Objective
Considerable international investment in hospital electronic prescribing (ePrescribing) systems has been made, but despite this, it is proving difficult for most organizations to realize safety, quality, and efficiency gains in prescribing. The objective of this work was to develop policy-relevant insights into the optimization of hospital ePrescribing systems to maximize the benefits and minimize the risks of these expensive digital health infrastructures.
Methods
We undertook a systematic scoping review of the literature by searching MEDLINE, Embase, and CINAHL databases. We searched for primary studies reporting on ePrescribing optimization strategies and independently screened and abstracted data until saturation was achieved. Findings were theoretically and thematically synthesized taking a medicine life-cycle perspective, incorporating consultative phases with domain experts.
Results
We identified 23,609 potentially eligible studies from which 1367 satisfied our inclusion criteria. Thematic synthesis was conducted on a data set of 76 studies, of which 48 were based in the United States. Key approaches to optimization included the following: stakeholder engagement, system or process redesign, technological innovations, and education and training packages. Single-component interventions (n = 26) described technological optimization strategies focusing on a single, specific step in the prescribing process. Multicomponent interventions (n = 50) used a combination of optimization strategies, typically targeting multiple steps in the medicines management process.
Discussion
We identified numerous optimization strategies for enhancing the performance of ePrescribing systems. Key considerations for ePrescribing optimization include meaningful stakeholder engagement to reconceptualize the service delivery model and implementing technological innovations with supporting training packages to simultaneously impact on different facets of the medicines management process.
Key Words: patient safety, quality, efficiency, ePrescribing, health IT, medicines management
In most health systems globally, replacing traditional paper-based processes and pathways with digital systems and services is now considered a strategic priority to modernize and optimize the efficiency and safety of healthcare. Previous implementation experiences have highlighted that this is usually a complex long-term process, involving multiple stakeholders, with different expectations and priorities.1 Electronic prescribing (also known as computerized physician order entry [CPOE] with or without computerized decision support [CDS] and henceforth referred to as ePrescribing) is an area of health-system digitization, which can provide a range of benefits—from more efficient medication ordering and administration processes to alerting, error prevention, and improved patient safety.2 ePrescribing systems also have the potential to promote prescriber adherence with evidence-based guidelines, facilitate cost-conscious prescribing, and enable changes in the medicines use process.3
Realizing the benefits of ePrescribing is largely dependent on optimizing these systems, so that the available functionalities are fully enabled, appropriately used, integrated with other relevant health information technology (IT), and embedded with clinical priorities and workflows. Substantial and cost-effective reductions in clinically important medication errors can be achieved with the implementation of ePrescribing systems, but these are not guaranteed. Successful deployment is contingent on the context of the ePrescribing implementation, and the use of identical software can lead to very different results in different hospitals, in part because these systems are highly configurable at the local level.4 ePrescribing systems are becoming increasingly complex, requiring iterative development and commitment to a life-cycle perspective.5 Emergent research focuses on ensuring these large-scale and expensive health IT infrastructures now deliver the promised clinical improvements through the process of systems optimization.6
The aim of this work was to map the landscape of optimization strategies within the field of hospital ePrescribing and develop an evidence-informed policy-focused overview of the range of approaches that have been deployed to maximize the patient, provider, and organizational benefits of ePrescribing systems.
METHODS
Overview of Methods
We used the 6-stage framework proposed by Arksey and O’Malley7 and then further refined by Levac et al8 to undertake a systematic scoping review of the international literature. Our methods are detailed in full in the published protocol and summarized hereinafter.9 Embracing the iterative nature of conducting a scoping protocol, a feature strongly endorsed by Levac et al,8 the inclusion and exclusion criteria from the initial protocol have been refined (Table 1). An essential requirement for included studies was the implementation of an optimization strategy within an ePrescribing system. Systems optimization was defined as “the activity of enhancing system capabilities and integration of subsystem elements to the extent that all components operate at or above user expectations.”10 ePrescribing was defined as “the utilization of electronic systems to facilitate and enhance the communication of a prescription or medicine order, aiding the choice, administration and supply of a medicine through knowledge and decision support, and providing a robust audit trail for the entire medicines use process.”11
TABLE 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| Primary studies or systematic reviews with a clearly defined methodology that describe an approach/approaches that are implemented to optimize an ePrescribing system. |
| The study must take part in a healthcare context that is applicable to learning for UK NHS hospitals. |
| The study should be set in a high-income country, as defined by the OECD. |
| Exclusion criteria |
| Study does not describe an optimization strategy implemented in an ePrescribing system. |
| The study is an opinion piece or a review without a clearly defined methodology. |
| Study takes place in a healthcare context that is not applicable to learning for UK NHS hospitals. |
| The country of the study is not within the OECD. |
OECD, Organization for Economic Co-operation and Development; NHS, National Health Service.
Systematic Scoping Review
Abstracts and full texts were independently screened by 2 reviewers, with conflicts moderated by a third member of the research team (J.W., S.M., C.H., M.B., M.H., U.P.). In view of the substantial body of evidence uncovered, we decided to focus on the most recently published articles using principles of data saturation. Saturation is a methodological principle taken from qualitative research where it is mostly used as a criterion for discontinuing data analysis or collection.12 Although the concept of saturation still seems to be evolving, we identify with the 2016 definition by Given,13 who considered saturation as the point at which “additional data do not lead to any new emergent themes.” This involved conducting full-text screening (J.W., S.M., C.H., M.B.) and data extraction (J.W., S.M., M.B.) where we added a code for every new optimization strategy encountered during extraction. It was agreed that saturation would be achieved when no new codes emerged for optimization strategies from 10 consecutive studies.
Interpretation of Findings
Extracted data were synthesized by applying thematic analysis to a theoretical approach that considered the medication management life cycle and the propensity for errors at each stage of this process.14 ePrescribing optimization strategies were then thematically analyzed based on where the intervention lay within the ePrescribing process (Fig. 1).
FIGURE 1.
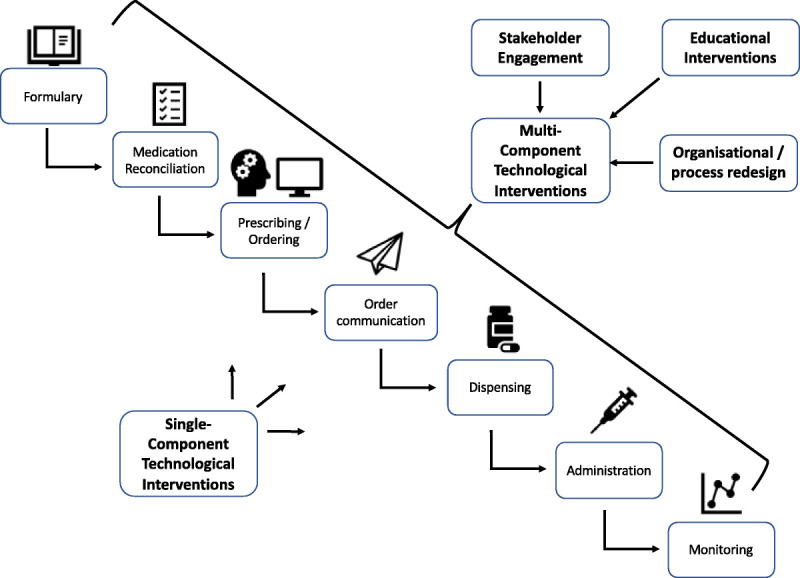
Theoretical approach showing a conceptualization of the medicines use process and the various stages with potential for optimization within an ePrescribing system.
Consultative phases with domain experts and patient and public involvement representatives were integrated throughout this work. The feedback from 2 round-table events with international and UK experts on ePrescribing, which took place in June 2019 and January 2020, were used to discuss and refine the thematic synthesis applied to our results.
RESULTS
Included Studies
We identified 30,214 potentially eligible studies, which, after the removal of duplicate studies, resulted in 23,609 studies eligible for abstract screening. The abstract screening stage led to the identification of an initial set of 1367 records eligible for full-text screening. Studies were then screened and extracted in reverse chronological order, with 817 studies not screened because of reaching data saturation. Of the 550 studies screened, we excluded 336 leaving 214 included studies (Fig. 2).
FIGURE 2.
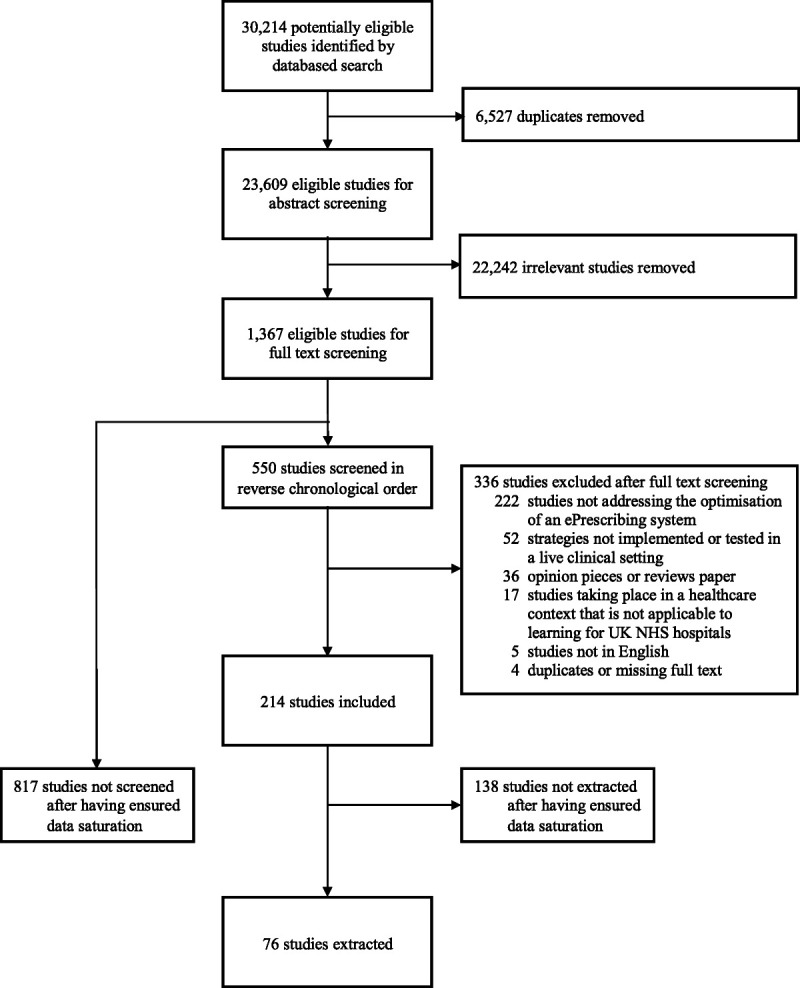
Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
We applied criteria of inductive thematic saturation to the set of included studies, until no new optimization approaches were identified. We considered saturation to have been achieved when 10 consecutive studies did not yield any new approaches to optimization. Data extraction was accordingly discontinued after extracting 76 articles, published in the period from November 01, 2017, to November 06, 2019, when no new approaches to optimization emerged from 10 consecutive studies (Fig. 3).
FIGURE 3.
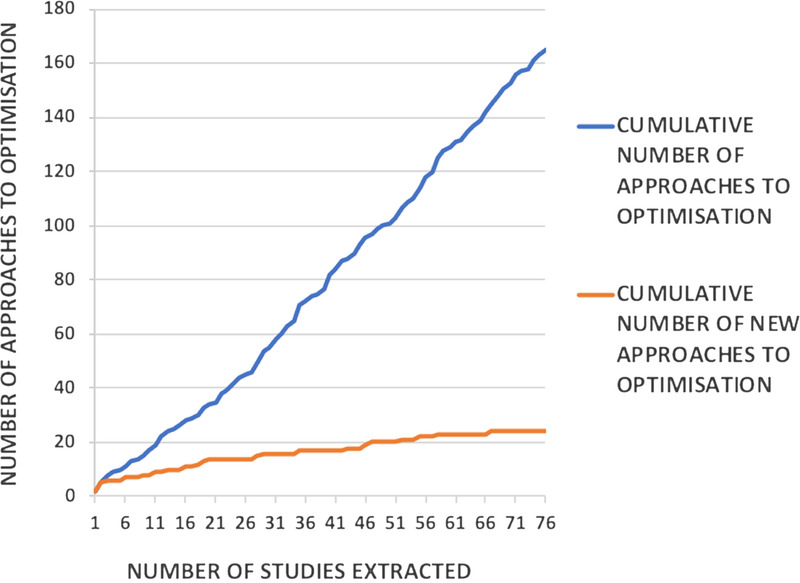
Saturation of new approaches to optimization when extracting data from the most recently published studies. Data extraction was discontinued after extracting 76 articles, when it seemed that new approaches to optimization were no longer emerging.
Characteristics of Included Studies
The majority of the 76 included articles described U.S.-based studies (n = 48), with studies originating from Spain coming a distant second (n = 6; Fig. 4).
FIGURE 4.
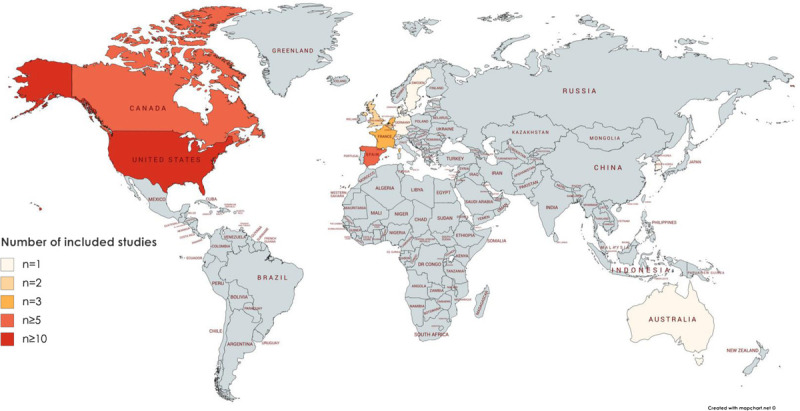
Heat map of the world showing countries based on the frequency of included studies.
Optimization strategies implemented in the hospital setting (n = 61) accounted for most included studies and ranged from hospital-wide strategies (n = 29) and strategies encompassing multiple wards and departments (n = 12) to highly targeted, specialty-specific interventions (n = 20). A small number of studies were based on the optimization of ePrescribing systems in community healthcare (n = 13). A further study was conducted in both the hospital and community, and another was conducted in a clinical research setting.
Of the studies providing sufficient detail, the ePrescribing landscape was dominated by commercial ePrescribing software systems with Epic being the most frequently reported (n = 11) system. Many optimization strategies were reliant on the addition of third-party software packages or apps (n = 24).
Some studies related to single optimization strategies taking place at a very specific stage of the prescribing process. In contrast, other studies combined a number of optimization strategies, working in synergy in an attempt to achieve prescribing goals and support clinical outcomes. These can thus be viewed as multicomponent interventions, using multiple technological optimization strategies or by supplementing technological interventions with other strategies, such as educational interventions, stakeholder engagement, and organizational transformation.
Single-Component Interventions
A number of studies (n = 26) described optimization strategies, implemented as single-component interventions (Table 2).15–40 Table 2 summarizes the various single-component optimization strategies used in the included studies, highlighting the stage of the ePrescribing process being optimized and the overall reported effectiveness of the intervention on the primary outcome.
TABLE 2.
Single-Component Interventions Classified According to the Aspect of the ePrescribing Process Being Optimized
| Stage of the ePrescribing Process Being Optimized | Study | Country | Single-Component Intervention | Study Design/Evaluation Method | Care Setting | Study Context | Primary Outcome Measure | Effect of Intervention |
|---|---|---|---|---|---|---|---|---|
| Formulary | Gayoso-Rey et al15 (2020) | Spain | Formulary changes | Prospective study | Secondary care | Standardizing an oncology drug database to reduce prescribing errors | Rate of prescribing errors | + |
| Medication reconciliation | Jurado et al16 (2018) | France | Transferring ePrescribing data across boundaries of care | Pilot study | Secondary care | Suitability of new medication reconciliation tool when patients admitted to hospital | Establishing an accurate medication profile | + |
| Prescribing/ordering | Epstein et al17 (2019) | Israel | CDSS | Pre/post study | Secondary care | The impact of CDSS in COPD management after discharge | Rate of adherence with discharge recommendations/guidelines | + |
| Daniels et al18 (2019) | United States | Refining alerts | Quality improvement report | Secondary care | Improving drug-drug interaction alert effectiveness | No. interruptive drug-drug interaction alerts | + | |
| Wai et al19 (2019) | United States | CDSS | Retrospective cohort study | Secondary care | Improving correct prescribing of thiamine in patients with alcohol use disorder | Proportion of patients receiving high-dose parenteral thiamine | + | |
| Gupta et al20 (2019) | Canada | CDSS | Interrupted time series | Primary care | Improving asthma management in primary care | Proportion of patients with written asthma action plan | + | |
| Orchard et al21 (2018) | Australia | CDSS | Pre/post study | Primary care | Improving guideline-recommended therapy in atrial fibrillation | Proportion of patients appropriately prescribed anticoagulants | + | |
| Figueroa et al22 (2019) | Spain | Refining alerts | Pre/post study | Secondary care | Improving appropriate pharmacological prophylaxis | Prescribing of pharmacological prophylaxis | + | |
| Gaweda et al23 (2018) | United States | CDSS | Retrospective case-control study | Secondary care | Improving anemia management in dialysis patients | Percentage of hemoglobin concentrations between 10 and 12 g/dL | + | |
| Akhloufi et al24 (2018) | The Netherlands | CDSS | Mixed methods | Secondary care | Using CDSS to support intravenous to oral antibiotic switch | Clinical relevance and usefulness of CDSS | N/A | |
| Pendharkar et al25 (2018) | Canada | Order set | Step-wedge study | Tertiary care | Improving management of acute exacerbation of COPD | Hospital length of stay | / | |
| Simpao et al26 (2018) | United States | Data dashboard | Quality improvement report | Tertiary care | Developing an electronic antibiogram | No outcome measure—descriptive/feasibility report | N/A | |
| Order communication | Choi et al27 (2019) | Korea | Prescription validation/intervention | Retrospective study | Secondary care | Impact of pharmacist interventions on physicians’ acceptance of CDSS recommendations | The no. dosing alerts and physicians’ acceptance rates | + |
| Jourdan et al28 (2018) | France | Prescription validation/intervention | Prospective study | Secondary care | Impact of pharmacist interventions on clinical outcome and cost | No. avoided hospitalization days and associated cost avoidance | + | |
| Groppi et al29 (2018) | United States | Prescription validation/intervention | Descriptive research report | Secondary care | Streamlining the documentation process to capture pharmacist interventions | No outcome measure—descriptive/feasibility report | N/A | |
| Durvasula et al30 (2018) | United States | Alerts | Retrospective study | Secondary care | High-cost medications triggered an electronic alert to a review committee | Retrospective cost savings | + | |
| Medication dispensing | Berdot et al31 (2019) | France | Robotic dispensing technology | Mixed methods | Secondary care | Evaluation of an automated-dispensing system after implementation and upgrade | Return on investment and quality improvement metrics | + |
| Lupi et al32 (2020) | United States | Automated dispensing cabinet | Pre/post study | Tertiary care | Optimization of automated dispensing cabinets by clinical pharmacists | No. dispenses from central pharmacy, no. stockouts, inventory cost | + | |
| Campmans et al33 (2018) | The Netherlands | Alerts | Cross-sectional study | Community pharmacy | Reducing medication dispensing errors | Evaluating the experience of pharmacists using a survey | N/A | |
| Rodriguez-Gonzalez et al34 (2019) | Spain | Robotic dispensing technology | Pre/post study | Tertiary care | Impact of implementing a robotic dispensing system | Frequency of medication dispensing errors | + | |
| Beobide Telleria35 (2018) | Spain | Automated tablet dispensing and packaging system | Pre/post study | Nursing home | Impact of implementing an ATDPS | No. medication dispensing errors | + | |
| Bhakta et al36 (2018) | United States | Automated robotic compounding technology | Quasi-experimental study | Tertiary care | Impact of implementing ARCT in a satellite oncology pharmacy | Turnaround time for preparations | + | |
| Medication administration | Campbell et al37 (2018) | United States | Data dashboard | Pre/post study | Secondary care | Implementing a dashboard allowing nurses to visualize their individual near-miss medication error | No. near-miss medication administration events | + |
| Monitoring | Pereboom et al38 (2019) | The Netherlands | CDSS | Pre/post study | Secondary care | Improving adequate dosing of gentamicin and vancomycin | Plasma concentrations measured within 72 h | + |
| Beeler (2019)39 | Switzerland | CDSS | Cluster randomized control trial | Tertiary care | The impact of CDSS in potentially serious potassium-increasing drug-drug interactions | The frequency of potassium-monitoring intervals >72 h | / | |
| Kim (2018)40 | United States | CDSS | Post hoc analysis | Research setting | Using pharmacogenetic testing to identify drug therapy problems in polypharmacy patients | No. drug therapy problems per patient | / |
+ = intervention significantly improved primary outcome; / = intervention effects were null.
ATDPS, automated tablet dispensing and packaging system; ARCT, automated robotic compounding technology; CDSS, computerized decision support system; N/A, intervention did not measure objective outcomes.
All of the single-component optimization strategies were technological interventions targeting a single step in the prescribing process. The optimization strategies most frequently implemented were those at the prescribing interface (n = 10), and the most frequently deployed single-component optimization strategy was CDS systems (n = 9).
Formulary Changes
In 1 study, targeting formulary changes, active pharmaceutical ingredients in the electronic formulary of a Spanish hospital were standardized and implemented within an oncology outpatient clinic.15 This optimization work was associated with a decrease in medication errors. One of the key factors in the work was the implementation of “tall-man letters” in the electronic prescribing system with the aim of reducing errors relating to similarly named medications (e.g., DOBUTamine and DOPamine).
Medication Reconciliation
Medication reconciliation represents a crucial step in the prescribing process for hospitalized patients that aims to reduce the risk of iatrogenic harm through a comprehensive review of patients’ medication history. A study conducted in a hospital diabetes service in Toulouse, France, used the Electronic Pharmaceutical Record to summarize patients’ medication history, including over-the-counter drugs, dispensed by community pharmacies over the previous 4 months.16 By using national health insurance card details and linking to a secure Electronic Pharmaceutical Record server, the study reported improved medication reconciliation accuracy. In almost 30% of patients, medication errors that had not previously been identified were able to be detected and rectified.
Prescribing
At the prescriber interface of ePrescribing systems, CDS,17,19–21,23,24 order sets,25 alerts,18,22 and a data dashboard26 were all deployed as optimization strategies. Computerized decision support was often applied as an optimization strategy to aid the management of clinical conditions with standardized treatment algorithms amenable to decision support.17,19–21 Computerized decision support was also used for broader clinical goals, such as to promote switching of antibiotics from the intravenous to oral route to support antimicrobial stewardship,24 and for anemia management in dialysis patients.23
Reflecting the increasing body of literature around alert fatigue, and the risks associated with this optimization strategy, some studies attempted to optimize the existing alerts within their ePrescribing systems.18,22 For example, Daniels et al18 sought to optimize drug-drug interaction alerts at a pediatric hospital in Tennessee either through suppression or by using patient-specific data from the electronic health record to contextualize and filter alerts. The number of interruptive drug-drug interaction alerts was reportedly reduced by 40% for all clinicians.
Order Communications
A frequently deployed strategy was to empower pharmacists to intercept and validate prescriptions at the order communication stage (n = 3). Some of the studies applied this intervention as a second check to capture errors and thereby improve the quality and safety of prescribing,27–29 whereas a couple of the studies used this strategy to reduce medication costs.28,30
A caveat to relying on assessment of prescriptions is the substantial volume of electronic prescriptions that are ordered throughout hospitals on a daily basis. To tackle this issue, Jourdan et al28 used a third-party software called PHARMA to identify specific high-risk medications or restricted usage drugs for pharmacist review. By optimizing the pharmacist review process, the research group estimated that they would have prevented 73 intensive care admission days, 74 continuous monitoring unit hospitalization days, and 66 days of conventional hospitalization over the 6-month study period. This equated to €5.09 of public health savings for every euro invested in the prescription review activity.
Dispensing
Single-component interventions optimizing the medication dispensing process were dominated by large capital expenditures in robotics and automation, namely, automated dispensing cabinets,32 robotic dispensing technology,31,34 automated tablet dispensing and packaging systems,35 and automated robotic compounding technology.36 These studies mainly focused on increased efficiency and cost savings as well as improving patient safety by reducing medication dispensing errors.
A significant reduction in dispensing errors was achieved by implementing centralized robotic dispensing systems in hospital pharmacies31,34 and by installing an automated tablet dispensing and packaging system in 7 Spanish geriatric nursing homes.35 Cost savings were achieved32 at an 800-bed academic medical center by reducing the inventory cost across 65 automated dispensing cabinets. Through careful analysis and optimization of the medications held in the automated dispensing cabinets, they were also able to reduce the number of out of stock medications and the number of dispenses from central pharmacy. Meanwhile, implementing automated robotic compounding technology in a satellite oncology pharmacy significantly decreased the turnaround time for medications, and a return on investment within 8.6 years was projected through supply cost savings.36
Administration
To optimize medication administration, Campbell et al37 used real-time data, extracted from the electronic health record, to warn nurses of their individual “near-miss medication error risk” delivered through a visual dashboard. By correlating near-miss medication errors reported through the Barcode Medication Administration System with environmental data related to call lights, patient count, medication count, hours worked, sepsis scores, and task count for a 25-bed unit, the authors performed a regression analysis to identify factors predisposing to errors on an individual practitioner and unit-wide basis. During this pilot study, the intervention reported a reduction in near-miss events.
Monitoring
Optimization strategies for monitoring prescribed medications attempted to identify and reduce adverse drug reactions40 or drug-drug interactions.39,40 In 1 study, CDS was combined with pharmacogenetic data to help identify drug therapy problems in polypharmacy patients,40 whereas in another study, 5 features of an existing CDS were optimized to deliver patient-specific decision support for potassium-increasing drug-drug interactions. Neither of these studies were reported as having a positive effect on their respective primary end points. In contrast, Pereboom et al38 reported successfully using CDS to improve plasma concentration monitoring, thereby supporting appropriate dosing of antibiotics with pharmacokinetic dosing rules, such as gentamicin and vancomycin.
Multicomponent Interventions
Most studies (n = 50) reported on interventions that used more than 1 approach to optimize ePrescribing systems (Table 3).4,41–94 Table 3 summarizes the various combinations of optimization strategies and intervention components that were used in the included studies and the overall reported effectiveness of the intervention on the primary outcome.
TABLE 3.
Multicomponent Interventions Classified According to the Combination of Intervention Strategies Deployed
| Optimization Strategies Used | Study | Country | Specific Intervention Components | Study Design/Evaluation Method | Care Setting | Stage of ePrescribing Process Being Optimized | Primary Outcome Measure | Effect of Intervention |
|---|---|---|---|---|---|---|---|---|
| 1 | Burkoski et al41 (2019) | Canada | BCMA, closed-loop medication system | Interrupted time series | Secondary care | Whole prescribing process | Medication errors and adverse drug events | + |
| Pettit et al42 (2019) | United States | CDSS, CPOE | Pre/post study | Secondary care | Prescribing/ordering | Medication prescribing errors | + | |
| Bowdle et al43 (2018) | United States | BCMA, CDSS, smart infusion pumps | Pre/post study | Secondary care | Prescribing/ordering, dispensing, administration | Medication administration errors | + | |
| Ashburner et al44 (2018) | United States | Alerts, CDSS | Randomized controlled trial | Primary care | Prescribing/ordering | Medication prescribing rates | / | |
| Risor (2018)45 | Denmark | Automated dispensing cabinets, BCMA, CPOE | Pre/post study | Secondary care | Administration | Medication administration errors | / | |
| Schnock et al46 (2018) | United States | BCMA, smart infusion pumps | Pre/post study | Secondary care | Administration | Medication administration errors | + | |
| Desmedt et al47 (2018) | Belgium | Alerts, CDSS | Pre/post study | Secondary care | Prescribing/ordering | Prescription dose appropriateness | / | |
| Karlsson et al48 (2018) | Sweden | Alerts, CDSS | Cluster randomized controlled trial | Primary care | Prescribing/ordering | Adherence to prescribing guidelines | + | |
| Gunn et al49 (2018) | United States | Alerts, CDSS indication-based prescribing | Prospective cohort study | Secondary care | Prescribing/ordering | Provider-initiated medication order | + | |
| Macias et al50 (2018) | Spain | BCMA, CPOE | Pre/post study | Tertiary care | Administration | Medication administration errors | + | |
| Ni et al51 (2018) | United States | BCMA, CDSS, drug monitoring, refining alerts, smart infusion pumps | Prospective cohort study | Tertiary care | Administration, monitoring | Detection of dosing-related medication administration errors | + | |
| Rosa et al52 (2018) | United States | Alerts, order set | Interrupted time series | Secondary care | Prescribing/ordering, administration | Compliance and timing of care | + | |
| 1, 2 | Ilcewicz et al53 (2019) | United States | Order set, prescriber education | Retrospective cohort study | Secondary care | Prescribing/ordering, administration, monitoring | Glycemic control over 72 h | + |
| MacMaster et al54 (2019) | United States | Automated dispensing cabinet, BCMA, nurse education | Observational study | Secondary Care | Administration | Medication administration errors | + | |
| Thompson et al55 (2018) | United States | BCMA, staff education | Pre/post study | Secondary care | Administration | Medication administration errors | + | |
| Mathioudakis et al56 (2018) | United States | CDSS, prescriber education | Quality improvement study | Secondary care | Prescribing/ordering | Optimization design process | N/A | |
| Mainous et al57 (2018) | United States | CDSS, prescriber education | Pre/post study | Primary care | Prescribing/ordering | Iron test ordering | + | |
| Gulati et al58 (2018) | United States | Order set, prescriber education | Retrospective cohort study | Secondary care | Prescribing/ordering | Cumulative steroid use | + | |
| O’Sullivan et al59 (2018) | United States | CDSS, prescriber education | Retrospective cohort study | Secondary care | Prescribing/ordering | Compliance rate with intraoperative antibiotic redosing criteria | + | |
| Connor et al60 (2018) | United States | CDSS, data dashboard, prescriber education | Pre/post study | Secondary care | Prescribing/ordering | Single-unit blood transfusion rates | + | |
| Tamblyn et al61 (2018) | Canada | CPOE, data dashboard, prescriber education | Pre/post study | Tertiary care | Whole prescribing process | Medication reconciliation completion rates | + | |
| Pontefract et al4 (2018) | United Kingdom | CDSS, CPOE | Pre/post study | Secondary care | Prescribing/ordering | Medication prescribing errors | N/A | |
| 1, 3 | Muhlenkamp et al62 (2019) | United States | Refining alerts, stakeholder engagement | Pre/post study | Secondary care | Prescribing/ordering | Change in dosage alerts | + |
| Kawamanto63 (2018) | United States | CDSS, refining alerts, stakeholder engagement | Pre/post study | Secondary care | Prescribing/ordering | Alert appropriateness | + | |
| Crespo et al64 (2018) | Canada | CDSS, stakeholder engagement | Multicenter observational study | Secondary care | Whole prescribing process | Identification of medication discrepancies | N/A | |
| 1, 4 | Gill et al65 (2019) | United States | CDSS, prescriber feedback | Prospective randomized study | Primary care | Monitoring | Hemoglobin A1C levels | + |
| Quintens et al66 (2019) | Belgium | CDSS, prescription validation/intervention | Retrospective cohort study | Secondary care | Whole prescribing process | No. alerts resulting in pharmacist intervention, physician acceptance rates | + | |
| Carver et al67 (2018) | United States | Alerts, CDSS, prescription validation/intervention | Multicenter retrospective study | Secondary care | Whole prescribing process | Incidence of IV to oral therapy conversion, associated cost savings | + | |
| Kang et al68 (2018) | United States | Alerts, CDSS, prescription validation/intervention | Pre/post study | Secondary care | Prescribing/ordering, order communication | Formulary medication utilization | + | |
| Christ et al69 (2018) | United States | CDSS, prescription validation/intervention | Pre/post study | Secondary care | Prescribing/ordering, order communication | Attainment of analgesia at 24 h from admission | / | |
| Amor-Garcia70 (2018) | Spain | CPOE, prescription validation/intervention | Pre/post study | Secondary care | Prescribing/ordering, order communication | Implementation of additional pharmacist checks and CPOE | N/A | |
| Howell et al71 (2019) | United States | CDSS, prescription validation/intervention | Prospective study | Secondary care | Whole prescribing process | No. alerts, no. interventions | N/A | |
| Lesselroth et al72 (2018) | United States | Data dashboard, drug monitoring, prescription validation/intervention | Randomized controlled trial | Primary care | Monitoring, order communication | Medication reconciliation rates | / | |
| Horng73 (2018) | United States | Automated dispensing cabinet, CPOE, prescription validation/intervention | Pre/post study | Secondary care | Dispensing, administration, order communication | Time from antibiotic order entry to medication administration | + | |
| Peters-Strickland et al74 (2018) | United States | Data dashboard, drug monitoring, prescriber feedback | Human factor/validity study | Primary care | Monitoring | Performance task failure rates | + | |
| Harvin et al75 (2018) | United States | Data dashboard, prescription validation/intervention, drug monitoring | Pre/post study | Secondary care | Order communication | Time to intervention | + | |
| Kummer et al76 (2018) | United States | Order set, transferring ePrescribing data across boundaries of care | Pre/post study | Tertiary care | Whole prescribing process | Administration of tissue plasminogen activator | + | |
| 1–3 | Biltoft et al77 (2018) | United States | Smart infusion pumps, prescriber education, stakeholder engagement | Case study | Secondary care | Administration | Patient safety, revenue-generation gains | + |
| 1, 2, 4 | Gabel et al78 (2019) | United States | CDSS, prescriber education, prescriber feedback | Pre/post study | Secondary care | Prescribing/ordering, administration | Incidence of postoperative nausea and vomiting | + |
| Nguyen et al79 (2020) | United States | CDSS, prescriber education, prescription validation/intervention | Quality improvement study | Secondary care | Prescribing/ordering, order communication | Monthly spending on intravenous acetaminophen | + | |
| Gulliford et al80 (2019) | United States | CDSS, prescriber education, prescriber feedback | Cluster randomized controlled trial | Primary care | Prescribing/ordering | Rate of antibiotic prescriptions for respiratory tract infections | + | |
| Adeola et al81 (2018) | United States | Alerts, CDSS, order set, formulary changes, prescriber education | Pre/post study | Tertiary care | Prescribing/ordering | Exposure to target medications | + | |
| Gong et al82 (2019) | United States | CDSS, mandatory free-text justification of a prescription, prescriber feedback, prescriber education | Longitudinal study | Primary care | Prescribing/ordering | Cost-effectiveness and QALYs | + | |
| Shea et al83 (2018) | United States | Alerts, formulary changes, pharmacist education, prescription validation/intervention | Retrospective cohort study | Secondary care | Whole prescribing process | Medication error rate | + | |
| Muluneh et al84 (2018) | United States | Calculating adherence using medication possession ratio, closed-loop medication system, patient education, prescription validation/intervention | Pre/post study | Tertiary care | Whole prescribing process | Patient knowledge tests, adherence, molecular response rate | + | |
| Muth et al85 (2018) | United States | CDSS, Prescriber education, Prescription validation/intervention | Cluster randomized controlled trial | Primary care | Whole prescribing process | Mean Medication Appropriateness Index sum score | / | |
| Peyko et al86 (2018) | United States | Drug monitoring, prescriber and pharmacist education, prescription validation/intervention | Pre/post study | Secondary care | Prescribing/ordering, order communication | Timing of vancomycin level assessment | + | |
| 1–4 | Lane et al51 (2019) | United States | Data dashboard, staff and patient education, stakeholder engagement | Case study | Tertiary care | Whole prescribing process | Implementation of an antimicrobial stewardship program | N/A |
| Conners et al87 (2018) | United States | Order set, pay for performance bonuses, prescriber education, prescriber feedback, stakeholder engagement | Pre/post study | Secondary care | Prescribing/ordering | Asthma order set usage frequency | + | |
| Weiner et al88 (2019) | United States | Alerts, CDSS, order set, prescriber education, stakeholder engagement, transferring ePrescribing data across boundaries of care | Quality improvement study | Primary and secondary care | Whole prescribing process | No. opioid prescriptions per month | + |
1 = technological intervention; 2 = educational intervention; 3 = stakeholder engagement; 4 = organizational/process redesign. + = intervention significantly improved primary outcome; / = intervention effects were null.
IV, intravenous; QALY, quality-adjusted life year; N/A, intervention did not measure objective outcomes.
Many multicomponent interventions targeted a single step in the prescribing process, such as drug prescribing/ordering, medication administration, medication dispensing, and monitoring. In contrast, other multicomponent interventions targeted multiple stages in the prescribing process. In 1 study, a multifaceted approach included changes to many aspects of the CPOE system, alongside integration of data from regional databases and substantial work on culture change through education and targeted meetings, involving both clinical and IT professionals.88 In other cases, the focus of the multilevel intervention was much narrower in scope, for example, by targeting changes in technical systems to support barcode medicines administration.94
A multitude of optimization strategies were adopted within these 50 multicomponent interventions. The specific components implemented varied considerably between studies; however, these could be broadly categorized into 4 distinct intervention strategies, namely, technological innovations (such as installation of automated dispensing equipment), educational packages (such as formal training sessions for prescribers/pharmacists), organizational/process redesign interventions (such as formulary changes and additional personnel procedures during the medication reconciliation process), and stakeholder engagement (consisting of involving relevant staff in the design and adaptation of specific intervention strategies). These strategies were used in different combinations within the identified studies.
Some form of technological innovation was deployed in every multicomponent study. Of these, CDS was the most commonly used technology (n = 27) often with alerts (n = 13). Although some studies implemented alerts as an optimization strategy, many also sought to refine and modify these to reduce alert fatigue. For example, Kawamoto et al92 targeted alert fatigue by refining alerts in Epic using stakeholder engagement to determine how best to alter alert functionality. Specifically, an expert governance group was consulted and high-frequency, low-value alerts were identified and disabled or modified. This resulted in reported significant reductions in alerts per visit and overall alert volume over the 3-year period from baseline to posttest. In addition, alerts leading to a discontinuation of the triggering medication within 1 hour increased by a reported 17%. Five studies implemented a technological intervention in conjunction with some form of stakeholder engagement. Additional technological innovations included barcode medication administration (BCMA, n = 8), CPOE (n = 7), order sets (n = 7), visualization tools/data dashboards (n = 6), smart infusion pumps (n = 4), computerized medication reconciliation software (n = 2), and closed-loop medication systems (n = 2).
Education was a consistently implemented strategy across the included studies, being applied in some form within 23 of the multicomponent interventions. Education often coincided with the implementation/optimization of new ePrescribing technology and focused on adequately training staff to use the new systems/software. One study, for example, used a strategy of training clinical staff, while at the same time supporting cultural change to empower pharmacists to enforce restrictions within the prescribing process.79 Again, in some cases, the educational component was focused on effecting organizational change, whereas, in one example, it was provided by the ePrescribing system vendor to enable clinical users of the system to manage changes to the CDS.57
Eighteen studies used some form of organizational/process redesign. As with interventions involving education or stakeholder engagement, organizational/process redesign interventions typically accompanied some form of technological intervention. Small-scale changes, such as pharmacist-led prescription validation procedures, comprised the majority of these studies. However, a small number of studies involved more innovative, larger-scale interventions, such as the implementation of an antimicrobial stewardship program,51 a closed-loop, pharmacist-led oral chemotherapy program,84 integration of ePrescribing systems across boundaries of care,76,88 or the addition of mandatory free-text justification for a medication.82 There were also some examples of changes to organizational data governance within existing ePrescribing systems because of the integration of data from national and regional databases. This occurred, for example, in the case of a national database of drug-drug interactions66 and a state-level database of prescription drug monitoring programs.88
Relatively few studies directly measured aspects of patient harm (such as adverse drug events or mortality; Kim, 201840; Biltoft et al,77 2018; Gabel et al,78 2019). Instead, the majority used surrogate outcomes as proxy measures for patient safety or efficiency, such as medication error rates, hospital length of stay, blood sampling or timing of medication administration, among others (Tables 2, 3).
DISCUSSION
Principal Findings
There is now a substantial body of published work, mainly from the United States, that has targeted a range of medication management processes, using both relatively simple and more complex multifaceted interventions to enhance ePrescribing systems. Technological innovations comprised the majority of the strategies reported in the literature. However, technology-driven interventions were also often deployed in combination with behavioral components, including education, stakeholder engagement, and organizational change.
The fact that many studies are now focusing on refining alerts to reduce the burden of alert fatigue reflects the potential risks that can be introduced with digitization of prescribing processes. The healthcare context is also an important consideration for ePrescribing optimization strategies, with integrated healthcare systems aiding the detection of medication overuse, errors, and interactions. This is well illustrated by Jurado et al16 who used integrated data from community pharmacies to help detect medication discrepancies on admission to hospital and also Weiner et al88 who developed integrated information exchange between statewide emergency departments to counter opioid overuse. Conversely, nonintegrated healthcare systems will undoubtedly struggle to implement these optimization strategies.
Many strategies that successfully filter or contextualize data have shown evidence of success, particularly when such strategies focus on clinical impact, either by identifying high-risk patients or high-risk medications.28 Some studies have demonstrated that advanced capabilities can be achieved through the optimization of ePrescribing systems. The optimization of an ePrescribing system was central to the development of an antimicrobial stewardship program in an integrated healthcare system spanning many hospitals, with clinical decision support, data dashboards, and development of methods to track and report antimicrobial stewardship interventions. Likewise, highly integrated and contextualized environmental data from a ward setting allowed Campbell et al37 to provide real-time warnings to nursing staff about their risk of medication administration errors through a visual dashboard.
Achieving a return on investment through ePrescribing optimization was most frequently reported in the context of medication dispensing. Optimization with robotics and automation, whilst representing significant investments, seem to deliver a return on investment with quantifiable improvements to the safety and quality of the medication process.31,32,34–36
All of the single-component optimization strategies that we identified focused on technological interventions. This is likely to be a direct result of our search strategy and also our inclusion and exclusion criteria. Our searches combined broad terms, such as “quality improvement” and “audit”; however, our searches also focused on known optimization strategies, such as “CDS” and “alerts.” As such, to constitute optimization of an ePrescribing system, single interventions almost always had a technological aspect to ensure that the work was clearly relevant to ePrescribing systems. It is also possible that some of the single optimization strategies actually had more components to the intervention than were reported in the published study. For example, interventions may have been implemented with staff education; however, the educational component may not have been fully described. Of the articles that did describe the intervention, it was generally apparent that interventions with more components were also more likely to target multiple stages of the prescribing process, reflecting the complexities involved in optimizing numerous aspects of an ePrescribing system, and the need for a multifaceted approach.
Multicomponent interventions were overwhelmingly implemented within secondary care settings. Of these, all used either a quasi-experimental or observational study design, finding the intervention to be effective at optimizing the ePrescribing system in most studies (21/24 of studies measuring objective outcomes). Interestingly, results of multicomponent interventions within the primary care setting were more equivocal, with only 7 of the 10 studies reporting positive findings. Furthermore, all of the studies reporting null effects in the primary care setting were randomized controlled trials,44,72,85 possibly indicating that bias associated with nonrandomized and observational study designs may be inflating the observed effect estimates in secondary care settings.94 Inflated positive results, where randomization and blinding are not possible, also raise the possibility of the Hawthorne effect where subjects modify their behavior in response to being observed or measured. Conversely, it may be that the primary care setting presents barriers to the optimization of ePrescribing systems that are not present in secondary care. The primary care setting often provides continuity of care, with the reconciliation and arbitration of prescribed medications from a variety of other settings. The extent of integration and data sharing between hospitals, primary care, and community pharmacies within a healthcare system will either facilitate or impede the detection of medication errors and interactions. Where strong integration and transfer of data exist, optimization of ePrescribing systems may not alter what are already deemed to be well-managed patients and safe prescribing practices.44,85 However, as no formal appraisal of study quality was undertaken (as per usual practice in scoping reviews), it cannot be assumed that these results were not merely due to study quality issues, and the points discussed previously merit further investigation.
While a scarcity of experimental research was a finding of note in this review, so too is the abundant use of surrogate outcomes. The use of surrogate outcomes to determine the effectiveness of the included studies is unsurprising given the rarity of adverse events, which lead to clinically significant harm, coupled with the large sample sizes required to detect statistically significant changes to this outcome. However, although outcomes, such as medication error rates, do correlate relatively well with patient harm, the relationship between patient harm and other outcomes, such as efficiency of medication administration, or prescriber satisfaction is less clear. Therefore, interventions reporting positive results for such outcomes do not definitively demonstrate an improvement to patient safety and should be interpreted with caution.
Strengths and Limitations
By following the principles of inductive thematic saturation,13 working backward chronologically, this has allowed the scoping review to succinctly map the most emergent and technologically relevant optimization strategies in this diverse field of literature. In this study, we describe a novel application of data saturation within a scoping review. Although we have carefully linked the concept of saturation to a central objective of the scoping review, namely, to describe the range of approaches to optimization, we acknowledge that by working backward through the most recently published literature, this may have introduced bias. By reaching saturation and only capturing the most recent optimization strategies, our scoping review could be missing fundamental optimization strategies in the ePrescribing journey that have been historically reported.
Assessing the impact of the various optimization strategies described in the literature is also challenging. Many studies measured the success of their interventions by using surrogate measures without addressing important clinical outcome measures. Even when studies did consider both process and outcome measures, improving ePrescribing processes with optimization strategies did not guarantee success in terms of clinical outcomes. For example, Figueroa et al22 successfully optimized ePrescribing alerts, resulting in improved prescribing of low molecular weight heparin; however, this did not have a significant impact on the incidence of venous thromboembolic events. Vice versa, Pendharkar et al25 reduced the length of acute hospital admissions in chronic obstructive pulmonary disease (COPD) patients but also reported a low uptake of the order set that was critical to their optimization strategy.
Another limitation of this scoping review is that optimization strategies that are easy to measure or that are amenable to research and publication will be overrepresented in the literature. This publication bias may have led to strategies that are difficult to measure or publish not appearing in the literature. We postulate that this may relate to some of the fundamental elements of system maintenance that may not be considered “worthy” of publication and also elements of optimization that are difficult to measure such as cyber security, software updates, and data storage and backup.
Implications for Policy, Practice, and Research
Pharmacists, clinicians, nurses, hospital management, and health IT specialists are all key stakeholders in driving forward improvements in medicine management. Consulting and working with key stakeholders were identified in many studies as an important facilitator in the ePrescribing optimization journey.87,88 Pharmacists, as custodians of many of the stages within the medication use process, are rightly reflected in the literature as playing a central role in many of the optimization strategies encountered. Patients were, however, notably exempt as stakeholders—an oversight that will need to be addressed to ensure that the voices of the main beneficiaries are adequately heard.
It also seems that pharmacists, doctors, and nurses use ePrescribing systems as customizable tools to deliver localized and specialty-specific quality improvement aims. These optimization strategies can be powerful, achieving strong clinical buy-in and ownership, while also allowing ePrescribing systems and workflows to be customized extensively to local clinical and specialty-specific needs.81 Although localized innovation may be an effective method to improve usability and relevance of ePrescribing systems, optimization at scale will be dependent on success stories being cascaded and efficiently applied elsewhere. Poorly managed localized customization has the risk of leading to increasingly divergent systems and workflows, making policy deliberations and large-scale interventions difficult to manage. Policy-focused interventions will need to strike a balance between being sensitive to local needs, while delivering interventions that can drive tangible improvements in clinical outcome measures across large patient populations.
When extracting data, we aimed to link the optimization strategies being deployed with ePrescribing systems, third-party software, and apps. Unfortunately, the scoping review revealed a paucity of technical data and many studies neglected to name the ePrescribing software (n = 53) let alone describe the system specifications and capabilities. When describing changes, improvements, and optimization strategies relating to ePrescribing systems, authors should endeavor to name the software system and the version being used. Journals could play a role in mandating minimum levels of critical information and providing authors with appendices to document technical specifications.
CONCLUSIONS
There is now substantial knowledge on approaches to optimize and potentially enhance the beneficial impacts of hospital ePrescribing systems. These include targeting single or multiple facets of the medicines management process. These approaches can be categorized as those focusing primarily on understanding and responding to stakeholder needs, reconceptualizing the medicines management process of care, technological innovations, and educational and training packages. Simultaneously deploying a combination of these approaches is likely to have the greatest impact on realizing the benefits of this increasingly ubiquitous technology.
ACKNOWLEDGMENTS
The authors thank the following members of the research team who assisted with the literature screening: Erica Mason, Sena Nur Arduc, Abdul Haseeb, and Ghadah Assiri. The authors acknowledge the support of colleagues in the Department of Health and Social Care, the National Health Service and the Medicines and Healthcare products Regulatory Agency (MHRA): Ann Slee, Jason Cox, Richard Cattell, Helen Causley, Paul Stonebrook, Mick Foy, Kathryn Ord, and Graeme Kirkpatrick. The authors also thank our patient and public representatives, Antony Chuter and Jillian Beggs, as well as Donna Watson, Academic Support Librarian at the University of Edinburgh.
Footnotes
D.W.B. is the Editor-in-Chief of the Journal of Patient Safety. A.S. is supported by the Medical Research Council through its funding of Health Data Research UK. This study/project is funded by the National Institute for Health Research (NIHR, Optimizing ePrescribing in Hospitals [PR-ST-01-10001]/Policy Research Program). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care The other authors disclose no conflict of interest.
The study was conceived and designed by A.S. and D.W.B. The study protocol was written by J.W., A.S. and D.W.B. The literature search strategy was written by J.W. and A.S. Abstracts and full texts were screened by J.W., S.M., C.H., M.B., M.H., and U.P. Data were extracted by J.W., S.M., C.H., and M.B. All authors contributed equally to data analysis and interpretation. All authors contributed equally to writing the manuscript.
Contributor Information
Stephen Malden, Email: Stephen.Malden@ed.ac.uk.
Catherine Heeney, Email: Catherine.Heeney@ed.ac.uk.
Matt Bouamrane, Email: Matt.Bouamrane@ed.ac.uk.
Mike Holder, Email: M.W.Holder@sms.ed.ac.uk.
Uditha Perera, Email: Uditha.Perera@ed.ac.uk.
David W. Bates, Email: DBATES@PARTNERS.ORG.
Aziz Sheikh, Email: aziz.sheikh@ed.ac.uk.
REFERENCES
- 1.Catwell L, Sheikh A. Information technology (IT) system users must be allowed to decide on the future direction of major national IT initiatives. But the task of redistributing power equally amongst stakeholders will not be an easy one. Inform Prim Care. 2009;17:1–4. [DOI] [PubMed] [Google Scholar]
- 2.Black AD Car J Pagliari C, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med. 2011;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sittig DF, Stead WW. Computer-based physician order entry: the state of the art. J Am Med Inform Assoc. 1994;1:108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pontefract SK Hodson J Slee A, et al. Impact of a commercial order entry system on prescribing errors amenable to computerised decision support in the hospital setting: a prospective pre-post study. BMJ Qual Saf. 2018;27:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock N, Williams R. E-infrastructures: how do we know and understand them? Strategic ethnography and the biography of artefacts. Comput Support Coop Work. 2010;19:521–556. [Google Scholar]
- 6.NHS England . Global digital exemplars. Available at: https://www.england.nhs.uk/digitaltechnology/connecteddigitalsystems/exemplars/. Accessed December 13, 2020.
- 7.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 8.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams J, Bates DW, Sheikh A. Optimising electronic prescribing in hospitals: a scoping review protocol. BMJ Health Care Inform. 2020;27:e100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidd TT. Handbook of research on technology Project Management, planning, and operations: 9781605664002: Library & Information Science Books | IGI global. Available at: https://www.igi-global.com/book/handbook-research-technology-project-management/510#table-of-contents. Accessed December 13, 2020.
- 11.NHS Connecting for Health . Patient safety through e-prescribing: E-health insider and British computer society health informatics forum, 2008. Available at: https://www.digitalhealth.net/includes/images/Document_Library0282/E-prescribingRoundTableReaderFeedback.pdf. Accessed December 13, 2020.
- 12.Saunders B Sim J Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Given LM. 100 questions (and answers) about qualitative research. California: SAGE Publications; 2015. pg 135. [Google Scholar]
- 14.Bates DW Cullen DJ Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE prevention study group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 15.Gayoso-Rey M Romero-Ventosa EY Leboreiro-Enríquez B, et al. Standardization consensus of a hospital drug database: an efficient tool. Ther Innov Regul Sci. 2020;54:85–92. [DOI] [PubMed] [Google Scholar]
- 16.Jurado C Calmels V Lobinet E, et al. The electronic pharmaceutical record: a new method for medication reconciliation. J Eval Clin Pract. 2018;24:681–687. [DOI] [PubMed] [Google Scholar]
- 17.Epstein D Barak-Corren Y Isenberg Y, et al. Clinical decision support system: a pragmatic tool to improve acute exacerbation of COPD discharge recommendations. COPD. 2019;16:18–24. [DOI] [PubMed] [Google Scholar]
- 18.Daniels CC Burlison JD Baker DK, et al. Optimizing drug-drug interaction alerts using a multidimensional approach. Pediatrics. 2019;143:e20174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wai JM Aloezos C Mowrey WB, et al. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S Price C Agarwal G, et al. The electronic asthma management system (eAMS) improves primary care asthma management. Eur Respir J. 2019;53:1802241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orchard J Neubeck L Freedman B, et al. eHealth tools to provide structured assistance for atrial fibrillation screening, management, and guideline-recommended therapy in metropolitan general practice: the AF-SMART study. J Am Heart Assoc. 2019;8:e010959. Available at: 10.1161/JAHA.118.010959. Accessed April 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa R Alfonso A López-Picazo J, et al. Improvement of appropriate pharmacological prophylaxis in hospitalised cancer patients with a multiscreen e-alert system: a single-Centre experience. Clin Transl Oncol. 2019;21:805–809. [DOI] [PubMed] [Google Scholar]
- 23.Gaweda AE Jacobs AA Aronoff GR, et al. Individualized anemia management in a dialysis facility—long-term utility as a single-center quality improvement experience. Clin Nephrol. 2018;90:276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhloufi H Hulscher M van der Hoeven CP, et al. A clinical decision support system algorithm for intravenous to oral antibiotic switch therapy: validity, clinical relevance and usefulness in a three-step evaluation study. J Antimicrob Chemother. 2018;73:2201–2206. [DOI] [PubMed] [Google Scholar]
- 25.Pendharkar SR Ospina MB Southern DA, et al. Effectiveness of a standardized electronic admission order set for acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2018;18:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpao AF Ahumada LM Larru Martinez B, et al. Design and implementation of a visual analytics electronic antibiogram within an electronic health record system at a tertiary pediatric hospital. Appl Clin Inform. 2018;9:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi KS, Lee E, Rhie SJ. Impact of pharmacists’ interventions on physicians’ decision of a knowledge-based renal dosage adjustment system. Int J Clin Pharmacol. 2019;41:424–433. [DOI] [PubMed] [Google Scholar]
- 28.Jourdan J-P Muzard A Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharmacol. 2018;40:1474–1481. [DOI] [PubMed] [Google Scholar]
- 29.Groppi JA Ourth H Morreale AP, et al. Advancement of clinical pharmacy practice through intervention capture. Am J Health Syst Pharm. 2018;75:886–892. [DOI] [PubMed] [Google Scholar]
- 30.Durvasula R Kelly J Schleyer A, et al. Standardized review and approval process for high-cost medication use promotes value-based care in a large academic medical system. Am Health Drug Benefits. 2018;11:65–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Berdot S Korb-Savoldelli V Jaccoulet E, et al. A centralized automated-dispensing system in a French teaching hospital: return on investment and quality improvement. International J Qual Health Care. 2019;31:219–224. [DOI] [PubMed] [Google Scholar]
- 32.Lupi KE Day KM Gilmore JF, et al. Evaluation of a clinical pharmacist-led automated dispensing cabinet stewardship program at a tertiary academic medical center. J Pharm Pract. 2020;33:576–579. [DOI] [PubMed] [Google Scholar]
- 33.Campmans Z van Rhijn A Dull RM, et al. Preventing dispensing errors by alerting for drug confusions in the pharmacy information system—a survey of users. PLoS One. 2018;13:e0197469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Gonzalez CG Herranz-Alonso A Escudero-Vilaplana V, et al. Robotic dispensing improves patient safety, inventory management, and staff satisfaction in an outpatient hospital pharmacy. J Eval Clin Pract. 2019;25:28–35. [DOI] [PubMed] [Google Scholar]
- 35.Beobide Telleria I. Impacto de la automatización en la seguridad de la dispensación de medicamentos a centros. Farm Hosp. 2018;04:141–146. [DOI] [PubMed] [Google Scholar]
- 36.Bhakta SB Carmine Colavecchia A Coffey W, et al. Implementation and evaluation of a sterile compounding robot in a satellite oncology pharmacy. Am J Health Syst Pharm. 2018;75(11 suppl 2):S51–S57. [DOI] [PubMed] [Google Scholar]
- 37.Campbell AA, Harlan T, Campbell M. Using real-time data to warn nurses of medication administration errors using a nurse situational awareness dashboard. Stud Health Technol Inform. 2018;250:140–141. [PubMed] [Google Scholar]
- 38.Pereboom M Mulder IJ Verweij SL, et al. A clinical decision support system to improve adequate dosing of gentamicin and vancomycin. Int J Med Inf. 2019;124:1–5. [DOI] [PubMed] [Google Scholar]
- 39.Beeler PE Eschmann E Schneemann M, et al. Negligible impact of highly patient-specific decision support for potassium-increasing drug-drug interactions – a cluster-randomised controlled trial. Swiss Med Wkly. 2019;149:w20035. [DOI] [PubMed] [Google Scholar]
- 40.Kim K Magness JW Nelson R, et al. Clinical utility of pharmacogenetic testing and a clinical decision support tool to enhance the identification of drug therapy problems through medication therapy management in polypharmacy patients. J Manag Care Spec Pharm. 2018;24:1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burkoski V Yoon J Solomon S, et al. Closed-loop medication system: leveraging technology to elevate safety. Can J Nurs Leadersh. 2019;32(SP):16–28. [DOI] [PubMed] [Google Scholar]
- 42.Pettit NN Han Z Choksi A, et al. Reducing medication errors involving antiretroviral therapy with targeted electronic medical record modifications. AIDS Care. 2019;31:893–896. [DOI] [PubMed] [Google Scholar]
- 43.Bowdle TA Jelacic S Nair B, et al. Facilitated self-reported anaesthetic medication errors before and after implementation of a safety bundle and barcode-based safety system. Br J Anaesth. 2018;121:1338–1345. [DOI] [PubMed] [Google Scholar]
- 44.Ashburner JM Atlas SJ Khurshid S, et al. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. J Gen Intern Med. 2018;33:2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risør BW, Lisby M, Sørensen J. Complex automated medication systems reduce medication administration errors in a Danish acute medical unit. Int J Qual Health Care. 2018;30:457–465. [DOI] [PubMed] [Google Scholar]
- 46.Schnock KO Dykes PC Albert J, et al. A multi-hospital before–after observational study using a point-prevalence approach with an infusion safety intervention bundle to reduce intravenous medication administration errors. Drug Saf. 2018;41:591–602. [DOI] [PubMed] [Google Scholar]
- 47.Desmedt S Spinewine A Jadoul M, et al. Impact of a clinical decision support system for drug dosage in patients with renal failure. Int J Clin Pharmacol. 2018;40:1225–1233. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson LO Nilsson S Bång M, et al. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: a cluster-randomized trial in a Swedish primary care setting (the CDS-AF study). PLoS Med. 2018;15:e1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunn L, Tunney R, Kelly K. Nonmodal clinical decision support and antimicrobial restriction effects on rates of fluoroquinolone use in uncomplicated infections. Appl Clin Inform. 2018;09:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macias M Bernabeu-Andreu FA Arribas I, et al. Impact of a barcode medication administration system on patient safety. Oncol Nurs Forum. 2018;45:E1–E13. [DOI] [PubMed] [Google Scholar]
- 51.Lane MA Hays AJ Newland H, et al. Development of an antimicrobial stewardship program in an integrated healthcare system. Am J Health Syst Pharm. 2019;76:34–43. [DOI] [PubMed] [Google Scholar]
- 52.Rosa R Zavala B Cain N, et al. Antimicrobial stewardship program implementation of a quality improvement intervention using real-time feedback and an electronic order set for the management of Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2018;39:346–349. [DOI] [PubMed] [Google Scholar]
- 53.Ilcewicz HN, Hennessey EK, Smith CB. Evaluation of the impact of an inpatient hyperglycemia protocol on glycemic control. J Pharm Pharm Sci. 2019;22:85–92. [DOI] [PubMed] [Google Scholar]
- 54.MacMaster HW Gonzalez S Maruoka A, et al. Development and implementation of a subcutaneous insulin pen label bar code scanning protocol to prevent wrong-patient insulin pen errors. Jt Comm J Qual Patient Saf. 2019;45:380–386. [DOI] [PubMed] [Google Scholar]
- 55.Thompson KM Swanson KM Cox DL, et al. Implementation of bar-code medication administration to reduce patient harm. Mayo Clin Proc Innov Qual Outcomes. 2018;2:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathioudakis N Jeun R Godwin G, et al. Development and implementation of a subcutaneous insulin clinical decision support tool for hospitalized patients. J Diabetes Sci Technol. 2019;13:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mainous AG Carek PJ Lynch K, et al. Effectiveness of clinical decision support based intervention in the improvement of care for adult sickle cell disease patients in primary care. J Am Board Fam Med. 2018;31:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gulati S Zouk AN Kalehoff JP, et al. The use of a standardized order set reduces systemic corticosteroid dose and length of stay for individuals hospitalized with acute exacerbations of COPD: a cohort study. Int J Chron Obstruct Pulmon Dis. 2018;13:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Sullivan CT Rogers WK Ackman M, et al. Implementation of a multifaceted program to sustainably improve appropriate intraoperative antibiotic redosing. Am J Infect Control. 2019;47:74–77. [DOI] [PubMed] [Google Scholar]
- 60.Connor JP Raife T Medow JE, et al. The blood utilization calculator, a target-based electronic decision support algorithm, increases the use of single-unit transfusions in a large academic medical center. Transfusion (Paris). 2018;58:1689–1696. [DOI] [PubMed] [Google Scholar]
- 61.Tamblyn R Winslade N Lee TC, et al. Improving patient safety and efficiency of medication reconciliation through the development and adoption of a computer-assisted tool with automated electronic integration of population-based community drug data: the RightRx project. J Am Med Inform Assoc. 2018;25:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muhlenkamp R Ash N Ziegenbusch K, et al. Effect of modifying dose alerts in an electronic health record on frequency of alerts. Am J Health Syst Pharm. 2019;76(suppl 1):S1–S8. [DOI] [PubMed] [Google Scholar]
- 63.Kawamanto K Flynn MC Kukhareva P, et al. A pragmatic guide to establishing clinical decision support governance and addressing decision support fatigue: a case study. AMIA Annu Symp Proc. 2018;2018:624–633. [PMC free article] [PubMed] [Google Scholar]
- 64.Crespo A Redwood E Vu K, et al. Improving the safety and quality of systemic treatment regimens in computerized prescriber order entry systems. J Oncol Pract. 2018;14:e393–e402. [DOI] [PubMed] [Google Scholar]
- 65.Gill J Kucharski K Turk B, et al. Using electronic clinical decision support in patient-centered medical homes to improve management of diabetes in primary care: the DECIDE study. J Ambul Care Manag. 2019;42:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quintens C De Rijdt T Van Nieuwenhuyse T, et al. Development and implementation of “check of medication appropriateness” (CMA): advanced pharmacotherapy-related clinical rules to support medication surveillance. BMC Med Inform Decis Mak. 2019;19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carver KH Burgess LH Cooper M, et al. Use of clinical decision support to identify i.v.-to-oral conversion opportunities and cost savings. Am J Health Syst Pharm. 2018;75(23 suppl 4):S82–S86. [DOI] [PubMed] [Google Scholar]
- 68.Kang A Thompson A Rau J, et al. Effects of clinical decision support and pharmacist prescribing authority on a therapeutic interchange program. Am J Health Syst Pharm. 2018;75(17 suppl 3):S77–S81. [DOI] [PubMed] [Google Scholar]
- 69.Christ TN Villadolid JJ Choksi A, et al. Impact of a clinical decision support tool on cancer pain management in opioid-tolerant inpatients. Hosp Pharm. 2018;53:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amor-García MÁ Ibáñez-García S Díaz-Redondo A, et al. Multidisciplinary strategy to reduce errors with the use of medical gases. Farm Hosp Organo Of Expresion Cient Soc Espanola Farm Hosp. 2018;42:103–107. [DOI] [PubMed] [Google Scholar]
- 71.Howell CK, Jacob J, Mok S. Remote antimicrobial stewardship: a solution for meeting the joint commission stewardship standard? Hosp Pharm. 2019;54:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lesselroth B Adams K Church V, et al. Evaluation of multimedia medication reconciliation software: a randomized controlled, single-blind trial to measure diagnostic accuracy for discrepancy detection. Appl Clin Inform. 2018;09:285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horng M Brunsman AC Smoot T, et al. Using lean methodology to optimize time to antibiotic administration in patients with sepsis. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2018;75(5 Suppl 1):S13–S23. [DOI] [PubMed] [Google Scholar]
- 74.Peters-Strickland T Hatch A Adenwala A, et al. Human factors evaluation of a novel digital medicine system in psychiatry. Neuropsychiatr Dis Treat. 2018;14:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvin A Mellett JJJ Knoell D, et al. Implementation of a prioritized scoring tool to improve time to pharmacist intervention. Am J Health Syst Pharm. 2018;75:e50–e56. [DOI] [PubMed] [Google Scholar]
- 76.Kummer B Lerario M Navi B, et al. Clinical information systems integration in New York City’s first mobile stroke unit. Appl Clin Inform. 2018;9:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biltoft J, Finneman L. Clinical and financial effects of smart pump–electronic medical record interoperability at a hospital in a regional health system. Am J Health Syst Pharm. 2018;75:1064–1068. [DOI] [PubMed] [Google Scholar]
- 78.Gabel E Shin J Hofer I, et al. Digital quality improvement approach reduces the need for rescue antiemetics in high-risk patients: a comparative effectiveness study using interrupted time series and propensity score matching analysis. Anesth Analg. 2019;128:867–876. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen LP, Nguyen L, Austin JP. A quality improvement initiative to decrease inappropriate intravenous acetaminophen use at an academic medical center. Hosp Pharm. 2020;55:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gulliford MC Prevost AT Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;12:l236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adeola M Azad R Kassie GM, et al. Multicomponent interventions reduce high-risk medications for delirium in hospitalized older adults: reducing high-risk medications in older adults. J Am Geriatr Soc. 2018;66:1638–1645. [DOI] [PubMed] [Google Scholar]
- 82.Gong CL Zangwill KM Hay JW, et al. Behavioral economics interventions to improve outpatient antibiotic prescribing for acute respiratory infections: a cost-effectiveness analysis. J Gen Intern Med. 2019;34:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shea KM Hobbs ALV Shumake JD, et al. Impact of an antiretroviral stewardship strategy on medication error rates. Am J Health Syst Pharm. 2018;75:876–885. [DOI] [PubMed] [Google Scholar]
- 84.Muluneh B Schneider M Faso A, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14:e324–e334. [DOI] [PubMed] [Google Scholar]
- 85.Muth C Uhlmann L Haefeli WE, et al. Effectiveness of a complex intervention on Prioritising multimedication in multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open. 2018;8:e017740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peyko V, Friedman-Jakubovics M. Novel approach to vancomycin level monitoring: impact of a multidisciplinary monitoring system on timing of vancomycin levels. Am J Health Syst Pharm. 2018;75:121–126. [DOI] [PubMed] [Google Scholar]
- 87.Conners GP Fowler MA Lee BR, et al. A quality improvement bundle including pay for performance for the standardization of order set use in moderate asthma. Pediatr Emerg Care. 2018;34:740–742. [DOI] [PubMed] [Google Scholar]
- 88.Weiner SG Price CN Atalay AJ, et al. A health system–wide initiative to decrease opioid-related morbidity and mortality. Jt Comm J Qual Patient Saf. 2019;45:3–13. [DOI] [PubMed] [Google Scholar]
- 89.Horng M Brunsman AC Smoot T, et al. Using lean methodology to optimize time to antibiotic administration in patients with sepsis. Am J Health Syst Pharm. 2018;75(5 suppl 1):S13–S23. [DOI] [PubMed] [Google Scholar]
- 90.Ibáñez-García S. Estrategia multidisciplinar para reducir errores en el uso de los gases medicinales. Farm Hosp. 2018;3:103–107. [DOI] [PubMed] [Google Scholar]
- 91.Kawamoto K Flynn MC Kukhareva P, et al. A pragmatic guide to establishing clinical decision support governance and addressing decision support fatigue: a case study. AMIA Annu Symp Proc. 2018;2018:624–633. [PMC free article] [PubMed] [Google Scholar]
- 92.Ni Y Lingren T Hall ES, et al. Designing and evaluating an automated system for real-time medication administration error detection in a neonatal intensive care unit. J Am Med Inform Assoc. 2018;25:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Risør BW, Lisby M, Sørensen J. Complex automated medication systems reduce medication administration errors in a Danish acute medical unit. International J Qual Health Care. 2018;30:457–465. [DOI] [PubMed] [Google Scholar]
- 94.MacLehose RR Reeves BC Harvey IM, et al. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess. 2000;4:1–154. [PubMed] [Google Scholar]


