Abstract
Remote interventions are increasingly utilized in transplant medicine but have rarely been rigorously evaluated. We investigated a remote intervention targeting immunosuppressant management in pediatric lung transplant recipients. Patients were recruited from a larger multisite trial if they had a Medication Level Variability Index (MLVI) ≥ 2.0, indicating worrisome tacrolimus level fluctuation. The manualized intervention included 3 weekly phone calls and regular follow-up calls. A comparison group included patients who met enrollment criteria after the sub-protocol ended. Outcomes were defined before the intent-to-treat analysis. Feasibility was defined as ≥ 50% of participants completing the weekly calls. MLVI was compared pre- and 180 days post-enrollment and between intervention and comparison groups. Of 18 eligible patients, 15 enrolled. Seven additional patients served as the comparison. Seventy-five percent of participants completed ≥ 3 weekly calls; average time on protocol was 257.7 days. Average intervention group MLVI was significantly lower (indicating improved blood level stability) at 180 days post-enrollment (2.9 ± 1.29) compared to pre-enrollment (4.6 ± 2.10), p=0.02. At 180 days, MLVI decreased by 1.6 points in the intervention group, but increased by 0.6 in the comparison group (p=0.054). Participants successfully engaged in a long-term remote intervention, and their medication blood levels stabilized. NCT02266888.
1. Introduction
Remote communications (e.g., telephone, video, or text messaging) can improve access to medical services.1 More recently, clinicians and institutions have turned to remote communication to minimize contagion during the COVID-19 pandemic.2
Remote contacts may have particular appeal in the long-term management of transplant recipients. First, reducing exposure to pathogens is especially important in immunosuppressed individuals,3 and indeed, transplant programs have rapidly turned to telehealth in the context of COVID-19.4–6 Second, many transplant recipients travel extensively for their care,7,8 and remote technology may reduce this burden. Third, immunosuppressant nonadherence remains the leading cause of preventable graft failure,9–15 and frequent remote encounters could facilitate adherence. But, there are challenges. Remote interventions may be less effective at communicating with patients than in-person encounters, confidentiality must be maintained, and some patients may not have the means or comfort with technology to participate.16,17
Key components of a robust evaluation of telehealth interventions include assessing pre-defined outcomes in prospective multisite trials.18 Yet, despite its promise, there is a dearth of such investigations into telehealth in pediatric populations.19–21 Additionally, telehealth interventions must maintain patient contact over time. This is particularly important for pediatric transplant recipients whose immunosuppression regimens may last a full lifetime, but research points toward potential challenges sustaining such engagment.22
Over the last decade, we developed a remote telemetric intervention targeting psychological (in particular, avoidance behaviors associated with transplant-related posttraumatic stress) and other barriers to pediatric patients’ engagement in post-transplant care. The intervention manual was piloted over multiple rounds of iterative development, application, and modification (e.g. NCT01960322, NCT02320422)23–26; the focus on avoidance is based on extensive work showing that avoidance could lead to nonadherence,23 is prevalent,25,27–29 and can be addressed effectively and safely in vulnerable patients.23,30
To select patients with potential risk for nonadherence, we use the Medication Level Variability Index (MLVI), the standard deviation (SD) of consecutive outpatient immunosuppressant (tacrolimus) blood levels.31–33 Tacrolimus blood level fluctuation as a marker of post-transplant risk was first described about 20 years ago23,31 and has been shown to predict rejection and other adverse outcomes in pediatric and adult organ transplant recipients: liver33–35, kidney36, lung37, heart38, bone marrow39, and mixed populations.40 Most research describes a linear relationship between MLVI and poor outcomes (higher MLVI is correlated with higher risk, for example, of rejection). A few larger trials calculated a threshold beyond which there seems to be a “step up” in risk,33,34 but risk may continue to increase even beyond this threshold.
We investigated the feasibility of this remote intervention strategy as a sub-protocol of the NIAID-sponsored Clinical Trials in Organ Transplantation in Children consortium (CTOTC-08; B Cell Induction in Pediatric Lung Transplantation, clinicaltrials.gov NCT02266888), which examined rituximab use in pediatric lung transplant recipients. Our primary hypothesis was that a remote intervention targeting nonadherence in pediatric transplant recipients whose MLVI is at or above the identified risk threshold would be feasible as demonstrated by at least 50% of participants and families completing the three core intervention calls. A secondary aim was to evaluate whether MLVI decreased post-intervention.
2. Methods
2.1. Participants
Participants in the active and the comparison groups were drawn from CTOTC-08, a collaboration of 7 pediatric lung transplant centers across the U.S: Children’s Hospital Boston, Cincinnati Children’s Hospital, Children’s Hospital of Philadelphia, Nationwide Children’s Hospital, Stanford University, Texas Children’s Hospital, and Washington University in St. Louis. The first participant was enrolled in the remote intervention sub-protocol in December 2015; enrollment ended in April 2018. We aimed to recruit at least 15 subjects, with an average follow-up of 3 months. Sample size was determined based on our estimate of potential recruitment in the parent study.
Eligible participants for CTOTC-08 (the parent protocol) were candidates for a lung transplant who were less than 21 years old. In addition, eligible participants for the sub-protocol must have received a transplant, have had at least three outpatient tacrolimus levels drawn at least three months posttransplant (effectively recruiting patients close to 6 months posttransplant or longer), speak either English or Spanish, and have an MLVI ≥ 2.0, indicating substantial variability in medication levels over time.32,33
In determining the threshold for intervention eligibility in the present trial, investigators drew on analyses from the MALT (Medication Adherence in pediatric Liver Transplant recipients) trial.33 MALT determined that a “step up” in risk occurs at a threshold of 2.0. Lacking robust data for lung recipients, we selected the MALT study33 MLVI threshold, knowing that this might be a conservative threshold, to ensure patients who might benefit from the intervention were not excluded, while accepting the possibility that some who received it may not need it.
See ClinicalTrials.gov (NCT02266888) for inclusion / exclusion criteria of the parent study.
2.2. Design and Procedures
The study was approved by the Icahn School of Medicine at Mount Sinai’s Institutional Review Board (IRB ID: STUDY-16–01340-CR001) and by the review boards of each enrolling site/center. Patients in CTOTC-08 were eligible for the sub-protocol if they had an MLVI ≥ 2.0 as determined from at least three consecutive outpatient tacrolimus blood levels. A written consent was obtained from a parent/guardian and assent was obtained from the patient at the site (not by the interventionist) prior to enrollment in the parent study. Additionally, re-confirmation of consent and assent was obtained upon enrollment in the remote intervention sub-protocol. The intervention was administered to patients as a supplement to standard care and not in place of it.
2.3. Remote Intervention
2.3.1. Call frequency and duration.
Participants completed three weekly “protocol calls” followed by regular follow-up calls. These calls could be completed across a longer period of time if the interventionist was unable to establish immediate contact with the participant. The frequency of subsequent follow-up calls was established collaboratively between the interventionist and patient based on the patient’s preferences; they typically occurred every four to eight weeks for as long as the sub-protocol was active (up to 2.5 years), or until the participant asked not to be called. The protocol calls generally lasted approximately 30 to 45 minutes, and follow-up calls typically lasted about 5 to 10 minutes. The calls were generally held between the patient and interventionist; parents were also involved in at least a portion of the calls, depending on the age and identified needs of the patient.
2.3.2. Call content.
A manual described the required content of each call and was used to assess fidelity to the intervention. During the first call, participants were asked to provide information about themselves, their transplant, and their medication regimens, and identify potential barriers to adherence to the post-transplant regimen. In the second protocol call, the patient was introduced to the relationship between avoidance (reluctance to take the medications due to distress when thinking about it) and nonadherence,41,42 and the patient’s own possible avoidance reactions were explored. Barriers to adherence were again assessed, and problem-solving strategies were discussed. During the third call, barriers were addressed. Subsequent follow-up calls included the same general content as the third call. All calls were recorded. A sample of the recorded sessions were reviewed by a supervisor; recordings were destroyed following review. Interventionists were advanced-level PhD clinical psychology candidates who received training in the use of the manual. A random sample of 15% of the tapes were rated for adherence to the manual, with a requirement of 80% or more adherence (using a checklist).
2.4. Outcome Measures
The primary outcome measure was feasibility, assessed by the percentage of patients who followed study procedures (completed at least the three protocol calls over a period of at least three weeks). We pre-defined “successful engagement” as ability to engage at least 50% of the participants and their families in at least the three protocol sessions delivered over the course of engagement. Exploratory feasibility outcome measures included the number of consenting versus declining patients, the total amount of time on protocol, and attrition. Acceptability was assessed through a review of patients’/caregivers’ responses to targeted questions about their experiences with the intervention, done by the interventionist, typically during the last call.
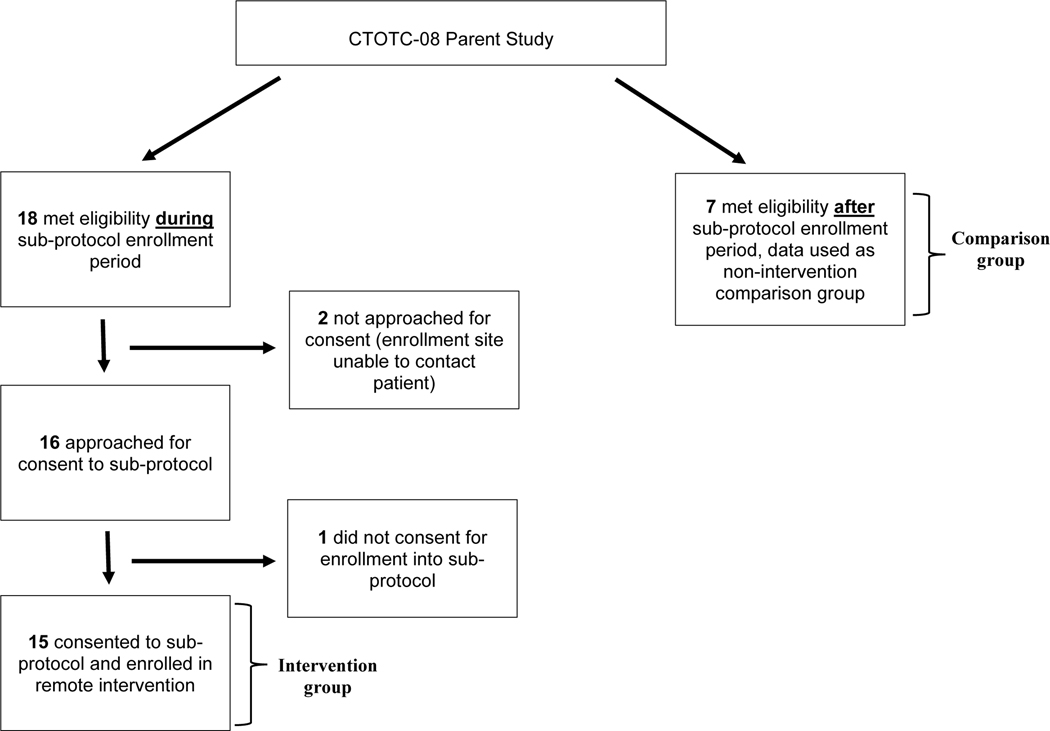
Another secondary outcome measure was MLVI in patients who did and did not participate in the intervention. We have identified two groups: those who met enrollment criteria and participated (“intervention group”) and those who would have met criteria, were enrolled in the same parent study, and came from the same centers, but were not approached because the intervention sub-protocol had ended already (comparison group; Figure 1). For the intervention group, we calculated mean MLVI before enrollment, 180 days post-enrollment, and 180 days after the final intervention session. The comparison group had only 180 days of information after meeting enrollment criteria. Outcome measures, comparison timeframe, and analytic strategies were defined prior to data lock. Change in MLVI over time was examined within the intervention group, the comparison group, and between these two groups.
Figure 1. Participant recruitment flowchart.
MLVI was calculated as the SD of at least three consecutive outpatient tacrolimus trough blood levels, obtained as part of routine care at the site, for the period of interest. Extensive prior research33 established that adjustments for “non-trough” levels or timing of blood tests are not necessary, possibly because a non-trough level or more or less frequent testing (as compared with usual practice) may indicate nonadherence in and of themselves. Similarly, we previously observed that dose changes, absorption, and metabolic nuances were not associated with MLVI33; therefore, adjustments to MLVI calculations based on these factors were not attempted in the current protocol.
2.5. Statistical analysis
Descriptive statistics are presented to assess feasibility. Within the intervention group, paired t-tests were used to 1) compare MLVI pre-enrollment to MLVI 180 days post-enrollment and 2) compare MLVI pre-enrollment to MLVI 180 days after the last intervention session, in an intent-to-treat paradigm. Independent samples t-tests were used to compare change in MLVI from pre-enrollment to 180 days post-enrollment for the intervention group to change in MLVI from the time patients met eligibility criteria to 180 days later for the comparison group. An alpha level (p-value) of 0.05 or less was selected as the level of statistical significance. All statistical analyses were performed using IBM SPSS Statistics package, 25th edition, and SAS v9.4 (SAS Institute, Cary, NC). Acceptability was examined qualitatively by reviewing feedback obtained by participants and/or their caregivers.
3. Results
3.1. Demographic Information
Twenty-seven participants were enrolled in the parent study; 15 enrolled in the sub-protocol intervention and 7 were post-hoc controls who met sub-protocol criteria after the sub-protocol reached its expected recruitment target. Baseline characteristics of the intervention (n=15) and control (n=7) cohorts are presented in Table 1 and participant ages are included in Table 2. Remote intervention participants were predominantly female (n=10, 66.7%) and white (n=11, 73.3%). On average, participants were 13.3 years old (± 4.40) at transplant. For the comparison group, average age at transplant was 12.3 years (± 2.43); the majority identified as male (n=5, 71.4%), and white (n=6, 85.7%). Differences were not statistically significant (Table 1).
Table 1.
Sample Demographic Information
| Enrolled in intervention (n=15) | Never flagged for intervention (n=7) | p-valueb | |
|---|---|---|---|
|
| |||
| Age (years) at transplant | 0.590 | ||
| Mean (SD) | 13.3 (4.40) | 12.3 (2.43) | |
| Median | 14 | 12 | |
| Min, max | 2, 20 | 9, 15 | |
| Gender (n, %) | 0.172 | ||
| Male | 5 (33) | 5 (71) | |
| Female | 10 (67) | 2 (29) | |
| Race (n, %) | 0.805 | ||
| White | 11 (73) | 6 (86) | |
| Black or African American | 1 (7) | 0 | |
| More than one race | 1 (7) | 0 | |
| Unknown/Not Reported | 2 (13) | 1 (14) | |
| Ethnicity (n, %) | 0.455 | ||
| Hispanic or Latino | 3 (20) | 2 (29) | |
| Not Hispanic or Latino | 9 (60) | 5 (71) | |
| Unknown/Not Reported | 3 (20) | 0 | |
| Months from transplanta | 0.158 | ||
| Mean (SD) | 7.5 (3.09) | 5.4 (3.19) | |
| Median | 6.9 | 4.0 | |
| Min, max | 3.7, 12.9 | 3.4, 12.3 | |
Time to enrollment in intervention presented for the 15 subjects enrolled; time to initial MLVI ≥ 2.0 presented for the 7 subjects never flagged for the intervention.
P-value results from T-test for continuous variables and Fisher’s Exact test or Cochran-Mantel-Haenszel test for categorical variables.
Table 2.
Participant Feedback Summary
| Age (Yrs)a | Feedback – Caregiver | Feedback – Participant | Positive Y/N | Negative Y/N |
|---|---|---|---|---|
|
| ||||
| 20 | N/A | N/A | N/A | N/A |
| 11 | N/A | Liked the calls | Y | N |
| 15 | N/A | Liked the calls, did not find them disruptive to schedule. | Y | N |
| 18 | N/A | N/A | N/A | N/A |
| 17 | N/A | Did not mind talking on phone, liked speaking with another person in healthcare field. Felt able to independently manage medication, but believed calls would be helpful for others. | Y | N |
| 15 | Once weekly calls were too frequent for child who does not like talking on the phone. | N/A | N | Y |
| 15 | Content of calls was developmentally appropriate. Appreciated flexibility of calls. | Liked flexibility of call scheduling. Calls were not a burden to complete. | Y | N |
| 17 | N/A | N/A | N/A | N/A |
| 10 | Calls important in engaging the participant, helpful in facilitating communication with clinical team. | Does not like to speak on the phone, “nothing personal.” | Y – Care-giver | Y – subject |
| 16 | Liked how patient took ownership of progress during the calls and that patient learned how to talk about medical condition with others. Appreciative of flexibility with call scheduling. | Liked calls and having someone other than family checking in at home. | Y | N |
| 13 | N/A | N/A | N/A | N/A |
| 3 | Appreciated the support. Speaking of avoidance reaction helped to understand those feelings, address them, and improve care of patient. Appreciated problem-solving barriers to adherence and working on strategies to improve medication administration. | N/A | Y | N |
| 9 | N/A | N/A | N/A | N/A |
| 15 | Appreciated opportunity to learn more about patient’s thoughts and feelings regarding transplant. | Helpful to speak about experience with someone knowledgeable about transplantation. | Y | N |
| 16b | N/A | Liked the calls, could be helpful. | Y | N |
Age at time of enrollment;
Patient was not able to be reached for an exit interview. However, they provided feedback about the intervention during non-exit interview calls; therefore, their comments are included in this table.
3.2. Fidelity to the manual
Interventionists displayed greater than 90% fidelity to the manual; there was no need for remedial actions.
3.3. Feasibility
3.3.1. Consenting vs. declining.
Eighteen patients were possibly eligible during the enrollment period. Of those, 2 were not approached because the enrollment site was not able to contact them. Of the 16 approached patients, 15 consented (93.8% enrollment rate; Figure 1).
3.3.2. Percentage of participants following study procedures.
The majority of intervention participants (n=12, 75%) completed all three calls. One patient, who was enrolled early in the trial, met inclusion criteria twice in the span of several years and was enrolled in the study twice (the patient completed the three weekly calls once); we did not incorporate a second set of MLVIs or patient feedback into the analyses because of concern about repeated-measures correlation that would complicate the interpretation of the tests.
3.3.3. Attrition.
Most intervention participants (n=11, 73.3%) continued to be called until the intervention study ended, long after the initial weekly calls transitioned to follow-ups. Three participants opted to stop the phone calls prior to the end of the sub-protocol but allowed continued collection of their clinical data. One participant stopped answering the calls, which were discontinued.
3.3.4. Time on protocol.
Participants were enrolled in the intervention for an average of 257.7 (±146.3) days. Total possible participation time varied based on enrollment date. Eleven of the 15 intervention participants continued the calls until the intervention study ended, receiving them for the maximum amount of time possible.
3.4. Acceptability
Feedback, obtained for 10 of the 15 enrolled participants, is detailed in Table 2. Four participants had feedback from both the participant and the caregiver, 4 had feedback from the participant only, and 2 had feedback from the caregiver only. Overall, participants commented that they found the calls helpful and liked speaking with another person about their transplant experiences.
Two responses included negative feedback about the intervention. One respondent, the caregiver of a participant who chose to end the calls early, expressed that once weekly calls were too frequent for the child who did not like speaking on the phone. The other participant who provided negative feedback similarly described discomfort with speaking on the phone. Feedback sessions were not conducted with 3 participants who opted to end the phone calls early.
3.5. MLVI
3.5.1. Change in MLVI for intervention group.
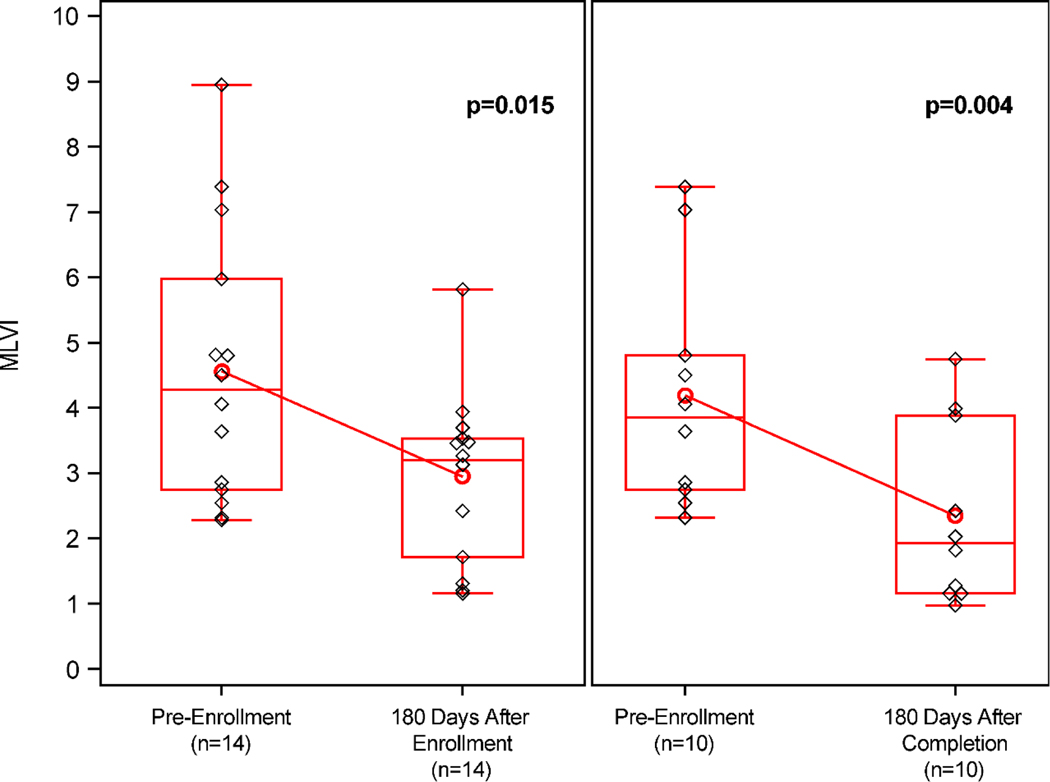
Within the intervention group (Figure 2), MLVI was significantly lower (−1.62; 95% CI: −2.87, −0.37; p=0.02) 180 days post-enrollment (2.9 ± 1.29) compared to pre-enrollment (4.6 ± 2.10) for all participants for whom we had complete MLVI data (n=14). For those participants with data available 180 days after completion of their last intervention session (n=10), MLVI was significantly lower (−1.84; 95% CI: −2.95, −0.74; p=0.004) after this time period (2.3 ± 1.38) compared to pre-enrollment (4.2 ± 1.80).
Figure 2. Change in MLVI pre-enrollment, 180 days post-enrollment, and 180 days after final session.
Paired analysis of change in MLVI from pre-enrollment to 1) 180 days post-enrollment (n=14, p=0.015) and 2) 180 days after final session (n=10, p=0.004). Boxes represent interquartile ranges. Whiskers extend to minimum/maximum values. Red horizontal lines represent group medians; red circles represent group means. Black diamonds indicate individual subject MLVIs.
3.5.2. Change in MLVI in the comparison group.
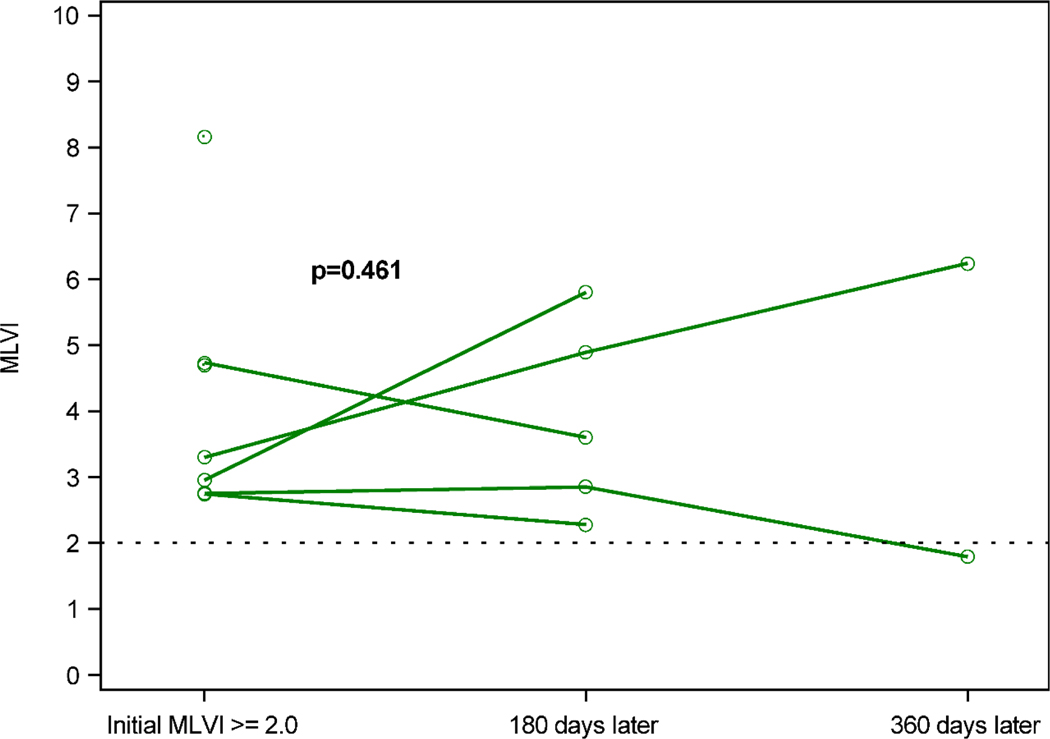
Figure 3 shows the progress of MLVIs over time for the patients who met eligibility after the enrollment period ended and, therefore, did not receive the intervention (n=7). For the 5 patients for whom complete MLVI data for the 180-day benchmark was available, there was no significant difference (0.59; 95% CI: −1.42, 2.60; p=0.46) in MLVIs 180 days after meeting eligibility criteria (3.9 ± 1.45) compared to when eligibility criteria were met (3.3 ± 0.84).
Figure 3. Change in MLVI for eligible patients flagged after enrollment ended (the comparison group).
Comparison subjects’ MLVI over time. Green circles represent MLVI when initial MLVI ≥ 2.0 and 180 and 360 days later. Data for individual subjects (n=5) are connected by a line. MLVI did not differ when criteria were met (3.3 ± 0.84) compared to 180 days after (3.9 ± 1.45), p=0.461.
3.5.3. Change in MLVI in intervention vs. comparison group.
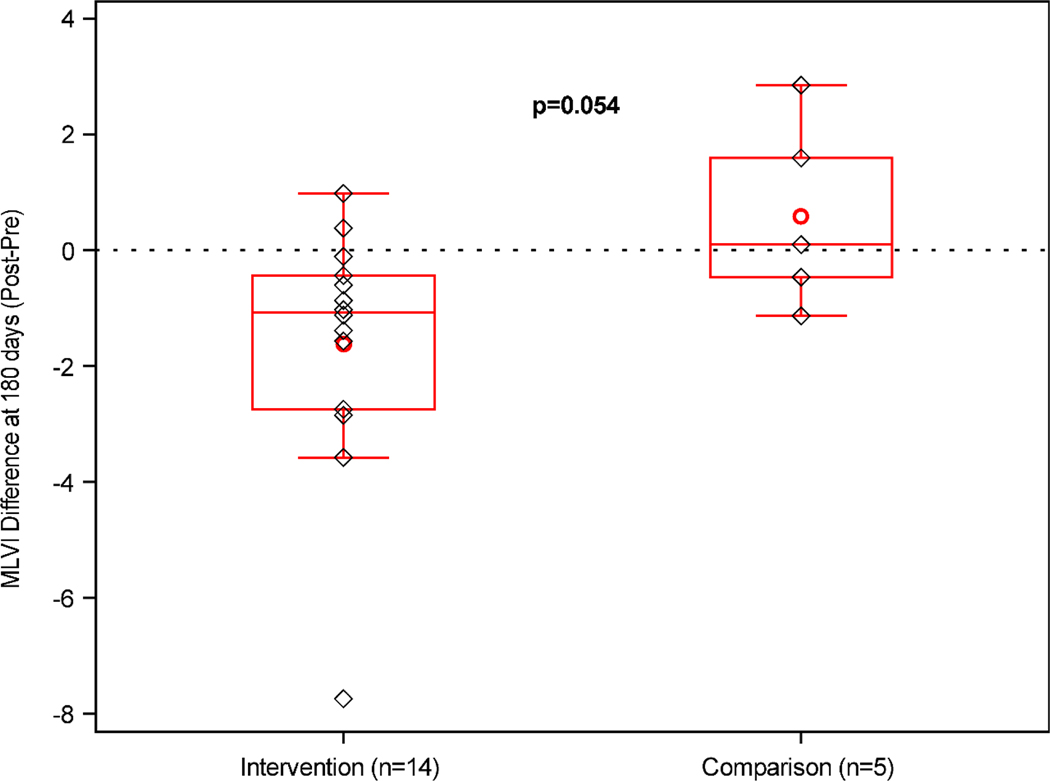
On average, MLVI decreased from pre-enrollment to 180 days post-enrollment by 1.6 points (± 2.16) for the intervention group, but increased by 0.6 points (± 1.62) 180 days after meeting eligibility criteria for the comparison group. The difference between those groups reached borderline significance (−2.21; 95% CI: −4.46, 0.04; p=0.054, Figure 4).
Figure 4. Comparison of change in MLVI between intervention participants and patients eligible after enrollment ended (the comparison group).
Average change in MLVI at the 180-day benchmark in the intervention (n=14, −1.6 ± 2.16) versus comparison (n=5, 0.6 ± 1.62) group. Boxes represent interquartile ranges. Whiskers extend to minimum/maximum values. Red horizontal lines represent group medians; red circles represent group means. Black diamonds indicate individual subject change in MLVI.
4. Discussion
This multisite prospective study using pre-defined outcome measures and a comparison group was designed to investigate long-term outcomes of a remote intervention protocol. We found it is possible to successfully engage patients and/or their parents, even though they had not met the interventionist in person. Almost all eligible patients consented to participate, three-quarters of the participants completed the baseline number of calls, and the majority continued the calls through the end of the sub-protocol. Observed MLVI improvement within the intervention, but not the comparison, group suggests that the intervention was not only feasible and acceptable, but also appeared to stabilize medication blood levels over time. We also surmise that, if no intervention occurs, blood level fluctuation does not decrease or may worsen, which is consistent with prior observations.32,33 These findings are especially salient nowadays, as the pandemic has fueled increased interest in telehealth interventions.43,44
Evidence for the feasibility and acceptability of this intervention, along with the potential impact on clinical outcomes, is particularly encouraging given the limited evidence available concerning the effectiveness of remote interventions, even beyond the transplant field. To illustrate: a recent review of pediatric telehealth45 cited five references,46–50 none of which compared medical outcomes over time with controls or considered engagement beyond the initial period. In pediatric oncology, a systematic review of 21 digital health interventions identified mixed evidence for effectiveness, but these studies suffered from several methodological limitations.51 A recently published pilot study52 investigating a remote monitoring intervention in adult lung transplant recipients determined they could be engaged long term (i.e., enter monitoring data at least weekly for approximately two years). Survival rates did not differ between the monitored and control groups, but in a retrospective review, the intervention was associated with lower hospital readmissions and hospital costs. Our current findings complement those important insights and show that successful long-term engagement and improved clinical outcomes through telemedicine is possible in pediatrics, in a prospective study with pre-defined outcomes.
Our manual emphasized addressing avoidance behaviors and providing problem-solving tools. While the intervention was grounded in an established theoretical framework and substantial background information from pilot trials, we cannot definitively conclude which, if any, specific components were necessary. Only dismantling studies could inform such a determination, and such studies are beyond this consortium’s mandate. It is possible that, as intended, the intervention addressed avoidance behaviors in patients and/or their caregivers, decreased distress, and increased medication adherence. Alternatively, it is possible that establishing a relationship with an interventionist encouraged positive behavior change – indeed, such a mechanism would be consistent with psychotherapy research that underscores the therapeutic relationship as a central determinant of outcomes.53 Or, the fact that the calls were placed, whether or not they were answered, may have served as a strong reminder of the importance of medication taking. Given the small sample size and study design, we are unable to test which of these hypothesized mechanisms explains the MLVI stabilization in the intervention group. However, an alternative explanation that the intervention substantially altered sites’ efforts is unlikely, as there was no manual-driven interaction component between interventionists and sites except in cases of emergency.
We found that routine follow-up with patients (and/or their parents) who measured excessive fluctuation in immunosuppressant blood levels is possible even when the interventionist is not known to the patient, is not a medical expert in transplantation, and is not a member of the treatment team. It is possible that strict adherence to our specific manual is not necessary, and operationalization of our approach might include following our methods but not necessarily our detailed manual: monitoring MLVI (via an electronic medical record algorithm) and implementing a “regimen” of enhanced calls focused on adherence and management of barriers to those patients who measure wide fluctuation in drug levels until such fluctuation is reduced. Such an approach would minimize the need for additional resources by targeting only vulnerable patients (rather than providing support to all patients indiscriminately) and could employ clinicians who are not necessarily trained psychologists.
4.1. Strengths and Limitations
The strengths of this study are its prospective, multisite design, pre-defined robust outcome measures, and long term follow up – elements embraced by the National Institutes of Health’s expert conference on telemedicine research.18
A caveat is that participants were drawn from a group of patients already participating in another study (CTOTC-08). It is conceivable that such patients are more likely to participate in our intervention as compared with transplant patients more broadly. However, given the different aims of the primary study (i.e., examining the effects of B cell targeted induction therapy on survival) versus those of our sub-protocol, this may not have been an important contributor to our high enrollment rate, though the overall sample size was small. A second limitation is that feedback sessions were not available for most of the participants who chose to end the calls early, who may have reported some negative feedback. Third, we did not standardize care across sites, including the frequency of contact with patients. Fourth, we acknowledge that the comparison group, which offered us an unanticipated opportunity to look at the trajectory of MLVI in the absence of an intervention, was defined post-hoc. It consisted of CTOTC-08 patients who met inclusion criteria after the intervention ended, not during the same time that the intervention was delivered, and the allocation was not through randomization.
The participants in our study demonstrated more stable tacrolimus levels over time and research shows that more consistent levels are associated with fewer rejection episodes.23,31,33–38,40 The present study, however, was not powered to evaluate how more stable drug levels affected the incident rates of rejection or mortality. Only larger trials would be better powered to investigate whether our approach reduces post-transplant rejection.
Future Directions
Our study is the first controlled trial showing that MLVI is a modifiable risk factor that is responsive to behavioral intervention. It is unclear whether a variability of 2.0 is the most appropriate threshold at which to administer the intervention. It is possible that different thresholds may be optimal for different kinds of transplants – or even that the optimal threshold for intervention varies somewhat between different programs or recipients. For example, consistent with many biological variables, transplant outcomes are associated with patient demographics,54 and the threshold beyond which there is a “step up” in risk may vary by these factors, or transplant site. Since our study commenced, research in heart transplant suggests 3.0 may be the optimal threshold for that population.38 In clinical applications of our surveillance and intervention paradigm, it is possible that a higher threshold (e.g., 3.0) in the lung transplant setting would be sufficiently inclusive. Additional long-term cohort trials are needed to discern the threshold appropriate for specific transplant settings, but those are costly and lengthy. Until specific results are available, we believe it is prudent to leave the decision about acceptable thresholds to individual centers who are more familiar with their unique population and setting of care.
Additionally, in the current study, parents were involved in the calls based on interventionists’ clinical judgment. While we believe this flexible approach is beneficial, decisions about mandatory versus optional parental involvement could be based on standardized measures (e.g., the REFILS,55 which assesses allocation of responsibility for illness management behaviors).
4.2. Conclusions
A remote intervention paradigm was successful in engaging pediatric patients in addressing aspects of post-transplant care, in particular consistent medication-taking behavior, over time. As compared with standard care controls, the intervention group had more consistent medication blood levels. We conclude that it is possible to engage pediatric patients in remote interventions and that the MLVI is a modifiable risk factor, impacted by a behavioral intervention. Our approach could be a novel addition to existing strategies that transplant programs can employ to reduce organ loss.
Acknowledgements/Funding
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children, a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases. The work was supported by Grant U01AI077810 “B Cell Targeted Induction to Improve Outcomes in Pediatric Lung Transplantation” (PI: Lara Danziger-Isakov and Stuart Sweet) and UM2AI117870 (Rho) from the Division of Allergy, Immunology and Transplantation of the National Institutes of Health, NIDDK award R01DK080740 (PI: Eyal Shemesh), and NICHD award F31HD096946 (PI: Sarah Duncan-Park).
Abbreviations:
- CTOTC
Clinical Trials in Organ Transplantation in Children consortium
- MLVI
Medication Level Variability Index
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data availability statement
De-identified clinical and mechanistic data will be made available through ImmPort (https://www.immport.org), a NIH, NIAID, and DAIT funded data repository.
References
- 1.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154–161. doi: 10.1056/NEJMra1601705 [DOI] [PubMed] [Google Scholar]
- 2.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020. doi: 10.1056/NEJMp2003539 [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928 [DOI] [PubMed] [Google Scholar]
- 4.Yadav A, Caldararo K, Singh P. Optimising the use of telemedicine in a kidney transplant programme during the coronavirus disease 2019 pandemic. J Telemed Telecare. 2020. doi: 10.1177/1357633X20942632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JH, Diop M, Burgos YL, et al. Telehealth in outpatient management of kidney transplant recipients during COVID-19 pandemic in New York. Clin Transplant. 2020;00:e14097. doi: 10.1111/ctr.14097 [DOI] [PubMed] [Google Scholar]
- 6.Santos-Parker KS, Santos-Parker JR, Highet A, et al. Practice change amidst the COVID-19 pandemic: harnessing the momentum for expanding telehealth in transplant. Clin Transplant. 2020;34(7):e13897. doi: 10.1111/ctr.13897 [DOI] [PubMed] [Google Scholar]
- 7.Schucht J, Davis EG, Jones CM, Cannon RM. Does distance to transplant center affect post kidney transplant readmission rates? Am Surg. 2019;85(8):834–839. [PubMed] [Google Scholar]
- 8.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276–2288. doi: 10.2215/CJN.04940610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertram A, Fuge J, Suhling H, et al. Adherence is associated with a favorable outcome after lung transplantation. PLoS One. 2019;14(12):e0226167. doi: 10.1371/journal.pone.0226167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Hear Lung Transplant. 1999;18(6):549–562. doi: 10.1016/S1053-2498(98)00044-8 [DOI] [PubMed] [Google Scholar]
- 11.Didlake RH, Dreyfus K, Kerman RH, Van Buren CT, Kahan BD. Patient noncompliance: a major cause of late graft failure in cyclosporine-treated renal transplants. Transpl Proc. 1988;20(3 Suppl 3):63–69. [PubMed] [Google Scholar]
- 12.Shneider C, Dunphy C, Shemesh E, Annunziato RA. Assessment and treatment of nonadherence in transplant recipients. Gastroenterol Clin North Am. 2018;47(4):939–948. doi: 10.1016/j.gtc.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Shemesh E, Shneider BL, Emre S. Adherence to medical recommendations in pediatric transplant recipients: time for action. Pediatr Transplant. 2008;12(3):281–283. doi: 10.1111/j.1399-3046.2008.00920.x [DOI] [PubMed] [Google Scholar]
- 14.Yazigi NA. Adherence and the pediatric transplant patient. Semin Pediatr Surg. 2017;26(4):267–271. doi: 10.1053/j.sempedsurg.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 15.Chisholm-Burns MA, Spivey CA. The “cost” of medication nonadherence: consequences we cannot afford to accept. J Am Pharm Assoc. 2012;52(6):823–826. doi: 10.1331/JAPhA.2012.11088 [DOI] [PubMed] [Google Scholar]
- 16.Burke BL, Hall RW, the Section on Telehealth Care. Telemedicine: pediatric applications. Pediatrics. 2015;136(1):e293–308. doi: 10.1542/peds.2015-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brophy PD. Overview on the challenges and benefits of using telehealth tools in a pediatric population. Adv Chronic Kidney Dis. 2017;24(1):17–21. doi: 10.1053/j.ackd.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 18.Puskin DS, Cohen Z, Ferguson AS, Krupinski E, Spaulding R. Implementation and evaluation of telehealth tools and technologies. Telemed e-Health. 2010;16(1):96–102. doi: 10.1089/tmj.2009.0182 [DOI] [PubMed] [Google Scholar]
- 19.Bian J, Cristaldi KK, Summer AP, et al. Association of a school-based, asthma-focused telehealth program with emergency department visits among children enrolled in South Carolina Medicaid. JAMA Pediatr. 2019;173(11):1041–1048. doi: 10.1001/jamapediatrics.2019.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson CA, McSwain SD, Curfman AL, Chuo J. The current pediatric telehealth landscape. Pediatrics. 2018;141(3):e20172334. doi: 10.1542/peds.2017-2334 [DOI] [PubMed] [Google Scholar]
- 21.Totten AM, Womack DM, Eden KB, et al. (Pacific Northwest Evidence-based Practice Center, Portland, OR). Telehealth: mapping the evidence for patient outcomes from systematic reviews. Technical Brief 26. Rockville, MD: Agency for Healthcare Research and Quality: 2016. 125 p. Report No.: 16-EHC034-EF. Contract No.: 290–2015-0. [PubMed] [Google Scholar]
- 22.Fleming JN, Taber DJ, McElligott J, McGillicuddy JW, Treiber F. Mobile health in solid organ transplant: the time is now. Am J Transplant. 2017;17(9):2263–2276. doi: 10.1111/ajt.14225 [DOI] [PubMed] [Google Scholar]
- 23.Shemesh E, Lurie S, Stuber ML, et al. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics. 2000;105(2):e29. doi: 10.1542/peds.105.2.e29 [DOI] [PubMed] [Google Scholar]
- 24.Shemesh E, Annunziato RA, Shneider BL, et al. Improving adherence to medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):316–323. doi: 10.1111/j.1399-3046.2007.00791.x [DOI] [PubMed] [Google Scholar]
- 25.Shemesh E, Rudnick A, Kaluski E, et al. A prospective study of posttraumatic stress symptoms and nonadherence in survivors of a myocardial infarction (MI). Gen Hosp Psychiatry. 2001;23(4):215–222. doi: 10.1016/S0163-8343(01)00150-5 [DOI] [PubMed] [Google Scholar]
- 26.Shemesh E, Koren-Michowitz M, Yehuda R, et al. Symptoms of posttraumatic stress disorder in patients who have had a myocardial infarction. Psychosomatics. 2006;47(3):231–239. doi: 10.1176/appi.psy.47.3.231 [DOI] [PubMed] [Google Scholar]
- 27.Annunziato RA, Rubinstein D, Murgueitio M, et al. Psychiatric symptom presentation in ethnically diverse cardiology patients. Ethn Dis. 2009;19:271–275. [PubMed] [Google Scholar]
- 28.Arnaboldi P, Lucchiari C, Santoro L, Sangalli C, Luini A, Pravettoni G. PTSD symptoms as a consequence of breast cancer diagnosis: clinical implications. Springerplus. 2014;3(1):e392. doi: 10.1186/2193-1801-3-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23(3):409–448. doi: 10.1016/S0272-7358(03)00031-X [DOI] [PubMed] [Google Scholar]
- 30.Shemesh E, Annunziato RA, Weatherley BD, et al. A randomized controlled trial of the safety and promise of cognitive-behavioral therapy using imaginal exposure in patients with posttraumatic stress disorder resulting from cardiovascular illness. J Clin Psychiatry. 2011;72(2):168–174. doi: 10.4088/JCP.09m05116blu [DOI] [PubMed] [Google Scholar]
- 31.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010;14(8):940–943. doi: 10.1111/j.1399-3046.2010.01396.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shemesh E, Duncan S, Anand R, et al. Trajectory of adherence behavior in pediatric and adolescent liver transplant recipients: The Medication Adherence in Children who had a Liver Transplant cohort. Liver Transplant. 2018;24(1):80–88. doi: 10.1002/lt.24837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shemesh E, Bucuvalas JC, Anand R, et al. The Medication Level Variability Index (MLVI) predicts poor liver transplant outcomes: a prospective multi-site study. Am J Transplant. 2017;17(10):2668–2678. doi: 10.1111/ajt.14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supelana C, Annunziato RA, Schiano TD, et al. Medication Level Variability Index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transplant. 2014;20:1168–1177. doi: 10.1002/lt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieber SR, Helcer J, Leven E, et al. Pretransplant psychosocial risk factors may not predict late nonadherence and graft rejection in adult liver transplant recipients. Exp Clin Transplant. 2018;16(5):533–540. doi: 10.6002/ect.2016.0349 [DOI] [PubMed] [Google Scholar]
- 36.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85(6):1404–1411. doi: 10.1038/ki.2013.465 [DOI] [PubMed] [Google Scholar]
- 37.Gallagher HM, Sarwar G, Tse T, et al. Erratic tacrolimus exposure, assessed using the standard deviation of trough blood levels, predicts chronic lung allograft dysfunction and survival. J Hear Lung Transplant. 2015;34(11):1442–1448. doi: 10.1016/j.healun.2015.05.028 [DOI] [PubMed] [Google Scholar]
- 38.Sirota M, Heyrend C, Ou Z, Masotti S, Griffiths E, Molina KM. Tacrolimus trough variability - predictors and outcomes in pediatric heart transplantation. J Hear Lung Transplant. 2020;39(4S):S206. doi: 10.1016/j.healun.2020.01.822 [DOI] [PubMed] [Google Scholar]
- 39.Skeens MA, Dietrich MS, Ryan-Wenger N, Gilmer MJ, Mulvaney SA, Akard TF. The Medication Level Variability Index (MLVI) as a potential predictive biomarker of graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Pediatr Transplant. 2019;23(5):e13451. doi: 10.1111/petr.13451 [DOI] [PubMed] [Google Scholar]
- 40.Pollock-BarZiv SM, Finkelstein Y, Manlhiot C, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14(8):968–975. doi: 10.1111/j.1399-3046.2010.01409.x [DOI] [PubMed] [Google Scholar]
- 41.Supelana C, Annunziato RA, Kaplan D, Helcer J, Stuber ML, Shemesh E. PTSD in solid organ transplant recipients: current understanding and future implications. Pediatr Transplant. 2016;20(1):23–33. doi: 10.1111/petr.12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taggart Wasson L, Shaffer JA, Edmondson D, et al. Posttraumatic stress disorder and nonadherence to medications prescribed for chronic medical conditions: a meta-analysis. J Psychiatr Res. 2018;102(July):102–109. doi: 10.1016/j.jpsychires.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Medical Association. Press Release: AMA supports telehealth initiative to improve health care access [Internet]. Chicago (IL): American Medical Association; 2020. Mar 19 [cited 2020 Apr 23]. Available from: https://www.ama-assn.org/press-center/press-releases/ama-supports-telehealth-initiative-improve-health-care-access [Google Scholar]
- 44.Portnoy J, Waller M, Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract. 2020;8(5):1489–1491. doi: 10.1016/j.jaip.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utidjian L, Abramson E. Pediatric telehealth: opportunities and challenges. Pediatr Clin North Am. 2016;63(2):367–378. doi: 10.1016/j.pcl.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 46.Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):e3. doi: 10.1542/peds.109.1.e3 [DOI] [PubMed] [Google Scholar]
- 47.Marcin JP, Ellis J, Mawis R, Nagrampa E, Nesbitt TS, Dimand RJ. Using telemedicine to provide pediatric subspecialty care to children with special health care needs in an underserved rural community. Pediatrics. 2004;113(1):1–6. doi: 10.1542/peds.113.1.1 [DOI] [PubMed] [Google Scholar]
- 48.Richter GM, Sun G, Lee TS, et al. Speed of telemedicine versus ophthalmoscopy for retinopathy of prematurity diagnosis. Am J Ophthalmol. 2009;148(1):136–149. doi: 10.1016/j.ajo.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reese RM, Jamison R, Wendland M, et al. Evaluating interactive videoconferencing for assessing symptoms of Autism. Telemed e-Health. 2013;19(9):671–677. doi: 10.1089/tmj.2012.0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fogel AL, Teng JMC. Pediatric teledermatology: a survey of usage, perspectives, and practice. Pediatr Dermatol. 2015;32(3):363–368. doi: 10.1111/pde.12533 [DOI] [PubMed] [Google Scholar]
- 51.Ramsey WA, Heidelberg RE, Gilbert AM, Heneghan MB, Badawy SM, Alberts NM. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psychooncology. 2020;29(1):17–37. doi: 10.1002/pon.5280 [DOI] [PubMed] [Google Scholar]
- 52.Schenkel FA, Barr ML, McCloskey CC, et al. Use of a Bluetooth tablet-based technology to improve outcomes in lung transplantation: a pilot study. Am J Transplant. 2020;20(12):3649–3657. doi: 10.1111/ajt.16154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert MJ, Barley DE. Research summary on the therapeutic relationship and psychotherapy outcome. Psychotherapy. 2001;38(4):357–361. doi: 10.1037/0033-3204.38.4.357 [DOI] [Google Scholar]
- 54.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant. 2020;20(6):1597–1605. doi: 10.1111/ajt.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annunziato RA, Bucuvalas JC, Yin W, et al. Self-management measurement and prediction of clinical outcomes in pediatric transplant. J Pediatr. 2018;193:128–133. doi: 10.1016/j.jpeds.2017.09.069 [DOI] [PMC free article] [PubMed] [Google Scholar]