Aim
There is lack of evidence regarding the screening role of ECG for sudden cardiac death (SCD) prevention. Our aim was to evaluate the prevalence of ECG abnormalities among teenagers according to sport participation and competitive status.
Methods
Eleven thousand nine hundred and forty-nine Italian pupils from 179 secondary schools (13–19 years) were consecutively enrolled. ECG abnormalities were divided into minor and major. Medical history, clinical examination and sport activity information were acquired. Further evaluations were suggested in case of major ECG abnormalities. Follow-up was performed at 2 years.
Results
N = 1945 (16%) pupils had ECG abnormalities. Major ECG abnormalities were detected in 13% of the cohort, minor in 34%. ECG abnormalities were more common in nonathletes compared with athletes. A diagnosis of cardiac disease was reached in 25 (1.6%) of the pupils with major ECG abnormalities.
Conclusion
ECG abnormalities are common among young populations and more prevalent in nonathletes. Among pupils with major ECG abnormalities 1.6% had a cardiac disease diagnosis. Our results are in line with the data supporting ECG screening in the general young population.
Keywords: competitive athletes, ECG, noncompetitive athletes, screening, sudden cardiac death, teenagers
Introduction
Sudden cardiac death in the young (SCD-Ys) is a shocking event and represents an unresolved public health problem. The incidence of SCD-Ys ranges from 0.46 to 3.7 per 100 000 persons.1 Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, coronary artery abnormalities, acute myocarditis and cardiac ion channelopathies represent the leading causes of SCD-Ys and are frequently under-detected before the occurrence of the fatal event.1 SCD often occurs without any previous warning symptoms.
A large body of evidence has demonstrated the usefulness of ECG in the early diagnosis of heart disease associated with SCD.2–6 Nevertheless, ECG screening programs for the general young population are still being debated.7 According to the European Society of Cardiology (ESC)3,4 and the International Olympic Committee,8 an ECG is recommended for the evaluation of young competitive athletes in order to prevent SCD, while routine ECG in young competitive athletes is not recommended by the American Heart Association.7
Recently, the UK national screening program in the general young population found that the inclusion of an ECG increases the diagnostic yield for cardiac disease by five-fold and it is associated with a significant cost reduction.6
Maron et al.9 analyzed retrospectively the Minnesota registry observing that SCD was eight-fold more common in nonathletes than athletes. A comparison of the prevalence of ECG abnormalities between competitive athletes, noncompetitive athletes and sedentary subjects is lacking.
The aim of the present study is to evaluate the prevalence of ECG abnormalities among an unselected population of Italian students (teenagers) and to evaluate the difference between nonathletes, competitive athletes, and noncompetitive athletes.
Methods
Study population
Sudden Cardiac Death Prevention Program in the young is a prospective observational registry funded by the ‘Italian Heart and Circulation Foundation’ and ‘Fondazione Roma’ created in order to screen Italian pupils by ECG.
Over an interval time of 4 years, 11 949 Italian pupils from 179 secondary schools (lower and upper school, aged 13–19 years) among 10 different regions (Veneto, Abruzzo, Calabria, Campania, Lazio, Lombardia, Piemonte, Puglia, Sicilia, Toscana) were consecutively enrolled in the study and underwent ECG screening. There were no exclusion criteria. Informed consent was obtained by the student if at least 18 years old or by parents in the case of minors. The study was approved by the hospital Ethical Review Board at the Policlinico ‘Umberto I’, University Hospital of ‘Sapienza’ University of Rome, according to the Declaration of Helsinki.
ECG acquisition and analysis
Standard 12-lead ECGs were performed in the supine position using the handheld Mortara ELI-10 Mobile paperless electrocardiograph. ECG tracing was achieved at 25 mm/s paper speed and 10 mm/mV. Low pass filtering was set at 40 mHz.
Acquisition was performed in the schools by a group of 10 volunteer cardiologists in order to guarantee high-quality ECG trace and the possibility to evaluate the ECG immediately after the acquisition. A second ECG was acquired whether the first ECG showed one of the following conditions: PR interval greater than 200 ms, ECG was acquired after hyperventilation; abnormal T-wave inversion in leads V2–V4, ECG was repeated at 50 mm/s paper speed in order to detect epsilon waves; type III Brugada pattern in leads V1 or V2, ECG was repeated with leads V1 and V2 placed in the third or second intercostal space.
Each ECG was then transmitted to the ECG analysis center at Sapienza University of Rome, where two dedicated cardiologists evaluated all the ECGs defining them as ‘normal’ or ‘abnormal’, blinded to sport information. Furthermore, ECG abnormalities were divided into ‘minor’ and ‘major’ according to the definition reported in Table 1 and adapted from the latest recommendations for electrocardiographic interpretation in athletes.10 The classification of ECG findings was modified as the ECG analysis was carried out blinded to sport information and the same criteria were used for the whole population.
Table 1.
Definition of abnormal electrocardiographic findings
| Abnormal ECG finding | Definition |
| Major abnormal ECG findings | |
| T-wave inversion | More than 1 mm in depth from baseline in two or more adjacent leads not including aVR or V1 |
| ST-segment depression | At least 1 mm in depth in two or more adjacent leads |
| Pathological Q waves | More than 3 mm in depth or more than 0.04 s in duration in two or more leads |
| Complete left bundle branch block | QRS more than 0.12 s, predominantly negative QRS complex in lead V1 (QS or rS), and upright monophasic R wave in leads I and V6 |
| Complete right bundle branch block | QRS more than 0.12 s, terminal R wave in lead V1 (rsR’) and wide terminal S wave in leads I and V6 |
| Left anterior fascicular block | Frontal plane axis between −45° and −90°; qR pattern in lead aVL; R-peak time in lead aVL of 45 ms or more; QRS duration less than 0.12 s |
| Left posterior fascicular block (LPFB) | Frontal plane axis between 90° and 180° in adults; rS pattern in leads I and aVL; qR pattern in leads III and aVF; QRS duration less than 0.12 s |
| Left atrial enlargement | Prolonged P-wave duration of more than 0.12 s in leads I or II with negative portion of the P wave at least 1 mm in depth and at least 0.04 s in duration in lead V1 |
| Left axis deviation | −30° to −90° |
| Right atrial enlargement | High/pointed P wave at least 2.5 mm in leads II and III or V1 |
| Right axis deviation | +90° and +180° |
| Mobitz type II 2° AV block | Intermittently nonconducted P waves not preceded by PR prolongation and not followed by PR shortening |
| 3° AV block | Complete heart block |
| Ventricular preexcitation WPW | PR interval less than 0.12 s with a δ wave (slurred upstroke in the QRS complex) |
| Long QT interval | QTc at least 0.47 s (99% men) QTc at least 0.48 s (99% women) QTc at least 0.50 s (unequivocal LQTS) |
| Short QT interval | QTc 0.34 s or less |
| Brugada-like ECG pattern | High take-off and downsloping ST-segment elevation in V1–V3 |
| Epsilon wave | Small negative deflection just beyond the QRS in V1 or V2 |
| Profound sinus bradycardia | Less than 30 bpm or sinus pauses at least 3 s |
| Atrial tachyarrhythmias | Supraventricular tachycardia, atrioventricular nodal re-entrant tachycardia, atrial fibrillation, atrial flutter |
| Premature ventricular contractions | At least two per tracing |
| Ventricular arrhythmias | Couplets, triplets, nonsustained ventricular tachycardia |
| Left ventricular hypertrophy | RaVL at least 11 mm R wave in V5 or V6 plus S wave in V1 > 35 mm; SV2 > 30 mm + SV3 > 25mm; |
| Right ventricular hypertrophy | Right axis deviation at least 120°, tall R wave in V1+persistent precordial S waves (R–V1+S–V5 > 10.5 mm) |
| J wave | J waves were defined as an upward deflection, slurs as a conduction delay beginning on the QRS downstroke |
| Wandering pacemaker | At least three different P-wave morphologies and a ventricular rate less than 100 bpm |
| Atrial premature beats | At least two per tracing |
| First degree AV- block | PQ greater than 0.20 s |
| Mobitz type I 2° AV block | Intermittently nonconducted P waves preceded by PR prolongation |
| Short PQ interval | PQ less than 0.12 s |
| Rhythm sinus coronary | P waves that are inverted in leads II, III, and aVF with a normal or prolonged P-R interval |
| Sinus bradycardia | Heart rate less than 60 bpm |
| Sinus tachycardia | Heart rate more than 100 bpm |
| Incomplete right bundle branch block | QRS greater than 0.10 and less than 0.12 s, terminal R wave in lead V1 (rsR’) and wide terminal S wave in leads I and V6 |
| Early repolarization | With the PR interval as the isoelectric line and the amplitude criteria as at least 0.1 mV, J waves were defined as an upward deflection, slurs as a conduction delay beginning on the QRS downstroke, and ST-segment elevation at the end of the QRS complex. The criterion requiring ST-segment elevation in two contiguous leads in any lead group (inferior: II, III, and AVF; lateral: I, aVL, and V4–V6; anterior: V1–V3) was applied. (references) |
| Sinus arrhythmia | Increase in heart rate that occurs during inspiration |
AV, atrioventricular.
For the ECG analysis, all the following evaluations were performed manually with the use of the calipers: heart rate; QRS axis; P-wave, Q-wave, R-wave, S-wave, and T-wave voltages; ST-segments; QRS duration; PR interval and QT interval. The QT interval was measured by means of the tangent method and corrected for heart rate with Bazett's formula.
In case a second ECG was acquired during the first level of analysis, the first ECG was marked in a dedicated box, in order to inform the ECG analysis center to analyze the second ECG.
Pupils who had major abnormal ECG findings had been informed and referred to the local cardiologist for further evaluation.
Medical history and clinical examination
The presence of risk factors, past medical history and sporting activity were investigated by a questionnaire.
Competitive athletes were considered ‘individuals who are involved in regular (usually intense) training in organized individual or team sports, with an emphasis on competition and performance’. They ‘span the age spectrum and can compete at the youth, high school, academy, university, semi-professional, professional, national, international, and Olympic levels’. Usually, they exercise more than 6 h/week.
Noncompetitive athletes were defined as individuals ‘engaged in sports for pleasure and leisure-time activity’.11 Nonathletes constituted sedentary individuals.
Follow-up
A follow-up at 12 and 24 months was performed by phone call in order to assess the incidence of overall death, cardiac death, resuscitated cardiac arrest or diagnosis of cardiac disease. For unavailable students, the schools were directly contacted in order to assess if any death had occurred in the period under investigation.
Statistical analysis
Baseline characteristics were reported as median and interquartile range (IQR) for continuous variables and frequencies and proportions for categorical variables. In order to examine the normality distribution of the data, Shapiro–Wilk test was used. For the ECG findings, prevalence estimates were calculated in all the subgroups of students considered in this study. Chi-square test with continuity correction was used to examine the association between categorical variables. Kruskal–Wallis test was used to compare numerical variables, as the outcome variable was not normally distributed. Post hoc analysis was performed to evaluate the differences among the three groups (competitive athletes, noncompetitive athletes and nonathlete populations). Bonferroni's correction was applied to correct for multiple testing. Multivariate multinomial logistic regression was performed to adjust odds ratios in an examination of the impact of sport on the presence of minor or major ECG abnormalities. Variables statistically significant at univariate analysis were selected. A significance level of 0.05 was used for all tests. All the analyzes were performed using R version 3.6.2 (https://www.R-project.org/).
Results
Baseline characteristics
We enrolled 11 949 students, the mean age was 15.6 ± 3.5 years and 47.2% were boys. Baseline characteristics and information of sporting activity are shown in Table 2.
Table 2.
Baseline characteristics of the cohort
| Overall population | Nonathletes (A) | Noncompetitive athletes (B) | Competitive athletes (C) | P-value (A vs. B) | P-value (A vs. C) | P-value (B vs. C) | |
| N (%) | 11 949 | 4548 (38.1) | 446 (41.4) | 2455 (20.5) | |||
| Male [n (%)] | 5643 (47.2) | 1660 (36.5) | 2.421 (48.9) | 1562 (63.6) | <0.001 | <0.001 | <0.001 |
| Age (years) | 17 (12–18) | 17 (11–18) | 18 (16–18) | 17 (12–18) | <0.001 | <0.001 | 0.001 |
| BMI (kg/m2) | 20.6 (18.7–22.8) | 20.4 (18.4–22.6) | 20.7 (19.0–23.0) | 20.7 (18.7–22.9) | <0.001 | 0.013 | 0.022 |
| SBP (mmHg) | 120 (110–120) | 115 (110–120) | 120 (110–120) | 120 (110–120) | <0.001 | 0.013 | 0.039 |
| DBP (mmHg) | 70 (60–80) | 70 (60–80) | 70 (60–80) | 70 (60–80) | 0.885 | 0.976 | 0.982 |
Numerical variables are expressed as median (IQR); categorical variable are expressed as frequencies (percentage).
∗Chi-square (gender) and Kruskal–Wallis test (age, BMI, SBP, DBP).
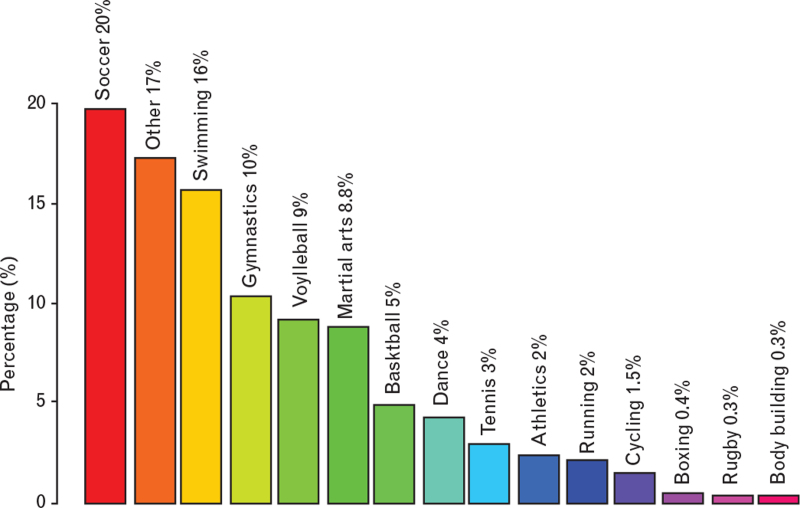
The percentage of students who practiced sport at a competitive level was 21%; noncompetitive athletes were 41% whereas nonathletes were 38%. The list of sports included more than 35 disciplines (Fig. 1).
Fig. 1.
Prevalence of sport disciplines. Illustration of the distribution of sport activities practiced by the students.
ECG analysis
Overall, abnormal ECGs (major and/or minor) were found on 16.3% (n = 1945) of the total cohort and are shown in Table 3. The prevalence of major abnormalities was 13% (n = 1547) whereas the prevalence of minor was 34% (n = 4035).
Table 3.
ECG findings
| Total population (n = 11 949) | Nonathletes (n = 4548) (A) | Noncompetitive athletes (n = 4.946) (B) | Competitive athletes (n = 2455) (C) | |||||||||
| ECG finding | n | % | n | % | n | % | n | % | P-value | P-value (A vs. B) | P-value (A vs. C) | P-value (B vs. C) |
| Major abnormalities | ||||||||||||
| T-wave inversion/ST-segment depression | 422 | 3.5 | 178 | 3.9 | 170 | 3.4 | 74 | 3.0 | 0.135 | – | – | – |
| Complete left bundle branch block | 1 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | 0.493 | – | – | – |
| Complete right bundle branch block | 203 | 1.7 | 61 | 1.3 | 101 | 2 | 41 | 1.7 | 0.030 | 0.032 | 0.965 | 0.94 |
| Left anterior fascicular block/left posterior fascicular block | 26 | 0.2 | 9 | 0.2 | 14 | 0.3 | 3 | 0.1 | 0.352 | – | – | – |
| Left/right atrial enlargement | 239 | 2 | 124 | 2.7 | 83 | 1.7 | 32 | 1.3 | <0.001 | <0.001 | <0.001 | 0.779 |
| Left axis deviation | 100 | 0.8 | 36 | 0.8 | 42 | 0.8 | 22 | 0.9 | 0.893 | – | – | – |
| Right axis deviation | 315 | 2.6 | 132 | 2.9 | 119 | 2.4 | 64 | 2.6 | 0.319 | – | – | – |
| Ventricular preexcitation (WPW) | 12 | 0.1 | 4 | 0.1 | 7 | 0.1 | 1 | 0.04 | 0.412 | – | – | – |
| Long QT interval | 56 | 0.5 | 20 | 0.4 | 20 | 0.4 | 16 | 0.7 | 0.319 | – | – | – |
| Short QT interval | 127 | 1.1 | 49 | 1.1 | 43 | 0.9 | 35 | 1.4 | 0.089 | – | – | – |
| Brugada-like ECG pattern | 97 | 0.8 | 68 | 1.5 | 19 | 0.4 | 10 | 0.4 | <0.001 | <0.001 | <0.001 | 1 |
| Premature ventricular contractions | 60 | 0.5 | 32 | 0.7 | 18 | 0.4 | 10 | 0.4 | 0.049 | 0.096 | 0.512 | 1 |
| Left ventricular hypertrophy | 241 | 2.0 | 81 | 1.8 | 90 | 1.8 | 70 | 2.9 | 0.004 | 1 | 0.013 | 0.016 |
| Right ventricular hypertrophy | 30 | 0.3 | 8 | 0.2 | 14 | 0.3 | 8 | 0.3 | 0.411 | – | – | – |
| Minor abnormalities | ||||||||||||
| First degree AV block | 33 | 0.3 | 19 | 0.4 | 9 | 0.2 | 5 | 0.2 | 0.068 | – | – | – |
| Short PQ interval | 310 | 2.6 | 136 | 3.0 | 132 | 2.7 | 42 | 1.7 | 0.005 | 1 | 0.005 | 0.039 |
| Rhythm sinus coronary | 61 | 0.5 | 19 | 0.4 | 26 | 0.5 | 16 | 0.7 | 0.415 | – | – | – |
| Junctional rhythm | 25 | 0.2 | 7 | 0.2 | 13 | 0.3 | 5 | 0.2 | 0.509 | – | – | – |
| Atrial premature beats | 117 | 1.0 | 47 | 1.0 | 48 | 1.0 | 22 | 0.9 | 0.854 | – | – | – |
| Sinus bradycardia | 578 | 4.8 | 127 | 2.8 | 226 | 4.6 | 225 | 9.2 | <0.001 | <0.001 | <0.001 | <0.001 |
| Sinus tachycardia | 632 | 5.3 | 333 | 7.3 | 226 | 4.6 | 73 | 3 | <0.001 | <0.001 | <0.001 | 0.004 |
| Incomplete right bundle branch block | 1878 | 15.7 | 918 | 20.2 | 625 | 12.6 | 335 | 13.6 | <0.001 | <0.001 | <0.001 | 0.714 |
| Early repolarization | 589 | 4.9 | 232 | 5.1 | 197 | 4.0 | 160 | 6.5 | <0.001 | 0.03 | 0.048 | <0.001 |
| Sinus arrhythmia | 2291 | 19.2 | 1.084 | 23.8 | 817 | 16.5 | 390 | 15.9 | <0.001 | <0.001 | <0.001 | 1 |
ECG abnormalities presented in Table 1 were not reported if no cases were founded. Post hoc analysis results presented in case of overall P-value less than 0.05.
The more common major abnormalities were T-wave inversion/ST-segment depression (3.5%), right axial deviation (2.6%), left ventricular hypertrophy (2%), atrial enlargement (2%) and complete right bundle branch block (1.7%).
The analysis of the prevalence of each ECG abnormality in the three groups (nonathletes, noncompetitive athletes and competitive athletes) revealed that incomplete right bundle branch block (respectively, 20.2, 12.6 and 13.6%, P < 0.001), sinus tachycardia (7.3, 4.6 and 3%, P < 0.001), short PQ interval (3, 2.7 and 1.7%, P = 0.005), atrial enlargement (2.7, 1.7 and 1.3%, P < 0.001), Brugada-like ECG pattern (1.5, 0.4 and 0.4%, P < 0.001) and premature ventricular contraction (0.7, 0.4 and 0.4%, P = 0.049) were more common in nonathletes compared with the athletic groups. As expected, sinus bradycardia (respectively, nonathletes, noncompetitive athletes and competitive athletes: 2.8, 4.6 and 9.2%, P < 0.001), early repolarization (5.1, 4 and 6.5%, P < 0.001) and left ventricular hypertrophy (1.8, 1.8 and 2.9%, P = 0.004) were more common in competitive athletes. Complete right bundle branch block was more common in noncompetitive athletes (respectively, nonathletes, noncompetitive athletes and competitive athletes: 1.3, 2 and 1.7%, P = 0.030).
As the three groups were heterogeneous in terms of age and sex distribution, a multinomial logistic regression was performed. Results from the regression showed that ECG abnormalities were more common in nonathletes compared with noncompetitive athletes and competitive athletes (Table 4).
Table 4.
Multinomial logistic regression
| Minor | |||
| OR | 95% CI | P-value | |
| Intercept | 0.037 | 0.029–0.048 | <0.001 |
| Sex (male) | 1.401 | 1.287–1.525 | <0.001 |
| Age | 1.221 | 1.204–1.238 | <0.001 |
| Noncompetitive sport | 0.812 | 0.739–0.893 | <0.001 |
| Competitive sport | 0.801 | 0.713–0.900 | <0.001 |
| Major | |||
| OR | 95% CI | P-value | |
| Intercept | 0.017 | 0.012–0.024 | <0.001 |
| Sex (male) | 2.182 | 1.938–2.457 | <0.001 |
| Age | 1.222 | 1.199–1.246 | <0.001 |
| Noncompetitive sport | 0.851 | 0.747–0.971 | 0.016 |
| Competitive sport | 0.796 | 0.678–0.934 | 0.005 |
Reference group for all analyses is no anomalies. CI, confidence interval; OR, odds ratio.
The prevalence of ECG abnormalities was stratified by age groups (≤15, 16–17 and ≥18 years) and the variables presenting statistical difference are reported in Table 5. Specifically, the younger classes presented lower prevalence compared with other two older classes of age in terms of the following ECG abnormalities: T-wave inversion, complete RBB, right axial deviation, short QT interval, Brugada-like ECG pattern, short PQ interval, sinus bradycardia, early repolarization and sinus arrhythmias. Differences between the groups 16 and 17 and at least 18 years of age were observed only for Brugada-like ECG patterns and early repolarization (more prevalent in the older group) and sinus tachycardia (less prevalent in the older group).
Table 5.
ECG analysis stratified by age groups
| Age ≤ 15 years, n = 4.055 (A) | Age 16–17 years, n = 2.974 (B) | Age ≥ 18 years, n = 4920 (C) | ||||||||
| ECG finding | n | % | n | % | n | % | P-value | P-value A vs. B | P-value A vs. C | P-value B vs. C |
| Major abnormalities | ||||||||||
| T-wave inversion/ST-segment depression | 86 | 2.1 | 126 | 4.2 | 210 | 4.3 | <0.001 | <0.001 | <0.001 | 1 |
| Complete right bundle branch block | 23 | 0.6 | 65 | 2.2 | 115 | 2.3 | <0.001 | <0.001 | <0.001 | 1 |
| Right axis deviation | 15 | 0.4 | 110 | 3.7 | 190 | 3.9 | <0.001 | <0.001 | <0.001 | 1 |
| Short QT interval | 7 | 0.2 | 44 | 1.5 | 76 | 1.5 | <0.001 | <0.001 | <0.001 | 1 |
| Brugada-like ECG pattern | 0 | 0 | 21 | 0.7 | 76 | 1.5 | <0.001 | <0.001 | <0.001 | 0.005 |
| Right ventricular hypertrophy | 6 | 0.1 | 15 | 0.5 | 9 | 0.2 | 0.006 | 0.038 | 1 | 0.062 |
| Minor abnormalities | ||||||||||
| First degree AV block | 4 | 0.1 | 10 | 0.3 | 19 | 0.4 | 0.028 | 0.156 | 0.04 | 1 |
| Short PQ interval | 32 | 0.8 | 113 | 3.8 | 165 | 3.4 | <0.001 | <0.001 | <0.001 | 1 |
| Atrial premature beats | 24 | 0.6 | 27 | 0.9 | 66 | 1.3 | 0.002 | 0.472 | 0.002 | 0.325 |
| Sinus bradycardia | 127 | 3.1 | 156 | 5.2 | 295 | 6 | <0.001 | <0.001 | <0.001 | 0.577 |
| Sinus tachycardia | 234 | 5.8 | 185 | 6.2 | 213 | 4.3 | 0.001 | 1 | 0.006 | 0.001 |
| Early repolarization | 37 | 0.9 | 168 | 5.6 | 384 | 7.8 | <0.001 | <0.001 | <0.001 | 0.001 |
| Sinus arrhythmia | 180 | 4.4 | 736 | 24.7 | 1375 | 27.9 | <0.001 | <0.001 | <0.001 | 0.008 |
AV, atrioventricular.
One third of the students (33%) complained of symptoms: 20% palpitation; 11% chest pain; 7% dyspnea; 6.4% syncope. Among students with major ECG abnormalities 17% (n = 263) complained of palpitations, 8% (n = 128) chest pain, 6% (n = 89) dyspnea and 5% (n = 78) syncope. Among the students with ECG abnormalities, only 66 (1.1%) had a positive family history of SCD.
Follow-up analysis
Follow-up was achievable for 90% (n = 10 754) of enrolled students and no events were reported. For the remaining 10% (n = 1195) of unavailable students, the schools were directly contacted in order to assess if overall death, cardiac death, resuscitated cardiac arrest or diagnosis of cardiac disease occurred in the period under investigation and no cases of deaths were recorded.
Among the students who were referred to the local cardiologist for major ECG, 25 (1.6%) were finally diagnosed with cardiac disease, both structural or electrical: preexcitation syndrome 0.8% (n = 12); long QT syndrome 0.3% (n = 4); Brugada syndrome 0.2% (n = 3); hypertrophic cardiomyopathy 0.1% (n = 2); dilated cardiomyopathy 0.1% (n = 2); myocarditis 0.1% (n = 2).
Discussion
This is the largest prospective ECG screening study restricted to teenagers (13–19 years). The original aspect of the study is the comparison of the prevalence of ECG abnormalities between competitive athletes, noncompetitive athletes and nonathletes. ECGs were analyzed irrespectively of the athletic status. We found that ECG abnormalities are common in the general young population (16.3%) with higher prevalence in nonathletes compared with noncompetitive athletes or competitive athletes if adjusted for age and sex.
Subjects with major ECG abnormalities underwent further evaluation and 1.6% were found to have a cardiac disease at follow-up. No deaths occurred at 2 years, follow-up.
There are conflicting results on the utility of ECG as a screening program in the general population. The lack of agreement is partly because of different national regulations and programs and partly to different methodologies in recruitment and analysis.
Corrado et al.12 for the first time showed that in Italy the implementation of the preparticipation screening program for sport eligibility, based on ECGs, resulted in a 90% decrease in SCD in athletes, whereas the rate of SCD did not change significantly among unscreened nonathletes. Subsequent studies confirmed the incremental value of ECG for early identification of asymptomatic athletes who have potentially lethal heart disorders in athletes in different age groups.13–21 The role of ECG as a screening tool was supported also in studies involving the general population.22–26
Data supporting the role of ECG as a screening tool comes from the UK national screening programs. Chandra et al.5 performed ECG screening in young subjects (aged 14–35 years) and compared athletes’ and nonathletes’ ECG findings using Corrado's ECG Classification.27 Specifically, 21.8% of nonathletes and 33% of athletes (P < 0.001) had potentially pathological ECGs. This study included a wider range of ages compared with our cohort; the enrollment was based on voluntary participation and analyzed together nonathletes and noncompetitive athletes.
More recently, 26 900 young individuals (aged 14–35 years) were evaluated using a health questionnaire and ECGs and with secondary investigations in case of abnormal results. Authors found that the addition of an ECG to a health questionnaire increases the likelihood of identifying disease associated with SCD by five times and it is more cost effective for identifying serious disease compared with screening using a health questionnaire alone.6
On the other hand, evidence from the USA demonstrated the failure of the screening program to detect cardiovascular disease at risk of SCD in the athletes and low statistical sensitivity of ECGs.28–30 Furthermore, the utility of ECG was criticized by the Israeli experience as they did not find any difference in mortality after the introduction of the ECG screening.31 However, this study was based on data collected by newspapers only.
Differences in results are related to different methodological aspects. First is the timing of the ECG screening. Among the aforementioned studies, some of them were focused on children,32 some of them on adolescents20,21 and others included a wide range of adults including elderly people.18 It is important to underline that some abnormal ECG findings are rarely detected before the age of 12 years and they can be identified later during adolescence whereas older people can reveal ECG changes related to coronary artery disease.
Another critical difference is the criteria used for the ECG analysis and the acquisition of the ECG. This aspect makes it difficult to compare prevalence of ECG abnormalities across different studies. However, data of our study are in keeping with results from the Chandra's screen program.5 In our study, the ECG was acquired by cardiologists and the analysis was entrusted to expert cardiologists, decreasing the false-positive rates.
The ECG screening had several benefits. First of all, it permitted the diagnosis of occult disease known to be at risk of SCD and the subjects were recommended to refrain from training. Secondly, ECG abnormalities can identify subjects with phenotype negative at the screening time but with the need for future follow-up (students with major abnormality without evidence of structural heart disease by imaging modalities). In addition, the benefit of screening goes beyond the identification of the student at risk of SCD as it often triggers the evaluation of first-degree relatives who may also be at risk from inherited cardiac diseases.
Study limitation
The study is based on voluntary participation; accordingly selection bias could not be excluded. No students with known cardiac disease were present in the cohort. Accordingly, we could not exclude the possibility of underestimating the real prevalence of ECG abnormalities and cardiac diseases. Our results are based mainly on a Caucasian cohort (97.5%) and may not reflect the findings in other ethnicities. Data collection on sport activity was limited to sport category and competitive vs. noncompetitive status while the number of hours of training per week was not recorded. The same ECG definitions were applied to the entire cohort without differences among subgroups. Although this approach could slightly increase the detection of minor abnormalities, it did not have any impact on major ECG abnormalities. Moreover, this approach avoided underestimating the prevalence of ECG abnormalities in the subgroup of students exposed to less intense sport and with shorter duration. The follow-up is limited to up to 24 months and including only phone calls, possibly accounting for the absence of SCD observed in this study. The prevalence of the symptoms reported was collected using a questionnaire. This could explain the relatively high prevalence of symptoms reported compared with the low prevalence of cardiovascular diseases diagnosed.
Conclusion
This study assessed the prevalence of ECG abnormalities in the general young population comparing three different groups: sedentary subjects, noncompetitive athletes and competitive athletes. We found that, among a population of 11 949 consecutive teenagers, 13% had major ECG abnormalities requiring further investigations and leading to a diagnosis of cardiac disease potentially associated with SCD in 1.6%. Nonathletes had a higher incidence of ECG abnormalities compared with the athletic groups. Our results are in line with the data supporting ECG screening in the general young population.
Acknowledgements
The authors deeply appreciate the contribution of the ‘Italian Heart and Circulation Foundation’ and ‘Fondazione Roma’.
Funding: This work was supported by the ‘Italian Heart and Circulation Foundation’ and ‘Fondazione Roma’.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Massimo Mancone and Viviana Maestrini contributed equally to this manuscript.
References
- 1.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015; 36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Eur Heart J 2005; 26:516–524. [DOI] [PubMed] [Google Scholar]
- 3.Pelliccia A, Fagard R, Bjornstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the WG of Cardiac Rehabilitation and Exercise Physiology and the WG of Myocardial and Pericardial Diseases of the ESC. Eur Heart J 2005; 26:1422–1445. [DOI] [PubMed] [Google Scholar]
- 4.Mont L, Pelliccia A, Sharma S, et al. Preparticipation cardiovascular evaluation for athletic participants to prevent sudden death: position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Europace 2017; 19:139–163. [DOI] [PubMed] [Google Scholar]
- 5.Chandra N, Bastiaenen R, Papadakis M, et al. Prevalence of electrocardiographic anomalies in young individuals: relevance to a nationwide cardiac screening program. JACC 2014; 63:2028–2034. [DOI] [PubMed] [Google Scholar]
- 6.Dhutia H, Malhotra A, Finnochiaro G, et al. Diagnostic yield and financial implications of a nationwide electrocardiographic screening programme to detect cardiac disease in the young. Europace 2021; 23:1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maron BJ, Friedman RA, Kligfield P, et al. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age). Circulation 2014; 130:1303–1334. [DOI] [PubMed] [Google Scholar]
- 8.Ljungqvist A, Jenoure P, Engebretsen L, et al. The International Olympic Committee (IOC) Consensus Statement on periodic health evaluation of elite athletes March 2009. Br J Sports Med 2009; 43:631–643. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Haas TS, Duncanson ER, et al. Comparison of the frequency of sudden cardiovascular deaths in young competitive athletes versus nonathletes: should we really screen only athletes? Am J Cardiol 2016; 117:1339–1341. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J 2018; 39:1466–1480. [DOI] [PubMed] [Google Scholar]
- 11.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021; 42:17–96. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 2006; 296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 13.Brosnan M, La Gerche A, Kalman J, et al. The Seattle Criteria increase the specificity of preparticipation ECG screening among elite athletes. Br J Sports Med 2014; 48:1144–1150. [DOI] [PubMed] [Google Scholar]
- 14.Fuller C, Scott C, Hug-English C, Yang W, Pasternak A. Five-year experience with screening electrocardiograms in National Collegiate Athletic Association Division I Athletes. Clin J Sport Med 2016; 26:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister GC, Puffer JC, Maron BJ. Preparticipation cardiovascular screening for US collegiate student-athletes. JAMA 2000; 283:1597–1599. [DOI] [PubMed] [Google Scholar]
- 16.Baggish AL, Hutter AM, Jr, Wang F, et al. Cardiovascular screening in college athletes with and without electrocardiography: a cross-sectional study. Ann Intern Med 2010; 152:269–275. [DOI] [PubMed] [Google Scholar]
- 17.Bessem B, Groot FP, Nieuwland W. The Lausanne recommendations: a Dutch experience. Br J Sports Med 2009; 43:708–715. [DOI] [PubMed] [Google Scholar]
- 18.Hevia AC, Fernandez MM, Palacio JM, Martín EH, Castro MG, Reguero JJ. ECG as a part of the preparticipation screening programme: an old and still present international dilemma. Br J Sports Med 2011; 45:776–779. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MG, Basavarajaiah S, Whyte GP, Cox S, Loosemore M, Sharma S. Efficacy of personal symptom and family history questionnaires when screening for inherited cardiac pathologies: the role of electrocardiography. Br J Sports Med 2008; 42:207–211. [DOI] [PubMed] [Google Scholar]
- 20.Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden cardiac death. Med Sci Sport Ex 1997; 29:1131–1138. [DOI] [PubMed] [Google Scholar]
- 21.Fudge J, Harmon KG, Owens DS, et al. Cardiovascular screening in adolescents and young adults: a prospective study comparing the Preparticipation Physical Evaluation Monograph 4th Edition and ECG. Br J Sports Med 2014; 48:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng CT, Ong HY, Cheok C, et al. Prevalence of electrocardiographic abnormalities in an unselected young male multiethnic South-East Asian population undergoing preparticipation cardiovascular screening. Europace 2012; 14:1018–1024. [DOI] [PubMed] [Google Scholar]
- 23.Uhm JS, Hwang IU, Oh YS, et al. Prevalence of electrocardiographic findings suggestive of sudden cardiac death risk in 10,867 apparently healthy young Korean men. PACE 2011; 34:717–723. [DOI] [PubMed] [Google Scholar]
- 24.Kobza R, Cuculi F, Abacherli R, et al. Twelve-lead electrocardiography in the young: physiologic and pathologic abnormalities. Heart rhythm 2012; 9:2018–2022. [DOI] [PubMed] [Google Scholar]
- 25.Kobza R, Toggweiler S, Dillier R, et al. Prevalence of preexcitation in a young population of male Swiss conscripts. Pacing Clin Electrophysiol 2011; 34:949–953. [DOI] [PubMed] [Google Scholar]
- 26.Marek J, Bufalino V, Davis J, et al. Feasibility and findings of large-scale electrocardiographic screening in young adults: data from 32,561 subjects. Heart rhythm 2011; 8:1555–1559. [DOI] [PubMed] [Google Scholar]
- 27.Corrado D, Pelliccia A, Heidbuchel H, et al. Section of Sports Cardiology, European Association of Cardiovascular Prevention and Rehabilitation. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 2010; 31:243–259. [DOI] [PubMed] [Google Scholar]
- 28.Maron BJ, Bodison SA, Wesley YE, Tucker E, Green KJ. Results of screening a large group of intercollegiate competitive athletes for cardiovascular disease. J Am Coll Cardiol 1987; 10:1214–1221. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 2006; 114:1633–1644. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Haas TS, Ahluwalia A, Rutten-Ramos SC. Incidence of cardiovascular sudden deaths in Minnesota high school athletes. Heart rhythm 2013; 10:374–377. [DOI] [PubMed] [Google Scholar]
- 31.Steinvil A, Chundadze T, Zeltser D, et al. Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death proven fact or wishful thinking? JACC 2011; 57:1291–1296. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Yoshinaga M, Anan R, et al. Usefulness and cost effectiveness of cardiovascular screening of young adolescents. Med Sci Sports Ex 2006; 38:2–6. [DOI] [PubMed] [Google Scholar]