Background:
As a referral center for chronic pain, we see many patients with “idiopathic” shoulder pain and limited range of motion. The combination of mild or subclinical carpal tunnel syndrome and cubital tunnel syndrome may be an underrecognized etiology of symptoms in such patients. Here, we report our treatment algorithm and results for such patients.
Methods:
Of patients with a chief complaint of shoulder pain, we identified 56 consecutive patients who had pain or tingling with median nerve compression at the proximal wrist crease and positive Tinel’s around the cubital tunnel. They were first provided a night-time wrist orthosis. If still symptomatic, nerve blocks were given to median and ulnar nerves under ultrasound guidance. If symptoms recurred after nerve blocks, nerve conduction studies and surgical release of affected nerves were performed.
Results:
Six patients had 60% or more pain relief with orthosis (mean 4.7 ± 0.8 (SD) to 2.2 ± 0.8). Twenty-three patients had nerve blocks and had persistent pain relief (6.0 ± 1.7 to 2.1 ± 1.9) and significant shoulder motion improvement. Twenty-seven patients only had temporal relief and required surgery but postoperatively had persistent pain relief (6.2 ± 2.0 to 1.2 ± 1.0) and improved shoulder motion. qDASH improved from 33.4 ± 20.1 preoperatively to 12.2 ± 7.4 postoperatively.
Conclusions:
All patients had substantial improvement in shoulder pain and motion with compressive neuropathy treatments. Targeted physical examination can identify these patients, who can have significant improvement with appropriate diagnosis and treatment. The study sheds light on an underrecognized cause of shoulder dysfunction.
Takeaways
Question: What is a possible etiology of “idiopathic” shoulder pain/stiffness in patients without apparent pathology around the shoulder?
Findings: At our referral center for chronic pain, 56 consecutive “idiopathic” shoulder pain patients had pain/tingling induced by proximal wrist compression and Tinel’s at cubital tunnel. They had good relief of shoulder symptoms with conservative treatment, nerve blocks, or surgical release of carpal and cubital tunnels
Meaning: Many patients with “idiopathic” shoulder pain and stiffness may have unrecognized carpal tunnel and cubital tunnel syndromes, and treating the entrapment neuropathies can significantly improve shoulder function.
INTRODUCTION
Any provider treating shoulder problems will encounter patients with shoulder pain or limited range of motion (ROM) without any apparent active shoulder or cervical pathology. They may have no identifiable shoulder pathology, or they may have pathology but continue to have dysfunction despite appropriate treatment such as physical therapy or rotator cuff repair. The apparent lack of correlation between objective findings and perceived disability, possibly related to psychological and sociological factors, can be frustrating and challenging for both the provider and patients.1
Mild, subclinical compressive neuropathy may be an underrecognized cause of shoulder dysfunction in such patients. Carpal tunnel syndrome (CTS) has been long known to cause shoulder pain but can be easily overlooked, especially when patients report no symptoms in the hand.2–6 To our knowledge, there has been no report in the literature which describes shoulder pain as a symptom of cubital tunnel syndrome (CuTS).
As a referral center for chronic pain and dysfunction in the extremities, we evaluate many patients with chief complaint of shoulder pain and limited ROM. We have identified a significant subset of these patients who have both undiagnosed CTS and CuTS, often mild or subclinical. In this study, we report our experience with a series of 56 such patients. We hypothesized that physical examination can identify this subset of patients and that treatment of compressive neuropathies will lead to significant improvement in shoulder pain and ROM.
MATERIALS AND METHODS
Subjects
This article conforms to the Declaration of Helsinki. We included all patients who presented to our hospital in 2018 who met the following three criteria:
Had a chief complaint of shoulder pain. All patients had shoulder X-rays to check for arthritis. Patients’ shoulders were examined and tested for rotator cuff pathology (by Jobe empty can test, Gerber’s lift off test, Hornblower’s sign), for shoulder impingement (by Hawkins-Kennedy impingement sign), or for biceps pathology (by Speed’s test). If any test was positive, shoulder MRI was obtained. Patients were excluded if MRI showed full thickness rotator cuff tear, proximal biceps pathology, arthritis, or labral tear.
Had pain or tingling with manual compression of median nerve at the proximal wrist crease for 10 seconds.
Had Tinel’s sign along the ulnar nerve, anywhere from arcade of Struthers to the cubital tunnel. Exclusion criteria were positive Spurling test and pain with neck ROM.
Treatment Algorithm
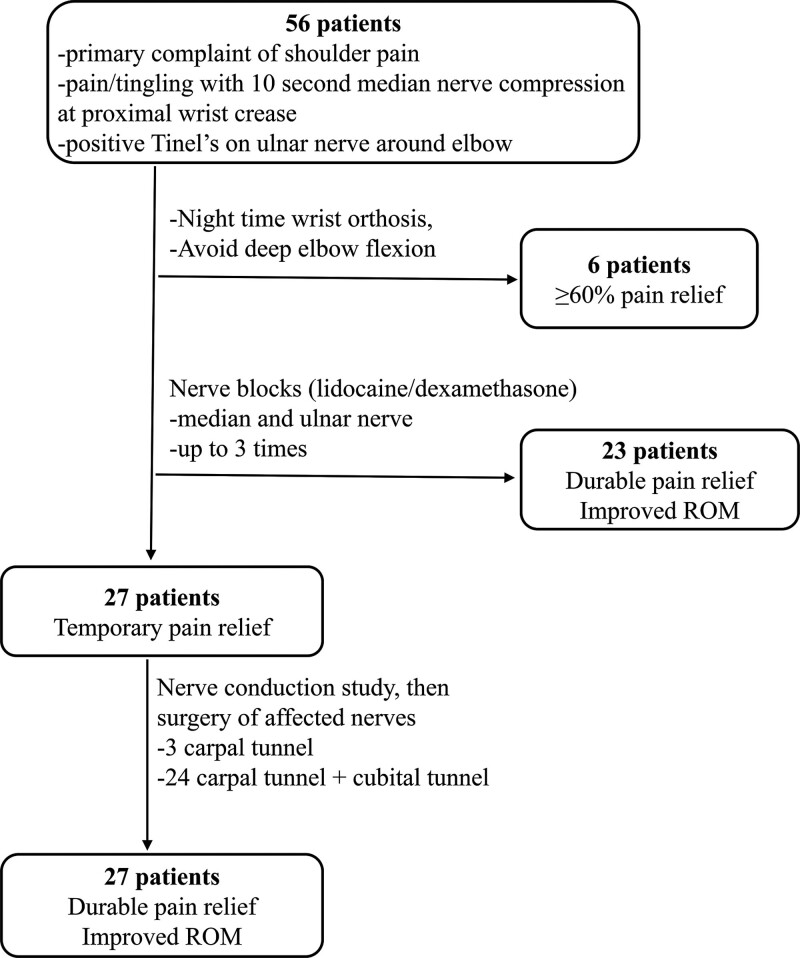
Our treatment algorithm is summarized in Figure 1.
Fig. 1.
Flowchart summarizing the treatment algorithm and results.
Nonsteroidal antiinflammatory drugs (loxoprofen or celecoxib) and pregabalin (25–75 mg at bedtime) were prescribed as needed for symptom relief throughout the course of treatment. Opioids were not used. Patients were treated as follows:
Therapy 1: For patients without difficulties in activities of daily living (ADL) or patients who did not want injections, we recommended wearing a wrist brace while sleeping and avoidance of deep elbow flexion.
Therapy 2: When therapy 1 was insufficient (patient had <60% relief of pain) or when patient had substantial problems with ADL (such as difficulty in wearing clothes, difficulty with sleep due to nocturnal pain, etc.), nerve blocks were performed under ultrasonography guidance (details below).
Therapy 3: For patients who only had temporary relief from nerve blocks, a nerve conduction study (NCS) was performed, followed by surgical decompression of affected nerves.
Nerve Blocks
Nerve blocks were performed under ultrasonographic guidance.7–9 The injections were performed around the median nerve at the distal forearm and around the ulnar nerve approximately 8 cm proximal to the medial epicondyle. Each nerve was hydrodissected with 5 mL of 1% lidocaine and 4 mg of dexamethasone to achieve a doughnut-shaped circumferential distribution of the medication around each nerve on short axis ultrasonography. If symptoms recurred, repeat nerve blocks were performed up to two more times.
Nerve Conduction Study
Diagnostic criteria from the American Association of Neuromuscular and Electrodiagnostic Medicine were used to diagnose CTS based on NCS results.10 CTS was diagnosed using either (1) comparison of sensory nerve conduction velocities between the median and ulnar nerves in the ring finger or between the median and radial nerves in the thumb,11 or (2) comparison of motor nerve distal latencies of the second lumbrical muscle (innervated by the median nerve) and the second palmar interosseous muscle (innervated by the ulnar nerve).12 CuTS was diagnosed if a decrease in velocity of 15% or more was seen in either compound muscle or sensory nerve action potential of the ulnar nerve above and below the elbow.
Surgical Procedure
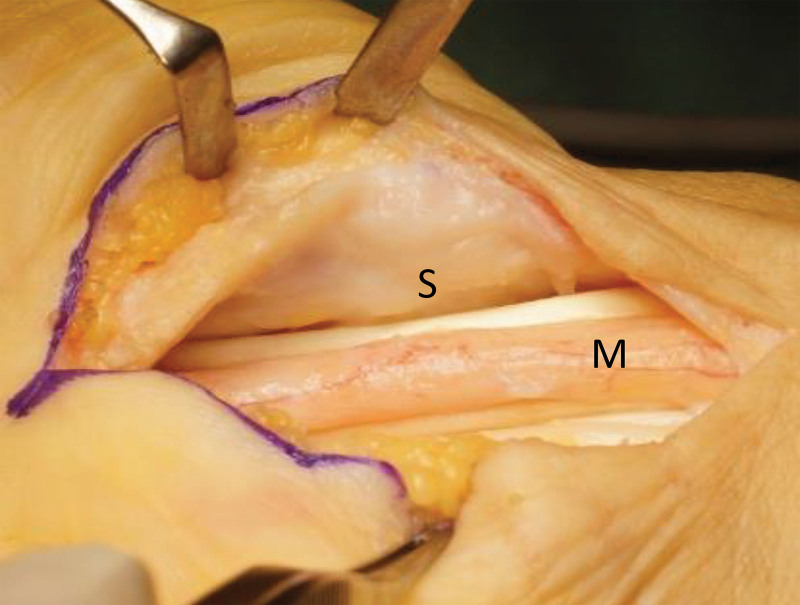
For CTS, complete release of the transverse carpal ligament was performed through an open volar approach. Additionally, flexor retinaculum and flexor tendon adhesions to the nerve were released (Fig. 2).
Fig. 2.
Intraoperative photographs for right CTS. The tendon was freed from the nerve. Improved circulation was noted in the perineurium of the median nerve (M) after resection of synovium and release of tendon adhesions. The synovium under the flexor retinaculum (S) was also subsequently resected.
For CuTS, the medial antebrachial cutaneous nerve was first identified and protected. The ulnar nerve was released proximally from the arcade of Struthers all the way distal to the proximal fascia of the flexor carpi ulnaris (FCU) and flexor digitorum superficialis (FDS). Blood vessels accompanying the nerve were preserved. The intermuscular septum was excised, and muscle bellies of the FCU and FDS were incised near their proximal origin, allowing the muscles to advance distally. Then, the ulnar nerve was transposed near the median nerve submuscularly into the space created by the advancement of the FCU and FDS muscles.
Outcome Measures
Shoulder active range of motion (AROM; forward flexion, abduction, and external rotation) was measured by physicians or therapists in all patients at the initial and follow-up visits. During each visit, patients were asked to rate their pain on a numeric rating scale from 0 to 10, with 10 being the worst imaginable pain.13 Furthermore, the empty14 and full can tests15 were performed to assess the supraspinatus. The Phalen elbow flexion shoulder abduction test,16,17 in which loads are simultaneously applied to the median and ulnar nerves, was performed for 30 seconds. The results were rated as good (patients can perform the test without any problems), fair (patients are symptomatic but can perform the test), and poor (patients cannot perform the test). After treatment, shoulder AROM was measured in the same manner. Patients rated their improvement in pain after treatment on a percentage scale.
Additionally, in the surgery group, grip strengths were measured before and after surgery, and the Japanese version of the Quick Disability of the Arm, Shoulder, and Hand (qDASH) questionnaire18 was administered. The patients were evaluated for 1 year after the last treatment session.
Statistical Analysis
A paired t test was performed to compare pretreatment and posttreatment measurements. Alpha was set at 0.05.
RESULTS
Approximately 1100 new patients were seen at our outpatient clinic in 2018. All patients who met the three inclusion criteria agreed to participate, and these 56 patients (17 men, 39 women, left 20, right 36) were included in the study. The mean age was 60.0 years [range 27–83]. The mean time from onset of symptoms to the initial presentation to our hospital was 12.5 months [0 day–21 years] with an SD of 33.7 months.
1) After orthosis therapy, six patients had 60% or more pain relief (NRS 4.7 (SD 0.8) to 2.2 (SD 0.8). These patients had normal shoulder AROM before and after treatment.
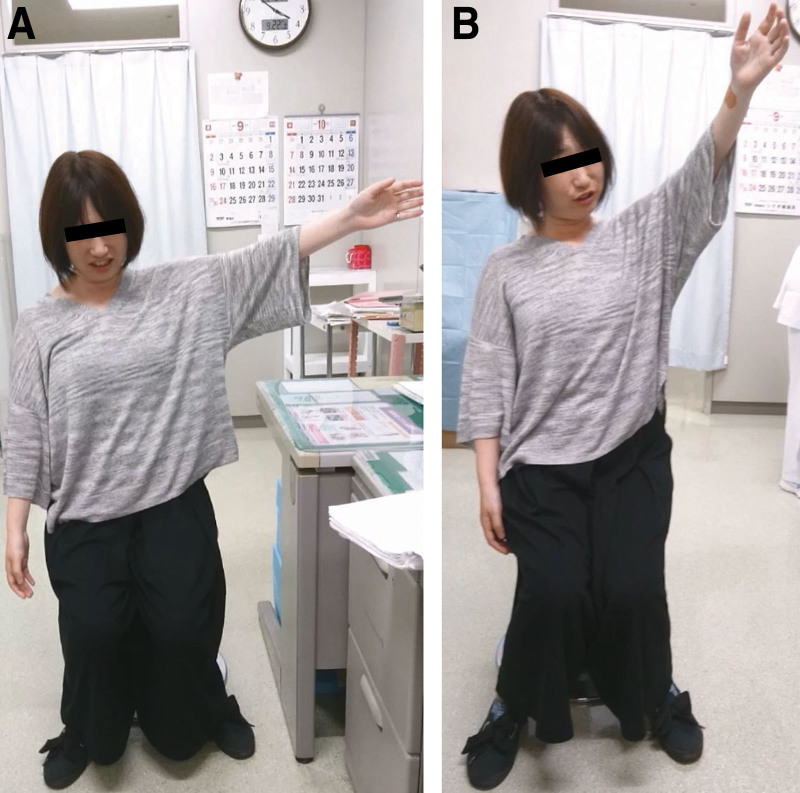
2) In 23 patients, nerve blocks led to persistent symptom relief. Shoulder AROM improved (Table 1). Pain improved from 6.0 (SD 1.7) to 2.1 (SD 1.9). A representative case is shown in Figure 3.
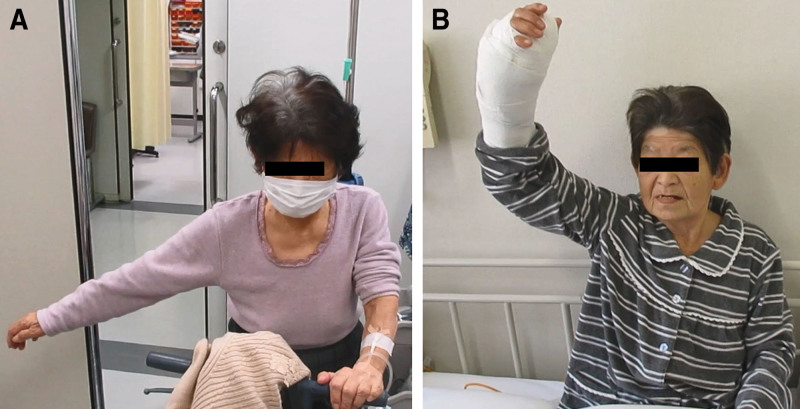
3) In 27 patients, shoulder AROM and pain improved with nerve blocks, but the improvement was temporary. NCS was performed in the 27 patients, and 21 patients were positive for CTS with at least one of the diagnostic criteria, and 18 patients were positive for cubital tunnel syndrome with at least one of the diagnostic criteria. Three underwent surgery for CTS alone; 24 underwent simultaneous surgeries for CTS and CuTS. There were statistically significant improvements in active forward flexion, abduction, and external rotation (Table 2). Pain improved from 6.2 (SD 2.0) to 1.2 (SD 1.0). The mean preoperative grip strength on the affected side was 19.2 (SD 9.3) kgf, which was lower than the mean grip strength of 28.7 (SD 9.4) kgf on the unaffected side. The mean qDASH score was 33.4 (SD 20.9). At final follow-up at 1 year, the mean grip strength and qDASH were 26.4 (SD 8.2) kgf and 12.2 (SD 7.4), respectively. A representative case is shown in Figure 4.
Table 1.
Shoulder Active Range of Motion at Initial and Final Visits in the Injection Group (n = 23)
| Shoulder ROM | Mean | SD | Minimum | Maximum | P |
|---|---|---|---|---|---|
| FL | |||||
| Initial visit | 152.6 degrees | 38.2 degrees | 90 degrees | 180 degrees | |
| After injection | 170.4 degrees | 20.1 degrees | 110 degrees | 180 degrees | 0.0048* |
| ABD | |||||
| Initial visit | 147.8 degrees | 43.2 degrees | 60 degrees | 180 degrees | |
| After injection | 167.0 degrees | 24.8 degrees | 100 degrees | 180 degrees | 0.01* |
| ER | |||||
| Initial visit | 53.3 degrees | 14.2 degrees | 10 degrees | 70 degrees | |
| After injection | 57.1 degrees | 8.6 degrees | 40 degrees | 70 degrees | 0.10 |
ABD, abduction; ER, external rotation; FL, flexion.
*indicates statistical significance.
Fig. 3.
Representative clinical photographs of a patient treated with nerve blocks. She is demonstrating maximal active abduction of the left shoulder before nerve block (A) and shortly after nerve blocks (B). She previously had an MRI elsewhere, which showed no rotator cuff tear.
Table 2.
Shoulder Active Range of Motion at Initial and Final Visits in the Surgery Group (n = 27)
| Shoulder ROM | Mean | SD | Minimum | Maximum | P |
|---|---|---|---|---|---|
| FL | |||||
| Initial visit | 137.6 degrees | 48.3 degrees | 30 degrees | 180 degrees | |
| After surgery | 175.4 degrees | 12.5 degrees | 120 degrees | 180 degrees | 0.0001* |
| ABD | |||||
| Initial visit | 134.4 degrees | 51.3 degrees | 40 degrees | 180 degrees | |
| After surgery | 175.7 degrees | 13.9 degrees | 110 degrees | 180 degrees | 0.0001* |
| ER | |||||
| Initial visit | 49.6 degrees | 16.3 degrees | 0 degrees | 60 degrees | |
| After surgery | 58.5 degrees | 10.3 degrees | 10 degrees | 70 degrees | 0.0009* |
ABD: abduction; ER: external rotation; FL: flexion.
*indicates statistical significance.
Fig. 4.
Representative clinical photographs of a patient treated with surgery. She is demonstrating maximal active abduction of the right shoulder before surgery (A) and on postoperative day 1 (B). She previously had an MRI elsewhere, which showed a large rotator cuff tear, but had not received any shoulder surgeries.
DISCUSSION
Cervical pathologies are well known to cause pain and limited function in the shoulder. Many physicians evaluating shoulder pain will include physical examination of the neck and cervical spine. However, wrists and elbows may not be routinely examined on patients with isolated shoulder complaints. Our results suggest that physical examination can identify a subset of patients who have unrecognized CTS and CuTS as causes of isolated shoulder pain and stiffness and that these patients have significant improvement when these compressive neuropathies are addressed.
Compression neuropathies such as CTS or CuTS typically cause pain and tingling at or distal to the compression site. However, they can also cause symptoms proximal to the compression site. Kendall recognized this as early as 1960 and wrote that sensory symptoms in the arm or shoulder from CTS have “commonly led to the erroneous diagnosis of ‘costo-clavicular syndrome’ when in fact the trouble is occurring at the wrist.”2 Torebjörk et al found that intraneural stimulation of median nerve at the wrist in healthy subjects can cause proximal pain in the arm, axilla, or chest.19 Marchettini et al performed a similar study for ulnar nerve at the wrist and found that pain can be referred to the posterior arm, lateral chest wall, and scapular region.20
Padua et al reported that proximal symptoms of CTS tend to be pain rather than sensory symptoms.21 Kummel et al found significant improvement in symptoms after carpal tunnel release in 11 patients who presented with shoulder pain.5 Maki et al reported that 20%–30% of surgically treated patients with CTS complained of pain, of whom 20%–30% had pain in the forearm or shoulder. Pain in these patients quickly resolved after surgery.22 More recently, del Piñal has presented a series of patients with “irritative carpal tunnel syndrome” who have CRPS-like symptoms often with limitation in elbow and shoulder function. These patients had significant improvement after carpal tunnel release.23,24
To our knowledge, there has been no terminology used in the literature that specifically refers to symptoms proximal to nerve compression site, although some clinicians have used less-specific terms such as “proximal radiation of pain” or “bi-directional pain.” Therefore, we have been using the term “retrograde neuropathic pain” (RNP).
Recognition of RNP can be challenging when patients present with pain and limited ROM only in the shoulder. We have observed RNP in both upper and lower extremities, sometimes leading to diagnosis by others as “idiopathic pain” or “complex regional pain syndrome.” The patients often do not report any symptoms distally, even when they are specifically asked. This lack of distal symptoms is likely due to the nerve compressions being mild or subclinical. Therefore, targeted physical examination is key to identifying these compressive neuropathies. We have found compression at proximal wrist crease (slightly more proximal than the original compression test directly over carpal tunnel, as described by Durkan25) and Tinel’s around cubital tunnel to be reliable tests to identify shoulder RNP patients.
Other physical examination maneuvers may be useful, especially if other sites of nerve compression are suspected. Scratch/sensory collapse test,26,27 which may be related to stimulation of specialized cutaneous Schwann cells that sense noxious stimuli,28 may be helpful. There are various other potential nerve compressions in the upper extremity, including Guyon’s canal, proximal forearm, dorsal scapula nerve, and quadrilateral space syndrome. It should be noted that our surgical treatment for cubital tunnel was submuscular transposition, which involves release of lacertus (bicipital) aponeurosis and some advancement of flexor-pronator mass. Therefore, the surgery may have provided some decompression of median nerve at proximal forearm.
How can the combination of mild or subclinical CTS and CuTS cause significant symptoms? The spared nerve injury model for neuropathic pain described by Decosterd et al may offer a clue.29 They have shown in animal models that transection or crush injury of two out of three branches of the sciatic nerve (tibial and common peroneal nerves) leads to hypersensitivity in the remaining branch (sural nerve). Interestingly, hypersensitivity was also seen in the saphenous nerve, which is not a direct branch of sciatic nerve but shares the L3 root with the sciatic nerve.29 Their findings suggest that an injury in a nerve branch can affect other nerve branches that share proximal origins. We theorized that similar to the above model, compressions at CTS and CuTS made patients more susceptible to neuropathy of other nerve branches in the upper extremity. Of note, this is a distinct phenomenon from the well-described “double crush syndrome” where there are compressions at a proximal site and a distal site along the course of a single nerve.30 For distinction, we suggest the term “parallel crush syndrome” to describe the increased susceptibility of nerves caused by compression of other nerves running in parallel.
Significant improvement in shoulder ROM was seen after treatment in our patients. Previous studies have shown that strain in the median and ulnar nerves increases during shoulder elevation.31,32 One possible explanation for the improved shoulder ROM is the reduced irritability of the median and ulnar nerves after surgical decompression, which in turn allowed the nerves to withstand traction better.
A major limitation of the study is that not all patients had imaging study such as MRI or CT scan to evaluate the shoulder or the cervical spine because the disorders of these were ruled out based on physical findings alone.
However, the significant improvement in pain and ROM after treatment suggest that compressive neuropathy was a major cause of shoulder dysfunction in our cohort of patients. At our hospital, ultrasound-guided nerve injections could be readily performed by orthopedic surgeons in the outpatient clinic. Therefore, injections were quick, effective tools that were both diagnostic and therapeutic.
At other clinical practices, ultrasound-guided nerve injections may not be readily available, and patients may need to be referred elsewhere to receive injections. Even for clinicians in such settings, our results are relevant. Wrist brace and counseling on avoiding elbow flexion and pressure can be provided, and recognizing CTS and CuTS as potential causes of shoulder dysfunction may relieve symptoms around the shoulder caused by CTS and CuTS.
In summary, this study shows that patients presenting with a chief complaint of shoulder pain or stiffness may be having symptoms from unrecognized nerve compressions at the carpal tunnel and cubital tunnel. Physical examination (manual compression of median nerve and Tinel’s sign around the cubital tunnel) can help identify such patients. When appropriately recognized, simple interventions such as orthosis, nerve blocks, and surgery can significantly improve these symptoms.
ACKNOWLEDGEMENTS
Dr. Yuji Inada was an orthopedic surgeon who has devoted his career to treatment of complex, challenging chronic pain. He sadly passed away in February 2020 at the age of 60. He was a key contributor to this research and would have been a co-author of this article.
PATIENT CONSENT
The patients provided written consent for the use of their images.
Footnotes
Published online 17 February 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Ring D1, Gus SD, Malhotra L, Jupiter JB. Idiopathic arm pain. J Bone Joint Surg Am. 2004;86:1387–1391. [DOI] [PubMed] [Google Scholar]
- 2.Kendall D. Aetiology, diagnosis, and treatment of paraesthesiae in the hands. Br Med J. 1960;2:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crymble B. Brachial neuralgia and the carpal tunnel syndrome. Br Med J. 1968;3:470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phalen GS. The carpal-tunnel syndrome. Clinical evaluation of 598 hands. Clin Orthop Relat Res. 1972;83:29–40. [DOI] [PubMed] [Google Scholar]
- 5.Kummel BM, Zazanis GA. Shoulder pain as the presenting complaint in carpal tunnel syndrome. Clin Orthop Relat Res. 1973;92:227–230. [DOI] [PubMed] [Google Scholar]
- 6.Richard BR, Jose O. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. First ed. Stoneham, MA: Butterworth-Heinemann; 1993:37–38. [Google Scholar]
- 7.Nakanishi Y, Omokawa S, Kobata Y, et al. Ultrasound-guided selective sensory nerve block for wide-awake forearm tendon reconstruction. Plast Reconstr Surg Glob Open. 2015;3:e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingerman M. Regional anesthesia for outpatient hand surgery: ultrasound-guided peripheral nerve block. J Hand Surg Am. 2011;36:532–4; quiz 534. [DOI] [PubMed] [Google Scholar]
- 9.Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand. 2008;52:727–737. [DOI] [PubMed] [Google Scholar]
- 10.American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918–922. [DOI] [PubMed] [Google Scholar]
- 11.Ralph MB, Nathan DP. Manual of Nerve Conduction Studies. 2nd ed. New York, N.Y.: Demos Medical Publishing; 2006:144–160. [Google Scholar]
- 12.Preston DC, Logigian EL. Lumbrical and interossei recording in carpal tunnel syndrome. Muscle Nerve. 1992;15:1253–1257. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. [DOI] [PubMed] [Google Scholar]
- 14.Jobe FW, Moynes DR. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med. 1982;10:336–339. [DOI] [PubMed] [Google Scholar]
- 15.Kelly BT, Kadrmas WR, Speer KP. The manual muscle examination for rotator cuff strength. An electromyographic investigation. Am J Sports Med. 1996;24:581–588. [DOI] [PubMed] [Google Scholar]
- 16.Shima H, Kida H, Sugano W, et al. Peripheral nerve problems following venipuncture - SFPD (The sum of factors concerning peripheral disorders) hypothesis and its prevention – Part 1. A case report of 10 cases that complained neurological symptoms and required treatment in hospitals after blood donation. J Jpn B Program. 2012;34:591–594. [Google Scholar]
- 17.Owaki Y, Nishi A, Sato C, et al. The introduction of SAEFP test as a self-check. J Jpn B Program. 2014;37:589–592. J-GLOBAL ID:201502240904044962 [Google Scholar]
- 18.Imaeda T, Toh S, Wada T, et al. ; Impairment Evaluation Committee, Japanese Society for Surgery of the Hand. Validation of the Japanese Society for Surgery of the Hand version of the Quick Disability of the Arm, Shoulder, and Hand(QuickDASH-JSSH) questionnaire. J Orthop Sci. 2006;11:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torebjörk HE, Ochoa JL, Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain. 1984;18:145–156. [DOI] [PubMed] [Google Scholar]
- 20.Sedal L, McLeod JG, Walsh JC. Ulnar nerve lesions associated with the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1973;36:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padua L, Coraci D, Erra C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15:1273–1284. [DOI] [PubMed] [Google Scholar]
- 22.Maki Y, Tsubokawa N, Narisawa H. Pain as a symptom of carpal tunnel syndrome. J Jpn Soc Surg Hand. 2017;33:863–866. [Google Scholar]
- 23.del Piñal F. American Society for Surgery of the Hand_meeting Instructional Course Lectures. Available at https://www.asshannualmeeting.org/servlet/servlet.FileDownload?file=00P0a00000mMoGUEA0. Accessed August 10, 2021.
- 24.Francisco del Piñal. Irritative Carpal Tunnel Syndrome. American Society for Surgery of the Hand_HAND.e. 2020. Available at https://www.assh.org/hande/s/watch?id=aBP0a000000MWUKGA4. Accessed August 10, 2021
- 25.Durkan JA. A new diagnostic test for carpal tunnel syndrome. J Bone Joint Surg Am. 1991:73:535–538. [PubMed] [Google Scholar]
- 26.Kahn LC, Yee A, Mackinnon SE. Important details in performing and interpreting the scratch collapse test. Plast Reconstr Surg. 2018;141:399–407. [DOI] [PubMed] [Google Scholar]
- 27.Čebron U, Curtin CM. The scratch collapse test: a systematic review. J Plast Reconstr Aesthet Surg. 2018:71:1693–1703. [DOI] [PubMed] [Google Scholar]
- 28.Abdo H, Clalvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019:365:695–599. [DOI] [PubMed] [Google Scholar]
- 29.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. [DOI] [PubMed] [Google Scholar]
- 30.Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359–362. [DOI] [PubMed] [Google Scholar]
- 31.Byl C, Puttlitz C, Byl N, et al. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg Am. 2002;27:1032–1040. [DOI] [PubMed] [Google Scholar]
- 32.Ochi K, Horiuchi Y, Horiuchi K, et al. Shoulder position increases ulnar nerve strain at the elbow of patients with cubital tunnel syndrome. J Shoulder Elbow Surg. 2015;24:1380–1385. [DOI] [PubMed] [Google Scholar]