Abstract
Klebsiella pneumoniae strains with the transferable carbapenem-hydrolyzing metallo-β-lactamases, which include IMP- and VIM-type enzymes, remain extremely rare. To investigate whether IMP- or VIM-producing K. pneumoniae isolates had spread at a university medical center in Taiwan, a total of 3,458 clinical isolates of K. pneumoniae consecutively collected in 1999 and 2000 were tested by the agar diffusion method, colony hybridization, PCR, and nucleotide sequencing. A total of 40 isolates (1.2%), or 17 nonrepetitive isolates, from 16 patients were found to carry blaIMP-8, a metallo-β-lactamase gene recently identified from a K. pneumoniae strain in Taiwan. Carriage of blaVIM or other blaIMP genes was detected in none of the remaining isolates. Of the 17 nonrepetitive blaIMP-8-positive isolates, 15 isolates (88.2%) appeared susceptible to imipenem (MICs, ≤4 μg/ml) and meropenem (MICs, ≤1 μg/ml), indicating the difficulty in detecting blaIMP-8 in K. pneumoniae by routine susceptibility tests; 14 isolates (82.4%) produced SHV-12 as well; and 14 isolates (82.4%) were also resistant to fluoroquinolones. The organisms caused wound infections in eight patients and bloodstream infections in three patients. They were not directly associated with the death of nine patients. Before the recovery of the blaIMP-8-positive isolates, all 16 patients had undergone various surgical procedures, and 15 patients had been admitted to the surgical intensive care unit, suggesting a nosocomial outbreak. Two major patterns were observed by pulsed-field gel electrophoresis for 14 of the 17 nonrepetitive isolates, indicating that the clonal spread was mainly responsible for the outbreak.
The emergence of acquired metallo-β-lactamases (MBLs) in gram-negative bacilli is becoming a therapeutic challenge because the enzymes usually possess a broad hydrolysis profile that includes carbapenems and extended-spectrum β-lactams (13). Two major groups of MBLs have been described: IMP- and VIM-type enzymes (13). IMP-1 was the first identified acquired MBL (17) and has spread among Enterobacteriaceae, Pseudomonas aeruginosa, and other nonfastidious gram-negative nonfermenters in Japan (8, 9, 22, 23). In the past three years, a number of acquired MBLs were identified in Europe (12, 19, 21) and the Far East (7, 10, 27, 28, 30). IMP-2 was identified from a clinical isolate of Acinetobacter baumannii in Italy (21). VIM-1 was identified from a clinical isolate of P. aeruginosa in Italy (12), and outbreaks of the VIM-1-producing P. aeruginosa isolates have been recognized in Greece (26) and Italy (4). VIM-2 was first identified from a clinical isolate of P. aeruginosa in France (19) and has been found in P. aeruginosa in Japan (N. Shibata, Y. Arakawa, H. Kurokawa, Y. Doi, and K. Shibayama, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. C-524, p. 273, 2001) and in Pseudomonas and Acinetobacter spp. in Korea recently (K. Lee, J. B. Lim, J. Yum, D. Yong, J. R. Choi, Y. Chong, J. M. Kim, and D. M. Livermore, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2003, p. 123, 2000). All acquired MBL genes found so far were inserted in integrons (1, 7, 10, 11, 12, 19, 21, 28, 30).
Both IMP- and VIM-type MBLs have been detected in Taiwan (27, 28). Both IMP-1 and VIM-2 were found in Pseudomonas putida and Pseudomonas stutzeri (27), and a variant of the VIM-2 enzyme, VIM-3, was found in P. aeruginosa (27). A variant of the IMP-2 enzyme, IMP-8, was identified from a clinical isolate of Klebsiella pneumoniae, which produced the SHV-12-type extended-spectrum β-lactamase (ESBL) and TEM-1 as well (28). Reports of MBL-producing K. pneumoniae isolates remain rare. In Japan, only one IMP-1-producing K. pneumoniae isolate was detected in two surveys of gram-negative bacilli (8, 23). Outside Japan, there was only one confirmed report of an IMP-1-producing K. pneumoniae isolate collected from Singapore (T. H. Koh, G. S. Babini, N. Woodford, L.-H. Sng, L. M. C. Hall, and D. M. Livermore, Letter, Lancet 353:2162, 1999). Since K. pneumoniae is notorious as a host of resistance plasmids and is one of the major causes of nosocomial infections (5), the present study was carried out in order to investigate the prevalence of K. pneumoniae producing IMP- or VIM-type MBLs in a university medical center in Taiwan. A nosocomial outbreak of K. pneumoniae carrying blaIMP-8 in the intensive care units (ICUs) was recognized, and thus a retrospective analysis of the cases from which the IMP-8-producing isolates were recovered was also conducted.
MATERIALS AND METHODS
Bacterial strains, clinical isolates and patients.
A total of 3,458 clinical isolates of K. pneumoniae were consecutively collected at the National Cheng Kung University Medical Center, a 900-bed university hospital in southern Taiwan, from January 1999 to December 2000. Of these isolates, 1,622 and 1,836 isolates were collected in 1999 and 2000, respectively. All these isolates were identified by using the conventional techniques (5) and/or the API 20E system (bioMérieux, Marcy l'Etoile, France). MBLs have been shown to confer resistance to ceftazidime and cephamycins; however, susceptibilities to carbapenems for MBL producers covered a wide range (4, 7, 8, 10, 12, 13, 17, 19, 21–23, 27, 28, 30). Thus, only the isolates that met the following criteria on the basis of the results of the disk diffusion method were selected for further experiments: reduced susceptibilities to imipenem (inhibition zone diameter, <16 mm) or meropenem (inhibition zone diameter, <16 mm), or resistance to both ceftazidime (inhibition zone diameter, ≤14 mm) and cefoxitin (inhibition zone diameter, ≤14 mm). The medical records of the patients with MBL-producing isolates were reviewed. The bacterial strains used as controls for colony hybridization and PCR included blaVIM-1-containing P. aeruginosa VR-143/97 (12), blaVIM-2-carrying P. putida NTU-91/99 (27), blaVIM-3-containing P. aeruginosa NTU-26/99 (27), blaIMP-1-carrying P. putida NTU-92/99 (27), blaIMP-2-containing A. baumannii AC-54/97 (21), and blaIMP-8-carrying K. pneumoniae KPO787 (28).
Colony blot hybridization.
Colony blot hybridization was performed as described elsewhere (6, 27). The DNA probes generated by PCR amplification of the entire blaVIM-1, blaVIM-2, blaIMP-1, and blaIMP-2 were prepared as described previously (27) and were labeled with [α-32P]dCTP (Amersham Pharmacia Biotech, Hong Kong, China) by the random priming technique with a commercial kit (Gibco BRL, Life Technologies, Gaithersburg, Md.). Since there are only two nucleotide differences between blaVIM-2 and blaVIM-3 (27) and four nucleotide differences between blaIMP-2 and blaIMP-8 (28), the VIM-3- and IMP-8-producing control strains can also be hybridized with the blaVIM-2- and blaIMP-2-specific probes, respectively.
PCR amplification and DNA sequencing.
Plasmids from clinical isolates were prepared by a rapid alkaline lysis procedure (24). PCR assays were performed to amplify the entire sequences of the blaIMP-1-, blaIMP-2-, blaVIM-1-, blaVIM-2-, blaSHV-, and blaTEM-related genes as described previously (27, 28). The amplicons were purified with PCR cleanup kits (Roche Molecular Biochemicals, Mannheim, Germany) and were sequenced on an ABI PRISM 310 sequencer analyzer (Applied Biosystems, Foster City, Calif.). The PCR and sequencing primers used were described elsewhere (27, 29). blaIMP-8 and blaVIM-3 from the control strains were successfully amplified with the primers for blaIMP-2 and blaVIM-2, respectively.
Transfer of resistance.
Conjugation experiments were performed as described previously (20, 29) with streptomycin- and rifampin-resistant Escherichia coli C600 as the recipient (2). Tryptic soy agar plates supplemented with 500 μg of streptomycin (Sigma Chemical Company, St. Louis, Mo.) per ml or 64 μg of rifampin (Sigma) per ml and 10 μg of ceftazidime (Glaxo Group Research Ltd., Greenford, United Kingdom) per ml were used to select transconjugants. Plasmids from E. coli transconjugants were digested with EcoRI (Roche Molecular Biochemicals). Digested DNA samples were analyzed by electrophoresis on 0.8% agarose gels. The gels were stained with ethidium bromide (Sigma), and plasmid bands were visualized under UV light. The plasmid sizes of transconjugants were estimated by adding up restriction fragments.
Analytical IEF.
Crude preparations of β-lactamases were obtained by sonication (3) and were subjected to analytical isoelectric focusing (IEF) as described previously (14, 27, 29). β-Lactamase activity was detected by overlaying the gels with 0.5 mM nitrocefin (Oxoid, Basingstoke, United Kingdom) in 50 mM HEPES (pH 7.5) supplemented with 2 mM ZnCl2 (21, 27).
Susceptibility tests.
The MICs of five β-lactam agents were determined by the agar dilution method, and the susceptibilities to eight non-β-lactam antibiotics were determined by the disk diffusion method. Both tests were performed and interpreted according to the National Committee for Clinical Laboratory Standards (NCCLS) (15, 16). The antimicrobial agents used for the agar dilution tests and their sources are as follows: aztreonam, Bristol-Myers Squibb, New Brunswick, N.J.; cefoxitin, Sigma Chemical Company; ceftazidime, Glaxo Group Research Ltd.; cefotaxime, Hoechst-Roussel Pharmaceuticals, Inc., Somerville, N.J.; imipenem, Merck Sharp & Dohme, West Point, Pa.; and meropenem, Sumitomo Pharmaceuticals Ltd., Osaka, Japan. Antimicrobial disks were all obtained from Becton Dickinson Microbiology Systems, Cockeysville, Md., including amikacin, chloramphenicol, ciprofloxacin, gentamicin, ofloxacin, pefloxacin, tobramycin, and trimethoprim-sulfamethoxazole.
PFGE analysis.
Genomic DNAs prepared by the procedure of Piggot et al. (18) were digested overnight with 10 U of XbaI (New England Biolabs, Beverly, Mass.) as recommended by Tenover et al. (25) and were subjected to pulsed-field gel electrophoresis (PFGE) with the Pulsaphor plus system (Amersham Pharmacia Biotech) as described previously (29). DNA fragments were separated in 1% agarose gels in 0.5× Tris-borate-EDTA buffer at 180 V for 30 h, with pulse times ranging from 5 to 35 s. The results were interpreted according to the criteria of Tenover et al. (25)
RESULTS
Screening of isolates.
On the basis of the NCCLS criteria, only five of the 3,458 isolates showed reduced susceptibilities to imipenem (inhibition zone diameter, <16 mm) or meropenem (inhibition zone diameter, <16 mm). The five isolates also demonstrated resistance to ceftazidime (inhibition zone diameter, ≤14 mm) and cefoxitin (inhibition zone diameter, ≤14 mm). One hundred and thirty-five isolates exhibited resistance to both ceftazidime and cefoxitin but were susceptible to imipenem and meropenem. These 140 isolates were selected for further experiments.
IMP-8 producers and clinical features.
The blaIMP-2-specific probe yielded a strong hybridization signal with 40 of the 140 isolates in colony hybridization experiments. Of the 40 isolates, 36 were found to carry blaIMP-8, blaSHV-12, and blaTEM-1 by PCR and nucleotide sequencing, and four were found to harbor blaIMP-8, blaSHV-11, and blaTEM-1. Nine and 31 blaIMP-8-positive isolates were collected in 1999 and 2000, respectively. The blaVIM and blaIMP-1 genes were not detected in the remaining 100 isolates in both colony hybridization experiments and PCR assays.
The 40 blaIMP-8-positive isolates were recovered from 16 patients. Twenty of these isolates were recovered from wound specimens, 12 were from sputa, five were from blood samples, two were from tips of central venous tips, and one was from a urine sample (Table 1). Of these 40 samples, 29 were submitted from the surgical ICU, 6 were from the medical ICU, and 4 were from the surgical wards. Prior to the isolation of IMP-8 producers, all 16 patients had undergone various surgical procedures, and all patients except patient 14 had been admitted to the surgical ICU. The underlying diseases and causes of admission of the 16 patients are summarized in Table 1. Before isolation of the IMP-8 producers, five patients had received extended-spectrum β-lactams and one of them had also received meropenem. blaIMP-8-positive isolates were associated with wound infections in eight patients and caused bloodstream infections in three patients. After isolation of the IMP-8 producers from blood samples, patients 7 and 8 received imipenem and patient 14 received meropenem. Nine patients died during the ICU stay due to multiorgan failure not directly related to infections with IMP-8 producers.
TABLE 1.
Origins of blaIMP-8-containing K. pneumoniae isolates and clinical characteristics of 16 patients with these isolates
| Patient no. | Age (yr)/sexa | Diseaseb | Isolate | Collection date (day/mo/yr) | Wardc | Sourced | Type of infection | Previous therapye | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 79/M | CAD, DM | 99w853 | 23/7/99 | SICU | Urine | Colonization | None | Death |
| 2 | 70/M | Laryngeal cancer | 99a300 | 1/9/99 | SICU | Sputum | Colonization | CAZ | Death |
| 99b203 | 11/9/99 | MICU | Sputum | Colonization | |||||
| 3 | 73/M | PPU, COPD | 99c025 | 20/9/99 | SICU | Sputum | Colonization | None | Recovered |
| 4 | 35/F | Placenta percreta complicated by perforation of urinary bladder | 99c196 | 22/9/99 | SICU | Wound | Wound infection | None | Recovered |
| 99e790 | 23/10/99 | Surgical ward | Wound | Wound infection | |||||
| 5 | 65/M | Traumatic ICH | 99c515 | 27/9/99 | SICU | Sputum | Colonization | None | Recovered |
| 99c893 | 30/9/99 | SICU | Wound | Wound infection | |||||
| 6 | 79/M | PPU, DM | 00e843 | 25/10/99 | SICU | Wound | Wound infection | CTX, ATM | Death |
| 7 | 47/M | Traumatic ICH, DM, liver cirrhosis | 3396/00 | 1/1/00 | SICU | Blood | Bactermia | None | Death |
| 3397/00 | 1/1/00 | SICU | Blood | Bacteremia | |||||
| 8 | 69/F | Traumatic ICH, multiple fractures | 00k621 | 3/1/00 | SICU | Wound | Wound infection | None | Recovered |
| 00k622 | 3/1/00 | SICU | Wound | Wound infection | |||||
| 3599/00 | 4/1/00 | SICU | Blood | Bacteremia | |||||
| 3606/00 | 4/1/00 | SICU | Blood | Bacteremia | |||||
| 00k908 | 5/1/00 | SICU | Wound | Wound infection | |||||
| 001060 | 7/1/00 | SICU | Wound | Wound infection | |||||
| 001330 | 10/1/00 | SICU | Wound | Wound infection | |||||
| 001545 | 12/1/00 | SICU | Wound | Wound infection | |||||
| 001650 | 13/1/00 | SICU | Wound | Wound infection | |||||
| 9 | 68/M | Traumatic multiple fractures | 00m393 | 26/1/00 | Surgical ward | Sputum | Colonization | None | Death |
| 00p177 | 24/2/00 | SICU | Sputum | Colonization | |||||
| 00p251 | 25/2/00 | SICU | Sputum | Colonization | |||||
| 00q795 | 13/3/00 | SICU | Wound | Wound infection | |||||
| 00r091 | 17/3/00 | SICU | Sputum | Colonization | |||||
| 00r134 | 17/3/00 | SICU | Sputum | Colonization | |||||
| 00r149 | 17/3/00 | SICU | Wound | Wound infection | |||||
| 10 | 73/F | Urinary tract cancer | 00m749 | 26/1/00 | SICU | Sputum | Colonization | None | Recovered |
| 11 | 69/F | PPU, liver cirrhosis | 00m869 | 27/1/00 | SICU | Wound | Wound infection | CTX, ATM | Death |
| 00n070 | 31/1/00 | SICU | Wound | Wound infection | |||||
| 00o044 | 11/2/00 | SICU | Wound | Wound infection | |||||
| 00o227 | 24/2/00 | SICU | Wound | Wound infection | |||||
| 12 | 71/M | Lung cancer | 00t801 | 17/4/00 | SICU | Wound | Wound infection | None | Death |
| 13 | 73/M | CAD, DM | 00d401 | 2/8/00 | MICU | Sputum | Colonization | CAZ | Death |
| 00f195 | 22/8/00 | MICU | Sputum | Colonization | |||||
| 14 | 95/F | Parkinsonism, COPD | 00h908 | 20/9/00 | MICU | CVC tip | Colonization | CAZ, ATM | Death |
| 2907/00 | 23/9/00 | MICU | Blood | Catheter-associated bacteremia | MEM | ||||
| 15 | 76/F | Cholangitis, cholelithiasis | 00j910 | 12/10/00 | Surgical ward | Wound | Wound infection | None | Recovered |
| 00j911 | 12/10/00 | Surgical ward | Wound | Wound infection | |||||
| 16 | 76/F | CAD, congestive heart failure | 00m578 | 13/11/00 | MICU | CVC tip | Colonization | None | Recovered |
M, male; F, female.
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ICH, intracranial hemorrhage; PPU, perforated peptic ulcer.
MICU, medical intensive care unit; SICU, surgical intensive care unit.
CVC, central venous catheter.
Only broad-spectrum β-lactams are listed. ATM, aztreonam; CAZ, ceftazidime; CTX, cefotaxime; MEM, meropenem.
Analytical IEF.
On IEF gels, all 40 IMP-8-producing isolates had three major bands with pIs of 5.4, 7.6, and 8.2. The pI 7.6 band probably represented the chromosomal SHV β-lactamase of K. pneumoniae, the pI 5.4 band might represent the TEM-1 β-lactamase, and the pI 8.2 band might represent either IMP-8 alone or a mixture of IMP-8 and SHV-12 (29).
PFGE.
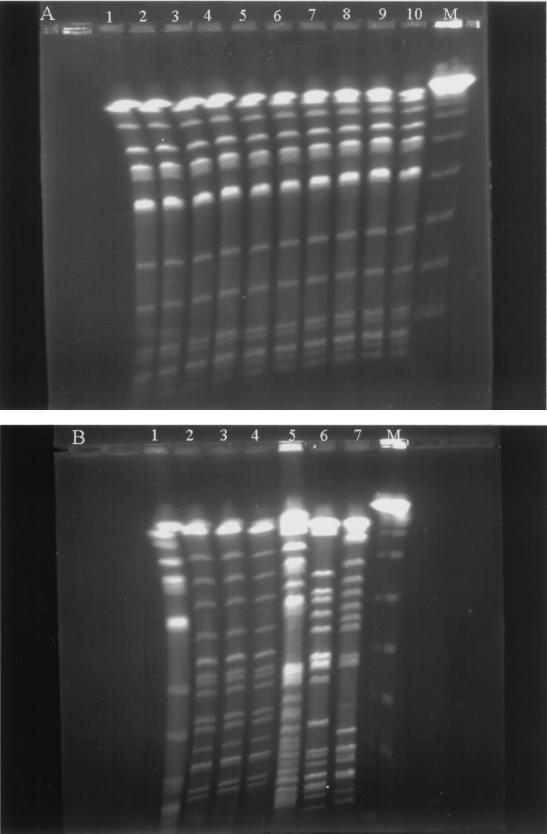
Except for two isolates from patient 13, the repetitive IMP-8-producing isolates recovered from the same patient had identical PFGE patterns. Thus, there were 17 nonrepetitive isolates identified in the outbreak. The PFGE results are summarized in Table 2 and are shown in Fig. 1. Five PFGE patterns were identified. Of the 14 isolates producing both IMP-8 and SHV-12, 11 isolates had pattern A. Isolates 3599/00, 00d401, and 99w853 had distinct patterns B, D, and E, respectively. Although isolate 00m869 exhibited a resistance phenotype different from those of isolate 3396/00 and 00t801, all three isolates had pattern C.
TABLE 2.
Antimicrobial susceptibilities and PFGE patterns of 17 nonrepetitive K. pneumoniae isolates and their transconjugants
| Genotypes of β-lactamases, isolate and transconjugant | MIC (μg/ml)a
|
Non-β-lactam antibioticsb to which resistance is shown | PFGE pattern | |||||
|---|---|---|---|---|---|---|---|---|
| CAZ | CTX | FOX | ATM | IPM | MEM | |||
| Isolate | ||||||||
| IMP-8 + SHV-12 + TEM1 | ||||||||
| 99a300 | >256 | 64 | >256 | >256 | 0.5 | 0.25 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 99c025 | >256 | 16 | >256 | 256 | 1 | 0.5 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 99c196 | >256 | 128 | >256 | >256 | 1 | 1 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 99c893 | 256 | 32 | >256 | >256 | 1 | 0.5 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 00m393 | >256 | 256 | >256 | >256 | 0.5 | 0.25 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 00m749 | >256 | 64 | >256 | >256 | 0.5 | 0.25 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 2907/00 | >256 | 32 | >256 | 64 | 0.5 | 0.25 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 00j910 | >256 | 128 | >256 | >256 | 1 | 0.5 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 00m578 | >256 | 64 | >256 | >256 | 4 | 1 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | A |
| 99e843 | >256 | 128 | >256 | 128 | 1 | 0.5 | CHL, SXT, GEN, TOB | A |
| 00f195 | >256 | 64 | >256 | 64 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | A |
| 3599/00 | >256 | 128 | >256 | >256 | 2 | 0.5 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | B |
| 00m869 | >256 | 128 | >256 | >256 | >256 | 16 | CHL, SXT, GEN, TOB, PEF, OFX, CIP | C |
| 00d401 | >256 | >256 | >256 | >256 | >256 | 8 | CHL, SXT, GEN, TOB | D |
| IMP-8 + SHV-11 + TEM1 | ||||||||
| 99w853 | >256 | 64 | >256 | 0.25 | 2 | 0.5 | CHL, SXT, GEN, TOB, AMK, PEF, OFX, CIP | E |
| 3396/00 | >256 | 32 | >256 | 0.06 | 0.25 | 0.25 | CHL, SXT, TOB, AMK, PEF, OFX, CIP | C |
| 00t801 | >256 | 32 | >256 | 0.25 | 0.5 | 0.5 | CHL, SXT, TOB, AMK, PEF, OFX, CIP | C |
| Transconjugant | ||||||||
| IMP-8 + SHV-12 + TEM1 | ||||||||
| 99a300 | 256 | 64 | >256 | 16 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 99c025 | >256 | 16 | >256 | 32 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 99c196 | 256 | 32 | 256 | 16 | 1 | 0.25 | CHL, SXT, GEN, TOB | |
| 99c893 | 128 | 16 | >256 | 32 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 00m393 | 128 | 16 | 128 | 8 | 0.25 | 0.13 | CHL, SXT, GEN | |
| 00m749 | 256 | 64 | 256 | 16 | 0.25 | 0.25 | CHL, SXT, GEN, TOB | |
| 2907/00 | >256 | 32 | 256 | 16 | 1 | 0.25 | CHL, SXT, GEN, TOB | |
| 00m578 | 256 | 16 | >256 | 32 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 99e843 | 256 | 64 | >256 | 16 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 00f195 | 128 | 32 | 128 | 8 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| 00d401 | 128 | 32 | 256 | 16 | 0.5 | 0.25 | CHL, SXT, GEN, TOB | |
| IMP-8 + TEM-1 | ||||||||
| 00m869 | 256 | 32 | >256 | 0.13 | 0.5 | 0.25 | SXT, GEN, TOB | |
| 99w853 | 256 | 64 | >256 | 0.06 | 0.5 | 0.5 | CHL, SXT, GEN, TOB | |
CAZ, ceftazidime; CTX, cefotaxime; FOX, cefoxitin; ATM, aztreonam; IPM, imipenem; MEM, meropenem.
CHL, chloramphenicol; SXT, trimethoprim-sulfamethoxazole; GEN, gentamicin; TOB, tobramycin; AMK, amikacin; PEF, pefloxacin; OFX, ofloxacin; CIP, ciprofloxacin.
FIG. 1.
PFGE of XbaI-digested genomic DNAs from 17 nonrepetitive blaIMP-8-positive K. pneumoniae isolates. (A) Lane M, bacteriophage lambda DNA concatemers (Gibco BRL), which served as molecular size markers; lanes 1 to 10, pattern A, isolates 99a300, 99c025, 99c196, 99c893, 99e843, 00m393, 00m749, 00f195, 2907/00, and 00j910. (B) Lane M, bacteriophage lambda DNA concatemers; lane 1, pattern A, isolate 00m578; lanes 2 to 4, pattern C, isolates 00m869, 3396/00, and 00t801; lane 5, pattern D, isolate 00d401; lane 6, pattern E, isolate 99w853; lane 7, pattern B, isolate 3599/00. Isolates 00f195 and 00d401 were from the same patient.
Susceptibility tests.
The susceptibilities of the nonrepetitive blaIMP-8-positive isolates to various β-lactams are summarized in Table 2. All 40 isolates were resistant to ceftazidime, cefotaxime, and cefoxitin. Thirty-six isolates, or 14 nonrepetitive isolates, which all carried blaIMP-8, blaSHV-12, and blaTEM1, were also resistant to aztreonam (MICs, ≥64 μg/ml), while only four isolates, or three nonrepetitive isolates, which harbored blaIMP-8, blaSHV-11, and blaTEM-1 were susceptible to this agent (MICs, 0.06 to 0.25 μg/ml). Only five isolates, or two nonrepetitive isolates, exhibited resistance to imipenem (MICs, >256 μg/ml) and reduced susceptibilities to meropenem (MICs, 8 to 16 μg/ml). All the other isolates appeared susceptible to carbapenems. The agar diffusion tests revealed that all 40 isolates studied were also resistant to non-β-lactam antibiotics (Table 2).
Conjugation experiments.
The 17 nonrepetitive blaIMP-8-positive isolates were subjected to conjugation experiments. The blaIMP-8-containing plasmids were successfully transferred from 13 of 17 isolates to E. coli C600. PCR and nucleotide sequencing showed that 11 transconjugants carried blaIMP-8, blaSHV-12, and blaTEM1 and that two transconjugants carried blaIMP-8 and blaTEM-1. Expression of blaIMP-8 by the transconjugants was confirmed by IEF analysis. Resistance to chloramphenicol, trimethoprim-sulfamethoxazole and aminoglycosides was also transferred to E. coli from all K. pneumoniae isolates except isolate 00m869, in which chloramphenicol resistance was not transferable (Table 2). The sizes of the plasmids transferred to E. coli were all >100 kb.
DISCUSSION
The present study indicates that MBLs in K. pneumoniae remained uncommon in the university medical center. Of the 3,458 K. pneumoniae isolates, only 40 isolates (1.2%) from 16 patients were found to carry blaIMP-8. Neither the other blaIMP genes nor the blaVIM genes were detected in the remaining isolates. Nine IMP-8-producing isolates from six patients were identified among 1,622 isolates collected in 1999 (0.6%), and 31 blaIMP-8-positive isolates from 10 patients were recognized from 1,836 isolates collected in 2000 (1.7%), suggesting an increasing prevalence rate of blaIMP-8-positive K. pneumoniae in the university hospital. The increased prevalence rate could be in part due to the nosocomial outbreak.
Correlation between carriage of blaIMP-8 and of carbapenem resistance in our isolates was imperfect (Table 2). Only a total of five blaIMP-8-containing isolates, or two nonrepetitive isolates, were resistant to carbapenems. After conjugation, all E. coli transconjugants appeared susceptible to carbapenems. Similar findings have also been described in many reports of MBLs (4, 7, 8, 10, 12, 13, 17, 19, 22–23, 27, 28, 30). In addition, it is also noted that the cloned IMP or VIM enzymes confer only low-level resistance to carbapenems in E. coli (7, 10, 12, 17, 19, 21, 28, 30). Thus, there have been two speculations about the imperfect correlation: either the MBL genes are not expressed, or substantive resistance might require reduced uptake of the carbapenem as well as the presence of MBLs (13). Koh et al. (T. H. Koh, L. H. Sng, G. S. Babini, N. Woodford, D. M. Livermore, and L. M. C. Hall, Letter, Antimicrob. Agents Chemother. 45:1939–1940, 2001) described the loss of a major 39-kDa outer membrane protein in an IMP-1-producing K. pneumoniae isolate with high-level resistance to carbapenems recently, supporting the speculation that high-level resistance to carbapenems demands impermeability and an IMP enzyme. This model might also apply to our isolates. The fact that most of our blaIMP-8-positive isolates were susceptible to carbapenems indicates the difficulty in detecting K. pneumoniae isolates with the IMP enzymes by using routine susceptibility tests. Therefore, determinations of carbapenemase activity or molecular biology techniques are needed for the purposes of epidemiology and, perhaps, patients' treatment.
Monobactams are stable to hydrolysis by MBLs (7, 10, 12, 17, 19, 21, 30); however, 36 of the total of 40 blaIMP-8-containing isolates (90.0%), or 14 of the 17 nonrepetitive isolates (82.4%), were resistant to aztreonam. Production of the SHV-12 ESBL in addition to IMP-8 by these isolates should be responsible for their resistance to aztreonam. Of greater concern is that all the IMP-8-producing isolates were also resistant to most non-β-lactam agents (Table 2). All of them were resistant to at least one kind of aminoglycosides, and 37 of the total of 40 isolates (92.5%), or 14 of the 17 nonrepetitive isolates (82.4%), were resistant to fluoroquinolones (Table 2). Therefore, strict infection control measures should be implemented with the appearance of such multidrug-resistant isolates to prevent their spread.
Five patterns were obtained by PFGE. Eleven and 3 of the 17 nonrepetitive isolates exhibited PFGE patterns A and C, respectively, indicating that the nosocomial outbreak of blaIMP-8-containing K. pneumoniae was mainly due to clonal spread. The isolates from patient 14 exhibited the same PFGE and plasmid profiles as those from the surgical ICU, suggesting that the blaIMP-8-positive strain in the medical ICU might have been spread from the surgical ICU. Isolates 00d401 and 00f195, both of which were recovered from patient 13, gave different PFGE patterns, suggesting horizontal transfer of the resistance plasmid between these two isolates. Isolates 00m869, 3396/00, and 00t801 all had PFGE pattern C (Table 2). Isolates 99e843, 00f195, and 00m869 had resistance phenotypes different from those of isolates with the same PFGE patterns. The discrepancy could be due to loss or acquisition of resistance genes in the epidemiologically related isolates under various selective pressures.
In an outbreak caused by IMP-1-producing gram-negative rods at a hospital in Japan (8), 53.8% of the IMP-1-producing P. aeruginosa isolates were recovered from patients with malignant diseases, suggesting that malignancy is a risk factor for the acquisition of IMP-1-producing isolates. The present study suggests that surgery is another important risk factor for the acquisition of MBL producers. Each one of our patients had received some kind of surgery, and eight patients (50%) had wound infections associated with the isolation of IMP-8 producers. In contrast, only 3 of the 16 patients (18.8%) had malignant diseases. It is noteworthy that many of our patients with wound infections stayed at the surgical ICU during the same period (Table 2), implying that the blaIMP-8-positive strains could have been spread by the health care staff. Only 1 of the 16 patients (6.3%) had received carbapenems, while 5 patients (31.3%) had been administered extended-spectrum β-lactams prior to the isolation of the blaIMP-8-positive isolates. The data were consistent with those obtained by Hirakata et al. (8) and with the suggestion that the selective pressure from carbapenems was not required for the acquisition of MBL producers. Nine of the 16 patients finally died, but none of them died of the infections caused by blaIMP-8-positive isolates. Serial wound cultures indicated that blaIMP-8-positive isolates could colonize in the wounds for up to approximately 2 months (Table 1). These findings indicate that even though the blaIMP-8-containing isolates were not necessarily responsible for the high mortality of our patients, the morbidity they caused is still a big problem. Three patients with bloodstream infections were administered carbapenems after the recovery of blaIMP-8-positive isolates on the basis of the in vitro susceptibility tests. Two of them eventually died, although they had all survived the infections. Because of the limited numbers of patients, it is not clear whether the favorable response was due to the treatment regimens that included carbapenems. Despite susceptibilities of the blaIMP-8-positive strains to carbapenems, the use of carbapenems for the treatment of infections with MBL-producing strains should be restricted to prevent the possibility of emergence of more potent IMP enzymes under the selective pressure from carbapenems.
In conclusion, the present study indicates the emergence of infections caused by blaIMP-8-positive K. pneumoniae in Taiwan. These blaIMP-8-positive isolates were all multidrug resistant and usually produced an ESBL as well. The nosocomial outbreak identified in a university hospital was largely caused by genetically related strains. Although the blaIMP-8-positive isolates were still confined to the ICUs and spread at a low prevalence rate in the university hospital, strict infection control measures against such isolates should be implemented in order to prevent their further dissemination.
ACKNOWLEDGMENTS
We kindly thank G. M. Rossolini, Dipartimento di Biologia Molecolare, Sezione Di Microbiologia, Università di Siena, Siena, Italy, for provision of the IMP-2-containing A. baumannii strain AC-54/97 and the VIM-1-containing P. aeruginosa strain VR-143/97.
This work was partially supported by grants NCKUH90-031 from National Cheng Kung University Hospital and NSC89-2320-B-006-149 from the National Science Council, Republic of China.
REFERENCES
- 1.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann B J, Low K B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980;44:1451–1456. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 4.Cornaglia G, Mazzariol A, Lauretti L, Rossolini G M, Fontana R. Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-β-lactamase. Clin Infect Dis. 2000;31:1119–1125. doi: 10.1086/317448. [DOI] [PubMed] [Google Scholar]
- 5.Farmer J J., III . Enterobacteriaceae: introduction and identification. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 438–449. [Google Scholar]
- 6.Grunstein M, Hogness D S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA. 1975;72:3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkey P M, Xiong J, Ye H, Li H, M'Zali F H. Occurrence of a new metallo-beta-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People's Republic of China. FEMS Microbiol Lett. 2001;194:53–57. doi: 10.1111/j.1574-6968.2001.tb09445.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirakata Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, Matsuda J, Nakano M, Tomono K, Maesaki S, Kaku M, Yamada Y, Kamihira S, Kohno S. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–2011. doi: 10.1128/aac.42.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinical isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyobe S, Kusadokoro H, Ozaki J, Matsumura N, Minami S, Haruta S, Sawai T, O'Hara K. Amino acid substitutions in a variant of IMP-1 metallo-β-lactamase. Antimicrob Agents Chemother. 2000;44:2023–2027. doi: 10.1128/aac.44.8.2023-2027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauretti L, Riccio M L, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini G M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore D M, Woodford N. Carbapenemases: a problem in waiting? Curr Opin Microbiol. 2000;3:489–495. doi: 10.1016/s1369-5274(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 14.Matthew M, Harris M, Marshall M J, Rose G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 7th ed. Approved standard M2–A7. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolates of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piggot P J, Amjad M, Wu J J, Sandoval H, Castro J. Genetic and physical maps of Bacillus subtilis 168. In: Harwood C R, Cutting S M, editors. Molecular biology methods for bacillus. West Sussex, England: John Wiley & Sons Ltd.; 1990. pp. 493–543. [Google Scholar]
- 19.Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo J D, Nordmann P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000;44:891–897. doi: 10.1128/aac.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provence D L, Curtiss R., III . Gene transfer in gram-negative bacteria. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 319–347. [Google Scholar]
- 21.Riccio M L, Franceschini N, Boschi L, Caravelli B, Cornaglia G, Fontana R, Amicosante G, Rossolini G M. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob Agents Chemother. 2000;44:1229–1235. doi: 10.1128/aac.44.5.1229-1235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984;20:608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsakris A, Pournaras S, Woodford N, Palepou M-F I, Babini G S, Douboyas J, Livermore D M. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000;38:1290–1292. doi: 10.1128/jcm.38.3.1290-1292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan J J, Hsueh P R, Ko W C, Luh K T, Tsai S H, Wu H M, Wu J J. Metallo-β-lactamases among clinical isolates of Pseudomonas in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob Agents Chemother. 2001;45:2224–2228. doi: 10.1128/AAC.45.8.2224-2228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J J, Ko W C, Wu J J. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:2368–2371. doi: 10.1128/AAC.45.8.2368-2371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan J J, Wu S M, Tsai S H, Wu J J, Su I J. Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum β-lactamase and identification of a novel AmpC Enzyme (CMY-8) in southern Taiwan. Antimicrob Agents Chemother. 2000;44:1438–1442. doi: 10.1128/aac.44.6.1438-1442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yano H, Kuga A, Okamoto R, Kitasato H, Kobayashi T, Inoue M. Plasmid-encoded metallo-beta-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob Agents Chemother. 2001;45:1343–1348. doi: 10.1128/AAC.45.5.1343-1348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]