Abstract
Background:
Despite the significant adverse clinical consequences of RBC alloimmunization, our understanding of the signals that induce immune responses to transfused RBCs remains incomplete. Though RBC storage has been shown to enhance alloimmunization in the hen egg lysozyme, ovalbumin, and human Duffy (HOD) RBC alloantigen mouse model, the molecular signals leading to immune activation in this system remain unclear. Given that the nonclassical major histocompatibility complex (MHC) Class I molecule CD1D can bind to multiple different lysophospholipids and direct immune activation, we hypothesized that storage of RBCs increases lysophospholipids known to bind CD1D, and further that recipient CD1D recognition of these altered lipids mediates storage-induced alloimmunization responses.
Study Design and Methods:
We used a mass spectrometry-based approach to analyze the changes in lysophospholipids that are induced during storage of mouse RBCs. CD1D knockout (CD1D-KO) and wild-type (WT) control mice were transfused with stored HOD RBCs to measure the impact of CD1D deficiency on RBC alloimmunization.
Results:
RBC storage results in alterations in multiple lysophospholipid species known to bind to CD1D and activate the immune system. Prior to transfusion, CD1D-deficient mice had lower baseline levels of polyclonal immunoglobulin (IgG) relative to WT mice. In response to stored RBC transfusion, CD1D-deficient mice generated similar levels of anti-HOD IgM and anti-HOD IgG.
Conclusion:
Although storage of RBCs leads to alteration of several lysophospholipids known to be capable of binding CD1D, storage-induced RBC alloimmunization responses are not impacted by recipient CD1D deficiency.
Keywords: CD1D, lysophospholipids, red blood cell alloimmunization, storage
1 |. INTRODUCTION
RBC alloimmunization occurs when patients are exposed to foreign RBCs through either transfusion or pregnancy, driving the generation of antibodies against surface proteins or carbohydrates that are either polymorphic or differently expressed on donor RBCs.1 For those patients who have made an initial anti-RBC protein response, re-exposure to the antigen via subsequent transfusion may induce a rapid recall response and increase circulating alloantibody levels. The resulting alloantibodies often bind to the transfused RBCs and induce their destruction in a process known as a hemolytic transfusion reaction (HTR).2 In addition to destroying the clinically needed transfused RBCs, the widespread immune and coagulation activation induced during an HTR can lead to the development of shock, disseminated intravascular coagulation, renal failure, and in extreme cases, death. Accordingly, much of blood banking practice is dedicated to the detection of anti-RBC alloantibodies and providing alloimmunized patients with antigen-negative blood. RBC alloimmunization can be a significant problem for patients who require chronic transfusions. In those who generate either multiple alloantibodies or antibodies to common antigens, providing sufficient units of compatible antigen-negative RBCs can be both time and resource intensive. This can lead to significant morbidity due to delays in locating sufficient compatible units to treat symptoms in a timely manner and, in rare cases, death due to inability to identify sufficient units.3
Despite the medical importance of RBC alloimmunization, our understanding of the fundamental immune mechanisms that govern anti-RBC alloantibody generation is limited. In particular, the molecular signals that stimulate innate immune cell activation in response to RBC transfusion are unknown. Specifically, innate immunity evolved to respond to moieties not found in humans known as pathogen-associated molecular patterns (PAMPs),4 and sterile RBCs have no obvious source of PAMPs. Alternatively, immune activation can occur in the absence of clear infectious etiology via a different class of patterns known as damage-associated molecular patterns (DAMPs),5 though no RBC-derived DAMP has been described. DAMPs are endogenous molecules that are released from damaged cells and are recognized by specific receptors that can in turn stimulate the immune system. RBC storage has been shown to induce a wide variety of biophysical and molecular changes,6 and several reports have demonstrated that storage of human RBCs leads to production of biologically active lipids capable of activating neutrophils.7,8 Thus, potential sources of DAMPs are bioactive lipids such as lysophospholipids.
Clinically, all RBCs are stored for some time at 4°C prior to their transfusion. Although most RBC units are transfused between 10 and 21 days, the storage time allowed by current FDA standards is 35–42 days depending on storage solution. We have previously demonstrated that extending storage of allogenic mouse RBCs to a date, which mimics the extremes of allowable recovery characteristics set out by the FDA, leads to enhancement of alloimmunization in the HOD mouse model system.9,10 Enhancement of alloimmunization to donor RBCs as a function of storage time has also been reported in recipient patients with sickle cell disease (SCD) who require transfusion.11 It is important to note that similar effects were not observed in general transfusion practice.12,13 Although its clinical applicability may be restricted to certain patient populations or to certain blood group antigens, the enhancement observed in the stored HOD model provides an experimentally tractable system to investigate potential molecular drivers of alloimmunization. Indeed, mechanistic studies in the murine model have shown that extended storage of mouse RBCs leads to (i) increases in uptake by multiple different innate cell phagocytes including macrophages and dendritic cells,14,15 (ii) increases in circulating levels of multiple different innate cell-produced cytokines,10,16 and ultimately (iii) increases in adaptive antibody production.9,10 Our working model is that extended RBC storage induces DAMP production by stored HOD RBCs, which in turn stimulates both the innate and adaptive immune system.
One potential source of DAMPs is altered endogenous lipids that occur during RBC storage. It has become increasingly clear that the immune system has evolved the ability to recognize and respond to changes in various lipid moieties through a number of innate-like T-cell subsets.17 Chief among these are NK T cells (NKT), which are able to respond to changes in lipid species via their recognition of a wide variety of self and non-self lysophospholipid species bound to the nonclassical MHC molecule CD1D.18,19 NKT recognition of lipids bound to CD1D has been shown to play a role in controlling immune responses in multiple different contexts including atheroschlerosis,20–22 multiple models of autoimmunity,23 cancer,24 as well as bacterial, mycobacterial, and fungal infections.19,25 NKT recognition of altered phospholipids bound to CD1D typically leads to activation of NKT cells. Activated NKT cells can in turn provide help to B cells via cytokines such as IL-21 and direct CD40-CD40L interactions, thereby inducing B-cell activation and antibody secretion.26 Herein, we demonstrate that storage of mouse RBCs leads to dramatic alterations in multiple phospholipid species capable of binding to CD1D. Given that NKT recognition of lipids bound to CD1D is known to drive immune activation and antibody production, we tested the hypothesis that CD1D plays a functional role in the immune recognition of transfused RBCs and regulates anti-RBC alloantibody production in a mouse model.
2 |. MATERIALS AND METHODS
2.1 |. Mice
FVB, C57BL/6J wild-type (WT), and CD1D-KO mice were purchased from Jackson Laboratories (strain IDs 001800, 000664, and 008881, respectively). CD1D-KO mice have been described previously.27 As detailed on the Jackson Laboratories website, the CD1 locus was targeted by a neomycin cassette in 129S6/SvEvTac-derived TC1 embryonic stem (ES) cells. Correctly targeted ES cells were injected into recipient blastocysts, and chimeric mice were bred to C57BL/6J mice to establish the CD1-mutant colony. Mice were backcrossed to C57BL/6J mice for 12 generations. HOD transgenic mice express a triple fusion construct of Hen egg lysozyme, Ovalbumin, and the human Duffy red blood cell antigen selectively on the surface of RBCs, and were generated on the FVB background as described previously.28 All mice were bred and maintained at the animal facilities of the University of Virginia and Bloodworks Northwest. They were transfused between 8 and 10 weeks of age. Experimental groups were age and sex matched. All procedures were approved by Institutional Animal Care and Use Committees at the University of Virginia and Bloodworks Northwest.
2.2 |. Blood collection and storage
Blood from donor FVB or HOD mice was collected by cardiac puncture under sterile conditions into 20% CPDA-1 (Boston Bioproducts, IBB-420). The blood was leukoreduced with white blood cell filters (Pall, AP-4851), hematocrit was set to 75%, and blood was either transfused fresh or stored at 4°C for 12 days.
2.3 |. Transfusion and phlebotomy of mice
Recipient mice were transfused with 100 μL (volume adjusted equivalent of 1 unit of packed RBCs in humans) of stored HOD blood via retro-orbital injection, as previously described.10 Blood from transfused mice was collected by submandibular vein puncture 7 and 14 days post-transfusion (dpt) and allowed to clot. Samples were then spun at 5000 rpm for 5 min, and the supernatant was transferred to new tubes. This process was then repeated for the second time to get sera with no RBC contamination. Sera were stored at −20°C until analysis.
2.4 |. Flow cross-match
Sera samples from untransfused controls or transfused WT and CD1D-KO mice were diluted at 1:10 in FACS buffer (phosphate-buffered saline (PBS) with 0.5% bovine serum albumin (BSA), 2% fetal bovine serum, and 0.1% sodium azide) and cross-matched with HOD or WT antigen-negative FVB RBCs as control. HOD or FVB RBCs were plated in 96-well plates (Costar 3795) and incubated with diluted serum for 20 min at room temperature. The cells were then washed with FACS buffer and stained using secondary antibodies: phycoerythrin-conjugated anti-mouse immunoglobulin M (IgM) (Invitrogen, M31504), Alexa Fluor 647-conjugated anti-mouse immunoglobulin G (IgG) (Invitrogen, A21236), phycoerythrin-conjugated anti-mouse IgG1 (Invitrogen, 31862), Alexa Fluor 647-conjugated anti-mouse IgG2b (Invitrogen, A21242), or fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG2c (SouthernBiotech, 1077-02) at 1:200 dilution in FACS buffer for 20 min at room temperature. Antibody binding was detected using an Attune NxT flow cytometer, and data were analyzed using the FlowJo Software to obtain mean fluorescence intensity (MFI). RBC-specific MFIs (HOD MFI minus FVB MFIs) are shown throughout.
2.5 |. HEL-specific enzyme-linked immunosorbent assay
Antibody responses to the Hen egg lysozyme (HEL) portion of the HOD antigen were measured by HEL-specific enzyme-linked immunosorbent assay (ELISA), as previously described.10 Briefly, high-binding polystyrene plates (Corning 9018) were coated overnight at 4°C with 10 μg/ml HEL (Sigma-Aldrich, L6876) in PBS. Plates were then washed (0.05% Tween-20 in PBS) and incubated with blocking buffer (2% BSA and 0.05% Tween-20 in PBS). Sera samples were serially diluted (starting at 1:50) in blocking buffer and incubated in coated plates for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-mouse IgM or IgG-specific antibody (Jackson ImmunoResearch, 115-035-075 or 115-035-008, respectively) was then used as a secondary stain at a 1:5000 dilution for 1 h at room temperature. Wells were developed using 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (SeraCare, 52-00-03) and quenched with 2 N H2SO4 after 10 min. Optical densities were measured at 450 nm. End-point titers were calculated using GraphPad Prism through interpolation of the cutoff value from the fit of the optical density versus (1/serum dilution) curve for each sample using the “plateau followed by one-phase exponential decay” model. The cutoff value was defined as the average plus 3 standard deviations (SDs) of signals from background wells (i.e., signal values from wells incubated with blocking buffer alone). IgG subtypes were determined by single dilution ELISAs. Sera samples were diluted at 1:1000 or 1:100 depending whether they were obtained from mice transfused with stored or fresh HOD, respectively. Serum dilutions were incubated on HEL-coated high-binding plates (Corning 9018) for 1 h at room temperature. After being washed, plates were incubated with the secondary antibodies (HRP-conjugated anti-mouse IgG1, IgG2b, or IgG2c, Jackson Immuno-Research, 115-035-205, 115-035-207, or 115-035-208, respectively) at 1:5000 for 1 h at room temperature. Plates were developed using TMB for 10 min, quenched with 2 N H2SO4, and then optical densities were measured at 450 nm.
2.6 |. Total IgG and IgM ELISA
Sera samples from 8-week-old unimmunized WT or CD1D-KO mice were diluted at 1:20,000 and total IgG and IgM were measured using the ELISA kits, according to manufacturer’s instructions (Invitrogen 88–50400 and 88–50470, respectively). Briefly, ELISA plates were coated with captured antibodies and incubated overnight at 4°C. After blocking, diluted sera samples were added, as well as serially diluted standards. Following incubation, detection antibody was added. Plates were developed with the substrate solution. All reagents were provided with the kits.
2.7 |. Analysis of lysophospholipids
Lysophospholipids in fresh and stored RBCs from FVB mice were analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) as described previously.29 Briefly, analytes were extracted by 80% methanol (vol/vol) with an internal standard mixture containing lyso-platelet-activating factor C-16-d4 (LPAF 16:0-d4, Cayman Chemical); 17:1 lysophosphatidylcholine (LPC 17:1), 17:1 lysophosphatidylethanolamine (LPE 17:1), and 17:1 lysophosphatidylserine (LPS 17:1) from Avanti Polar Lipids. LC–MS analysis was performed on a QTrap 6500 mass spectrometer (AB Sciex) coupled with an Acquity I-class UPLC (Waters). Analytes were detected using multiple reaction monitoring (MRM) in the negative-ion mode. Data were analyzed by using the MultiQuant software (AB Sciex) and peak areas were used for relative quantification.
2.8 |. Statistical analysis
Statistical analysis and graphing were performed using the GraphPad Prism software. One-way Kruskal–Wallis analysis of variance was initially performed followed by Mann–Whitney test for post hoc comparisons. A value of p < .05 was considered to be statistically significant and assigned *, whereas p < .01, p < .001, and p < .0001 were assigned **, ***, and ****, respectively.
3 |. RESULTS
WT mice were transfused with either (i) fresh HOD RBCs or (ii) stored HOD RBCs. Two weeks post transfusion, serum was isolated from each mouse and tested for anti-HOD IgG. Consistent with previously published reports, stored HOD RBCs induced a substantially increased level of anti-HOD IgG compared with fresh HOD RBCs, as measured by either flow cross-match (Figure 1A) or limiting dilution ELISA (Figure 1B). These findings confirmed that the storage conditions used in the current studies recapitulated the previously reported phenomenon indicating storage of HOD RBCs leads to production of factors that enhance immunity.9,10
FIGURE 1.
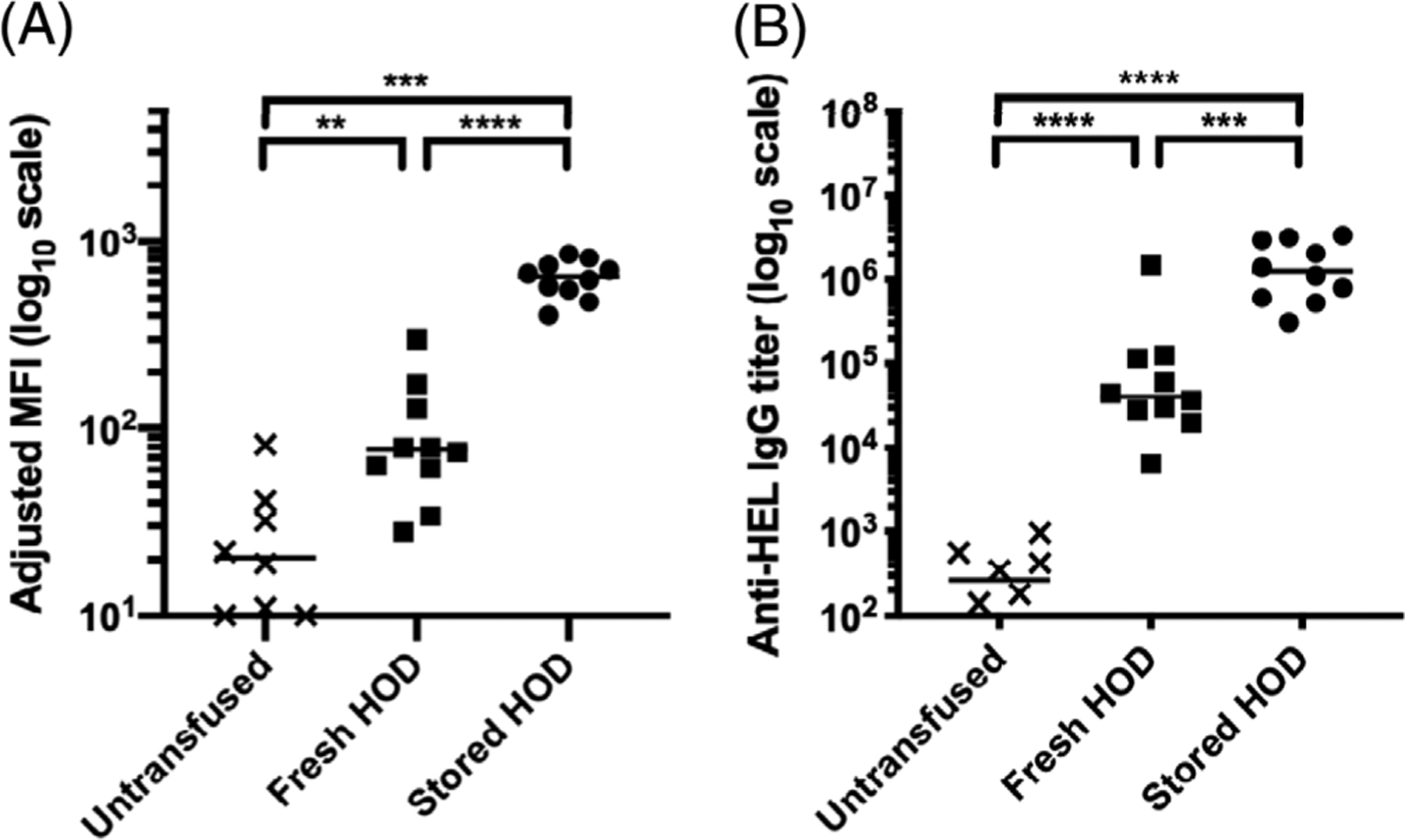
Transfusion of stored HOD blood results in the production of higher levels of anti-RBC IgG compared to fresh blood. WT mice were either untransfused, transfused with fresh HOD RBCs, or stored HOD RBCs. Serum was collected 14 dpt and the levels of anti-HOD IgG generation were measured by (A) flow cross-match presented as adjusted MFI and (B) anti-HEL limiting dilution ELISA presented as antibody titer. Each dot represents one mouse. Bars on scatter plots are median values. Figure shows a representative experiment out of 4. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. dpt, days post-transfusion; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity; WT, wild type
To better understand how storage might be driving immune activation, we next focused on the generation of bioactive lipids. Lysophospholipids are an important class of bioactive lipids that are known to have multiple different downstream effects on immune cell function, and other bioactive lipids have been shown to be altered during RBC storage.7,8 We employed a mass spectrometry-based approach to characterize the changes in lysophospholipids that are induced during storage of RBCs from the background strain of HOD mice, FVB. Although there were small but reproducible alterations for many of the lipid species measured (Figure 2), four lysophospholipid species demonstrated greater than twofold changes in their abundance after RBC storage. Two different species of LPAF were increased greater than twofold, with LPAF (16:0) increased 2.3-fold and LPAF (18:1) increased 2.8-fold. In contrast, one LPC species and one LPE species were decreased greater than twofold, with LPC (20:4) decreased 2.1-fold and LPE (18:0) decreased 3.8-fold.
FIGURE 2.
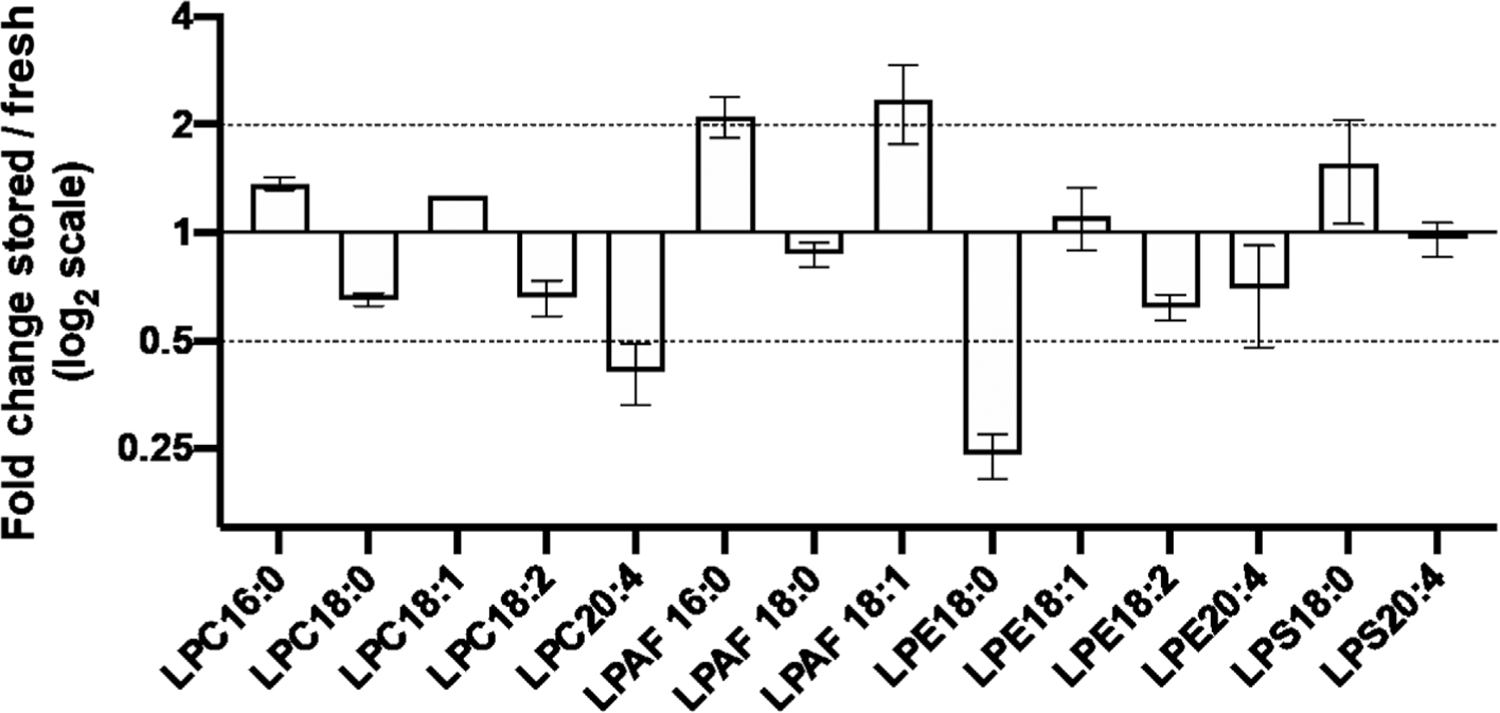
Storage of RBCs leads to alterations in multiple different lysophospholipid species. Specific lysolipid species were quantified in FVB RBCs within 24 h of collection (fresh) and after 7 days of storage (stored) by targeted mass spectrometry. Average fold changes (stored/fresh) of three independent experiments are presented scaled by log2 with observed standard deviations presented with error bars. Specific lysophosphatidylcholine (LPC), lyso-platelet activating factor (LPAF), lysophosphatidylethanolamine (LPE), and lysophosphatidylserine (LPS) species with different acyl chain lengths are represented
Given that CD1D has been shown to bind multiple different phospholipid species18 including LPAF30 and LPC,30 we hypothesized that CD1D-mediated binding of altered phospholipids mediated storage-associated anti-RBC alloantibody responses. Mice with a targeted disruption of the CD1D gene (CD1D-KO) were used to assess if CD1D in the recipient is required for the increased IgG response to stored RBCs. Baseline characterization of naïve CD1D-KO mice prior to transfusion demonstrated similar total levels of circulating polyclonal IgM antibodies compared to WT mice (Figure 3A), but significantly reduced levels of circulating polyclonal IgG (Figure 3B), consistent with CD1D playing a general role in class switching of immunoglobulin.26 This confirmed that CD1D does indeed control class-switched IgG antibody production globally in our specific pathogen free (SPF) housed mouse colony, and presumably reflects a functional role for CD1D responses generated to our SPF microflora.
FIGURE 3.
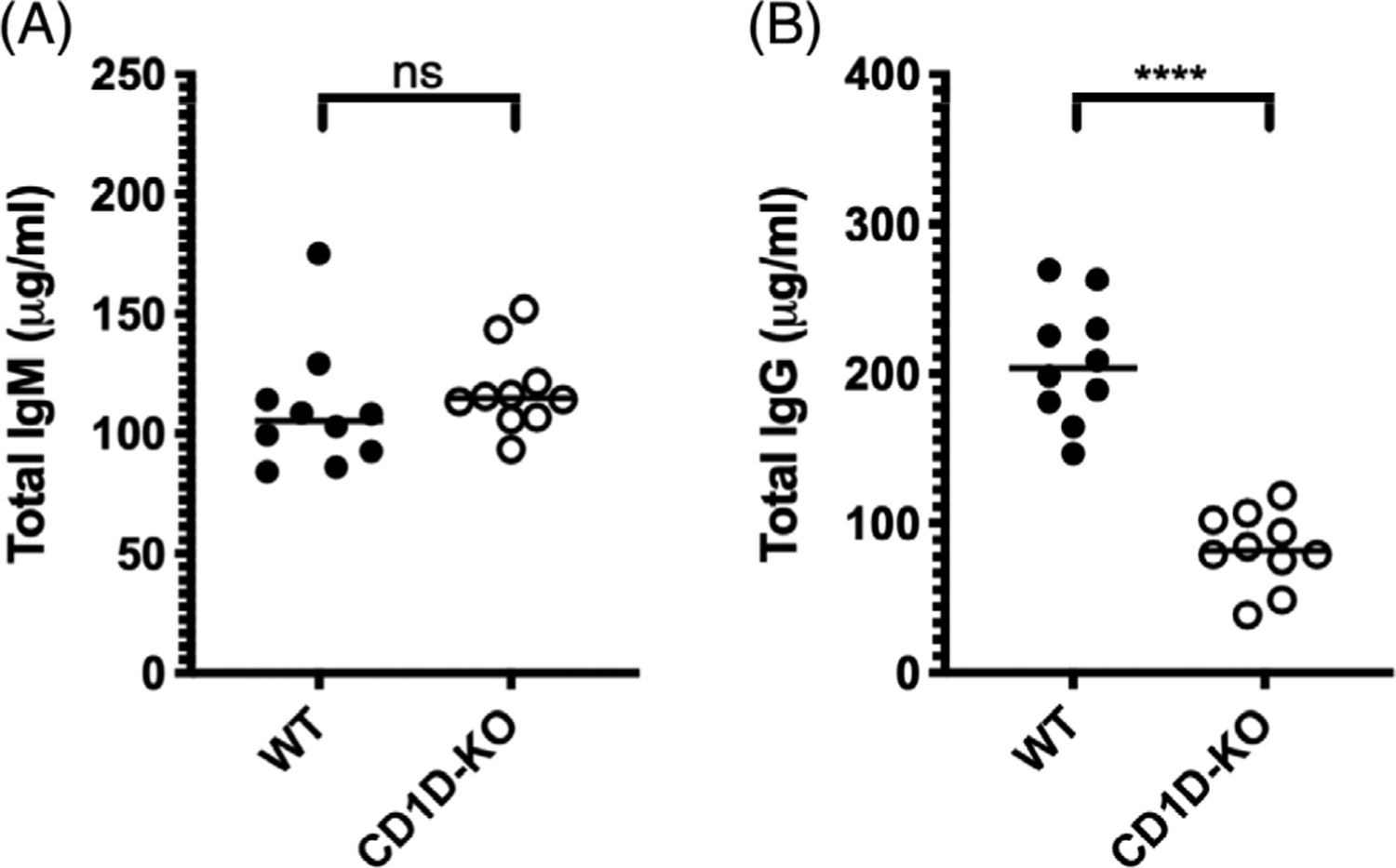
CD1D-deficient mice have similar levels of circulating total IgM and lower levels of circulating total IgG compared to WT controls prior to transfusion. Serum was collected from unimmunized 8-week-old WT and CD1D-KO mice. (A) Total IgM and (B) total IgG were measured by ELISA. Each dot represents one mouse. Bars on scatter plots represent median values. Figure shows a representative experiment out of 4. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. IgG, immunoglobulin G; IgM, immunoglobulin M; ELISA, enzyme-linked immunosorbent assay; WT, wild type
To determine whether CD1D was necessary for the response to stored RBC transfusion, we transfused WT and CD1D-KO mice with 1 “mouse unit” (100 μl) of stored HOD blood. Transfusion of stored HOD blood resulted in similar anti-RBC IgM levels in both WT and CD1D-KO mice as measured by both flow cross-match (Figure 4A) and HEL-specific ELISA (Figure 4B). Thus, the absence of CD1D does not impact IgM secretion induced by stored HOD transfusion. Like IgM, no difference was observed in anti-HOD IgG in response to stored RBCs in WT compared with CD1D-KO mice, as measured by flow cross-match or ELISA (Figure 5). To test whether deletion of CD1D alters IgG subclass, IgG1-, IgG2b-, and IgG2c-specific secondary antibodies were used to test recipient sera. No reproducibly significant differences were found between WT and CD1D-KO mice in the production of the measured IgG subclasses (Figure 6). These data indicate that recipient CD1D is not required for the increase in immunogenicity of stored HOD RBCs and that its deletion does not alter the overall quantity of anti-HOD IgG or distribution of anti-HOD IgG subclass. This stands in sharp contrast to the overall levels of IgG in these mice, as pre-transfusion CD1D-KO mice have significantly lower total polyclonal IgG levels.
FIGURE 4.
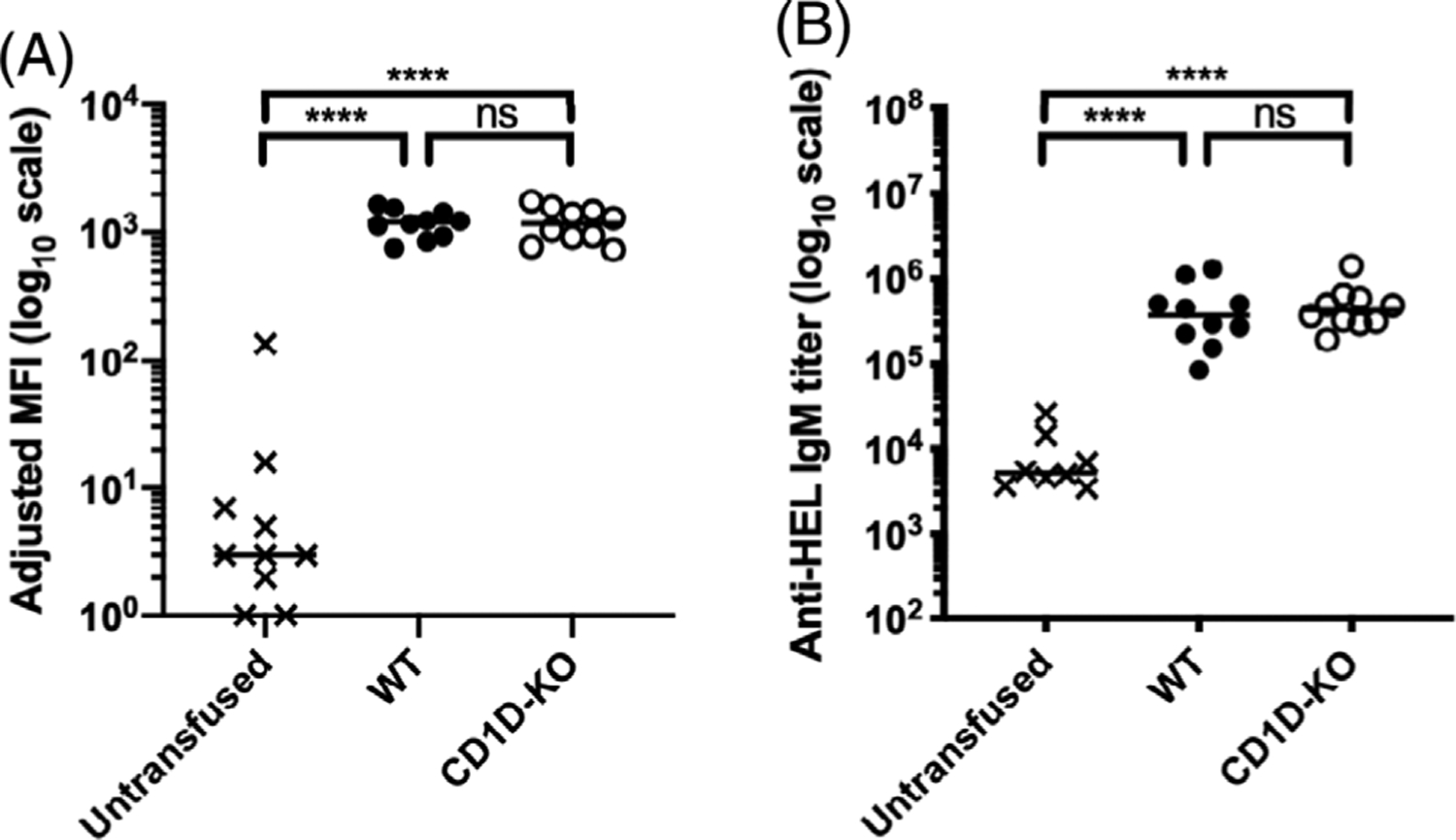
CD1D-deficient mice generate similar anti-RBC IgM alloantibody levels compared with WT mice in response to transfused stored RBCs. WT and CD1D-KO mice were transfused with stored HOD RBCs. Serum was collected 7 dpt and the levels of anti-HOD alloantibody generation were measured by (A) flow cross-match presented as adjusted MFI and (B) limiting dilution ELISA presented as IgM titer. Each dot represents one mouse. Bars on scatter plots represent median values. Figure shows a representative experiment out of 4. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. dpt, days post-transfusion; IgM, immunoglobulin M; ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity; WT, wild type
FIGURE 5.
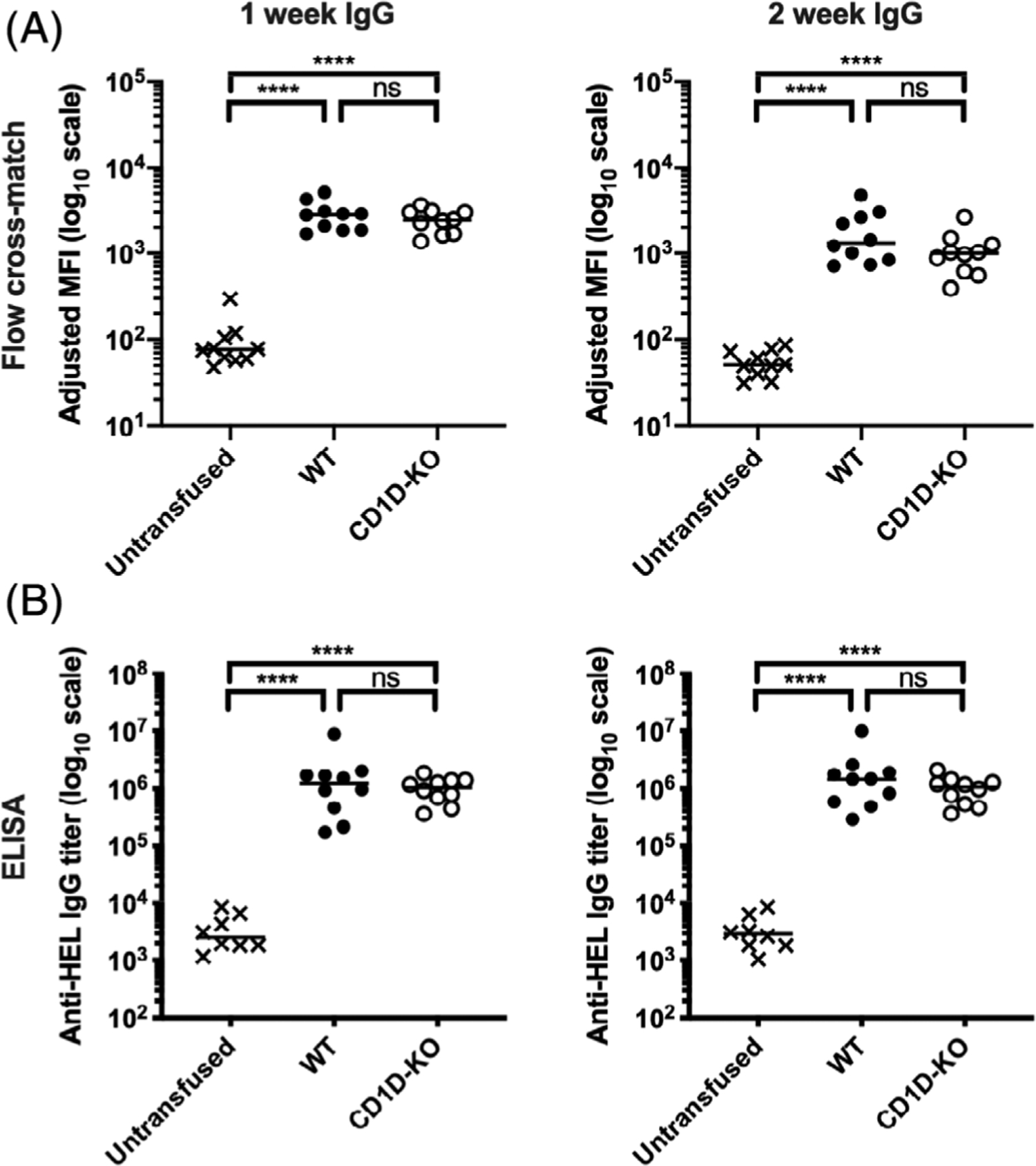
CD1D-deficient mice generate similar anti-RBC IgG alloantibody levels compared with WT mice in response to transfused stored RBCs. WT and CD1D-KO mice were transfused with stored HOD RBCs. Serum was collected 7 and 14 dpt and the levels of anti-HOD alloantibody generation were measured by (A) flow cross-match presented as adjusted MFI and (B) limiting dilution ELISA presented as IgG titer. Each dot represents one mouse. Bars on scatter plots represent median values. Figure shows a representative experiment out of 4. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. dpt, days post-transfusion; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity; WT, wild type
FIGURE 6.
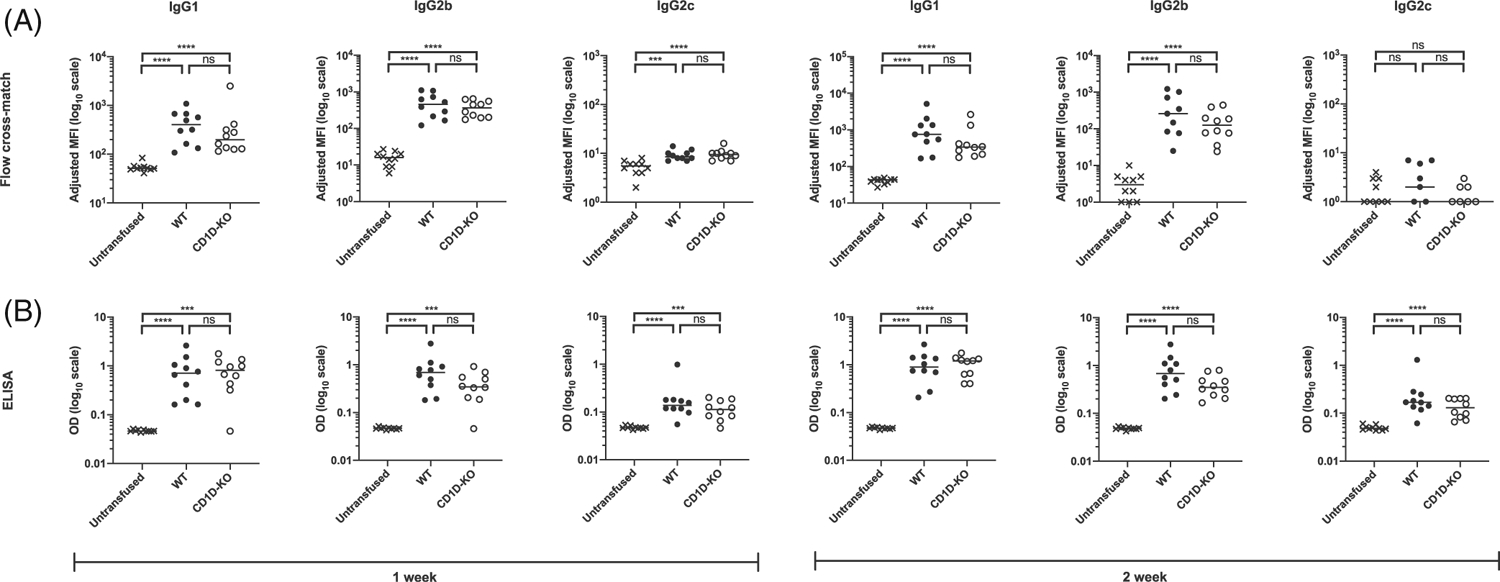
CD1D-deficient mice generate similar anti-RBC IgG subclass alloantibody levels compared with WT mice in response to transfused stored RBCs. WT and CD1D-KO mice were transfused with stored HOD RBCs. Serum was collected 7 and 14 dpt and the levels of IgG1, IgG2b, and IgG2c anti-HOD alloantibody levels were measured by (A) flow cross-match presented as adjusted MFI and (B) anti-HEL ELISA presented as OD measured at 1:1000 dilution. Each dot represents one mouse. Bars on scatter plots represent median values. Figure shows a representative experiment out of 4. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. dpt, days post-transfusion; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity; OD, optical density; WT, wild type
To address the possibility that CD1D might be playing a functional role in response to freshly collected RBCs rather than stored RBCs, we compared anti-HEL antibody levels generated in WT and CD1D-KO mice in response to fresh HOD RBC transfusion (Figure 7). Similar to what we observed in response to stored RBCs, both WT and CD1D-KO mice generated similar levels of anti-HOD IgM, total anti-HOD IgG, and anti-HOD IgG subclasses in response to fresh RBC transfusion. Thus, CD1D is not required for the generation of anti-RBC alloantibodies in response to fresh RBC transfusion.
FIGURE 7.
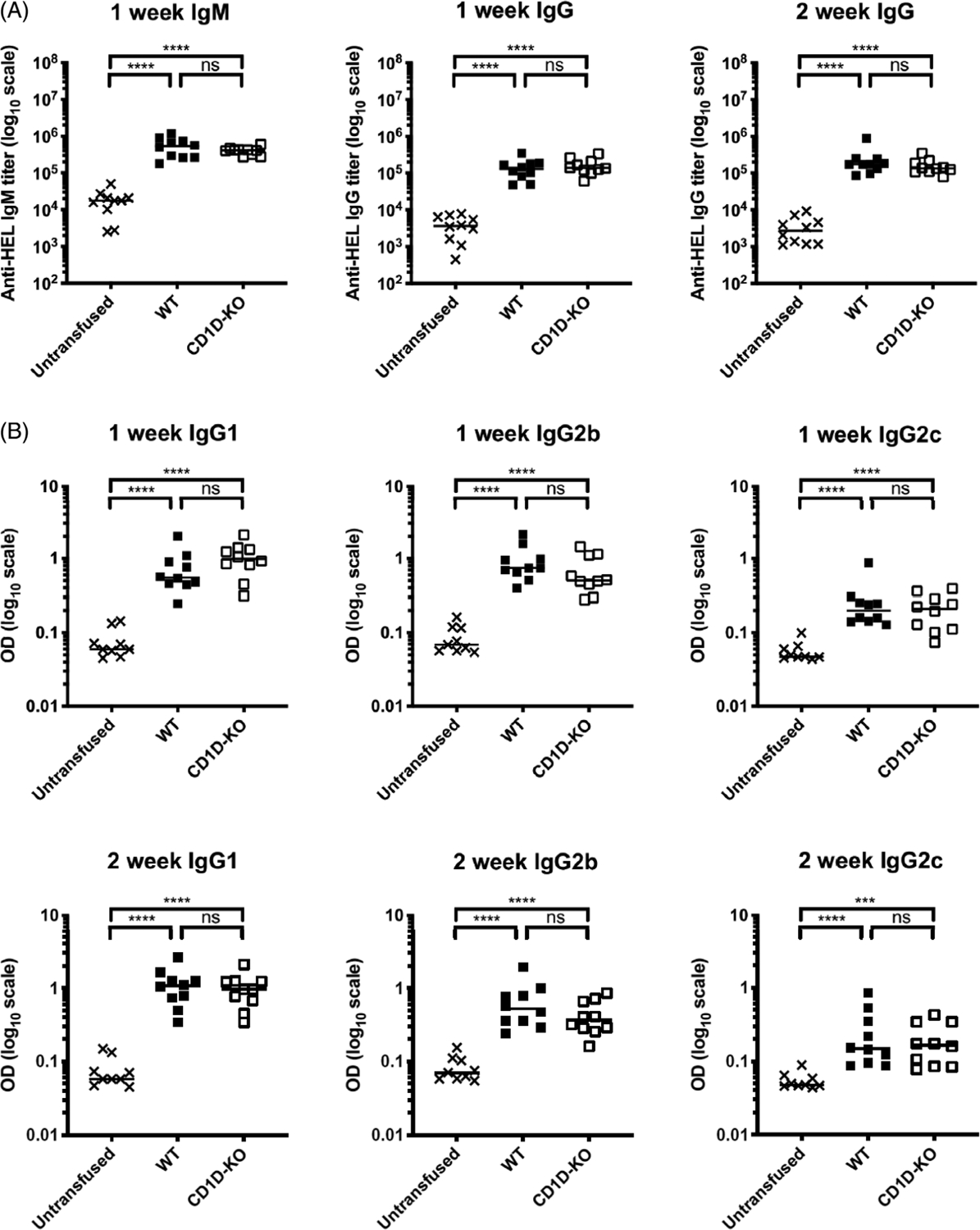
CD1D-deficient mice generate similar anti-RBC alloantibody levels compared with WT mice in response to transfused fresh RBCs. WT and CD1D-KO mice were transfused with fresh HOD RBCs. Serum was collected 7 and 14 dpt. (A) The levels of anti-HEL IgM and IgG generation were measured by limiting dilution ELISA and presented as IgM or IgG titer. (B) The levels of IgG subtypes were measured by single dilution ELISA at 1:100 dilution and presented as OD. Each dot represents one mouse. Bars on scatter plots represent median values. Figure shows a representative experiment out of 2. *p < .05, **p < .01, ***p < .001, ****p < .0001, ns p > .5. dpt, days post-transfusion; IgG, immunoglobulin G; IgM, immunoglobulin M; ELISA, enzyme-linked immunosorbent assay; OD, optical density; WT, wild type
4 |. DISCUSSION
We have used MS to characterize the changes in specific lysophospholipid species induced during storage of RBC from the FVB strain. Two different species of LPAF were increased greater than twofold during storage: LPAF (16:0) and LPAF (18:1). One species of lysophosphatidylcholine (LPC) was decreased twofold: LPC (20:4), and one species LPE was decreased fourfold: LPE (18:0). Although there are several publications that have looked at lipid profiles of stored RBCs,6–8,31–34 we have uniquely quantified a wide range of different lysophospholipid species of different acyl chain lengths. Collectively, our MS data demonstrate that storage of RBCs induces a range of changes in multiple different lysophospholipid species with some increasing while others decreasing.
Given the known biological activity of many of these species, we were interested in determining how these alterations might lead to immune recognition and activation. CD1D has been shown to bind multiple different phospholipid species with different acyl chain lengths18 including LPAF30 and LPC.30 Importantly, CD1D-bound lysophospholipids can be recognized by NKT cells, and this recognition can lead to NKT activation.19 NKT cell activation via recognition of CD1D bound lysophospholipids can drive NKT cell activation. Activated NKT cells can then go on to support B cell antibody secretion via both NKT secretion of cytokines such as IL-21 and direct co-stimulation via CD40-CD40L interactions.26 Thus, based on the existing literature, it is reasonable to hypothesize that CD1D-mediated binding of altered phospholipids mediates the storage-associated enhancement anti-RBC alloantibody responses by driving NKT cell activation, which in turn drives B-cell antibody secretion. To address this hypothesis, we compared immune antibody responses in WT versus CD1D-deficient animals. Prior to transfusion, we first characterized the baseline polyclonal total antibody levels in WT and CD1D-deficient mice. We found that they had similar levels of circulating IgM, but CD1D-KO mice had significantly lower levels of circulating total IgG at baseline. This presumably reflects the fact that CD1D plays an important role in the generation of IgG against normal flora in our SPF mouse colony, and is consistent with previous reports demonstrating CD1D control of antibody responses to various infectious agents.23–25
Having established that at baseline CD1D-deficient mice express lower IgG for the same level of IgM, we next asked whether they responded differently to stored RBC transfusion. We anticipated that CD1D-deficient mice would similarly demonstrate similar anti-RBC IgM responses but lower anti-RBC IgG responses to stored RBC transfusion. We were surprised to find that CD1D-deficient mice have similar anti-RBC alloantibody responses across the board for anti-HOD IgM, anti-HOD IgG, and anti-HOD IgG subclass-specific responses. CD1D-deficient mice also had similar anti-HOD IgM, anti-HOD IgG, and anti-HOD IgG subclass responses to fresh HOD RBC transfusion. Overall, our data demonstrate that CD1D deficiency functions differently in response to either fresh or stored RBC transfusion compared with that it does in response to either commensal microbiota35 or influenza infection.36
There are several potential interpretations of our results. One is that the lysophospholipid alterations induced during RBC storage do not play a functional role in the stimulation of anti-RBC immune responses. Alternatively, it is possible that lipid alterations induced by storage do play a role in driving anti-RBC alloantibodies, but that that they do so by activating CD1D independent pathways. The current data only show that CD1D is not required, but we cannot rule out that it is still involved for two reasons. First, there may be a redundant pathway capable of compensating for the loss of CD1D. Second, lipids are a complex family of molecules, often with opposing actions, and so the observed effects may be a balance of stimulatory and/or inhibitory pathways that both act through CD1D. Indeed, CD1D is known to bind to both LPAF that we observe being increased by storage, and also LPC that is decreased during storage. Thus, CD1D may have opposing effects in response to the specific mix of lipids, which could be deconvoluted by testing the specific effects of different lipid mixtures. Given the diverse molecular mechanisms driven by immune recognition of lipid species,37–39 future studies should investigate whether specific lipids or other lipid responsive regulatory pathways might be responsible for driving anti-RBC alloantibodies in response to transfusion of stored blood.
ACKNOWLEDGMENTS
The authors would like to thank other members of the Luckey lab: Dr. Abhinav Arneja and Anupam Prakash, for many helpful discussions. These studies were supported by grants to C.J.L. from the National Institutes of Health (NHLBI R01 HL134691), the UVA Cancer Center (P30CA044579) and the UVA Department of Pathology, C.J.L., J.C.Z. and J.E.H. from the National Institutes of Health (NHLBI P01 HL132819), and J.C.Z. and X.F. from the National Institutes of Health (NHLBI R01HL148151) and Bloodworks NW.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: P30 CA044579, P01 HL132819, R01 HL134691, R01 HL148151
Abbreviations:
- CPDA-1
citrate-phosphate-dextrose-adenine
- DAMPS
damage-associated molecular patterns
- dpt
days post transfusion
- ELISA
enzyme-linked immunosorbent assay
- FACS
flourecent activated cell sorting
- FDA
Food and Drug Administration
- HOD
hen egg lysozyme, ovalbumin, and human Duffy
- HTR
hemolytic transfusion reaction
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL
interleukin
- KO
knock out
- LPAF
lyso-platelet-activating factor
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- LPS
lysophosphatidylserine
- MHC
major histocompatibility complex
- NKT
natural killer T cells
- PAMPS
pathogen associated molecular patterns
- SCD
sickle cell disease
- SPF
specific pathogen free
- WT
WT
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Zimring JC, Welniak L, Semple JW, Ness PM, Slichter SJ, Spitalnik SL, et al. Current problems and future directions of transfusion-induced alloimmunization: summary of an NHLBI working group. Transfusion. 2011;51:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimring JC, Spitalnik SL. Pathobiology of transfusion reactions. Annu Rev Pathol. 2015;10:83–110. [DOI] [PubMed] [Google Scholar]
- 3.Nickel RS, Hendrickson JE, Fasano RM, Meyer EK, Winkler AM, Yee MM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–14. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loi MM, Kelher M, Dzieciatkowska M, Hansen KC, Banerjee A, West FB, et al. A comparison of different methods of red blood cell leukoreduction and additive solutions on the accumulation of neutrophil-priming activity during storage. Transfusion. 2018;58:2003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arneja A, Salazar JE, Jiang W, Hendrickson JE, Zimring JC, Luckey CJ. Interleukin-6 receptor-alpha signaling drives anti-RBC alloantibody production and T-follicular helper cell differentiation in a murine model of red blood cell alloimmunization. Haematologica. 2016;101:e440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai PC, Deal AM, Pfaff ER, Qaqish B, Hebden LM, Park YA, et al. Alloimmunization is associated with older age of transfused red blood cells in sickle cell disease. Am J Hematol. 2015;90:691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalpuri S, Schonewille H, Middelburg R, van de Watering L, de Vooght K, Zimring J, et al. Effect of storage of red blood cells on alloimmunization. Transfusion. 2013;53:2795–800. [DOI] [PubMed] [Google Scholar]
- 13.Dinardo CL, Fernandes FLA, Sampaio LR, Sabino EC, Mendrone A. Transfusion of older red blood cell units, cytokine burst and alloimmunization: a case–control study. Rev Bras Hematol Hemoter. 2015;37:320–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojczyk BS, Kim N, Bandyopadhyay S, Francis RO, Zimring JC, Hod EA, et al. Macrophages clear refrigerator storage-damaged red blood cells and subsequently secrete cytokines in vivo, but not in vitro, in a murine model. Transfusion. 2014;54:3186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabro S, Gallman A, Gowthaman U, Liu D, Chen P, Liu J, et al. Bridging channel dendritic cells induce immunity to transfused red blood cells. J Exp Med. 2016;213:887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felley L, Gumperz JE. Are human iNKT cells keeping tabs on lipidome perturbations triggered by oxidative stress in the blood? Immunogenetics. 2016;68:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen-Smith RM, Salio M, Cerundolo V. CD1d-dependent endogenous and exogenous lipid antigen presentation. Curr Opin Immunol. 2015;34:116–25. [DOI] [PubMed] [Google Scholar]
- 19.Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. 2010;22:79–86. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Kanellakis P, Hosseini H, Cao A, Deswaerte V, Tipping P, et al. A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE−/− mice by reducing lesion necrosis and inflammation. Cardiovasc Res. 2016;109:305–17. [DOI] [PubMed] [Google Scholar]
- 21.Getz GS, Reardon CA. Natural killer T cells in atherosclerosis. Nat Rev Cardiol. 2017;14:304–14. [DOI] [PubMed] [Google Scholar]
- 22.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson G̈K, et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AK, Tripathi P, Cardell SL. Type II NKT cells: an elusive population with Immunoregulatory properties. Front Immunol. 2018;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair S, Dhodapkar MV. Natural killer T cells in cancer immunotherapy. Front Immunol. 2017;8:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen NR, Tatituri RVV, Rivera A, Watts GFM, Kim EY, Chiba A, et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty DG, Melo AM, Moreno-Olivera A, Solomos AC. Activation and regulation of B cell responses by invariant natural killer T cells. Front Immunol. 2018;9:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SMA, Carter QL, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110: 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114: 2315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56:2560–70. [DOI] [PubMed] [Google Scholar]
- 30.Maricic I, Girardi E, Zajonc DM, Kumar V. Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J Immunol. 2014;193: 4580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 32.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Investig. 1998;101:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelher MR, McLaughlin NJD, Banerjee A, Elzi DJ, Gamboni F, Khan SY, et al. LysoPCs induce Hck- and PKCδ-mediated activation of PKCγ causing p47phox phosphorylation and membrane translocation in neutrophils. J Leukoc Biol. 2017;101: 261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowds CM, Blumberg RS, Zeissig S. Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota. Clin Immunol. 2015;159:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Navia AW, et al. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell. 2018;172:517–533.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McWilliam HE, Villadangos JA. MR1: a multi-faceted metabolite sensor for T cell activation. Curr Opin Immunol. 2020;64: 124–9. [DOI] [PubMed] [Google Scholar]
- 38.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plüddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–17. [DOI] [PubMed] [Google Scholar]


