OBJECTIVES:
High-flow nasal cannula is widely used in acute hypoxemic respiratory failure due to coronavirus disease 2019, yet data regarding its effectiveness is lacking. More evidence is needed to guide patient selection, timing of high-flow nasal cannula initiation, and resource allocation. We aimed to assess time to discharge and time to death in severe coronavirus disease 2019 in patients treated with high-flow nasal cannula compared with matched controls. We also evaluated the ability of the respiratory rate-oxygenation ratio to predict progression to invasive mechanical ventilation.
DESIGN:
Time-dependent propensity score matching was used to create pairs of individuals who were then analyzed in a Cox proportional-hazards regression model to estimate high-flow nasal cannula’s effect on time to discharge and time to death. A secondary analysis excluded high-flow nasal cannula patients intubated within 6 hours of admission. A Cox proportional-hazards regression model was used to assess risk of invasive mechanical ventilation among high-flow nasal cannula patients stratified by respiratory rate-oxygenation.
SETTING:
The five hospitals of the Johns Hopkins Health System.
PATIENTS:
All patients who were admitted with a laboratory-confirmed diagnosis of coronavirus disease 2019 were eligible for inclusion.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
High-flow nasal cannula was associated with longer median time to discharge: 10.6 days (interquartile range, 7.1–15.8 d) versus 7.8 days (interquartile range, 4.9–12.1 d). Respiratory rate-oxygenation index performed poorly in predicting ventilation or death. In the primary analysis, there was no significant association between high-flow nasal cannula and hazard of death (adjusted hazard ratio, 0.79; 95% CI, 0.57–1.09). Excluding patients intubated within 6 hours of admission, high-flow nasal cannula was associated with reduced hazard of death (adjusted hazard ratio, 0.67; 95% CI, 0.45–0.99).
CONCLUSIONS:
Among unselected patients with severe coronavirus disease 2019 pneumonia, high-flow nasal cannula was not associated with a statistically significant reduction in hazard of death. However, in patients not mechanically ventilated within 6 hours of admission, high-flow nasal cannula was associated with a significantly reduced hazard of death.
Keywords: comparative effectiveness research, coronavirus disease 2019, critical care outcomes, noninvasive ventilation, pneumonia, viral, respiratory distress syndrome, adult
Patients with coronavirus disease 2019 (COVID-19) pneumonia frequently develop acute hypoxemic respiratory failure (AHRF) (1). High-flow nasal cannula (HFNC) is widely used with the goal of preventing or minimizing duration of invasive mechanical ventilation (IMV) (2). While one report suggests that HFNC may reduce need for IMV (3), evidence for HFNC in COVID-19 is limited.
Before COVID-19, two large randomized controlled trials of HFNC did not demonstrate a statistically significant reduction in the need for endotracheal intubation when compared with standard supplemental oxygen therapy (4, 5), although one showed a reduction in 90-day mortality (4). Meta-analyses, on the other hand, suggest that HFNC reduces the need for IMV (6, 7). Concerns persist that a subset of patients on HFNC may suffer worsened outcomes from delayed intubation (8, 9). Large tidal volumes and excessive work of breathing are hypothesized to cause volutrauma, harmful swings in transpulmonary pressures, and worsened edema through increased pulmonary transvascular pressures (10). Additionally, waiting until patients are more hypoxemic or in greater respiratory distress may exacerbate respiratory muscle fatigue and increase peri-intubation cardiac events (8). These concerns parallel those related to noninvasive ventilation, where intubation after more than 48 hours has been associated with higher mortality (11).
As the benefits of HFNC may be outweighed by the harms associated with delayed intubation, further study should define its optimal use. Consequently, prognostic indices have been developed to predict HFNC “failure” (i.e., progression to IMV). Perhaps the best known index is the respiratory rate-oxygenation (ROX) ratio, the ratio of oxygen saturation/Fio2 (Spo2/Fio2) to respiratory rate (RR), an index developed specifically for AHRF secondary to pneumonia (12). In a validation prospective multicentered cohort, ROX index less than 3.85 after 12 hours had an area under the curve (AUC) of 0.75 for predicting need for IMV (9).
We conducted a retrospective review of 504 patients with severe COVID-19 treated with HFNC to compare their outcomes with matched controls who never received HFNC or received HFNC only after weaning from IMV. We also examined the ROX index’s ability to predict progression to IMV.
METHODS
Study Participants
This retrospective cohort study examined patients admitted to the five hospitals of the Johns Hopkins Health System (JHHS). This system includes two academic hospitals and three community hospitals with 2,513 beds (354 ICU beds). The institutional review board of these hospitals (IRB-3) approved this study as minimal risk and waived consent requirements (IRB00250975).
All patients admitted with a diagnosis of COVID-19 were eligible for inclusion. Diagnosis of COVID-19 required detection of severe acute respiratory syndrome coronavirus 2 nucleic acid or antigen and an International Classification of Diseases, 10th Revision code indicating symptomatic disease (Supplemental Table 1, http://links.lww.com/CCM/G792). Patients who were discharged or died within 24 hours of admission were excluded as they would be unlikely to benefit from HFNC in the presence of either mild (early discharge) or terminal disease (death within 24 hr).
Data Collection
The primary data source was Johns Hopkins registry of patients with suspected or confirmed COVID-19 (JH-CROWN): The COVID-19 Precision Medicine Analytics Platform (PMAP) Registry. The Johns Hopkins PMAP collects demographic characteristics, medical history, symptoms, vital signs, respiratory events, laboratory results, and medications. Some patients have been included in other descriptions of the cohort (1, 13–19).
Outcomes
The primary outcome was time to death within 28 days from HFNC initiation. Patients discharged alive any time before 28 days were right censored at 28 days. Based on the experience of other cohorts of critically ill COVID-19 patients, these patients were assumed to be alive at 28 days (20). The secondary outcome was time to discharge. Time to IMV could not be compared as some control patients were already ventilated at the time of matching.
Our primary analysis included all patients with any HFNC exposure unless the exposure occurred only after a period of IMV. In a secondary analysis, patients intubated within 6 hours of admission orders were also excluded. A 6-hour cutoff was chosen to exclude patients intubated prior to or immediately upon arrival to the ICU. In all analyses, both the primary and secondary outcomes were stratified by degree of hypoxemia (Spo2/Fio2 < 200). An adapted version of the ROX index was evaluated to determine its ability to predict a composite outcome of ventilation or death 1 day and 7 days from HFNC initiation. This composite outcome was chosen to both include do not intubate (DNI) patients (for whom HFNC “failure” would result in death) and to adjust for death as a competing risk for those patients who died while on HFNC.
Statistical Analyses
To account for the nonrandomized administration of HFNC and variation in timing of HFNC initiation, time-dependent propensity score matching was used to create pairs of individuals (21, 22). Pairs consisted of one HFNC patient and a control patient who most closely matched this patient at the time of HFNC initiation (defined as time from admission to start of HFNC). Both time-invariant (fixed) and time-dependent (varying) covariates before the HFNC start time (for HFNC patients) and before right-censoring or last follow-up date (for control group patients) were used in a Cox regression model as predictors to obtain parameter estimates for the propensity score matching.
Time-invariant covariates included race, age, sex, body mass index (BMI), Charlson Comorbidity Index, and code status (i.e., “do not resuscitate”/”DNI” order). Time-dependent covariates included measures of disease severity such as Spo2/Fio2 ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, temperature, RR, and the nonrespiratory components of the Sequential Organ Failure Assessment (SOFA) score (23). Other time-dependent variables included laboratory results, such as C-reactive protein, absolute lymphocyte count, platelet count, WBC count, hemoglobin, albumin, alanine aminotransferase, estimated glomerular filtration rate, and d-dimer. To adjust for secular trends (beyond just remdesivir and dexamethasone receipt), patients were also matched according to whether they were among the first or second half of all patients treated (i.e., admitted before or after June 1, 2020). Early June marked the beginning of widespread corticosteroid use that coincided with a sharp reduction in mortality rates (24).
Beginning from hospital admission day (day 0), a sequential 1:1 greedy matching without replacement was conducted (13). A patient who started HFNC at a given day t was matched with a control patient at day t if they had similar propensity scores. Day t refers to the day of hospitalization, starting with day 0. Analyses were then performed on the matched sets. Cox proportional-hazards regression models were applied to estimate the effect of HFNC on time to discharge and time to death. The same set of demographic, clinical, and laboratory variables were included in both models. Remdesivir and corticosteroid exposure were included as covariates.
In addition, four sensitivity analyses were performed. First, hazard ratios were stratified by site (academic versus community). Second, to explore HFNC’s effect on patients who progressed to IMV, hazard ratios were calculated for these patients compared with their matched controls. Third, to assess the effect of excluding patients who died within 24 hours, hazard ratios for death were recalculated with their inclusion. Finally, 90-day inhospital mortality was compared (death vs discharge alive) to include deaths in patients hospitalized beyond 28 days. In this analysis, inhospital death was compared with discharge alive as postdischarge vital status was inaccessible and no assumption of 90-day survival was made. Sensitivity analyses were performed with the primary analysis and included all patients with HFNC exposure except as previously specified.
To assess the ROX index in predicting ventilation or death, a Cox proportional-hazards regression model was applied to HFNC patients starting at HFNC initiation. ROX at 12 hours post-HFNC start was used. If it was not available, the nearest ROX value from the preceding 5 hours was used. Other pertinent clinical, demographic, and laboratory variables were selected using the least absolute shrinkage and selection operator regularization method (25). Three-hundred forty-six of 462 patients (75%) were randomly selected as training data to fit the model, and the rest were used to draw the receiver operating characteristic curve and test model performance. This process was repeated 100 times to determine average performance.
Missing values were imputed using the last observation carried forward if the last observation was within 3 days of the missing data, otherwise, using multiple imputation by chained equations with predictive mean matching method (26). p values were not adjusted for multiple comparisons. All secondary analyses are therefore intended to be hypothesis generating. Data were analyzed using R, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
RESULTS
Patients Characteristics
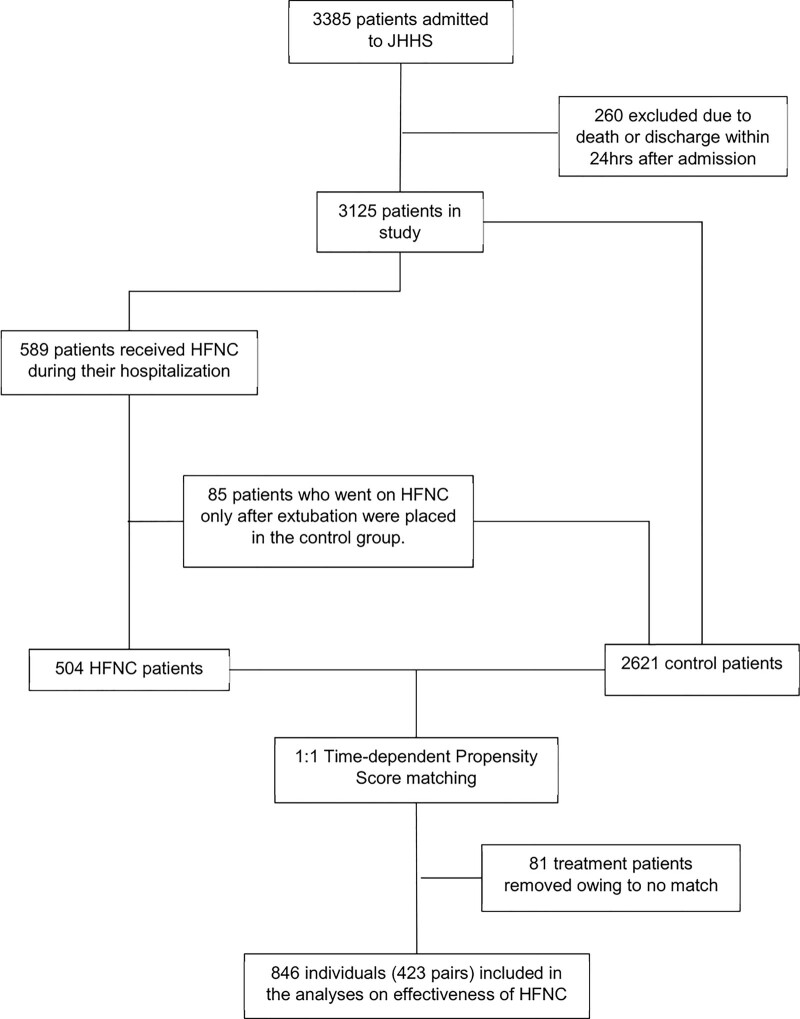
Of 3,385 patients admitted to JHHS between March 4, 2020, and November 28, 2020, with COVID-19, 260 were excluded due to death or discharge within 24 hours from admission. The remaining 3,125 patients were analyzed. There were 589 patients (18.8%) who received HFNC during their admission. Of these, 85 (14.4%) were ventilated before receiving HFNC (i.e., received HFNC only after extubation) and thus were included in the control group (Fig. 1). Median time to initiation of HFNC from admission was 0.8 days (interquartile range [IQR], 0.1–2.7 d). After time-dependent propensity score matching, 423 patients (83.9%) in the HFNC group were matched successfully, and together with their matched controls were selected for the primary analysis. After excluding patients intubated within 6 hours of admission (166 patients), 327 (67.0%) were matched successfully. Baseline characteristics of all patients and of HFNC and control patients on day of matching are shown in Table 1. Nearly half had diabetes and/or obesity. Almost 70% were from underrepresented minorities (35% non-LatinX Black and 25% LatinX). Intubation rates were similar in both groups: 48.0% (203 patients) of HFNC patients and 42.8% (181 patients) of matched controls required intubation. Among patients who required IMV, median time from admission to IMV was longer for HFNC patients (2.7 d; IQR, 1.1–5.0 d) than for control patients (0.8 d; IQR, 0–1.3 d). Among HFNC patients, average maximum Fio2 was 0.80 (sd 0.22) and average maximum flow rate was 42.5 L (sd 13.7 L).
Figure 1.
Patient cohort and controls. HFNC = high-flow nasal cannula, JHHS = Johns Hopkins Health System.
Table 1.
Patient Characteristics
| Characteristics | All Patientsa | Propensity Score-Matched Patientsb | ||
|---|---|---|---|---|
| All HFNC (n = 504) | All Control (n = 2,621) | Matched HFNC (n = 423) | Matched Control (n = 423) | |
| Demographics | ||||
| Female, n (%) | 220 (43.7) | 1,316 (50.2) | 190 (44.9) | 181 (42.8) |
| Black, n (%) | 176 (34.9) | 944 (36) | 146 (34.5) | 147 (34.8) |
| Hispanic, n (%) | 128 (25.4) | 618 (23.6) | 106 (25.1) | 98 (23.2) |
| Body mass index, median (IQR) | 29.7 (25.6–35.3) | 28.6 (23.3–33.8) | 29.6 (4.9) | 29.3 (4.8) |
| Age, median (IQR) | 64 (52–73) | 60 (44–74) | 64 (10.2) | 65 (13) |
| Nonrespiratory Sequential Organ Failure Assessment score, mean (sd) | 3.3 (2.9) | 2.0 (2.3) | 3.5 (3.0) | 3.6 (3.5) |
| Do not resuscitate/do not intubate, n (%) | 166 (32.9) | 492 (18.8) | 143 (33.8) | 150 (35.5) |
| Vital signs, mean (sd) | ||||
| Fio2 | 0.58 (0.29) | 0.31 (0.19) | 0.8 (0.22) | 0.64 (0.29) |
| Oxygen saturation/Fio2 | 311.4 (124.2) | 427.6 (98.7) | 251.6 (93.6) | 254.2 (103) |
| Systolic BP (mm Hg) | 107.1 (18.9) | 110.9 (19) | 101 (18.1) | 102 (19.7) |
| Diastolic BP (mm Hg) | 59 (11.3) | 61.3 (11.7) | 55.3 (11) | 55.8 (11.2) |
| Pulse (beats/min) | 100.4 (19.4) | 96.2 (19.5) | 103.2 (20.5) | 102.9 (22.6) |
| Laboratory results, mean (sd) | ||||
| C-reactive protein (mg/dL) | 13.6 (8.7) | 8 (7.9) | 13.8 (9) | 12.8 (10.3) |
| Absolute lymphocyte count (K cells/mm3) | 0.9 (0.9) | 1.3 (6) | 1 (1) | 1 (0.7) |
| Platelets count (K cells/mm3) | 212.3 (90.1) | 212.7 (91.2) | 231.1 (100) | 226.1 (94) |
| WBC count (K cells/mm3) | 8.4 (4.2) | 8.1 (9.1) | 9.2 (4.5) | 9.1 (4.7) |
| Hemoglobin (g/dL) | 12.4 (2.2) | 12.2 (2.3) | 11.9 (2.2) | 11.9 (2.2) |
| Albumin (g/dL) | 3.3 (0.6) | 3.6 (0.6) | 3.1 (0.5) | 3.1 (0.6) |
| Alanine aminotransferase (U/L) | 48.8 (55.2) | 49.5 (160.3) | 49.8 (56.4) | 50.4 (61.1) |
| Estimated glomerular filtration rate (mL/min) | 69.8 (33.5) | 75.5 (35) | 73.5 (34.5) | 73.1 (35.1) |
| d-dimer (mg/L fibrinogen equivalent units) | 2.4 (5.4) | 2.1 (4.2) | 2.9 (5.6) | 2.9 (5.3) |
| Past diagnoses, n (%) | ||||
| Hypertension | 338 (67.1) | 1,537 (58.6) | 288 (68.1) | 273 (64.5) |
| Coronary artery disease | 252 (50) | 1,044 (39.8) | 215 (50.8) | 219 (51.8) |
| Congestive heart failure | 161 (31.9) | 490 (18.7) | 141 (33.3) | 108 (25.5) |
| Chronic kidney disease | 120 (23.8) | 554 (21.1) | 105 (24.8) | 96 (22.7) |
| Diabetes | 251 (49.8) | 944 (36) | 216 (51.1) | 181 (42.8) |
| Asthma | 76 (15.1) | 347 (13.2) | 64 (15.1) | 55 (13) |
| Chronic obstructive pulmonary disease/chronic lung disease | 177 (35.1) | 672 (25.6) | 149 (35.2) | 128 (30.3) |
| Cancer | 54 (10.7) | 304 (11.6) | 48 (11.3) | 58 (13.7) |
| Liver disease | 76 (15.1) | 330 (12.6) | 67 (15.8) | 61 (14.4) |
| Charlson Comorbidity Index | ||||
| 0 | 105 (20.8) | 737 (28.1) | 82 (19.4) | 84 (19.9) |
| 1–4 | 332 (65.9) | 1,599 (61) | 283 (66.9) | 280 (66.2) |
| ≥ 5 | 67 (13.3) | 285 (10.9) | 58 (13.7) | 59 (13.9) |
| Concomitant medications, n (%) | ||||
| Hydroxychloroquine | 76 (15.1) | 337 (12.9) | 70 (16.5) | 95 (22.5) |
| Azithromycin | 243 (48.2) | 829 (31.6) | 204 (48.2) | 199 (47.0) |
| Corticosteroids | 318 (63.1) | 802 (30.6) | 249 (58.9) | 190 (44.9) |
| Remdesivir | 257 (51) | 539 (20.6) | 194 (45.9) | 133 (31.4) |
BP = blood pressure, HFNC = high-flow nasal cannula, IQR = interquartile range.
aData shown is from day 0 of hospital admission.
bData shown is from the day of HFNC initiation or matched day.
Overall, patients receiving HFNC were more likely to be male, had more comorbidities, and were more likely to receive remdesivir and corticosteroids. Differences in clinical characteristics were minimal among propensity score-matched patients, although matched HFNC patients were modesty more likely to receive corticosteroids and remdesivir than matched controls.
Mortality
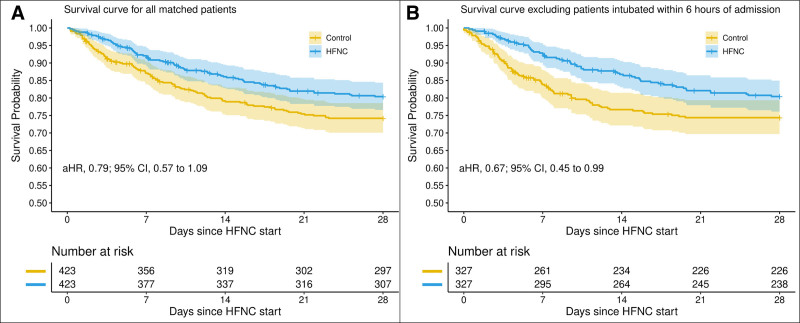
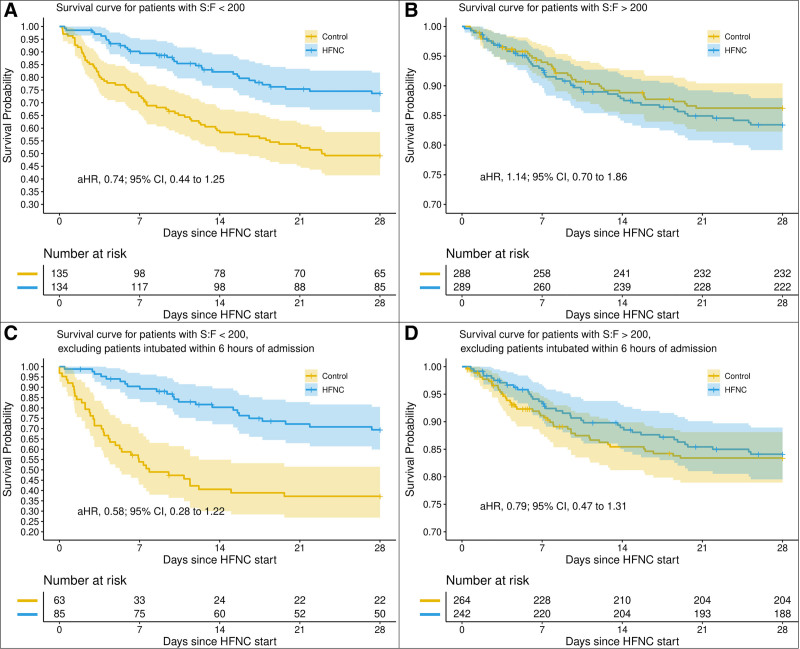
Patients in the HFNC group had a 28-day mortality of 18.7% (79 deaths) compared with 25.1% (106 deaths) among the matched controls. In the primary analysis, HFNC was associated with a trend toward reduced hazard of death that was not statistically significant (adjusted hazard ratio [aHR], 0.79; 95% CI, 0.57–1.09; median 6.9 d [IQR, 2.6–12.2 d] compared with 8.5 d [IQR, 4.3–15.0 d]) (Fig. 2). This trend was most evident in patients with Spo2/Fio2 ratio less than 200 (aHR, 0.74; 95% CI, 0.44–1.25). In the secondary analysis, in which patients who were intubated within 6 hours of admission were excluded, there was a significant decrease in the hazard of death (aHR, 0.67; 95% CI, 0.45–0.99). Subgroup analysis suggests that this association may be more pronounced in patients with Spo2/Fio2 less than 200 (aHR, 0.58; 0.95% CI, 0.28–1.22) (Fig. 3).
Figure 2.
Primary analyses of mortality according to high-flow nasal cannula (HFNC) exposure. These Kaplan-Meier curves depict cumulative survival in (A) patients with any HFNC exposure and their matched controls as well as in (B) HFNC patient not intubated within 6 hr of admission and their matched controls. p value of less than 0.05 for comparison between HFNC patients not intubated within 6 hr of admission and their matched controls. aHR = adjusted hazard ratio.
Figure 3.
Primary analyses of mortality according to high-flow nasal cannula (HFNC) exposure, stratified by degree of hypoxemia. Similar to Figure 2, these Kaplan-Meier curves depict cumulative survival in patients with any HFNC exposure and in HFNC patients not intubated within 6 hr of admission as well as their respective matched controls. However, in this iteration, patients have also been matched by their degree of hypoxemia (ratio of oxygen saturation/Fio2 [S/F] > 200 or < 200). p value of less than 0.05 for comparison between the more hypoxemic HFNC patients not intubated within 6 hr of admission and their matched controls. aHR = adjusted hazard ratio.
In the sensitivity analyses, hazard ratios were similar across hospital type (academic vs community), when only patients who progressed to IMV were examined, and with the inclusion of patients who died within 24 hours of admission (Supplemental Figs. 1–3, http://links.lww.com/CCM/G792). Finally, the hazard ratio for inhospital death versus discharge alive before 90 days was also similar (Supplemental Fig. 7, http://links.lww.com/CCM/G792).
Time to Discharge
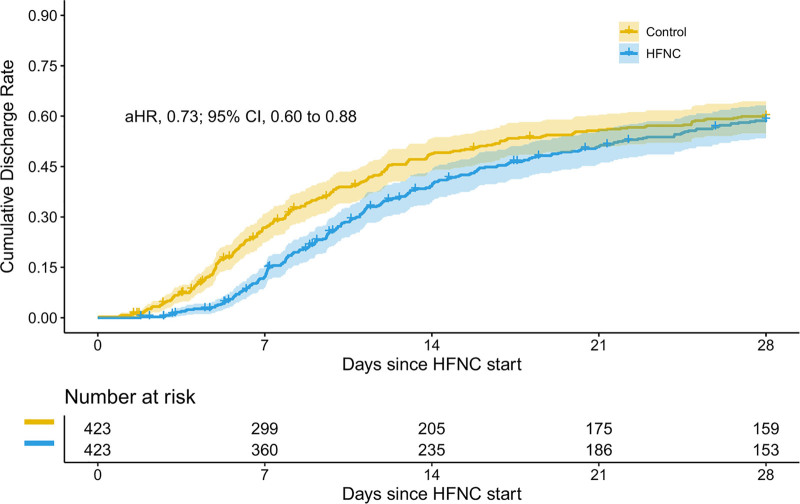
In the primary analysis, median time to discharge from treatment initiation was 10.6 days (IQR, 7.1–15.8 d) for HFNC patients and 7.8 days (IQR, 4.9–12.1 d) for matched controls (aHR, 0.73; 95% CI, 0.60–0.88) (Fig. 4). In the secondary analysis, median time to discharge from treatment initiation was 10.2 days (IQR, 7.1–16.0 d) for HFNC patients and 7.1 days (IQR, 4.4–11.4 d) for controls (aHR, 0.57; 95% CI, 0.46–0.70). Hazard ratios were similar across all sensitivity analyses (Supplemental Figs. 3–5, http://links.lww.com/CCM/G792).
Figure 4.
Cumulative discharge rates. This figure demonstrates that time to discharge was significantly longer among high-flow nasal cannula (HFNC) patients. However, cumulative discharge rates begin to converge between days 14 and 21. aHR = adjusted hazard ratio.
Predictive Ability of ROX Index
Of 504 HFNC patients, 462 patients (91.7%) had a ROX recorded within 5–12 hours from HFNC initiation. Three-hundred eighty-eight patients (84.0%) had a ROX index greater than 3.85 at 5–12 hours following HFNC initiation. The rate of ventilation or death at 28 days in this group was 54.6% compared with 73.0% among patients with ROX less than 3.85. A ROX index greater than 3.85 in the 12 hours following HFNC initiation was associated with a significantly lower hazard of ventilation or death (aHR, 0.60; 95% CI, 0.41–0.87). However, this association did not translate to high discrimination using ROX as a stand-alone measure, irrespective of the cutoff chosen. A model only using ROX index (ROX > 3.85 or not) had an AUC for predicting ventilation or death at 1 day and at 7 days of 0.58 and 0.55, respectively. A model using ROX index as well as demographic, clinical, and laboratory variables had higher AUCs for predicting ventilation or death by 1 day (0.73 d) and 7 days (0.71 d) from HFNC initiation (Supplemental Fig. 6, http://links.lww.com/CCM/G792).
DISCUSSION
In our primary analysis, the use of HFNC was not associated with a reduction in the hazard of death at 28 days. However, if patients intubated within 6 hours of admission were excluded, there was a statistically significant reduction in the hazard of death in the patients who received HFNC (aHR, 0.67; 95% CI, 0.45–0.99). These findings suggest that HFNC may be associated with a mortality benefit in properly selected patients (i.e., patients with severe hypoxemia but who are at low risk for immediate progression to IMV). In contrast to several earlier reports of HFNC in COVID-19–associated AHRF (27–31), we found that a ROX index less than 3.85 was a poor predictor of progression to IMV. However, the ROX index did have some utility in predicting progression to IMV when incorporated into a model with other clinical, laboratory, and demographic variables.
Interestingly, we observed lower mortality in the HFNC group despite similar rates of IMV and longer length of stay. While we were unable to calculate a hazard ratio of progression to IMV in the control group, the similar rates of IMV in both groups make it unlikely that any reduction in mortality was due to a reduced need for IMV. The seemingly paradoxical findings of reduced mortality with similar intubation rates and increased length of stay suggest the possibility that HFNC may improve outcomes by simply delaying intubation until it is medically necessary. These findings contradict those of a retrospective single-center observational study by Kang et al (8) that examined HFNC use in ICU patients with a variety of causes of respiratory failure. That group found that late HFNC “failure” (i.e., progression to IMV after more than 48 hr of HFNC therapy) was associated with higher ICU mortality compared with patients who progressed from HFNC to IMV in less than 48 hours. In contrast to their study, our study focused on a more homogenous cohort of patients with acute respiratory distress syndrome (ARDS), and we employed time-dependent propensity score matching. In addition, we examined all HFNC patients, regardless of whether or they not they progressed to IMV. Interestingly, in our cohort, HFNC was still associated with reduced mortality in a sensitivity analysis restricted to patients who progressed to IMV. This was true even though it was common for these patients to be treated with HFNC for greater than 48 hours.
There are multiple physiologically plausible reasons for which delaying intubation, when safe, may be beneficial. For example, COVID-19 patients may be at higher risk for ventilator-induced lung injury (VILI) (32) in the early “exudative” phase of ARDS. A notable feature of the “exudative” phase is surfactant dysfunction caused by alveolar edema and by alveolar epithelial cell necrosis. Postponing IMV until there is incipient alveolar type II cell hyperplasia (“proliferative” phase) associated with an improved epithelial barrier and increased surfactant (33) may minimize ventilator-associated atelectotrauma. Thus, postponing IMV could reduce VILI and its downstream effects including mortality. This question merits further investigation. At the very least, we would challenge the notion that progression to IMV be characterized as HFNC “failure.”
It should be noted, however, that patients in the HFNC group had longer times to discharge. Thus, while HFNC receipt may improve survival, its use may also contribute to longer hospital stays. Longer hospital stays may be due in part to greater survival in the HFNC group as ARDS survivors have longer hospital stays than nonsurvivors (34).
To our knowledge, this is the largest cohort study investigating outcomes related to HFNC use in COVID-19. The rates of intubation and death for patients receiving HFNC in our cohort were 47.0% and 22.4%, respectively, which are comparable to those of previously published cohorts of patients with AHRF due to COVID-19 (3, 27–31, 35). These cohorts include a retrospective cohort reported by Demoule et al (3) that also used propensity score matching to compare outcomes between HFNC and matched controls. Interestingly, despite similar rates of IMV and death in their HFNC group, they found that while HFNC use was associated with a decreased risk of intubation, it was not associated with decreased mortality. Compared with their cohort, our cohort had a modestly higher BMI and significantly higher reported rates of congestive heart failure and chronic lung disease.
The strengths of our study include its size and the rigorous time-dependent propensity score matching done for a wide range of clinical parameters including SOFA score, a validated ICU prognostic score. To control for secular trends, patients were matched by whether they were among the first or second half of patients admitted in the study period. To control for other treatments that may affect mortality, exposure to remdesivir and corticosteroids were included as covariates. In addition, sensitivity analyses were conducted to investigate differences between hospitals and to explore the effect of HFNC in the subset of patients who progressed to IMV.
This study has several limitations. It is retrospective and limited to a single healthcare system. It did not assess longitudinal data relating to putative HFNC physiologic benefits (e.g., improvements in oxygenation, RR, breathing pattern) or subjective benefits (e.g., relief of dyspnea). In addition, time from symptom onset could not be universally determined and thus was not incorporated in the matching. As control patients could be matched after intubation, we were unable to calculate hazard ratios for risk of intubation. Furthermore, some of the parameters upon which matching occurred (e.g., RR, Spo2/Fio2 ratio, SBP, DBP) were affected by ventilation itself as well as concomitant sedation. However, on the whole, these interventions (e.g., application of positive end-expiratory pressure) likely improved these variables, making the ventilated patients appear less ill.
Finally, while intubation was never delayed due to a paucity of staff or ventilators, the threshold for intubation likely changed over the course of the study period as evidence mounted that HFNC was not associated with excessive aerosolization and infectious risk (36) and as clinicians became more comfortable with managing COVID-19 patients with HFNC. More conservative use of HFNC earlier in the pandemic may have limited its effectiveness in reducing the need for IMV or in reducing mortality, thus biasing against a treatment effect.
CONCLUSIONS
In a retrospective analysis of HFNC in 504 patients with severe COVID-19, treatment with HFNC was not associated with reduced hazard of death within 28 days. However, in a secondary analysis excluding patients who were intubated within 6 hours of admission, HFNC receipt was significantly associated with reduced hazard of death. Prospective randomized controlled trials of HFNC in COVID-19 and other viral pneumonias are needed to further explore this association.
ACKNOWLEDGMENTS
The data used for this publication were part of the JH-CROWN: The COVID PMAP Registry, which is based on the contribution of many patients and clinicians. We would like to thank the Johns Hopkins Health System and its surrounding communities for working together to provide outstanding patient care and to keep each other safe during these extraordinary times.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Impact Statement: This study adds much needed data about and analysis of the effectiveness of high-flow nasal cannula (HFNC) for acute hypoxemic respiratory failure in severe coronavirus disease 2019 pneumonia. HFNC is widely used for this indication despite uncertainty surrounding patient selection, timing, and resource allocation. The results of this study support the use of HFNC in appropriately selected patients.
Supported, in part, by funding from Hopkins inHealth, the Johns Hopkins Precision Medicine Program through JH-CROWN and the Coronavirus Disease 2019 Administrative Supplement for the Health and Human Services Region 3 Treatment Center from the Office of the Assistant Secretary for Preparedness and Response.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Garibaldi BT, Fiksel J, Muschelli J, et al. : Patient trajectories among persons hospitalized for COVID-19: A cohort study. Ann Intern Med. 2021; 174:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W, Moller MH, Arabi YM, et al. : Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020; 49:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demoule A, Vieillard Baron A, Darmon M, et al. : High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020; 202:1039–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network: High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E, Lemiale V, Mokart D, et al. : Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: The HIGH randomized clinical trial. JAMA. 2018; 320: 2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreyro BL, Angriman F, Munshi L, et al. : Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: A systematic review and meta-analysis. JAMA. 2020; 324:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochwerg B, Granton D, Wang DX, et al. : High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019; 45:563–572 [DOI] [PubMed] [Google Scholar]
- 8.Kang BJ, Koh Y, Lim CM, et al. : Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015; 41:623–632 [DOI] [PubMed] [Google Scholar]
- 9.Roca O, Caralt B, Messika J, et al. : An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019; 199:1368–1376 [DOI] [PubMed] [Google Scholar]
- 10.Marini JJ, Gattinoni L: Management of COVID-19 respiratory distress. JAMA. 2020; 323:2329–2330 [DOI] [PubMed] [Google Scholar]
- 11.Moretti M, Cilione C, Tampieri A, et al. : Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000; 55:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roca O, Messika J, Caralt B, et al. : Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016; 35:200–205 [DOI] [PubMed] [Google Scholar]
- 13.Ignatius EH, Wang K, Karaba A, et al. : Tocilizumab for the treatment of COVID-19 among hospitalized patients: A matched retrospective cohort analysis. Open Forum Infect Dis. 2021; 8:ofaa598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery RK, Chiang TP, Marr KA, et al. : Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: A retrospective cohort. Am J Transplant. 2020; 21:2498–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaba SM, Jones G, Helsel T, et al. : Prevalence of co-infection at the time of hospital admission in COVID-19 patients, a multicenter study. Open Forum Infect Dis. 2021; 8:ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen KM, Mehta HB, Palamuttan N, et al. : Association between chronic use of immunosuppressive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: A retrospective cohort study in a large US health system. Clin Infect Dis. 2021;ciaa1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wongvibulsin S, Garibaldi BT, Antar AAR, et al. : Development of severe COVID-19 adaptive risk predictor (SCARP), a calculator to predict severe disease or death in hospitalized patients with COVID-19. Ann Intern Med. 2021; 174:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garibaldi BT, Wang K, Robinson ML, et al. : Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021; 4:e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulanger M, Molina E, Wang K, et al. : Peripheral plasma cells associated with mortality benefit in severe COVID-19: A marker of disease resolution. Am J Med. 2021; 134:1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators: Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020; 180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B: Propensity score matching with time-dependent covariates. Biometrics. 2005; 61:721–728 [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Bowe B, Li T, et al. : Risk of death among users of proton pump inhibitors: A longitudinal observational cohort study of United States veterans. BMJ Open. 2017; 7:e015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 24.Bennett TD, Moffit RA, Hajagos JG, et al. : The National COVID Cohort Collaborative: Clinical characterization and early severity prediction. medRxiv. Preprint posted online January 23, 2021. doi: 10.1101/2021.01.12.21249511 [Google Scholar]
- 25.Tibshirani R: The lasso method for variable selection in the Cox model. Stat Med. 1997; 16:385–395 [DOI] [PubMed] [Google Scholar]
- 26.Van Buuren S, Groothuis-Oudshoorn K: mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011; 45:1–67 [Google Scholar]
- 27.Panadero C, Abad-Fernández A, Rio-Ramirez MT, et al. : High-flow nasal cannula for acute respiratory distress syndrome (ARDS) due to COVID-19. Multidiscip Respir Med. 2020; 15:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J, Zhang Y, Ni L, et al. : High-flow nasal oxygen in coronavirus disease 2019 patients with acute hypoxemic respiratory failure: A multicenter, retrospective cohort study. Crit Care Med. 2020; 48:e1079–e1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucman N, Mullaert J, Roux D, et al. ; Contributors: Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020; 46:1924–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel M CJ, Mills N, Marron R, et al. : ROX index predicts intubation in patients with COVID-19 pneumonia and moderate to severe hypoxemic respiratory failure receiving high flow nasal therapy. medRxiv. Preprint posted online July 3, 2020. doi: 10.1101/2020.06.30.20143867 [Google Scholar]
- 31.Calligaro GL, Lalla U, Audley G, et al. : The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine. 2020; 28:100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med. 2014; 370:980. [DOI] [PubMed] [Google Scholar]
- 33.Matthay MA, Zemans RL, Zimmerman GA, et al. : Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 35.Ferrando C, Mellado-Artigas R, Gea A, et al. ; COVID-19 Spanish ICU Network: Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: A multicenter, adjusted cohort study. Crit Care. 2020; 24:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loh NW, Tan Y, Taculod J, et al. : The impact of high-flow nasal cannula (HFNC) on coughing distance: Implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020; 67:893–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.