Abstract
Purpose
To compare the efficacy and safety between paclitaxel coated balloon (PCB) angioplasty and conventional balloon (CB) angioplasty in the treatment of dysfunctional arteriovenous fistula (AVF).
Methods
We searched four major electronic databases (PubMed, EMBASE, Web of Science and the Cochrane Library) for randomized controlled trials (RCTs) published from inception through November 28, 2021. Outcomes of interest included target lesion primary patency (TLPP), technical success and all-cause mortality. The STATA package version 15.1 was utilized to undertake meta-analyses.
Results
Fourteen RCTs totaling 1535 patients were analyzed. The available data showed that there were no significant differences of TLPP rates at 3, 6, 9 and 12 months between the PCB group and the CB group (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.93–1.07, p = 1.000, I2 = 33.5%, Cochrane Q test p = 0.185, fixed-effect model; RR 1.17, 95% CI 0.99–1.39, p = 0.065, I2 = 75.4%, Cochrane Q test p = 0.000, random-effect model; RR 0.81, 95% CI 0.35–1.89, p = 0.625, I2 = 62.8%, Cochrane Q test p = 0.045, random-effect model; RR 1.19, 95% CI 0.97–1.47, p = 0.096, I2 = 40.5%, Cochrane Q test p = 0.071, random-effect model). In addition, two groups had similar technical success rates (RR 1.00, 95% CI 0.97–1.03, p = 1.000, I2 = 0.0%, Cochrane Q test p = 0.596, fixed-effect model) and all-cause mortality rates (RR 1.00, 95% CI 0.54–1.84, p = 1.000, I2 = 0.0%, Cochrane Q test p = 0.599, fixed-effect model).
Conclusions
PCB angioplasty did not appear to convey any obvious advantage over CB angioplasty in the treatment of dysfunctional AVF. However, further multi-center, large-scale and well-designed RCTs are needed to prove outcomes.
Keywords: Paclitaxel, angioplasty, balloon, arteriovenous fistula, meta-analysis
Introduction
Reliable vascular access is known as the lifeline of maintenance hemodialysis patients. There are several commonly used permanent hemodialysis vascular access types such as autologous arteriovenous fistula (AVF), tunnel-cuffed catheter (TCC) and arteriovenous graft (AVG). However, the extensive application of AVG in clinical practice has not yet been realized due to its high price and technological problems. Note that TCC was chosen only when AVF could not be established or patients were expected to have a relatively short survival time. It has been found that the patients with AVF had a better survival rate compared with patients with other access types [1]. As a consequence, AVF is currently the preferred choice for vascular access. And its functional status directly affects the dialysis efficiency and quality of life of patients undergoing maintenance hemodialysis. Nevertheless, the persistence of AVF was not satisfactory enough and the most prevalent causes of dysfunctional AVF were thrombosis and vascular stenosis [2]. Therefore, long-term patency preservation of the fistula tract presented an urgent clinical problem to be solved. In the past era, dysfunctional AVF was generally treated with surgical methods. With the rapid advancement of intraluminal interventional techniques, it has emerged as a primary therapeutic approach in the treatment of this disease. Conventional balloon (CB) angioplasty was thought to be the gold standard for the treatment of dysfunctional dialysis access, either AVF or AVG. But Haskal’s study showed that the incidence of patency of the treatment area and the access circuit in the CB group was only 23% and 20%, respectively [3]. Compared with CB, high-pressure balloons and cutting balloons are able to improve the patients’ prognosis, but the stenosis rate is still high in the short term [4,5]. Accordingly, the emergence of paclitaxel coated balloon (PCB) is expected to be useful for solving the foregoing issues. The role of PCB in coronary artery diseases and peripheral arterial diseases has been widely recognized [6,7]. However, whether PCB angioplasty outperforms CB angioplasty in the treatment of dysfunctional AVF is still in controversy.
Several studies confirmed a benefit of PCB angioplasty [8–16] while the others showed they were equivalent in target lesion primary patency (TLPP) [17–20]. Moreover, the results of a randomized controlled trial (RCT) showed that the TLPP after PCB angioplasty was even worse [21]. As the safety and benefits of PCB angioplasty remain unknown, we aimed to conduct a meta-analysis to reevaluate the results.
Materials and methods
The present meta-analysis was reported referring to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement [22].
Search strategy
A systematic search of relevant literature available on PubMed, EMBASE, Web of Science and the Cochrane Library containing several keywords “arteriovenous fistula,” “dialysis fistula,” “drug-coated balloon,” “drug-eluting balloon” and “paclitaxel” published from their date of inception to November 28, 2021, was carried out (Appendix 1). We did not use any language or data restrictions, although we used only English search terms. References of these articles were also searched to find potential relevant articles.
Inclusion and exclusion criteria
Titles, abstracts and the full texts of all retrieved studies were preliminarily filtrated by a pair of authors to determine the inclusion (LC and LM). Disagreements from the two authors were solved by consensus or by appeal to a third review author (JJ). Inclusion criteria: (1) RCTs with two parallel arms; (2) Hemodialysis patients with dysfunctional AVFs; (3) Patients were treated with PCB angioplasties or CB angioplasties; (4) TLPP rates, technical success rates or all-cause mortality rates of both methods were provided in the literature; (5) Clinical follow-up of at least 6 months. Exclusion criteria: (1) Observational studies, animal studies, in vitro tests, reviews, comments, editorials, case reports and series, protocols, letters, conference abstracts, crossover trials and single-arm tests; (2) Repeated reporting; (3) Full text not available; (4) AVF and AVG data reported together; (5) Use of a stent.
Outcomes of interest and data extraction
The endpoint events were defined in accordance with the Society of Interventional Radiology (SIR) criteria for percutaneous interventional procedures in dialysis access [23] and the previous literature [12,15,19]. TLPP was adjudicated as freedom from clinically-driven target lesion revascularization (CD-TLR) or access circuit thrombosis during the follow-up period. TLPP ended when any one of the followings occurred: (1) decreased access blood flow (<500mL/min, 25% decrease in flow); (2) elevated venous pressures; (3) decreased dialysis dose (Kt/V); (4) abnormal physical exam included: i. diminished or abnormal thrill (focal, systolic only, etc); ii. pulsatility; iii. flaccid access; iv. abnormal bruit; v. arm or hand swelling; (5) prolonged bleeding; (6) difficult puncture; (7) infiltration; (8) recirculation; (9) pulling clots. Technical success was defined as successful completion of the angioplasty procedure with <30% residual stenosis by visual estimate and a palpable thrill. All-cause mortality was reported through 12 months. Data were separately extracted by two review authors (LC and LM).
Risk of bias and quality assessment
Methodological quality appraisal was conducted by two independent reviewers (LC and LM). In the event of a discrepancy between the two authors, a third author will decide (JJ). The quality evaluation of selected studies was performed using 7 elements from the recommended Cochrane Collaborations tool: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias [24]. Publication bias was assessed through visual inspection of funnel charts, whereby an asymmetric funnel diagram indicated the presence of publication bias.
Statistical analysis
Statistical heterogeneity was assessed via the I2 statistic and Cochrane’s Q test. If evident heterogeneity (I2 > 50% or p < 0.1), the random-effect model was employed for analysis; if not, the fixed-effect model. We conducted sensitivity analyses and subgroup analyses to search for the potential sources of heterogeneity. A value of p < 0.05 was accepted as a statistically significant difference. Meta-analyses were processed with STATA software version 15.1 (StataCorp, College Station, TX, USA) to calculate risk ratios (RRs) and their 95% confidence intervals (CIs). The kappa coefficients were computed by use of SPSS 25.0 (SPSS Inc., Chicago, IL, USA) to assess the degree of concordance between the two investigators.
Results
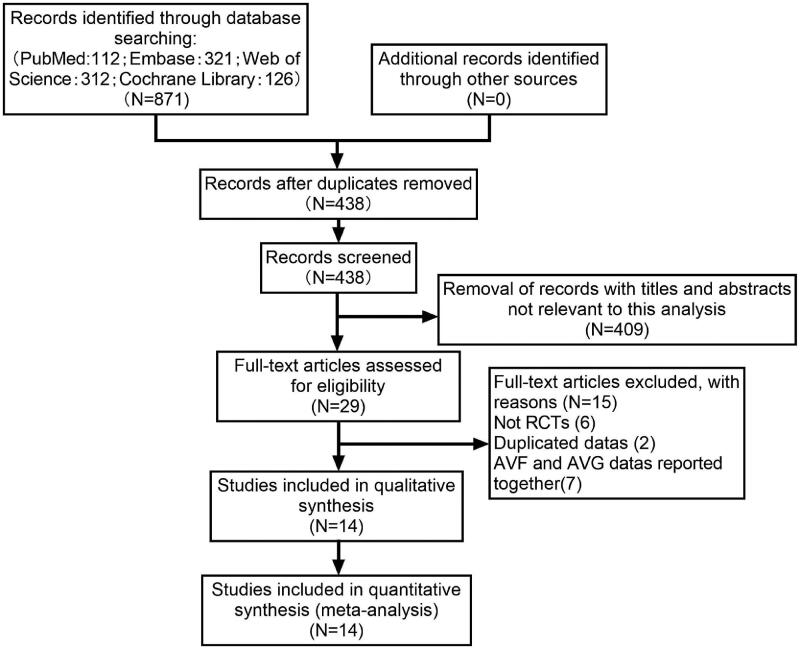
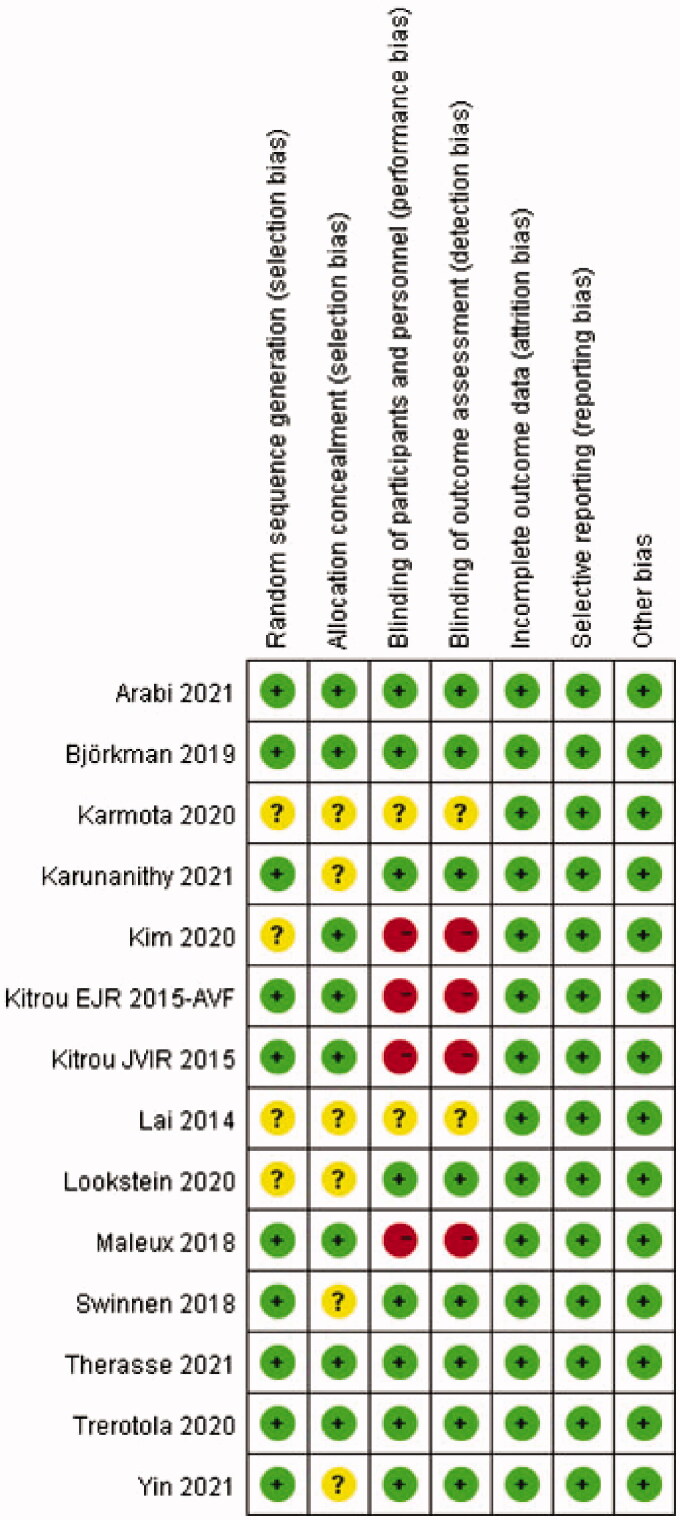
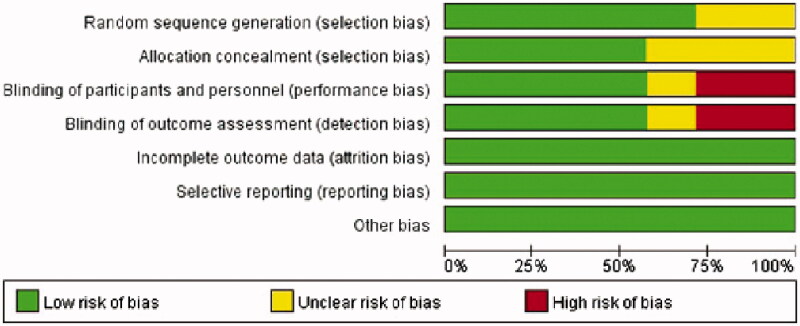
We identified 871 potentially eligible studies. After deduplication, 438 documents remained. Of these abstracts, 409 were excluded based upon the inclusion and exclusion criteria. Then, a total of 29 potentially relevant full-texts were retrieved and subjected to further review. Studies excluded and reasons for exclusion after full-text screening were provided in Table 1. Eventually, fourteen RCTs [8–21], including eight multi-center trials [8,12–16,18,20], with 1535 patients fulfilled the criteria for inclusion. A moderately high level of agreement between two independent investigators was observed at the title and abstract review (kappa = 0.695) and full-text evaluation (kappa = 0.861) stages. The detailed steps of the study search and selection process were outlined in Figure 1 and the baseline characteristics of the selected trials were summarized in Table 2. Two of the fourteen studies included patients with AVF and AVG, but we extracted data only for patients with AVF [9,14]. As for the methodological quality assessment, all studies scored three to seven points, among which four articles scored seven points [14,15,17,21]. The kappa values of agreement during the quality appraisal and data extraction were 0.863 and 1.000, respectively. The detailed score of each item for each article was described in Figure 2 and the proportion of each item in the methodological evaluation was shown in Figure 3.
Table 1.
List of records excluded after full-text reading.
| Author, year | Reason for exclusion |
|---|---|
| Ali 2020 [25] | 1 |
| Bjorkman 2021 [26] | 2 |
| Eldmarany 2020 [27] | 3 |
| Irani 2018 [28] | 1 |
| Karnabatidis 2021 [29] | 3 |
| Katsanos 2012 [30] | 1 |
| Moreno-Sánchez 2019 [31] | 1 |
| Pang 2021 [32] | 1 |
| Patanè 2019 [33] | 3 |
| Rai 2019 [34] | 3 |
| Roosen 2017 [35] | 1 |
| Teo 2013 [36] | 1 |
| Trerotola 2018 [37] | 2 |
| Verbeeck 2016 [38] | 3 |
| Yildiz 2019 [39] | 3 |
1: arteriovenous fistula and graft datas reported together; 2: duplicated datas; 3: not randomized controlled trials.
Figure 1.
Study selection flow diagram.
Table 2.
Baseline characteristics of included studies.
| Author, year | Cases |
Male% |
Age |
Diabetes mellitus% |
Hypertension% |
Device type | Inflation time | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB | CB | PCB | CB | PCB | CB | PCB | CB | PCB | CB | |||
| Arabi, 2021 [17] | 12 | 11 | 41.7 | 54.5 | 68.7 ± 10.1 | 66.8 ± 13.7 | 91.7 | 72.7 | 100 | 90.9 | PCB: Lutonix 035, Bard (2 µg/mm2) Control: unspecified |
120 sec |
| Björkman, 2019 [21] | 21 | 18 | 55.6 | 72.2 | 67.4 (46–87) | 67.0 (28–82) | 61.1 | 61.1 | 88.9 | 77.8 | PCB: IN. PACT Admiral, Medtronic (3.5 µg/mm2) Control: unspecified |
90 sec |
| Karmota, 2020 [8] | 30 | 30 | 43.3 | 53.3 | 54.7 ± 13.2 | 49.2 ± 11.5 | 63.3 | 50.0 | 50.0 | 56.7 | PCB: Lutonix 035, Bard (dose unspecified) Control: unspecified |
180 sec |
| Karunanithy, 2021 [18] | 106 | 106 | 63.2 | 57.5 | 66.9 ± 12.7 | 64.1 ± 13.3 | 54.7 | 43.4 | – | – | PCB: Lutonix 035, Bard (2 µg/mm2) Control: Ultraverse, Bard |
Unspecified |
| Kim, 2020 [19] | 20 | 19 | 60.0 | 47.4 | 60.7 ± 12.2 | 63.7 ± 11.8 | 80.0 | 78.9 | – | – | PCB: IN. PACT Admiral, Medtronic (dose unspecified) CB: Mustang, Boston Scientific |
120 sec |
| Kitrou, EJR, 2015-AVF [9] | 7 | 7 | 75 | 70 | 65.7 ± 13.2 | 62.5 ± 15.4 | 20 | 20 | 15 | 10 | PCB: IN. PACT Admiral, Medtronic (3 µg/mm2) Control: HPB |
Unspecified |
| Kitrou, JVIR, 2015 [10] | 20 | 20 | 60 | 70 | 64.3 ± 14.5 | 57 ± 14.2 | 20 | 35 | 15 | 15 | PCB: IN. PACT Admiral, Medtronic (3 µg/mm2) Control: HPB |
90 sec |
| Lai, 2014 [11] | 10 | 10 | 40 | 67.2 ± 9.4 | 50 | 40 | PCB: SeQuent Please, B.Braun (dose unspecified) CB: unspecified |
60 sec | ||||
| Lookstein, 2020 [12] | 170 | 160 | 65.9 | 63.1 | 65.8 ± 13.1 | 65.5 ± 13.4 | 62.9 | 68.8 | 91.2 | 94.4 | PCB: IN. PACT Admiral, Medtronic (3.5 µg/mm2) Control: uncoated balloon |
Unspecified |
| Maleux, 2018 [20] | 33 | 31 | 72.7 | 58.1 | 69.3 ± 14.9 | 66.9 ± 17 | – | – | – | – | PCB: IN. PACT Admiral, Invatec/Medtronic (dose unspecified) Control: Admiral Extreme, Invatec/Medtronic |
Unspecified |
| Swinnen, 2018 [13] | 68 | 60 | 61.8 | 61.7 | 65.2 ± 13.6 | 64.5 ± 13.9 | 55.9 | 65.0 | – | – | PCB: IN. PACT Admiral/Pacific, Medtronic (3 µg/mm2) Control: uncoated angioplasty balloon of the operator’s choice |
120 sec |
| Therasse, 2021-AVF [14] | 60 | 60 | 83.3 | 83.3 | 63.5 ± 12.6 | 66.6 ± 12.6 | 61.7 | 71.7 | 86.7 | 81.7 | PCB: Passeo-18 Lux, Biotronik (3 µg/mm2) CB: same type without drug |
60 sec |
| Trerotola, 2020 [15] | 141 | 144 | 61.7 | 59 | 64 ± 15 | 61 ± 13 | 58.2 | 65.3 | 94.3 | 98.6 | PCB: Lutonix 035, Bard (2 µg/mm2) Control: control balloon of similar design but without drug coating |
Unspecified |
| Yin, 2021 [16] | 78 | 83 | 56.4 | 50.6 | 56 ± 13 | 54 ± 13 | 34.6 | 34.9 | 84.6 | 84.3 | PCB: APERTO, Cardionovum (3 µg/mm2) Control: Ohicho II HPBs, Kaneka Corp |
120–180 sec |
PCB: paclitaxel coated balloon; CB: conventional balloon; sec: seconds; -: missing data.
Figure 2.
Risk of bias summary in included studies.
Figure 3.
Risk of bias graph in included studies.
3-Month TLPP
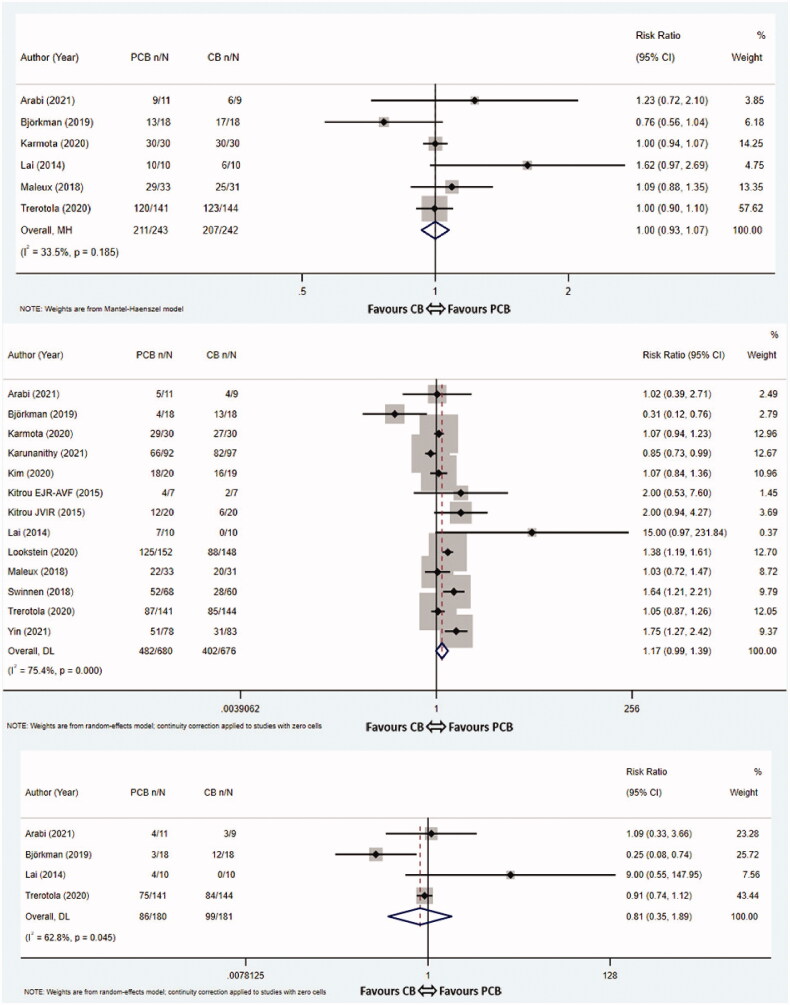
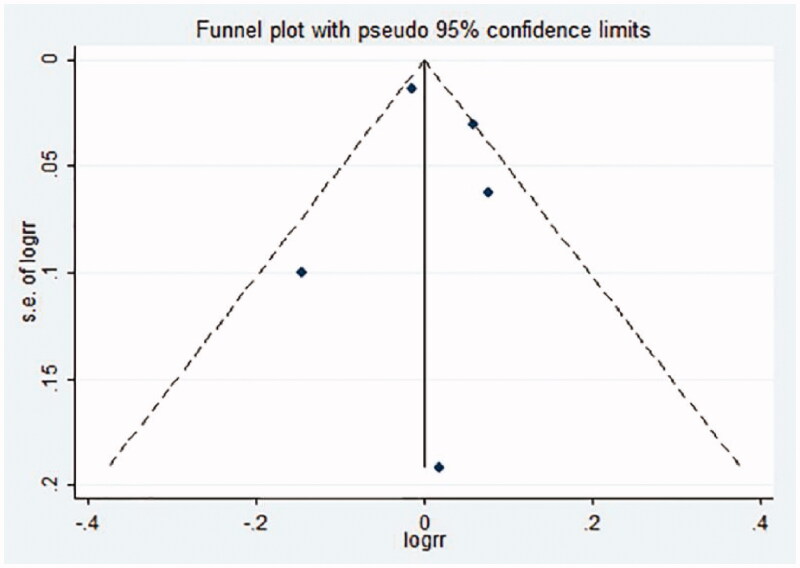
Six studies [8,11,15,17,20,21] evaluated the 3-month TLPP. The pooled rates for the PCB group and CB group were 86.8% (211/243) vs 85.5% (207/242), respectively. The meta-analysis showed that the difference of 3-month TLPP rates between two groups was not statistically significant (RR 1.00, 95% CI 0.93–1.07, p = 1.000, I2 = 33.5%, Cochrane Q test p = 0.185, fixed-effect model, Figure 4(A)). The funnel graph was roughly symmetrical which indicated the absence of significant publication bias (Egger’s test p = 0.563, Appendix 2).
Figure 4.
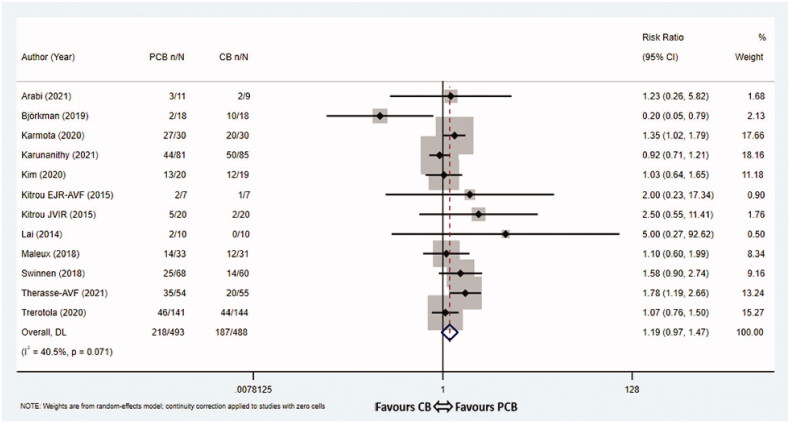
Meta-analysis of TLPP at 3 months (A), 6 months (B), 9 months (C) and 12 months (D). TLPP: target lesion primary patency; PCB: paclitaxel coated balloon; CB: conventional balloon; CI: confidence interval.
6-Month TLPP
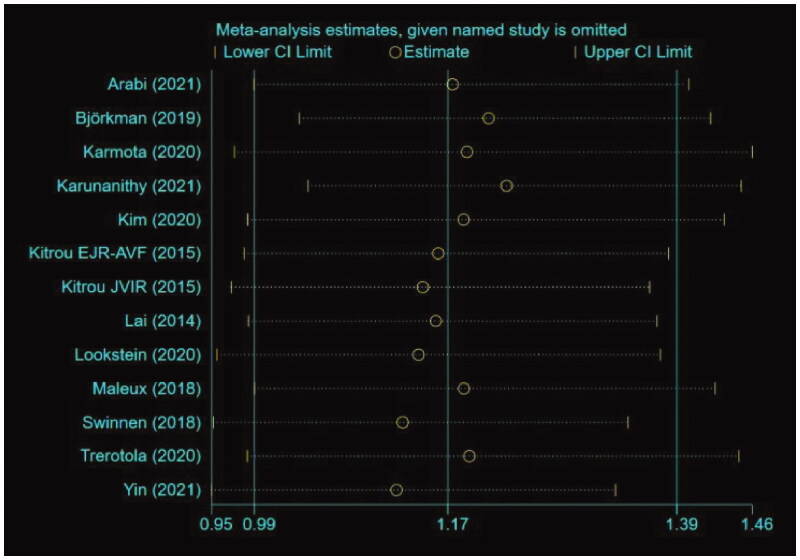
Thirteen studies [8–13,15–21] investigated the 6-month TLPP. The pooled event rates at 6 months in the PCB group and the CB group were 70.9% (482/680) and 59.5% (402/676), respectively. While the PCB group did improve the 6-month TLPP rates, the difference did not reach statistical significance (RR 1.17, 95% CI 0.99–1.39, p = 0.065, I2 = 75.4%, Cochrane Q test p = 0.000, random-effect model, Figure 4(B)). In order to estimate the possible sources of heterogeneity, sensitivity analysis and subgroup analyses based on PCB type, paclitaxel dose and inflation time were performed for this outcome. It is a pity that subgroup analyses of study characteristics did not find any factors that accounted for the heterogeneity (Table 3). The results of a subgroup analysis according to PCB type indicated that the APERTO (Cardionovum) balloons significantly outperformed CBs in terms of 6-month TLPP (RR 1.75, 95% CI 1.27–2.42, p = 0.001, fixed-effect model), whereas the Lutonix 035 (Bard), IN.PACT Admiral (Medtronic) and SeQuent Please (B.Braun) balloons did not improve 6-month TLPP rates compared with CBs (RR 0.97, 95% CI 0.87–1.08, p = 0.557, I2 = 47.2%, Cochrane Q test p = 0.128, fixed-effect model; RR 1.22, 95% CI 0.96–1.57, p = 0.110, I2 = 67.1%, Cochrane Q test p = 0.006, random-effect model; RR 1.00, 95% CI 0.48–2.10, p = 1.000, fixed-effect model). A subgroup analysis stratified by paclitaxel dose revealed that treatment with standard-dose PCBs (3.0 μg/mm2 and 3.5 μg/mm2) were significantly effective than CBs at improving 6-month TLPP rates for dysfunctional AVFs (RR 1.43, 95% CI 1.07–1.91, p = 0.016, I2 = 65.2%, Cochrane Q test p = 0.013, random-effect model). The subgroup analysis based on inflation time showed that the dilation time of PCBs greater than 120 s did significantly improve 6-month TLPP rates compare with the controls (RR 1.29; 95% CI 1.03–1.63, p = 0.029, I2 = 68.9%, Cochrane Q test p = 0.012, random-effect model). We also did not find the source of heterogeneity through the sensitivity analysis. After removing one study, the estimates did not change significantly, which means these results were relatively robust in this meta-analysis (Appendix 2). The funnel graph was symmetrically distributed, and publication bias was not evident (Egger’s test p = 0.443, Appendix 2).
Table 3.
Subgroup analyses.
| Parameters | Factors | Subgroup | No. of trials | Effect estimate and 95% CI | I2 (%) | P value for Q statistic | P value | Effect model |
|---|---|---|---|---|---|---|---|---|
| 6-month TLPP | PCB type | Lutonix 035, Bard | 4 | 0.97 (0.87, 1.08) | 47.2 | 0.128 | 0.557 | Fixed-effect model |
| IN.PACT Admiral, Medtronic | 7 | 1.22 (0.96, 1.57) | 67.1 | 0.006 | 0.110 | Random-effect model | ||
| SeQuent Please, B.Braun | 1 | 1.00 (0.48, 2.10) | 0.0 | – | 1.000 | Fixed-effect model | ||
| APERTO, Cardionovum | 1 | 1.75 (1.27, 2.42) | 0.0 | – | 0.001 | Fixed-effect model | ||
| Paclitaxel dose | Low-dose (2 μg/mm2) | 3 | 0.95 (0.84, 1.08) | 35.5 | 0.212 | 0.428 | Fixed-effect model | |
| Standard-dose (3 μg/mm2 and 3.5 μg/mm2) | 6 | 1.43 (1.07, 1.91) | 65.2 | 0.013 | 0.016 | Random-effect model | ||
| Unspecified | 4 | 1.00 (0.87, 1.15) | 41.5 | 0.163 | 1.000 | Fixed-effect model | ||
| Inflation time | ≥120 sec | 5 | 1.29 (1.03, 1.63) | 68.9 | 0.012 | 0.029 | Random-effect model | |
| <120 sec | 3 | 1.48 (0.26, 8.48) | 85.0 | 0.001 | 0.662 | Random-effect model | ||
| Unspecified | 5 | 1.08 (0.85, 1.38) | 80.5 | 0.000 | 0.513 | Random-effect model | ||
| 12-month TLPP | PCB type | Lutonix 035, Bard | 4 | 1.06 (0.88, 1.27) | 23.9 | 0.268 | 0.535 | Fixed-effect model |
| IN.PACT Admiral, Medtronic | 6 | 1.12 (0.83, 1.50) | 44.5 | 0.109 | 0.467 | Fixed-effect model | ||
| Passeo-18 Lux, Biotronik | 1 | 1.78 (1.19, 2.66) | 0.0 | – | 0.005 | Fixed-effect model | ||
| SeQuent Please, B.Braun | 1 | 1.00 (0.25, 4.00) | 0.0 | – | 1.000 | Fixed-effect model | ||
| Paclitaxel dose | Low-dose (2 μg/mm2) | 3 | 1.00 (0.80, 1.23) | 0.0 | 0.764 | 0.978 | Fixed-effect model | |
| Standard-dose (3 μg/mm2 and 3.5 μg/mm2) | 5 | 1.33 (0.72, 2.48) | 57.8 | 0.050 | 0.367 | Random-effect model | ||
| Unspecified | 4 | 1.00 (0.80, 1.24) | 47.3 | 0.127 | 1.000 | Fixed-effect model | ||
| Inflation time | ≥120 sec | 4 | 1.33 (1.04, 1.71) | 0.0 | 0.676 | 0.023 | Fixed-effect model | |
| <120 sec | 4 | 1.24 (0.37, 4.09) | 70.1 | 0.018 | 0.730 | Random-effect model | ||
| Unspecified | 4 | 1.01 (0.83, 1.24) | 0.0 | 0.801 | 0.898 | Fixed-effect model |
CI: confidence interval; TLPP: target lesion primary patency; PCB: paclitaxel coated balloon; CB: conventional balloon; sec: seconds.
9-Month TLPP
Data on 9-month TLPP were extracted from four articles [11,15,17,21]. The pooled rates for the PCB group and CB group were 47.8% (86/180) vs 54.7% (99/181), respectively. No statistically significant differences were observed between the two groups (RR 0.81, 95% CI 0.35–1.89, p = 0.625, I2 = 62.8%, Cochrane Q test p = 0.045, random-effect model, Figure 4(C)). There were too few RCTs to perform sensitivity analysis, subgroup analysis and publication bias test.
12-Month TLPP
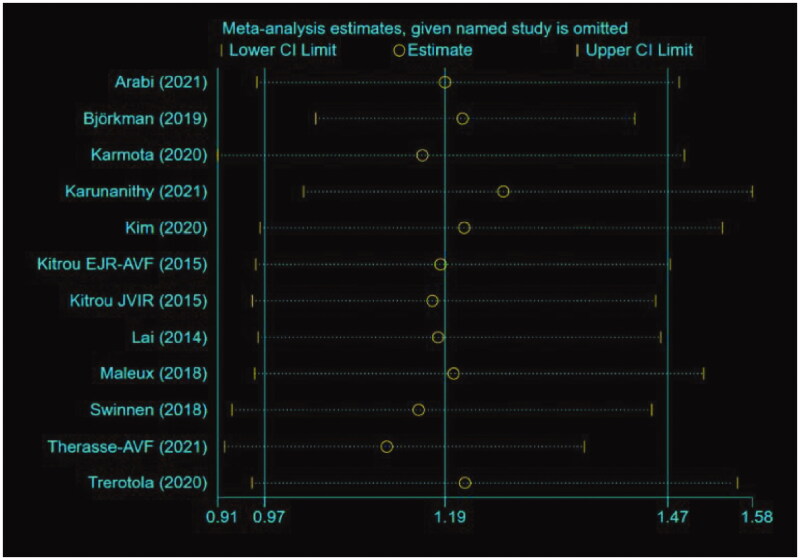
Of twelve trials [8–11,13–15,17–21] reporting the 12-month TLPP or having sufficient data for extrapolation, the pooled 12-month TLPP rates were 44.2% (218/493) in the PCB group vs 40.4% (197/488) in the CB group, respectively. PCB angioplasty was not correlated with 12-month TLPP (RR 1.19, 95% CI 0.97–1.47, p = 0.096, I2 = 40.5%, Cochrane Q test p = 0.071, random-effect model, Figure 4(D)). Almost all of the subgroup analyses had no influence on the heterogeneity of the pooled analysis (Table 3). The outcome of subgroup analysis according to PCB types demonstrated that the Passeo-18 Lux (Biotronik) balloons were favored over CBs in terms of 12-month TLPP (RR 1.78, 95% CI 1.19–2.66, p = 0.005, fixed-effect model), whereas the Lutonix 035 (Bard), IN.PACT Admiral (Medtronic) and SeQuent Please (B.Braun) balloons did not improve 12-month TLPP compared with CBs (RR 1.06, 95% CI 0.88–1.27, p = 0.535, I2 = 23.9%, Cochrane Q test p = 0.268, fixed-effect model; RR 1.12, 95% CI 0.83–1.50, p = 0.467, I2 = 44.5%, Cochrane Q test p = 0.109, fixed-effect model; RR 1.00, 95% CI 0.25–4.00, p = 1.000, fixed-effect model). A subgroup analysis based on inflation time demonstrated that 12-month TLPP rates were significantly higher in the group with the PCB inflation time ≥120 s compared with the controls (RR 1.33, 95% CI 1.04–1.71, p = 0.023, I2 = 0.0%, Cochrane Q test p = 0.676, fixed-effect model). Additional sensitivity analysis was performed by eliminating each included study step by step, showing that the studies were reliable and robust (Appendix 2). No significant publication bias existed in the studies evaluating the 12-month TLPP rates (Egger’s test p = 0.740, Appendix 2).
Technical success
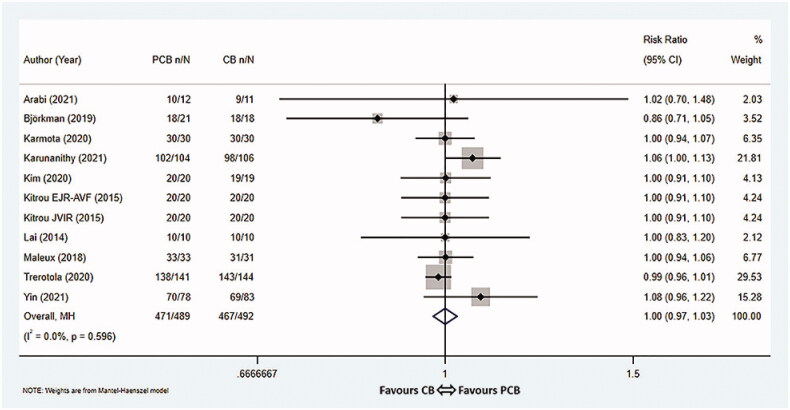
Altogether, eleven studies [8–11,15–21] reported the technical success rates in a total of 981 patients, with 489 assigned to the PCB group and 492 assigned to the CB group. The pooled rates for the PCB group and CB group were 96.3% (471/489) vs 94.9% (467/492), respectively. The results revealed that there was no statistically significant difference in technical success rates between the two groups (RR 1.00, 95% CI 0.97–1.03, p = 1.000, I2 = 0.0%, Cochrane Q test p = 0.596, fixed-effect model, Figure 5). The funnel plot was symmetrically distributed, indicating no remarkable publication bias in these studies (Egger’s test p = 0.751, Appendix 2).
Figure 5.
Meta-analysis of technical success rate. PCB: paclitaxel coated balloon; CB: conventional balloon; CI: confidence interval.
All-cause mortality
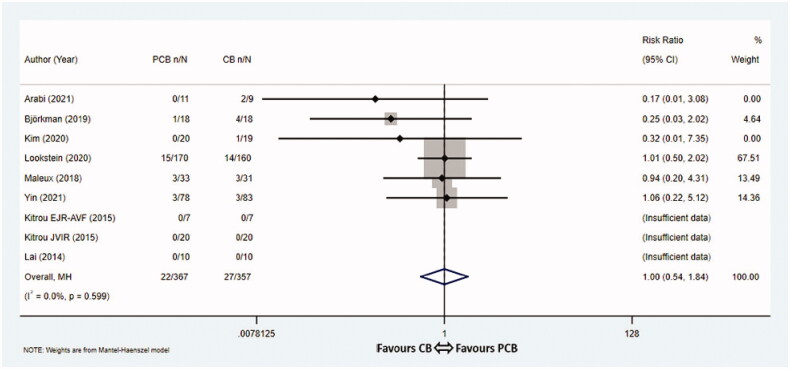
12-month mortality rates were documented in nine studies [9–12,16,17,19–21], among which three studies [9–11] documented zero death. These nine articles involved 724 patients, with 367 assigned to the PCB group and 357 assigned to the CB group. Overall, the pooled 12-month mortality rates were 6.0% (22/367) in the PCB group vs 7.6% (27/357) in the CB group, respectively. The statistical analysis showed no significant differences between the two groups with respect to 12-month mortality rates (RR 1.00, 95% CI 0.54–1.84, p = 1.000, I2 = 0.0%, Cochrane Q test p = 0.599, fixed-effect model, Figure 6). The associated funnel plot was basically symmetrical, suggesting no obvious publication bias (Egger’s test p = 0.055, Appendix 2).
Figure 6.
Meta-analysis of all-cause mortality. PCB: paclitaxel coated balloon; CB: conventional balloon; CI: confidence interval.
Discussion
In this meta-analysis, we conducted a comprehensive search for all studies regarding PCB angioplasty versus CB angioplasty in dysfunctional AVF. A total of fourteen articles containing 1535 subjects were included, and results showed no significant differences between the two groups in TLPP rates after 3, 6, 9 and 12 months of treatment (RR 1.00, 95% CI 0.93–1.07, p = 1.000, I2 = 33.5%; RR 1.17, 95% CI 0.99–1.39, p = 0.065, I2 = 75.4%; RR 0.81, 95% CI 0.35–1.89, p = 0.625, I2 = 62.8%; RR 1.19, 95% CI 0.97–1.47, p = 0.096, I2 = 40.5%). In addition, there were no significant differences observed in the technical success rates (RR 1.00, 95% CI 0.97–1.03, p = 1.000, I2 = 0%) and 12-month mortality rates (RR 1.00, 95% CI 0.54–1.84, p = 1.000, I2 = 0%) between the two groups. Moreover, a cost-effectiveness analysis conducted by Diehm et al revealed that the catheter material costs for PCBs and CBs were 2008 and 464 Swiss Francs per patient, respectively [40]. This is because while an uncoated balloon was used in the CB angioplasty, both an uncoated balloon for predilation and a PCB were required in the PCB angioplasty. From the above results, it appears that PCB angioplasty is neither more effective nor much safer and is more costly. Thereby, PCB angioplasty seems to be not cost-effective compared to CB angioplasty from an economic point of view.
Since the first RCT using PCB in AVF was conducted by Lai et al. [11], the debates on the benefits of PCB angioplasty for the treatment of dysfunctional AVF have never ceased. A number of meta-analyses evaluating the efficacy and safety of PCB angioplasty versus CB angioplasty for the treatment of dysfunctional AVF have been published [41–43]. In line with our findings, a recent meta-analysis by Liao et al demonstrated no significant improvement of TLPP rates in the PCB group, either for that at 6 months (RR 0.75, 95% CI 0.56–1.01, p = 0.06) or 12 months (RR 0.89, 95% CI 0.79–1.00, p = 0.06) [41]. Similar results have also been reported by Abdul Salim et al and Lazarides et al. [42,43]. In comparison to the meta-analyses described above, the strength of this paper lies in the incorporation of the most recent studies with large sample sizes into the final meta-analysis, making the results more persuasive.
Obvious heterogeneity existed in the included literature. We noticed that there was one study that obviously deviated from the axis of symmetry in the forest graph, which might have a great influence on heterogeneity [21]. Bjorkman et al suggested that the target lesion revascularization-free survival after the PCB angioplasty was clearly worse with 1-year follow-up, which was opposite to other studies [21]. In order to find out the source of heterogeneity, sensitivity analyses were performed by omitting one study at a time. The results of sensitivity analyses showed that there were no significant changes in the overall effect measures, indicating the results were relatively reliable.
In extension, we performed subgroup analyses based on paclitaxel dose, PCB type and inflation time. Of interest, the 6-month outcomes of the endovascular invention utilizing PCBs for dysfunctional AVFs were linked with the doses of paclitaxel delivered to vessels. We found that standard-dose PCBs (3.0 μg/mm2 and 3.5 μg/mm2) were significantly more effective compared with CBs in improving 6-month TLPP rates, while there were no significant differences between low-dose PCBs (2.0 μg/mm2) and CBs at 6-month TLPP rates. Katsanos and colleagues, pooling data from eleven RCTs, showed that standard-dose PCBs (3.0 μg/mm2 and 3.5 μg/mm2) were superior to low-dose PCBs (2.0 μg/mm2) in reducing the rates of restenosis and target lesion revascularization (TLR) in the femoropopliteal artery, a finding that was generally consistent with the conclusions of our meta-analysis [44]. For these, the current study recommended that priority should be given to standard-dose PCBs (3.0 μg/mm2 and 3.5 μg/mm2) in the treatment of dysfunctional AVFs.
Meanwhile, we also found that APERTO (Cardionovum) balloons significantly outperformed CBs in terms of 6-month TLPP, whereas the Lutonix 035 (Bard), IN.PACT Admiral (Medtronic) and SeQuent Please (B.Braun) balloons did not improve 6-month TLPP rates compared with CBs. And Passeo-18 Lux (Biotronik) balloons were favored over CBs in terms of 12-month TLPP, whereas the Lutonix 035 (Bard), IN.PACT Admiral (Medtronic) and SeQuent Please (B.Braun) balloons did not improve 12-month TLPP rates compared with CBs. We speculated that this may be explained by the different doses of drug delivered to vessels.
Another interesting finding was that 6- and 12-month TLPP rates were significantly higher in the group with the PCB inflation time ≥120 s compared with the controls. However, no significant differences were detected between the group with the PCB inflation time <120 s and the control group. In agreement with this study, Rhee and colleagues also reported that fully optimized PCB angioplasty with prolonged inflation time plays an important role in reducing target lesion failure after PCB angioplasty [45].
Although all of the enrolled studies were RCTs, allowing our findings to be reliable, several limitations should be acknowledged. Firstly, because of the macroscopic differences between PCBs and CBs, investigators cannot be unaware of the treatment assignment. None of the included RCTs was double-blinded, ineluctably increasing the risk of bias. Secondly, heterogeneity existed in the included literature. Sensitivity analyses and subgroup analyses according to PCB type, paclitaxel dose and inflation time were conducted to find the source of heterogeneity, compensating for this deficiency to some extent. However, the subgroup analysis based on AVF age was not performed in this meta-analysis. TLPP has been shown to have a positive correlation with AVF age in a previous study by Irani et al [28]. Thirdly, not all included articles reported the six outcomes and the data used for meta-analysis were incomplete, despite our efforts to contact authors of the included studies. Fourthly, all follow-ups were clinically driven. That is to say, not all patients have undergone ultrasound examinations before endovascular interventions, therefore causing a potential bias. Fifthly, unpublished results were not available, which inevitably produced publication bias. Finally, it should be acknowledged that the present study was not registered, with the possibility of a small offset. But it has to be pointed out that our meta-analysis was conducted strictly in compliance with the process of a systematic review.
In conclusion, this meta-analysis showed that there was insufficient evidence to support the distinct superiority of PCB angioplasty over CB angioplasty in the treatment of dysfunctional AVF. Due to the heterogeneity across studies, the findings from our study should be dealt with with some caution, although sensitivity analyses and subgroup analyses were performed to compensate for this deficiency to some extent. Thus, more multi-center, large-scale and well-designed RCTs are required to confirm our conclusions in the future.
Acknowledgments
Not applicable.
Appendix 1. The table that illustrates search terms
| PubMed | |
|---|---|
| ((((((((("Arteriovenous Fistula"[Mesh]) OR (((((((((((((((((((((fistula, arteriovenous[Title/Abstract]) OR (fistulas, arteriovenous[Title/Abstract])) OR (aneurysm, arteriovenous[Title/Abstract])) OR (anastomosis arteriovenosa[Title/Abstract])) OR (arterial venous anastomosis[Title/Abstract])) OR (arterio venous anastomosis[Title/Abstract])) OR (arterio venous aneurysm[Title/Abstract])) OR (arterio-venous fistula[Title/Abstract])) OR (arterio-venous fistulae[Title/Abstract])) OR (arterio-venous fistulas[Title/Abstract])) OR (arteriovenous anastomosis[Title/Abstract])) OR (arteriovenous aneurysm[Title/Abstract])) OR (arteriovenous crossing[Title/Abstract])) OR (arteriovenous fistulas[Title/Abstract])) OR (arteriovenous fistulae[Title/Abstract])) OR (artery vein fistula[Title/Abstract])) OR (av anastomosis[Title/Abstract])) OR (av aneurysm[Title/Abstract])) OR (AV fistula[Title/Abstract])) OR (AV fistulae[Title/Abstract])) OR (AV fistulas[Title/Abstract]))) OR (arteriovenous access[Title/Abstract])) OR (hemodialysis fistulas[Title/Abstract])) OR (hemodialysis access[Title/Abstract])) OR (dialysis fistulas[Title/Abstract])) OR (dialysis access[Title/Abstract])) OR (dialysis fistula[Title/Abstract])) OR (dialysis fistulae[Title/Abstract])) AND ((("Paclitaxel"[Mesh]) OR (((((((((((((((((((((((((((((((((((((((((((((((((((((((((((7-epi-taxol[Title/Abstract]) OR (7 epi taxol[Title/Abstract]))) OR (abi 007[Title/Abstract])) OR (abi007[Title/Abstract])) OR (abraxane[Title/Abstract])) OR (albumin bound paclitaxel[Title/Abstract])) OR (albumin-bound paclitaxel[Title/Abstract])) OR (anzatax[Title/Abstract])) OR (apealea[Title/Abstract])) OR (asotax[Title/Abstract])) OR (biotax[Title/Abstract])) OR (bms 181339[Title/Abstract])) OR (bms181339[Title/Abstract])) OR (bmy 45622[Title/Abstract])) OR (bmy45622[Title/Abstract])) OR (bris taxol[Title/Abstract])) OR (bristaxol[Title/Abstract])) OR (britaxol[Title/Abstract])) OR (coroxane[Title/Abstract])) OR (dts 301[Title/Abstract])) OR (dts301[Title/Abstract])) OR (endotag-1[Title/Abstract])) OR (formoxol[Title/Abstract])) OR (genexol[Title/Abstract])) OR (genexol pm[Title/Abstract])) OR (hunxol[Title/Abstract])) OR (ifaxol[Title/Abstract])) OR (infinnium[Title/Abstract])) OR (intaxel[Title/Abstract])) OR (mbt 0206[Title/Abstract])) OR (mbt0206[Title/Abstract])) OR (medixel[Title/Abstract])) OR (mitotax[Title/Abstract])) OR (nab paclitaxel[Title/Abstract])) OR (nanoparticle albumin bound paclitaxel[Title/Abstract])) OR (nsc-125973[Title/Abstract])) OR (nsc 125973[Title/Abstract])) OR (nsc 673089[Title/Abstract])) OR (nsc125973[Title/Abstract])) OR (nsc673089[Title/Abstract])) OR (oas pac 100[Title/Abstract])) OR (oaspac100[Title/Abstract])) OR (oncogel[Title/Abstract])) OR (onxol[Title/Abstract])) OR (paclitaxel, (4 alpha)-Isomer[Title/Abstract])) OR (pacitaxel[Title/Abstract])) OR (paclitaxel nab[Title/Abstract])) OR (pacxel[Title/Abstract])) OR (padexol[Title/Abstract])) OR (parexel[Title/Abstract])) OR (paxceed[Title/Abstract])) OR (paxene[Title/Abstract])) OR (paxus[Title/Abstract])) OR (pazenir[Title/Abstract])) OR (praxel[Title/Abstract])) OR (sb 05 (terpenoid)[Title/Abstract])) OR (sb05 (terpenoid)[Title/Abstract])) OR (taxocris[Title/Abstract]))) OR ((((((((((((drug eluting balloons[Title/Abstract]) OR (drug-eluting balloon[Title/Abstract])) OR (balloon, drug-eluting[Title/Abstract])) OR (balloons, drug-eluting[Title/Abstract])) OR (balloons, drug eluting[Title/Abstract])) OR (drug-coated balloons[Title/Abstract])) OR (drug coated balloons[Title/Abstract])) OR (drug-coated balloon[Title/Abstract])) OR (balloon, drug-coated[Title/Abstract])) OR (balloons, drug-coated[Title/Abstract])) OR (balloons, drug coated[Title/Abstract])) OR (Passeo-18 Lux[Title/Abstract]))) | |
| Web of Science | |
| #1 | TS=(Arteriovenous Fistula OR fistula, arteriovenous OR fistulas, arteriovenous OR aneurysm, arteriovenous OR anastomosis arteriovenosa OR arterial venous anastomosis OR arterio venous anastomosis OR arterio venous aneurysm OR arterio-venous fistula OR arterio-venous fistulae OR arterio-venous fistulas OR arteriovenous anastomosis OR arteriovenous aneurysm OR arteriovenous crossing OR arteriovenous fistulas OR arteriovenous fistulae OR artery vein fistula OR av anastomosis OR av aneurysm OR AV fistula OR AV fistulae OR AV fistulas OR arteriovenous access OR hemodialysis fistulas OR hemodialysis access OR dialysis fistulas OR dialysis access OR dialysis fistula OR dialysis fistulae) |
| #2 | TS=(drug eluting balloons OR drug-eluting balloon OR balloon, drug-eluting OR balloons, drug-eluting OR balloons, drug eluting OR drug-coated balloons OR drug coated balloons OR drug-coated balloon OR balloon, drug-coated OR balloons, drug-coated OR balloons, drug coated OR Passeo-18 Lux) |
| #3 | TS=(Paclitaxel OR 7-epi-taxol OR 7 epi taxol OR abi 007 OR abi007 OR abraxane OR albumin bound paclitaxel OR albumin-bound paclitaxel OR anzatax OR apealea OR asotax OR biotax OR bms 181339 OR bms181339 OR bmy 45622 OR bmy45622 OR bris taxol OR bristaxol OR britaxol OR coroxane OR dts 301 OR dts301 OR endotag-1 OR formoxol OR genexol OR genexol pm OR hunxol OR ifaxol OR infinnium OR intaxel OR mbt 0206 OR mbt0206 OR medixel OR mitotax OR nab paclitaxel OR nanoparticle albumin bound paclitaxel OR nsc-125973 OR nsc 125973 OR nsc 673089 OR nsc125973 OR nsc673089 OR oas pac 100 OR oaspac100 OR oncogel OR onxol OR paclitaxel, (4 alpha)-Isomer OR pacitaxel OR paclitaxel nab OR pacxel OR padexol OR parexel OR paxceed OR paxene OR paxus OR pazenir OR praxel OR sb 05 (terpenoid) OR sb05 (terpenoid) OR taxocris OR taxol OR taxol A OR taxol, bris OR taxus (drug) OR taycovit OR yewtaxan) |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 |
| Embase | |
| #1 | 'arteriovenous fistula'/exp |
| #2 | 'fistula, arteriovenous':ab,ti OR 'fistulas, arteriovenous':ab,ti OR 'aneurysm, arteriovenous':ab,ti OR 'anastomosis arteriovenosa':ab,ti OR 'arterial venous anastomosis':ab,ti OR 'arterio venous anastomosis':ab,ti OR 'arterio venous aneurysm':ab,ti OR 'arterio-venous fistula':ab,ti OR 'arterio-venous fistulae':ab,ti OR 'arterio-venous fistulas':ab,ti OR 'arteriovenous anastomosis':ab,ti OR 'arteriovenous aneurysm':ab,ti OR 'arteriovenous crossing':ab,ti OR 'arteriovenous fistulas':ab,ti OR 'arteriovenous fistulae':ab,ti OR 'artery vein fistula':ab,ti OR 'av anastomosis':ab,ti OR 'av aneurysm':ab,ti OR 'av fistula':ab,ti OR 'av fistulae':ab,ti OR 'av fistulas':ab,ti OR 'arteriovenous access':ab,ti OR 'hemodialysis fistulas':ab,ti OR 'hemodialysis access':ab,ti OR 'dialysis fistulas':ab,ti OR 'dialysis access':ab,ti OR 'dialysis fistula':ab,ti OR 'dialysis fistulae':ab,ti |
| #3 | #1 OR #2 |
| #4 | 'drug-coated balloon'/exp |
| #5 | 'drug eluting balloons':ab,ti OR 'drug-eluting balloon':ab,ti OR 'balloon, drug-eluting':ab,ti OR 'balloons, drug-eluting':ab,ti OR 'balloons, drug eluting':ab,ti OR 'drug-coated balloons':ab,ti OR 'drug coated balloons':ab,ti OR 'balloon, drug-coated':ab,ti OR 'balloons, drug-coated':ab,ti OR 'balloons, drug coated':ab,ti OR 'passeo-18 lux':ab,ti |
| #6 | #4 OR #5 |
| #7 | 'paclitaxel'/exp |
| #8 | '7-epi-taxol':ab,ti OR '7 epi taxol':ab,ti OR 'abi 007':ab,ti OR 'abi007':ab,ti OR 'abraxane':ab,ti OR 'albumin bound paclitaxel':ab,ti OR 'albumin-bound paclitaxel':ab,ti OR 'anzatax':ab,ti OR 'apealea':ab,ti OR 'asotax':ab,ti OR 'biotax':ab,ti OR 'bms 181339':ab,ti OR 'bms181339':ab,ti OR 'bmy 45622':ab,ti OR 'bmy45622':ab,ti OR 'bris taxol':ab,ti OR 'bristaxol':ab,ti OR 'britaxol':ab,ti OR 'coroxane':ab,ti OR 'dts 301':ab,ti OR 'dts301':ab,ti OR 'endotag-1′:ab,ti OR 'formoxol':ab,ti OR 'genexol':ab,ti OR 'genexol pm':ab,ti OR 'hunxol':ab,ti OR 'ifaxol':ab,ti OR 'infinnium':ab,ti OR 'intaxel':ab,ti OR 'mbt 0206':ab,ti OR 'mbt0206':ab,ti OR 'medixel':ab,ti OR 'mitotax':ab,ti OR 'nab paclitaxel':ab,ti OR 'nanoparticle albumin bound paclitaxel':ab,ti OR 'nsc-125973':ab,ti OR 'nsc 125973':ab,ti OR 'nsc 673089':ab,ti OR 'nsc125973':ab,ti OR 'nsc673089':ab,ti OR 'oas pac 100':ab,ti OR 'oaspac100':ab,ti OR 'oncogel':ab,ti OR 'onxol':ab,ti OR 'paclitaxel, (4 alpha)-isomer':ab,ti OR 'pacitaxel':ab,ti OR 'paclitaxel nab':ab,ti OR 'pacxel':ab,ti OR 'padexol':ab,ti OR 'parexel':ab,ti OR 'paxceed':ab,ti OR 'paxene':ab,ti OR 'paxus':ab,ti OR 'pazenir':ab,ti OR 'praxel':ab,ti OR 'sb 05 (terpenoid)':ab,ti OR 'sb05 (terpenoid)':ab,ti OR 'taxocris':ab,ti OR 'taxol':ab,ti OR 'taxol a':ab,ti OR 'taxol, bris':ab,ti OR 'taxus (drug)':ab,ti OR 'taycovit':ab,ti OR 'yewtaxan':ab,ti |
| #9 | #7 OR #8 |
| #10 | #6 OR #9 |
| #11 | #3 AND #10 |
| Cochrane Library | |
| #1 | (Arteriovenous Fistula):ti,ab,kw OR (fistula, arteriovenous):ti,ab,kw OR (fistulas, arteriovenous):ti,ab,kw OR (aneurysm, arteriovenous):ti,ab,kw OR (anastomosis arteriovenosa):ti,ab,kw OR (arterial venous anastomosis):ti,ab,kw OR (arterio venous anastomosis):ti,ab,kw OR (arterio venous aneurysm):ti,ab,kw OR (arterio-venous fistula):ti,ab,kw OR (arterio-venous fistulae):ti,ab,kw OR (arterio-venous fistulas):ti,ab,kw OR (arteriovenous anastomosis):ti,ab,kw OR (arteriovenous aneurysm):ti,ab,kw OR (arteriovenous crossing):ti,ab,kw OR (arteriovenous fistulas):ti,ab,kw OR (arteriovenous fistulae):ti,ab,kw OR (artery vein fistula):ti,ab,kw OR (av anastomosis):ti,ab,kw OR (av aneurysm):ti,ab,kw OR (AV fistula):ti,ab,kw OR (AV fistulae):ti,ab,kw OR (AV fistulas):ti,ab,kw OR (arteriovenous access):ti,ab,kw OR (hemodialysis fistulas):ti,ab,kw OR (hemodialysis access):ti,ab,kw OR (dialysis fistulas):ti,ab,kw OR (dialysis access):ti,ab,kw OR (dialysis fistula):ti,ab,kw OR (dialysis fistulae):ti,ab,kw |
| #2 | (drug eluting balloons):ti,ab,kw OR (drug-eluting balloon):ti,ab,kw OR (balloon, drug-eluting):ti,ab,kw OR (balloons, drug-eluting):ti,ab,kw OR (balloons, drug eluting):ti,ab,kw OR (drug-coated balloons):ti,ab,kw OR (drug coated balloons):ti,ab,kw OR (drug-coated balloon):ti,ab,kw OR (balloon, drug-coated):ti,ab,kw OR (balloons, drug-coated):ti,ab,kw OR (balloons, drug coated):ti,ab,kw OR (Passeo-18 Lux):ti,ab,kw |
| #3 | (Paclitaxel):ti,ab,kw OR (7 epi taxol):ti,ab,kw OR (abi 007):ti,ab,kw OR (abi007):ti,ab,kw OR (abraxane):ti,ab,kw OR (albumin bound paclitaxel):ti,ab,kw OR (albumin-bound paclitaxel):ti,ab,kw OR (anzatax):ti,ab,kw OR (apealea):ti,ab,kw OR (asotax):ti,ab,kw OR (biotax):ti,ab,kw OR (bms 181339):ti,ab,kw OR (bms181339):ti,ab,kw OR (bmy 45622):ti,ab,kw OR (bmy45622):ti,ab,kw OR (bris taxol):ti,ab,kw OR (bristaxol):ti,ab,kw OR (britaxol):ti,ab,kw OR (coroxane):ti,ab,kw OR (dts 301):ti,ab,kw OR (dts301):ti,ab,kw OR (endotag-1):ti,ab,kw OR (formoxol):ti,ab,kw OR (genexol):ti,ab,kw OR (genexol pm):ti,ab,kw OR (hunxol):ti,ab,kw OR (ifaxol):ti,ab,kw OR (infinnium):ti,ab,kw OR (intaxel):ti,ab,kw OR (mbt 0206):ti,ab,kw OR (mbt0206):ti,ab,kw OR (medixel):ti,ab,kw OR (mitotax):ti,ab,kw OR (nab paclitaxel):ti,ab,kw OR (nanoparticle albumin bound paclitaxel):ti,ab,kw OR (nsc-125973):ti,ab,kw OR (nsc 125973):ti,ab,kw OR (nsc 673089):ti,ab,kw OR (nsc125973):ti,ab,kw OR (nsc673089):ti,ab,kw OR (oas pac 100):ti,ab,kw OR (oaspac100):ti,ab,kw OR (oncogel):ti,ab,kw OR (onxol):ti,ab,kw OR (pacitaxel):ti,ab,kw OR (paclitaxel nab):ti,ab,kw OR (pacxel):ti,ab,kw OR (padexol):ti,ab,kw OR (parexel):ti,ab,kw OR (paxceed):ti,ab,kw OR (paxene):ti,ab,kw OR (paxus):ti,ab,kw OR (pazenir):ti,ab,kw OR (praxel):ti,ab,kw OR (taxocris):ti,ab,kw OR (taxol):ti,ab,kw OR (taxol A):ti,ab,kw OR (taxol, bris):ti,ab,kw OR (taycovit):ti,ab,kw OR (yewtaxan):ti,ab,kw |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 |
Appendix 2.
Figure A1.
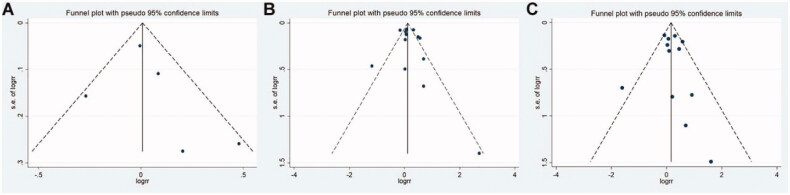
Funnel plot of TLPP at 3 months (A), 6 months (B) and 12 months (C). TLPP: target lesion primary patency.
Figure A2.
Sensitivity analysis of TLPP at 6 months. TLPP: target lesion primary patency; CI: confidence interval.
Figure A3.
Funnel plot of technical success rate.
Figure A4.
Sensitivity analysis of TLPP at 12 months. TLPP: target lesion primary patency; CI: confidence interval.
Funding Statement
This work was supported by the Construction of Key Projects by Zhejiang Provincial Ministry under Grant number WKJ-ZJ-2017, the Zhejiang Province Chinese Medicine Modernization Program under Grant number 2020ZX001, the Key Project of Scientific Research Foundation of Chinese Medicine under Grant number 2022ZZ002 and the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China under Grant number LHDMZ22H050001.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
Not applicable.
References
- 1.Kim DH, Park JI, Lee JP, et al. The effects of vascular access types on the survival and quality of life and depression in the incident hemodialysis patients. Ren Fail. 2020;42(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Kruska L.. Future directions for vascular access for hemodialysis. Semin Dial. 2015;28(2):107–113. [DOI] [PubMed] [Google Scholar]
- 3.Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362(6):494–503. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Nadkarni GN, Yacoub R, et al. Comparison of cutting balloon angioplasty and percutaneous balloon angioplasty of arteriovenous fistula stenosis: a meta-analysis and systematic review of randomized clinical trials. J Interv Cardiol. 2015;28(3):288–295. [DOI] [PubMed] [Google Scholar]
- 5.Rajan DK, Platzker T, Lok CE, et al. Ultrahigh-pressure versus high-pressure angioplasty for treatment of venous anastomotic stenosis in hemodialysis grafts: is there a difference in patency? J Vasc Interv Radiol. 2007;18(6):709–714. [DOI] [PubMed] [Google Scholar]
- 6.Yerasi C, Case BC, Forrestal BJ, et al. Drug-coated balloon for de novo coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(9):1061–1073. [DOI] [PubMed] [Google Scholar]
- 7.Manzi M, Cester G, Palena LM.. Paclitaxel-coated balloon angioplasty for lower extremity revascularization: a new way to fight in-stent restenosis. J Cardiovasc Surg. 2010;51(4):567–571. [PubMed] [Google Scholar]
- 8.Karmota AG. Paclitaxel coated-balloon (PCB) versus standard plain old balloon (POB) fistuloplasty for failing dialysis access. Ann R Coll Surg Engl. 2020;102(8):601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitrou PM, Katsanos K, Spiliopoulos S, et al. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial (NCT01174472). Eur J Radiol. 2015;84(3):418–423. [DOI] [PubMed] [Google Scholar]
- 10.Kitrou PM, Spiliopoulos S, Katsanos K, et al. Paclitaxel-coated versus plain balloon angioplasty for dysfunctional arteriovenous fistulae: one-year results of a prospective randomized controlled trial. J Vasc Interv Radiol. 2015;26(3):348–354. [DOI] [PubMed] [Google Scholar]
- 11.Lai CC, Fang HC, Tseng CJ, et al. Percutaneous angioplasty using a paclitaxel-coated balloon improves target lesion restenosis on inflow lesions of autogenous radiocephalic fistulas: a pilot study. J Vasc Interv Radiol. 2014;25(4):535–541. [DOI] [PubMed] [Google Scholar]
- 12.Lookstein RA, Haruguchi H, Ouriel K, et al. Drug-Coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med. 2020;383(8):733–742. [DOI] [PubMed] [Google Scholar]
- 13.Swinnen JJ, Hitos K, Kairaitis L, et al. Multicentre, randomised, blinded, control trial of drug-eluting balloon vs sham in recurrent native dialysis fistula stenoses. J Vasc Access. 2019;20(3):260–269. [DOI] [PubMed] [Google Scholar]
- 14.Therasse E, Caty V, Gilbert P, et al. Safety and efficacy of paclitaxel-eluting balloon angioplasty for dysfunctional hemodialysis access: a randomized trial comparing with angioplasty alone. J Vasc Interv Radiol. 2021;32(3):350–359. [DOI] [PubMed] [Google Scholar]
- 15.Trerotola SO, Saad TF, Roy-Chaudhury P.. The lutonix AV randomized trial of paclitaxel-coated balloons in arteriovenous fistula stenosis: 2-Year results and subgroup analysis. J Vasc Interv Radiol. 2020;31(1):1–14.e5. [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, Shi Y, Cui T, et al. Efficacy and safety of paclitaxel-coated balloon angioplasty for dysfunctional arteriovenous fistulas: a multicenter randomized controlled trial. Am J Kidney Dis. 2021;78(1):19–27. [DOI] [PubMed] [Google Scholar]
- 17.Arabi M, Salman R, Alharbi A, et al. Paclitaxel-coated balloons compared to plain balloon angioplasty in the management of dysfunctional arteriovenous fistulae: a single-center randomized clinical trial. Saudi J Kidney Dis Transpl. 2021;32(1):118–127. [DOI] [PubMed] [Google Scholar]
- 18.Karunanithy N, Robinson EJ, Ahmad F, et al. A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit for arteriovenous fistulas. Kidney Int. 2021;100(2):447–456. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Kim JH, Byun SS, et al. Paclitaxel-coated balloon versus plain balloon angioplasty for dysfunctional autogenous radiocephalic arteriovenous fistulas: a prospective randomized controlled trial. Korean J Radiol. 2020;21(11):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maleux G, Mijnsbrugge WV, Henroteaux D, et al. Multicenter, randomized trial of conventional balloon angioplasty versus paclitaxel-coated balloon angioplasty for the treatment of dysfunctioning autologous dialysis fistulae. J Vasc Interv Radiol. 2018;29(4):470–475.e3. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkman P, Weselius EM, Kokkonen T, et al. Drug-Coated versus plain balloon angioplasty in arteriovenous fistulas: a randomized, controlled study with 1-Year Follow-Up (the drecorest Ii-Study). Scand J Surg. 2019;108(1):61–66. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ, Sacks D, Martin LG, et al. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol. 2003;14(9 Pt 2):S433–S442. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali MA, Abdulrahaman MA, Hamza M.. Comparative study between drug-coated balloon angioplasty vs plain balloon angioplasty in management of venous stenosis in hemodialysis access circuit. Egypt J Surg. 2020;39(3):603–612. [Google Scholar]
- 26.Bjorkman P, Weselius EM, Venermo M.. No difference in mid-term and long-term mortality after vascular paclitaxel exposure. Ann Vasc Surg. 2021;72:253–260. [DOI] [PubMed] [Google Scholar]
- 27.Eldmarany HA, Khalefa SA.. El bahaey A. Drug-coated balloon angioplasty for failing arteriovenous fistulae: feasibility and short-term outcomes. Egypt J Surg. 2020;39(4):932–938. [Google Scholar]
- 28.Irani FG, Teo T, Tay KH, et al. Hemodialysis arteriovenous fistula and graft stenoses: randomized trial comparing drug-eluting balloon angioplasty with conventional angioplasty. Radiology. 2018;289(1):238–247. [DOI] [PubMed] [Google Scholar]
- 29.Karnabatidis D, Kitrou PM, Ponce P, et al. A multicenter global registry of paclitaxel drug-coated balloon in dysfunctional arteriovenous fistulae and grafts: 6-month results. J Vasc Interv Radiol. 2021;32(3):360–368. [DOI] [PubMed] [Google Scholar]
- 30.Katsanos K, Karnabatidis D, Kitrou P, et al. Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. J Endovasc Ther. 2012;19(2):263–272. [DOI] [PubMed] [Google Scholar]
- 31.Moreno-Sanchez T, Moreno-Ramirez M, Machancoses FH, et al. Efficacy of paclitaxel balloon for hemodialysis stenosis fistulae after one year compared to high-pressure balloons: a controlled, multicenter, randomized trial. Cardiovasc Intervent Radiol. 2020;43(3):382–390. [DOI] [PubMed] [Google Scholar]
- 32.Pang S, Au-Yeung K, Liu G, et al. Randomized controlled trial for paclitaxel-coated balloon versus plain balloon angioplasty in dysfunctional hemodialysis vascular access: 12-month outcome from a nonsponsored trial. Ann Vasc Surg. 2021;72:299–306. [DOI] [PubMed] [Google Scholar]
- 33.Patane D, Failla G, Coniglio G, et al. Treatment of juxta-anastomotic stenoses for failing distal radiocephalic arteriovenous fistulas: drug-coated balloons versus angioplasty. J Vasc Access. 2019;20(2):209–216. [DOI] [PubMed] [Google Scholar]
- 34.Rai A, Sobhiyeh M.. Comparison of the efficacy of using paclitaxel-eluting balloon and plain balloon angioplasty for arteriovenous fistula in hemodialysis patients. Biomed Res Ther. 2019;6(5):3151–3155. [Google Scholar]
- 35.Roosen LJ, Karamermer Y, Vos JA, et al. Paclitaxel-coated balloons do not prevent recurrent stenosis in hemodialysis access fistulae: results of a randomized clinical trial. Ital J Vasc Endovasc Surg. 2017;24(2):35–40. [Google Scholar]
- 36.Teo T, Tan BS, Yin W, et al. Prospective randomized trial comparing drug-eluting balloon versus conventional percutaneous transluminal angioplasty (DEBAPTA) for the treatment of hemodialysis arteriovenous fistula or arteriovenous graft stenoses – interim report of first 30 patients. J Vasc Interv Radiol. 2013;24(4):S40–S41. [Google Scholar]
- 37.Trerotola SO, Lawson J, Roy-Chaudhury P, et al. Drug coated balloon angioplasty in failing AV fistulas: a randomized controlled trial. Clin J Am Soc Nephrol. 2018;13(8):1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbeeck N, Pillet J, Toukouki A, et al. Paclitaxel-coated balloon angioplasty of venous stenoses in native dialysis fistulas: primary and secondary patencies at 6 and 12 months. J Belg Soc Radiol. 2016;100(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz I. The efficacy of paclitaxel drug-eluting balloon angioplasty versus standard balloon angioplasty in stenosis of native hemodialysis arteriovenous fistulas: an analysis of clinical success, primary patency and risk factors for recurrent dysfunction. Cardiovasc Intervent Radiol. 2019;42(5):685–692. [DOI] [PubMed] [Google Scholar]
- 40.Diehm N, Schneider H.. Cost-effectiveness analysis of paclitaxel-coated balloons for endovascular therapy of femoropopliteal arterial obstructions. J Endovasc Ther. 2013;20(6):819–825. [DOI] [PubMed] [Google Scholar]
- 41.Liao MT, Chen MK, Hsieh MY, et al. Drug-coated balloon versus conventional balloon angioplasty of hemodialysis arteriovenous fistula or graft: a systematic review and meta-analysis of randomized controlled trials. PLOS One. 2020;15(4):e231463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul Salim S, Tran H, Thongprayoon C, et al. Comparison of drug-coated balloon angioplasty versus conventional angioplasty for arteriovenous fistula stenosis: systematic review and meta-analysis. J Vasc Access. 2020;21(3):357–365. [DOI] [PubMed] [Google Scholar]
- 43.Lazarides MK, Christaina E, Antoniou GA, et al. Plain versus paclitaxel-coated balloon angioplasty in arteriovenous fistula and graft stenosis: an umbrella review. J Vasc Access. 2021;00(0):112972982110052. [DOI] [PubMed] [Google Scholar]
- 44.Katsanos K, Spiliopoulos S, Paraskevopoulos I, et al. Systematic review and meta-analysis of randomized controlled trials of paclitaxel-coated balloon angioplasty in the femoropopliteal arteries: role of paclitaxel dose and bioavailability. J Endovasc Ther. 2016;23(2):356–370. [DOI] [PubMed] [Google Scholar]
- 45.Rhee TM, Lee JM, Shin ES, et al. Impact of optimized procedure-related factors in drug-eluting balloon angioplasty for treatment of in-Stent restenosis. JACC Cardiovasc Interv. 2018;11(10):969–978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.