Abstract
Hepatocellular carcinoma (HCC) is the most common type of liver malignant tumor, with high recurrence and mortality rates. Mesenchymal stem cells (MSCs) are multipotent cells that can be recruited into the tumor microenvironment (TME). What is known, TME plays a vital part in tumor progression. In recent years, accumulating studies have found that MSCs have a dual role of promotion and inhibition in the occurrence and development of HCC. In this review, we analyzed the role of MSCs in TME and summarized the relationship between MSCs and liver cancer stem cells, the molecular signaling pathway mechanisms of MSCs promoting and inhibiting HCC, and the latest research progress of MSCs in the treatment of HCC.
Keywords: combination therapy, hepatocellular carcinoma, liver cancer stem cells, mesenchymal stem cells, signaling pathway, tumor microenvironment
The relationship between mesenchymal stem cells (MSCs) and hepatocellular carcinoma (HCC) is revealed comprehensively, including the molecular signaling pathway mechanisms of MSCs promoting and inhibiting HCC, the relationship between MSCs and liver cancer stem cells, and the latest research progress of MSCs in the treatment of HCC.
1. INTRODUCTION
Primary liver cancer is the seventh most common cancer and the second most common cause of cancer death in the world. 1 Globally, hepatocellular carcinoma (HCC) is the main histological type of liver cancer, accounting for more than 75% of the total number of liver cancers. 2 Liver cancer stem cells (LCSCs), recognized by certain surface markers, are responsible for the tumorigenesis, recurrence, metastasis, chemotherapy resistance and poor prognosis of HCC. 3
Increasing evidence supports the great effects of the tumor microenvironment (TME) on the generation, development and metastasis of HCC. TME is composed of diversified cells and non‐cellular components. 4 Mesenchymal stem cells (MSCs), an integral part of TME, are considered to be a key factor in tumor progression and metastasis. MSCs are also referred to as “mesenchymal stromal cells”, which can further be induced to differentiate into osteoblasts, chondrocytes, adipocytes, and other cells in vitro. 5 Recently, massive research has been devoted to exploring the association between HCC and MSCs. The function of MSCs in the occurrence, development and treatment of HCC is quite controversial. A growing body of research illustrated that MSCs have the dual characteristics of suppressing and promoting tumors through different molecular signaling mechanisms. Even in different stages of HCC, MSCs also play a contradictory role. In the early stage of HCC, they can reduce DNA damage and ROS accumulation to play a tumor suppressor effect. However, in the late stage, MSCs manifested as a tumor promoter on HCC by promoting the stem cell‐like properties and epithelial‐mesenchymal transition (EMT). 6
Abundant studies results in vitro or in vivo suggest that MSCs increase the number and tumorigenicity of CSCs in varied tumors, including human ovarian tumors, 7 breast cancer, 8 prostate cancer, 9 Ewing's sarcoma, 10 and colorectal Tumor, 11 etc. Undoubtedly, HCC is no exception. In addition, the intrinsic ability of MSCs to treat HCC has been reported. Therefore, in this review, we stressed the role of MSCs in TME, the links between MSCs and LCSCs, the molecular signaling pathway mechanisms of MSCs acting on HCC (Table 1), and the current research status of MSCs in the treatment of HCC (Table 2).
TABLE 1.
Mesenchymal stem cells (MSCs) promote or inhibit hepatocellular carcinoma (HCC) progression
| Dual function | MSCs | Cell line | Impact on biological behavior | Mechanism | Reference |
|---|---|---|---|---|---|
| Promotion | hBMSCs | Bel7407, Huh7, LM3, Hep3B | Promote growth, migration and invasion | MAPK pathway, EMT, ITGA5 | 12 |
| hADMSCs | Hep3B, Huh7, HCCLM3 | Inhibit proliferation, promote migration and invasion | ROS/MAPK/HIF‐1α signaling pathway | 13 | |
| hUCMSCs | 7402, Hep3b | Promote growth | COX2/PGE2/EP4 axis, YAP, AKT/mTOR/SREBP1 pathway | 14 | |
| Canine ADMSCs | AZACH | Promote proliferation and invasion | TGFβ1, EGF, HGF, PDGFβ, VEGFA, IGF2 | 15 | |
| HCC‐MSCs | 293T, Hep3B, PLC, Huh7, HepG2, MHCC‐97L, HCC‐LM3, SMMC‐7721 | Promote EMT and tumorigenesis | lncRNA‐MUF, ANXA2, miR‐34a, Wnt/β‐catenin | 16 | |
| hBMSCs | Bel‐7404, HepG2 | Promote migration and invasion | IL‐6/STAT3 pathway | 17 | |
| BMSCs | SNU‐398 | Promote migration and invasion | AQP1 | 18 | |
| hBMSCs | SNU‐398 | Promote migration and invasion | CXCR4 | 19 | |
| UCMSCs | HCCLM3 | Promote migration and invasion | TGF‐β | 20 | |
| Inhibition | UCMSCs | H7402 | Inhibit growth, migration and angiogenesis | — | 21 |
| BMSCs, UCMSCs | HepG2 | Inhibit growth and proliferation | — | 22 | |
| hUCMSCs | CCl4‐induced mouse liver tumor | Inhibit growth | anti‐oxidation | 23 | |
| UCMSCs | HepG2 | Inhibit growth, promote apoptosis | AFP, Bcl‐2, Survivin | 24 | |
| hAMSCs | HepG2 | Inhibit proliferation, promote apoptosis | Wnt/β‐catenin and IGF‐1R/PI3K/AKT pathway | 25 | |
| MenSCs | HepG2, HuH‐7 | Inhibit growth | PI3K/AKT and MAPK pathway | 26 | |
| ADMSCs | HepG2/C3A/HB‐8065, PLC‐PRF‐5/CRL‐8024 | Inhibit proliferation, migration and invasion, promote apoptosis | P53, retinoblastoma, c‐Myc, hTERT, TIMP‐1/2/3 | 27 | |
| Continued inhibition | |||||
| Mouse BMSCs | The hepatic metastasis model of colorectal carcinoma | Suppress development, and promoted the anti‐tumor immunity | p65, iNOS, CD8+ T cells | 28 | |
TABLE 2.
Mesenchymal stem cells (MSCs) combination therapy for hepatocellular carcinoma (HCC)
| Combination therapy | Processing method | MSCs form | Mechanism | Effect | Reference |
|---|---|---|---|---|---|
| Combination chemotherapy | Sorafenib | MSCs | IL‐1, TNF–α, IL‐10 | Promote apoptosis and inhibit proliferation | 29, 30 |
| Adriamycin | MSC‐sFlt1 | Anti‐angiogenesis | Prevent growth and induce apoptosis | 31, 32 | |
| Sorafenib | MSCs‐CM | — | Inhibit growth | 33 | |
| Adriamycin | ADMSC‐Exo‐199a | mTOR pathway | Improve chemotherapy sensitivity | 34 | |
| Sorafenib | MSC‐Exo‐siGRP78 | Targeting GRP78 | Inhibit growth and invasion, reverse drug resistance | 35 | |
| Combination radiotherapy | Radiotherapy | AT‐MSCs | IFITM1, STAT3, MMPs, P53, P21, caspases | Inhibit growth, migration and invasion, and enhance the effect of RT treatment | 36 |
| I131 | SMAD‐NIS‐ MSCs | TGFB1 | Delay growth and prolong survival | 37, 38 | |
| Combination other therapy | Oncolytic adenovirus | MSCs | Extend virus cycle and improve safety | Enhance the efficacy of anti‐liver cancer | 39 |
| rAd‐Apoptin | MSCs/MSCs‐CM | — | Inhibit proliferation | 40 | |
| Mel | MSCs | Induces apoptosis and inhibits inflammation and oxidative stress | Promote the therapeutic potential of MSCs | 41 | |
| Vitamin D | MSCs | Inhibit TGF‐β pathway | Improve pathological images, liver function, and promote the recovery of liver parenchyma | 42 | |
| GPC3‐ENG | MSCs | Activate T cells and produce IL2 | Promote the killing of gpc3‐positive tumor cells | 43 | |
| rAd‐NK4 | MSCs | Erk1/2 | Inhibit growth and migration and tumor angiogenesis | 44 |
2. MSCs IN THE TME
As a member of TME, MSCs have been reported in various tumor tissues such as pancreatic cancer, colon cancer, breast cancer, and gastric cancer. 45 , 46 , 47 , 48 A large number of studies have found that MSCs can also home to liver TME. 13 , 16 MSCs usually exist in a variety of human tissues, including bone marrow, adipose tissue, liver, intestine, lung, connective tissue, spleen, skin, placenta, umbilical cord and other tissues. 49 Therefore, the homing property of MSCs makes the source of MSCs in TME diversified, including cells not only in liver situ but also recruited from a distance. Perhaps relatedly easier access, the current research on HCC‐related MSCs mainly obtained from bone marrow, fat, and umbilical cord.
The homing mechanisms of MSCs have been reported widely (Figure 1). Many soluble molecules secreted by hepatoma cells can induce MSCs migration toward HCC. Bayo et al. found that the migration of MSC to HCC is related to the chemotaxis axis of CXCL8/IL‐8, CXCL1‐2‐3/GRO, CCL2/MCP‐1 and AMF. Factors secreted by HCC regulate the chemotactic potential and gene profile of MSCs, thereby promoting their recruitment. 50 CCL15, secreted by human HCC, has a chemotactic effect on hMSCs in vivo and in vitro via the CCL15/CCR1 axis. 51 Lejmi et al. reported that MIP‐1δ and MIP‐3α are involved in the migration of pluripotent mesenchymal cells induced by liver cancer cells which may be related to the migration and evolution of MSCs to myofibroblasts around the tumor. 52 CXCR4/ CXCL12 and TGF‐β/TGF‐βR signals also play an important role in the migration of MSCs to HepG2 cells. 53 Interestingly, thyroid hormones can also increase hMSC migration to HCC stroma via integrin αvβ3. 54 Interestingly, Chengying et al. found that HCC cells up‐regulated the expression of EGF, CXCL9, CCL25, and MMP‐9 in vivo and in vitro, which promoted the preferential transplantation of MSCs to the metastatic area rather than the primary tumor site. 55 In addition, the induction of hypoxia and hyperthermia is also the reason for the enhancement of MSCs’ recruitment ability. 56 , 57 Although the phenomenon of pluripotent mesenchymal stem cells homing to tumors has been confirmed in succession, its tropism mechanism still needs to be further elucidated.
FIGURE 1.
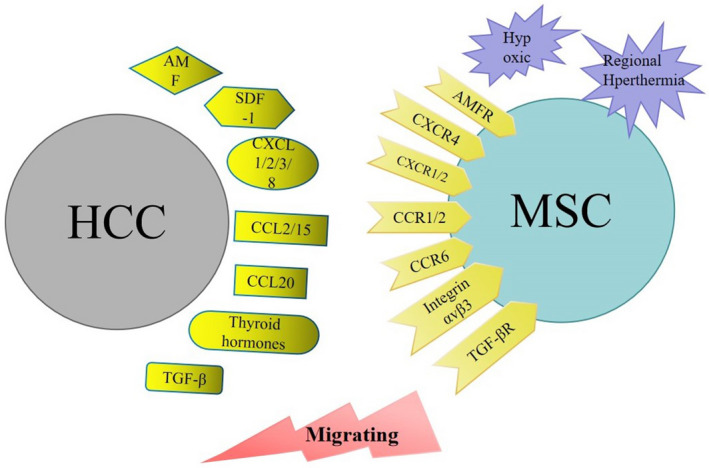
Chemotaxis mechanisms mediating mesenchymal stem cells (MSCs) migration to the hepatocellular carcinoma (HCC) microenvironment. MSCs. Chemokines in the TME (dark yellow) and external physical factors (purple) promote the migration of MSCs to the HCC microenvironment
More and more evidence shows that MSCs can promote immune suppression by secreting varieties of cytokines in TME, such as IL‐10, TGFβ, nitric acid, indoleamine 2,3 dioxygenase and prostaglandin E2. 58 , 59 IFN‐γ and TNF‐α also play important roles in the immunosuppression of MSCs, for example, inhibiting the differentiation of dendritic cells (DC) 60 and promoting the polarization of M2 macrophages, 61 , 62 , 63 thereby promoting tumor growth. 64 , 65 The direct cell‐to‐cell interaction between MSCs and natural killer cells (NK) changes the phenotype and inhibits proliferation and cytokine secretion of NK cells. 66 In addition, the soluble factors secreted by MSCs have been shown to inhibit the proliferation of T cells and b cells, while increasing the apoptosis of activated T cells. 67 , 68 , 69 Meaningfully, Tumor secreted factors induce MSCs to differentiate into CAF phenotype through the TGF‐β/Smad signaling pathway. 70 , 71 CAFs inhibit lymphocyte tumor infiltration, increase the activity of immunosuppressive regulatory T cells, and induce monocyte apoptosis to promote immune tolerance to the tumor environment. 72 , 73 , 74 In addition, it has been reported that CAFs damage the anti‐tumor function of T cells by activating neutrophils. 75 A large amount of evidence indicates that MSCs can suppress the immune response to support tumor growth, whether it is induced by direct cell interaction or indirect differentiation.
Interestingly, MSCs also play a role in suppressing tumors by positively regulating the immune response. MSCs inhibit tumor growth by increasing the infiltration of monocytes and granulocytes in TME. 76 In addition, bone marrow mesenchymal stem cells (BMSCs), which stimulate resting T cells and act as antigen‐presenting cells, activated by toll‐like receptor 3 (TLR3) enhance neutrophil function. 77 , 78 MSCs may also function in recruiting different immune groups into TME, changing the ratio of Treg and myeloid‐derived suppressor cells to CD8+ T cells, and shifting the balance to an anti‐tumor state. 79 GPC3‐ENG MSCs redirected T cells to gpc3‐positive tumor cells and induced antigen‐dependent tumor cell killing, and MSCs overexpressing sirt1 also attracted CD8+ T cells approach. 28 , 43
2.1. The relationship between MSCs and LCSCs
CSCs could be isolated from tumor cell lines transformed from MSCs, speculating the possibility of CSCs origin from mutant MSCs. 80 , 81 The mutual transformation and influential mechanism of MSCs and CSCs have been reported in various tumors. For instance, the soluble mediator secreted by adipose‐derived mesenchymal stem cells (ADMSCs) advance cell proliferation and the phosphorylation of PI3K/Akt and MAPK signal‐related proteins in bladder cancer stem cell‐like cells. 82 Lung adenocarcinoma stem cells are positively controlled by MSCs derived from Wharton´s Jelly (WJMSCs) through a paracrine mechanism. 83
The relationship between MSCs and LCSCs has also been demonstrated. Studies have found that the phenotype and genotype of BMSCs are changed through HCC cells paracrine effects, generating a population of spherical stem cells with CSCs characteristics termed cancer‐induced stem cells (CiSCs). The Wnt/β‐catenin signaling pathway and TGF‐β may exert an enormous function on the transformation of BMSCs to CiSCs. 84 SK cells are considered to be a human liver cancer cell line with properties of mesenchymal origin. Not only can they differentiate into osteoblasts and adipocytes like BMSCs and ADMSCs, but they show the relative homogeneity of CSCs, suggesting the feasibility of normal MSCs polarized into LCSCs with metastasis and self‐renewal capabilities. 85 HCC cells turn into a more aggressive phenotype when co‐cultured with MSCs, which promotes HCC stemness and tumor metastasis. 16 It has been reported that the lncRNA HULC and lncRNA MALAT‐1 play a certain part in both LCSCs and MSCs. LCSCs growth is boosted by both lncRNAs, and the synergistic effect of the two is more noticeable. 86 MSCs overexpressed HULC represent stronger capabilities of proliferation, migration and invasion. Meaningfully, the proliferation, angiogenesis and immunosuppressive properties of MSCs are prominent by MALAT1 inducing VEGF and IDO. 87 , 88 Therefore, HULC and MALAT‐1 may be potential mechanisms in the interaction of MSCs and LCSCs. In addition, Hou et al. documented that irradiated MSCs (IR‐MSCs) can maintain the stemness of LCSCs through the Wnt/β‐catenin signaling pathway. 89 In addition to HCC, exosomal miR‐126 derived from hepatoblastoma cells promotes the occurrence of hepatoblastoma by inducing BMSCs to differentiate into CSCs. 90
3. MECHANISMS OF MSCs PROMOTING THE PROGRESSION OF HCC
3.1. The MAPK signaling pathway
The activation of the MAPK signaling pathway has been found in the progression of HCC. Human mesenchymal stem cells (hMSCs) promote the growth of HCC by activating the MAPK pathway to increase the expression of proliferation‐related proteins Ki‐67, pHH3, and PCNA, and boost the metastasis of HCC through EMT and ITGA5. Additionally, tissues treated with hMSCs showed a significant decrease of NK cell marker CD56 expression and an increase of TNF‐α and IL‐6 expression, which may contribute to tumor growth and metastasis. 12 Of course, the effect between MSCs and HCC is mutually influential. When MSCs migrate to TME, MSCs are induced evolution into tumor‐associated mesenchymal stem cells (TA‐MSCs) by tumor cells through paracrine. After exposure to HCC conditioned medium (HCC‐CM), hADMSCs inhibit cell proliferation and enhance glycolysis by blocking the cell cycle and activating the mitochondrial apoptotic pathway, which can be reversed by withdrawing from HCC‐CM. Interestingly, the migration and invasion ability of hADMSCs is irreversibly enhanced under the action of HCC‐CM. On mechanism, changes in the phenotype and metabolism and permanent tumor‐promoting properties of hADMSCs are controlled by the ROS/MAPK/HIF‐1α signaling pathway. 13
3.2. The Wnt/β‐catenin signaling pathway
Numerous research has been done on the transduction of the Wnt/β‐catenin signal as the pathogenic basis of the occurrence and development of HCC. 91 The vital role of the Wnt/β‐catenin signaling pathway between MSCs and HCC also cannot be ignored. Moreover, various lncRNAs have been revealed to advance the progress of HCC. Yan et al. discovered a new type of lncRNA named lncRNA‐MUF, which is highly expressed in HCC tissues and strongly associated with poor prognosis. MSCs contribute to HCC's biological function through the interaction of lncRNA‐MUF with ANXA2 and miR‐34a. The Wnt/β‐catenin signaling pathway and EMT are activated by the bind between LncRNA‐MUF and ANXA2. In addition, lncRNA‐MUF, as a ceRNA competing with miR‐34a, leads to the up‐regulation of Snail1 and the activation of EMT, and ultimately promotes the malignancy of HCC. 16
3.3. The IL‐6/STAT3 signaling pathway
The IL‐6/STAT3 signaling pathway is also involved in the beneficial behavior of MSCs to HCC. Large amounts of IL‐6 are secreted from BMSCs to stimulate IL‐6/STAT3 pathway signal transduction and significantly increase the invasion rate of liver cancer cells, which can be reversed by anti‐IL‐6 antibodies. Therefore, MSCs may strengthen the metastasis and invasion of HCC by activating the IL‐6/STAT3 signaling pathway. 17
3.4. YAP related signaling pathways
Studies have found that the stimulation of YAP exerts a pivotal part in the effect of MSCs on HCC. Intriguingly, extensive literature indicated the active role of adipose tissue, adipose cells or ADMSCs in liver cancer progression. The progression of HCC can be induced by Hypoxic MSCs via activating YAP and regulating YAP‐mediated adipogenesis via the COX2/PGE2/EP4 axis. The mechanism is that hypoxia increases the expression of COX2 in MSCs and the secretion of PGE2, which then provokes YAP in HCC cells, leading to the proliferation of HCC cell lines and the growth of xenograft tumors. Simultaneously, adipogenesis in HCC cell lines is accelerated by YAP up‐regulating the AKT/mTOR/SREBP1 axis. Importantly, EP4 mediates the effect of a low concentration of MSCs on YAP activation and lipogenesis of HCC cells. 14 Moreover, some phosphorylated kinases and NF‐κB signaling pathways, which are activated by exosomes from liver cancer cells, make adipocytes differentiated from MSCs have tumor‐promoting properties. 92 Teshima et al. also elucidated the positive influence of AT‐MSCs soluble factors, such as TGFβ1, EGF, HGF, PDGFβ, VEGFA, IGF2, on the proliferation and invasion of canine hepatocellular carcinoma cells. 15
3.5. Other molecular mechanisms
AQP1 is a known water channel that promotes metastasis and angiogenesis. Researchers found that the AQP1 level of liver cancer cells increased after exposure to BMSCs‐CM. In contrast, under the condition of AQP1 inhibitors, the migration and invasion of BMSCs‐mediated tumor cells were blocked, indicating that the HCC cell malignant behavior may be caused by the recruitment of BMSCs into the TME through AQP1 participation. 18 Besides, CXCR4 also exerts a regulating role to hBMSCs and umbilical cord mesenchymal stem cells (UCMSCs) on HCC. 19 3D culture experiments have confirmed that TGF‐β participates in the metastasis and invasion progress of HCC advanced by UCMSCs that make no effect on liver cancer cell growth, drug resistance, and stem cell‐related gene expression. 20 However, the reasons behind this apparent discrepancy need to be further investigated.
Most research is verified by conditioned medium or co‐cultivation experiments, so we should pay more attention to the interaction between MSCs and HCC. As researchers have discovered, HCC cells recruit MSCs and induce the phenotype of CA‐MSCs by expressing various cytokines that enhance migration. Liver cancer cells are empowered with excellent proliferation and migration by CA‐MSCs supernatant, demonstrating that MSCs may have a direct paracrine effect on tumor cells, thereby strengthening tumor angiogenesis, invasion and metastasis.
4. MECHANISMS OF MSCs INHIBITING THE PROGRESSION OF HCC
4.1. The anti‐tumor mechanism of UCMSCs
In recent years, the inhibitory effect of UCMSCs in the initiation and development of HCC has been demonstrated consecutively. Liu et al. described the inhibition effect of UCMSCs and UCMSCs‐CM on the growth, migration, metastasis and angiogenesis of HCC cells. 21 The anti‐tumor characteristic of BMSCs and UCMSCs to HCC, especially UCMSCs, is also pointed out by Alshareeda et al. 22 Mechanism studies disclosed that exosomes derived from hUCMSCs (hucMSC‐Ex) reduce the oxidative stress level of liver tumors, thereby suppressing the acute liver injury and fibrosis induced by CCl4 and the growth of liver tumors, and significantly reducing tumor size and inflammation infiltration area. 23 Tang et al. found that UCMSCs may restrain the growth of co‐cultured hepatocarcinoma cells by down‐regulating AFP, Bcl‐2 and Survivin, and accelerate cell apoptosis which is related to the apoptosis signal pathway. Furthermore, UCMSCs function in a time‐dependent and cell‐number‐dependent manner. 24
4.2. The anti‐tumor mechanism of ADMSCs
Interestingly, numerous previous literature reported the promotion function of ADMSCs on liver cancer. However, Serhal et al. investigation highlighted its tumor suppressor effect on HCC. When HCC cells are co‐cultured with ADMSCs or treated with ADMSCs‐CM, the apoptosis rate of HCC increases and the proliferation is significantly hindered, which is accompanied by the up‐regulation of P53 and retinoblastoma mRNA, as well as the down‐regulation of c‐Myc and hTERT mRNA levels. Notably, ADMSCs and ADMSCs ‐CM inhibit the expression of two important HCC carcinogenic markers‐ AFP and DCP. Furthermore, the level of migration and invasion of liver cancer cells was remarkably reduced, which may be due to the increased expression of TIMP‐1/2/3. 27
4.3. Tumor suppressor signaling pathways of other types of MSCs
In addition, varieties of signaling pathways are involved in the process of MSCs inhibiting HCC. Previous literature showed that MSCs could regulate HCC proliferation negatively through NF‐κB, Notch1, Akt, and TGF‐β signaling pathways. 93 , 94 , 95 , 96 Currently, other signaling pathways have also been discovered consecutively. Human amniotic mesenchymal stem cells (hAMSCs) have notable anti‐tumor effects both in vivo and in vitro. hAMSCs paracrine a variety of cytokines, such as DKK‐3, DKK‐1 and IGFBP‐3, which suppress the proliferation and promote the apoptosis of liver cancer cells by blocking the Wnt/β‐catenin and IGF‐1R/PI3K/AKT signaling pathways. 25 Factors released by MSCs, such as DKK‐1, compete with Wnt for binding to LRP5/6, thereby inhibiting the Wnt signaling pathway. 97 , 98 The abundance and distribution of 5‐hmC and 5‐mC in the regulatory region are regulated by Human menstrual blood‐derived stem cells (MenSCs) to silence PI3K/AKT and MAPK carcinogenic pathways and chemotherapy resistance‐related genes including ID4 and HMGA1 and activate tumor suppressors. The inactivation of the MAPK pathway further destroys the c‐Myc‐mediated EMT. Briefly, HCC growth is controlled by MenSCs, especially function in enhancers and promoters. 26 The tumor suppressor effect of some genes on MSCs has also been confirmed. For example, MSCs overexpressing the pro‐inflammatory regulator sirt1 secrete chemokines to repress iNOS in an inflammatory environment, which further attract CD8+ T cells to approach without inhibiting their proliferation. Ultimately, the anti‐tumor effect is driven by its pro‐inflammatory ability. 28
5. THE MSCs‐BASED HCC THERAPIES
Accumulating studies with in vitro experiments and animal models have shown the ability of MSCs homing to TME. The migration capacity of MSCs is raised by highly tumorigenic HCC cells, which makes MSCs an ideal carrier for targeted therapy of liver cancer. 99 Compared with anti‐tumor monotherapy, the superiorities of synergistic combination therapy are including promoting the efficiency and specificity of MSCs‐mediated anti‐cancer, reversing drug resistance, improving radiotherapy potential and safety.
5.1. The combination therapy of MSCs and sorafenib
Contrasted to sorafenib alone, sorafenib combined with MSCs in the treatment of liver cancer gives rise to HCC cell apoptosis and attenuates tumor cell proliferation. Mechanism studies have discovered that MSCs reduced and increased the concentrations of tumor‐promoting factors such as IL‐1 and TNF‐α, and tumor suppressor IL‐10 respectively. Conclusionally, the anti‐tumor and anti‐metastatic features of MSCs are retained by combined with sorafenib. 29 , 30 The inhibitory effect of MSCs‐CM on the growth of liver cancer cells has been reported in several pieces of literature. Seyhoun et al. further found that sorafenib combined with MSCs‐CM also has a negative result on the growth of liver cancer cells. Meaningfully, the synergistic effect of sorafenib and 80% MSCs‐CM in HCC cells is of clinical significance, allowing the use of lower doses of sorafenib and being more effective than currently single use. 33 More precise drug‐resistant targets have already arisen. For example, GRP78, overexpressed in sorafenib‐resistant liver cancer cells, is used as a therapeutic target of siGRP78‐modified MSCs exosomes combined with sorafenib in HCC cells, thereby weakening the growth and invasion of cancer cells in vivo and in vitro, reversing sorafenib resistance. 35
5.2. The combination therapy of MSCs and adriamycin
sFlt1 is a promising VEGF inhibitor, which is becoming a new method to destroy angiogenesis. After genetic engineering, MSCs that secrete sFlt1 possess a marked anti‐angiogenesis effect in HCC, thereby hindering tumor growth. The growth of liver cancer and cell apoptosis can be prevented and induced separately by combined treatment of sFlt1 genetically engineered MSCs and continuous low‐dose adriamycin. Importantly, the effectiveness and safety of this combination therapy are verified through in vivo experiments. 31 , 32 MiR‐199a‐3p, the third most highly expressed miRNA in the normal liver, is down‐regulated in almost all HCC cells and associated with poor prognosis. And mTOR has been identified as the direct target of miR‐199a‐3p. Besides, exosomes derived from MSCs have tumor suppressor effects. 100 Hence, Lou et al. observed the adriamycin sensitivity of HCC was reinforced by mir‐199a modified adipose tissue‐derived mesenchymal stem cell exosomes (ADMSC‐Exo‐199a) through the mTOR pathway. 34
5.3. The combination therapy of MSCs and radiotherapy
ADMSCs can enhance the potential of radiotherapy (RT) for liver cancer. ADMSCs significantly destroy the ability of liver tumors growth, migration and invasion, and enhances the tumor regression effect of RT therapy by inhibiting IFITM1 gene expression, which mechanism is attributed to the down‐regulation of STAT3 and MMPs and the up‐regulation of P53 and caspases. 36 The effectiveness of the combination treatment of NIS genetically modified MSCs and I131 in HCC has also been confirmed. SMAD‐NIS‐MSCs have a high recruitment rate in the tumor stroma. And the SMAD promoter activity induced by TGFB1 takes with a strong biological targeted NIS transgene expression in subcutaneous HuH7 tumors. The noticeable therapeutic effects, including a delay in tumor growth and prolonged survival, are gained by the systemic application of SMAD‐NIS‐MSCs followed by I131 injection. This remarkable therapeutic effect is believed to be largely related to TGFB1 which leads to highly selective and focused amplification of MSCs‐based NIS expression in the tumor environment. 37 , 38
5.4. Therapy of genetic modified MSCs
Apoptin from the chicken anemia virus is a protein with an inherent ability to dissolve cancer cells without harming normal cells. By modifying the adenovirus (Ad) vector, therapeutic gene expression with low toxicity and high transferability can be achieved. The proliferation of liver cancer cells can be significantly restrained by both apoptin‐modified MSCs and CM in a dose‐dependent manner. 40 In addition, investigators described T cells were redirected to GPC3+ tumor cells by GPC3‐ENG MSCs, which may become a GPC3‐targeted treatment method. GPC3‐ENG MSCs express CD80 and 41BBL, which activate human T cells to produce IL2 in an antigen‐dependent manner and facilitate the proliferation of T cells, thereby killing the GPC3+ tumor cells. 43 NK4 can not only limit the growth, metastasis and invasion of tumor cells induced by HGF, but suppress tumor angiogenesis independent of the HGF/c‐Met pathway. MSCs modified by rAd‐NK4 apply negative effects on the growth and migration of liver cancer cells and tumor angiogenesis, which are associated with the inhibition of Erk1/2 phosphorylation, providing a new strategy for HCC targeted therapy. 44 HMGA1 and BYSL have also been found to be potential targets of genetically modified MSCs for liver cancer treatment. 26
5.5. Other combination therapies
MSCs‐mediated systemic delivery of oncolytic adenovirus (oAd) is a promising strategy that can strengthen the efficacy of anti‐liver cancer and improve safety at the same time. The virus replication in the MSCs vector is active. oAd accumulates specifically due to the homing of MSCs to tumors, and because of the cargo‐protective of MSCs, the circulation of virus particles in the blood is extended. Safety is improved by reducing liver sequestration and liver toxicity, and the elimination of MSCs is advanced through viral replication. 39 El‐Magd et al. have elucidated that Melatonin (Mel) maximizes the survival and therapeutic potential of MSCs in HCC possibly by inducing cell apoptosis and eliminating inflammation and oxidative stress. 41 In chemically induced HCC rats, the combination treatment of MSCs and vitamin D improves liver function and the recovery of liver parenchyma with better pathological images by hindering the TGF‐β signaling pathway. 42
6. CONCLUSION
MSCs not only can be recruited into the TME to become the HCC cell source, but launch the inhibition or promotion effects on HCC progression through multiple molecular signaling pathways (Figure 2). Yet, in spite of considerable research data highlight the prospect of MSCs and their secreted exosomes be modified or directly used to treat HCC (Figure 3), but the two‐way effect of MSCs on tumor vicious process still should not be ignored. When MSCs are used for treatment, the possibility that they may induce tumor recurrence exists. 101 , 102 Moreover, the close relationship between MSCs and LCSCs and their critical roles in drug resistance may lead to continued tumor relapse. 103 , 104 Therefore, we should be more cautious in MSCs‐based therapies for HCC and dedicated to revealing the mechanism of the MSCs bidirectional effect on HCC. More data need to be acquired on whether MSCs functions are in correlation with MSCs sources or HCC subtypes and what are the connections and differences between MSCs and TA‐MSCs? Intriguingly, the interaction of MSCs and LCSCs may exert an enormous function on MSCs accelerating the progress of HCC. However, research about it needs further to be done. In terms of treatment, we still need to further disclose the molecular mechanism of MSCs migration to improve recruitment efficiency and the effectiveness of targeted therapy. The interaction between MSCs and infiltrating immune cells also provides a new perspective for future HCC treatment. Foremost, blocking the malignant transformation and tumor promotion of MSCs in HCC targeted therapy is the key to making MSCs an ideal therapy for HCC. We believe that based on the outstanding properties of homing and inhibiting HCC, MSCs or their exosomes may constitute a compelling treatment or adjuvant therapy for HCC in the future.
FIGURE 2.
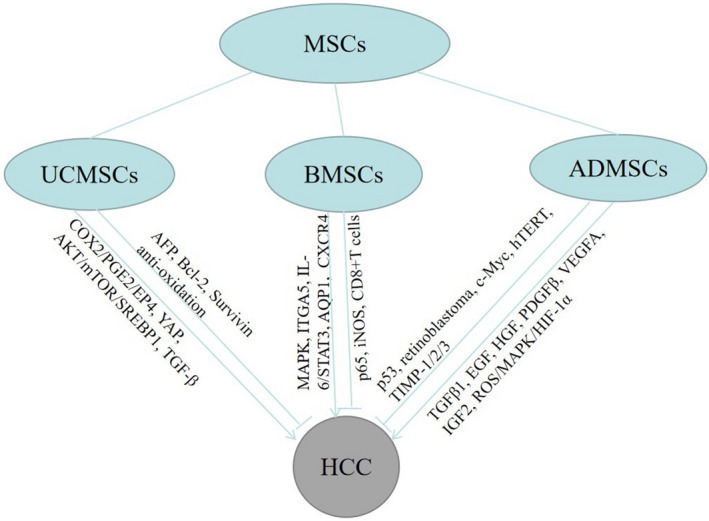
The molecular signaling mechanism of three main mesenchymal stem cells acting on HCC. MSCs, mesenchymal stem cells; UCMSCs, umbilical cord mesenchymal stem cells; BMSCs, bone marrow mesenchymal stem cells; ADMSCs, adipose‐derived mesenchymal stem cells; HCC, hepatocellular carcinoma
FIGURE 3.
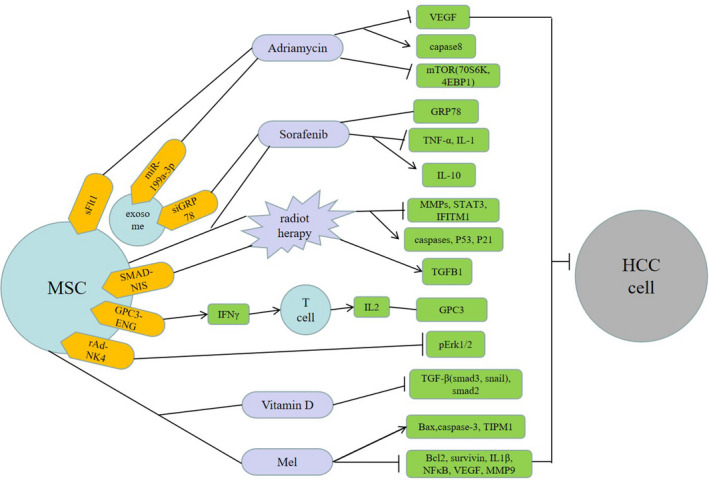
The combination therapies of mesenchymal stem cells (MSCs) for hepatocellular carcinoma (HCC). MSCs or their exosomes can be genetically modified (yellow) or combined with other treatments (purple) to inhibit the proliferation and progression of HCC cells through a variety of molecular signaling mechanisms (green)
CONFLICT OF INTEREST
The authors report no conflict of interest in this work.
AUTHOR CONTRIBUTIONS
Xiaoli Zhang contributed to literature analysis and manuscript writing. Na Li contributed to literature search. Ying Zhu contributed to supervision and revise. Wei Wen contributed to revise.
ETHICAL APPROVAL
Ethical approval was not required for this review article.
Zhang X, Li N, Zhu Y, Wen W. The role of mesenchymal stem cells in the occurrence, development, and therapy of hepatocellular carcinoma. Cancer Med. 2022;11:931–943. doi: 10.1002/cam4.4521
Funding information
This work was funded by the National Natural Science Foundation of China, grant number 81673728.
Contributor Information
Ying Zhu, Email: zhuyingsh52@126.com.
Wei Wen, Email: w11.5@163.com.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCE
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer. 2020;147:317‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan LH, Luk ST, Ma S. Turning hepatic cancer stem cells inside out – a deeper understanding through multiple perspectives. Mol Cells. 2015;38:202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tahmasebi Birgani M, Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molina ER, Smith BT, Shah SR, Shin H, Mikos AG. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release. 2015;219:107‐118. [DOI] [PubMed] [Google Scholar]
- 6. Zong C, Zhang H, Yang X, et al. The distinct roles of mesenchymal stem cells in the initial and progressive stage of hepatocarcinoma. Cell Death Dis. 2018;9:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206‐3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo J, Ok Lee S, Liang L, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33:2768‐2778. [DOI] [PubMed] [Google Scholar]
- 10. Riggi N, Suvà ML, De Vito C, et al. EWS‐FLI‐1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma X, Liu J, Yang X, et al. Mesenchymal stem cells maintain the stemness of colon cancer stem cells via interleukin‐8/mitogen‐activated protein kinase signaling pathway. Exp Biol Med. 2020;245:562‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Ji T, Wu DI, et al. Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin α5 in hepatocellular carcinoma. Cell Death Dis. 2019;10:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang C, Hu J, Chen Z, et al. Reversibility of hAT‐MSCs phenotypic and metabolic changes after exposure to and withdrawal from HCC‐conditioned medium through regulation of the ROS/MAPK/HIF‐1α signaling pathway. Stem Cell Res Ther. 2020;11:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Ren H, Zhou Y, et al. The hypoxia conditioned mesenchymal stem cells promote hepatocellular carcinoma progression through YAP mediated lipogenesis reprogramming. J Exp Clin Cancer Res. 2019;38:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teshima T, Matsumoto H, Koyama H. Soluble factors from adipose tissue‐derived mesenchymal stem cells promote canine hepatocellular carcinoma cell proliferation and invasion. PLoS One. 2018;13:e0191539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan X, Zhang D, Wu W, et al. Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA‐MUF interaction with ANXA2 and miR‐34a. Cancer Res. 2017;77:6704‐6716. [DOI] [PubMed] [Google Scholar]
- 17. Mi F, Gong L. Secretion of interleukin‐6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep. 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelagalli A, Nardelli A, Fontanella R, Zannetti A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow‐derived mesenchymal stem cells. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fontanella R, Pelagalli A, Nardelli A, et al. A novel antagonist of CXCR4 prevents bone marrow‐derived mesenchymal stem cell‐mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016;370:100‐107. [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Liu Y, Xu XX, Guo X, Sun GW, Ma XJ. Mesenchymal stem cells enhance the metastasis of 3D‐cultured hepatocellular carcinoma cells. BMC Cancer. 2016;16:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Shi Y, Han J, Zhang Y, Cao Z, Cheng J. Quantitative tracking tumor suppression efficiency of human umbilical cord‐derived mesenchymal stem cells by bioluminescence imaging in mice hepatoma model. Int J Stem Cells. 2020;13:104‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alshareeda AT, Alsowayan B, Almubarak A, Alghuwainem A, Alshawakir Y, Alahmed M. Exploring the potential of mesenchymal stem cell sheet on the development of hepatocellular carcinoma in vivo. J Vis Exp. 2018;(139):57805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang W, Tan Y, Cai M, et al. Human umbilical cord MSC‐derived exosomes suppress the development of CCl(4)‐induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang Y‐M, Bao W‐M, Yang J‐H, et al. Umbilical cord‐derived mesenchymal stem cells inhibit growth and promote apoptosis of HepG2 cells. Mol Med Rep. 2016;14:2717‐2724. [DOI] [PubMed] [Google Scholar]
- 25. Liu Q‐W, Li J‐Y, Zhang X‐C, et al. Human amniotic mesenchymal stem cells inhibit hepatocellular carcinoma in tumour‐bearing mice. J Cell Mol Med. 2020;24:10525‐10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu Y, Chen X, Zhao Y, Wang Y, Li Y, Xiang C. Genome‐wide DNA methylation and hydroxymethylation analysis reveal human menstrual blood‐derived stem cells inhibit hepatocellular carcinoma growth through oncogenic pathway suppression via regulating 5‐hmC in enhancer elements. Stem Cell Res Ther. 2019;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serhal R, Saliba N, Hilal G, et al. Effect of adipose‐derived mesenchymal stem cells on hepatocellular carcinoma: in vitro inhibition of carcinogenesis. World J Gastroenterol. 2019;25:567‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye F, Jiang J, Zong C, et al. Sirt1‐overexpressing mesenchymal stem cells drive the anti‐tumor effect through their pro‐inflammatory capacity. Mol Ther. 2020;28:874‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajighasemlou S, Nikbakht M, Pakzad S, et al. Sorafenib and mesenchymal stem cell therapy: a promising approach for treatment of HCC. Evid Based Complement Alternat Med. 2020;2020:9602728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seyhoun I, Hajighasemlou S, Muhammadnejad S, et al. Combination therapy of sorafenib with mesenchymal stem cells as a novel cancer treatment regimen in xenograft models of hepatocellular carcinoma. J Cell Physiol. 2019;234:9495‐9503. [DOI] [PubMed] [Google Scholar]
- 31. Niu J, Wang Y, Wang J, Bin L, Hu X. Delivery of sFIT‐1 engineered MSCs in combination with a continuous low‐dose doxorubicin treatment prevents growth of liver cancer. Aging. 2016;8:3520‐3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li G, Miao F, Zhu J, Chen Y. Anti‐angiogenesis gene therapy for hepatocellular carcinoma via systemic injection of mesenchymal stem cells engineered to secrete soluble Flt‐1. Mol Med Rep. 2017;16:5799‐5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seyhoun I, Hajighasemlou S, Ai J, et al. Novel combination of mesenchymal stem cell‐conditioned medium with sorafenib have synergistic antitumor effect of hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2019;20:263‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lou G, Chen L, Xia C, et al. MiR‐199a‐modified exosomes from adipose tissue‐derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone‐marrow‐derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol. 2018;16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu L, Tang Q, Yin X, et al. The therapeutic potential of adipose tissue‐derived mesenchymal stem cells to enhance radiotherapy effects on hepatocellular carcinoma. Front Cell Dev Biol. 2019;7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schug C, Urnauer S, Jaeckel C, et al. TGFB1‐driven mesenchymal stem cell‐mediated NIS gene transfer. Endocr Relat Cancer. 2019;26:89‐101. [DOI] [PubMed] [Google Scholar]
- 38. Schug C, Kitzberger C, Sievert W, et al. Radiation‐induced amplification of TGFB1‐induced mesenchymal stem cell‐mediated sodium iodide symporter (NIS) gene (131)I therapy. Clin Cancer Res. 2019;25:5997‐6008. [DOI] [PubMed] [Google Scholar]
- 39. Yoon A‐R, Hong J, Li Y, et al. Mesenchymal stem cell‐mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res. 2019;79:4503‐4514. [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Hou L, Wu X, et al. Inhibitory effect of genetically engineered mesenchymal stem cells with Apoptin on hepatoma cells in vitro and in vivo. Mol Cell Biochem. 2016;416:193‐203. [DOI] [PubMed] [Google Scholar]
- 41. El‐Magd MA, Mohamed Y, El‐Shetry ES, et al. Melatonin maximizes the therapeutic potential of non‐preconditioned MSCs in a DEN‐induced rat model of HCC. Biomed Pharmacother. 2019;114:108732. [DOI] [PubMed] [Google Scholar]
- 42. Saad El‐Din S, Fouad H, Rashed LA, Mahfouz S, Hussein RE. Impact of mesenchymal stem cells and vitamin D on transforming growth factor beta signaling pathway in hepatocellular carcinoma in rats. Asian Pac J Cancer Prev. 2018;19:905‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szoor A, Vaidya A, Velasquez MP, et al. T cell‐activating mesenchymal stem cells as a biotherapeutic for HCC. Mol Ther Oncolytics. 2017;6:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai C, Hou L, Zhang J, et al. The inhibitory effect of mesenchymal stem cells with rAd‐NK4 on liver cancer. Appl Biochem Biotechnol. 2017;183:444‐459. [DOI] [PubMed] [Google Scholar]
- 45. Cao H, Xu W, Qian H, et al. Mesenchymal stem cell‐like cells derived from human gastric cancer tissues. Cancer Lett. 2009;274(1):61‐71. [DOI] [PubMed] [Google Scholar]
- 46. Yan X‐L, Fu C‐J, Chen L, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132(1):153‐164. [DOI] [PubMed] [Google Scholar]
- 47. Lin J‐T, Wang J‐Y, Chen M‐K, et al. Colon cancer mesenchymal stem cells modulate the tumorigenicity of colon cancer through interleukin 6. Exp Cell Res. 2013;319(14):2216‐2229. [DOI] [PubMed] [Google Scholar]
- 48. Waghray M, Yalamanchili M, Dziubinski M, et al. GM‐CSF mediates mesenchymal‐epithelial cross‐talk in pancreatic cancer. Cancer Discov. 2016;6(8):886‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hassanzadeh A, Rahman HS, Markov A, et al. Mesenchymal stem/stromal cell‐derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bayo J, Real A, Fiore EJ, et al. IL‐8, GRO and MCP‐1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow‐derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2017;8(46):80235‐80248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gao Y, Zhou Z, Lu S, et al. Chemokine CCL15 mediates migration of human bone marrow‐derived mesenchymal stem cells toward hepatocellular carcinoma. Stem Cells. 2016;34(4):1112‐1122. [DOI] [PubMed] [Google Scholar]
- 52. Lejmi E, Perriraz N, Clément S, et al. Inflammatory chemokines MIP‐1δ and MIP‐3α are involved in the migration of multipotent mesenchymal stromal cells induced by hepatoma cells. Stem Cells Dev. 2015;24(10):1223‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mardomi A, Sabzichi M, Hussein Somi M, et al. Trafficking mechanism of bone marrow‐derived mesenchymal stem cells toward hepatocellular carcinoma HepG2 cells by modulating Endoglin, CXCR4 and TGF‐β. Cell Mol Biol (Noisy‐le‐grand). 2016;62:81‐86. [PubMed] [Google Scholar]
- 54. Schmohl KA, Müller AM, Wechselberger A, et al. Thyroid hormones and tetrac: new regulators of tumour stroma formation via integrin αvβ3. Endocr Relat Cancer. 2015;22(6):941‐952. [DOI] [PubMed] [Google Scholar]
- 55. Xie C, Yang Z, Suo Y, et al. Systemically infused mesenchymal stem cells show different homing profiles in healthy and tumor mouse models. Stem Cells Transl Med. 2017;6(4):1120‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tutter M, Schug C, Schmohl KA, et al. Regional hyperthermia enhances mesenchymal stem cell recruitment to tumor stroma: implications for mesenchymal stem cell‐based tumor therapy. Mol Ther. 2021;29(2):788‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Batten P, Sarathchandra P, Antoniw JW, et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo‐responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12(8):2263‐2273. [DOI] [PubMed] [Google Scholar]
- 59. Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T‐cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228‐234. [DOI] [PubMed] [Google Scholar]
- 60. Gao W‐X, Sun Y‐Q, Shi J, et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathew E, Brannon AL, Del Vecchio A, et al. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia. 2016;18(3):142‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Q‐Z, Su W‐R, Shi S‐H, et al. Human gingiva‐derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Selleri S, Bifsha P, Civini S, et al. Human mesenchymal stromal cell‐secreted lactate induces M2‐macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193‐30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Han Z, Tian Z, Lv G, et al. Immunosuppressive effect of bone marrow‐derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J Cell Mol Med. 2011;15(11):2343‐2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837‐3844. [DOI] [PubMed] [Google Scholar]
- 66. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74‐85. [DOI] [PubMed] [Google Scholar]
- 67. Di Nicola M, Carlo‐Stella C, Magni M, et al. Human bone marrow stromal cells suppress T‐lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838‐3843. [DOI] [PubMed] [Google Scholar]
- 68. Akiyama K, Chen C, Wang D, et al. Mesenchymal‐stem‐cell‐induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Connor BP, Vogel LA, Zhang W, et al. Imprinting the fate of antigen‐reactive B cells through the affinity of the B cell receptor. J Immunol. 2006;177(11):7723‐7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barcellos‐de‐Souza P, Comito G, Pons‐Segura C, et al. Mesenchymal stem cells are recruited and activated into carcinoma‐associated fibroblasts by prostate cancer microenvironment‐derived TGF‐β1. Stem Cells. 2016;34(10):2536‐2547. [DOI] [PubMed] [Google Scholar]
- 71. Shangguan L, Ti X, Krause U, et al. Inhibition of TGF‐β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma‐associated fibroblasts and abolishes their protumor effects. Stem Cells. 2012;30(12):2810‐2819. [DOI] [PubMed] [Google Scholar]
- 72. Ji J, Eggert T, Budhu A, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology (Baltimore, MD). 2015;62(2):481‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhao W, Su W, Kuang P, et al. The role of hepatic stellate cells in the regulation of T‐cell function and the promotion of hepatocellular carcinoma. Int J Oncol. 2012;41(2):457‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao W, Zhang L, Yin Z, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129(11):2651‐2661. [DOI] [PubMed] [Google Scholar]
- 75. Cheng Y, Li H, Deng Y, et al. Cancer‐associated fibroblasts induce PDL1+ neutrophils through the IL6‐STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell‐mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75(3):248‐255. [DOI] [PubMed] [Google Scholar]
- 77. Cassatella MA, Mosna F, Micheletti A, et al. Toll‐like receptor‐3‐activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29(6):1001‐1011. [DOI] [PubMed] [Google Scholar]
- 78. Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon‐gamma‐stimulated marrow stromal cells: a new type of nonhematopoietic antigen‐presenting cell. Blood. 2006;107(6):2570‐2577. [DOI] [PubMed] [Google Scholar]
- 79. Zheng H, Zou W, Shen J, et al. Opposite effects of coinjection and distant injection of mesenchymal stem cells on breast tumor cell growth. Stem Cells Transl Med. 2016;5(9):1216‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qian H, Ding X, Zhang J, et al. Cancer stemness and metastatic potential of the novel tumor cell line K3: an inner mutated cell of bone marrow‐derived mesenchymal stem cells. Oncotarget. 2017;8:39522‐39533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu X, Qian H, Zhu W, et al. Isolation of cancer stem cells from transformed human mesenchymal stem cell line F6. J Mol Med (Berl). 2010;88:1181‐1190. [DOI] [PubMed] [Google Scholar]
- 82. Maj M, Kokocha A, Bajek A, Drewa T. The effects of adipose‐derived stem cells on CD133‐expressing bladder cancer cells. J Cell Biochem. 2019;120:11562‐11572. [DOI] [PubMed] [Google Scholar]
- 83. Vulcano F, Milazzo L, Ciccarelli C, et al. Wharton's jelly mesenchymal stromal cells have contrasting effects on proliferation and phenotype of cancer stem cells from different subtypes of lung cancer. Exp Cell Res. 2016;345:190‐198. [DOI] [PubMed] [Google Scholar]
- 84. El‐Badawy A, Ghoneim MA, Gabr MM, et al. Cancer cell‐soluble factors reprogram mesenchymal stromal cells to slow cycling, chemoresistant cells with a more stem‐like state. Stem Cell Res Ther. 2017;8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eun JR, Jung YJ, Zhang Y, et al. Hepatoma SK Hep‐1 cells exhibit characteristics of oncogenic mesenchymal stem cells with highly metastatic capacity. PLoS One. 2014;9:e110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu M, Lin Z, Li X, et al. HULC cooperates with MALAT1 to aggravate liver cancer stem cells growth through telomere repeat‐binding factor 2. Sci Rep. 2016;6:36045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li X, Wang J, Pan Y, et al. Long non‐coding RNA HULC affects the proliferation, apoptosis, migration, and invasion of mesenchymal stem cells. Exp Biol Med. 2018;243:1074‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li X, Song Y, Liu F, et al. Long non‐coding RNA MALAT1 promotes proliferation, angiogenesis, and immunosuppressive properties of mesenchymal stem cells by inducing VEGF and IDO. J Cell Biochem. 2017;118:2780‐2791. [DOI] [PubMed] [Google Scholar]
- 89. Hou J, Zhao N, Zhu P, Chang J, Du Y, Shen W. Irradiated mesenchymal stem cells support stemness maintenance of hepatocellular carcinoma stem cells through Wnt/β‐catenin signaling pathway. Cell Biosci. 2020;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hu Y, Zai H, Jiang W, Yao Y, Ou Z, Zhu Q. miR‐126 in extracellular vesicles derived from hepatoblastoma cells promotes the tumorigenesis of hepatoblastoma through inducing the differentiation of BMSCs into cancer stem cells. J Immunol Res. 2021;2021:6744715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vilchez V, Turcios L, Marti F, Gedaly R. Targeting Wnt/β‐catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22:823‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang S, Xu M, Li X, et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor‐promoting properties. J Hematol Oncol. 2018;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD. NF‐kappaB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin. 2008;29:333‐340. [DOI] [PubMed] [Google Scholar]
- 94. Abdel Aziz MT, Khaled HM, El Hindawi A, et al. Effect of mesenchymal stem cells and a novel curcumin derivative on Notch1 signaling in hepatoma cell line. Biomed Res Int. 2013;2013:129629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao W, Ren G, Zhang L, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm. 2012;27:606‐613. [DOI] [PubMed] [Google Scholar]
- 96. Li G‐C, Ye Q‐H, Xue Y‐H, et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97‐H cell line. Cancer Sci. 2010;101:2546‐2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk‐1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67‐77. [DOI] [PubMed] [Google Scholar]
- 98. Hou L, Wang X, Zhou Y, et al. Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumour Biol. 2014;35:1239‐1250. [DOI] [PubMed] [Google Scholar]
- 99. Endaya B, Guan SP, Newman JP, et al. Human mesenchymal stem cells preferentially migrate toward highly oncogenic human hepatocellular carcinoma cells with activated EpCAM signaling. Oncotarget. 2017;8:54629‐54639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Alzahrani FA, El‐Magd MA, Abdelfattah‐Hassan A, et al. Potential effect of exosomes derived from cancer stem cells and MSCs on progression of DEN‐induced HCC in rats. Stem Cells Int. 2018;2018:8058979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chaput B, Foucras L, Le Guellec S, Grolleau JL, Garrido I. Recurrence of an invasive ductal breast carcinoma 4 months after autologous fat grafting. Plast Reconstr Surg. 2013;131:123e‐124e. [DOI] [PubMed] [Google Scholar]
- 102. Perrot P, Rousseau J, Bouffaut A‐L, et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010;5:e10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma‐associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 2016;7:6916‐6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Daverey A, Drain AP, Kidambi S. Physical intimacy of breast cancer cells with mesenchymal stem cells elicits trastuzumab resistance through Src activation. Sci Rep. 2015;5:13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


