved by all appropriate institutional review boards and ethics committees (Appendix 1, http://links.lww.com/CCM/G718).
Biomarker Analysis
Biomarkers in peripheral blood were assessed at baseline to examine their potential prognostic value and potential predictive value for tocilizumab efficacy. Efficacy was determined by clinical status assessed on the seven-category ordinal scale at day 28 (primary), mortality at day 28, time to hospital discharge, and requirement for mechanical ventilation by day 28 (in patients not receiving it at randomization). These endpoints were selected to ensure a robust and consistent signal across different, clinically meaningful outcomes. Imputation rules were assigned as published (7). Prespecified candidate biomarkers included IL-6 and CRP as markers of hyperinflammation, ferritin as an acute-phase protein and as a marker of macrophage activation, LDH as a marker of tissue damage, lymphocytes as markers of dysregulated immune response, and d-dimer as a marker of coagulopathy. The potential for neutrophil, monocyte, platelet, and WBC counts as biomarkers was also explored. IL-6 was measured using an immunoassay validated at QPS (Quantikine ELISA; R&D Systems, Minneapolis, MN). CRP was measured using an in vitro diagnostic method validated at PPD (Roche Cobas; Roche Diagnostics, Rotkreuz, Switzerland). All other biomarkers were assessed using standard clinical chemistry and hematology methods at local clinical laboratories.
Statistical Analysis
Biomarkers were assessed in the modified intention-to-treat population (any randomly assigned patients who received study medication). Subgroup analysis was conducted in patients in ordinal scale categories 4 (ICU or non-ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen) and 5 (ICU, requiring intubation and mechanical ventilation) at baseline. Histograms, scatterplots, and tables were generated to assess the completeness of data by treatment arm at baseline, determine the balance of biomarker levels at baseline, and identify outliers. Biomarkers not normally distributed (d-dimer, ferritin, IL-6, lymphocytes [absolute], neutrophil-to-lymphocyte ratio, and WBCs) were log-transformed before analysis. Pearson correlation coefficient was calculated between outcomes and biomarkers, between baseline covariates and biomarkers, and between individual biomarkers. Six candidate biomarkers assessed (IL-6, CRP, ferritin, LDH, lymphocytes, d-dimer) were prespecified in a biomarker analysis plan. No adjustments were made for multiple comparisons.
Prognostic modeling was assessed in the placebo arm only, controlling for the following covariates: mechanical ventilation status at randomization, antiviral use, steroid use, age, sex, and region (Europe/North America). No endpoints were considered primary because the motivation was to identify a robust, consistent signal across endpoints. Sensitivity analyses were performed on the placebo arm only using unadjusted analysis, on the tocilizumab and placebo arms adjusting for the same covariates plus treatment arm, and on the tocilizumab and placebo arms controlling only for treatment arm. Candidate prognostic biomarkers were assessed using a proportional odds model with the ordinal scale score at day 28 as a dependent variable and biomarker and covariates (depending on the model) as independent variables. Odds ratios, CIs, and p values were reported, and proportional odds assumptions were assessed graphically. A Fine-Gray model was fit for time to discharge, with death as a competing risk. A Cox proportional hazards model was fit as a sensitivity analysis. A binomial model with outcome as a dependent variable, biomarkers as independent variables, and covariates (depending on the model) was used for binary outcomes (death, discharge, mechanical ventilation).
Candidate predictive biomarkers were modeled as for candidate prognostic biomarkers, with addition of an interaction term between continuous biomarker levels and treatment. All reported predictive p values rely on the interaction p value term except those generated in the multivariate model assessing multiple biomarkers. Tertile analysis of predictive biomarkers was performed by creating vectors for each tertile (low, medium, high) and fitting a single model with interaction terms for medium and high tertiles with treatment. Treatment effects within each tertile were calculated from the estimates. No cut point optimization was performed, but analysis and visualization were performed using tertiles and quartiles. Combined predictive biomarkers were assessed by dichotomizing the biomarkers using median values as cutoffs.
Supportive analysis was conducted using data from the placebo arm of COVACTA and data from a phase 2 trial of tocilizumab in moderate-to-severe COVID-19 pneumonia (ClinicalTrials.gov: NCT04363736; MARIPOSA) (Appendix 2 and Table S1, http://links.lww.com/CCM/G718).
RESULTS
Biomarkers at Baseline
Baseline biomarker levels (Fig. S1A, http://links.lww.com/CCM/G718), except IL-6 and CRP, were generally balanced between treatment arms in COVACTA (Table 1; and Fig. S1B, http://links.lww.com/CCM/G718). Median levels were higher in the tocilizumab than the placebo arm for IL-6 (88.1 vs 70.3 ng/L) and CRP (169.3 vs 151.9). Median levels of IL-6, CRP, ferritin, d-dimer, LDH, and neutrophils (absolute level and percentage) were above normal, and lymphocytes (absolute level and percentage) were below normal (neutrophil-to-lymphocyte ratio was elevated). Monocytes (percentages), platelet counts, and WBC counts were within normal ranges. Limited correlation was observed between prespecified biomarkers (Fig. S1C, http://links.lww.com/CCM/G718).
Table 1.
Biomarker Levels at Baseline (Modified Intention-to-Treat Population)
| Biomarker | Normal Range | Placebo | Tocilizumab |
|---|---|---|---|
| Interleukin-6, ng/mLa | ≤ 0.007 | n = 99 | n = 233 |
| Mean (sd) | 192.2 (368.7) | 201.9 (418.4) | |
| Median (range) | 70.3 (3.1–2,810) | 88.1 (3.1–4,020) | |
| C-reactive protein, mg/L | ≤ 5 | n = 126 | n = 256 |
| Mean (sd) | 177.8 (117.1) | 187.8 (119.8) | |
| Median (range) | 151.9 (1.6–500) | 169.3 (1.1–500) | |
| Ferritin, pmol/La | 27 to ≤ 337 (women) | n = 124 | n = 240 |
| Mean (sd) | 27 to ≤ 674 (men) | 3,792 (7,463) | 3,069 (3,113) |
| Median (range) | 2,168 (96.9–75,300) | 2,250 (3.6–24,045) | |
| d-dimer, μg/mL fibrinogen equivalent unitsa | ≤ 0.5 | n = 66 | n = 131 |
| Mean (sd) | 4.2 (7.6) | 4.6 (8.4) | |
| Median (range) | 1.2 (0.3–46.7) | 1.3 (0.2–58.1) | |
| Lactate dehydrogenase, IU/L | 105–333 | n = 121 | n = 243 |
| Mean (sd) | 469.7 (291.7) | 479.4 (303.5) | |
| Median (range) | 422 (1.3–2,323) | 430 (0.7–3,282) | |
| Leukocytes, 109/La | 4.5–11 | n = 140 | n = 280 |
| Mean (sd) | 9.2 (4.1) | 9.3 (4.5) | |
| Median (range) | 8.5 (2.4–22.4) | 8.3 (2.7–28.2) | |
| Lymphocytes, 109/La | 0.9–2.9 | n = 139 | n = 288 |
| Mean (sd) | 0.96 (0.83) | 0.98 (0.57) | |
| Median (range) | 0.9 (0–8.9) | 0.9 (0–5.4) | |
| Lymphocytes, % | 20–40 | n = 133 | n = 268 |
| Mean (sd) | 13 (10) | 12 (7) | |
| Median (range) | 11 (0–55) | 11 (0–48) | |
| Neutrophils, 109/L | 1.7–7 | n = 139 | n = 291 |
| Mean (sd) | 7.5 (3.9) | 7.6 (4.1) | |
| Median (range) | 7.2 (0.9–23.1) | 6.8 (1.0–24.6) | |
| Neutrophils, % | 40–60 | n = 134 | n = 268 |
| Mean (sd) | 79 (12) | 79 (9) | |
| Median (range) | 82 (24–98) | 81 (44–99) | |
| Neutrophils/lymphocytes, ratioa | 1–3 | n = 132 | n = 265 |
| Mean (sd) | 65.0 (566.5) | 64.8 (683.6) | |
| Median (range) | 7.1 (0.4–6,509) | 7.5 (1.2–10,463) | |
| Monocytes, % | 2–8 | n = 131 | n = 269 |
| Mean (sd) | 6 (4) | 6 (4) | |
| Median (range) | 5 (0–19) | 5 (0–32) | |
| Platelets, 109/L | 150–400 | n = 142 | n = 295 |
| Mean (sd) | 262.0 (117.7) | 265.7 (113.3) | |
| Median (range) | 240 (53–814) | 253 (10.2–825) |
aLog-transformed for all additional analyses.
Evaluation of Prognostic Biomarkers
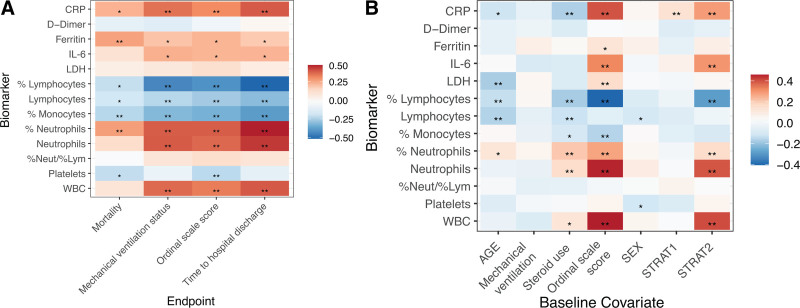
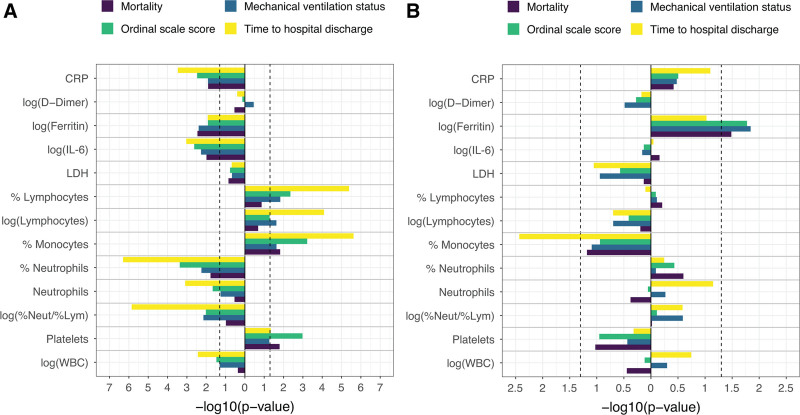
All biomarkers—except LDH, d-dimer, and neutrophil-to-lymphocyte ratio—correlated with clinical outcomes, including mortality, mechanical ventilation, ordinal scale score, and time to hospital discharge, at day 28 (Fig. 1A). Some biomarkers correlated with baseline covariates; IL-6, CRP, neutrophils (absolute level and percentage), and WBC count were positively correlated with higher baseline ordinal scale category (Fig. 1B). Adjusting for covariates, IL-6, CRP, ferritin, lymphocytes, monocytes, neutrophils, platelets, and WBCs showed robust evidence across sensitivity analyses, supporting their prognostic value, but LDH and d-dimer did not show prognostic value (Fig. S2, http://links.lww.com/CCM/G718). Prognostic modeling showed a consistent direction of effect across all clinical outcomes at day 28 for mortality, mechanical ventilation, ordinal scale score, and time to hospital discharge (Fig. 2A; and Fig. S2, http://links.lww.com/CCM/G718). Analysis of the ordinal scale at day 28 according to baseline continuous ferritin revealed a potential prognostic effect of ferritin in the placebo arm (p = 0.01; n = 124).
Figure 1.
Correlation between baseline biomarkers and clinical outcomes (A) and biomarkers and baseline covariates (B). p values are based on Pearson correlation unadjusted for covariates, placebo arm only for A and for placebo and tocilizumab arms for B. *p ≤ 0.05; **p ≤ 0.01. CRP = C-reactive protein, IL-6 = interleukin-6, LDH = lactate dehydrogenase, %Lym = % lymphocytes, %Neut = % neutrophils, STRAT = stratification factor.
Figure 2.
Efficacy outcomes. Placebo-adjusted, scaled prognostic model (A) and predictive model (B). Model adjusted for the stratification factors of region (Europe, North America) and mechanical ventilation (yes, no) as well as age, sex, baseline antiviral use (yes, no), and baseline steroid use (yes, no). Vertical dashed lines indicate p = 0.05. Outcomes are at day 28 and have been aligned, so that bars pointing in the same direction have the same direction of effect. Last observation carried forward was used for ordinal scale score at day 28. CRP = C-reactive protein, IL-6 = interleukin 6, LDH = lactate dehydrogenase, %Lym = % lymphocytes, %Neut = % neutrophils
Evaluation of Predictive Biomarkers
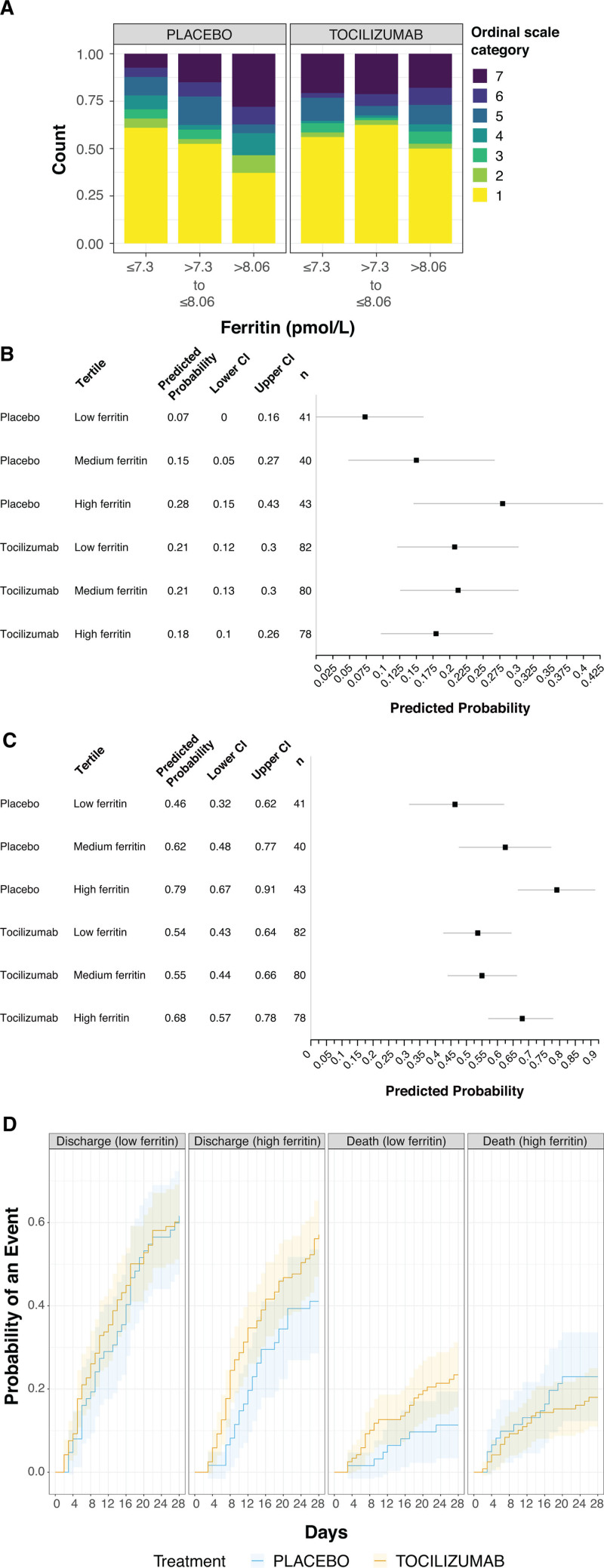
Predictive modeling identified ferritin as a consistent and significant predictive biomarker for the effects of tocilizumab in COVACTA, whereas other biomarkers, including IL-6, CRP, d-dimer, LDH, lymphocytes, monocytes, neutrophils, platelets, and WBC, did not show consistent or significant predictive value for the effects of tocilizumab across endpoints (Fig. 2B; and Fig. S3, http://links.lww.com/CCM/G718). Analysis of the ordinal scale at day 28 according to continuous baseline ferritin values showed that placebo patients with higher baseline levels had worse clinical status, whereas clinical outcomes at day 28 were similar across baseline ferritin tertiles in tocilizumab patients (n = 364; predictive effect interaction, p = 0.02; visualized using tertiles in Fig. 3A). Modeled data showed a predictive effect for ferritin as a biomarker for tocilizumab effects on mortality by day 28 (predictive interaction, p = 0.03) (Fig. 3B) and mechanical ventilation by day 28 (predictive interaction, p = 0.01) (Fig. 3C). Tocilizumab treatment decreased the probability of mechanical ventilation and mortality by day 28 compared with placebo in patients with elevated ferritin levels based on continuous assessment across a range of ferritin levels. Although time to hospital discharge was nominally shorter with tocilizumab than placebo in COVACTA (7), ferritin was not a significant predictive biomarker for the effect of tocilizumab on time to hospital discharge (p = 0.09) (Fig. 3D); however, the direction of effect was consistent with that observed for ordinal scale and mortality outcomes.
Figure 3.
Ferritin as a predictor for efficacy outcomes. A, Day 28 all-comers, B, unadjusted tertiles model-predicted probability of death to day 28, C, unadjusted tertiles model-predicted probability of mechanical ventilation to day 28 (in patients who did not receive mechanical ventilation at randomization), and D, cumulative incidence function plot of hospital discharge and death to day 28. Seven-category ordinal scale: 1, discharged or ready for discharge; 2, non-ICU hospital ward, not requiring supplemental oxygen; 3, non-ICU hospital ward, requiring supplemental oxygen; 4, ICU or non-ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, death. Ferritin values were log-transformed (sample size, n = 364). Ferritin tertile cutoff values were 3.63 pmol/L (minimum), 1,480.77 pmol/L, 3,150.29 pmol/L, and 75,299.67 pmol/L (maximum) in an unadjusted model showing predicted probability and 95% CI (B, C). Cumulative incidence was determined by Aalen-Johansen estimator, with an arbitrary median cut point of 7.7 on logarithmic scale (D).
Analysis comparing data from patients with severe disease in the tocilizumab 8 mg/kg arm from MARIPOSA with data from the placebo arm of COVACTA supported the predictive value of ferritin as a biomarker for mortality (ATT estimand based, p = 0.044) and mechanical ventilation status (Fig. S4, http://links.lww.com/CCM/G718).
Additional sensitivity analyses were performed on the negative signals observed for the biomarkers of interest: IL-6 and CRP. Analysis of the ordinal scale and mortality at day 28 confirmed that baseline IL-6 is not a predictive marker for the effects of tocilizumab (Fig. S5, http://links.lww.com/CCM/G718). Predictive analysis of CRP at a cutoff of 75 mg/L (17) was not significant for time to hospital discharge (p = 0.46), mortality (p = 0.92), or ordinal scale (p = 0.78) (data not shown), which is consistent with the tertiles analysis (Fig. S3, http://links.lww.com/CCM/G718). Analysis of combinations of the biomarkers such as ferritin, IL-6, and CRP (n = 257) as well as ferritin, LDH, and d-dimer (n = 165) did not show any synergistic effect between biomarkers (Table S2, http://links.lww.com/CCM/G718), further suggesting that ferritin alone was the strongest predictive biomarker for the effects of tocilizumab in COVACTA.
Subgroup Analyses
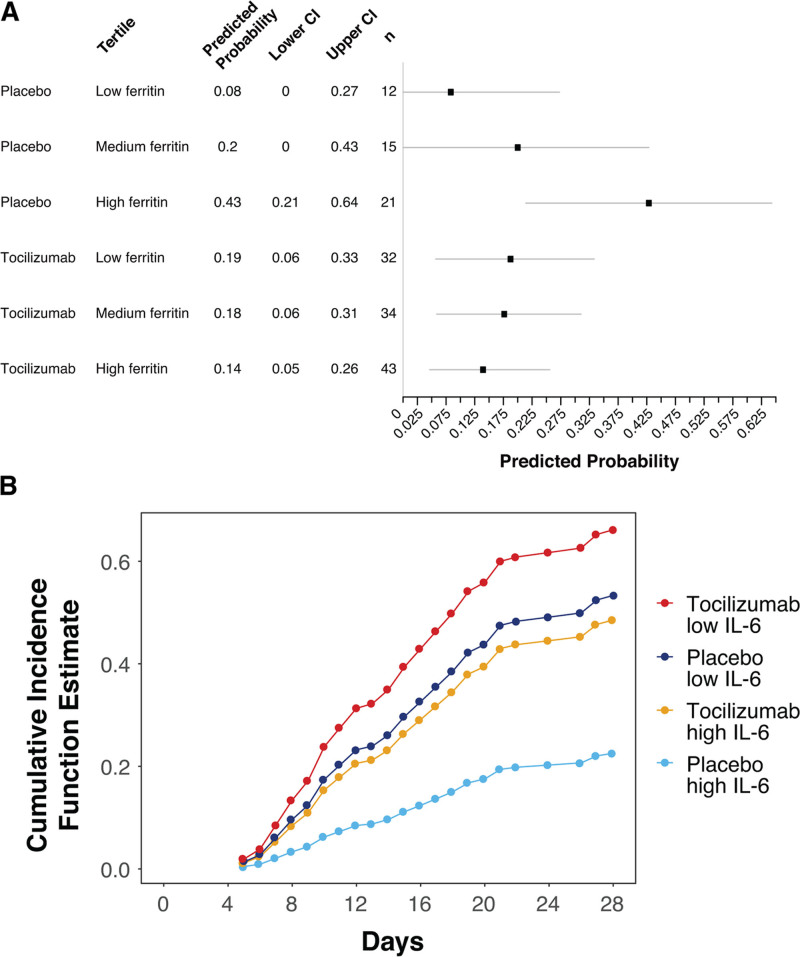
Patients who required high-flow oxygen or noninvasive or invasive mechanical ventilation without other advanced life support (ordinal scale categories 4 and 5) at baseline had higher baseline ferritin levels than patients in all other ordinal scale categories (Fig. S6 and Table S3, http://links.lww.com/CCM/G718), and those in the tocilizumab arm had better clinical outcomes than those in the placebo arm. Analysis of this subgroup using a continuous scale for baseline ferritin levels also demonstrated a predictive association of baseline ferritin levels and the effects of tocilizumab for mortality (interaction, p = 0.02). Tertile analysis was also performed (Fig. 4A; and Fig. S7, http://links.lww.com/CCM/G718). The predictive signal of ferritin as a continuous biomarker was significant for clinical status by ordinal scale at day 28 (interaction, p = 0.01) and incidence of mechanical ventilation by day 28 in patients who were not on mechanical ventilation at randomization (interaction, p = 0.01).
Figure 4.
Predictive biomarker assessment for the subset of patients in ordinal scale categories 4 and 5 at baseline in COVACTA (n = 157). A, Death by day 28 according to baseline ferritin levels. Ferritin values were log-transformed. Shown are predicted probabilities for death within each tertile of ferritin (tertile cutoff values were 3.63 pmol/L [minimum], 1,480.77 pmol/L, 3,150.29 pmol/L, and 75,299.67 pmol/L [maximum]) showing predicted probability and 95% CI based on an unadjusted model (calculated based on all-comers) fit using samples restricted to those for patients in ordinal scale categories 4 and 5 at baseline. B, Time to hospital discharge by baseline interleukin (IL)–6 levels. Figure shows the cumulative incidence function for time to hospital discharge based on the median IL-6 cutoff value.
Baseline IL-6 levels were predictive for tocilizumab effects in this subgroup for time to hospital discharge only (Fig. 4B; and Fig. S8, http://links.lww.com/CCM/G718) (adjusted Fine-Gray, p = 0.03), but this was not statistically significant in sensitivity analysis (Cox proportional hazards adjusted, p = 0.09). Furthermore, baseline IL-6 levels were not predictive for tocilizumab effects on hospital discharge (yes vs no; p = 0.13) or any other outcome in this subgroup. There was no evidence that baseline CRP levels were significantly predictive of tocilizumab effects based on the continuous or tertiles analysis (Fig. S7, http://links.lww.com/CCM/G718). Baseline CRP based on a cutoff of 75 mg/L was predictive for time to hospital discharge (Fine-Gray model, p = 0.03; Cox proportional hazards sensitivity analysis, p = 0.05) (data not shown) but not for the other outcomes.
DISCUSSION
Trials of tocilizumab in COVID-19 have produced mixed results. The COVACTA study did not show a mortality benefit of tocilizumab over standard care, but potential clinical benefits including shortened hospital stay and time to clinical improvements were observed (7). Tocilizumab did not demonstrate any mortality benefit above standard care in another randomized controlled trial (18), but the Evaluating Minority Patients with Actemra study showed tocilizumab reduced the likelihood of progression to mechanical ventilation or death (19). The open-label Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia study showed improved outcomes, including survival, above standard care in critically ill patients treated with tocilizumab (20), and the open-label Randomized Evaluation of Covid-19 Therapy study demonstrated that tocilizumab was associated with reduced all-cause mortality across all levels of respiratory support, higher probability of hospital discharge alive, and reduced need for mechanical ventilation (17).
Our exploratory analysis of the COVACTA trial is the first, to our knowledge, to report on prognostic and predictive biomarkers from a global, multicenter, randomized controlled trial in patients hospitalized with COVID-19 pneumonia. The candidate biomarkers we assessed—IL-6, CRP, ferritin, LDH, lymphocytes, neutrophils, monocytes, d-dimer, and platelets—are among the biomarkers previously identified in case series and observational studies as potential biomarkers for disease outcomes in COVID-19 (9, 12, 21).
IL-6, CRP, ferritin, LDH, lymphocyte, neutrophil, and d-dimer levels were abnormal at baseline in COVACTA, consistent with manifestations of hyperinflammation, macrophage activation, tissue damage, dysregulated immune cells, and coagulopathy in patients with severe COVID-19 (4, 9–13). Only a modest correlation was observed between biomarkers representing different manifestations, potentially reflecting the overlapping yet differentiated pathogenic pathways in COVID-19. Elevated levels of IL-6, CRP, ferritin, and neutrophils and decreased levels of lymphocytes demonstrated robust and consistent prognostic value as biomarkers of poor disease outcomes across all clinical outcomes assessed. Monocytes (percentage) and platelet counts also demonstrated prognostic value, although at baseline they were within normal ranges. Elevated LDH levels appeared to have prognostic value, but this was not robust across sensitivity analyses. Our findings support the hypothesis that systemic hyperinflammation, tissue damage, and dysregulated immune responses are associated with poor disease outcomes in patients with severe COVID-19 (4, 9, 10). Elevated d-dimer levels as a marker of coagulopathy showed weak and inconsistent prognostic signals in COVACTA. However, there were potential confounding factors for d-dimer assessment in COVACTA, including higher levels among survivors compared with other studies (10) (Table S4, http://links.lww.com/CCM/G718), disproportionate missing data by region (59.2%, Europe; 40.8%, North America), an association with the stratification factor region that was not evident for any other biomarkers tested, and an unadjusted p value of 0.023 for mortality in prognostic modeling compared with an adjusted p value of 0.54 for region. Thus, the prognostic value of d-dimer levels in COVID-19 remains controversial (12, 22, 23).
Ferritin was the only biomarker in this exploratory analysis that predicted the effects of tocilizumab treatment in COVACTA across multiple clinical outcomes. During infection and inflammation, ferritin, as a marker of myeloid cell activation in blood and inflamed tissue, is released from activated macrophages and hepatocytes to protect cells from oxidative damage and sequester iron from pathogens (24, 25). Once released, it can further activate macrophages to produce cytokines such as IL-6 and IL-1β, driving a positive feedback loop of ferritin-inducing proinflammatory cytokine release (24, 25). Altered myeloid cell activation, elevated IL-6 levels, and elevated ferritin levels were reported in COVID-19 and correlated with increased severity of COVID-19 disease (1, 26). Furthermore, elevated ferritin levels decreased significantly after tocilizumab administration in patients with severe COVID-19 (12), suggesting a possible mechanism underlying the predictive value of ferritin for tocilizumab treatment effects in this patient population. Further investigation of downstream mechanisms of IL-6 and ferritin signaling interaction, such as single-cell analysis of pulmonary cells, might elucidate how elevated ferritin levels predict benefit from inhibition of IL-6 activity in patients with severe COVID-19.
Although IL-6 and CRP demonstrated strong prognostic value as disease biomarkers in COVID-19, elevated levels of IL-6 or CRP at baseline were not predictive for tocilizumab treatment effects in the overall COVACTA population. IL-6 only demonstrated a trend for predictive value among the subgroup of patients who required high-flow oxygen and noninvasive or invasive mechanical ventilation (ordinal scale categories 4 and 5) at baseline. Similarly, baseline CRP using a cutoff of 75 mg/L, the same cutoff used in RECOVERY, was predictive for tocilizumab effect on time to hospital discharge but not for other endpoints. CRP was not predictive in continuous or tertile analyses. This is consistent with the findings of the REMAP-CAP study, in which CRP levels at baseline did not predict tocilizumab efficacy (19). These results might be counterintuitive given that tocilizumab inhibits IL-6 signaling; however, it is unclear how IL-6 activity in blood is associated with tocilizumab efficacy in complex immunologic diseases (27, 28). For patients with COVID-19, investigation into how peripheral IL-6 and CRP levels are associated with tocilizumab effects on hyperinflammation and dysregulated immune responses in affected tissue, in particular the lung, is warranted.
Because the COVACTA trial did not meet its primary endpoint of improved clinical status in patients with COVID-19–associated pneumonia or its key secondary endpoint of reduced patient mortality, identifying a subgroup of patients who might have derived clinical benefit (patients with elevated ferritin levels at baseline) also revealed a subgroup potentially harmed (patients without highly elevated ferritin levels at baseline); however, data interpretation was limited because of the relatively small sample size. In RECOVERY, a large platform study, mortality benefit was observed consistently in the tocilizumab group in all patients with hypoxia, regardless of their level of respiratory support (17). Additional studies are required to confirm the predictive value of ferritin for tocilizumab effects in patients with COVID-19 and to identify a clinically useful threshold at which ferritin levels could predict potential enhanced benefit. These studies, however, must consider factors such as variability between patient cohorts and assay methods. Another limitation of this study is that laboratory results were measured locally, entailing more variability than uniform test runs in a central laboratory. Local laboratory results for ferritin, however, were confirmed by retesting all samples at a central laboratory using an in vitro diagnostic method (Roche Cobas; Roche Diagnostics).
In conclusion, markers of hyperinflammation, macrophage activation, and dysregulated immune cells appear to be important prognostic biomarkers in COVID-19. Ferritin, as an acute-phase protein and a macrophage activation marker, might have potential as a predictive biomarker to identify patients with severe COVID-19 who respond to tocilizumab. Results from ongoing studies of tocilizumab in COVID-19 are needed to validate these findings.
Supplementary Material
Footnotes
*See also p. 510.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The first draft of the article was written by Dr. Cai and Dr. Tom, employees of Genentech. Dr. Tom contributed to data analysis and interpretation. Dr. Bao contributed to study design and to data collection, analysis, and interpretation. Dr. Tsai contributed to study design and data interpretation. Drs. Qamra and Summers contributed to data analysis, figure creation, and data interpretation. Dr. Carrasco-Triguero contributed to biomarker analysis. Dr. McBride contributed to study design and to data analysis and interpretation. Dr. Rosenberger contributed to the biomarker study design and data interpretation. Dr. Lin contributed to design of the MARIPOSA trial and to data analysis and interpretation. Dr. Stubbings contributed to the MARIPOSA trial clinical science team and to data analysis and interpretation of the MARIPOSA trial. Dr. Blyth contributed to enrollment of patients and to data collection and interpretation. Drs. Carratalà, François, and Bonfanti contributed to data collection. Drs. Benfield and van der Leest contributed to enrollment of patients and data collection. Dr. Haslem was a study site principal investigator. Dr. Rohatgi contributed to data collection and interpretation. Dr. Luyt contributed to enrollment of patients and data collection. Dr. Kheradmand contributed to data interpretation. Dr. Cai contributed to the literature search, generation of the biomarker strategy, planning and implementation of the biomarker analysis, and data collection, analysis, and interpretation. All authors were involved in writing the article or revising it critically for important intellectual content and approving the final version for publication, and all verify that they had access to and take responsibility for all the data in the article.
Supported by F. Hoffmann-La Roche Ltd and, in part, by federal funds received from the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, and Biomedical Advanced Research and Development Authority, under grant number HHSO100201800036C.
Drs. Tom, Bao, Tsai, Carrasco-Triguero, and Cai received funding from the Biomedical Advanced Research and Development Authority (BARDA) under OT number HHSO100201800036C. Drs. Tom, Bao, Tsai, Qamra, Summers, Carrasco-Triguero, McBride, Rosenberger, Lin, Stubbings, and Cai disclosed they are employees of Genetech/Roche. Drs. Tom and Cai disclosed that they have a patent pending to Genetech for biomarkers for predicting response to an interleukin (IL)–6 antagonist (P36367-US). Drs. Bao, Tsai, and van der Leest received funding from Roche/Genetech. Dr. Bao received support for article research from BARDA. Drs. Bao and Tsai disclosed a patent pending for a method for treating pneumonia, including coronavirus disease 2019 pneumonia with an IL-6 antagonist (EFS ID 38946141). Drs. Bao and Blyth disclosed government work. Drs. Bao, Tsai, Qamra, Summers, Carrasco-Triguero, McBride, Stubbings, Haslem, and Cai disclosed the off-label product use of tocilizumab (Actemra). Dr. Qamra received funding from Hoffman-La Roche Canada. Dr. Summers received funding from Roche Products Ltd. Drs. McBride, Rosenberger, and Lin disclosed they own stock/stock options of Genetech. Drs. McBride, Rosenberger, and Cai disclosed work for hire. Dr. Stubbings is an employee of F Hoffmann-La Roche AG. Dr. Blyth received funding from Rocket Medical UK Ltd. Dr. Carratalà’s institution received funding from Gilead and Roche. Dr. François received funding from Aridis, AM-Pharma, Asahi Kasei, Inotrem, GlaxoSmithKline, Enlivex, Polyphor, Takeda, Transgene, and Biomérieux. Dr. Benfield received funding from Novo Nordisk, Simonsen, GlaxoSmithKline, Pfizer, Gilead, Lundbeck, Kai Hansen Foundation and personal fees from GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Gilead, and Merck Sharp & Dohme outside the submitted work. Dr. Bonfanti received funding from Viiv, Gilead, and Janssen. Dr. van der Leest received funding from Bristol Myers Squibb, Merck Sharpe & Dohme, AbbVie, Boehringer Ingelheim, and AstraZeneca. Dr. Luyt’s institution received funding from Roche; he received funding from Correvio, Bayer Healthcare, Aerogen, ThermoFisher Brahms, Merck Sharpe & Dohme, Carmat, and Biomérieux. Dr. Luyt reports a grant from Roche to the Institute of Cardiometabolism and Nutrition, Sorbonne Université, Hôpital de la Pitié Salpêtrière, Assistance Publique - Hôpitaux de Paris for the COVACTA trial and a grant from Correvio and personal fees from Bayer Healthcare, Aerogen, ThermoFisher Brahms, Merck Sharp & Dohme, and Biomérieux outside the submitted work. Dr. Kheradmand received support for article research from the National Institutes of Health. Dr. Rosas received funding from Boehringer Ingelheim, Bristol Myers Squibb, and Immunomet. He reports a grant from Roche for the COVACTA trial and a grant and personal fees from Genentech/Roche outside the submitted work. Dr. Cai’s institution received funding from F. Hoffman-La Roche Ltd and the U.S. Department of Health and Human Services, the Office of the Assistant Secretary for Preparedness and Response. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The corresponding author had full access to the study data and had final responsibility for the decision to submit for publication.
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, access https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
REFERENCES
- 1.Vabret N, Britton GJ, Gruber C, et al. ; Sinai Immunology Review Project: Immunology of COVID-19: Current state of the science. Immunity. 2020; 52:910–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. : Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020; 27:992–1000.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzoni A, Salvati L, Maggi L, et al. : Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020; 130:4694–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Pang J, Ji P, et al. : Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2020; 93:35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye AG, Siegel R: The efficacy of IL-6 inhibitor tocilizumab in reducing severe COVID-19 mortality: A systematic review. PeerJ 2020; 8:e10322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. : Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020; 2:e474–e484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas IO, Bräu N, Waters M, et al. : Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021; 384:1503-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health: Coronavirus Disease 2019 (COVID-2019) Treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed April 29, 2021 [PubMed]
- 9.Herold T, Jurinovic V, Arnreich C, et al. : Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020; 146:128–136.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohse A, Klopfenstein T, Balblanc JC, et al. : Predictive factors of mortality in patients treated with tocilizumab for acute respiratory distress syndrome related to coronavirus disease 2019 (COVID-19). Microbes Infect. 2020; 22:500–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrozier T, Lohse A, Balblanc JC, et al. : Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID-19): Results of a multidisciplinary collaboration. Clin Exp Rheumatol. 2020; 38:742–747 [PubMed] [Google Scholar]
- 13.Luo P, Liu Y, Qiu L, et al. : Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020; 92:814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok LSC, Farahi N, Juss JK, et al. : Effects of tocilizumab on neutrophil function and kinetics. Eur J Clin Invest. 2017; 47:736–745 [DOI] [PubMed] [Google Scholar]
- 15.Gibiansky L, Frey N: Linking interleukin-6 receptor blockade with tocilizumab and its hematological effects using a modeling approach. J Pharmacokinet Pharmacodyn. 2012; 39:5–16 [DOI] [PubMed] [Google Scholar]
- 16.Fonseca JE, Santos MJ, Canhão H, et al. : Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009; 8:538–542 [DOI] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group: Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021; 397:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators: Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020; 383:2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salama C, Han J, Yau L, et al. : Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021; 384:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon AC, Mouncey PR, Al-Beidh F, et al. : Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry BM, de Oliveira MHS, Benoit S, et al. : Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med. 2020; 58:1021–1028 [DOI] [PubMed] [Google Scholar]
- 22.Ye W, Chen G, Li X, et al. : Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020; 21:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisman DE, Ronner L, Pinotti R, et al. : Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020; 8:1233–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappert K, Jahić A, Tauber R: Assessment of serum ferritin as a biomarker in COVID-19: Bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers. 2020; 25:616–625 [DOI] [PubMed] [Google Scholar]
- 25.Ruscitti P, Di Benedetto P, Berardicurti O, et al. : Pro-inflammatory properties of H-ferritin on human macrophages, ex vivo and in vitro observations. Sci Rep. 2020; 10:12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulte-Schrepping J, Reusch N, Paclik D, et al. ; Deutsche COVID-19 OMICS Initiative (DeCOI): Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020; 182:1419–1440.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teachey DT, Lacey SF, Shaw PA, et al. : Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016; 6:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy EH, De Benedetti F, Takeuchi T, et al. : Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020; 16:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.