Summary
The ability to directly observe leukocyte behavior in vivo has dramatically expanded our understanding of the immune system. Zebrafish are particularly amenable to the high-resolution imaging of leukocytes during both homeostasis and inflammation. Due to its natural transparency, intravital imaging in zebrafish does not require any surgical manipulation. As a result, zebrafish are particularly well-suited for the long-term imaging required to observe the temporal and spatial events during the onset and resolution of inflammation. Here, we review major insights about neutrophil and macrophage function gained from real time imaging of zebrafish. We discuss neutrophil reverse migration, the process whereby neutrophils leave sites of tissue damage and resolve local inflammation. Further, we discuss the current tools available for investigating immune function in zebrafish and how future studies that simultaneously image multiple leukocyte subsets can be used to further dissect mechanisms that regulate both the onset and resolution of inflammation.
Keywords: Wound Healing, Inflammation, Resolution, Neutrophils, Reverse Migration, Chemotaxis, Zebrafish
Introduction
Innate immune inflammation is critical for host defense and tissue repair. In response to injury both neutrophils and macrophages are rapidly recruited to interstitial sites of tissue damage where they clear away cellular debris.1–3 Live imaging in situ has been instrumental in advancing our understanding of the initial events that mediate leukocyte recruitment and the onset of inflammation. However, less is known about the mechanisms governing the resolution of inflammation needed to restore tissue homeostasis. Addressing this gap is particularly important due to the contribution of uncontrolled inflammation to tissue damage and disease. Herein, we discuss how live imaging of inflammation has uncovered fundamental new information about how inflammation starts and stops. We also discuss the importance of using transparent model organisms like zebrafish to observe the temporal and spatial dynamics of cell behavior in the tissue microenvironment in normal and diseased tissues after injury.
The field of cellular immunology was enabled by the in vivo observation of leukocytes. Long before the development of fluorescent probes, 19th century investigators uncovered fundamental immunological processes by imaging either extremely thin or naturally transparent samples and organisms. Julius Cohnheim detailed the leukocyte diapedesis cascade through blood vessels by imaging frog tongues stretched taut over pieces of corkboard.4 Élie Metchnikoff, often credited as the “father of cellular immunology”, made his seminal discoveries on chemotaxis and phagocytosis using optically transparent organisms amenable to live imaging. He observed the phagocytosis of dead cellular material in developing tadpoles5, the phagocytosis of fungi in water fleas6, and phagocytosis of foreign material in starfish larvae, which he described “as transparent as water”.7,8
In recent decades, the zebrafish (danio rerio) has emerged as a popular model organism across multiple disciplines for in vivo observation of biological processes.9,10 Zebrafish possess the same attributes that facilitated Metchnikoff’s early discoveries – optical transparency and external fertilization. Even in the absence of any fluorescent cell lineage markers, leukocytes are readily detectable in the blood and crawling through tissues by simple light microscopy in zebrafish. For example, the migration of macrophages to form a granuloma in response to mycobacterial infection in zebrafish was first demonstrated using light microscopy.11 However, it is difficult to distinguish different leukocyte subsets or perform long-term cell fate mapping by simple light microscopy. Thus, new insights about leukocyte function have largely relied on the use of transgenic animals with fluorescent protein expression restricted to the leukocyte subpopulation of interest.
Neutrophil reverse migration – an unexpected insight from zebrafish
To understand the behavior of neutrophils in tissues required the neutrophil-specific expression of fluorescent markers. The first neutrophil labeled lines (tg(zMPO:GFP)) were simultaneously generated in our lab and the Renshaw lab in 2006.12,13 Shortly thereafter, the lysozyme C (LyzC) promoter was also used to label neutrophils.14,15 The transparency of zebrafish larvae enabled the high-resolution imaging of the dynamic behavior of neutrophils within tissues in response to injury in an intact organism, for the first time. The robust recruitment of neutrophils shortly after injury of the tailfin was visualized using real time imaging. These findings were consistent with the early work done by Metchnikoff showing the robust recruitment of “phagocytes” (macrophage-like cells) to injury or foreign objects, and more recent work by Herbomel and colleagues imaging macrophage behavior in zebrafish.16 Metchnikoff had also described the restitution of tissues after injury in transparent organisms, and found that phagocytes “perish” and are “englobed” by other phagocytes in the days after the injury8.
By tracking the fate of fluorescent neutrophils over time during resolution of damage in zebrafish, our lab made a surprising discovery. Traditional dogma was that neutrophils, after entering sites of injury, undergo apoptosis and are subsequently cleared by macrophages to resolve local inflammation. What we observed was something completely different: neutrophils leave sites of tissue damage and migrate back into the vasculature to resolve the local inflammatory response.12 These studies showed that neutrophils can resolve a local response by “reverse migration” and their subsequent reverse transendothelial migration into the vasculature17, with minimal if any neutrophil apoptosis in these tissues. While this observation was unexpected, it did corroborate earlier work that had demonstrated in a rodent model of glomerulonephritis that the majority of invading neutrophils do not undergo apoptosis in the tissues and their loss from tissues was postulated to be due their re-entering the circulation.18
It is important to note that it took over a decade for the reverse migration of neutrophils to be directly demonstrated in mice using photoconversion for cell fate tracking.19 As Metchnikoff said so elegantly in 1893: simple model organisms use “infinitely simpler and more primitive conditions than those in man and vertebrata” and this “really furnishes the key to the comprehension of the complex pathological phenomena which are of special interest in medical science”.8 Neutrophil reverse migration and their reverse transendothelial migration into the circulation has now been extensively documented by both our lab and other labs in zebrafish and mice alike.19–25 Furthermore, human neutrophils undergo reverse migration through endothelial layers in vitro.26
This observation of reverse migration prompted an immediate question: what do neutrophils do upon exiting the tissue? To specifically track reverse migrated neutrophils, our lab used transgenic zebrafish expressing the photoconvertible Dendra2 fluorophore under the mpx promoter. We found that, after reverse transendothelial migration back into the vasculature, wound-sensitized neutrophils dispersed into peripheral tissues and also travelled back to the caudal hematopoietic tissue.17 In rodent models, reverse migrated neutrophils were found to travel to the lung and ultimately ended up in the bone marrow.19 Neutrophils have been described to deliver pathogen-derived peptides for the priming of bone marrow CD8+ T cells.19,24 Indeed, additional studies in mouse models have shown that the reverse migration of neutrophils from sterile liver injury19 and the reverse transmigration of neutrophils into the circulation25 can contribute to the development of systemic inflammation. For example, with increased vascular permeability, after exiting the circulation neutrophils remain associated with the endothelial layer and rapidly undergo abluminal-to-luminal reverse transendothelial migration (rTEM), in the absence of interstitial reverse migration. This rapid, reverse transendothelial migration occurs in response to disrupted chemokine gradients following excessive inflammation-induced vascular permeability and is associated with systemic dissemination of neutrophils.27 Thus, neutrophil reverse migration and rTEM appears to not just function as a strategy for resolving local tissue inflammation, but also as a mechanism for activating the other arms of the immune response.
What regulates neutrophil reverse migration?
Several recent reports have addressed the signals that mediate neutrophil reverse migration. Similar to neutrophils, macrophages infiltrate peripheral tissues in response to damage and infection, albeit with slightly delayed kinetics.23 By simultaneously imaging neutrophils and macrophages following sterile tail transection, we were able to determine that macrophages characteristically interacted with neutrophils at the wound immediately prior to reverse migration.23 In the absence of macrophages, neutrophils lingered at wound edge for significantly longer and exhibited altered migratory phenotypes.23 These findings are consistent with the recently reported cloaking behavior of macrophages that prevents neutrophil-mediated inflammatory damage in rodent models.28 While these data identified macrophages as being a regulator of neutrophils, neutrophils can still reverse migrate in the absence of macrophages, pointing towards macrophage-independent mechanisms that mediate neutrophil reverse migration.23 One way in which macrophages influence neutrophil reverse migration is through PGE2 production29, but other soluble factors are likely to be involved. Along these lines, we investigated the role of IL-8 and the IL-8 receptors, Cxcr1 and Cxcr2, in neutrophil forward and reverse migration following wounding. Cxcr1 (functional equivalent of human Cxcr2) and zebrafish Cxcr2 (functional equivalent of human Cxcr1), are known to regulate the in vivo motility of neutrophils.30–32 Interestingly, while IL-8a signaling through Cxcr1 mediated neutrophil recruitment to sterile transection injuries, IL-8b signaling through Cxcr2 promoted chemokinesis and reverse migration.33,34 Cxcr1 is promptly internalized in recruited neutrophils, which prevents excessive congregation at wound sites.34 However, Cxcr2 plasma membrane localization and signaling is highly sustained in responding neutrophils, which promotes eventual neutrophil dispersal from wound sites.34 Cumulatively, these results indicate there are multiple distinct pathways that drive neutrophil reverse migration and promote resolution of inflammation.
Advances in the tools used for imaging inflammation in Zebrafish
Imaging methods have advanced dramatically in recent years, and optically transparent organisms are no longer required for intravital imaging. Multi-photon imaging approaches have permitted exquisite in vivo observation of leukocyte recruitment in rodents and other organisms that would be otherwise poorly suited for in vivo imaging.35 However, the spatial resolution possible in vertebrate systems like mice is limited by the normal opacification of the tissues, especially the skin. In addition, these models are generally not amenable to long term imaging in situ. There is therefore a gap in understanding how tissue repair normally evolves over days, as this process requires longer term imaging, which is not feasible in rodent models. A recent focus of our lab has been investigating the inflammatory and tissue response to different types of injuries, including thermal injury and infection over the timescale of days after the initial insult.36 Additionally, live imaging of the extracellular matrix has enabled us to observe remodeling of collagen fibers over 10 hours after injury.37 Continual advances in live imaging, such as these, demonstrate the benefit of using zebrafish to model temporal and spatial tissue dynamics after injury.
The ease of intravital imaging in the larval zebrafish is due, in part, to the absence of pigmentation early in development. Wild-type zebrafish become increasingly pigmented as they develop their namesake stripes, but specimens remain highly amenable to imaging in the embryonic and larval stages. For investigators interested in imaging beyond the earliest developmental time points, mutant zebrafish lines with defective pigmentation have been described. Zebrafish possess three distinct classes of pigment cells – black melanophores, reflective iridophores, and yellow xanthophores.38 Nacre zebrafish have mutations in the mitfa gene that disrupts melanphore formation39, while roy orbison mutants lack reflective iridophores due to mutations in the mpv17 gene.40 In 2008, the Zon lab reported that crossing the nacre and roy orbison mutants results in fish that maintain optical transparency throughout development and into adulthood, which they termed casper.41 Casper mutants have been widely used since for intravital imaging in larval, juvenile, and adult fish.
As a model to facilitate the live imaging of leukocytes, optical transparency and external fertilization are the most noteworthy traits of zebrafish. However, the ease and accessibility of genetic manipulation also contribute to the usefulness of zebrafish as a model organism. Approximately 70% of human genes have at least one obvious orthologue in the zebrafish.42 Due to their high fecundity, zebrafish are amenable to high-throughput mutagenesis and forward genetic screens for investigating protein-coding gene function43,44 and the development of mutants that recapitulate clinical hallmarks of human disease.45 Both TALEN and CRISPR/Cas9 technologies have been used extensively to disrupt gene function in zebrafish.46,47 For investigations at the larval stage, genes of interest can be depleted by morpholino injection or transient CRISPR or “crispants”.48 More recently, CRISPR/Cas9 technology has been used to make zebrafish knockouts specifically in neutrophils or macrophages by driving Cas9 expression with tissue-specific promoters.15,49 While this technology is still relatively new, it is poised to facilitate the development of cell type specific knockouts across a wide variety of leukocyte subpopulations.
One of the challenges in the field is to understand how multicellular immune cell interactions form. Resolution of inflammation is critically dependent on these types of heterotypic intercellular interactions between leukocytes. As discussed above, macrophage interactions with neutrophils play a role in prompting reverse migration.23 To properly investigate the resolution of inflammation thus requires the ability to simultaneously image multiple distinct cell populations. New transgenic zebrafish lines have been developed in the past decade to facilitate this type of investigation. The mpeg1 and mfap4 promoters specifically label macrophages but not neutrophils in the zebrafish system.50,51 Using double transgenic zebrafish to simultaneously image neutrophils and macrophages has become standard practice for determining their unique contributions and interactions during wound healing and cancer.52–55 While there has been substantial focus on neutrophil and macrophage migration and function, transgenic zebrafish lines exist for the visualization of other leukocyte populations. The Lck promoter has been used to label and visualize bulk T cell populations in vivo56,57, and fluorescent expression under the CD4.1 or Foxp3 promoters allows for the visualization of helper or regulatory T cell subsets, respectively.58,59 Some laboratories have used weak GFP expression in the tg(Lck:GFP) fish to purify B cells, but this approach has not been used for live intravital imaging of B cell dynamics.60 However, the promoter and upstream regulatory elements of CD79, a component of the B cell receptor complex, have been used to drive GFP expression in B cell populations to facilitate in vivo imaging.61 Additionally, a transgenic fish expressing GFP under the regulatory elements of the major immunoglobulin M (IgM) has been used to image zebrafish B cell in vivo.62 A subpopulation of GFP+ cells in the tg(lck:GFP) fish express genes specific to natural killer (NK) cells, but no techniques presently exist to distinguish NK cells from the more abundant T cells for live imaging experiments.63,64 As in mammals, CD4.1 also labels some tissue-resident macrophages, which has permitted investigation into the ontogeny and function of Langerhans cells in the zebrafish system.58,65 Dendritic cells have been described in zebrafish66–68, but currently no transgenic lines allow for their specific in vivo observation. However, the regulatory elements of a class II major histocompatibility gene have been used to label all professional antigen-presenting cells, including dendritic cells.67
In addition to using cell lineage markers, reporters for cell activation state have also provided new insights in situ. For example, combining a macrophage lineage marker with a reporter for the pro-inflammatory cytokine, tumor necrosis factor alpha (TNFα), has permitted the simultaneous tracking of macrophage motility and activation status.69 While imperfect, the presence or absence of the TNFα reporter signal has been used to distinguish pro-inflammatory (M1) macrophages from pro-resolution (M2) macrophages.36 One can imagine taking a similar approach for other leukocyte populations like T cells. CD4+ T cells classically differentiate into various effector populations (Th1/Th2/Th17) depending on the nature of the immunological challenge, each with characteristic cytokine expression profiles.70 Combining the tg(lck:GFP) reporter with a reporter of lineage-defining cytokines like IFNγ, IL-4 or IL-17, will enable the imaging of T cell differentiation in vivo in real time. The ability to track leukocyte activation and differentiation in vivo will not only expand our understanding of how activation occurs normally, but also how these signals become dysregulated in chronic inflammation where molecules like TNFα and IL-17 are known to be aberrantly expressed.71,72
Imaging cell signaling in situ
In addition to labelling leukocyte populations, genetic tools exist to investigate the spatial dynamics of intracellular signaling in leukocytes and other cells in vivo. The GCaMP family of genetic Ca2+ sensors have been used in the zebrafish system to investigate calcium signaling in neurons and other cell types.73–76 Our laboratory has investigated calcium signaling following wounding77, and others have expressed GCaMP specifically in neutrophils to demonstrate polarized calcium signaling at the leading edge during migration.78 It has also been shown that neutrophil clustering is associated with sustained intracellular calcium signals that mediate synthesis of the leukotriene chemoattractant LTB4 and neutrophil swarming.79,80 Swarming behavior is initiated when a pioneer neutrophil recognizes either tissue damage or the presence of microbes and attracts subsequent waves of neutrophils.81 Interestingly, release of cellular components by the initial pioneer neutrophil in the form of neutrophil extracellular traps (NETs), also mediates this neutrophil swarming behavior.82
Fluorescent biosensors for various inositol lipids to investigate the role of phosphoinositide 3-kinases (PI(3)K) and SHIP phosphatases in interstitial leukocyte motility have also been revealing.83,84 Our laboratory has also made use of fluorescent probes that differentially label stable and dynamic pools of actin to understand how they uniquely contribute to interstitial leukocyte migration.83 Some groups have even used FRET-based tension sensors to study mechanical forces in endothelial cells in vivo85, opening the door for future studies to investigate the mechanobiology of leukocytes in the zebrafish. Cumulatively, these tools allow investigators to use the zebrafish system to link molecular intracellular processes to leukocyte motility and function in real time.
Modeling human neutrophil disorders in zebrafish.
There has been long standing interest in modeling human disease in zebrafish including cancer, infectious disease, and genetic disorders.86 The development of specific neutrophil disease models has also helped to elucidate disease pathogenesis and identify new treatments. For example, a model of chronic neutrophil inflammation in epidermal tissues87,88 has been useful in screening for new anti-inflammatory drugs and for the study of anti-inflammatory effects of glucocorticoids.89
The use of zebrafish to model human neutrophil disorders has provided new insight into cell signaling and disease pathogenesis. For example, the dynamics of the interaction between the neutrophil chemokine receptor CXCR4 and its receptor ligand (stromal-derived factor-1; SDF-1) was live imaged in a zebrafish model of congenital neutropenia, warts, hypo-gammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, which demonstrated neutropenia and susceptibility to infection.90 The findings revealed that CXCR4 WHIM mutations increased neutrophil adhesion in hematopoietic tissue, and this was regulated by signaling through the small GTPase Rac1. A model of leukocyte adhesion deficiency due to dominant inhibitory mutations in Rac2 also revealed that CXCR4-Rac2 signaling regulates neutrophil actin cytoskeletal dynamics, resulting in neutrophil retention in hematopoietic tissue.91 In addition to providing new insight into disease mechanisms, these models have also been used to provide new understanding about how neutrophils affect fungal and mycobacterial infection92–94 and cancer progression95. Additional genetic models of immune dysregulation have been developed including a model of Chronic Granulomatous disease (CGD) that exhibits robust neutrophil inflammation and susceptibility to specific fungal disease.96 Taken together, these studies highlight the conservation of the innate immune system in zebrafish and ability to model specific human immune disorders.
Cell signaling and neutrophil motility during wound healing
The wound healing response is characterized by the rapid influx of leukocytes, with neutrophils typically being the first responding cell population. As noted above, zebrafish neutrophils are similar in form and function to their mammalian counterparts, and the zebrafish model is amenable to imaging on the timescale of days.97–100 Neutrophils begin infiltrating the damaged tissue rapidly and are most abundant 1-hour after tail transection.23 Macrophages, in contrast, show a slightly delayed but more prolonged influx into the damaged tissue.23. Because of the ease in imaging, genetic manipulation, and drug treatment in the zebrafish system, a tremendous amount of work has gone into uncovering the signals that guide neutrophils and macrophages into the wound environment. A genetically encoded H2O2 sensor was used to demonstrate that epithelial cells at the wound edge produce H2O2 within minutes of caudal fin transection, generating a gradient that extends for hundreds of microns into the tissue.101 Further, disrupting this H2O2 gradient was shown to block neutrophil recruitment to the wound edge.101 H2O2 is a pleiotropic signaling molecule, and our laboratory set out to determine the mechanism by which leukocytes sense H2O2 gradients. We found that pharmacologically or genetically interfering with the Src family kinase Lyn blunted neutrophil recruitment to wounds, similar to interfering with H2O2 gradients.102 Lyn was able to directly sense H2O2 gradients through oxidation of a single cysteine residue, C466.102 Other investigators utilizing the zebrafish system have determined that similar H2O2 gradients promote early neutrophil and macrophage recruitment to transformed cells during oncogenesis.103 While H2O2 clearly promotes leukocyte migration to wounds and transformed cells, the same was not true for infections. In a zebrafish inner ear infection with Pseudomonas aeruginosa, neutrophils are recruited to the infected tissue where they phagocytose the pathogen. However, neutrophil recruitment to the site of infection remained fully intact when H2O2 gradients were disrupted, pointing to important chemoattraction differences between sites of damage or transformation and sites of infection.104
More recent studies have elucidated the contribution of cellular swelling to sensing tissue damage. Metchnikoff documented tissue level swelling following wounding8, and cellular swelling following injury has been extensively documented, especially in astrocytes following brain injury.105–108 Cellular swelling has been proposed to contribute to inflammation through the leakage of cytoplasmic factors into the extracellular space during cell lysis.109 However, other studies have demonstrated that cellular swelling can have pro-inflammatory effects through NLRP3 activation independent of cell lysis.110,111 Many epithelial barriers, including the oral cavity, esophagus, and lungs, separate an interstitial environment from a relatively hypotonic external environment.111–113 The skin of freshwater animals like the zebrafish creates a barrier between hypertonic interstitial fluid and hypotonic water.111 Damage to this epithelial barrier thus results in notable cellular swelling in cells near the wound edge.111,114 Cellular and nuclear swelling following tail fin damage results in the translocation of cytosolic phospholipase A2 and 5-lipoxygenase to the nuclear envelope and their subsequent activation.114 Activation of these enzymes results in the production of pro-inflammatory eicosanoids, thus linking mechanical changes from osmotic stress to the recruitment of leukocytes.114 When zebrafish tails are wounded in media isotonic to the interstitial tissue, there is a sharp reduction in leukocyte recruitment relative to normal hypotonic media.111 Taken together, these findings reveal the power of the zebrafish system to identify tissue scale signaling mechanisms that regulate neutrophil recruitment. A future challenge is to understand these tissue scale mechanisms during the resolution phase of the inflammatory response.
Regulation of interstitial leukocyte migration
While the specific chemoattractive signals that mediate neutrophil recruitment to damage, cancer and infection may differ, all contexts appear to rely heavily upon PI(3)K activity to drive interstitial migration. PI(3)K phosphorylates the 3 position of inositol membrane lipids to convert PI(4,5)P2 to PI(3,4,5)P3. These inositol species subsequently serve as docking sites for adaptor proteins to drive signaling, actin polymerization, and motility.115,116 Several studies had demonstrated that PI(3,4,5)P3 is specifically localized to the leading edge in motile dictyostelium and neutrophils in vitro, but PI(3,4,5)P3 was notably non-polarized in various non-hematopoietic zebrafish cells migrating in vivo.117–120 We therefore set out to determine if the in vivo interstitial motility of neutrophils either relies upon PI(3)K activity or employs a polarized PI(3,4,5)P3 gradient at the leading edge. To detect a PI(3,4,5)P3 gradient, we expressed the GFP-tagged pleckstrin homology (PH) domain of AKT, which binds indiscriminately to both PI(3,4,5)P3-PI(3,4)P2, as well as cytoplasmic mCherry specifically in zebrafish neutrophils using the MPO promoter and performed ratiometric imaging.83 PI(3,4,5)P3-PI(3,4)P2 primarily localizes to the leading-edge during motility in vivo and this polarization depended upon PI3K activity.83 This polarization was especially prominent during directed migration.83 However, the inability to distinguish between PI(3,4,5)P3 and PI(3,4)P2 localization limited the interpretation. While the PH domain of AKT binds promiscuously to both PI(3,4,5)P3-PI(3,4)P2, these lipid species can distinguished the PH domain of GRP1, which interacts only with PI(3,4,5)P3, and the PH domain of TAPP1, which interacts only with PI(3,4)P2. Using these more specific bioprobes with the same ratiometric imaging approach, it was determined that while PI(3,4)P2 localizes to both the leading and trailing edges, PI(3,4,5)P3 preferentially accumulated at the leading edge.84 The conversion of PI(3,4,5)P3 to PI(3,4)P2 is mediated by the SHIP family of phosphatases, which remove the 5 position phosphate group from the inositol ring. To determine if SHIP activity regulates neutrophil interstitial activity, we employed a morpholino-based knockdown approach. Interestingly, depleting SHIP increased neutrophil recruitment to wounds and significantly elevated speeds during random motility.84 The increased accumulation at the wound edge could be returned to baseline with low dose administration of PI3K inhibitors, indicating that SHIP proteins regulate neutrophil motility by regulating PI3K signaling.84 Likewise, overexpression of the SHIP phosphatase domain in neutrophils dampened neutrophil motility.84 These findings addressed some of controversy regarding PI3K signaling in regulating neutrophil chemotaxis and directional sensing, and suggested that it is a key regulator of the actin machinery during neutrophil interstitial motility in vivo.
Despite navigating the exact same interstitial tissue environment during tissue injury responses, neutrophils and macrophages utilize distinct modes of motility.55 Neutrophils utilize a classic ameboid mode of motility with more compact cell morphology and significantly higher velocities than macrophages, which utilize a more mesenchymal mode of motility with highly elongated cell morphologies and paxillin-rich puncta.55 Similar to other mesenchymal cells, macrophages are highly dependent upon protease activity for interstitial motility, whereas neutrophils are not.55 Pharmacological inhibition of Arp2/3 function inhibits the motility of both cell populations, but the effect is more profound for neutrophils.55 Likewise, macrophage motility is more sensitive to ROCK inhibition than neutrophils. These migratory differences appear to have functional consequences in different wound contexts.55 Following thermal injury, collagen fiber organization is disrupted in the tail fin.36,37 While neutrophils readily migrate into regions of disrupted collagen organization, macrophage migration is impaired in these regions, indicating that neutrophil motility is less dependent upon extracellular matrix composition and structure for their interstitial motility.52
Identification of new mechanisms that regulate inflammation resolution
A critical question in the area of inflammation resolution is: what is it that normally restores tissue homeostasis after injury? The zebrafish sterile tail transection has been an invaluable tool for studying both the onset and resolution of inflammation, as tissue homeostasis is fully restored after damage. This transition from inflammatory to resolving stages is often dysregulated in humans, resulting in the presence of chronic wounds and inflammation.121 What causes a normally self-limiting acute wound to become chronic? What kind of signals or processes fail to occur in these patients? Infection is the single most likely cause of delayed or defective wound healing.122 In the zebrafish system, a concomitant tail transection and infection with Listeria monocytogenes (Lm) leads to substantial defects in tail regrowth compared to transection alone.36 We therefore sought to identify transcriptional changes that differ between neutrophils responding to sterile tissue damage versus infection.123 Myeloid-derived Growth Factor (MYDGF) was identified as a target gene upregulated in both neutrophils and macrophages in response to wounding, but not infection.124 To determine if MYDGF regulates proper wound healing, we generated MYDGF-deficient mutant zebrafish and performed sterile tail transection experiments. MYDGF-deficient zebrafish larvae displayed increased neutrophil recruitment, decreased neutrophil reverse migration, and defective tissue regeneration following transection relative to WT larvae.124 A similar defect in neutrophil reverse migration has been reported in zebrafish deficient in HIF-1α.20 Cumulatively these data point towards MYDGF and HIF-1α playing critical roles in mediating normal tissue homeostasis after damage. Future studies should continue to address the fundamental question about what the normal processes are that maintain tissue homeostasis and inhibit inflammation after injury, to prevent the transition to chronic tissue inflammation.
Looking Forward: Zebrafish and Adaptive Immunity
Real time imaging of immunity in zebrafish has primarily been focused on myeloid cells. The relative paucity of research on lymphocyte function is due, in part, to technical considerations. Macrophages and neutrophils are present and functional early in development, capable of phagocytosis by 30 hours post-fertilization (hpf) and respiratory bursts by 72 hpf.98 Lymphocytes, in contrast, emerge much later in development. Recombination-activating genes (RAGs) and TCRα transcripts are detectable by 4 days post-fertilization (DPF).125,126 However, mature T cells are rare in the peripheral tissues until approximately 10 DPF.58 The standard AB strain of zebrafish is highly pigmented by this age, making intravital imaging of T cells challenging. As described above, several mutant strains with defective pigmentation have been developed to facilitate intravital imaging at these later stages. The most often used is the Casper mutant published by the Zon lab in 2008.41
While intravital imaging experiments of lymphocytes are rare, a growing body of work indicates that adaptive immunity is highly conserved between zebrafish and mammals. Zebrafish possess the same cadre of professional antigen-presenting cells as mammals. MHCII-expressing B cells62, macrophages67,97, and dendritic cells66 have all been described in the zebrafish.68 Studies have determined that zebrafish dendritic cells can process and present antigen to activate T cells as in mammals.66 Zebrafish possess both αβ and γδ T cells, and T cell development occurs in the thymus.56,127,128 The transcription factors that regulate T cell differentiation in mammals are well-conserved in the zebrafish, and their deletion often results in similar phenotypes.129 Loss of Foxp3, for instance, results in prominent lymphocyte expansion, splenomegaly, and multi-organ inflammation similar to foxp3-deficient “scurfy” mice.130–133
In the same way that the simultaneous imaging neutrophils and macrophages lent itself to unexpected findings in the context of wound healing23, we anticipate co-imaging lymphocytes with other leukocyte populations will result in novel insights regarding cellular interactions in the context of wound healing, infection, and cancer. One report has already demonstrated that T cells undergoing thymic egress interact dynamically and intimately with a resident macrophage population as they migrate.58 We have demonstrated the imaging of T cells in the liver of a zebrafish model of hepatocellular carcinoma and found an inverse correlation of the presence of neutrophils and T cells in the liver in a model of high fat diet. This model also demonstrated that metformin reduces neutrophil numbers and restored T cell surveillance in the liver, suggesting interactions between neutrophils and T cells in HCC.53 We anticipate future studies will reveal extensive interactions between lymphocytes and various myeloid cells in a wide variety of contexts.
Towards a More Complete Model of Wound Healing: Imaging dynamic behaviors in immune systems
Much of our insight into wound healing in zebrafish is owed to the simplicity of the larval zebrafish tail transection model. The fact that neutrophils and macrophages are largely absent from the skin at rest, but rapidly infiltrate upon injury, makes the model highly amenable to the analysis of cellular motility and cell-cell interactions (Figure 1A, D, H). While the simplicity of the model makes interpretation straightforward, it is worth noting that the complete absence of hematopoietic cells in the skin is never observed outside of the earliest stages of development. By the time mice or humans are born, the skin is heavily populated by various T cell subsets, a variety of tissue-resident macrophages, and dendritic cells (Figure 1B). Due to their positioning at border sites, these cells are the first leukocyte subsets to detect tissue damage following wounding, and they each contribute to the wound healing response. The network of leukocytes involved in the initiation and resolution of inflammation following wounding quickly becomes dizzying. Among the cell populations present in the skin at rest that contribute meaningfully to either tissue homeostasis or the wound healing response, there are: commensal-specific CD4+ or CD8+ T cells134–136, Foxp3-expressing regulatory T cells137,138, epidermal γδ T cells139–143 skin-resident memory T cells144–146, Langerhans cells147–149, dermal macrophages121,150–153, and dendritic cells154. Following tissue damage, these resident cell populations are joined by neutrophils, monocyte-derived macrophages, effector T cells, and NK cells, all of which can contribute to wound healing.155–158
Figure 1. Complex leukocyte interactions during the wound healing response in zebrafish and mice.
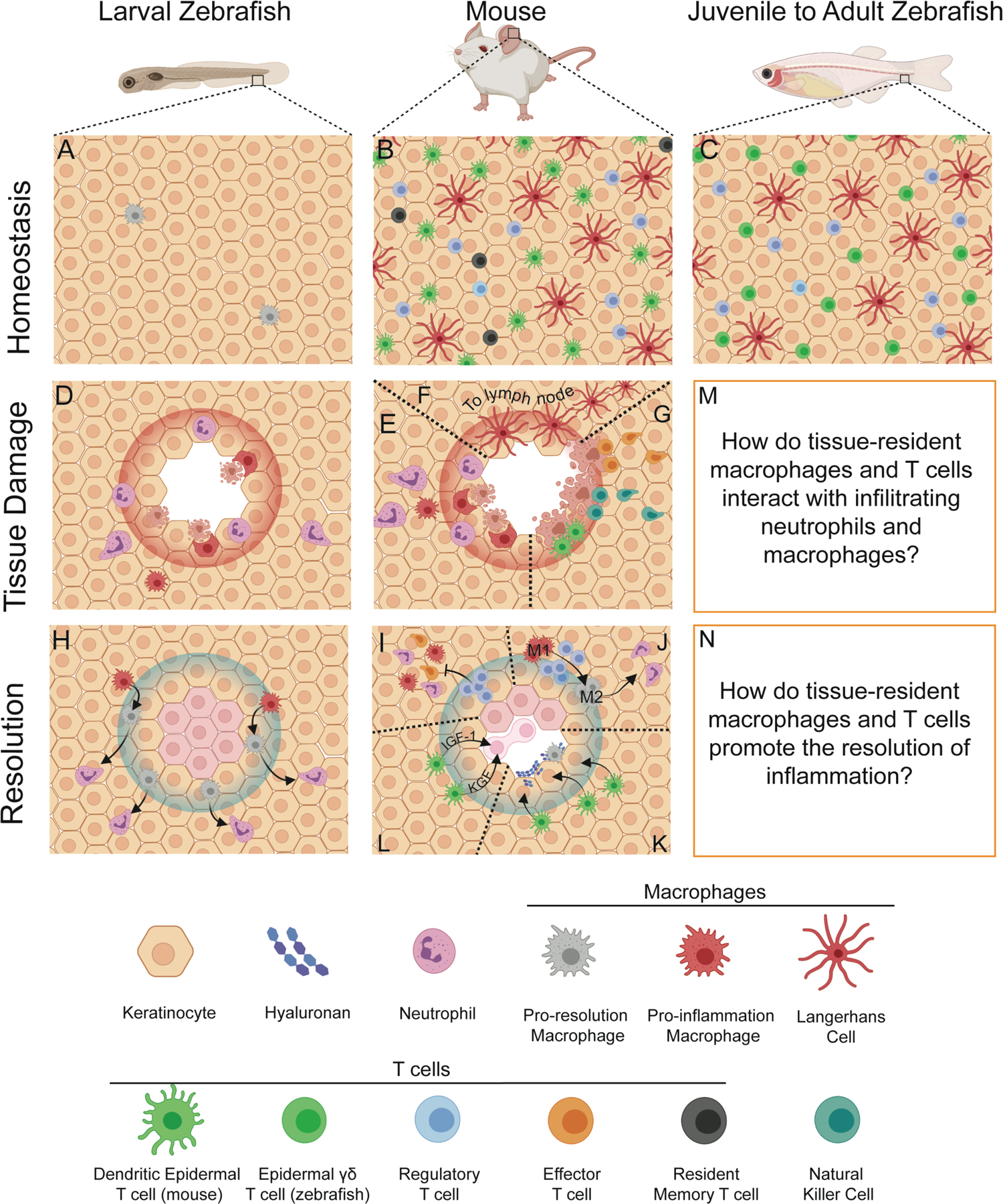
A-C. Comparing the homeostatic leukocyte composition of larval and mature zebrafish to that of mice. A. During the early larval stages of development, the epidermal layer of the zebrafish is largely devoid of leukocytes besides some migratory macrophages and resident mpeg+ cells. B. As early as birth, the epidermis of mouse skin is highly populated with diverse tissue-resident leukocyte populations. These include Langerhans cells and dendritic epidermal T cells. Shortly after birth, in response to commensal microbe exposure, Foxp3-expressing regulatory T cells colonize the skin to promote tolerance. Throughout life, these populations are joined by skin-resident memory T cell populations that develop after the resolution of infections. These cell populations are the first to detect tissue damage in the skin and regulate the inflammatory response, its resolution, and tissue homeostasis. C. The leukocyte composition in the skin of zebrafish becomes similar to that of mice during development. As in mice, Langerhans cells and other tissue-resident macrophages called metaphocytes (not shown) form a network in the epidermis. T cells are apparent in the skin by 10 DPF and are abundant in the skin of adults. This skin-resident T cell population is enriched for γδ T cells, but it is presently unclear if they assume the dendritic morphology of their mouse counterparts. As in mice, Foxp3-expressing regulatory T cells are abundant in the epidermis of adult zebrafish. D-E. In both larval zebrafish and mice, tissue damage prompts the rapid influx of neutrophils and macrophages infiltrate the damaged tissue to clear away cellular debris and destroy invading microbes. F. In mice, sentinel antigen-presenting cells like Langerhans cells and dermal macrophages respond to pathogen-derived products by migrating to the draining lymph node where they present antigen to T cells. G. In some cases, CD8+ cytotoxic T cells will enter into the tissue to kill infected cells. Natural killer cells and dendritic epidermal T cells can also directly kill target cells expressing stress-induced ligands. H. As the wound healing response progresses, responding macrophages will transition from a pro-inflammatory phenotype (M1) to a pro-healing phenotype (M2) and subsequently drive the reverse migration of neutrophils to promote the resolution of inflammation. I. In mice, regulatory T cells resident in the skin and those that enter following damage play a critical role in resolving the inflammatory response. Regulatory T cells first prevent the further influx of pro-inflammatory cells including neutrophils, macrophages, and effector T cells. J. These same regulatory T cells can promote the polarization of responding macrophages towards the pro-healing M2 phenotype. K-L. Skin-resident dendritic epidermal T cells promote wound healing by acting directly upon keratinocytes at the wound. These T cells can prompt keratinocytes to produce hyaluronan, which serves as a scaffold for migrating macrophages. Through the production of IGF-1 and KGF, dendritic epidermal T cells promote keratinocyte proliferation, which drives tissue regeneration. M-N. While it is clear if mature zebrafish harbor the same Langerhans cells, regulatory T cells, and epidermal γδ T cells networks as in mice and humans, these fish have not been yet been used to investigate the complex intercellular interactions that drive both the initial inflammatory response and the subsequent resolution. We anticipate that co-imaging regulatory T cells and epidermal γδ T cells with infiltrating neutrophils and macrophages will provide new insights into how tissue-resident T cell populations restore tissue homeostasis following wounding. Created with BioRender.com.
Is it possible to use the zebrafish untangle the web of leukocyte interactions involved during a wound healing response? While most of these cell populations are either infrequent or entirely absent in larval stage zebrafish, the skin composition in juvenile and adult fish is remarkably similar to mice and humans (Figure 1C). T cells are abundant in the tail fin by 12 DPF, and Foxp3-expressing regulatory T cells are readily detected in the epidermis in adult fish.57,59 Just as in mice, the proportion of γδ T cells in the skin of zebrafish is notably higher than it is in hematopoietic tissues like the spleen or head kidney.128 The skin of juvenile or adult zebrafish is also replete with Langerhans cells, other skin-resident macrophage populations, and dendritic cells.65,66,159,160 While it is unclear if zebrafish produce skin-resident memory T cells like mice and humans do, it is apparent that the same basic network of Langerhans cells, γδ T cells, and Foxp3-expressing T cells is present in the epidermis of mice and mature zebrafish alike (Figure 1B–C).
Larval zebrafish lack most of these skin-resident leukocyte populations, so their initial response to tissue damage involves neutrophils and macrophages infiltrating a tissue largely devoid of hematopoietic cells (Figure 1D). Due the presence of a fully-developed adaptive immune system and numerous tissue-resident leukocyte populations, the inflammatory phase of wound healing in mice is more complicated (Figure 1E–G). Just as in larval zebrafish, neutrophils and monocyte-derived macrophages rapidly infiltrate the damaged tissue (Figure 1E). Antigen-presenting cells resident in the skin that sense pathogen-derived products will migrate to the draining lymph nodes to present processed pathogen-derived peptides to T cells (Figure 1F).161,162 Depending on the nature of the damage, cytotoxic T cells may be recruited to the damaged site to kill infected cells (Figure 1G).163–165 NK cells and resident γδ T cells can also kill cells in the wound environment that have upregulated stress-induced ligands (Figure 1F). 166–168
During the resolution phase, resident T cell populations contribute notably to the wound healing response (Figure 1I–M). Regulatory T cells help initiate the shift from inflammation to resolution by first blocking the further influx of inflammatory macrophages, effector T cells, and neutrophils (Figure 1I).169,170 Regulatory T cells, through IL-10, IL4, and IL-13 secretion, can also promote macrophage skewing away from the pro-inflammatory M1 phenotype and towards the anti-inflammatory M2 phenotype (Figure 1J).171 Epidermal γδ T cells promote wound healing by acting directly on keratinocytes to promote the secretion hyaluronan, which provides a scaffold for macrophage motility into damaged tissue (Figure 1K).172 Further, epidermal γδ T cells produce the keratinocyte growth factors IGF-1 and KGF, which promotes keratinocyte proliferation and tissue regeneration (Figure 1L).139,141,173,174 The full scope of how regulatory T cells and epidermal γδ T cells regulate cutaneous wound healing is beyond the scope of this review, but is covered extensively by Boothby et al.175 and Havran et al.176. Given that mature zebrafish harbor both of these T cell populations (Figure 1C), future studies can dissect their role in wound healing by imaging their behavior and interactions with other leukocyte subsets in real time.
Challenges going forward
Future challenges include the simultaneous live imaging of different leukocyte populations during tissue damage and repair, to uncover new interactions and cellular dynamics. There is still much to be learned just by watching immune cells within tissues during damage and repair in different conditions. These transparent animals enable the high-resolution visualization of cellular dynamics, both temporally and spatially over extended durations. The application of increasingly sophisticated molecular tools to this in situ analysis is also likely to reveal fundamental new insights to normal resolution and how it is different in disease. The key challenge will be to try to harness these normal mechanisms to identify potential treatments for uncontrolled inflammation. The identification of MYDGF as a putative endogenous inhibitor of neutrophil inflammation helps to support this idea.124 Finally, understanding the plasticity of immune cells populations using reporters that detect the continuum of cellular activation like M1 or M2 macrophages, is a key future direction. These tools will be critical to uncover mechanisms of cellular plasticity as immunity evolves. Metchnikoff noted the plasticity of cells in the tissue microenvironment, after injury, with mobile “mesodermic phagocytes” becoming “fixed connective tissue cells” during tissue repair.8 This kind of plasticity of cells in damaged tissues remains to be uncovered. However, a recent report showed that macrophages develop E-cadherin expression and become “epitheloid” in mycobacterial granulomas as they mature.177 It is these types of intercellular events that need to be imaged to uncover new mechanisms of normal inflammation resolution and how it goes awry in disease.
Acknowledgements
We would like to thank Milka Sarris and Adam Horn for their thoughtful feedback and suggestions in the drafting of this article. This work was funded by NIH National Institute of General Medical Sciences R35 GM118027 (AH) and NIH National Heart, Lung, and Blood Institute T32 HL07899 (TFR).
Footnotes
Conflict of Interest
The authors declare no conflict of interest with this review
References
- 1.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 2016;16(6):378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weavers H, Martin P. The cell biology of inflammation: From common traits to remarkable immunological adaptations. J Cell Biol 2020;219(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manley HR, Potter DL, Heddleston JM, Chew T-L, Keightley MC, Lieschke GJ. Frontline Science: Dynamic cellular and subcellular features of migrating leukocytes revealed by in vivo lattice lightsheet microscopy. Journal of Leukocyte Biology 2020;108(2):455–468. [DOI] [PubMed] [Google Scholar]
- 4.Cohnheim J Neue untersuchungen über die entzündung Hirschwald; 1873. [Google Scholar]
- 5.Metchnikoff E Untersuchungen über die mesodermalen Phagocyten einiger Wirbeltiere. Biol Zentralbl 1883;3:560–565. [Google Scholar]
- 6.Metchnikoff E Immunity in infective diseases University Press; 1907. [Google Scholar]
- 7.Metchnikoff E My stay in Messina (Memories of the past, 1908). Souvenirs, Editions en Langues Etrangères Moscow 1959. [Google Scholar]
- 8.Metchnikoff E Lectures on the comparative pathology of inflammation delivered at the Pasteur Institute in 1891 Рипол Классик; 1893. [Google Scholar]
- 9.Anderson KV, Ingham PW. The transformation of the model organism: a decade of developmental genetics. Nat Genet 2003;33 Suppl:285–293. [DOI] [PubMed] [Google Scholar]
- 10.Grunwald DJ, Eisen JS. Headwaters of the zebrafish -- emergence of a new model vertebrate. Nat Rev Genet 2002;3(9):717–724. [DOI] [PubMed] [Google Scholar]
- 11.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-Time Visualization of Mycobacterium-Macrophage Interactions Leading to Initiation of Granuloma Formation in Zebrafish Embryos. Immunity 2002;17(6):693–702. [DOI] [PubMed] [Google Scholar]
- 12.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 2006;80(6):1281–1288. [DOI] [PubMed] [Google Scholar]
- 13.Renshaw SA, Loynes CA, Trushell DMI, Elworthy S, Ingham PW, Whyte MKB. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006;108(13):3976–3978. [DOI] [PubMed] [Google Scholar]
- 14.Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Developmental Biology 2007;7(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Cao L, Jeffries J, Zhu X, Staiger CJ, Deng Q. Neutrophil-specific knockout demonstrates a role for mitochondria in regulating neutrophil motility in zebrafish. Disease Models & Mechanisms 2018;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999;126(17):3735–3745. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. Journal of leukocyte biology 2011;89(5):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes J, Johnson RJ, Mooney A, Hugo C, Gordon K, Savill J. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am J Pathol 1997;150(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017;358(6359):111–116. [DOI] [PubMed] [Google Scholar]
- 20.Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011;118(3):712–722. [DOI] [PubMed] [Google Scholar]
- 21.Ellett F, Elks PM, Robertson AL, Ogryzko NV, Renshaw SA. Defining the phenotype of neutrophils following reverse migration in zebrafish. J Leukoc Biol 2015;98(6):975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson AL, Holmes GR, Bojarczuk AN, et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 2014;6(225):225ra229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauzin S, Starnes TW, Becker FB, Lam P-y, Huttenlocher A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. Journal of Cell Biology 2014;207(5):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy D, Perrin H, Abadie V, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity 2012;37(5):917–929. [DOI] [PubMed] [Google Scholar]
- 25.Woodfin A, Voisin M-B, Beyrau M, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011;12(8):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamza B, Wong E, Patel S, Cho H, Martel J, Irimia D. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr Biol (Camb) 2014;6(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen-Woods C, Joulia R, Barkaway A, et al. Local microvascular leakage promotes trafficking of activated neutrophils to remote organs. The Journal of Clinical Investigation 2020;130(5):2301–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell 2019;177(3):541–555.e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loynes CA, Lee JA, Robertson AL, et al. PGE(2) production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv 2018;4(9):eaar8320–eaar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das ST, Rajagopalan L, Guerrero-Plata A, et al. Monomeric and Dimeric CXCL8 Are Both Essential for In Vivo Neutrophil Recruitment. PLOS ONE 2010;5(7):e11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the Cytoplasmic Tails of CXCR1 and CXCR2 in Mediating Leukocyte Migration, Activation, and Regulation. The Journal of Immunology 2003;170(6):2904. [DOI] [PubMed] [Google Scholar]
- 32.Deng Q, Sarris M, Bennin DA, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. Journal of Leukocyte Biology 2013;93(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A. Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Reports 2017;19(8):1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coombs C, Georgantzoglou A, Walker HA, et al. Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish. Nature Communications 2019;10(1):5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoover EE, Squier JA. Advances in multiphoton microscopy technology. Nature Photonics 2013;7(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miskolci V, Squirrell J, Rindy J, et al. Distinct inflammatory and wound healing responses to complex caudal fin injuries of larval zebrafish. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeBert DC, Squirrell JM, Rindy J, et al. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development 2015;142(12):2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawls JF, Mellgren EM, Johnson SL. How the zebrafish gets its stripes. Dev Biol 2001;240(2):301–314. [DOI] [PubMed] [Google Scholar]
- 39.Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 1999;126(17):3757–3767. [DOI] [PubMed] [Google Scholar]
- 40.D’Agati G, Beltre R, Sessa A, et al. A defect in the mitochondrial protein Mpv17 underlies the transparent casper zebrafish. Dev Biol 2017;430(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White RM, Sessa A, Burke C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008;2(2):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496(7446):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kettleborough RN, Busch-Nentwich EM, Harvey SA, et al. A systematic genomewide analysis of zebrafish protein-coding gene function. Nature 2013;496(7446):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varshney GK, Carrington B, Pei W, et al. A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat Protoc 2016;11(12):2357–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gut P, Reischauer S, Stainier DYR, Arnaout R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiological Reviews 2017;97(3):889–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlem TJ, Hoshijima K, Jurynec MJ, et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet 2012;8(8):e1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang WY, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 2013;31(3):227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nature Genetics 2000;26(2):216–220. [DOI] [PubMed] [Google Scholar]
- 49.Ablain J, Durand Ellen M, Yang S, Zhou Y, Zon Leonard I. A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish. Developmental Cell 2015;32(6):756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011;117(4):e49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walton EM, Cronan MR, Beerman RW, Tobin DM. The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish. PLOS ONE 2015;10(10):e0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barros-Becker F, Squirrell JM, Burke R, et al. Distinct Tissue Damage and Microbial Cues Drive Neutrophil and Macrophage Recruitment to Thermal Injury. iScience 2020;23(11):101699–101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Oliveira S, Houseright RA, Graves AL, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. Journal of Hepatology 2019;70(4):710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golenberg N, Squirrell JM, Bennin DA, et al. Citrullination regulates wound responses and tissue regeneration in zebrafish. Journal of Cell Biology 2020;219(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barros-Becker F, Lam P-Y, Fisher R, Huttenlocher A. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. Journal of Cell Science 2017;130(22):3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langenau DM, Ferrando AA, Traver D, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proceedings of the National Academy of Sciences of the United States of America 2004;101(19):7369–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jerison ER, Quake SR. Heterogeneous T cell motility behaviors emerge from a coupling between speed and turning in vivo. eLife 2020;9:e53933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dee CT, Nagaraju RT, Athanasiadis EI, et al. CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2-and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. J Immunol 2016;197(9):3520–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui SP, Sheng DZ, Sugimoto K, et al. Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev Cell 2017;43(6):659–672.e655. [DOI] [PubMed] [Google Scholar]
- 60.Burroughs-Garcia J, Hasan A, Park G, Borga C, Frazer JK. Isolating Malignant and Non-Malignant B Cells from lck:eGFP Zebrafish. J Vis Exp 2019(144). [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Li YS, Shinton SA, et al. Zebrafish B Cell Development without a Pre-B Cell Stage, Revealed by CD79 Fluorescence Reporter Transgenes. J Immunol 2017;199(5):1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page DM, Wittamer V, Bertrand JY, et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 2013;122(8):e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmona SJ, Teichmann SA, Ferreira L, et al. Single-cell transcriptome analysis of fish immune cells provides insight into the evolution of vertebrate immune cell types. Genome Res 2017;27(3):451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Q, Iyer S, Lobbardi R, et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. Journal of Experimental Medicine 2017;214(10):2875–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin X, Zhou Q, Zhao C, Lin G, Xu J, Wen Z. An Ectoderm-Derived Myeloid-like Cell Population Functions as Antigen Transporters for Langerhans Cells in Zebrafish Epidermis. Dev Cell 2019;49(4):605–617.e605. [DOI] [PubMed] [Google Scholar]
- 66.Lugo-Villarino G, Balla KM, Stachura DL, Bañuelos K, Werneck MB, Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci U S A 2010;107(36):15850–15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wittamer V, Bertrand JY, Gutschow PW, Traver D. Characterization of the mononuclear phagocyte system in zebrafish. Blood 2011;117(26):7126–7135. [DOI] [PubMed] [Google Scholar]
- 68.Lewis KL, Del Cid N, Traver D. Perspectives on antigen presenting cells in zebrafish. Developmental and comparative immunology 2014;46(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen-Chi M, Laplace-Builhe B, Travnickova J, et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015;4:e07288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol 2020;38:705–725. [DOI] [PubMed] [Google Scholar]
- 71.Hadian Y, Bagood MD, Dahle SE, Sood A, Isseroff RR. Interleukin-17: Potential Target for Chronic Wounds. Mediators of Inflammation 2019;2019:1297675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4(3):321–325. [DOI] [PubMed] [Google Scholar]
- 73.Muto A, Ohkura M, Kotani T, Higashijima S-i, Nakai J, Kawakami K. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proceedings of the National Academy of Sciences 2011;108(13):5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turrini L, Fornetto C, Marchetto G, et al. Optical mapping of neuronal activity during seizures in zebrafish. Scientific Reports 2017;7(1):3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cong L, Wang Z, Chai Y, et al. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 2017;6:e28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruzzone M, Chiarello E, Albanesi M, et al. Whole brain functional recordings at cellular resolution in zebrafish larvae with 3D scanning multiphoton microscopy. Scientific Reports 2021;11(1):11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo SK, Freisinger CM, LeBert DC, Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol 2012;199(2):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beerman RW, Matty MA, Au GG, et al. Direct In Vivo Manipulation and Imaging of Calcium Transients in Neutrophils Identify a Critical Role for Leading-Edge Calcium Flux. Cell Rep 2015;13(10):2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poplimont H, Georgantzoglou A, Boulch M, et al. Neutrophil Swarming in Damaged Tissue Is Orchestrated by Connexins and Cooperative Calcium Alarm Signals. Curr Biol 2020;30(14):2761–2776.e2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lämmermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013;498(7454):371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng LG, Qin JS, Roediger B, et al. Visualizing the Neutrophil Response to Sterile Tissue Injury in Mouse Dermis Reveals a Three-Phase Cascade of Events. Journal of Investigative Dermatology 2011;131(10):2058–2068. [DOI] [PubMed] [Google Scholar]
- 82.Isles HM, Loynes CA, Alasmari S, et al. Pioneer neutrophils release chromatin within in vivo swarms. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Developmental cell 2010;18(2):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam P-y, Yoo SK, Green JM, Huttenlocher A. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. Journal of cell science 2012;125(Pt 21):4973–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lagendijk AK, Gomez GA, Baek S, et al. Live imaging molecular changes in junctional tension upon VE-cadherin in zebrafish. Nature Communications 2017;8(1):1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;8(5):353–367. [DOI] [PubMed] [Google Scholar]
- 87.Mathias JR, Dodd ME, Walters KB, et al. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J Cell Sci 2007;120(Pt 19):3372–3383. [DOI] [PubMed] [Google Scholar]
- 88.Carney TJ, von der Hardt S, Sonntag C, et al. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 2007;134(19):3461–3471. [DOI] [PubMed] [Google Scholar]
- 89.Xie Y, Meijer AH, Schaaf MJM. Modeling Inflammation in Zebrafish for the Development of Anti-inflammatory Drugs. Front Cell Dev Biol 2020;8:620984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood 2010;116(15):2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev Cell 2011;21(4):735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knox BP, Deng Q, Rood M, Eickhoff JC, Keller NP, Huttenlocher A. Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae. Eukaryot Cell 2014;13(10):1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan Q, van der Gast CJ, Yu Y. Bacterial community assembly and turnover within the intestines of developing zebrafish. PLoS One 2012;7(1):e30603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramakrishnan L Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 2012;12(5):352–366. [DOI] [PubMed] [Google Scholar]
- 95.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol 2016;37(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schoen TJ, Rosowski EE, Knox BP, Bennin D, Keller NP, Huttenlocher A. Neutrophil phagocyte oxidase activity controls invasive fungal growth and inflammation in zebrafish. J Cell Sci 2019;133(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001;98(10):3087–3096. [DOI] [PubMed] [Google Scholar]
- 98.Harvie EA, Huttenlocher A. Neutrophils in host defense: new insights from zebrafish. Journal of leukocyte biology 2015;98(4):523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nature Reviews Immunology 2016;16(6):378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mathias JR, Walters KB, Huttenlocher A. Neutrophil motility in vivo using zebrafish. Methods Mol Biol 2009;571:151–166. [DOI] [PubMed] [Google Scholar]
- 101.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459(7249):996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 2011;480(7375):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live Imaging of Innate Immune Cell Sensing of Transformed Cells in Zebrafish Larvae: Parallels between Tumor Initiation and Wound Inflammation. PLOS Biology 2010;8(12):e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng Q, Harvie EA, Huttenlocher A. Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cellular Microbiology 2012;14(4):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bullock R, Maxwell WL, Graham DI, Teasdale GM, Adams JH. Glial swelling following human cerebral contusion: an ultrastructural study. J Neurol Neurosurg Psychiatry 1991;54(5):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 1997;87(6):900–907. [DOI] [PubMed] [Google Scholar]
- 107.Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD. Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. Journal of Neurochemistry 2011;117(3):437–448. [DOI] [PubMed] [Google Scholar]
- 108.Marmarou CR, Liang X, Abidi NH, et al. Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain Res 2014;1581:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev 2011;243(1):191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Compan V, Baroja-Mazo A, López-Castejón G, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 2012;37(3):487–500. [DOI] [PubMed] [Google Scholar]
- 111.Enyedi B, Kala S, Nikolich-Zugich T, Niethammer P. Tissue damage detection by osmotic surveillance. Nat Cell Biol 2013;15(9):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilljam H, Ellin A, Strandvik B. Increased bronchial chloride concentration in cystic fibrosis. Scand J Clin Lab Invest 1989;49(2):121–124. [DOI] [PubMed] [Google Scholar]
- 113.Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis 1993;148(6 Pt 1):1633–1637. [DOI] [PubMed] [Google Scholar]
- 114.Enyedi B, Jelcic M, Niethammer P. The Cell Nucleus Serves as a Mechanotransducer of Tissue Damage-Induced Inflammation. Cell 2016;165(5):1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nature Reviews Molecular Cell Biology 2012;13(3):195–203. [DOI] [PubMed] [Google Scholar]
- 116.Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell 2009;101(1):13–29. [DOI] [PubMed] [Google Scholar]
- 117.Dumstrei K, Mennecke R, Raz E. Signaling pathways controlling primordial germ cell migration in zebrafish. J Cell Sci 2004;117(Pt 20):4787–4795. [DOI] [PubMed] [Google Scholar]
- 118.Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development 2008;135(15):2521–2529. [DOI] [PubMed] [Google Scholar]
- 119.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G Protein Signaling Events Are Activated at the Leading Edge of Chemotactic Cells. Cell 1998;95(1):81–91. [DOI] [PubMed] [Google Scholar]
- 120.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000;287(5455):1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Frontiers in Physiology 2018;9(419). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leaper D, Assadian O, Edmiston CE. Approach to chronic wound infections. Br J Dermatol 2015;173(2):351–358. [DOI] [PubMed] [Google Scholar]
- 123.Houseright RA, Rosowski EE, Lam P-Y, et al. Cell type specific gene expression profiling reveals a role for complement component C3 in neutrophil responses to tissue damage. Scientific Reports 2020;10(1):15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Houseright RA, Miskolci V, Mulvaney O, et al. Myeloid-derived growth factor regulates neutrophil motility in interstitial tissue damage. Journal of Cell Biology 2021;220(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trede NS, Zon LI. Development of t-cells during fish embryogenesis. Developmental & Comparative Immunology 1998;22(3):253–263. [DOI] [PubMed] [Google Scholar]
- 126.Danilova N, Hohman VS, Sacher F, Ota T, Willett CE, Steiner LA. T cells and the thymus in developing zebrafish. Dev Comp Immunol 2004;28(7–8):755–767. [DOI] [PubMed] [Google Scholar]
- 127.Meeker ND, Smith AC, Frazer JK, et al. Characterization of the zebrafish T cell receptor beta locus. Immunogenetics 2010;62(1):23–29. [DOI] [PubMed] [Google Scholar]
- 128.Wan F, Hu C-B, Ma J-X, Gao K, Xiang L-X, Shao J-Z. Characterization of γδ T Cells from Zebrafish Provides Insights into Their Important Role in Adaptive Humoral Immunity. Front Immunol 2017;7:675–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mitra S, Alnabulsi A, Secombes CJ, Bird S. Identification and characterization of the transcription factors involved in T-cell development, t-bet, stat6 and foxp3, within the zebrafish, Danio rerio. Febs j 2010;277(1):128–147. [DOI] [PubMed] [Google Scholar]
- 130.Kasheta M, Painter CA, Moore FE, et al. Identification and characterization of T reg-like cells in zebrafish. J Exp Med 2017;214(12):3519–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001;27(1):18–20. [DOI] [PubMed] [Google Scholar]
- 132.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001;27(1):68–73. [DOI] [PubMed] [Google Scholar]
- 133.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001;27(1):20–21. [DOI] [PubMed] [Google Scholar]
- 134.Linehan JL, Harrison OJ, Han S-J, et al. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 2018;172(4):784–796.e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naik S, Bouladoux N, Linehan JL, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015;520(7545):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harrison Oliver J, Linehan Jonathan L, Shih H-Y, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019;363(6422):eaat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nosbaum A, Prevel N, Truong HA, et al. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol 2016;196(5):2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scharschmidt TC, Vasquez KS, Truong HA, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015;43(5):1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science 2002;296(5568):747–749. [DOI] [PubMed] [Google Scholar]
- 140.Jameson JM, Cauvi G, Witherden DA, Havran WL. A Keratinocyte-Responsive γδ TCR Is Necessary for Dendritic Epidermal T Cell Activation by Damaged Keratinocytes and Maintenance in the Epidermis. The Journal of Immunology 2004;172(6):3573. [DOI] [PubMed] [Google Scholar]
- 141.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol 2005;6(1):73–79. [DOI] [PubMed] [Google Scholar]
- 142.Toulon A, Breton L, Taylor KR, et al. A role for human skin–resident T cells in wound healing. Journal of Experimental Medicine 2009;206(4):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu P, Fu X, Xiao N, et al. Involvements of γδT Lymphocytes in Acute and Chronic Skin Wound Repair. Inflammation 2017;40(4):1416–1427. [DOI] [PubMed] [Google Scholar]
- 144.Cheuk S, Wikén M, Blomqvist L, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 2014;192(7):3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gallais Sérézal I, Classon C, Cheuk S, et al. Resident T Cells in Resolved Psoriasis Steer Tissue Responses that Stratify Clinical Outcome. J Invest Dermatol 2018;138(8):1754–1763. [DOI] [PubMed] [Google Scholar]
- 146.Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest 2017;127(11):4031–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunologic Research 2013;57(1):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. Journal of Experimental Medicine 2009;206(13):2937–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deckers J, Hammad H, Hoste E. Langerhans Cells: Sensing the Environment in Health and Disease. Front Immunol 2018;9(93). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- 151.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184(7):3964–3977. [DOI] [PubMed] [Google Scholar]
- 152.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175(6):2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yanez DA, Lacher RK, Vidyarthi A, Colegio OR. The role of macrophages in skin homeostasis. Pflugers Arch 2017;469(3–4):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. Journal of Experimental Medicine 2010;207(13):2921–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X, Balaji S, Steen EH, et al. T Lymphocytes Attenuate Dermal Scarring by Regulating Inflammation, Neovascularization, and Extracellular Matrix Remodeling. Advances in Wound Care 2019;8(11):527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.D’Alessio FR, Kurzhagen JT, Rabb H. Reparative T lymphocytes in organ injury. The Journal of Clinical Investigation 2019;129(7):2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Q, Smith CW, Zhang W, Burns AR, Li Z. NK Cells Modulate the Inflammatory Response to Corneal Epithelial Abrasion and Thereby Support Wound Healing. The American Journal of Pathology 2012;181(2):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sobecki M, Krzywinska E, Nagarajan S, et al. NK cells in hypoxic skin mediate a trade-off between wound healing and antibacterial defence. Nature Communications 2021;12(1):4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kuil LE, Oosterhof N, Ferrero G, et al. Zebrafish macrophage developmental arrest underlies depletion of microglia and reveals Csf1r-independent metaphocytes. eLife 2020;9:e53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He S, Chen J, Jiang Y, et al. Adult zebrafish Langerhans cells arise from hematopoietic stem/progenitor cells. eLife 2018;7:e36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Otsuka M, Egawa G, Kabashima K. Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front Immunol 2018;9(1768). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol 2000;114(3):560–568. [DOI] [PubMed] [Google Scholar]
- 163.Kehren J, Desvignes C, Krasteva M, et al. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med 1999;189(5):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Novais FO, Carvalho AM, Clark ML, et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLOS Pathogens 2017;13(2):e1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015;15(6):388–400. [DOI] [PubMed] [Google Scholar]
- 166.Nitahara A, Shimura H, Ito A, Tomiyama K, Ito M, Kawai K. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol 2006;126(5):1052–1058. [DOI] [PubMed] [Google Scholar]
- 167.Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci 2011;68(14):2399–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9(5):503–510. [DOI] [PubMed] [Google Scholar]
- 169.Nosbaum A, Prevel N, Truong H-A, et al. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol 2016;196(5):2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mathur AN, Zirak B, Boothby IC, et al. Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 2019;50(3):655–667.e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A 2007;104(49):19446–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med 2005;201(8):1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A 1989;86(3):802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A 1992;89(15):6896–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boothby IC, Cohen JN, Rosenblum MD. Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair. Sci Immunol 2020;5(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol 2010;184(10):5423–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cronan MR, Beerman RW, Rosenberg AF, et al. Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection. Immunity 2016;45(4):861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]


