Abstract
Fate and transport of nitrogen (N) in urban coastal watersheds continues to draw research interest due to serious impacts of N pollution and complexities with N sources and transport pathways. In this study, we used multiple tracers for source identification of N pollution (15N isotope in nitrate and chemical sewage tracers in water) and waters (using isotopes of 18O and 2H in water) in a coastal northwest Florida U.S.A. urban bayou fed by two contrasting streams, namely Jackson Creek traversing a dense residential area and Jones Creek flowing mainly through a wetland preserve. Results showed that the slightly higher δ15N-NO3− values in Jones Creek and the bayou were insufficient to distinguish N sources; yet the different chemical sewage tracer concentrations (e.g., sucralose, carbamazepine and sulfamethoxazole) clearly demonstrated the major N source from leaking septic tanks in the Jackson Creek sub-basin but not in the Jones Creek sub-basin. The higher concentrations of nitrate, which constituted over 98% of dissolved inorganic N in Jackson Creek, support active nitrification in sandy soils and steep terrain while higher δ15N-NO3− and much lower nitrate in Jones Creek are likely associated with denitrification in dense vegetative wetland and riparian zones. Episodic high nitrate concentrations and δ18O values in Jackson Creek preceded by periods of little rainfall indicated that the creek was sustained by subsurface flow with a steady input of nitrate. This study demonstrated the connection of land use and stormwater runoff generation to the forms of N entering urban waterways, the utility of N sourcing approaches, and the value of watershed-scale assessments for developing strategies to limit N loadings in urban settings.
Keywords: Nitrogen pollution, Septic system, Stable isotope, Sewage tracer, Urban streams
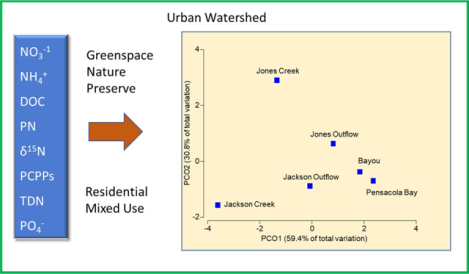
Graphical Abstract
1. Introduction
Nitrogen (N) pollution of water resources poses global environmental concern, causing degradation of freshwater, estuarine, and coastal ecosystems and significant adverse effects on human health (Howarth, 2008; Breitburg et al., 2018). Dissolved inorganic nitrogen (DIN) including ammonium (NH4+), nitrite (NO2−) and nitrate (NO3−) are the most common reactive forms of N available for biological uptake in terrestrial and aquatic ecosystems. Ingestion of nitrate and nitrite in drinking water is potentially harmful to humans, and may lead to methemoglobinemia in newborn infants, brain damage, and cancers (Camargo and Alonso, 2006). Excess reactive N compounds in the ambient environment are associated with many large-scale environmental problems, including contamination of groundwater, eutrophication of surface waters, toxic algae blooms, and hypoxia (Seitzinger et al., 2005; Breitburg et al., 2018). The associated social and economic effects are significant. For example, the combined 2008 value losses in recreational water usage, waterfront real estate, spending on recovery of threatened and endangered species, and drinking water in the U.S. were approximately $2.2 billion as a result of eutrophication in U.S. freshwaters (Dodds et al., 2008). Comparable benefit losses in recreational activities, economic development, and aesthetics are expected to be even higher in coastal urban landscapes (Howarth, 2008; Teurlincx et al., 2019).
Because N is one of the most essential elements for life and food production, anthropogenic sources of N pollution are often multifaceted, involving chemical fertilizer and manure applications in agriculture, urban stormwater with leaking septic and sewer systems, fertilization of lawns, wastewater discharges, and fossil fuel-related air emission and deposition of N compounds. Identification of N pollution sources, along with an understanding N fate and transport in terrestrial and aquatic environments, is critical for development and implementation of N pollution abatement programs. Several chemical tracer approaches are available to help identify sources of nitrogen pollution in a watershed. The nitrogen-15 (15N) technique is perhaps the most widely used one (see review by Heaton, 1986; Macko and Ostrom, 1994). Since the pioneering work by Kohl et al. (1971) using the technique to identify sources of nitrate in drainage waters of the Sangamon River (Illinois, USA), numerous applications for tracing sources and sinks of N in aquatic systems have followed. A few examples are Chang et al. (2002) determining nitrate sources among different land uses in the Mississippi River Basin; Rock and Mayer (2004) assessing sources of surface water nitrate within the Oldman River Basin, Southern Alberta, Canada; Sebilo et al. (2006) assessing nitrification and denitrification in the Seine River and Estuary, France; Fry et al. (2003) identifying anthropogenic N loading in four Pacific estuaries; Bruland and MacKenzie (2010) and Jones et al. (2018) applying the technique with aquatic biota, such as wetland plants and seagrasses for N source identification in coastal systems. The fundamental assumption of this technique is that the natural abundances of the heavier, stable nitrogen isotope 15N, expressed in delta (δ) notation relative to the 15N concentration in the air, may carry an isotopic signature of different sources. In other words, dissolved inorganic nitrogen (especially nitrate) inputs to watersheds have different δ15N values so the source of N can be tracked. In general, δ15N values of nitrate range from −11 to +4‰ for precipitation (Elliott et al., 2007), +4 to +8‰ for soil organic matter mineralized N, −4 to +8‰ for inorganic fertilizers, and +7 to +20‰ for urban sewage and livestock effluents (Kendall et al., 2007). It is obvious that δ15N values have substantial overlaps among different N sources.
Despite this widely accepted isotopic signature of different N sources, interpretation of δ15N data for source identification can be challenging. In addition to the well-recognized overlaps of δ15N values among different sources, the fate and transport of N in the environment can involve a wider range of biological and physical processes that cause isotopic fractionation (Kendall et al., 2007). For example, as different species of N move through watersheds, their δ15N values can become enriched through disproportionate loss of the lighter 14N isotopes (Cifuentes et al., 1989; Casciotti et al., 2003; Kendall et al., 2007). In particular, nitrification and denitrification, both of which are biologically mediated by bacteria, can alter the δ15N signature. The mixing of different sources of water through hydrological processes may further alter the δ15N signature, resulting in significant uncertainties or even errors in source assignment.
These challenges become especially pronounced in a coastal urban setting where land-based freshwater is tidally mixed with saltwater from the ocean (Silva et al., 2002: Pennino et al., 2016). While the complex hydrodynamic mixing creates significant spatial variability of N concentrations, identifying N sources in urban waterways can be further complicated by multiple sources (mentioned above) within a dynamic urban landscapes (Yang and Toor, 2016; Hobbie et al., 2017), as well as complex N transport pathways and biogeochemical turnover along the land-ocean interface (Dahnke et al., 2008). Identification of N sources in coastal urban waterways is often accomplished with multi-isotopes or multiple tracers along with ancillary hydrologic and chemical data (e.g., Sebilo et al., 2006; Wankel et al., 2006; Dahnke et al., 2008). A few tracer techniques other than 15N have been used. For example, whether nitrogen inputs are from human or animal waste may be differentiated by microbial source tracking which is based on co-evolution of bacterial gut flora with the host (Garcia-Aljaro et al., 2018). Chemical tracers of sewage such as artificial sweeteners or pharmaceutical and personal care products (PPCP) are recalcitrant compounds that persist through the human gut and wastewater treatment plants into waterways. They are mainly specific to human inputs (Kolpin et al., 2002; Lim et al., 2017). Similar chemical tracers could be used to detect animal waste from agriculture if any feed additives may likewise persist. In general, chemical tracers of sewage provide more rapid, stable, and source-specific detection than microbial markers. Therefore, selection of appropriate tracer approaches to identify nitrogen inputs in urban watersheds depends on a multitude of factors such as land use attributes, geomorphologic features, and hydrologic dynamics of the watershed of concern. Few studies, however, have concurrently employed 15N, PCPPs, and isotopes of water to evaluate N sources.
In this work, we compared uses of multiple tracers for identification of N pollution sources and N transport pathways in a coastal urban bayou watershed where Total Maximum Daily Loads (TMDLs) have been in place partly due to elevated N loading. Two contrasting urban streams feed the bayou, traversing dynamic landscapes and riparian conditions, namely heavily residential areas vs. wooded wetland preserve. Specifically, the objectives of this study were to (1) compare spatial variabilities of N speciation and concentrations along two coastal urban streams and the bayou the two streams feed; (2) identify N sources with a multi-tracer approach using the 15N isotope technique and chemical sewage tracers; and (3) explore N hydrological transport pathways by identification of source waters with isotopes of 18O and 2H in water. The results demonstrate the connection of land use and runoff generation to the forms of nitrogen entering urban waterways, the utility of nitrogen sourcing approaches, and the value of watershed-scale assessments for developing strategies to limit nitrogen loadings in urban settings.
2. Materials and methods
2.1. Study area and watershed delineation
Bayou Chico is part of the Pensacola Bay system located in northwest Florida and drains a largely urbanized landscape (Fig. 1). The bayou has been on the Clean Water Act 303(d) Listing of Impaired Waters since 1971. Total Maximum Daily Loads (TMDLs) have been approved by the Florida Department of Environmental Protection (FDEP) for fecal coliform bacteria and excess nutrients, especially nitrogen (FDEP, 2011). A Basin Management Action Plan (BMAP) was adopted in 2011 to address the TMDLs. The bayou once supported industrial activities including ship building, but now mainly contains marinas, light industries, parks, and residential shorelines. The regional climate is temperate, temperatures typically vary from 45 °F to 89 °F and are rarely below 31 °F or above 94 °F. The mean annual precipitation exceeds 160 cm, with July through August being the wettest months, having a 40% to 60% chance of a given day being a rainy day. The daily chance of rain from September to June is around 30%, and lowest at 20% during October (Weatherspark, 2021). Soils are primarily sandy or sandy loam with occasional clayey deposits (USDA, 2004).
Fig. 1.
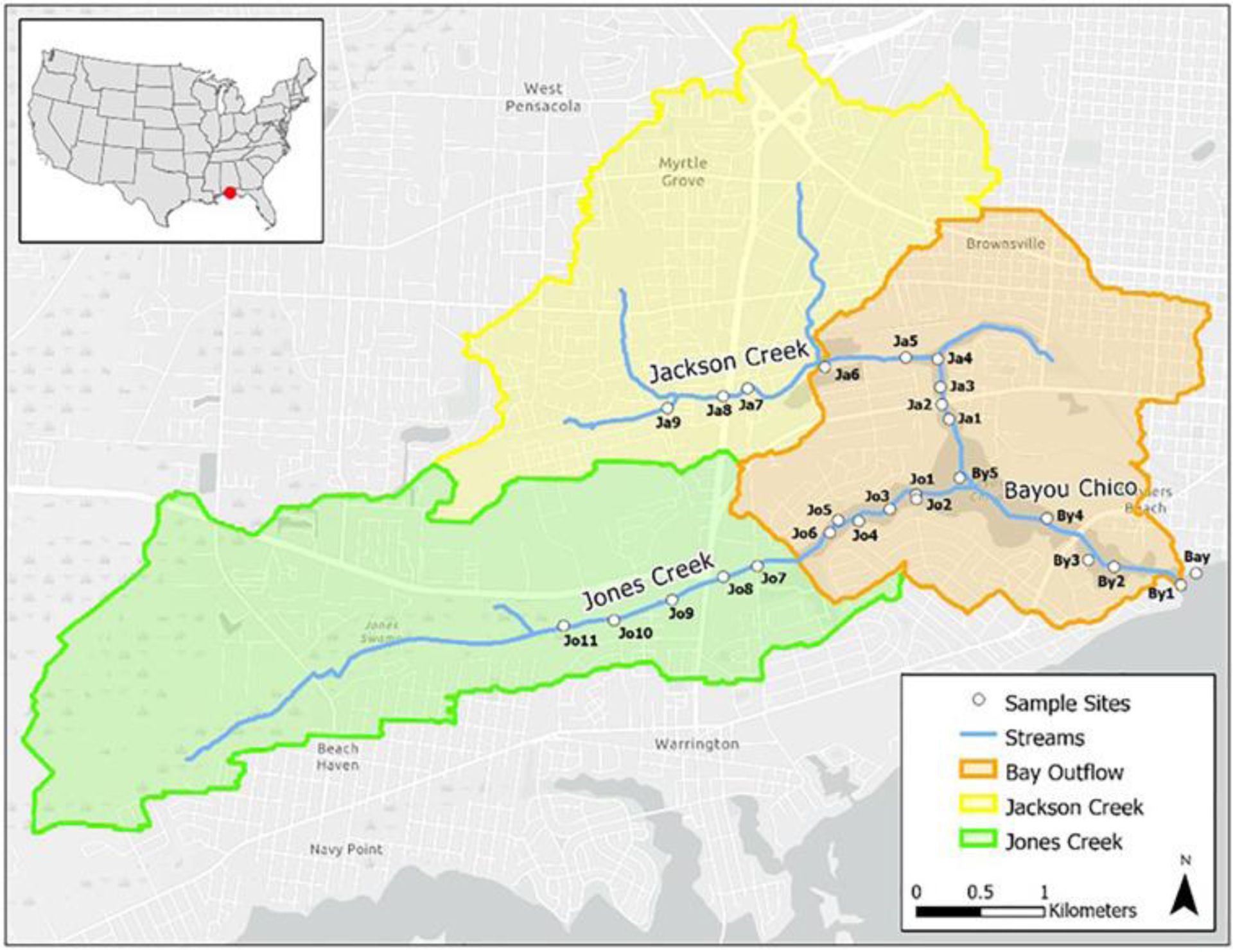
Bayou Chico watershed sub-basins and sampling locations (blue circles) along streams. The U.S. EPA Better Assessment Science Integrating Point and Nonpoint Sources (BASINS) modeling software (v. 4.1) was used for basin delineation. Elevation data (5-m resolution LiDAR data) and a stream network layer from the National Hydrography Dataset (NHD) were used as inputs.
The bayou has a surface area of 95 ha and a drainage basin of 2670 ha (Table 1). The Bayou Chico watershed contains three subbasins: the Jones and Jackson creek sub-basins to the west, and the tidal Bayou Outflow sub-basin to the east (Fig. 1). Jackson Creek drains the northwestern portion of the Bayou Chico watershed. The Jackson Creek sub-basin (826 ha) is heavily developed primarily with residential neighborhoods and some industrial and commercial parcels. Urban lands account for 97% of land uses while wetland and forest occupy 2%. Elevation north of Jackson Creek rises from 6 to 24 m above sea level with steep elevations dropping off into some sections of the stream bed. Slope averages about 5–10% in most of the subbasin.
Table 1.
Sub-basin characteristics of the Bayou Chico watershed. The area of the drainage basins (hectares), % land use based on the 2016 National Land Cover Database, slope (%) and elevation (m MSL) calculated with a digital elevation model based on 5 m resolution LiDAR data. Urban land use is largely residential.
| Area | Urban | Wetland forest | Slope | Elevation | |
|---|---|---|---|---|---|
| Jackson Creek | 97 | 97 | 2 | 5–10 | 18.2 |
| Jones Creek | 64 | 64 | 35 | 1–2 | 7.5 |
| Bayou Outflow | 95 | 95 | 4 | 1–5 | 8.9 |
Jones Creek drains the southwestern section of the Bayou Chico watershed and empties into the bayou well-separated from the Jackson Creek outflow. The Jones Creek sub-basin (1030 ha) is flat (1–2% slope) and ~7.5 m above sea level. Jones Creek originates in a nature preserve that has residential and business developments near its headwaters and along its outskirts. Sections of terrain in the nature preserve have been graded to improve stormwater drainage and reduce runoff. Past the preserve, Jones Creek flows through a residential and business district before emptying into the bayou. Land uses in the sub-basin are predominantly urban (64%) along with about 35% of wetland and forest (EPA, 2013).
The Bayou Outflow sub-basin drains an area of about 814 ha. Much of the area is urbanized, consisting of well-established residential subdivisions and industrial- and commercial-uses. The bayou connects to the Gulf of Mexico through Pensacola Bay.
Septic systems are in use throughout the Bayou Chico watershed with the greatest numbers in the northwest and northeast regions (FDEP, 2011; Fig. S1, Suppl. information). Recent efforts to reduce nutrient inputs into the bayou include an ongoing decade-long septic-to-sewer conversion program that largely eliminated septic system use in several neighborhoods, particularly along the western shores of the bayou.
2.2. Sample collection and preservation
A quarterly sampling schedule was followed from May 2016 through February 2018 with additional samples collected after rainfall events. The time between samplings ranged from ~3.5 to 12 weeks. A total of 26 sites were selected within Bayou Chico and along Jackson and Jones creeks (Fig. 1). Fifteen sites were accessed from dry land and the remaining 11 sites, from the mouth of the bayou into the creek outlets, were accessed via boat. Sampling sites were spaced along the creeks and throughout the bayou taking into consideration environmental, topological, and urban features which could influence nitrogen concentrations (Fig. 1). These stations include, from downstream to upstream, one in Pensacola Bay near the bayou outlet (Bay), five in the bayou proper (By1 through By5), eleven in Jones Creek (Jo1 though Jo11), and nine in Jackson Creek (Ja1 through Ja9). Not all stations were sampled with every sampling trip.
Surface water was collected using Niskin samplers or buckets. Water was poured from the sampler into opaque 2-L HDPE bottles with water temperature, pH, salinity, and conductivity measured with a YSI 63 handheld instrument (Yellow Springs, OH). The HDPE bottles were then stored on ice for transportation back to the laboratory where the water samples were processed within 2–4 h of collection. Pre-combusted (450 °C for 2–4 h) 0.5-μm pore size Whatman GF/F glass-fiber filters were used to prepare particulate and dissolved nutrient samples that were stored at −80 °C until analyzed.
2.3. Water quality and nutrient analyses
The glass fiber filters were analyzed to determine particulate carbon (PC), particulate nitrogen (PN), and particulate phosphorus (PP) concentrations. Filtered water was used to measure concentrations of dissolved organic carbon (DOC), total dissolved nitrogen (TDN), total dissolved phosphorus (TDP), nitrate/nitrite (NOx), ammonium (NH4+), and ortho-phosphate (PO43−). All analyses were done using established procedures (Suppl. information).
2.4. Stable isotope ratio analyses of NO3-N and water
Water samples collected in May and June 2017 were filtered through 0.22 μm pore size filters (Millipore; Sigma) to remove microorganisms and particulates. Filtered samples were stored in pre-cleaned vials and kept frozen until thawed for analysis. A modified version of the USGS bacterial denitrification method (Coplen et al., 2012, Suppl. information) was followed to convert nitrate (NO3−) in the samples to nitrous oxide (N2O) using Pseudomonas chlororaphis, subsp. aureofaciens (ATCC 13985). δ15N/14N ratio values were determined from the N2O gas produced by the denitrifying bacteria.
The samples were sent to the EPA National Risk Management Laboratory (NRML) in Cincinnati where they were analyzed on a Finnegan isotope ratio mass spectrometer. The nitrogen isotope values are reported as per mil δ values:‰δ‰=Rsample/Rstandard−1×1000where R = ratio of heavy to light isotopes of nitrogen (15N/14N) in the sample and in atmospheric air as the standard.
Water samples for stable isotope analysis (18O and 2H) were collected in 25 mL syringes rinsed with site water and filtered through 0.22 μm pore filters and analyzed at EPA NRML. Isotope values are reported in the δ notation relative to the internal V-SMOW standard.
2.5. Chemical tracers
Whole water samples were collected with a bucket or Niskin sampler and poured into new HDPE bottles and stored on ice. The samples were shipped overnight with cold packs to SGS AXYS Analytical Services Ltd. (B.C., Canada) where they were analyzed for sucralose and Group 1 acid-extracted pharmaceuticals and personal care products (PCPP) using high performance liquid chromatography with tandem mass spectrometry according to EPA Method 1694 (EPA, 2007).
2.6. Data analyses
Sampling locations were grouped into segments of Pensacola Bay (Bay), Bayou (Ba1 though Ba5), Jackson Outflow (Ja1 through Ja5), Jackson Creek (Ja6 through Ja9), Jones Outflow (Jo1 through Jo6), and Jones Creek (Jo7 through Jo11). This grouping was based on the geographic locations of sampling stations in the system and the source area of waters they represent (Fig. 1). A subset of samples and variables having no missing data was used to examine associations of segment water quality and chemical parameters. The subset contained 164 samples having three environmental parameters (temperature, salinity, and pH) and eight chemical measurements (NO2−, NO3−, DOC, TDN, PO43−, PC, PN, and SiO44−). Unfortunately, exclusion of missing data eliminated winter samples from this analysis. Because most analytes were not normally distributed, statistical differences of variables were evaluated with a Kruskal–Wallis rank sum test followed by a Dunn test for comparison among segments as implemented in R (R Core Team, 2014). For isotope data whereby sample numbers were not large enough for a feasible normality test, analysis of variance (ANOVA) followed by Least Significance Test (LSD) was conducted in Minitab (Version 19). Primer-e 7 with PERMANOVA+ software was used to visualize sample similarities based on normalized data by Principal Components Analysis (PCA) and Principal Coordinate Analysis (PCoA) in Euclidean space of centroids between segment data. The data set was also analyzed by PERMANOVA+ (Primer-e 7) to determine associations between segments and sampling season. PERMANOVA is a non-parametric permutation method and may be used with data sets that are not normally distributed (Anderson et al., 2008). Data normalized as a percent of total variable value was used for PERMANOVA with 9999 permutations.
The significance level of α = 0.05 was used in all statistical analyses.
2.7. Availability of data
Data obtained for this study are available through EPA Science Hub https://sciencehub.epa.gov/sciencehub/datasets/2842.
3. Results
3.1. Salinity, temperature, and pH
The flow of freshwater from the two creeks into the brackish bayou waters and the extent of tidal mixing can be demonstrated by transitions in salinities (Fig. 2). Although the two creeks have freshwaters, Jackson Creek sites always registered salinities of 0.1 psu with an average conductivity of 160 μS, whereas the most upstream Jones Creek sites had 0 psu salinities and average conductivity of 79 μS. Each creek flows into a separate outlet where average salinities ranged between 3 and 8 psu, indicative of mixing with the bay water. The outlets join the main body of the bayou where salinities increased from about 10 psu at the confluence to 15 psu toward the mouth of the bayou into Pensacola Bay. Average water temperatures ranged from 23 to 28 °C, with Jones Creek being cooler than Jackson Creek, and water pH averaged 6.1 to 7.8, with Jones Creek being the lowest (Fig. S2 in Supplemental information).
Fig. 2.
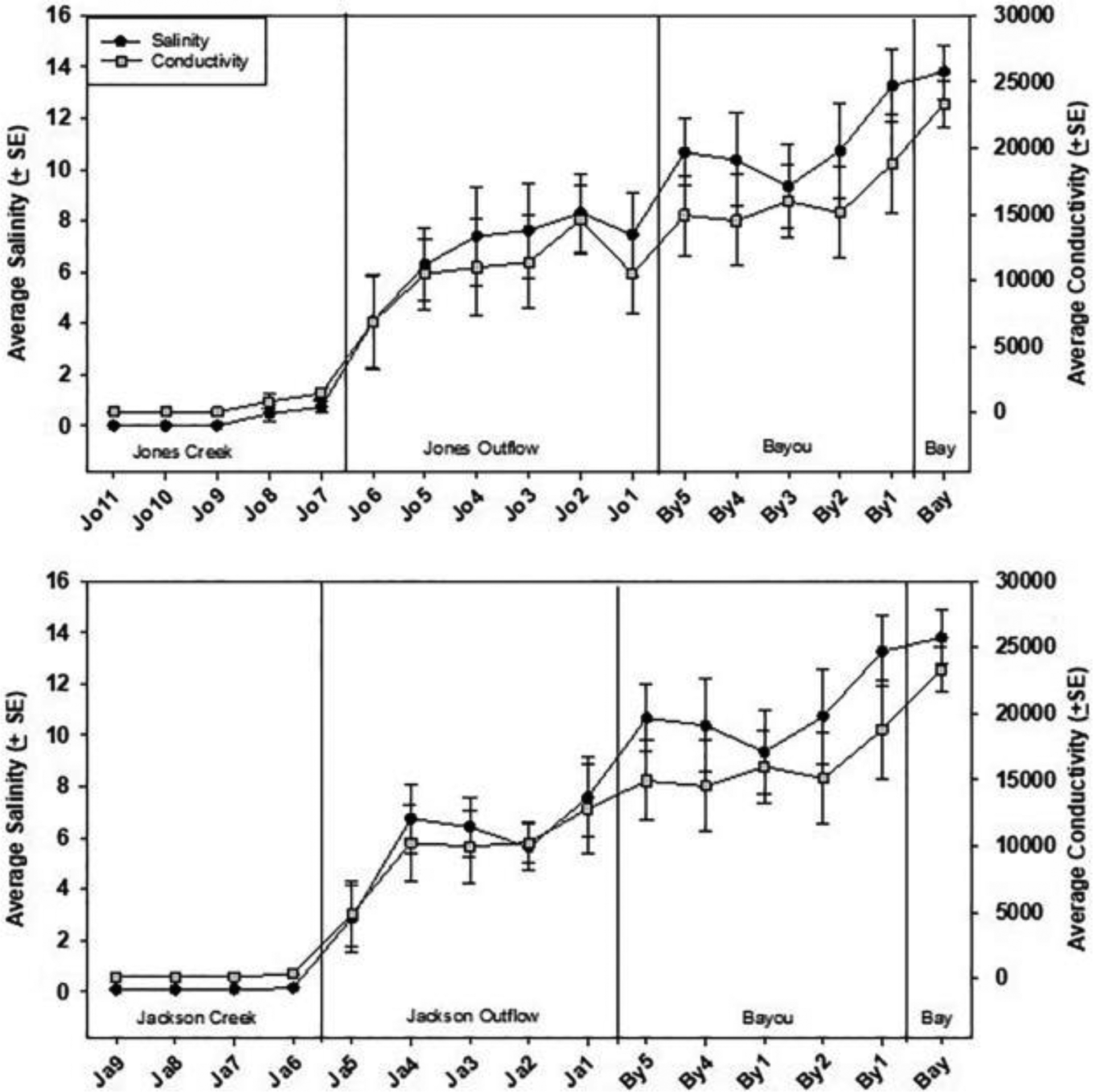
Salinity and conductivity of Jackson and Jones creeks and Bayou Chico. Sampling sites are arrayed independent of distance measurements on the horizontal axis from the most upstream locations on the left to the mouth of the bayou and an adjacent Pensacola Bay site on the right. Note the same data for the bayou sites are used in both graphs for demonstration. Error bars are standard error of all data collected at a specific site (N = 8 to 25).
3.2. Comparisons of segments and seasons
A PCA plot to evaluate effects of variables by creek segment and season is provided in Fig. 3A. PC1 explained 35% of the variation, PC2 25% of variation with an additional 13% explained by PC3. For PC1 pH, temperature, salinity, PC and PN had positive loading scores whereas TDN, PO43−, and NO3− had negative scores (Suppl. information). PC2 is characterized by a positive score for DOC and negative scores for NO2− and SiO44−. Season did not influence variability in the PCA as the same segments from different seasons plotted closely together (not shown). Samples from the two creeks plotted on the negative side of PC1. Jackson Creek samples plotted toward the lower quadrant suggesting a strong effect from TDN, PO43−, and NO3−, whereas Jones Creek in the upper right quadrant was associated with DOC (Fig. 3B). The bayou and bay sites were associated with high salinity, pH, temperature and PCN on the positive side of PC1. The outflow segments plotted roughly between the bayou/bay sites and their respective creeks. A clear separation of the creek and bayou segments was demonstrated by PCoA of Euclidean distances between the centroids of the PCA data clouds and explains 90% of the variability in the distances (Fig. 3C).
Fig. 3.
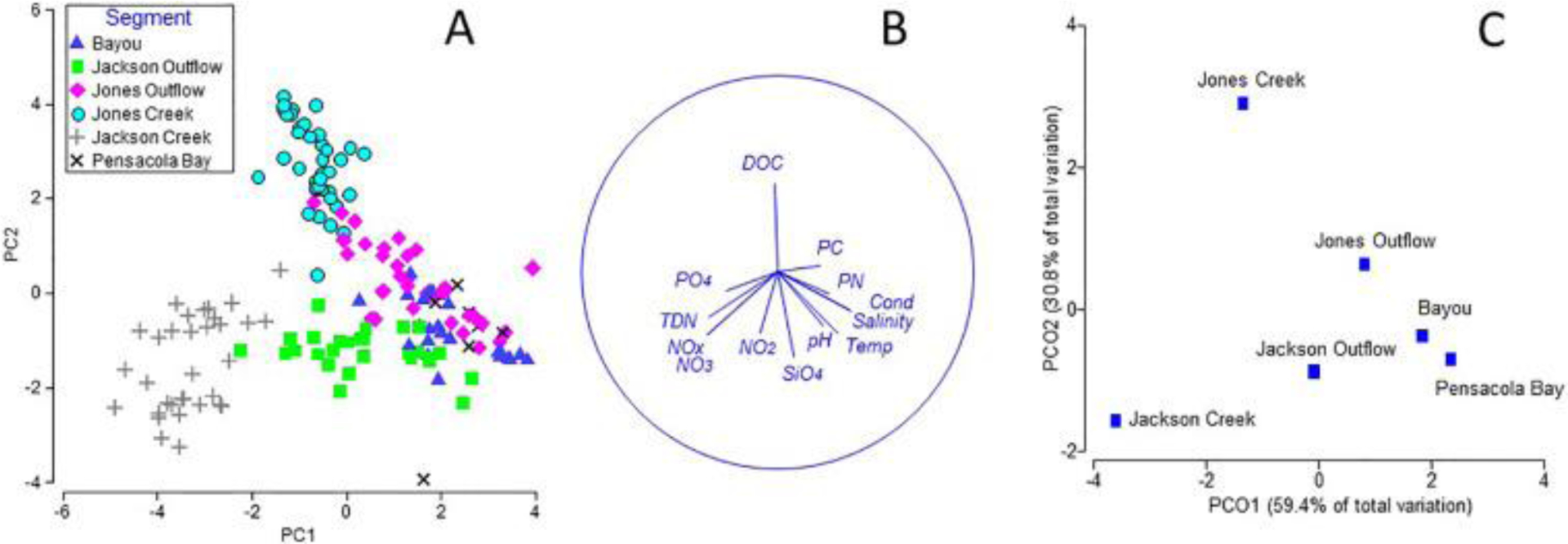
Interactions among water quality variables and clustering of segments and seasons. Data are grouped by segments of Jackson Creek, Jackson Outflow, Jones Creek, Jones Outflow, Bayou, and Pensacola Bay. A; Principal Components Analysis. B; Vector plot of parameters chosen to display a range of observed responses to the gradient, with the vector direction for each species reflecting the (Pearson) correlations of their (square root-transformed) counts with the two ordination axes and length giving the multiple correlation coefficient on the ordination points (the circle is a correlation of 1). C; Principal Coordinate Analysis of centroids of seqments in the PCA.
The differences among segments identified by the above PCA and PCoA are confirmed by PERMANOVA results. Additionally, significant difference between seasons was shown with PERMANOVA, P(perm) = 0.019, though data are lacking for the winter timeframe. Pairwise tests of segments indicated significant differences [P(perm) < 0.05] between all segments with the exceptions of the bayou vs. the bay site and the Jones Outflow vs. bayou sites (Table 2).
Table 2.
P values of PERMANOVA results for pairwise tests of segments. Values in bold italics indicate significant differences between pairs at P(perm) < 0.05.
| Groups | Bayou | Jones Outflow | Jones Creek | Jackson Outflow | Jackson Creek |
|---|---|---|---|---|---|
| Pensacola Bay | 0.0641 | 0.0212 | 0.0001 | 0.0638 | 0.0001 |
| Bayou | 0.0402 | 0.0001 | 0.0010 | 0.0001 | |
| Jones Outflow | 0.0002 | 0.0009 | 0.0002 | ||
| Jones Creek | 0.0002 | 0.0002 | |||
| Jackson Outflow | 0.0001 |
3.3. Nutrient distribution in creeks and bayou
Nutrient speciation and concentration data are grouped according to river segments of Jackson Creek, Jackson Outflow, Jones Creek, Jones Outflow, and Bayou Chico (Table 3). Jackson Creek had significantly higher median concentrations of NO2−, NO3−, TDN and PO43− than Bayou Chico or Jones Creek. In fact, at Jackson Creek, NO2−, NO3−, and TDN concentrations were five times higher, and PO43− concentrations were two times higher than the respective concentrations in the bayou or Jones Creek. NH4+ concentrations were a few μM at all sampling sites. NO3− accounted for >50% of TDN in Jackson Creek and its outflow branch, in comparison to between 2 and 20% in Jones Creek and the remainder of the bayou. Therefore, about half the dissolved nitrogen in Jackson Creek is inorganic nitrogen, whereas smaller fractions of dissolved nitrogen in Jones Creek and Bayou Chico are inorganic (~20%).
Table 3.
Nutrient concentrations in the Bayou Chico watershed. Concentrations are reported as μmol g−1 for PP, PN, and PC, and μmol L−1 for NOx, NO2−, NO3−, NH4+, SiO4+, TDN, DO, and PO4−. The same letter in a nutrient column indicates no significant difference between the median concentrations.
| NOx | NO2− | NO3− | NH4+ | SiO4+ | TDN | DOC | PO4 | TDP | PP | PN | PC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayou Chico | Average | 9.65 | 0.11 | 9.54 | 0.70 | 44.62 | 34.94 | 481.00 | 0.14 | 0.35 | 0.69 | 12.47 | 86.70 |
| ±SE | 1.53 | 0.04 | 1.53 | 0.12 | 1.65 | 4.19 | 46.70 | 0.01 | 0.03 | 0.07 | 0.90 | 5.68 | |
| Median | 8.14A | 0.05A | 7.82A | 0.47A | 46.56A,C | 35.6A | 472.90A,C | 0.13A | 0.38A,B | 0.7A | 12.23A,C | 80.65A,B | |
| Min | 0.01 | 0.00 | 0.01 | 0.10 | 11.88 | 6.40 | 165.20 | 0.09 | 0.07 | 0.18 | 5.64 | 46.42 | |
| Max | 28.85 | 0.98 | 28.67 | 2.86 | 52.52 | 79.35 | 969.40 | 0.19 | 0.64 | 1.20 | 23.52 | 157.76 | |
| Jones Outflow | Average | 12.65 | 0.05 | 12.60 | 0.89 | 36.36 | 44.77 | 730.90 | 0.20 | 0.36 | 0.77 | 15.47 | 121.50 |
| ±SE | 1.05 | 0.01 | 1.04 | 0.11 | 2.42 | 3.07 | 55.80 | 0.01 | 0.02 | 0.05 | 1.67 | 12.00 | |
| Median | 12.22A | 0.04A,C | 12.22A | 0.72A | 38.57C | 46.75A,D | 706.90C | 0.17C | 0.38A,B | 0.73A | 14.16A | 113.3B | |
| Min | 1.44 | 0.00 | 1.42 | 0.07 | 1.34 | 10.38 | 195.70 | 0.11 | 0.13 | 0.29 | 0.47 | 4.70 | |
| Max | 34.46 | 0.29 | 34.17 | 3.00 | 60.42 | 72.97 | 1386.30 | 0.37 | 0.59 | 1.35 | 68.33 | 516.70 | |
| Jones Creek | Average | 11.03 | 0.02 | 11.01 | 1.75 | 20.66 | 56.82 | 1510.20 | 0.19 | 0.31 | 0.42 | 6.80 | 84.96 |
| ±SE | 2.39 | 0.01 | 2.39 | 0.11 | 2.49 | 3.25 | 95.20 | 0.01 | 0.03 | 0.06 | 0.55 | 5.32 | |
| Median | 5.73A | 0.00C | 5.73A | 1.65B | 12.65B | 61.97D | 1620.70B | 0.15A,C | 0.26A | 0.3B | 5.84B,C | 76.94A,B | |
| Min | 0.73 | 0.00 | 0.73 | 0.21 | 1.87 | 10.36 | 358.30 | 0.07 | 0.12 | 0.06 | 3.34 | 37.69 | |
| Max | 73.12 | 0.25 | 73.07 | 4.10 | 71.46 | 89.01 | 2481.80 | 0.67 | 0.97 | 1.52 | 20.19 | 259.58 | |
| Jackson Outflow | Average | 61.05 | 0.44 | 60.60 | 2.10 | 49.13 | 106.90 | 384.70 | 0.15 | 0.33 | 0.77 | 15.58 | 115.10 |
| ±SE | 4.14 | 0.03 | 4.12 | 0.19 | 1.76 | 12.90 | 44.40 | 0.01 | 0.06 | 0.09 | 1.66 | 14.40 | |
| Median | 59.62B | 0.47B | 59.27B | 2.09B | 51.06A | 101.70C,D | 342.90A | 0.14A,C | 0.23A | 0.68A | 14.24A | 92.2A,B | |
| Min | 21.55 | 0.12 | 21.42 | 0.18 | 20.07 | 17.10 | 61.00 | 0.10 | 0.07 | 0.21 | 0.00 | 0.00 | |
| Max | 116.06 | 0.65 | 115.52 | 4.25 | 67.3 | 294.80 | 948.30 | 0.22 | 1.97 | 1.97 | 39.73 | 349.20 | |
| Jackson Creek | Average | 152.42 | 0.31 | 152.14 | 1.87 | 45.74 | 270.20 | 401.40 | 0.35 | 0.38 | 0.37 | 6.13 | 57.28 |
| ±SE | 5.72 | 0.03 | 5.72 | 0.18 | 3.78 | 18.80 | 45.20 | 0.03 | 0.04 | 0.05 | 0.92 | 6.42 | |
| Median | 161.98B | 0.32B | 161.82B | 1.51B | 45.51A,C | 281.70B | 330.80A | 0.34B | 0.35A,B | 0.36B | 4.04B | 46.92A | |
| Min | 69.65 | 0.00 | 62.20 | 0.53 | 1.30 | 32.70 | 56.00 | 0.08 | 0.04 | 0.03 | 0.00 | 0.00 | |
| Max | 214.24 | 0.67 | 213.92 | 4.52 | 104.34 | 403.50 | 1.00 | 1.51 | 1.32 | 19.87 | 130.48 | ||
In contrast to the high levels of dissolved nitrogen in Jackson Creek, PN concentrations were similar in the two creeks (6.13 μM in Jackson Creek and 6.80 μM in Jones Creek) and half the concentrations measured in the bayou and outflow segments (Table 3). The average PC to PN ratio for Jones Creek was 12, and 6–9 for Jackson Creek, the creek outlets and the bayou, suggesting that particulate matter in Jackson Creek and the bayou was highly degraded. Average DOC concentrations were very high in Jones creek (~1500 μM), roughly 3.5 times higher than in Jackson Creek (385 μM). Dissolved phosphorus concentrations were low at all sampling locations and the concentrations did not vary greatly over the study sites (median = 0.4 μM). Particulate phosphorus concentrations varied similarly with PC and PN following the sequence of creek outflows > bayou > creeks.
3.4. Stable isotopes
Values of δ15N-NO3− in the Bayou Chico watershed are provided in Table 4 and their variations with nitrate concentration and salinity are shown in Fig. 4. In May, average δ15N-NO3− were 7.0‰ (S.E. = 0.33‰) and 8.5‰ (S.E. = 0.21‰) for Jackson and Jones creek sites, respectively, and were significantly different (p = 0.004). Values of δ15N-NO3− in the creek outflows were generally higher than in the creeks, with the Jackson Creek outflow having greater δ15N values than the Bayou. No correlations were identified between δ15N and nitrate concentrations or salinity. However, the relatively lower δ15N values, higher nitrate concentrations and lower salinity in Jackson Creek contrasted well against the higher δ15N values, lower nitrate concentrations, and higher salinity in Jackson Creek outflow (Fig. 4).
Table 4.
Mean δ15N-NO3− ‰ (S.E) for creeks and bayou sections. Samples were collected during a dry period May and after a rain event in June of the same year (2016). n = 4–5 except for Jones Outflow in June which is from a single sample. N.D., not done.
| Site | May | June |
|---|---|---|
| Bayou | 9.5 (0.18) | N.D. |
| Jackson Outflow | 10.3 (0.82) | N.D. |
| Jackson Creek | 7.0 (0.33) | 6.2 (0.26) |
| Jones Outflow | 9.4 (0.32) | 8.7 |
| Jones Creek | 8.5 (0.21) | 6.1 (0.56) |
Fig. 4.
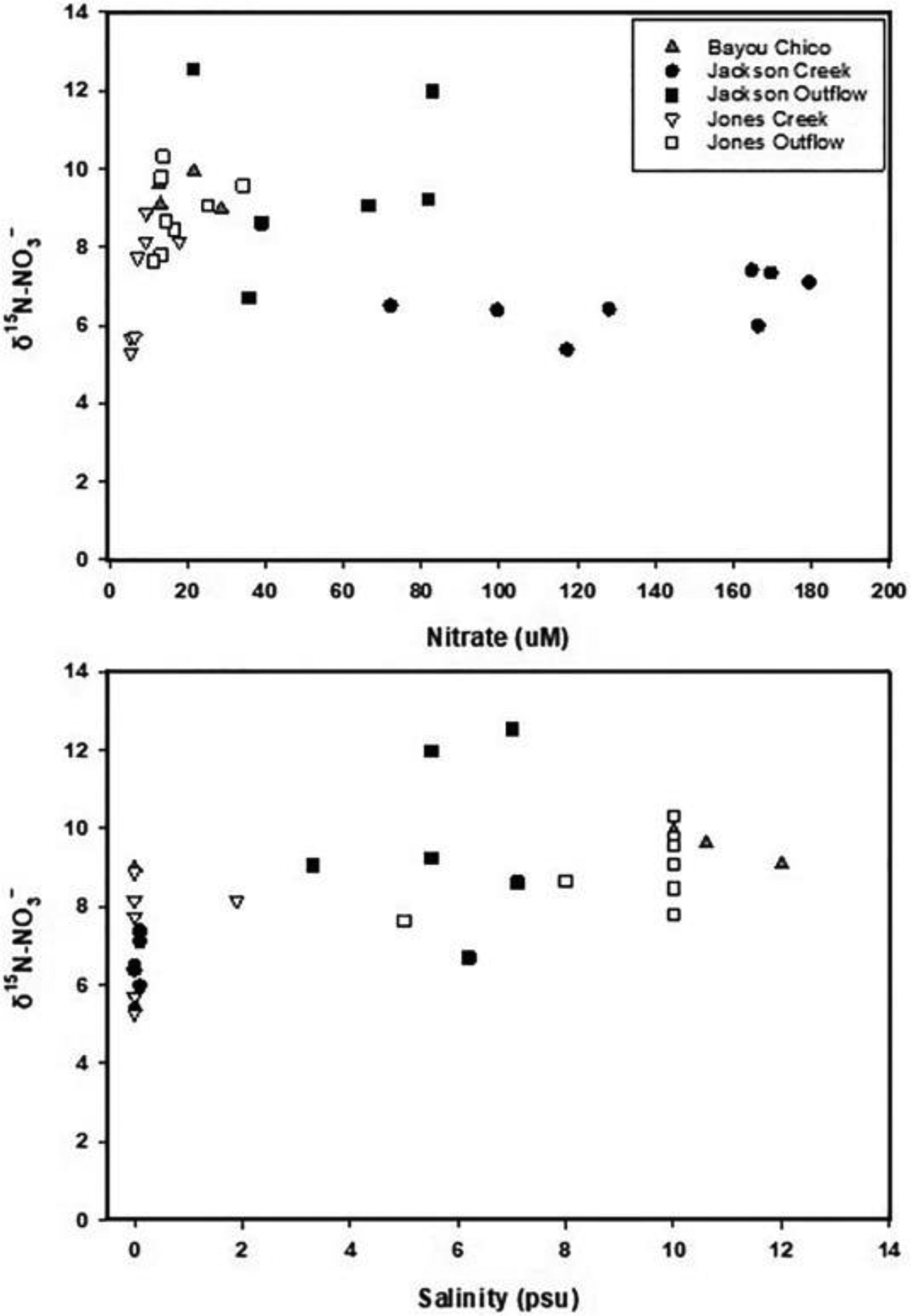
Changes of δ15N of NO3− with (a) nitrate concentrations and (b) salinity along the creeks and bayou.
δ2H and δ18O composition for water samples collected in this study ranged from −4.1 to −34.4‰ and from −1.7 to −5.4‰, respectively (Fig. 5). The creek waters are significantly depleted in 2H and 18O relative to seawater (where δ2H and δ18O are both zero). δ2H and δ18O values are smaller in Jackson Creek than in Jones Creek though not statistically different. In each creek-bayou continuum, the waters tend to be isotopically lighter at the headwater, and δ2H and δ18O values increase linearly with increasing salinity when moving from upstream stations to downstream in the bayou (Fig. 5a). Larger isotopic variations are observed in creek waters than in the bayou. In general, δ2H and δ18O values array along the global meteoric water line (MWL) with a slope of ~8 in H and O isotope space (Fig. 5b).
Fig. 5.
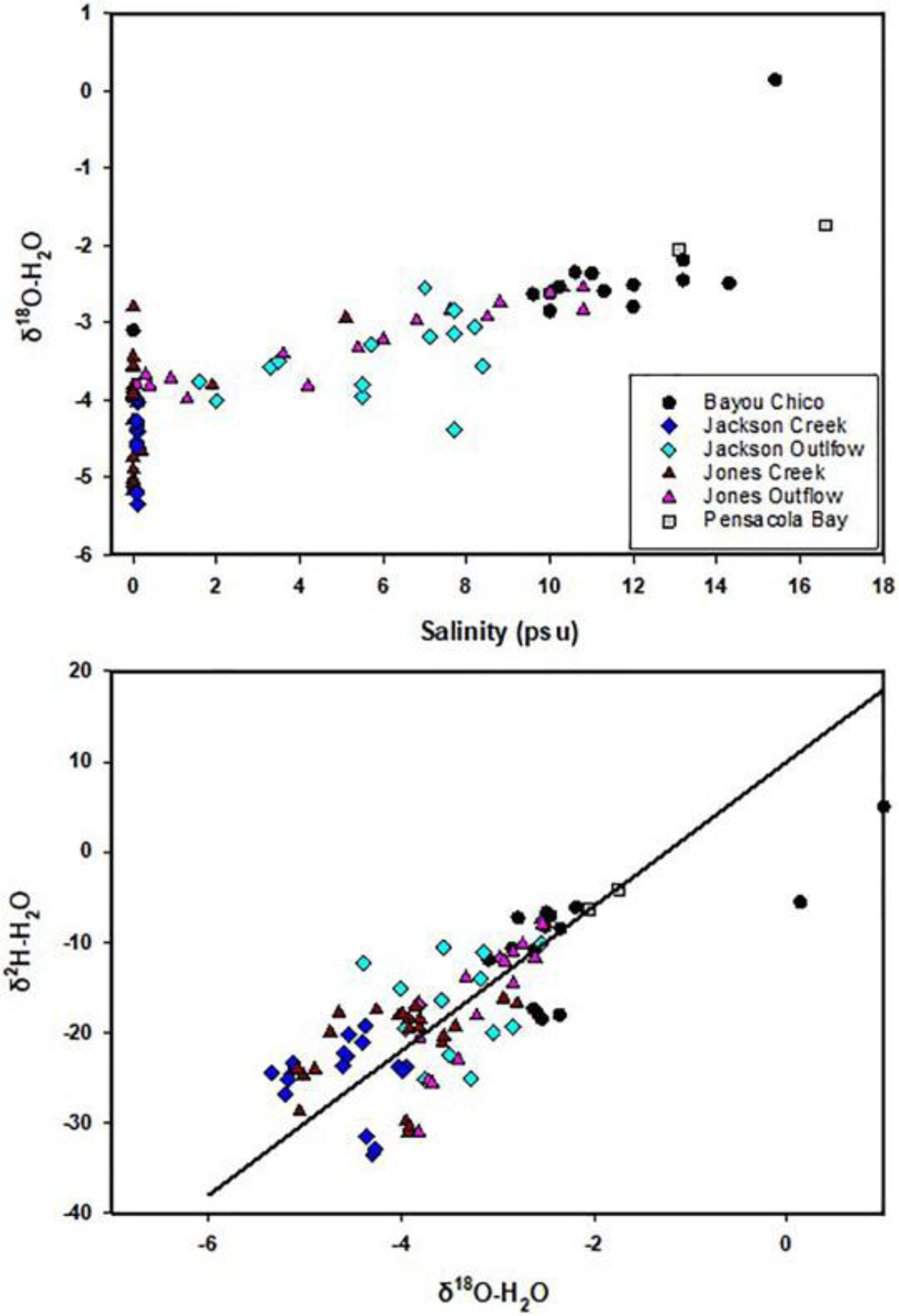
Change of δ18O and δ2H of H2O with salinity along the creeks and bayou. (b) Relation between δ2H and δ18O with the Global Meteoric Water Line: δ2H = 8 δ18O + 10.
3.5. Personal care and pharmaceutical products tracers of sewage inputs
Concentrations for major chemical tracers of sewage are provided in Fig. 6 and the rest in Table S1. Concentrations of sucralose in Jackson Creek ranged from 1350 ng/L to 2070 ng/L and were highest about mid-way in the creek headwater (Ja7). In contrast, sucralose concentrations in Jones creek were between 306 and 324 ng/L. Sucralose concentrations in the bayou were higher in the outlet from Jackson Creek than in the one from Jones creek and decreased with distance toward the opening into Pensacola Bay (Table 5). Caffeine, carbamazepine, erythromycin-H2O, and sulfamethoxazole were the only acid-extracted Group 1 PCPP detected. Caffeine was found throughout the waterways with concentrations ranging from about 10–30 ng/L in Jones and Jackson Creeks and about 35–40 ng/L in the bayou (Table S1). Carbamazepine and sulfamethoxazole were hardly detected in Jones Creek and at the bayou outlet. Within the bayou and Jackson Creek, carbamazepine and sulfamethoxazole concentrations ranged about 2–45 ng/L with a pattern similar for sucralose and nitrate (Fig. 6).
Fig. 6.
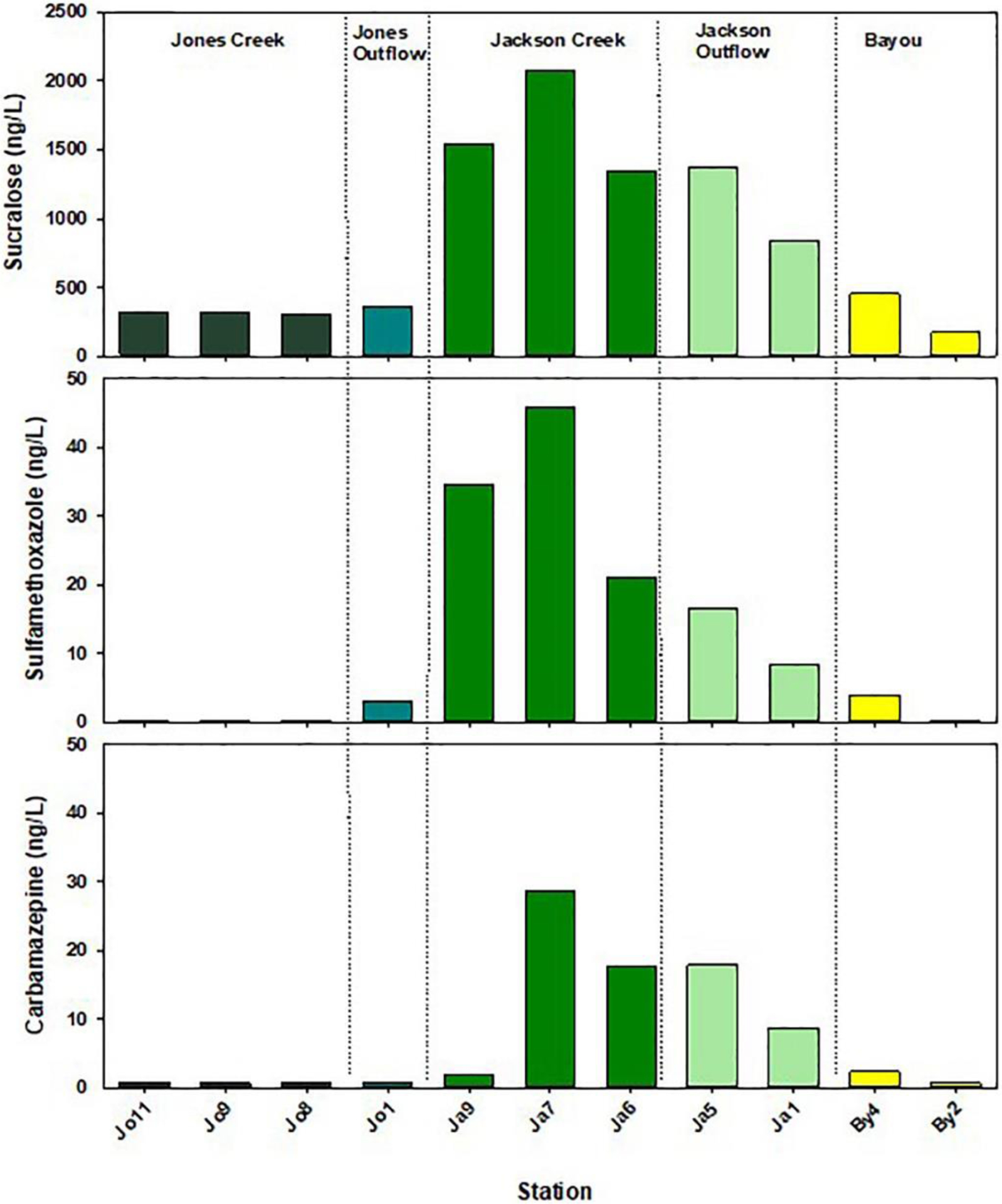
Concentrations of chemical tracers along the creeks and bayou. For Carbamazepine, when concentrations were reported lower than 1.5–1.7 ng/L, one half of the detection limit was used.
Table 5.
Personal care and pharmaceutical products in Bayou Chico and its watershed (ng/L).
| By2 | By4 | Ja1 | Ja5 | Ja6 | Ja7 | Ja9 | Jo1 | Jo8 | Jo9 | Jo11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucralose | 174 | 454 | 840 | 1370 | 1350 | 2070 | 1540 | 367 | 306 | 320 | 324 |
| Caffeine | 40.9 | 37.1 | 42.9 | 24.5 | 32.9 | 18.3 | <15.5 | 31.3 | 34.1 | 16.5 | 17.8 |
| Carbamazepine | <1.56 | 2.37 | 8.72 | 17.9 | 17.8 | 28.5 | 1.81 | <1.64 | <1.53 | <1.56 | <1.52 |
| Dehydronifedipine | <0.834 | <0.637 | <0.645 | <0.681 | <0.752 | 0.853 | 0.827 | <0.772 | <1.06 | <1.13 | <1.02 |
| Sulfamethoxazole | <0.622 | 3.95 | 8.43 | 16.7 | 21.2 | 45.8 | 34.7 | 3.03 | <0.612 | <0.625 | <0.607 |
<, not detected at reporting limit.
4. Discussion
The magnitude and speciation of nitrogen and nitrate isotopic signatures exhibited are indicative of the complex water quality drivers of urban watersheds (Peterson et al., 2001). The isotopic signature of nitrate among the creeks and bayou does not seem to signify a predominant nitrogen source in the system, and this may be linked to a multitude of factors such as varying land-based inputs, nitrogen biogeochemical transformation, as well as rainfall driven transport pathways and tidal mixing. Overlapping signatures of 15N-nitrate from degrading organic matter and sewage can confound identifying nitrogen sources (Kendall et al., 2007). The distinction between fertilizer and sewage or organic matter is clearer. However, sewage tracer concentrations clearly demonstrated the major nitrogen source is likely from septic tank leakage into Jackson Creek and then the bayou. Furthermore, distinct N speciation and their concentrations between creeks reflect the effects of different basin characteristics, supporting previous work linking spatial variations in water quality with contributing sources associated with domestic or industrial origins (e.g., Simeonov et al., 2003; Zhou et al., 2007; Li et al., 2011). These points are further discussed below.
4.1. Nitrogen source identification with sewage markers
The sewage chemical marker data offered more specificity with respect to sewage contribution than the 15N technique. Note that carbamazepine, an antiepileptic drug, and sucralose, an artificial sweetener, are both widely used as municipal wastewater tracers in aquatic ecosystems for their high resistance to degradation toward wastewater treatment and low adsorption by soils (Clara et al., 2004; Gasser et al., 2010; Oppenheirmer et al., 2011). Sulfamethoxazole, an antibiotic drug, was also detected in this study, though not widely recommended as a sewage tracer. The spatial variations in these tracer concentrations (Fig. 6) suggest that septic systems are the major source of nitrate in Jackson Creek and the bayou, but only a minor source in Jones Creek. Much lower sewage chemical marker concentrations in Jones Creek are consistent with recent large-scale conversion of aged/leaking septic systems in Jones Creek basins into sewers as part of the state-legislated Basin Management Action Plan. Because these chemical tracers are conservative, decreasing tracer concentration with increasing salinity along the Jackson Creek-Bayou continuum is mostly due to dilution by saltwater from the bay.
4.2. Nitrogen isotope and biogeochemical processes
While several studies have shown that the δ15N of nitrate is correlated with the percentage of wastewater inputs from urban areas (Mayer et al., 2002; Elliott and Brush, 2006), the overall spatial pattern of δ15N-NO3− values and nitrate concentration in this study does not provide a clear indication of the predominance of sewage over the other sources. For example, the sub-basin land uses, N speciation and concentrations, and sewage chemical tracer data indicate that N is primarily sourced from septic systems in Jackson Creek and organic matter decomposition in Jones Creek. However, the slightly higher δ15N-NO3− values in Jones Creek than in Jackson Creek are insufficient to distinguish nitrogen derived from decomposing leaf litter prevalent in Jones Creek from anthropogenic inputs contributing to the high nitrate levels in Jackson Creek. Furthermore, decreasing N concentrations along Jackson Creek into the bayou are consistent with the increasing tidal mixing of land-based N-laden freshwater with ocean-based N-depleted saltwater (Dahnke et al., 2008). The influences of the creeks, particularly Jackson Creek, extend to the upper reaches of the bayou in the outflow segments, and ultimately to the water quality of the bayou. However, spatial variation in δ15N-NO3− along Jones and Jackson Creeks and in the bayou does not conform to that of nitrate concentrations and salinity (Fig. 4).
Several points are worth noting regarding changes in δ15N-NO3− and related biogeochemical processes. First, the fact that nitrate constitutes the majority of DIN and very low NH4+ concentration across the entire system indicates that nitrification is the predominant process for N transport from land to the aquatic systems. The extent of 15N fractionation during nitrification is dependent on the fraction of the substrate pool (reservoir) that is consumed (Kendall et al., 2007). In Jackson Creek, the sandy nature of soils allows for rapid conversion of NH4+, including any from septic systems, to NO3−. In Jones Creek, nitrification of ammonia derived from the degradation of organic matter and its subsequent oxidation by nitrifying bacteria generated similar δ15N-NO3−, perhaps within only a few per mil (Kendall et al., 2007). Coffin and Cifuentes (1999) reported that δ15N of suspended particulate organic matter measured in river and creek water entering nearby Perdido Bay, 11 km west from Bayou Chico were 6.5 ± 3.7‰ (standard deviation), similar to δ15N-NO3− values of Jones Creek measured in June 2016. Also note that particulate N concentrations in the outflow segments are higher than those of the upstream creeks and the downstream bayou (Table 3), indicative of formation of an estuarine turbidity maximum. The relative enrichment of organic matter in the turbidity maximum zone can serve as a source of nitrate through nitrification (Dahnke et al., 2008). This can be one of the reasons for higher δ15N-NO3− values despite lower nitrate concentrations than in the creeks (Fig. 4). In the downstream bayou where a thick deposit of land-based, contaminated sediment has been of concern for many years (FDEP, 2011), nitrate derived from mineralization of benthic organic sediment may likely carry similar δ15N signatures with the creek waters.
Second, denitrification, which causes the δ15N of the residual nitrate to increase exponentially as nitrate concentrations decrease (Heaton, 1986), can be an active process modulating the δ15N and concentrations in the system. The sandy soils and steep slopes in the Jackson Creek basin do not support active denitrification during transport from land to the creek. However, higher δ15N-NO3− and much lower nitrate in Jones Creek are likely associated with denitrifications in dense vegetative wetland and riparian zones. The decrease in nitrate concentrations with an increase in δ15N-NO3− values along Jackson Creek into the bayou also suggests denitrification and biological assimilation as lighter isotopes are used preferentially in biogeochemical reactions (Heaton, 1986). Most of Jackson Creek has vegetated banks and natural beds that promote nitrogen removal processes, such as denitrification, and help prevent nitrogen inputs into the bayou from being any greater. Such “riparian” denitrification was also noted by Sebilo et al., 2003, Sebilo et al., 2006 in the Seine River Basin (France) where riparian denitrification removed up to 50% of the N exported from agricultural soils during the summer low flow season. Furthermore, denitrification can also occur in the estuarine, organic rich sediments of the bayou which are inducive for hypoxia development in the summer.
Third, nitrate in rainfall in this region typically has very low δ15N-NO3− values (Elliott et al., 2007). Thus, δ15N-NO3− in the creeks tends to be diluted by rainfall. The lower δ15N-NO3− values of Jackson Creek in June than in May reflect rainfall inputs depleted in δ15N (Dillon and Chanton, 2005). In the bayou where mixing with sea water is prevalent, slightly higher δ15N-NO3− values than in the creeks are likely due to denitrification or suggest that nitrate from the sea water carries a similar isotopic signature with the creek waters (An and Gardner, 2002). In contrast, Dahnke et al. (2008) noted a linear decrease of δ15N-NO3− values with increasing salinity in the Elbe estuary, Germany. These authors argued that this conservative mixing of freshwater with sea water is indicative of lack of denitrification due to large scale dredging of estuarine sediments.
4.3. Sources of water and N transport pathways
The distinct basin characteristics between Jones and Jackson creeks such as land use and land cover, elevation, slope, and impervious areas (Table 1) exhibit different sources of water and thus N transport pathways. The steep elevation to the north of Jackson Creek and dense residential development is evident in contrast to the comparatively flat terrain and wetland preserve of Jones Creek. Sources of waters in the creeks and bayou may consist of three main components: (1) “new water” driven by recent precipitation that has reached the creeks either by surface runoff or by rapid flow through the shallow subsurface; (2) “old water” representing groundwater that resides in the watershed for a long time; and (3) sea water. The relative contributions of these sources can be reflected by the δ2H and δ18O values. While the higher δ2H and δ18O in the bayou reflect increasing percentage of sea water as salinity increases toward the bay (Fig. 5), the much lower values and large variability of δ2H and δ18O in the creeks suggest changes in the isotopic compositions of the sources may reflect the influence of the drainage basin attributes and seasonal variations in precipitation (Kendall and Coplen, 2001). A time series plot of daily rainfall, nitrate concentration, and δ18O (Fig. 7) does not show a clear pattern of seasonality because heavy rains fall in this region year-round. However, higher nitrate concentrations and δ18O values did show up on the 11/10/16 sampling date, which was preceded by a period of little rainfall for three weeks, indicating that the creek was sustained by subsurface flow with a steady input of nitrate in the watershed. This subsurface flow is from the shallow surficial aquifer that generally experiences more evaporation of water in the soil zone than stormwater. These samples also reside to the right of the Meteoric Water Line (Fig. 5b), suggesting that this water has a relatively long residence time in the watershed before being discharged into the creek. Thus, nitrate from the leach field of the septic tanks would travel through this transport pathway. This sustained base flow carrying leachate from septic tanks may also explain why Jackson Creek sites always registered salinities of 0.1 psu with an average conductivity of 160 μS, whereas the most upstream Jones Creek sites had 0.0 psu salinities and average conductivity of 79 μS (Fig. 2). During rainfall events, stream flows contain mostly relatively “new water” with a mixture of surface and subsurface flow. The steeper slopes, sandy soils, higher elevation, and denser residential development are inducive to surface runoff and returned subsurface flow, which was visually observed during our field reconnaissance. Furthermore, runoff and flow rates are expected to be greater than in Jones Creek. The fact that NO3− accounted for 98% of DIN in Jackson Creek also suggests that nitrogen transport is carried out in an oxygen rich environment, i.e., surface or returned subsurface flows.
Fig. 7.
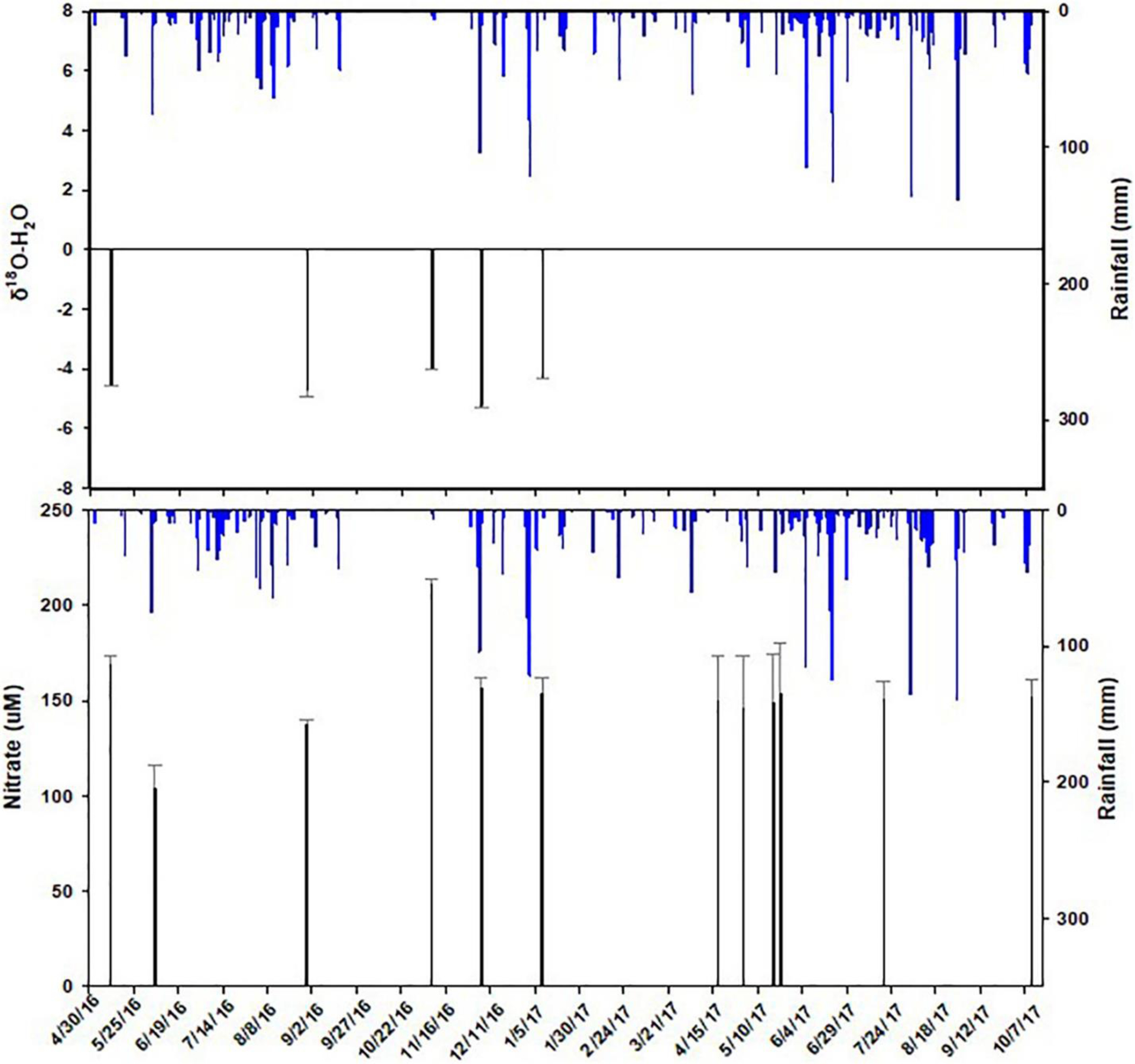
Temporal variation of δ18O and nitrate concentration (black bars) in response to rainfall (blue bars) in Jackson Creek. Error bars are standard deviation of the upstream four samples taken in the creek.
In contrast to Jackson Creek, nitrogen transport into Jones Creek is shaped by different hydrological forcings. The low elevation and gentle slope support formation of a very shallow aquifer that is closely connected with surface water. This is especially so in the upper Jones Creek where longer residence time is warranted for evaporation in the swamp, partly attributable to its slightly higher δ18O values than Jackson Creek. During a rainfall event, the “new water” can mix with the “old water” in the preserve and push these swamp waters into the creek through the saturated soil zone. This hydrological process facilitates transport of organic dissolved carbon and nitrogen to the stream. The fact that about 78% of TDN is organic in Jones Creek is indicative of the biogenic origin of N in association with the on-site stormwater treatment preserve. Similar N speciation and transport patterns were reported in south Florida where dense vegetative cover, flat terrain, high groundwater table, and humid subtropical climate are inducive for enhanced biological activities and cycling of carbon and nitrogen as well as rapid decomposition of organic matter (Wan et al., 2017).
4.4. Management implications
The importance of coastal waterways to the quality of city living is well recognized in the ecosystem services they provide. Recreational activities, economic development, stormwater management, and aesthetics are some benefits derived from creeks, streams, inlets, and bays situated in an urban landscape (Teurlincx et al., 2019). These benefits are lessened when water quality becomes degraded with nitrogen pollution. Management, sustainability, and restoration of urban waterbodies, whether natural or man-made, requires an understanding of nitrogen sources, flow paths, and cycling (Teurlincx et al., 2019). Differences in N concentration and speciation between Jackson Creek and Jones Creek (Table 3) provide information that can be helpful in the ongoing, site-specific basin management planning to reduce bacteria and nutrient loadings in accordance with state guidelines. An important factor influencing the fate and transport of N in urban stormwater is green space. Natural green spaces such as parks, riparian zones, and wetlands help control the flow of stormwater and clean the water by filtering out particulates and pollutants including N (Mayer et al., 2005). Green infrastructure can be incorporated to manage stormwater for environmental protection and restoration. However, knowing where nitrogen in urban streams originates can help planners and designers optimize the placement of green infrastructure. Vegetative waterways in the urban landscape seem to exhibit great benefits in N transformation in this study.
5. Conclusions
This study demonstrated the usefulness of a multi-tracer (isotopes and sewage markers) approach along with water quality and hydrological monitoring in source identification and characterization of nitrogen pollution in a coastal urban setting. While the similar isotopic signature of nitrate in the aquatic system does not signify a predominant nitrogen source in the system, likely due to a multitude of factors such as varying land-based inputs, nitrogen biogeochemical transformation, as well as rainfall driven transport pathways and tidal mixing, sewage tracers clearly demonstrated the linkage of septic tank leakage as a major nitrogen source with high nitrate concentrations. Distinct spatial patterns in nitrogen speciation and their concentrations reflected the effects of basin characteristics, nitrogen transport pathways, and management practices. Such analyses can be invaluable on many occasions for identifying anthropogenic nitrogen sources that influence water quality, exploring interactions between external drivers and nitrogen transformation processes, and developing technically sound nitrogen pollution abatement programs.
Supplementary Material
Highlights.
Urban creek nitrogen levels and speciation reflect basin land use.
Sewage tracers were more useful 15N isotopes for assessing septic N pollution.
High creek water nitrate was sourced to leaking septic tanks via subsurface flow.
Creekside vegetation and a natural preserve in creek headwaters benefit N levels.
Acknowledgements
We thank Chips Kirshcenfeld, Escambia County Natural Resources Management, and Brent Wipf, Dana Morton, Mollie Taylor, and staff of the Escambia County Water Quality and Land Management Division for field assistance and valued insights into the Bayou Chico watershed. We thank Madhav Machavaram (Pegasus Technical Services) for stable isotope analyses; and Jessica Aukamp, Joe James, David Beddick, Alex Amario, Brandon Jarvis (all with EPA Gulf Breeze), and Ryan Boylen (Oak Ridge Institute for Science and Education) for their support in field work, sample processing, and nutrient analyses, and Andrea Lamper for assisting with the figures. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Footnotes
CRediT authorship contribution statement
Richard Devereux conceived the study, obtained the funding, led the fieldwork, analyzed and curated data, wrote the manuscript, prepared figures, reviewed and edited the manuscript. Yongshan Wan analyzed and reviewed the data, wrote and edited the manuscript. Jennifer Rackley organized the field work, conducted laboratory analyses, was responsible for GIS visualization, wrote and edited the manuscript. Veronica Fasselt assisted in organizing the research, obtained the funding, reviewed the data, reviewed and edited the manuscript. Deborah N. Vivian analyzed data and prepared figures.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- An S, Gardner WS, 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (LagunaMadre/Baffin Bay, Texas). Mar. Ecol. Prog. Ser 237, 41–50. 10.3354/meps237041. [DOI] [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR, 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods PRIMER-E, Plymouth, UK. [Google Scholar]
- Breitburg D, Levin LA, Oschlies A, Gregoire M, Chavez FP, Conley DJ, Garcon V, Gilbert D, Gutierrez D, Isensee K, Jacinto GS, Limburg KE, Montes I, Naqvi SWA, Pitcher GC, Rabalais N, Roman MR, Rose KA, Seibel BA, Telszewski M, Yasuhara M, Zhang J, 2018. Declining oxygen in the global ocean and coastal waters. Science 359, 46. 10.1126/science.aam7240. [DOI] [PubMed] [Google Scholar]
- Bruland GL, MacKenzie RA, 2010. Nitrogen source tracking with d15N content of coastal wetland plants in Hawaii. J. Environ. Qual 39, 409–419. 10.2134/jeq2009.0005. [DOI] [PubMed] [Google Scholar]
- Camargo JA, Alonso A, 2006. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ. Int 32, 831–849. 10.1016/j.envint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Casciotti KL, Sigman DM, Ward BB, 2003. Linking diversity and stable isotope fractionation in ammonia-oxidizing bacteria. Geomicrobiol J. 20, 335–353. 10.1080/01490450303895. [DOI] [Google Scholar]
- Chang CCY, Kendall C, Silva SR, Battaglin WA, Campbell DH, 2002. Nitrate stable isotopes: tools for determining nitrate sources among different land uses in the Mississippi River Basin. Can. J. Fish. Aquat. Sci 59, 1874–1885. 10.1139/f02-153. [DOI] [Google Scholar]
- Cifuentes LA, Fogel ML, Pennock JR, Sharp JH, 1989. Biogeochemical factors that influence the stable nitrogen isotope ratio of dissolved ammonium in the Delaware Estuary. Geochim. Cosmochim. Acta 53, 2713–2721. 10.1016/0016-7037(89)90142-7. [DOI] [Google Scholar]
- Clara M, Strenn B, Kreuzinger N, 2004. Carbamazepine as a possible anthropogenic marker in the aquatic environment: investigations on the behaviour of carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 38, 947–954. 10.1016/j.watres.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Coffin RB, Cifuentes LA, 1999. Stable isotope analysis of carbon cycling in the Predido estuary, Florida. Estuaries 4, 917–926. [Google Scholar]
- Coplen TB; Qi H; Révész K; Casciotti K; and Hannon JE 2012, Determination of the δ15N and δ18O of nitrate in water; RSIL lab code 2900, chap. 17 of Stable isotoperatiomethods, sec. C of Révész K, and Coplen TB eds.,Methods of the Reston Stable Isotope Laboratory. U.S. Geological Survey Techniques and Methods, book 10, 35 p., available at https://pubs.usgs.gov/tm/2006/tm10c17/. [Google Scholar]
- Dahnke K, Bahlmann E, Emeis K, 2008. A nitrate sink in estuaries? An assessment by means of stable nitrate isotopes in the Elbe estuary. Limnol. Oceanogr 53, 1504–1511. 10.4319/lo.2008.53.4.1504. [DOI] [Google Scholar]
- Dillon KS, Chanton JP, 2005. Nutrient transformations between rainfall and stormwater runoff in an urbanized coastal environment: Sarasota Bay, Florida. Limnol. Oceanogr 50, 62–69. 10.4319/lo.2005.50.1.0062. [DOI] [Google Scholar]
- Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, Schloesser JT, Thornbrugh DJ, 2008. Eutrophication of US freshwaters: analysis of potential economic damages. Environ. Sci. Technol 43, 12–19. 10.1021/es801217q. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Brush GS, 2006. Sedimented organic nitrogen isotopes in freshwater wetlands record long-term changes in watershed nitrogen source and land use. Environ. Sci. Technol 26 (40), 2910–2916. 10.1021/es051587q. [DOI] [PubMed] [Google Scholar]
- EPA 2007. U.S. Environmental Protection AgencyMethod 1694: Pharmaceuticals and personal care products in water, soil, sediment, and biosolids by HPLC/MS/MS: USEPA, Washington, DC, EPA-821-R-08–008, 77 p. [Google Scholar]
- EPA 2013. Final Total Maximum Daily Loads for Dissolved Oxygen in Jones Creek WBID 846A, https://archive.epa.gov/pesticides/region4/water/tmdl/web/pdf/2e_final_tmdl_846a_do_3f.pdf Accessed October 16, 2019.
- Elliott EM, Kendall C, Wankel SD, Burns DA, Boyer EW, Harlin K, Bain DJ, Butler TJ, 2007. Nitrogen isotopes as indicators of NOx source contributions to atmospheric deposition across the Midwestern and Northeastern United States. Environ. Sci. Technol 41, 7661–7667. 10.1021/es070898t. [DOI] [PubMed] [Google Scholar]
- FDEP, 2011. Basin management action plan for the implementation of total maximum daily loads for fecal coliform adopted by the Florida Department of Environmental Protection in Bayou Chico (Pensacola Basin) https://floridadep.gov/sites/default/files/bayou-chico-bmap.pdf. (Accessed 14 September 2020).
- Fry B, Grace A, McClelland JW, 2003. Chemical indicators of anthropogenic nitrogen loading in four Pacific estuaries. Pac. Sci 57, 77–101. 10.1353/psc.2003.0004. [DOI] [Google Scholar]
- Garcia-Aljaro C, Blanch AR, Campos C, Jofre J, Lucena F, 2018. Pathogens, faecal indicators and human-specific microbial source-tracking markers in sewage. J. Appl. Microbiol 126, 701–717. 10.1111/jam.14112. [DOI] [PubMed] [Google Scholar]
- Gasser G, Rona M, Voloshenko A, Shelkov R, Tal N, Pankratov I, Elhanany S, Lev O, 2010. Quantitative evaluation of tracers for quantification of wastewater contamination of potable water sources. Environ. Sci. Technol 44, 3919–3925. 10.1021/es100604c. [DOI] [PubMed] [Google Scholar]
- Heaton THE, 1986. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review. Chem. Geol 59, 87–102. 10.1016/0168-9622(86)90059-X. [DOI] [Google Scholar]
- Hobbie SE, Finlay JC, Janke BD, Nidzgorski DA, Millet DB, Baker LA, 2017. Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban pollution. Proc. Natl. Acad. Sci 114, 4177–4182. 10.1073/pnas.1618536114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth RW, 2008. Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8, 14–20. 10.1016/j.hal.2008.08.015. [DOI] [Google Scholar]
- Jones BL, Cullen-Unsworth LC, Unsworth RKF, 2018. Tracking nitrogen source using δ15N reveals human and agricultural drivers of seagrass degradation across the British Isles. Front. Plant Sci 9, 133. 10.3389/fpls.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall C, Coplen TB, 2001. Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol. Process 15, 1363–1393. 10.1002/hyp.217. [DOI] [Google Scholar]
- Kendall C, Elliott EM,Wankel SD, 2007. Tracing anthropogenic inputs of nitrogen to ecosystems. In:Michener RM, Lajtha K (Eds.), Stable Isotopes in Ecology and Environmental Science Blackwell, Oxford, pp. 375–449 10.1002/9780470691854.ch12. [DOI] [Google Scholar]
- Kohl DH, Shearer GB, Commoner B, 1971. Fertilizer nitrogen: contribution to nitrate in surface water in a corn belt watershed. Science 174, 1331–1334. 10.1126/science.174.4016.1331. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT, 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol 36 (6), 1202–1211. 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Li S, Li J, Zhang Q, 2011. Water quality assessment in the rivers along the water conveyance system of the Middle Route of the South to North Water Transfer Project (China) using multivariate statistical techniques and receptor modeling. J. Hazard. Mater 195, 306–317. 10.1016/j.jhazmat.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Lim FY, Ong SL, Hu J, 2017. Recent advances in the use of chemical markers for tracing wastewater contamination in aquatic environment: a review. Water 9, 143. 10.3390/w9020143. [DOI] [Google Scholar]
- Macko SA, Ostrom NE, 1994. Molecular and pollution studies using stable isotopes. In: Lajtha K, Michner R (Eds.), Stable Isotopes in Ecology and Environmental Science Blackwell, Oxford, pp. 45–62. [Google Scholar]
- Mayer B, Boyer EW, Goodale C, Jaworski NA, van Breemen N, Howarth RW, Seitzinger S, Billen G, Lajtha K, Nadelhoffer K, Van Dam D, Hetling LJ, Nosal M, Paustian K, 2002. Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: isotopic constraints. Biogeochemistry 57, 171–197. 10.1023/A:1015744002496. [DOI] [Google Scholar]
- Mayer PM; Reynolds SK; Canfield TJ 2005. Riparian buffer width, vegetative cover, and nitrogen removal effectiveness: a review of current science and regulations Washington, DC: U.S. Environmental Protection Agency, EPA/600/R-0.5/118:1–40. [Google Scholar]
- Oppenheirmer J, Eaton A, Badruzzaman M, Haghani AW, Jacangelo JG, 2011. Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Res 45, 4019–4027. 10.1016/j.watres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Pennino MJ, Jaushal SS, Murthy SN, Blomquist JD, Cornwell JC, Harris LA, 2016. Sources and transformations of anthropogenic nitrogen along an urban river estuarine continuum. Biogeosciences 13, 6211–6288. 10.5194/bg-13-6211-2016. [DOI] [Google Scholar]
- Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Marti E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory S, Morrall DD, 2001. Control of nitrogen export from watersheds by headwater streams. Science 292, 86–90. 10.1126/science.1056874. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL. http://www.R-project.org/. [Google Scholar]
- Rock L, Mayer B, 2004. Isotopic assessment of sources of surface water nitrate within the Oldman River basin, Southern Alberta, Canada. Water Air Soil Pollut. Focus 4 (2), 545–562. 10.1023/B:WAFO.0000028377.94365.09. [DOI] [Google Scholar]
- Sebilo M, Billen G, Grably M, Mariotti A, 2003. Isotopic composition of nitrate nitrogen as a marker of riparian and benthic denitrification at the scale of the whole Seine River system. Biogeochemistry 63, 35–51. [Google Scholar]
- Sebilo M, Billen G, Mayer B, Billiou D, Grably M, Garnier J, Mariotti A, 2006. Assessing nitrification and denitrification in the Seine River and estuary using chemical and isotopic techniques. Ecosystems 9, 564–577. 10.1007/s10021-006-0151-9. [DOI] [Google Scholar]
- Seitzinger SP, Harrison JA, Dumont E, Beusen A, Bouwman AF, 2005. Sources and delivery of carbon, nitrogen, and phosphorus to the coastal zone: an overview of Global Nutrient Export from Watersheds (NEWS) models and their application. Glob. Biogeochem. Cycles 19, GB4S01. 10.1029/2005GB002606. [DOI] [Google Scholar]
- Silva SR, Ging PB, Lee RW, Ebbert JC, Tesoriero AJ, Inkpen EL, 2002. Forensic applications of nitrogen and oxygen isotopes of nitrate in an urban environment. Environ. Forensic 3, 125–130. 10.1006/enfo.2002.0086. [DOI] [Google Scholar]
- Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis Th., 2003. Assessment of the surface water quality in northern Greece. Water Res. 37, 4119–4124. 10.1016/S0043-354(03)00398-1. [DOI] [PubMed] [Google Scholar]
- Teurlincx S, Kuiper JJ, Hoevenaar ECM, Lurling M, Brederveld RJ, Veraat AJ, Janssen ABG, Mooij WM, de Senerpont Domis LN, 2019. Towards restoring urban waters: understanding the main pressures. Curr. Opin. Environ. Sustain 36, 49–58. 10.1016/j.cosust.2018.10.011. [DOI] [Google Scholar]
- USDA, 2004. Soil survey of Escambia County, Florida https://www.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/florida/FL033/0/fl_escambia.pdf. (Accessed 14 September 2020).
- Wan Y,Wan L, Li Y, Doering P, 2017. Decadal and seasonal trends of nutrient concentration and export from highly managed coastal catchments. Water Res 115, 180–194. 10.1016/j.watres.2017.02.068. [DOI] [PubMed] [Google Scholar]
- Wankel SD, Kendall C, Francis CA, Paytan A, 2006. Nitrogen sources and cycling in the San Francisco Bay Estuary: a nitrate dual isotope approach. Limnol. Oceanogr 51, 1654–1664. 10.4319/lo.2006.51.4.1654. [DOI] [Google Scholar]
- Weatherspark, 2021. Average weather in Pensacola. https://weatherspark.com/y/13877/Average-Weather-in-Pensacola-Florida-United-States-Year-Round. (Accessed 5 February 2021).
- Yang Y-Y, Toor GS, 2016. δ15N and δ18O reveal the sources of nitrate-nitrogen in urban residential stormwater runoff. Environ. Sci. Technol 50, 2881–2889. 10.1021/acs.est.5b05353. [DOI] [PubMed] [Google Scholar]
- Zhou F, Liu Y, Gou H, 2007. Application of multivariate statistical methods to water quality assessment of the water courses in northwestern New Territories, Hong Kong. Environ. Monit. Assess 132, 1–13. 10.1007/s10661-006-9497-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data obtained for this study are available through EPA Science Hub https://sciencehub.epa.gov/sciencehub/datasets/2842.


