Abstract
Breast cancer metastasis to the brain develops after a clinical latency of years to even decades, suggesting that colonization of the brain is the most challenging step of the metastatic cascade. However, the underlying mechanisms used by breast cancer cells to successfully colonize the brain’s microenvironment remain elusive. Reelin is an archetypal extracellular glycoprotein that regulates migration, proliferation, and lamination of neurons. It is epigenetically silenced in various cancers, and its expression in multiple myelomas is linked to poor patient survival. We found that Reelin expression was low in primary breast cancer tissue. However, its expression was significantly higher in Her2+ breast cancers metastasizing to the brain. In particular, Reelin was highly expressed in the tumor periphery adjacent to surrounding astrocytes. This augmented Reelin expression was seen in Her2+ metastases, but not in triple negative (TN) primary tumors or in TN breast to brain metastasis (BBM) cells co-cultured with astrocytes. Furthermore, the elevated expression was sustained in Her2+ cells grown in the presence of the DNA methyltransferase (DNMT) inhibitor 5-azacytidine, indicating epigenetic regulation of Reelin expression. The relative growth and rate of spheroids formation derived from Her2+ primary and BBM cells cocultured with astrocytes were higher than those of TN primary and BBM cells, and knockdown of both Reelin and Her2 suppressed the astrocyte-induced growth and spheroid forming ability of Her2+ cells. Collectively, our results indicate that within the neural niche, astrocytes epigenetically regulate Reelin expression and its interaction with Her2 leading to increased proliferation and survival fitness.
Keywords: Breast cancer, Brain Metastasis, Her2, ERBB2, Reelin, Astrocytes, Epigenetics, Triple Negative breast cancer, neural microenvironment
Introduction
Metastases are responsible for 90% of all cancer deaths, and patients diagnosed with brain metastases have a dismal one-year survival rate of only 20% [1]. The brain is often the site of the first recurrence of Her2-positive (Her2+) breast cancer, even when the disease is in remission in other sites [2,3]. This emerging clinical problem significantly limits the survival gains made from recent advances in systemic therapy for breast cancer [4].
Breast cancer brain metastases (BBMs) often develop years to decades after the initial diagnosis, even when early circulating tumor cells are present in the blood [5,6]. This clinical latency suggests that colonization of the brain is the most important step of the metastatic cascade [7]. The “seed and soil” hypothesis suggests that metastatic breast cancer cells require favorable interactions in the neuronal microenvironment to form metastases [8,9]. The rare tumor cells that traverse an intact blood-brain barrier (BBB) lack tumor neovasculature and likely rely on early proliferative cues from the brain microenvironment. However, the underlying mechanisms that empower breast cancer cells to colonize the brain’s microenvironment remain unknown.
Reelin is an archetypal extracellular glycoprotein in the brain that guides migration, proliferation, and lamination of neurons [10]. Acting through its downstream signaling cascade, Reelin establishes and maintains the molecular identity of neuronal cells and regulates their migration and positioning [11]. The reelin promoter contains GC-rich CpG islands and its expression in neural progenitor cells is controlled by promoter methylation and histone acetylation [12,13]. Although reelin is epigenetically silenced in primary breast tumors [14], we recently found that Reelin expression is increased in Her2+ BBMs [15]. In this study, we have confirmed that primary breast cancer tissue (both Her2+ and triple negative [TN, tumor that is estrogen receptor-negative, progesterone receptor-negative and HER2-negative]) has lower expression of Reelin compared to Her2+ BBMs. The greater Reelin expression in BBMs was seen in the tumor periphery, peritumor region, and tumor vasculature relative to the tumor core. We also found that low expression of Reelin in primary breast cancer did not correlate with Her2 expression. However, Reelin expression was significantly higher in BBM cells, and strongly correlated with Her2 expression in Her2+ BBM tissue and cells. Consistent with resected tumor tissue, augmented expression of Reelin was observed in Her2+ but not in TN primary or TN BBM cells.
This increased expression of Reelin in BBMs as compared to primary tumors raises the question of whether cells in the neural microenvironment epigenetically regulate Reelin expression. We found that co-culturing Her2+ BBM cells with astrocytes increased Reelin expression. In addition, this astrocyte-induced expression of Reelin was sustained in Her2+ BBM cells grown in the presence of the DNA methyltransferase (DNMT) inhibitor 5-azacytidine. We also found that, when co-cultured with astrocytes, the growth rates of Her2+ (primary and BBM) cells were greater than those of TN (primary and BBM) cells. Knockdown of both Reelin and Her2 suppressed the astrocyte-induced growth and spheroid formation of Her2+ BBM cells. Collectively, our results indicate that when Her2+ breast cancer cells are exposed to the neural niche, astrocytes epigenetically regulate Reelin expression and its interaction with Her2 to create a metastatic phenotype.
Materials and Methods
BBM cell cultures and treatment
Fresh Her2+ and TN human tumor samples (n = 13) were acquired from patients undergoing resection of the breast to brain metastases in accordance with a City of Hope institutional review board-approved protocol (COH IRB #05091). A portion of the specimen was cultured in collagen (Life Technologies)-coated T75 flasks to derive low-passage primary cell lines, called COH-BBM1 (BBM1), COH-BBM2 (BBM2) and COH-BBM3 (BBM3), COH-BBM12 (BBM12) in DMEM-F12 media (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 1% glutamax, and 1% Anti-Anti (Life Technologies) [15]. MDA-MB-231 and BT474 cells were also cultured in the above described DMEM-F12 media in T75 flasks. All cells were maintained at 37°C and 5% CO2. For treatment with 5-azacytidine, the cells were grown in either serum-free or serum-containing media for 24–48 h before the media was replaced with DMEM containing 10 μM 5-azacytidine and grown for 24–48 h.
Purification of reactive astrocytes and co-culture with BBM cells
For isolation of reactive astrocytes, fresh human breast to brain metastasis tumor with adjacent tissue samples were acquired from patients undergoing resection in accordance with a City of Hope institutional review board-approved protocol (IRB #05091). The reactive astrocytes were isolated from samples collected on the protocol described above using the procedure described by Zhang et al [16] and characterized using immunofluorescence staining (GFAP). In brief, the tissue was pre-cleaned by removing gray matter and blood clots and then chopped into small pieces (< 1 mm3). The chopped tissue was incubated in 20 unit/ml papain at 34°C for 100 min and washed with a protease inhibitor stock solution. The tissue slurry was gently triturated with serological pipettes in protease inhibitor stock solution, after which the cells were resuspended in PBS with BSA and DNase and passed through a Nitex filter. The single cell suspension was transferred to a plastic petri dishes pre-coated with reactive astrocyte-specific anti-HepaCAM antibody and incubated for 10–30 min at room temperature. Unbound cells were removed and the bound cells were rinsed 8 times with PBS to wash away loosely bound contaminating cell types. Astrocytes were cultured in Neurobasal-DMEM-based serum-free medium in a collagen-coated flask. Half of the volume was replaced with fresh medium every 3–4 days to maintain the cultures.
To obtain conditioned media (CM), astrocytes were grown in serum-free DMEM for 24 h before collecting and purifying the media by centrifugation at 4000 × g for 15 min. To grow the BBM cells in CM, cells were initially grown for 24 h in serum-free DMEM. The medium was removed and the cells were washed once with DMEM before adding the CM.
To isolate extracellular vesicles, astrocytes and BBM2 cells were cultured for 48 h in serum free medium without antibiotics. The conditioned media were collected and extracellular vesicles were isolated by differential centrifugation as previously described [17,18]
For co-culture with astrocytes, the astrocytes were initially grown on the inner side of thincert cell culture inserts (0.4μ pore size; Greiner bio-one) for 24 h. Simultaneously, the BBM cells were grown in collagen-coated 6-well plates overnight. Both the astrocytes and BBM cells were washed with DMEM before placing the insert in blank DMEM. The cells were co-cultured for the indicated time points.
Establishing Reelin knockdown stable cells
To create stable cell lines expressing Reelin-specific shRNA, a set of recombinant retroviral plasmids (pGFP-C-shLenti) incorporating different Reelin-specific shRNAs or a scrambled shRNA were procured commercially (OriGene). BBM1, BBM3, BT474, and MDA-MB-231 cells were Lipofectamine 2000 transfected with the different shRNA vectors separately and grown in puromycin (4 μg/mL) for 2 weeks. A secondary positive selection was done by GFP-guided cell sorting using FACS. Those cells positive for both GFP and puromycin were further analyzed by PCR and western blot for effective knockdown of Reelin expression. The two plasmids that produced the highest level of knockdown were used for subsequent transfections.
Spheroid formation assay
Control or Reelin knockdown BBM1, BBM3, BT474, and MDA-MB-231 cells were grown overnight in serum-containing DMEM media. Cells were harvested and washed three times with serum-free media. Five thousand cells in 20 μL serum-free media (containing B27 supplements and 20 nM of EGF and 20 nm of FGF) were placed as hanging drops on the inner side of the culture dish containing 10 ml PBS. These cells were cultured for up to 120 h and observed by microscopy at 24 h intervals to analyze spheroid formation. For treatment with astrocyte CM, BT474 cells were cultured in serum-free media (with supplements) in ultra-low attachment flasks for 96 h. The spheroids were harvested and transferred to a new flask containing astrocyte CM for an additional 24–48h
Real-time PCR and Western blot analysis
Total RNA was extracted using Trizol (Invitrogen) and treated with RNase-free DNase (Qiagen) as previously following supplier’s instruction. To analyze expression of Reelin and Her2, cDNA was synthesized using the iScript reverse transcription kit (Bio-Rad). Real-time quantification of gene expression was done using specific primers and SYBR select master mix for CFX (Applied Biosystems). Control PCR reactions were done using GAPDH and/or actin-specific TaqMan probe.
For Western blot analysis, total cell lysates were prepared in protein lysis buffer (50 mM TrisHCl, pH7.5; 100 mM NaCl; 1% TritonX 100; 1 mM EDTA; 1 mM EGTA, 50 mM β-glycerophosphoran, 1 mM dithiothreitol, 1 mM phenylmethanesulfonyl fluoride; 2 mM sodium orthovanadate, 10 μg/mL aprotinin; 10 μg/mL leupeptin and 10 μg/mL pepstatin A) by incubating the cells for 20 min at 4 °C followed by centrifugation (15000 ×g, 15 min, 4 °C). The protein extracts were analyzed by Western blot using antibodies specific to Reelin, Her2, p-Her2. Actin or tubulin was used as a loading control.
Immunocytochemistry
The breast cancer brain metastasis tissue resections were fixed in 4% paraformaldehyde immediately after surgery and gradually dehydrated in sucrose before sectioning. Primary breast cancer tissue microarray slides containing 12 different samples of Her2+ and TN subtypes (duplicates of each) were purchased from US Biomax Inc. The paraffin-embedded tissue microarray slides were immersed twice in xylene (10 min) and then sequentially immersed in 100, 95 and 70% ethanol (5 min each) to deparaffinized the tissue. Antigen retrieval was done by incubating the slide in 0.1 M sodium citrate buffer at 95 °C for 15 min.
For immunohistological staining of breast cancer brain metastasis tissue and the primary breast cancer tissue microarray, the tissue sections were incubated with 3% H2O2 for 15 min, washed three times with NaCl/Pi and blocked with blocking buffer containing normal goat serum. The slide was then incubated with Reelin or Her2 antibody overnight, washed three times in NaCl/Pi, and then incubated with biotinylated goat secondary antibody for 1.5 h. The slide was washed three times with NaCl/Pi, incubated with avidin–biotin complexes for 1.5 h, washed twice with NaCl/Pi and then twice with 0.1 M Tris/HCl (pH 7.4). The slide was incubated with a 2,4–diaminobutyric acid substrate kit (Vector Laboratories) for peroxidase labeling. The tissue microarray slide was dehydrated by sequential immersion in 70, 95 and 100% ethanol and then cleaned by sequential incubation in CitriSolv clearing agent. Tissue sections were mounted with DPX mounting solution, photographed, and examined under a microscope. For quantification of protein expression, the signal intensity of the IHC images were normalized using the white balance function of Adobe Photoshop CS2 software (Adobe Systems Inc) and the contrast enhancer of ImageJ software (NIH), set at 0.1% saturated pixels. DAB signals were extracted using ImageJ with IHC Profiler plugin. Relative intensity was calculated as the mean gray value of the regions of interest subtracted from the maximum intensity value and plotted in a scale of 0 (no intensity) to 10 (maximum intensity).
Immunofluorescence microscopy
Spheroids were fixed in 4% paraformaldehyde for 24 h and then gradually dehydrated in sucrose before mounting with O.C.T. The spheroid sections were washed with NaCl/Pi, blocked with normal goat serum for 1 h and immunostained with primary antibodies specific to Reelin (LifeSpan BioScience, LS-B12290) and Her2 (Invitrogen, MA5–14057) for 2 h. The spheroids were washed twice with NaCl/Pi for 5 min, incubated with fluorescein isothiocyanate or rhodamine (Jackson Immuno-Research Laboratories) conjugated secondary antibodies in normal goat serum for 1 h, washed three times with PBS, stained with 2, 6–diaminopimelic acid for nuclear staining, and visualized by fluorescence microscopy.
Statistical analysis
Each experiment was done in two or three separate treatment samples, and then cells were pooled (and treated as one replicate). Each experiment was repeated least thrice (n = 3). RNA and protein were extracted and used for RT-PCR and Western blot analysis; each experiment was repeated at least three times (n = 3). Real-time PCR analysis of samples was done using three replicates and repeated in three independent experiments (n = 3). For the spheroid formation assay, each treatment was done in twelve replicates (n = 2) and the experiment was repeated at least twice. Normally distributed data were analyzed by ANOVA, and non-normally distributed data were analyzed using Student’s t-tests (SPSS Inc., Chicago, IL). The treatments were considered significantly different if p ≤ 0.05.
Results
Reelin expression in brain metastases correlates with Her2.
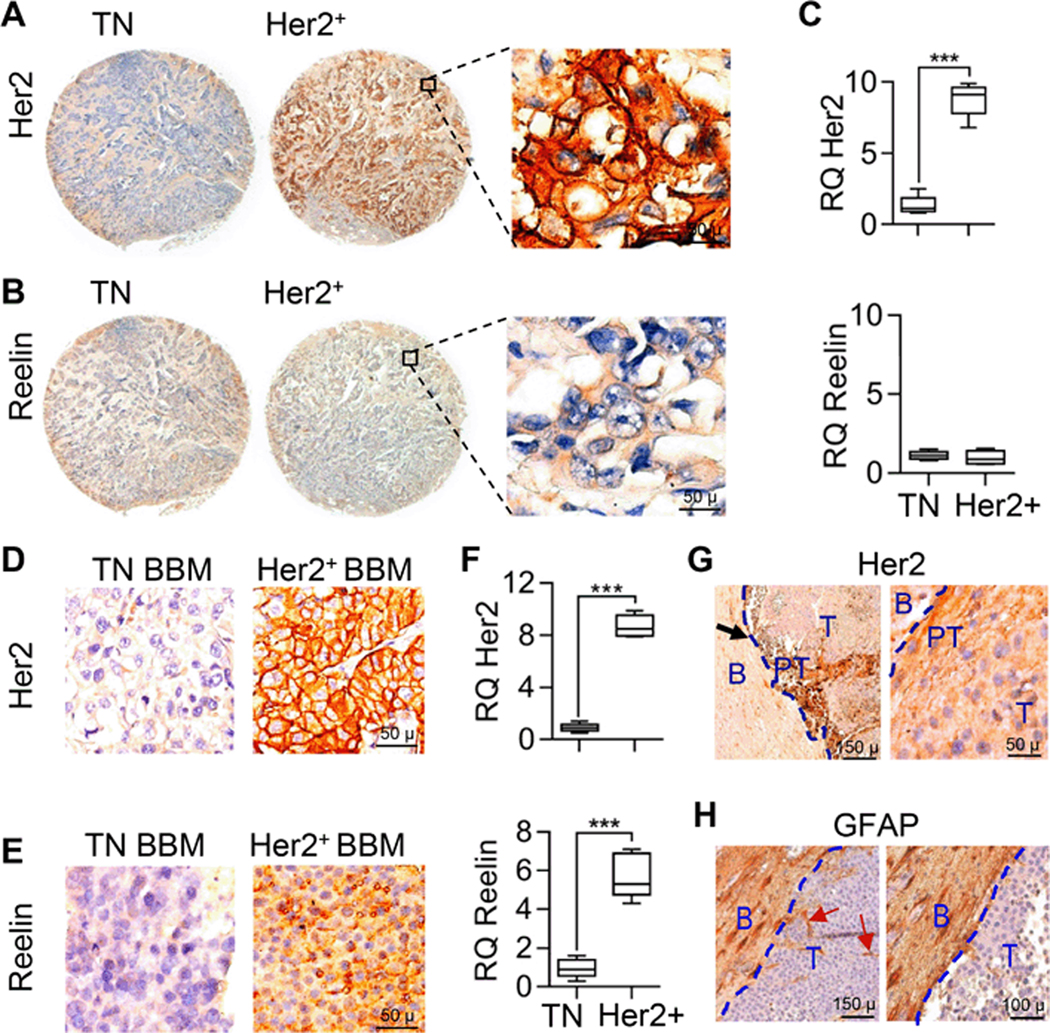
Recent studies showed Reelin expression is suppressed in gastric, pancreatic, and breast cancers. To investigate if Reelin expression correlates with Her2 expression in primary breast cancer, we obtained a set of breast cancer tissue microarrays containing both Her2+ (n = 31) and TN (n = 68) tissue specimens. The tissue array was immunohistochemically analyzed using Her2 and Reelin-specific antibodies (Figs. 1a–b). Quantification of immunohistochemical staining confirmed significantly higher expression of Her2 in Her2+ primary breast tumors and no/low expression in TN breast tumors (Figs. 1a–c). Expression of Reelin was low in both primary Her2+ and TN tumors (Fig. 1c).
Figure 1. Expression of Reelin in primary breast cancer and BBM cells is subtype and microenvironment specific.
(a-c) Immunohistochemical analysis of Reelin expression in Her2+ and TN primary breast cancer tissue. Quantification of Her2 and Reelin is shown on right (n = 12, p ≤ 0.01, bars indicate SEM). (d-f) Immunohistochemical analysis of Reelin expression in Her2+ and TN BBM patient specimens. Quantification of Her2 and Reelin is shown on right (n = 3, p ≤ 0.01, bars indicate SEM). (g) Immunohistochemical analysis of spatial distribution of Reelin within Her2+ BBM tumor and within tumor periphery. Tumor periphery and peritumor regions are separated by blue and red dashed lines. Triangle in lower panel indicates expression gradient of Reelin from tumor core to tumor periphery (B = brain, T = tumor, PT= peritumor regions). (h) Immunohistochemical analysis of astrocyte in tumor periphery and peritumor regions of BBM tumor. Tumor periphery is separated by blue dashed lines. Red arrow indicate presence of infiltrative astrocyte within BBM tumor (B = brain, T = tumor)
Immunohistochemical analysis of formalin-fixed BBM tissue [Her2+ (n = 6) and TN (n = 6)] confirmed higher expression of Her2 in Her2+ BBM tissue and low/no expression in TN BBM tissue (Figs. 1d–f). However, the expression of Reelin was significantly higher in Her2+ BBM tissue compared to TN BBM tissue (p < 0.01) (Fig. 1e). Comparative analysis showed that expression of Reelin in Her2+ BBM is significantly higher compared to Her2+ primary breast cancer tissue, TN BBM and TN primary breast cancer tissue (p < 0.01). Reelin expression in Her2+ BBM showed significant correlation with Her2 expression (r = 0.971, p < 0.01) (data not shown). Further analysis showed uniformed expression of Her2 throughout BBM tumors, while expression of Reelin was higher towards the tumor periphery adjacent to surrounding astrocytes (Fig. 1g–h). Thus, high Reelin levels in Her2+ BBM tumor cells, in particular for tumor cells associated with astrocytes, suggested that communication between breast cancer cells and astrocytes modulated Reelin expression
Astrocytes induce transactivation of Reelin.
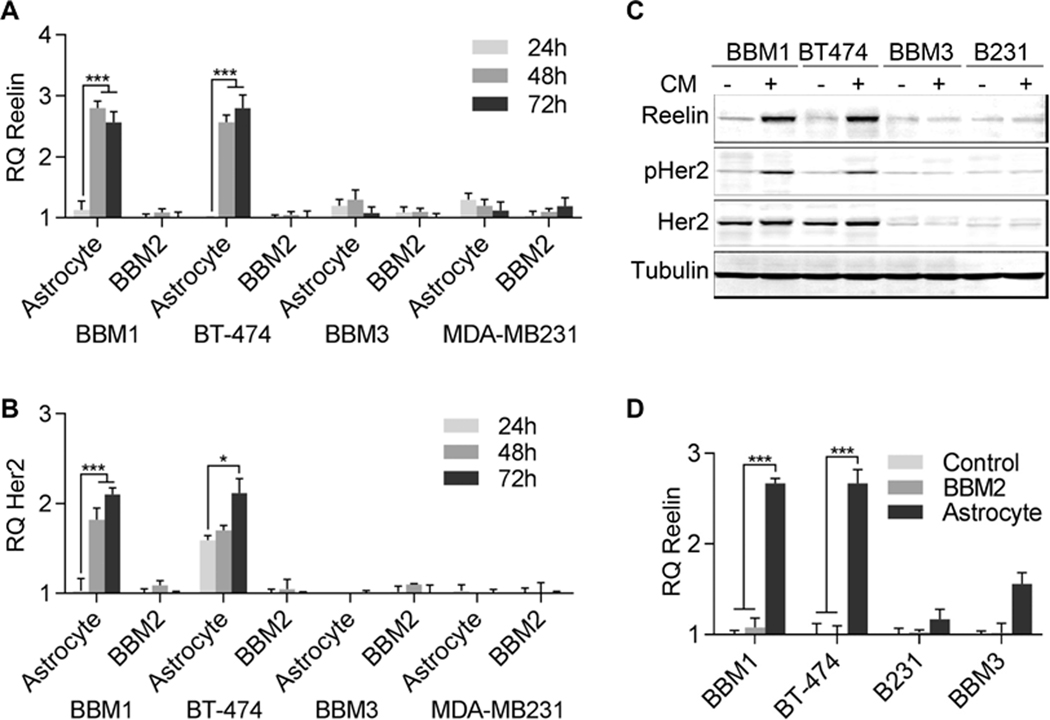
To understand whether astrocytes specifically regulate Reelin expression in Her2+ breast cancer cells, we exposed primary and BBM breast cancer cells to conditioned media from cultures of reactive astrocytes under serum-free conditions. Conditioned media (CM) culture experiments included established Her2+ (BT-474) and TN (MDA-MB-231) cell lines and Her2+ (BBM1) and TN (BBM3) BBM cell lines that were developed using freshly procured BBM tissue after neurosurgical resections at City of Hope. In control experiments, the cells were cultured in normal media. To confirm the specific functions of astrocytes or astrocyte-derived factors on Reelin expression we cultured the cells in CM from another Her2+ BBM culture (BBM2). Using real time-PCR quantification, we saw no significant difference in expression of Reelin and Her2 in BBM1, BT-474, BBM3, or MDA-MB-231 cells grown in normal media or in BBM2 CM. However, expression of Reelin was significantly higher in BBM1 and BT-474 cells cultured in astrocytes CM for 48 h (p ≤ 0.01) compared to the cells cultured in normal media. No changes in Reelin expression were observed in BBM3 and MDA-MB-231 cells (Fig. 2a) irrespective of culture conditions. Similar to the analysis of Reelin expression, Her2 was overexpressed in Her2+ BBM1 and BT474 cells compared to TN BBM3 and MDA-MB-231 cells. However, no changes in Her2 expression were observed in TN BBM3 and MDA-MB-231 cells cultured in normal media or in astrocytes CM (Fig. 2b).
Figure 2. Expression of Reelin is induced by astrocytes.
Primary breast cancer (BT474 and MDA-MB231) and BBM (BBM1 and BBM3) cells were cultured in astrocyte CM or BBM2 CM for 24–72h in serum-free media, after which cells were harvested for RNA and protein extraction. (A-B) Real-time PCR quantification of Reelin and Her2 expression in BBM1, BT474, BBM3, and MDA-MB-231 cells were plotted (RQ = relative quantification compared to Actin, n = 3, **p<0.05, ***p<0.01, bars indicate SEM). (C) Western blot analysis of Reelin, pHer2, and Her2 in BBM1, BT474, BBM3, and MDA-MB-231 cells co-cultured with astrocytes for 48 h. Tubulin was used as a loading control. (D) Exosomes (EVs) were extracted from astrocytes and BBM2 (Her2+) cells grown in serum-free media and used to treat BBM1, BT-474, BBM3, and MDA-MB-231 cells for 48 h. Real-time PCR quantification of Reelin expression is plotted (n = 6, ***p<0.01, bars indicate SEM).
Western blot analysis of Reelin indicates no significant difference in Reelin expression in cells cultured in BBM2 CM. However, Reelin expression was significantly higher in Her2+ BBM1 and BT-474 cells cultured in astrocytes CM. Western blot analysis showed no/low Her2 protein expression in TN BBM3 and MDA-MB231 cells. In contrast to the mRNA results, the difference in total Her2 protein levels in BBM1 and BT-474 cells were less between cells cultured in astrocyte CM compared to those cultured in BBM2 CM. However, phosphorylated Her2 (pHer2) levels were significantly higher in Her2+ BBM1 and BT-474 cells compared to cells cultured in BBM2 CM (Fig. 2c). These results indicate that astrocytes derived factors play critical roles in activation of Her2 by phosphorylation and transcriptional activation of Reelin and Her2 genes in Her2+ BBMs within neural microenvironment. Indeed treatment with astrocyte-derived exosomes induced Reelin expression in Her2+ BBM1 and BT-474 cells but not TN BBM3 and MDA-MB-231 cells. The control exosomes extracted from BBM2 cells had no effect on Reelin expression in any of the cell lines (Fig. 2d).
Astrocyte-derived factors increased proliferation of Her2+ BBM.
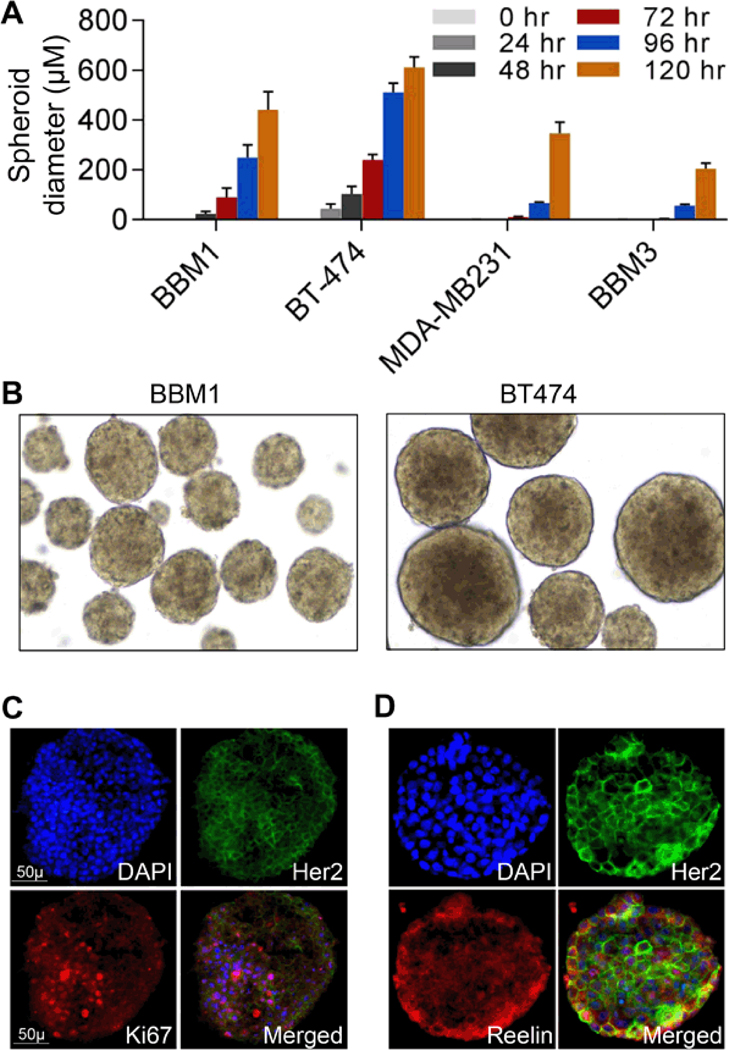
To understand how astrocytes affect BBM cells, we cocultured BBM1, BBM3, BT474, and MDA-MB-231 cells in serum-free astrocyte conditioned media for up to 120 h and analyzed their pro-metastatic behaviors such as cell proliferations and, spheroid formation. Control cells were cultured in serum-free media. The relative sizes and numbers of spheroids that formed were quantified at 24 h intervals. Our analysis showed except BT474 no cell lines formed spheroid at any time points when cultured in control media. However, all the cell lines formed spheroid at various time points when cultured in astrocyte conditioned media. The relative spheroid sizes and numbers were greater in Her2+ BBM1 and BT-474 cells as compared to TN BBM3 and MDA-MB-231 cells at all time points (Figs. 3a–b). Although BT-474 cells formed larger spheroids in earlier time points, BBM1 cells formed more spheroids over time (Fig. 3a). In addition, the relative size and numbers of BT-474 spheroids were higher when the cells are cultured in astrocyte conditioned media compared to control media. Immunofluorescence analysis of the spheroids showed Her2 and Reelin were present at higher levels in the peripheral regions of Her2+ BT474 spheroids; however, Ki67, a marker of proliferative cells, was distributed throughout the spheroids. These results indicates that astrocytes induced regulation of Reelin and Her2 potentially affects metastatic behavior of BBM cells
Figure 3. Astrocyte CM induced growth of tumor cell spheroids.
BBM1, BT-474, BBM3, and MDA-MB-231 cells were grown in serum-free astrocyte CM in low attachment flasks for 120 h. The cells were analyzed by light microscopy at 24 h intervals for the appearance of spheroids. (A) Relative sizes of spheroids (n = 12, Bars indicate SEM). (B) Spheroids derived from BBM1 and BT474 cells. (C-D) BT474 cells were cultured in serum-free media in a low attachment flask for 72 h. Spheroids were transferred to serum-free astrocyte CM for an additional 24 h before harvesting. Co-immunofluorescently stained spheroids using Her2 and Ki67 (C) and Her2 and Reelin (D) specific antibodies. Nuclear counterstaining was done with DAPI.
Epigenetic induction of Reelin expression.
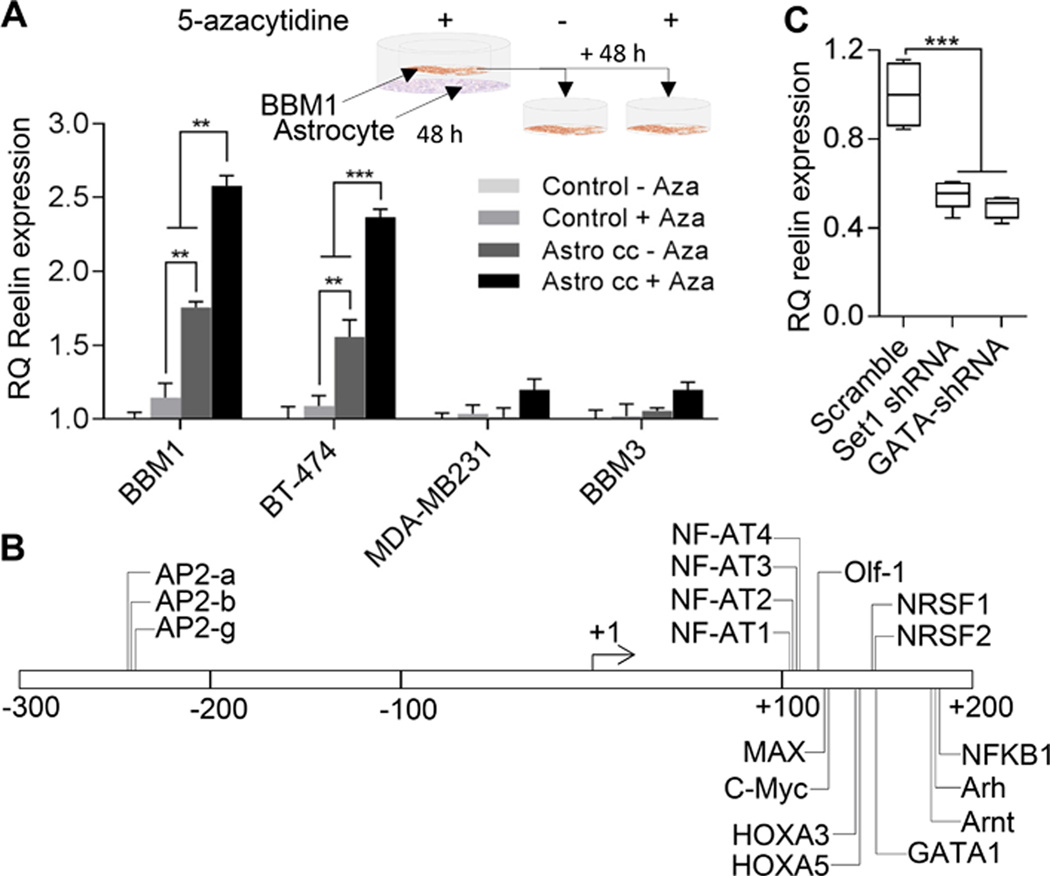
Suppression of Reelin expression in primary breast cancer is attributed to epigenetic silencing of the gene by DNA hypermethylation. To investigate if DNA methylation also plays a critical role in astrocyte-induced Reelin expression in Her2+ BBM1 cells, we cocultured BBM1, BT-474, BBM3, or MDA-MB-231 cells with astrocytes in serum-free medium containing the DNA methyltransferase (DNMT) inhibitor 5-azacytidine (10 μM) for 48 h. Then, cells were grown independently (in the absence of astrocytes) for an additional 48 h in normal DMEM (10% FBS) with or without 5-azacytidine (10 μM). Control cells were grown independently in serum-free media for 48 h and then in normal DMEM (10% FBS) with or without 5-azacytidine for an additional 48 h.
Real-time PCR analysis of RNA extracts from the cultured cells showed that treatment with 5-azacytidine alone did not increase Reelin expression in Her2+ or TN primary breast cancer and BBM cells as compared to controls. However, co-culture with astrocytes induced higher expression of Reelin in Her2+ BBM1 and BT-474 cells. However, this Astrocyte-induced elevated expression of Reelin decreases to basal level if the cells are grown then in normal media in subsequent generations. However, if the astrocyte co-cultured cells are subsequently grown in normal media containing DNMT inhibitor 5-azacytidine the Reelin expression remains continually elevated (Fig. 4a). These results suggest a potential role for DNA methylation by DNMT in suppressing Reelin expression.
Figure 4. Functions of astrocytic factors on Reelin transcription.
(A) Real-time PCR quantification of Reelin expression in Her2+ (BBM1) cells co-cultured with astrocytes (Astro cc) in the presence of 5-azacytidine for 24 h in serum-free media, followed by an additional 48 h of growth with or without 5-azacytidine (n = 3, **p<0.05, ***p<0.01, RQ = relative quantification compared to Actin). Bars indicate SEM. (B) Reelin promoter region showing cis-elements for transcription factors. (C) Set1 histone methyltransferase and GATA1 transcription factor were knocked down in BBM1 cells by shRNA and cocultured with astrocytes for 24 h. Real-time PCR quantification of Reelin expression (n = 3, ***p<0.01, bars indicate SEM).
To further define the mechanism of Reelin transcription within the neural niche, we performed in silico analysis of the promoter region of the Reelin gene to identify cis-elements using Tfsitescan. Our analysis showed that the −300 to +185 nt GC-rich region of the Reelin promoter contains cis-elements for several transcription factors that regulate neural microenvironment-specific gene transcription (Fig. 4b). To confirm the function of transcription factors that potentially play a critical function in epigenetically regulating Reelin gene activation under astrocyte co-culture conditions, we knocked down the transcription factor GATA1 and H3K4 Tri-methylase Set1 using shRNA in BBM1 cell lines using at least two different shRNA construct of each gene. Each of the individual shRNA constructs of all the genes was evaluated separately to confirm the specificity of the shRNAs to target genes. To confirm potential epigenetic regulation of Reelin transcription under neural niche, we knock down H3K4 trimethyltransferase Set1. Knockdown cells were then co-cultured with astrocytes. Real-time PCR analysis showed that knockdown of GATA1 and Set1 significantly suppressed astrocyte-induced Reelin expression in Her2+ BBM1 cells (Fig. 4c), indicating the critical role of histone modifications in Reelin gene transactivation.
Reelin knockdown suppresses astrocyte-induced Her2+ cell proliferation.
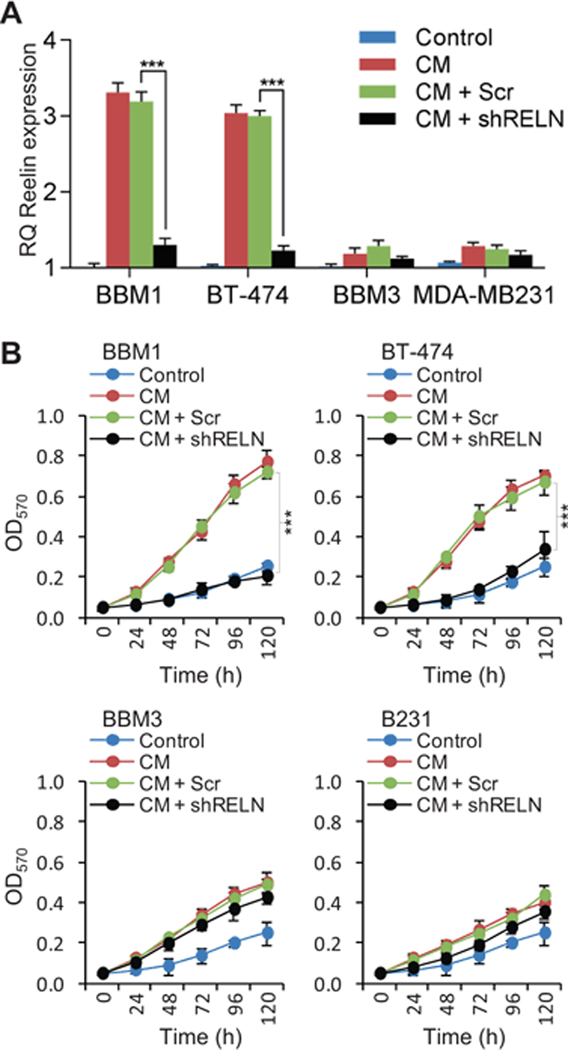
To investigate the function of Reelin on cell growth and proliferation we developed stable BBM1, BT-474, BBM3, and MDA-MB-231 cell lines that expressed Reelin shRNA (GFP-lentiviral constructs; control cells expressed scrambled shRNA). Real time-PCR analysis of Reelin expression confirmed successful knockdown of Reelin (>80% relative to scramble control, data not shown) in all four cell lines under normal growth conditions. To further confirm Reelin knockdown, the cells were grown in Astrocyte CM for 48 h before harvesting and real-time PCR analysis of Reelin expression. Astrocyte-induced Reelin expression was significantly suppressed in Reelin knockdown BBM1 and BT-474 cells (Fig. 5a). Similar to our previous results, we saw no significant changes in astrocyte-induced Reelin expression in TN BBM3 and MDA-MB231 cells.
Figure 5: Effect of Reelin knockdown on astrocyte CM-induced Reelin expression and cell proliferation.
Her2+ (BBM1 and BT-474) and TN (BBM3 and MDA-MB-231) cells were transfected with shRNA1 and shRNA2 specific to Reelin. Control cells were transfected with a lentiviral plasmid expressing scramble control (Scr). Cells were purified by FACS and Reelin knockdown was confirmed by RT-PCR and Western blot analysis. (A) Real-time PCR quantification of Reelin expression (n = 3, ***p<0.01, bars indicate SEM). (B) The cells were grown in CM or in normal DMEM (control) in 24-well plates (20,000 cells/per well) for up to 120 h. Numbers of viable cells were measured using MTT assay (n = 12, ***p<0.01, bars indicate SEM).
To analyze the effect of Reelin knockdown on astrocyte-induced cell growth, we cultured BBM1, BT-474, BBM3, and MDA-MB-231 cells in astrocyte conditioned media (CM) for 120 h and measured relative viable cell population at 24 h intervals using the MTT assay. Culture in astrocyte CM significantly increased the proliferation of BBM1 and BT474 cells over time. In addition, TN cells were less responsive to astrocyte CM than were Her2+ cells. Astrocyte CM induced proliferation was significantly suppressed in Reelin knockdown BBM1 and BT-474 cells (Fig. 5b). These results indicate that Reelin plays a critical role in astrocyte-induced proliferation of BBM cells within neural microenvironment.
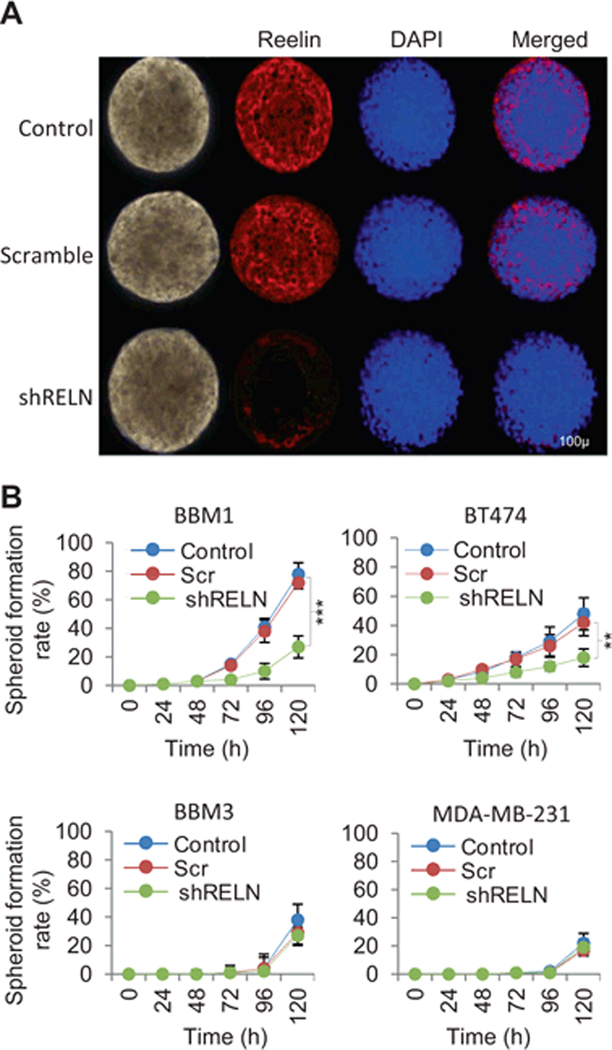
Reelin knockdown inhibits the spheroid-forming ability of Her2+ BBM cells.
To investigate the effect of Reelin expression on the metastatic properties of Her2+ and TN BBM cells, we analyzed the spheroid-forming ability of BBM1, BT-474, BBM3, and MDA-MB-231 cells expressing either Reelin shRNA or scrambled shRNA. Cells were grown in serum-free astrocyte CM in ultra low attached flask for 120 h and assessed for the formation of spheroids at 24h intervals. The relative spheroid size was not affected by Reelin knockdown in Her2+ BBM1 and BT-474 cells (Fig. 6a). However, knockdown of Reelin significantly inhibited the spheroid-forming ability of BBM1 and BT474 cells, resulting in fewer numbers of spheroid and spheroid forming cells compared to total cell count (Fig. 6b). However, this fewer spheroid numbers may be associated with fewer numbers of proliferative cells that survived under Reelin knockdown condition. Consistent with our previous findings, the lower numbers and smaller sizes of spheroids derived from TN BBM3 and MDA-MB231 cells were not significantly affected by Reelin knockdown.
Figure 6. Reelin knockdown affects spheroid formation by BBM cells.
BBM1, BT-474, BBM3, and MDA-MB-231 cells were transfected with Reelin shRNA-expressing lentiviral vectors. Control cells were transfected with scramble shRNA-expressing vectors (Scr). Cells were grown in serum-free media in low attachment flask for 120 h. (A) immunofluorescence staining of Reelin in BBM1 spheroids. Nuclear counterstaining was done using DAPI. (B) Relative numbers of cells in spheroids compared to the total cell in the flask at different time points (n = 12, ***p<0.01, **p<0.05 bars indicate SEM).
Discussion
The brain is an increasingly common metastatic site for patients with Her2+ breast cancer. To grow in this neural niche, breast cancer cells develop bi-directional interactions with components of the brain microenvironment [6,19]. Investigating the reciprocal interaction of invading tumor cells and resident brain cells requires incorporation of approaches from both neuroscience and cancer biology.
Reelin is an extracellular neuronal protein that plays a positive role in neuronal proliferation and differentiation and a negative role in neuronal migrations [20–22]. It stimulates neuronal differentiation by activating various downstream signaling events triggering neural development and function [20–22]. In the adult brain, Reelin expression is limited to specific cell types [23], and several recent studies reported higher Reelin expression in primary brain tumors of glial origin [24–27].
Consistent with this, previous studies showed a strong correlation of Reelin expression with various cancers. For example, high Reelin expression negatively correlates with progression-free survival and overall survival in multiple myeloma patients and positively correlates with tumor cell count in the bone marrow [28,29]. Reelin promotes adhesion of multiple myeloma cells to fibronectin and protects cells from drug-induced cell apoptosis [29]. Indeed, expression of Reelin is suppressed in many primary tumor types [30–32]. Consistent with these previous findings, we also found reduced Reelin levels in primary breast cancer compared to normal breast tissue. In addition, we found there is minimal to no Reelin expression in both Her2+ and TN primary breast cancers (Figure 1a). In contrast, Reelin was robustly expressed in Her2+ brain metastases, and this expression was lacking in brain metastases from TN breast cancer.
This led us to further investigate a possible interaction between Reelin and Her2 in brain metastases from Her2+ breast cancer patients. Consistent with recent findings by Biamonte et al., [33] that showed higher Reelin expression in the periphery of gliomas, we found the differential distribution of Reelin within BBM tumors, with higher expression in the tumor periphery. Thus our findings suggest that factors from the brain microenvironment drive Reelin expression in Her2+ BBM tumors.
In recent years, signal transmission through extracellular vesicles such as exosomes has emerged as a new aspect of transcellular signaling and is increasingly relevant for communication between glia and neurons, as well as invading tumor cells [34–36]. Exosomes carry cargo that includes membrane proteins, cytosolic proteins, and genetic material such as mRNAs and non-coding RNAs, such as microRNAs [37–39]. The observation that exosomes can transport such molecules within the nervous system opens a new possibility for cellular communication within the neural niche. Our findings showed that astrocyte-derived exosomes promote Reelin expression in Her2+ BBM cells via a bi-directional cellular communication between invading breast cancer cells and the brain cells.
Epigenetic regulation of transcription is critical for cell differentiation. The Reelin promoter is subjected to differential DNA methylation in the adult nervous system, and perturbations in Reelin promoter methylation correlate with alterations in neural development and function. Epigenetic silencing of Reelin occurs via promoter hypermethylation in breast, gastric and pancreatic cancers [30–32]. Our in silico analysis identified several cis-elements specific to neural transcription factors within close proximity to the Reelin promoter. The abundance of cis-elements in the GC-rich region of the Reelin promoter suggests that the expression of Reelin is finely tuned by an epigenetic mechanism both during development and disease. Indeed, our finding that showed DNMT inhibitor retention of astrocyte-induced Reelin expression indicates a reversal of DNA methylation in the reelin promoter within the neural niche.
Our findings that Reelin knockdown induced suppression of cell proliferation and spheroid forming efficiency of BBM1 cells indicates, a critical function of Reelin in breast cancer brain metastasis. Previous studies have shown the importance of Reelin in various aspects of malignancies [40,41]. In multiple cancer types, down-regulation of Reelin is associated with increased migratory ability and reduced survival of cancer cells, while overexpression of Reelin suppresses cell migration, invadopodia formation, and invasiveness of cancer cells [14,31,42,43]. In support, mice lacking Reelin expression have a dramatic reduction in breast cancer lung metastases [44,45]. Reelin expression in the host environment is critical for epithelial to mesenchymal transition of mammary carcinoma cells, indicating Reelin is an essential component supporting the transition of the primary tumor to a metastatic carcinoma [44,45]. Although the interplay between Reelin and breast cancer cells suggests a mechanism for the formation of breast cancer brain metastases, the functional role of Reelin in metastasizing Her2+ breast cancer cells remains largely unexplored.
In conclusion, our findings demonstrate that that breast cancer cells metastasizing to the brain develop bi-directional interactions with astrocytes. Astrocyte-derived induction of neural genes in colonizing tumor cells should provide a survival advantage and facilitate colonization of molecularly foreign brain microenvironment. As a contributor to brain metastasis formation, we provide evidence that astrocyte-induced Reelin expression in tumor cells facilitates the proliferation of Her2+ breast cancer cells.
ACKNOWLEDGEMENTS
We thank the members of the flow cytometry core, pathology core, and microscopy cores, for their valuable assistance in this project. We also thank Anders Persson for his advice on the manuscript.
FINANCIAL SUPPORT
R.J. is supported by Department of Defense (DOD) Grant (BC142323), The Margaret E. Early Medical Research Trust, National Institutes of Health (NIH) Grant K12 CA001727-16A1. Additional project support was provided by National Cancer Institute Grant P30 CA033572.
Footnotes
COMPETING INTEREST
The authors declare that no competing interests exist.
References
- 1.Kondziolka D, Kalkanis SN, Mehta MP, Ahluwalia M, Loeffler JS (2014) It is time to reevaluate the management of patients with brain metastases. Neurosurgery 75: 1–9. [DOI] [PubMed] [Google Scholar]
- 2.Honda Y, Goto R, Idera N, Horiguchi K, Kitagawa D, et al. (2014) Prognostic Significance of Subtypes and Gpa (Graded Prognostic Assessment) in Brain Metastases from Breast Cancer. Annals of Oncology 25. [Google Scholar]
- 3.Pestalozzi BC (2009) Brain metastases and subtypes of breast cancer. Annals of Oncology 20: 803–805. [DOI] [PubMed] [Google Scholar]
- 4.Percy DB, Ribot EJ, Chen Y, McFadden C, Simedrea C, et al. (2011) In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol 46: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccirilli M, Sassun TE, Brogna C, Giangaspero F, Salvati M (2007) Late brain metastases from breast cancer: clinical remarks on 11 patients and review of the literature. Tumori 93: 150–154. [DOI] [PubMed] [Google Scholar]
- 6.Termini J, Neman J, Jandial R (2014) Role of the neural niche in brain metastatic cancer. Cancer Res 74: 4011–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jandial R, Anderson A, Choy C, Levy ML (2012) Bidirectional Microevnironmental Cues Between Neoplastic and Stromal Cells Drive Metastasis Formation and Efficiency. Neurosurgery 70: N12–N13. [DOI] [PubMed] [Google Scholar]
- 8.Paget G (1889) Remarks on a Case of Alternate Partial Anaesthesia. Br Med J 1: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited-The role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer 128: 25272535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran T, D’Arcangelo G (1998) Role of reelin in the control of brain development. Brain Research Reviews 26: 285–294. [DOI] [PubMed] [Google Scholar]
- 11.Rice DS, Curran T (2001) Role of the Reelin signaling pathway in central nervous system development. Annual Review of Neuroscience 24: 1005–1039. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Obana EA, Radomski KL, Sukumar G, Wynder C, et al. (2016) Inhibition of the histone demethylase Kdm5b promotes neurogenesis and derepresses Reln (reelin) in neural stem cells from the adult subventricular zone of mice. Mol Biol Cell 27: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell CP, Chen Y, Kundakovic M, Costa E, Grayson DR (2005) Histone deacetylase inhibitors decrease reelin promoter methylation in vitro. J Neurochem 93: 483–492. [DOI] [PubMed] [Google Scholar]
- 14.Stein T, Cosimo E, Yu X, Smith PR, Simon R, et al. (2010) Loss of reelin expression in breast cancer is epigenetically controlled and associated with poor prognosis. Am J Pathol 177: 2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, et al. (2014) Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A 111: 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, et al. (2016) Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding CV, et al. (1996) B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine 183: 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronisz A, Wang Y, Nowicki MO, Peruzzi P, Ansari KI, et al. (2014) Extracellular Vesicles Modulate the Glioblastoma Microenvironment via a Tumor Suppression Signaling Network Directed by miR-1. Cancer Research 74: 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neman J, Choy C, Kowolik CM, Anderson A, Duenas VJ, et al. (2013) Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin Exp Metastasis 30: 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massalini S, Pellegatta S, Pisati F, Finocchiaro G, Farace MG, et al. (2009) Reelin affects chain-migration and differentiation of neural precursor cells. Mol Cell Neurosci 42: 341349. [DOI] [PubMed] [Google Scholar]
- 21.Becker J, Frohlich J, Perske C, Pavlakovic H, Wilting J (2012) Reelin signalling in neuroblastoma: migratory switch in metastatic stages. Int J Oncol 41: 681–689. [DOI] [PubMed] [Google Scholar]
- 22.Jossin Y, Cooper JA (2011) Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 14: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Arcangelo G (2014) Reelin in the Years: Controlling Neuronal Migration and Maturation in the Mammalian Brain. Advances in Neuroscience 2014-Article ID 597395: 19 pages. [Google Scholar]
- 24.Biamonte F, Scicchitano BB and Lama G. (2015) Evaluation of the Reelin signaling in cancer stem cells isolated from tumor and peritumor tissue of glioblastoma. Italian J. of Anat, Embriol. 120(1): 89. [Google Scholar]
- 25.Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, et al. (2009) MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J 23: 4276–4287. [DOI] [PubMed] [Google Scholar]
- 26.Hu HL, Deng F, Liu Y, Chen MF, Zhang XL, et al. (2012) Characterization and retinal neuron differentiation of WERI-Rb1 cancer stem cells. Molecular Vision 18: 2388–2397. [PMC free article] [PubMed] [Google Scholar]
- 27.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F (2007) Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis 13: 823–832. [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, Yan F, Zhao D, Lv M, Liang X, et al. (2016) Reelin promotes the adhesion and drug resistance of multiple myeloma cells via integrin beta1 signaling and STAT3. Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, Yan F, Zhao D, Lv M, Liang X, et al. (2016) Reelin promotes the adhesion and drug resistance of multiple myeloma cells via integrin beta1 signaling and STAT3. Oncotarget 7: 9844–9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui L, Wang Y, Ju LH, Chen M (2012) Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol Learn Mem 97: 425–440. [DOI] [PubMed] [Google Scholar]
- 31.Dohi O, Takada H, Wakabayashi N, Yasui K, Sakakura C, et al. (2010) Epigenetic silencing of RELN in gastric cancer. Int J Oncol 36: 85–92. [PubMed] [Google Scholar]
- 32.Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, et al. (2006) The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther 111: 272–286. [DOI] [PubMed] [Google Scholar]
- 33.Biamonte F, Scicchitano BM, Lama G (2015) Evaluation of the Reelin signaling in cancer stem cells isolated from tumor and peritumor tissue of glioblastoma. Italian Journal of Antomy and Embryology 120: 89. [Google Scholar]
- 34.Basso M, Bonetto V (2016) Extracellular Vesicles and a Novel Form of Communication in the Brain. Front Neurosci 10: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budnik V, Ruiz-Canada C, Wendler F (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, et al. (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34: 15482–15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, et al. (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu HY, Huang CM, Hung YF, Hsueh YP (2015) The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol 269: 202–212. [DOI] [PubMed] [Google Scholar]
- 39.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, et al. (2015) Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 17: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SA, Lee H, Pinney JR, Khialeeva E, Bergsneider M, et al. (2011) Development of Microfabricated Magnetic Actuators for Removing Cellular Occlusion. J Micromech Microeng 21: 54006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khialeeva E, Lane TF, Carpenter EM (2011) Disruption of reelin signaling alters mammary gland morphogenesis. Development 138: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Y, Chen H, Ma G, Cao X, Liu Z (2012) Reelin is involved in transforming growth factor-beta1-induced cell migration in esophageal carcinoma cells. PLoS One 7: e31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrone G, Vincenzi B, Zagami M, Santini D, Panteri R, et al. (2007) Reelin expression in human prostate cancer: a marker of tumor aggressiveness based on correlation with grade. Mod Pathol 20: 344–351. [DOI] [PubMed] [Google Scholar]
- 44.Khialeeva E, Chou JW, Carpenter EM (2015) Reelin signaling in tumor microenvironment is required for metastasis of mammary carcinoma cells. Cancer Research: DOI: 10.1158/1538-7445. [DOI] [Google Scholar]
- 45.Khialeeva E, Chou JW, Carpenter EM (2015) Exploring the relationship between reelin signaling and breast cancer metastasis. Cancer Research: DOI: 10.1158/1538-7445. [DOI] [Google Scholar]