Abstract
Objectives:
Typically, early childhood vaccination coverage in the U.S. is measured as the proportion of children by age 24 months who completed recommended vaccine series. However, these measures do not reflect whether vaccine doses were received at the ages recommended by the U.S. Advisory Committee on Immunization Practices, or whether children received vaccines concomitantly, per the ACIP recommended schedule. This study’s objective was to quantify vaccine timeliness and prevalence of specific patterns of undervaccination in U.S. children ages 0–19 months.
Methods:
Using 2017 National Immunization Survey-Child data, we calculated days undervaccinated for the combined 7-vaccine series and distinguished undervaccination patterns indicative of parental vaccine hesitancy, such as spreading out vaccines across visits (“shot-limiting”) or starting some but not all recommended vaccine series (“selective vaccination”), from other non-hesitancy patterns, such as missing final vaccine dose(s) or receiving all doses, with some or all late. We measured associations between demographic, socioeconomic and other characteristics with undervaccination patterns using multivariable log-linked binomial regression. Analyses accounted for the complex survey design.
Results:
Among n=15,333 U.S. children, only 41.2% received all recommended vaccine doses on-time by age 19 months. Approximately 20.9% of children had an undervaccination pattern suggestive of parental vaccine hesitancy, and 36.2% had other undervaccination non-hesitancy patterns. Uninsured children and those with lower levels of maternal education were more likely to exhibit undervaccination patterns suggestive of parental hesitancy. Lower levels of maternal education were also associated with other non-hesitancy undervaccination patterns.
Conclusions:
More than half of children in the U.S. are undervaccinated at some point by 19 months of age. Ongoing assessment of vaccine timeliness and immunization schedule adherence could facilitate timely and targeted public health interventions in populations with high levels of undervaccination.
Keywords: undervaccination, schedule, timeliness, childhood immunisation
Introduction
Providing vaccines to young children through an established immunization schedule has prevented millions of illnesses and deaths in the U.S. (1–4). The U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) currently recommends that children receive 10 vaccine series to protect against 14 diseases in the first two years of life (5). The ACIP recommends that by 19 months of age children receive four doses of diphtheria-tetanus-acellular pertussis (DTaP), three doses of poliovirus, one dose of measles-mumps-rubella (MMR), 3 doses of hepatitis B, three or four doses (depending on the brand) of Haemophilus influenzae type b (Hib), one dose of varicella, and four doses of pneumococcal conjugate vaccines (5). This 4:3:1:3*:3:1:4 series is also known as the combined 7-vaccine series, though the ACIP also recommends vaccinations for rotavirus, hepatitis A, and influenza during this time frame (5,6).
One metric used by the CDC to annually assess early childhood vaccine coverage is completion of the combined 7-vaccine series by age 19–35 months (6). As of 2019, the CDC started reporting this metric for children 24 months of age by birth cohort (7). CDC assesses this measure at national, state, and some local levels through the annual National Immunization Survey-Child (NIS-Child), an annual survey used for monitoring vaccine coverage among young children in the U.S. NIS-Child data from recent years estimate that about 70% of U.S. children have received all recommended vaccine doses in the combined 7-vaccine series by 19–35 months of age (6). However, this metric does not account for whether or not children received vaccine doses per the schedule recommended by the ACIP (8,9). Previous studies have shown that over half of U.S. children experience undervaccination in early childhood, meaning they are late in receiving or do not receive some or all recommended vaccine doses (8,10,11). Children who are behind on or missing recommended vaccine doses may be vulnerable to vaccine-preventable diseases (VPDs) and multiple VPD outbreaks have been linked to children experiencing undervaccination (12,13).
A 2015 National Vaccine Advisory Committee report emphasized the importance of measuring delays in receiving vaccine doses and recommended more focus on using timely vaccination as an indicator of vaccine confidence and a quality measure of immunization services (14). Previous studies have identified that between 13% and 23% of U.S. children may be vaccinated per an immunization schedule other than the one recommended by ACIP (9,15, 16). Several prior studies have used NIS-Child and other vaccination data sources to identify undervaccination patterns indicative of parental hesitancy, including spreading out vaccines across multiple visits (“shot-limiting”) and getting some recommended vaccines series but not others (“selective” vaccination) (9,16–18). However, not all children who fall behind or are missing vaccine doses fall into one of these patterns indicative of parental hesitancy (e.g., purposeful delay or refusal of one or more vaccines) (16,19,20). Therefore, in this study, we also sought to classify other undervaccination patterns not fully captured in previous work focusing on parental hesitancy.
The objectives of the current study were to 1) measure adherence to the ACIP-recommended immunization schedule, including timeliness of vaccine receipt, and 2) to quantify the prevalence of specific patterns of undervaccination among U.S. children ages 0–19 months using a nationally representative sample. Furthermore, we sought to determine whether socioeconomic, demographic, and other factors were associated with either patterns indicative of parental hesitancy or other types of undervaccination patterns.
Methods
Data source
We analyzed the 2017 NIS-Child public use data file for the current study (21). NIS-Child is a nationally representative annual telephone survey (using random-digit dialing) sponsored by the CDC that serves as the main surveillance system for monitoring early childhood vaccine coverage in the U.S. (22,23). Survey participants are parents of children ages 19–35 months. The survey includes a parent/guardian interview followed by a mailed questionnaire to the child’s medical providers to collect verified immunization histories for all vaccine doses the child received. Multi-stage weighting that accounts for sampling rate variation, nonresponse bias, and representativeness of the sample relative to the target population is used to ensure that data collected from the survey sample can be analyzed to represent vaccine coverage at national and state levels (22–24). More detail on NIS-Child sampling and weighting procedures have been previously published (24).
Per University of Montana Institutional Review Board protocol for secondary analysis of publicly available datasets, no review was required.
Study sample
The complete 2017 NIS-Child sample includes children who were ages 19–35 months at the time of household interview and from all 50 states, Washington, D.C., and Guam. Per NIS-Child recommendations for estimating vaccine coverage (25), we restricted our sample to the 15,333 children living in the U.S. (excluding territories) who had provider-verified vaccination data.
Vaccine series completion
The 2017 NIS-Child public use file includes variables that report the child’s age in days at each vaccination. We used these variables to estimate vaccine coverage at age 19 months, including whether or not children had received all recommended vaccine doses for the combined 7-vaccine series by age 19 months. As the ACIP recommends that all vaccine doses in the combined 7-vaccine series be completed by age 19 months, our study assessed vaccine timeliness and undervaccination patterns by this milestone. Additionally, the NIS-Child public use data file only reports three broad age categories for children based on their age when their parent/guardian was surveyed: 19–23 months, 24–29 months, and 30–35 months. By measuring vaccine coverage through 19 months of age, we were able to include all 15,333 children in our analytic sample. We also presented the percentage of children who had completed the following vaccine series by age 19 months: two doses of influenza, two or three doses (depending on the brand) of rotavirus during early childhood, and two doses of hepatitis A vaccines.
Vaccine timeliness
Using NIS-Child variables that provide the age in days at time of vaccination for each dose reported, we were able to calculate days undervaccinated (8–11) for each vaccine series in the combined 7-vaccine series through 19 months of age. Days undervaccinated is the difference between the age in days when a child received a vaccine dose and when the child should have received the dose per ACIP recommendations (8–11). For our calculations, days undervaccinated began accruing 30 days after a given dose was due. Details on the days undervaccinated calculations used in this study can be found in Table 1.
Table 1.
Specifications for calculating days undervaccinated for vaccine doses in the combined 7-vaccine seriesa
| Vaccine dose | Recommended age per ACIP | Minimum acceptable age in days, allowing for 4 day grace period | Minimum acceptable number of days between doses, allowing for 4 day grace period | Age in days when child is considered late |
|---|---|---|---|---|
| Diphtheria, tetanus, acellular pertussis (DTaP) | ||||
| Dose 1 | 2 months | 38 | -- | 93 |
| Dose 2 | 4 months | 66 | 24 | 154 |
| Dose 3 | 6 months | 94 | 24 | 215 |
| Dose 4 | 15–18 months | 361 | 179 | 580 |
| Inactivated poliovirus (IPV) | ||||
| Dose 1 | 2 months | 38 | -- | 93 |
| Dose 2 | 4 months | 66 | 24 | 154 |
| Dose 3 | 6–18 months | 94 | 24 | 580 |
| Measles, mumps, rubella (MMR) | ||||
| 1 dose | 12–15 months | 361 | -- | 489 |
| Hepatitis B | ||||
| Dose 1 | 0–2 months | 0 | -- | 93 |
| Dose 2 | 1–4 months | 24 | 24 | 154 |
| Dose 3 | 6–18 months | 164 | 38 | 580 |
| Haemophilus infleunzae type b (Hib) b,c | ||||
| Dose 1 | 2 months | 38 | -- | 93 |
| Dose 2 | 4 months | 66 | 24 | 154 |
| Dose 3 | 6 months | 94 | 24 | 215 |
| Dose 4 | 12–15 months | 361 | 52 | 580 |
| Varicella | ||||
| 1 dose | 12–15 months | 361 | -- | 489 |
| Pneumoccocal conjugate c | ||||
| Dose 1 | 2 months | 38 | -- | 93 |
| Dose 2 | 4 months | 66 | 24 | 154 |
| Dose 3 | 6 months | 94 | 24 | 215 |
| Dose 4 | 12–15 months | 361 | 52 | 580 |
Abbreviations: ACIP, Advisory Committee on Immunization Practices
These specifications were adapted from Glanz et al. (reference 9), which were adapted from Luman et al. (reference 8). For our calculations, we changed the minimum age of the 3rd dose of the Hepatitis B birth dose to 164 days from 176 days to account for ACIP guidelines that the final dose of this series be administered at age 24 weeks or later (24 × 7 days = 168 – 4 day grace period = 164 days) (Newcomer et al., reference 19; Robinson et al., reference 5).
If the first two Hib doses are PRP-OMP [PedvaxHib® or Comvax® brands], then a 6-month dose of the vaccine is not required.
Even though ACIP recommends the 4th dose of Hib and pneumococcal vaccines at age 12–15 months, we did not consider the 4th doses of Hib or pneumococcal conjugate vaccines late until age 580 days, which is approximately age 19 months. The combined DTaP-IPV-Hib vaccine (Pentacel® brand) is recommended to be given at ages 2,4,6 and 15–18 months, and pneumococcal, DTaP-containing, and Hib-containing vaccines are often concomitantly administered (Glanz et al., reference 9; Newcomer et al., reference 19; Sanofi Pentacel, reference 49).
Undervaccination patterns
We identified patterns of vaccination in the 2017 NIS-Child provider-verified sample. These patterns were assessed in a hierarchical manner, so that children were only reported within one group. First, we identified children with 0 days undervaccinated; these children received all vaccines on-time, per the ACIP schedule. Then, among children with ≥1 day undervaccinated, we identified three undervaccination patterns that have been previously found to be indicative of parental vaccine hesitancy: restrictive shot-limiting, defined as having at least 6 immunization visits with ≤3 vaccines at each visit before age 19 months (16,19), episodic shot-limiting, defined as having at least one immunization visit with 2 or fewer vaccines before age 15 months and no more than 2 immunization visits with 3 or more vaccines (17,19), and selective vaccination, defined as the child not starting at least one series in the combined 7-vaccine series before age 19 months (16,19). For the restrictive and episodic shot-limiting patterns, we considered all vaccine doses given on the same day, including those in the combined 7-vaccine series, as well as influenza, hepatitis A, and rotavirus (which is an orally-administered vaccine) doses. Combination vaccines were considered as one “shot” for assessing these shot-limiting patterns.
The next group identified were children who did not receive any doses of vaccines in the combined 7-vaccine series before age 3 months, which was classified as a delayed start pattern of undervaccination (19). While delayed start of vaccination could be due to parental choice to wait on initiating vaccine series, other reasons, such as illness with high fever or inability to access an immunization provider in time, may also influence this pattern. Therefore, we did not include children in the delayed start pattern in the group of patterns indicative of parental hesitancy described above, or in the group of other undervaccination patterns described next.
The remaining children in the dataset could be classified into two other undervaccination patterns: missing doses, which included children who had started all recommended series in the combined 7-vaccine series but were missing some doses needed for completion, and children who had received all doses, some or all late, indicating that they completed all recommended doses by age 19 months, but they received some or all doses later than recommended age(s) (19).
Data analysis
We described the population demographics and characteristics as well as vaccine coverage measures through age 19 months for the 2017 NIS-Child provider-verified sample. Using days undervaccinated calculations for individual vaccine series and the combined 7-vaccine series as a whole, we reported the number and percentage of children who 1) received all recommended vaccine doses on time, 2) received all recommended vaccine doses by 19 months but not all doses were received on time, and 3) those that did not complete all recommended vaccine doses by 19 months. Additionally, we reported the mean and standard error (SE), and the median and interquartile range (IQR), of days undervaccinated for each series and for the combined 7-vaccine series overall.
We also presented the number and percentage of children in each undervaccination pattern described above. Within each pattern, we calculated the mean (SE) and median (IQR) for the average number of days undervaccinated (ADU) at age 19 months (8,10). For ADU, we calculated the numerator as the sum of the number of days undervaccinated across series in the combined 7-vaccine series, and the denominator as the weighted sum of the number of vaccine series that should have been received by age 19 months (10,18,19). Since our analyses focused on the combined 7-vaccine series, the denominator was seven vaccines, weighted for the number of days that the child should have been vaccinated with those series through age 19 months. Additionally, we presented the number and percentage of children in each pattern that completed the combined 7-vaccine series by age 19 months.
We performed two separate multivariable log-linked binomial regression models. In one multivariable model, we estimated the association between demographic, socioeconomic and other characteristics and the outcome of being undervaccinated per a pattern indicative of parental hesitancy, which included the restrictive shot-limiting, episodic shot-limiting, and selective vaccination patterns as one group. The other multivariable model examined the outcome of being vaccinated per another pattern not indicative of parental hesitancy, which included starting all recommended series but missing doses and children who had received all doses, but some or all doses were late. The covariates in both models were identified a priori and included children’s age (at time of household interview) category, sex, combined race and ethnicity, census region, insurance status, types of immunization clinics accessed, history of receiving WIC benefits, and maternal education. In these multivariable analyses, we excluded 290 observations that were missing data for immunization clinic type. We conducted tests for multicollinearity in the two multivariable models (26). Unadjusted and adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) were reported.
All study analyses were conducted using SAS 9.4 (Cary, NC) and accounted for the stratified design and weighting of NIS-Child using SAS survey procedures. In the current study, we presented weighted proportions, means, and prevalence ratios (PRs) as appropriate.
Results
NIS-Child received a 26.1% overall (51.9% for landlines and 25.0% for cellphones) response rate to the 2017 telephone interview portion of the survey and adequate provider-reported vaccination data were available for 15,333 (53.9%) of children with a completed household interview (6). Overall, about 51.2% of children were male (Table 2). The majority of children were non-Hispanic White (47.0%), followed by children that were Hispanic, any race (26.8%). Non-Hispanic Black children accounted for 12.8% of the population. Most (55.7%) children had records indicating they accessed only private facilities for immunizations and about 53% had a history of ever receiving WIC benefits. The most common maternal education level was a college graduate education (37.7%), followed by more than 12 years of education, with no college graduation (22.4%). The percent of children that had completed all recommended doses for individual vaccine series in the combined 7-vaccine series by age 19 months ranged from 69.2% for DTaP and 90.3% for poliovirus, with 57.2% having completed the entire combined 7-vaccine series.
Table 2.
Demographic and socioeconomic characteristics, and vaccine coverage at age 19 months, 2017 National Immunization Survey-Child, United States
| Demographic and socioeconomic factors | n (weighted %) |
|---|---|
| Total sample with provider-verified immunization records n=15,333 | |
| Child’s age at time of survey interview | |
| 19–23 months | 4,518 (30.0) |
| 24–29 months | 4,540 (33.9) |
| 30–35 months | 6275 (36.1) |
| Child’s sex | |
| Male | 7,819 (51.2) |
| Female | 7,514 (48.8) |
| Race and Ethnicity | |
| White alone, Non-Hispanic | 8,958 (47.0) |
| Black alone, Non-Hispanic | 1,102 (12.8) |
| All other races alone and multiple races, | 2,104 (13.3) |
| Non-Hispanic | 3,169 (26.8) |
| Any race, Hispanic | |
| Census Region | |
| South | 6,012 (39.1) |
| Northeast | 2,986 (15.7) |
| Midwest | 3,071 (20.7) |
| West | 3,264 (24.4) |
| Insurance status | |
| Private Insurance only | 7,843 (41.6) |
| Any Medicaid | 5,714 (47.8) |
| Other Insurance | 1,337 (7.8) |
| Uninsured | 439 (2.7) |
| Vaccine provider facility type | |
| All public facilities | 1,708 (13.0) |
| All hospital facilities | 2,396 (13.7) |
| All private facilities | 8,318 (55.7) |
| All military/other facilities | 398 (3.6) |
| Mixed Types | 2,223 (13.9) |
| Missing | 290 |
| Child ever received WIC benefits | |
| Yes | 6,392 (53.0) |
| Not Yesa | 8,941 (47.0) |
| Maternal Education | |
| < 12 Years | 1,639 (16.0) |
| 12 Years | 2,503 (23.8) |
| > 12 years, Non-college Grad | 3,639 (22.4) |
| College Grad | 7,552 (37.7) |
| Vaccine coverage by age 19 months | |
| Vaccines in combined 7-vaccine series: | |
| Diphtheria-tetanus-acellular pertussis (DTaP), 4+ doses | 11,095 (69.2) |
| Poliovirus, 3 doses | 14,021 (90.3) |
| Measles-mumps-rubella, 1+ doses | 13,577 (88.3) |
| Haemophilus Influenzae type b (Hib), 2–3 doses | 11,457 (72.7) |
| Hepatitis B, 3+ doses | 13,665 (88.2) |
| Varicella, 1+ doses | 13,354 (87.0) |
| Pneumococcal conjugate, 4+ doses | 12,118 (76.9) |
| Combined 7-vaccine series | 9,357 (57.2) |
| Other vaccines recommended by | |
| ACIP: | 11,562 (71.0) |
| Rotavirus, 2–3 doses | |
| Hepatitis A | 8,225 (54.8) |
| 1 dose | 4,167 (24.9) |
| ≥2 doses | |
| Influenza | 10,488 (65.0) |
| ≥1 dose | 8,481 (51.0) |
| 2+ doses |
Timeliness of vaccine receipt
For vaccines in the combined 7-vaccine series, 7,139 children (41.7%) received all doses on time, 2,218 (15.6%) received all doses but some or all were late, and 5,976 (42.7%) were missing some or all doses required to complete one or more series by age 19 months (Table 3). Overall, 350 (1.5%) children received no vaccines through age 19 months. Among the individual vaccine series, the hepatitis B vaccine series (3 doses) had the highest percentage (83.1%) of children that received all doses on time. Vaccine series with four doses had lower percentages of children with on-time series completion, with the DTaP vaccine series having the lowest (56.1%).
Table 3.
Timeliness of vaccine receipt among U.S. children by 19 months of age, 2017 National Immunization Survey-Child, n=15,333
| Vaccine series: | Diphtheria-tetanus-acellular pertussis, 4 doses | Poliovirus, 3 doses | Measles-mumps-rubella, 1 dose | Haemophilus Influenzae type b, 3 or 4 doses, depending on brand | Hepatitis B, 3 doses | Varicella, 1 dose | Pneumococcal conjugate, 4 doses | Combined 7-vaccine series |
|---|---|---|---|---|---|---|---|---|
| Timing of vaccine receipt, no. of children (weighted %) | ||||||||
| All doses, on time | 9,387 (56.1) | 12,201 (75.6) | 12,819 (82.6) | 9,588 (57.2) | 12,994 (83.1) | 12,479 (80.3) | 9,754 (58.4) | 7,139 (41.7) |
| All doses, but not all on time | 1,708 (13.1) | 1,820 (14.7) | 758 (5.8) | 1,869 (15.5) | 671 (5.2) | 875 (6.6) | 2,364 (18.5) | 2,218 (15.6) |
| Missing some or all doses | 4,238 (30.8) | 1,312 (9.7) | 1,756 (11.7) | 3,876 (27.3) | 1,668 (11.8) | 1,979 (13.1) | 3,215 (23.1) | 5,976 (42.7) |
| Number of days undervaccinated | ||||||||
| Mean (SE) | 60.7 (2.3) | 41.1 (2.0) | 13.3 (0.5) | 67.3 (2.4) | 36.2 (2.0) | 15.0 (0.5) | 73.1 (2.6) | 102.6 (2.8) |
| Median (IQR) | 0 (0–19.7) | 0 (0–0) | 0 (0–0) | 0 (0–36.3) | 0 (0–0) | 0 (0–0) | 0 (0–49.7) | 0 (0–135.6) |
Abbreviations: SE, standard error; IQR, interquartile range
In the cohort overall, for each of the individual vaccine series in the combined 7-vaccine series, children had a median of 0 days undervaccinated. Most individual vaccine series had an interquartile range of 0 days undervaccinated, with the exception of the four-dose series: DTaP (0–19.7 days), Hib (0–36.3 days), and pneumococcal (0–49.7 days). The median number of days children were undervaccinated for any vaccine by 19 months of age was also 0 (IQR=0–135.6 days).
Patterns of undervaccination
While 7,139 (41.7%) children received all vaccines on time, 3,025 children (20.9%) had ≥1 day undervaccinated and had an undervaccination pattern indicative of parental choice to delay or refuse vaccination (Figure 1). Specifically, 761 (5.3%) and 583 (4.4%) children were categorized as falling into restrictive and episodic shot-limiting patterns of vaccination, respectively. Selective vaccination was identified in 1,681 (11.2%) children. Other undervaccination patterns not indicative of parental hesitancy were identified in 5,044 (36.2%) children (Figure 1), including 3,118 (22.6%) who started all recommended series but were missing dose(s) needed to complete series, and 1,926 (13.6%) who had completed recommended series by age 19 months, but some or all doses were received late.
Figure 1.
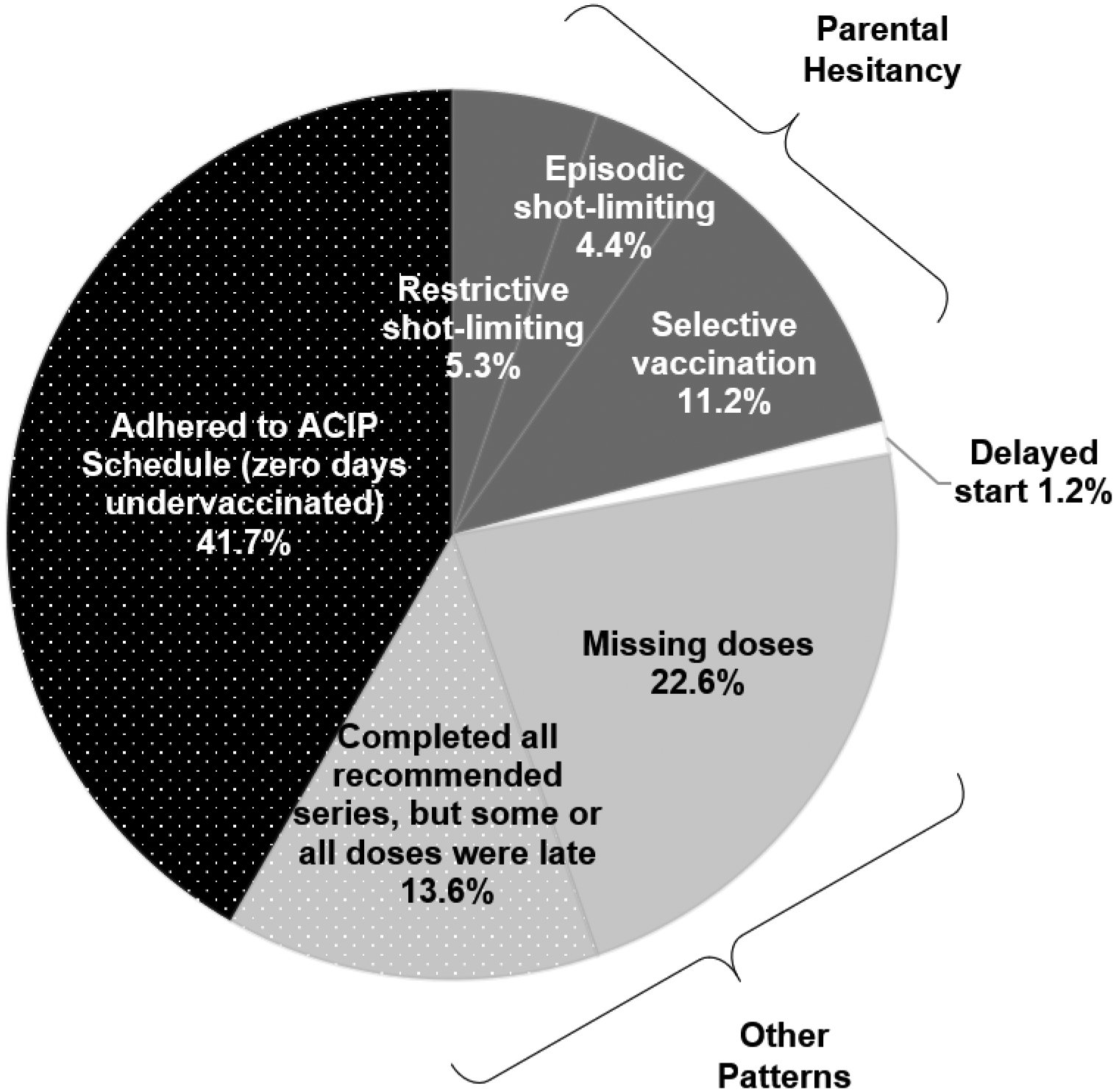
Vaccination patterns before age 19 months indicating adherence with the ACIP schedule, parental vaccine hesitancy, and other barriers too accessing immunization services, U.S., 2017 National Immunization Survey-Child.
Among patterns of undervaccination, mean ADU was highest for those with a delayed start at 264.4 days (SE=39.2), followed by those with selective vaccination patterns at 250.2 days (SE=7.6) (Table 4). Mean ADU was lowest for those who completed all recommended series, but some or all doses were late at 23.6 days (SE=1.1). Only 24.3% and 10.4% of children with a restrictive shot-limiting and episodic shot-limiting patterns, respectively, had completed the combined 7-vaccine series by 19 months of age.
Table 4.
Prevalence of patterns of undervaccination among U.S. children ages 0–19 months, 2017 National Immunization Survey-Child, n=15,333
| Overall frequency of undervaccination patterns | Completed combined 7-vaccine series by age 19 months | Average days undervaccinated for the combined 7-vaccine series | ||
|---|---|---|---|---|
| Pattern | n (column %)a | n (row %)a | Mean (±SE)b | Median (IQR) |
| n=15,333 | ||||
| All vaccines received on-time (zero days undervaccinated) | 7,139 (41.7) | 7,139 (100%) | 0 days (±0.0) | 0 (0–0) |
| Undervaccination patternsc indicating parental hesitancy | ||||
| Restrictive shot-limiting: | ||||
| At least 6 visits with ≤3 vaccines at each visit before age 19 months | 761 (5.3) | 182 (24.3%) | 80.8 days (±8.9) | 34.7 (15.3–115.2) |
| Episodic shot-limiting: | ||||
| At least 1 immunization visit with ≤2 vaccines before age 15 months and no more than 2 immunization visits with ≥3 vaccinesd | 583 (4.4) | 80 (10.4%) | 185.7 days (±11.9) | 164.5 (57.7–309.5) |
| Selective vaccination: | ||||
| Did not start a recommended vaccine series | 1,681 (11.2) | 0 (0%) | 250.2 days (±7.6) | 228.0 (47.0–487.4) |
| Undervaccination patterns indicating delayed start e | ||||
| Delayed start: | ||||
| Received first combined 7-vaccine series dose at age 3 months or later | 125 (1.2) | 30 (16.9%) | 264.4 days (±39.2) | 247.7 (104.6–442.4) |
| Other undervaccination patterns | ||||
| Missing doses: | ||||
| Started all series, missing dose(s) needed to complete series | 3,118 (22.6) | 0 (0%) | 45.4 days (±2.0) | 16.5 (0.3–63.9) |
| All doses received, some or all late: | ||||
| Completed all recommended series, but some or all doses were late | 1,926 (13.6) | 1,926 (100%) | 23.6 days (±1.1) | 14.0 (5.2–30.8) |
Abbreviations: SE, standard error
The unweighted number is presented alongside a weighted percentage
Mean (SE) presented are weighted
When classifying vaccination patterns, vaccine doses in the 7-combined vaccine series (diphtheria-tetanus-acellular pertussis, poliovirus, measles-mumps-rubella, Haemophilus Influenzae type b, hepatitis B, varicella, and pneumococcal conjugate vaccines) were considered, with the exception of the restrictive and episodic shot-limiting patterns. For the shot-limiting patterns, all vaccine doses given at immunization visits were considered, including oral rotavirus doses, and combination vaccines were counted as one vaccine.
For the episodic shot-limiting pattern, children had to have at least two immunization visits, and the requirement of at least 1 immunization visit with ≤2 vaccines did not include individual Hepatitis B vaccines given within 6 weeks of birth or influenza vaccines.
Delayed start patterns can indicate either parental hesitancy or other barriers to accessing immunization services
Factors associated with undervaccination patterns indicative of parental hesitancy
Factors associated with increased likelihood of exhibiting undervaccination patterns indicative of parental hesitancy included living in the Northeast (aPR=1.36, 95% CI: 1.18–1.56) compared to those located in the South, after adjusting for other population demographics and characteristics in our multivariable model. Compared to children with private insurance only, children with any Medicaid (aPR=1.31, 95% CI: 1.06–1.62) or no insurance (aPR=2.27, 95% CI: 1.78–2.88) had significantly higher likelihood of exhibiting undervaccination patterns indicative of parental vaccine hesitancy as well. Children with vaccine providers that were all hospital-based facilities (aPR=1.28; 95% CI: 1.03–1.60), compared to all public facilities, were also more likely to have undervaccination patterns indicative of parental hesitancy. Compared to children with mothers who were college graduates, having only 12 years of education (aPR=1.28; 95% CI: 1.04–1.57) was associated with increased likelihood of undervaccination patterns indicative of parental hesitancy (Table 5).
Table 5.
Factors associated with patterns of undervaccination indicative of parental hesitancya by 19 months of age, n=15,333
| Prevalence Ratio (95% CI) Unadjusted | Prevalence Ratio (95% CI) Adjustedb | |
|---|---|---|
| Child’s sex | ||
| Male | Referent | Referent |
| Female | 0.93 (0.82–1.05) | 0.92 (0.81–1.04) |
| Race and ethnicity | ||
| White alone, Non-Hispanic | Referent | Referent |
| Black alone, Non-Hispanic | 1.04 (0.88–1.25) | 1.01 (0.83–1.24) |
| All other races alone and multiple races, Non-Hispanic | 0.92 (0.76–1.10) | 0.92 (0.77–1.09) |
| Hispanic | 0.92 (0.77–1.09) | 0.87 (0.71–1.05) |
| Census region | ||
| South | Referent | Referent |
| Northeast | 1.28 (1.12–1.46) | 1.36 (1.18–1.56) |
| Midwest | 1.15 (1.01–1.31) | 1.15 (1.00–1.33) |
| West | 1.04 (0.84–1.29) | 1.12 (0.90–1.39) |
| Insurance status | ||
| Private Insurance only | Referent | Referent |
| Any Medicaid | 1.25 (1.09–1.44) | 1.31 (1.06–1.62) |
| Other Insurance | 1.23 (1.02–1.49) | 1.17 (0.93–1.47) |
| Uninsured | 2.42 (1.98–2.96) | 2.27 (1.78–2.88) |
| Vaccine provider facility type c | ||
| All public facilities | Referent | Referent |
| All hospital facilities | 1.24 (1.00–1.54) | 1.28 (1.03–1.60) |
| All private facilities | 0.99 (0.82–1.20) | 1.11 (0.90–1.36) |
| All military/other facilities | 1.38 (0.90–2.13) | 1.51 (0.98–2.31) |
| Mixed Types | 1.15 (0.91–1.46) | 1.22 (0.96–1.56) |
| Child ever received WIC benefits | ||
| Not Yes d | Referent | Referent |
| Yes | 1.04 (0.92–1.18) | 0.85 (0.70–1.03) |
| Maternal education | ||
| College Grad | Referent | Referent |
| < 12 Years | 1.32 (1.09–1.61) | 1.28 (0.97–1.68) |
| 12 Years | 1.34 (1.13–1.57) | 1.28 (1.04–1.57) |
| > 12 years, Non-college Grad | 1.16 (1.00–1.35) | 1.10 (0.92–1.31) |
Patterns of undervaccination indicative of parental hesitancy included restrictive shot-limiting, episodic shot-limiting, and restrictive vaccination patterns
Estimates are adjusted for all other variables in the table and age group of child at time of survey (19–23 months, 24–29 months, 30–35 months)
290 observations were excluded due to missing values for vaccine provider facility type
Not yes includes no, do not know, never heard of WIC, and refused to answer responses
Factors associated with other undervaccination patterns
After adjusting for covariates in our multivariable model, insurance status such as any Medicaid (aPR=1.22, 95% CI: 1.07–1.39) or “other” insurance (aPR=1.27, 95% CI: 1.10–1.47) remained positively associated with other undervaccination patterns, which included starting all but not completing all recommended vaccine series and completing all recommended series but receiving some or all doses late. Additionally, children who ever received WIC benefits were also more likely (aPR= 1.15, 95% CI: 1.02–1.30) to be identified as exhibiting these patterns as compared with children who had never received WIC or whose previous WIC benefit status was unknown. Children with mothers who had <12 years of education, compared to those with mothers who were at least college graduates, had increased likelihood of exhibiting these undervaccination patterns (aPR= 1.17, 95% CI: 1.01–1.36) (Table 6).
Table 6.
Factors associated with patterns of undervaccination indicative of other barriersa by 19 months of age, n=15,333
| Prevalence Ratio (95% CI) Unadjusted | Prevalence Ratio (95% CI) Adjustedb | |
|---|---|---|
| Child’s sex | ||
| Male | Referent | Referent |
| Female | 1.02 (0.93–1.11) | 1.00 [0.92–1.09) |
| Race and ethnicity | ||
| White alone, Non-Hispanic | Referent | Referent |
| Black alone, Non-Hispanic | 1.32 (1.15–1.51) | 1.11 (0.97–1.27) |
| All other races alone and multiple races, Non-Hispanic | 1.10 (0.95–1.26) | 1.02 (0.89–1.17) |
| Hispanic | 1.26 (1.13–1.40) | 1.01 (0.90–1.12) |
| Census region | ||
| South | Referent | Referent |
| Northeast | 0.94 (0.85–1.04) | 0.99 (0.89–1.09) |
| Midwest | 0.92 (0.84–1.01) | 0.96 (0.87–1.05) |
| West | 1.07 (0.94–1.23) | 1.10 (0.96–1.26) |
| Insurance status | ||
| Private Insurance only | Referent | Referent |
| Any Medicaid | 1.50 (1.36–1.65) | 1.22 (1.07–1.39) |
| Other Insurance | 1.40 (1.22–1.60) | 1.27 (1.10–1.47) |
| Uninsured | 1.06 (0.84–1.34) | 0.98 (0.77–1.24) |
| Vaccine provider facility type c | ||
| All public facilities | ||
| All hospital facilities | Referent | Referent |
| All private facilities | 0.84 (0.73–0.96) | 0.94 (0.82–1.09) |
| All military/other facilities | 0.75 (0.67–0.85) | 0.89 (0.79–1.01) |
| Mixed Types | 0.83 (0.62–1.12) | 0.90 (0.67–1.22) |
| 0.79 (0.68–0.92) | 0.88 (0.76–1.03) | |
| Child ever received WIC benefits | ||
| Not Yes d | ||
| Yes | Referent | Referent |
| 1.46 (1.33–1.59) | 1.15 (1.02–1.30) | |
| Maternal education | ||
| College Grad | Referent | Referent |
| < 12 Years | 1.51 (1.34–1.71) | 1.17 (1.01–1.36) |
| 12 Years | 1.36 (1.21–1.53) | 1.08 (0.95–1.23) |
| > 12 years, Non-college Grad | 1.26 (1.12–1.41) | 1.05 (0.93–1.19) |
Patterns of undervaccination indicative of other barriers including starting all series but missing doses needed to complete series, and completing all series by age 19 months but receiving some or all doses late
Estimates are adjusted for all other variables in the table and age group of child at time of survey (19–23 months, 24–29 months, 30–35 months)
290 observations were excluded due to missing values for vaccine provider facility type
Not yes includes no, do not know, never heard of WIC, and refused to answer responses
Discussion
In this study, we provided a comprehensive assessment of both vaccine timeliness and undervaccination patterns in U.S. children ages 0–19 months. Using data from the nationally representative 2017 NIS-Child survey, we found that only 41.7% of U.S. children received all vaccines on time for all recommended doses in the combined 7-vaccine series by age 19 months. Patterns of undervaccination indicative of parental choice to delay, spread out, or refuse vaccines were identified in 20.9% of U.S. children. While a previous analysis of undervaccination patterns with NIS-Child 2014 data focused on patterns indicative of parental hesitancy (16), we also categorized other undervaccination patterns. We found that in 2017, 36.2% of U.S. children had other undervaccination patterns, such as missing doses needed to complete multi-dose series or having received all recommended doses with some or all of the doses late.
Delay in receiving vaccines in early childhood is a documented concern in the U.S., both nationally and at the state level (14). Similar to our finding that over half of U.S. children in 2017 were undervaccinated by age 19 months, other studies using earlier years of NIS-Child surveys also found that undervaccination is common in young children. A previous study of 2003 NIS-Child data found that 3 in 4 U.S. children were late in receiving recommended vaccine doses at some point before age 24 months (8). A study of the 2012 NIS-Child sample found that 39% of children were undervaccinated for 7 months or more (11). In an analysis of 2014 NIS-Child data that investigated patterns of vaccination for the combined 7-vaccine series and the recommended doses of hepatitis A and rotavirus through 24 months of age, Hargreaves and colleagues found that 23% of children were undervaccinated per an “alternative” schedule, which included shot-limiting and selective vaccination patterns (16). Our current study sought to quantify the prevalence of undervaccination patterns with a more recent survey year, and to distinguish undervaccination patterns indicative of parental hesitancy from other undervaccination patterns.
While previous analyses of undervaccination patterns have largely focused on patterns indicative of parental hesitancy (9,15–19), children also experience vaccine delay due to reasons unrelated to hesitancy. The other patterns described in this study, including starting all series but missing final dose(s) and completing all series but late for some or all doses, may indicate other challenges preventing children from timely access to vaccine services. Furthermore, these patterns had the lowest mean ADU (45.4 days for those missing doses and 23.6 days for those who completed all doses, with some or all late) among all undervaccination patterns. A recent study that linked parents’ survey responses with immunization data from electronic health records showed that higher ADU (>61 days) was associated with medium or high levels of parental hesitancy, while lower ADU levels were associated with low or no parental hesitancy (18). Therefore, the undervaccination patterns of starting all series but missing final dose(s) and completing all series but being late in receiving some or all doses likely indicate barriers to timely vaccination other than parental hesitancy. There are a range of other barriers to accessing immunization services, including transportation barriers, a lack of immunization providers in medically-underserved areas, and parents not knowing when to bring children in for vaccines (27–29). Moreover, children may not be vaccinated on time if they were ill with a high fever during a well-child visit, or if their immunization clinic experienced a vaccine shortage.
Our finding that 20.9% of U.S. children were undervaccinated per patterns indicative of parental vaccine hesitancy is in line with earlier NIS-Child research (16,30,31), but higher compared to similar analyses in managed care populations (9) and surveys of parents (32,33). However, these studies all used different ways of measuring parental hesitancy and use of alternative schedules, limiting direct comparisons of results. To date, there has been no consistent tracking of the prevalence of children undervaccinated due to parental hesitancy in the U.S., which is vital for planning strategic public health interventions to target barriers to vaccination (14). Vaccine refusal, delayed vaccination, and missed doses all lead to an increased risk of VPDs (34–37). However, while parental refusal to vaccinate has been linked to measles and pertussis outbreaks across the U.S., a systematic review found that other barriers also play a role in VPD risk (13). Simply being behind on recommended 4-dose DTaP series, for any reason, is associated with increased pertussis risk, compared to children receiving vaccines on time (13). This is relevant to the current study’s finding that 22.6% of U.S. children initiated all vaccine series but were missing doses needed to complete multi-dose series. Previous studies have also found that missed opportunities to vaccinate and missed well-child visits have been obstacles to on-time series completion for multi-dose series, particularly the fourth DTaP dose (38,39).
Results from our multivariable models suggest that certain population demographics and characteristics may play a role in which children are more vulnerable to having undervaccination patterns that we identified in this study (9,15,16). Other studies examining NIS-Child data have also found similar associations between some of these factors, such as insurance status, vaccine provider facility type, and maternal education, and the likelihood of a child engaging in “alternate” or “unknown” vaccination patterns (16), as well as the likelihood of completing recommended immunization series (16,40,41). Our models indicate that some of these factors may play a role in undervaccination in general, whether it is due to parental hesitancy or other barriers. As compared to children with private insurance, children with any Medicaid were more likely to have an undervaccination pattern indicative of parental hesitancy and were also more likely to have an undervaccination pattern indicative of other barriers. Being uninsured was strongly associated with undervaccination patterns indicative of parental hesitancy. Parents and guardians of children with Medicaid or no insurance may be less likely to have consistent access to a regular primary care provider, and thus could be less likely to develop a trusting, long-term relationship with an immunization provider, which can impact vaccine education and confidence (42–45). Having Medicaid may also reflect a range of other barriers, such as transportation obstacles, that prevent timely vaccination at the multiple visits recommended in the first two years of life.
Similarly, our models showed that lower maternal education was associated with patterns indicative of both parental hesitancy (mothers with only 12 years of education compared to college graduates) and other barriers (mothers with <12 years compared to college graduates). Parental education is known to have conflicting associations with parental vaccine hesitancy in the existing literature (46). Data from the 2009 NIS-Child, which included questions to parents about whether they had ever refused or delayed a vaccine dose for their child, showed that a higher percentage of mothers who were college graduates reported doing so (30). Multiple studies have also found that parents with less formal education, or education that was less than 12 years, were more likely to report concerns about vaccines and their necessity, safety, and efficacy (33,46,47). However, others have found that parents with higher levels of education were more likely to be concerned about vaccines or to refuse them (46,48).
Limitations
Provider-verified NIS data are the current gold standard for estimating national vaccination coverage via public health surveillance. However, there are limitations of this data source. There is potential for selection bias among the telephone survey respondents due to low response as well as households without access to landlines or cell phones. Additionally, parents/guardians who are vaccine hesitant may be less likely to participate in a survey about their child’s immunization history. While this is a limitation, the sophisticated multi-stage weighting accounts for differences between participating and non-participating households. Previous examinations of NIS-Child data found that vaccine coverage is lower in weighted results as compared to unweighted results, suggesting that survey non-response is associated with lower vaccine coverage (23).
NIS-Child relies on parent/guardian report of all of the child’s vaccine providers, who are then expected to report and verify the child’s vaccination history. There is potential for parents/guardians to misreport the complete history of providers, and for providers to misreport a child’s vaccination history. In the current study, this could lead to misclassification of vaccine series completion, undervaccination status, and patterns of undervaccination. While the patterns identified (shot-limiting and selective vaccination) have been previously shown in prior research to be indicative of parental choice to delay, spread out, or refuse vaccines, it is still possible that individuals who fell into the parental hesitancy patterns experienced access barriers, and vice versa, or were misclassified (9,17,18,31). Provider behavior may also play a role in these patterns in certain cases. Finally, there are many reasons a child may exhibit undervaccination patterns indicative of other (non-hesitancy) barriers to receiving immunization services. For the current study, it is not possible to conclude the exact reasons children fall into these patterns based on immunization records alone.
Conclusion
Standard coverage metrics do not capture a full picture of immunization schedule adherence and undervaccination patterns. The systematic application of novel algorithms to quantify vaccine timeliness and to identify distinct undervaccination patterns can provide a comprehensive assessment of vaccine confidence and quality of immunization services, informing actionable steps towards improving early childhood vaccination rates.
Article highlights.
More than half of U.S. children experience undervaccination before 19 months of age
1 in 5 children had undervaccination patterns indicative of parental hesitancy
Series completion metrics do not capture a full picture of schedule adherence
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI165768. Ms. Freeman and Drs. Daley and Newcomer were also supported by a Center for Biomedical Research Excellence Award (1P20GM130418) from the National Institute of General Medical Sciences of the National Institutes of Health. The study sponsor did not have any role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- ADU
average days undervaccinated
- CI
confidence interval
- DTaP
diphtheria-tetanus-acellular pertussis
- Hib
Haemophilus influenzae type b
- PR
prevalence ratio
- MMR
measles, mumps, and rubella
- NIS
National Immunization Survey
- WIC
Special Supplemental Nutrition Program for Women, Infants, and Children
- VPD
vaccine-preventable disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era-United States, 1994–2013. MMWR Morb Mort Wkly Rep. 2014;63(16):352–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Orenstein WA, Ahmed R. Simply put: Vaccination saves lives. Proc Natl Acad Sci U S A. 2017;114(16):4031–4033. doi: 10.1073/pnas.1704507114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinshaw AS. The childhood immunization schedule and safety: stakeholder concerns, scientific evidence, and future studies. National Academies Press; 2013. [PubMed] [Google Scholar]
- 4.Smith JC. The structure, role, and procedures of the US Advisory Committee on Immunization Practices (ACIP). Vaccine. 2010;28:A68–A75. [DOI] [PubMed] [Google Scholar]
- 5.Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory Committee onn Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years of Younger - United States, 2020. Morbidity and Mortality Weekly Report (MMWR).; 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6905a3.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination Coverage Among Children Aged 19–35 Months - United States, 2017. MMWR Morb Mort Wkly Rep. 2018;67(40):1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination Coverage by Age 24 Months Among Children Born in 2016 and 2017—National Immunization Survey-Child, United States, 2017–2019. MMWR Morb Mort Wkly Rep. 2020;69(42):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed. JAMA. 2005;293(10):1204–1211. [DOI] [PubMed] [Google Scholar]
- 9.Glanz JM, Newcomer SR, Narwaney KJ, Hambidge SJ, Daley MF, Wagner NM, McClure DL, Xu S, Rowhani-Rahbar A, Lee GM, Nelson JC. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167(3):274–281. [DOI] [PubMed] [Google Scholar]
- 10.Glanz JM, Newcomer SR, Jackson ML, Omer SB, Bednarczyk RA, Shoup JA, DeStefano F, Daley MF, Goddard K, Panneton M, Groom H. White paper on studying the safety of the childhood immunization schedule in the Vaccine Safety Datalink. Vaccine. 2016;34:A1–A29. [DOI] [PubMed] [Google Scholar]
- 11.Kurosky SK, Davis KL, Krishnarajah G. Completion and compliance of childhood vaccinations in the United States. Vaccine. 2016;34(3):387–394. [DOI] [PubMed] [Google Scholar]
- 12.Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K. Measles outbreak—California, December 2014–February 2015. MMWR Morb Mort Wkly Rep. 2015;64(6):153. [PMC free article] [PubMed] [Google Scholar]
- 13.Phadke VK. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis Varun. JAMA. 2016;315(11):1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assessing the state of vaccine confidence in the United States: recommendations from the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 9, 2015 [published correction appears in Public Health Rep. 2016;131(1):218]. Public Health Rep. 2015;130(6):573–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadeau JA, Bednarczyk RA, Masawi MR, Meldrum MD, Santilli L, Zansky SM, Blog DS, Birkhead GS, McNutt LA. Vaccinating my way - Use of alternative vaccination schedules in New York State. J Pediatr. 2015;166(1):151–156.e1. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves AL, Nowak G, Frew P, Hinman AR, Orenstein WA, Mendel J, Aikin A, Nadeau JA, McNutt LA, Chamberlain AT, Omer SB. Adherence to Timely Vaccinations in the United States. Pediatrics. 2020;145(3). [DOI] [PubMed] [Google Scholar]
- 17.Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics. 2012;130(1):32–38. [DOI] [PubMed] [Google Scholar]
- 18.Daley MF, Reifler LM, Shoup JA, et al. Temporal trends in undervaccination: a population-based cohort study. Amer J Prev Med. 2021. Jul;61(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcomer SR, Freeman RE, Wehner BK, Anderson SL, Daley MF. Timeliness of Early Childhood Vaccinations and Undervaccination Patterns in Montana. Am J Prev Med. 2021. Jul;61(1):e21–e29. doi: 10.1016/j.amepre.2021.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daley MF, Shoup JA, Newcomer SR, Jackson ML, Groom HC, Jacobsen SJ, McLean HQ, Klein NP, Weintraub ES, McNeil MM, Glanz JM. Assessing Potential Confounding and Misclassification Bias When Studying the Safety of the Childhood Immunization Schedule. Acad Pediatr. 2018;18(7):754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services (DHHS). National Center for Immunization and Respiratory Diseases. The 2017 National Immunization Survey-Child, Atlanta, GA: : Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 22.Smith PJ, Battaglia MP, Huggins VJ, Hoaglin DC, Rodén AS, Khare M, Ezzati-Rice TM, Wright RA. Overview of the sampling design and statistical methods used in the National Immunization Survey. Am J Prev Med. 2001;20(4):17–24. [DOI] [PubMed] [Google Scholar]
- 23.Zell ER, Ezzati-Rice TM, Battaglia MP, Wright RA. National Immunization Survey: the methodology of a vaccination surveillance system. Public Health Rep. 2000;115(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolter KM, Smith PJ, Khare M, Welch B, Copeland KR, Pineau VJ, Davis N. Statistical methodology of the National Immunization Survey, 2005–2014. National Center for Health Statistics. Vital and Health Statistics 1(61). 2017. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC), National Center for Immunization and Respiratory Diseases (NCIRD). National Immunization Survey-Child: A User’s Guide for the 2017 Public-Use Data File. 2012. https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-PUF17-DUG.pdf. Accessed on April 20, 2021.
- 26.Craney TA, Surles JG. Model-dependent variance inflation factor cutoff values. Quality Engineering. 2002. Mar 25;14(3):391–403. [Google Scholar]
- 27.Grant R, Gracy D, Goldsmith G, Sobelson M, Johnson D. Transportation barriers to child health care access remain after health reform. JAMA Pediatr. 2014;168(4):385–386. [DOI] [PubMed] [Google Scholar]
- 28.Shipman SA, Lan J, Chang C-h, Goodman DC. Geographic maldistribution of primary care for children. Pediatrics. 2011;127(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vann JCJ, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst. Rev 2018. Jan 18;1(1):CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011. Jul;126(2_suppl):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilkey MB, McRee AL, Magnus BE, Reiter PL, Dempsey AF, Brewer NT. Vaccination confidence and parental refusal/delay of early childhood vaccines. PloS One. 2016. Jul 8;11(7):e0159087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011;128(5):848–856. [DOI] [PubMed] [Google Scholar]
- 33.Kempe A, Saville AW, Albertin C, Zimet G, Breck A, Helmkamp L, Vangala S, Dickinson LM, Rand C, Humiston S, Szilagyi PG.. Parental hesitancy about routine childhood and influenza vaccinations: A national survey. Pediatrics. 2020;146(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glanz JM, McClure DL, Magid DJ, et al. Daley MF, France EK, Salmon DA, Hambidge SJ. Parental Refusal of Pertussis Vaccination Is Associated With an Increased Risk of Pertussis Infection in Children. Pediatrics. 2009;123(6):1446–1451. [DOI] [PubMed] [Google Scholar]
- 35.Glanz JM, McClure DL, Magid DJ, Daley MF, France EK, Hambidge SJ. Parental refusal of varicella vaccination and the associated risk of varicella infection in children. Arch Pediatr Adolesc Med. 2010;164(1):66–70. [DOI] [PubMed] [Google Scholar]
- 36.Glanz JM, McClure DL, O’Leary ST, Narwaney KJ, Magid DJ, Daley MF, Hambidge SJ. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine. 2011;29(5):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran D, Terlinden A, Poirrier JE, Masseria C, Krishnarajah G. Vaccine Timeliness: A Cost Analysis of the Potential Implications of Delayed Pertussis Vaccination in the US. Pediatr Infect Dis J. 2016;35(5):542–547. [DOI] [PubMed] [Google Scholar]
- 38.Robison SG. Incomplete Early Childhood Immunization Series and Missing Fourth DTaP Immunizations; Missed Opportunities or Missed Visits? ISRN Prev Med. 2013;2013:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Smith PJ, Hill HA. Missed opportunities for simultaneous administration of the fourth dose of DTaP among children in the United States. Vaccine. 2017;35(24):3191–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Elam-Evans LD, Hill HA, Yankey D. Employment and Socioeconomic Factors Associated with Children’s Up-to-Date Vaccination Status. Clin Pediatr (Phila). 2017;56(4):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crouch E, Dickes LA. A Prediction Model of Childhood Immunization Rates. Appl Health Econ Health Policy. 2015;13(2):243–251. [DOI] [PubMed] [Google Scholar]
- 42.Cataldi JR, Kerns ME, O’Leary ST. Evidence-based strategies to increase vaccination uptake: a review. Curr Opin Pediatr. 2020. Feb 1;32(1):151–9. [DOI] [PubMed] [Google Scholar]
- 43.Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, DeVere V, Zhou C, Robinson JD. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013. Dec 1;132(6):1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Opel DJ, Zhou C, Robinson JD, Henrikson N, Lepere K, Mangione-Smith R, Taylor JA. Impact of childhood vaccine discussion format over time on immunization status. Acad Pediatr. 2018. May 1;18(4):430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brewer NT. What works to increase vaccination uptake. Acad Pediatr. 2021. May 1;21(4):S9–16. [DOI] [PubMed] [Google Scholar]
- 46.Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vaccines Immunother. 2013;9(8):1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gust DA, Kennedy A, Shui I, Smith PJ, Nowak G, Pickering LK. Parent attitudes toward immunizations and healthcare providers: The role of information. Am J Prev Med. 2005;29(2):105–112. [DOI] [PubMed] [Google Scholar]
- 48.Opel DJ, Taylor JA, Mangione-Smith R, Solomon C, Zhao C, Catz S, Martin D. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011. Sep 2;29(38):6598–605. [DOI] [PubMed] [Google Scholar]
- 49.Sanofi Pasteur, Pentacel website. https://www.pentacel.com. Accessed on March 30, 2021.


