Background:
Due to the wide spectrum of lower extremity defect presentation, various reconstructive techniques are available. Classic adipofascial flaps are still a second choice. The authors described a new multistage reconstructive approach with perforator-based pedicled adipofascial flap.
Methods:
This retrospective study analyzed data of 23 patients undergoing adipofascial flap reconstruction after distal leg trauma between June 2017 and January 2020. A reconstructive approach with an adipofascial flap followed by a skin graft was used in all patients. Patients were divided into two treatment groups, and in only one treatment group, an acellular dermal matrix was placed above the adipofascial flap during the first stage of the reconstruction. Negative pressure wound therapy was applied to both groups. Surgical technique, outcomes, and complications were discussed.
Results:
All patients achieved complete healing, and no flap loss was reported. Minor complications occurred in four patients; all were treated conservatively on outpatient basis. The surgical and aesthetic results were evaluated as satisfactory from both patients and professionals. However, the group treated with acellular dermal matrix showed a lower complication rate, and resulted significantly more satisfied with the overall results and in several domains of the questionnaire administered postoperatively (P < 0.05).
Conclusions:
The adipofascial flap is a safe and effective approach for the reconstruction of small-to-medium-sized defects of the distal lower extremity. Our two-stage reconstructive approach maximizes the pearls offered by the established technique; the dermal matrix guarantees a layered reconstruction optimizing the surgical and aesthetic outcomes of the skin graft with minimal donor site morbidity.
Takeaways
Question: The reconstruction of lower leg soft tissue defects is challenging. Due to the wide spectrum of lower extremity defect presentation, no universal algorithm exists for such defects.
Findings: Patients were divided in two treatment groups. In one group, a standard approach (adipofascial flap + skin graft) was performed; in the second group, an ADM was placed to achieve a layered reconstruction with an improvement of surgical and aesthetic outcomes.
Meaning: Adipofascial flap combined with ADM is a reliable reconstructive option with low morbidity at the donor site and satisfactory results in terms of surgical and aesthetic outcomes.
INTRODUCTION
Lower limb soft-tissue defects could represent a challenge for the reconstructive surgeon, especially in the distal third of the leg due to the anatomical characteristics of this region. Acute wounds are frequently characterized by exposure of deep structures such as tendons, bones, ligaments, and joints, and could be complicated by exposure of prosthetic devices.
Historically, these complicated defects have been successfully treated with local or free flaps, thus reducing the amputation rate.1,2 Nowadays, free flaps have a prominent role in leg reconstruction3–5; several flaps can be harvested based on size, extension, characteristic of the tissue defects, and surgeon’s experience.6,7 On the other hand, microsurgery has its drawbacks: it is expensive, time-consuming, and requires high surgical skills, adequate instruments, and a trained team; moreover, a minimal possibility of a free flap failure is reported with all the problems related to a re-exploration and secondary reconstructive procedure.8–11 To date, due to the wide spectrum of lower extremity defect presentations, no universal algorithm exists for their reconstruction; moreover, several studies showed no significant difference in terms of flap survival and complication rates between free and local flaps.4,7,12
The introduction of angiosome and perforasome theories have allowed plastic surgeons to reevaluate the role of local flaps in light of the perforator concept.13–17 Furthermore, the establishment of new treatment strategies, including negative pressure wound therapy (NPT) and acellular dermal matrix (ADM), has greatly improved the outcomes of limb reconstruction.18
Adipofascial flaps, first described for the reconstruction of lower limb defects by Lai,19 seem to obviate some of the limits of local flap reconstruction; however, results are not always satisfactory. In the present study, we reported our experience with a new multistage approach using the advantages of a perforator-based adipofascial flap and a dermal matrix substitute for small-to-medium sized soft-tissue defects of the lower leg.
MATERIALS AND METHODS
A retrospective study investigated patients who underwent lower leg soft tissue reconstruction with adipofascial flap after traumatic injury between June 2017 and January 2020. Twenty-three patients were identified; in all cases, after debridement an adipofascial perforator-based pedicled flap was used with immediate or delayed skin grafting. In 12 patients, after initial debridement, the defect was repaired with an adipofascial perforator-based flap with immediate full or split thickness skin graft in a single stage. In 11 patients, after debridement, a two-stage reconstruction was performed: a dermal matrix substitute (Integra Dermal Regeneration Template, Ethicon Inc, Somerville, N.J.) was applied over the flap during the first stage and covered with NPT (V.A.C., KCI Inc, San Antonio, Tex.); the NPT was interrupted 2 weeks after; then, during the second stage, full or split thickness skin graft was used to complete the reconstruction. Holes in the skin graft were made with a No.11 blade to avoid hematoma or seroma collection on the undersurface of the graft.
Data regarding age, sex, comorbidities, size of the defect, flap size, complications of both donor and recipient site, and healing time were recorded. These characteristics are summarized in Tables 1 and 2. Twenty-three patients were divided into two groups to compare surgical and aesthetic outcomes: patients treated only with adipofascial flap and immediate skin graft with NPT (Group I), and patients treated with adipofascial flap, ADM and NPT and delayed skin graft with NPT (Group II). In our practice, the approach gradually shifted to an ADM-combined protocol: in this series the last cases treated all belonged to group II. The authors’ surgical protocol (group II patients) and postoperative care are outlined in Figure 1.
Table 1.
Patients and Procedures
| Variable | Value (rate) |
|---|---|
| Patients | 23 |
| Age (y) | 37 ± 13.4 |
| Gender | |
| Women | 8 (35%) |
| Men | 15 (65%) |
| Tobacco use | 11 (48%) |
| Comorbidities | |
| Diabetes | 2 (8%) |
| Hypertension | 1 (4%) |
| Obesity | 3 (13%) |
| Cardiovascular pathology | 1 (4%) |
| Treatment protocol* | |
| Group I: flap + immediate skin graft + NPT | 12 |
| Group II: flap + ADM + NPT and delayed skin graft + NPT | 11 |
| Defect size (cm2) | |
| Group I | 30.8 ± 5.2 |
| Group II | 30 ± 6.4 |
| Flap size (cm2) | |
| Group I | 36 ± 5.3 |
| Group II | 34.3 ± 5.6 |
| Healing time (d) | |
| Group I | 59.6 ± 8.2 |
| Group II | 47 ± 5.8 |
*Debridement and NPT (7–10 days) was performed in both groups before flap harvesting.
Table 2.
Complications
| Group I | Group II | |
|---|---|---|
| Recipient site | ||
| Total flap necrosis | 0 | 0 |
| Partial flap necrosis | 1 | 0 |
| Total graft loss | 0 | 0 |
| Partial graft loss | 2 | 0 |
| Wound dehiscence | 1 | 0 |
| Infection | 0 | 0 |
| Donor site complications | 0 | 1 |
| Surgical revision | 0 | 0 |
Fig. 1.
The authors’ surgical protocol: adipofascial flap + ADM reconstruction is depicted; postoperative care is outlined. ABR: absolute bed rest.
An evaluation of the surgical outcome and aesthetic result was conducted through a questionnaire, scoring the reconstruction with a grade from 1 to 10 (1–2: very unsatisfied; 3–4: unsatisfied; 5–6: acceptable; 7–8: satisfied; 9–10: very satisfied) about six features: skin texture, color match, contour, recipient site appearance, donor site scar, and overall result satisfaction. The assessment was done by the surgical team, an external plastic surgeon, and by the patients at least 12 months from the last operation, and the results from the two groups were compared. The mean follow-up period was 15 ± 3.6 months.
Operative Technique
All the operations were performed by the senior author (EC). A debridement was performed in all traumatic injuries, and NPT was applied before flap reconstruction for 7–10 days. The reconstruction was performed in a later reconstructive stage (group I) or two subsequent reconstructive stages (group II). A preoperative hand-held Doppler was used in all cases to identify perforators adjacent to the defect. Under tourniquet ischemia, the skin incisions were performed, and a dermoepidermal flap was raised to expose the area where the perforators were located. It is important to preserve the dermal plexus of the skin flaps to ensure an adequate vascularization and avoid skin necrosis at the donor site (Fig. 2A). After the subcutaneous tissue was fully exposed, an adipofascial flap of adequate dimension was designed. Once a suitable perforator was found, the adipofascial flap was incised proximally and elevated in a proximal-to-distal fashion, until the chosen perforator (flap’s pivot point) was reached (Fig. 2B). The tourniquet was released to check the flap vascularization, and hemostasis was performed. The flap was then flipped (if multiple perforators were included in the flap) or rotated (if the flap was based on a single perforator) to achieve a tensionless inset. In group I patients, immediate skin grafting and NPT were applied, whereas in group II patients a dermal matrix was then placed over the flap, and the NPT was applied (50 mm Hg of continuous pressure with low intensity) (Figs. 3, 4). Primary closure of the donor site was always achievable. For group II patients, after 14 days a full thickness or a split thickness skin graft was placed over the revascularized dermal matrix (Fig. 5). NPT was applied over the skin graft to achieve better graft take. Hyaluronic acid and silver sulfadiazine-soaked gauzes were placed over ADM or skin graft before NPT was applied.
Fig. 2.
First reconstructive stage—intraoperative photographs. An adipofascial flap reconstruction is planned to cover the tendon exposure at the distal third of the leg. A, NPT and surgical debridement had already been accomplished. A dermoepidermal flap is raised to expose the subcutaneous tissue; it is important to preserve the subdermal plexus to avoid skin necrosis at the donor site. B, An adipofascial flap is raised based on the previously mapped perforator. After incision of the deep fascia, the previously mapped perforator from the posterior tibial artery is identified. The design of the flap is reevaluated based on the perforator position. The flap is then elevated in a proximal-to-distal fashion. The perforator marked with a star must be cauterized to allow the descent of the pivot point.
Fig. 3.
NPT (50 mmHg, continuous, low pressure) is immediately applied on the dermal matrix. Primary closure of the donor site is achieved.
Fig. 4.
Appearance of the recipient site after 14 days of NPT therapy; the bed is completely covered by granulation tissue and is ready for skin grafting.
Fig. 5.
A full thickness skin graft is applied over the dermal matrix.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics software package version 25 (IBM Corp. SPSS Statistics for Windows, N.Y.). Data for quantitative variables were provided as mean ± SD, if not differently stated. The values for categorical variables were analyzed by the two tailed Fisher’s exact test; the values for quantitative variables were analyzed by the two-tailed Mann–Whitney test. Significance was set at a value of P less than 0.05. ICC estimates were calculated based on a mean-rating (k = 3), absolute-agreement, two-way random-effects model to assess the reliability among raters. ICC values less than 0.5 are indicative of “poor” reliability, values between 0.5 and 0.75 indicate “moderate” reliability, values between 0.75 and 0.9 indicate “good” reliability, and values greater than 0.90 indicate “excellent” reliability.20
RESULTS
The mean age of the patients was 37 ± 13.4 years. The defect was located on the anterior aspect of the distal leg in 15 cases, and on the posterior aspect in eight cases. In four cases, bone exposure was reported. The flaps were based on perforators from the tibialis posterior vessels when the defect was located on the anterior aspect, and on perforators from the peroneal vessels when the defect was on the posterior leg. The average size of the defects was 30.8 ± 5.2 cm2 and 30 ± 6.4 cm2 respectively for groups I and II. The average flap size 36 ± 5.3 cm2 and 34.3 ± 5.6 cm2 respectively for groups I and II. All patients achieved complete healing of the soft tissue defect; the mean healing time was 59.6 ± 8.2 days and 47 ± 5.8 days for the two groups (Table 1).
Complications at the recipient site were observed in three patients; all cases were observed in group I. Total flap failure was not observed; however, a partial flap necrosis with limited wound dehiscence (<2 cm) developed in one case, and two cases of partial graft loss were observed; all of them were minor complications, and were treated conservatively with advanced dressings. One limited wound dehiscence (<2 cm) of the donor site was observed in a single patient of group II; it was treated conservatively. Overall complication rate was 17% (Table 2). The overall complication profile was not statistically different in the two groups. Anyway, we reported three of 12 patients with minor complications in group I, and one of 11 patients with a minor complication in group II.
Skin texture and color match had the lowest mean score in both groups’ questionnaires; this is related to the skin graft characteristics that differ from the surrounding skin. Donor site scar and contour instead had the highest score; this could be explained with the primary closure of the donor site and the possibility of harvesting a thin flap that does not modify the contour of the leg. Overall, the results were satisfying in both groups of patients; however, group II patients showed better outcomes related to skin texture, color match, contour, and recipient site appearance; the overall result was also better in group II (Table 3). The ICC was assessed for group I and group II. The ICC was 0.884 and 0.832, respectively. In both cases, the reliability among the three raters (equipe, external surgeon, and patients) was evaluated as “good.”20
Table 3.
Surgical and Aesthetic Outcomes Evaluation Conducted through a Questionnaire
| Equipe | External Surgeon | Patient | Mean | |
|---|---|---|---|---|
| Group I | ||||
| Skin texture | 7,2 | 7,1 | 7,0 | 7,1 |
| Color match | 7,3 | 7,3 | 6,8 | 7,1 |
| Contour | 8,0 | 7,9 | 7,5 | 7,8 |
| Recipient site appearance | 7,8 | 7,7 | 7,5 | 7,7 |
| Donor site scar | 8,8 | 8,7 | 8,5 | 8,8 |
| Overall result | 7,9 | 7,8 | 7,5 | 7,8 |
| Group II | ||||
| Skin texture | 8,1 | 7,8 | 7,8 | 7,9 |
| Color match | 8,0 | 7,8 | 7,7 | 7,8 |
| Contour | 8,9 | 8,7 | 8,5 | 8,8 |
| Recipient site appearance | 8,7 | 8,3 | 8,6 | 8,5 |
| Donor site scar | 9,0 | 8,8 | 8,7 | 8,8 |
| Overall result | 8,5 | 8,1 | 8,4 | 8,3 |
1–2 very unsatisfied; 3–4 unsatisfied; 5–6 acceptable; 7–8 satisfied; 9–10 very satisfied.
The patient reported satisfaction with the aesthetic outcome was further analyzed; group II patients reported significantly more satisfied with the overall results and also in every single domain of the questionnaire (all P < 0.05), except for the domain “donor site scar,” as we could expect (Table 4) (Figs. 6, 7).
Table 4.
Statistical Analysis: Patient Reported Outcomes of Group I versus Group II
| Group I | Group II | P | |
|---|---|---|---|
| Skin texture | 7 ± 0.7 | 7.8 ± 0.8 | 0.034 |
| Color match | 6.8 ± 0.6 | 7.7 ± 0.8 | 0.01174 |
| Contour | 7.5 ± 1 | 8.5 ± 0.8 | 0.02444 |
| Recipient site appearance | 7.5 ± 1.2 | 8.6 ± 0.9 | 0.03662 |
| Donor site scar | 8.5 ± 1 | 8.7 ± 0.8 | 0.64552 |
| Overall result | 7.5 ± 0.7 | 8.4 ± 0.8 | 0.02926 |
1–2 very unsatisfied; 3–4 unsatisfied; 5–6 acceptable; 7–8 satisfied; 9–10 very satisfied.
Bold values represent significant P values (<0.05).
Fig. 6.
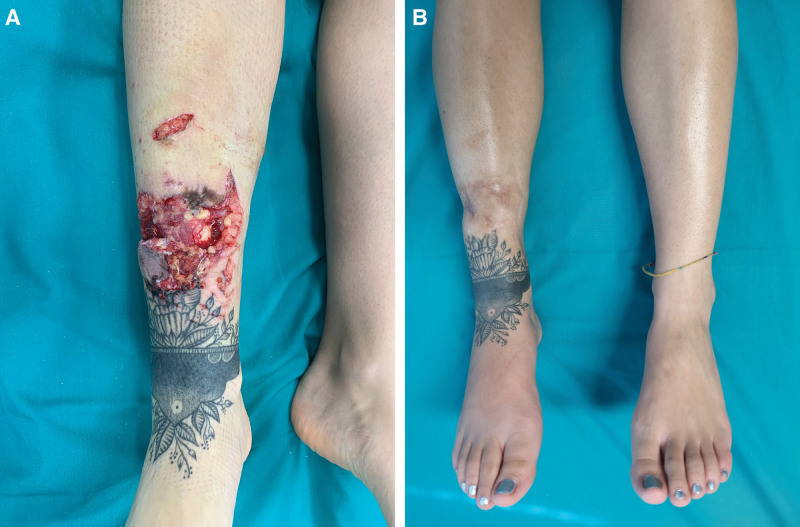
Preoperative and postoperative photographs - Group I patient. A, Preoperative photograph. Appearance of the lower leg of a young woman after a dog bite. Soft tissue defect with tibial bone exposure is evident. Debridement was performed, and NPT was administered before adipofascial flap reconstruction with concomitant skin grafting. B, Postoperative photograph. Frontal view of the leg 12 months after the single stage reconstruction without dermal matrix; the contour of the leg was not completely satisfactory for the patient.
Fig. 7.
Preoperative and postoperative photographs - Group II patient. A, Preoperative planning of a perforator-based adipofascial flap of the lower leg after trauma with tendon exposure. The perforator was mapped using Doppler ultrasound and flap was designed accordingly. Debridement was performed and negative pressure wound therapy was administered before reconstructive stages (authors’ protocol) could start. B, Postoperative photograph. Fifteen-month postoperative picture shows an overall satisfactory result in terms of both recipient site color match/skin texture, and contour.
DISCUSSION
Various techniques are available for the reconstruction of lower extremity defects, from local and regional flaps to free tissue transfer.21 Concerning indications, free flaps are clearly the first choice in larger and complex defects when multiple tissues are required, and in specific situations like osteomyelitis, diabetic foot, exposed fracture or extrusion of prosthetic devices. In small-to-medium sized defects, local flaps could be an easier alternative that require shorter operative time and no microsurgical skills; furthermore, local flaps have similar skin texture, color, and thickness to the defect site, thus providing a better aesthetic result.22, 23 Pitfalls of this reconstructive option are represented by the lack of local tissue, and the creation of a secondary defect in the already injured leg that often requires skin grafting.
Several types of local flaps, such as muscle,24,25 fasciocutaneous,26 adipofascial,19 perforator, and propeller skin flaps27,28 have been described. The sural flap, first described by Donski et al,26 is one of the most common and simple choices for distal leg reconstruction. This distally based flap relies mostly on septocutaneous perforators from the peroneal artery, and can cover defects of small-to-moderate size of the distal leg, the ankle, and the dorsum of the foot.29 As in most local flaps, partial flap necrosis or loss is not uncommon, and venous congestion is another common complication; a recent review of the literature regarding its use in the reconstruction of the lower third of the leg stated that the overall complication rate was 33.7%.30 Donor site direct closure is not always possible, thus requiring skin grafting for coverage; then, the aesthetic outcome is affected in two separate areas.
Muscle flaps, such as soleus25 or peroneus brevis flap,24 were also described for distal extremity reconstruction. The soleus muscle, as a distally based pedicled flap, has been described as capable of covering defects of the distal third of the leg; the flap receives its supply from the distal perforator of the posterior tibial vessels. However, as the authors reported, venous congestion is not uncommon, and distal flap necrosis is reported to occur in about 20% of the cases; excessive bulkiness of the flap, especially at the pivot point with an unpleasant aesthetic result, is usual.
Pedicled perforator flaps are based more frequently on perforators of the tibialis posterior artery and the peroneal artery, and less frequently on the perforators of the tibialis anterior artery.31–33 They indeed have some advantages: the source artery is left untouched and still available in case of flap loss for other local or free flaps; the underlying muscles are left untouched too, preserving their function, and there is no need for microsurgical anastomoses. Schaverien et al34 have carried out extensive studies regarding the perforators of the lower leg, providing anatomical basis for the elevation of pedicled perforator flap. As mentioned for the above-described local flaps, primary closure of the donor site is often not possible, and a skin graft is required. Moreover, the reported complication rate ranged from 12.5% to 50%.35,36
Adipofascial flaps for the reconstruction of the distal third of the leg were first described by Lai and colleagues,19 and various modifications of this procedure have been presented over time.37–41 In the first description for the reconstruction of the lower leg, an adipofascial flap was used as a random turnover flap with strict length-to-width ratio; soon after that, adipofascial flaps started to be raised as axial flaps based on the major vessel of the leg, especially the posterior tibial artery and their perforators.31, 42
These flaps have several advantages over the previously mentioned techniques: (1) they are easy and quick to harvest; (2) they have an abundant vascularization given by fascial plexus; (3) a direct closure of the donor site is always possible; (4) it is possible to harvest large flaps with a relatively long pedicle; (5) they have a wide arc of rotation; (6) they are thin, thus providing limited contour deformities; (7) there is no need to sacrifice major blood vessels or muscle; (8) there is no need for microsurgical instruments.37–40,43
Both dermal matrix substitute and negative pressure therapy (NPT) proved to be effective strategies in the management of acute and chronic wounds.44–46 Dermal matrix substitutes make the wound bed more graftable, allowing skin graft to be used even in complex defects. We used a double layer dermal matrix, with an internal layer made of gag and bovine collagen, and an external layer made of silicone. The internal porous layer acts as a scaffold for the regeneration of the dermis.
NPT has various effects on wound healing, including removal of fluid excess, stimulation of blood flow, angiogenesis, cell proliferation, reduction of bacterial load, and maintenance of a moist environment, which help wound healing. Moreover, NPT improves graft take by fluid removal, keeping the graft immobile, and promoting contact between it and the wound bed.47 In our opinion, in case an adipofascial flap is chosen, a tiny amount of liponecrosis should be taken into account, and NPT should be adopted as an ancillary but significant procedure to obtain likely uneventful healing. Moreover, NPT was demonstrated as safe and effective when combined with adipofascial flap reconstruction. Pontell et al48 applied NPT immediately after adipofascial flap reconstruction on four patients undergoing lower limb reconstruction. The authors did not report in detail the pressure and the intensity applied; however, no complications related to flap vascularization were reported. In our series, NPT was applied in all cases, and one case of partial flap necrosis was reported; anyway, it was a limited necrosis and healed successfully in outpatient setting. We believe that such specific complication could not be due to the NPT; however, we could not state it with certainty.
Jeschke et al49 and Molnar et al50 demonstrated a better and faster dermal matrix neovascularization with the use of NPT with a reduction of the infection rates; the same results were observed later by other authors.51 Given these reports, we applied those concepts to the reconstruction of soft tissue defects with perforator-based adipofascial flaps.
Group II showed lower complication rate and lower healing time, and that is consistent with the findings from Pontell et al48; however, they did not use NPT on their control group, thus creating heterogeneity between the two groups. In our study, NPT was used on both groups but with different timing. We observed two partial skin graft losses in group I (no ADM): the reconstruction of the dermis through the ADM allowed, in our opinion, a better take of the skin graft and an aesthetically more pleasant result.
The use of an ADM with an NPT device as a bridge therapy before the skin graft has, in our opinion, several advantages: it provides a more anatomical and aesthetically pleasing reconstruction because it allows for reconstruction of the normal anatomy of the skin with a subcutaneous layer (adipofascial flap), a dermal layer (ADM), and an epidermal layer (skin graft). Moreover, the dermal matrix substitute allows better graft take, thus permitting the use of a full thickness skin graft, optimizing the aesthetic outcome for the donor site of the graft.
The choice between a full-thickness and partial-thickness skin graft has been made based on the size of the wound, and also on the patients’ will; however, we believed that full thickness skin graft allowed for a more aesthetically pleasant result, and for the same reason, the skin graft was not meshed. A comparison between full-thickness and split-thickness skin graft outcomes has not been made.
Obviously, the use of a dermal matrix has some drawbacks, given the costs of the device and the need for further operation; however, this second reconstructive stage may be performed on local anesthesia. We are aware that ADM and NPT have increased the costs of the entire treatment; however, a comparative cost analysis was not performed. Our surgical strategy for small- to medium-sized lower limb soft tissue defects foresees one more hospitalization than the conventional approach. However, after debridement and first reconstructive stage, the later hospitalization lasted only 1 day or was a day-hospital program (Fig. 1).
The cost of ADM should be mentioned and taken into account. Still, we believe that our surgical and cosmetic results justify its use. In our series, the overall complication profile was not statistically different in the two groups; anyway, we reported a lower rate of minor complications in Group II. Indeed, ADM and NPT allow a better graft take, thus avoiding further costs of hospitalization due to surgical revisions. Finally, in our opinion, the advantages of a two-stage reconstruction are far more than the disadvantages.
The lower leg is a challenging area for the reconstructive surgeon, but it is also a well-exposed part of the body for our patients. Our approach matches the scientific trend of a tailor-made and aesthetic “friendly” reconstructive surgery that is far more aware and attentive of donor site morbidity and patients’ discomfort, along with their desire and expectations.52–55 Moreover, we believe that an aesthetically pleasing result, beside its own value, has a strong impact on patient’s self-confidence, preventing eventual mental distress with its associated social costs, especially considering that the setting of trauma is distressing itself for the patients.
This study is a retrospective analysis with its known shortcomings; however, there are no prospective studies, and this should be advocated. Furthermore, the small sample size is an evident limitation, but this can be explained with the narrow indication to post traumatic patients with small-to-medium soft tissue defects.
CONCLUSIONS
The adipofascial perforator-based flap is a highly reliable option for the reconstruction of small- to medium-sized defects of the distal lower extremity. Our two-stage reconstructive approach maximizes the pearls offered by the established technique; the dermal matrix, applied over the flap, guarantees a layered reconstruction optimizing the surgical and aesthetic outcomes of the skin graft with minimal donor site morbidity.
ACKNOWLEDGMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Published online 17 February 2022.
Disclosure: The authors have no financial interest in relation to the content of this article.
REFERENCES
- 1.Khouri RK, Shaw WW. Reconstruction of the lower extremity with microvascular free flaps: a 10-year experience with 304 consecutive cases. J Trauma. 1989;29:1086–1094. [DOI] [PubMed] [Google Scholar]
- 2.Hallock GG. Local fasciocutaneous flaps for cutaneous coverage of lower extremity wounds. J Trauma. 1989;29:1240–1244. [DOI] [PubMed] [Google Scholar]
- 3.Soltanian H, Garcia RM, Hollenbeck ST. Current concepts in lower extremity reconstruction. Plast Reconstr Surg. 2015;136:815e–829e. [DOI] [PubMed] [Google Scholar]
- 4.Bekara F, Herlin C, Somda S, et al. Free versus perforator-pedicled propeller flaps in lower extremity reconstruction: what is the safest coverage? A meta-analysis. Microsurgery. 2018;38:109–119. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez-Mujica J, Losco L, Aksoyler D, et al. Perforator-to-perforator anastomosis as a salvage procedure during harvest of a perforator flap. Arch Plast Surg. 2021;48:467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang MJ, Chung CH, Chang YJ, et al. Reconstruction of the lower extremity using free flaps. Arch Plast Surg. 2013;40:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cajozzo M, Toia F, Innocenti A, et al. Retrospective analysis in lower limb reconstruction: propeller perforator flaps versus free flaps. J Reconstr Microsurg. 2017;33:S34–S39. [DOI] [PubMed] [Google Scholar]
- 8.Benacquista T, Kasabian AK, Karp NS. The fate of lower extremities with failed free flaps. Plast Reconstr Surg. 1996;98:834–841. [DOI] [PubMed] [Google Scholar]
- 9.Losco L, Aksoyler D, Chen SH, et al. Pharyngoesophageal reconstruction with free jejunum or radial forearm flap as diversionary conduit: functional outcomes of patients with persistent dysphagia and aspiration. Microsurgery. 2020;40:630–638. [DOI] [PubMed] [Google Scholar]
- 10.Aksoyler D, Losco L, Bolletta A, et al. Three salvage strategies in microvascular fibula osteocutaneous flap for mandible reconstruction with vascular compromise and establishment of an algorithm. Microsurgery. 2021;41:223–232. [DOI] [PubMed] [Google Scholar]
- 11.Trignano E, Fallico N, Fiorot L, et al. Flap monitoring with continuous oxygen partial tension measurement in breast reconstructive surgery: a preliminary report. Microsurgery. 2018;38:402–406. [DOI] [PubMed] [Google Scholar]
- 12.Koh K, Goh TLH, Song CT, et al. Free versus pedicled perforator flaps for lower extremity reconstruction: a multicenter comparison of institutional practices and outcomes. J Reconstr Microsurg. 2018;34:572–580. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg. 2009;124:1529–1544. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102:599–616. [PubMed] [Google Scholar]
- 15.Maruccia M, Fallico N, Cigna E, et al. Suprafascial versus traditional harvesting technique for free antero lateral thigh flap: a case-control study to assess the best functional and aesthetic result in extremity reconstruction. Microsurgery. 2017;37:851–857. [DOI] [PubMed] [Google Scholar]
- 16.Hallock GG. A paradigm shift in flap selection protocols for zones of the lower extremity using perforator flaps. J Reconstr Microsurg. 2013;29:233–240. [DOI] [PubMed] [Google Scholar]
- 17.Cherubino M, Bolletta A, Baroni T, et al. Anatomical study and clinical application of ulnar artery proximal perforator flaps. J Reconstr Microsurg. 2021;37:201–207. [DOI] [PubMed] [Google Scholar]
- 18.Parrett BM, Matros E, Pribaz JJ, et al. Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg. 2006;117:1315–1323. [DOI] [PubMed] [Google Scholar]
- 19.Lai CS, Lin SD, Yang CC, et al. Adipofascial turn-over flap for reconstruction of the dorsum of the foot. Br J Plast Surg. 1991;44:170–174. [DOI] [PubMed] [Google Scholar]
- 20.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh HS, Lee JS, Hong JP. Consideration in lower extremity reconstruction following oncologic surgery: patient selection, surgical techniques, and outcomes. J Surg Oncol. 2016;113:955–961. [DOI] [PubMed] [Google Scholar]
- 22.Parrett BM, Talbot SG, Pribaz JJ, et al. A review of local and regional flaps for distal leg reconstruction. J Reconstr Microsurg. 2009;25:445–455. [DOI] [PubMed] [Google Scholar]
- 23.Maruccia M, Di Taranto G, Schonauer F, et al. Freestyle perforator puzzle flap for posterior trunk reconstruction. Ann Plast Surg. 2020;85:56–60. [DOI] [PubMed] [Google Scholar]
- 24.Abd-Al-Moktader MA. Distally based peroneus brevis muscle flap for large leg, ankle, and foot defects: anatomical finding and clinical application. J Reconstr Microsurg. 2018;34:616–623.. [DOI] [PubMed] [Google Scholar]
- 25.Song P, Pu LLQ. The soleus muscle flap: an overview of its clinical applications for lower extremity reconstruction. Ann Plast Surg. 2018;81:S109–S116. [DOI] [PubMed] [Google Scholar]
- 26.Donski PK, Fogdestam I. Distally based fasciocutaneous flap from the sural region. A preliminary report. Scand J Plast Reconstr Surg. 1983;17:191–196. [DOI] [PubMed] [Google Scholar]
- 27.Mendieta M, Cabrera R, Siu A, et al. Perforator propeller flaps for the coverage of middle and distal leg soft-tissue defects. Plast Reconstr Surg Glob Open. 2018;6:e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AlMugaren FM, Pak CJ, Suh HP, et al. Best local flaps for lower extremity reconstruction. Plast Reconstr Surg Glob Open. 2020;8:e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Follmar KE, Baccarani A, Baumeister SP, et al. The distally based sural flap. Plast Reconstr Surg. 2007;119:138e–148e. [DOI] [PubMed] [Google Scholar]
- 30.Daar DA, Abdou SA, Cohen JM, et al. Is the medial sural artery perforator flap a new workhorse flap? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143:393e–403e. [DOI] [PubMed] [Google Scholar]
- 31.Koshima I, Moriguchi T, Ohta S, et al. The vasculature and clinical application of the posterior tibial perforator-based flap. Plast Reconstr Surg. 1992;90:643–649. [DOI] [PubMed] [Google Scholar]
- 32.Hifny MA, Tohamy AMA, Rabie O, et al. Propeller perforator flaps for coverage of soft tissue defects in the middle and distal lower extremities. Ann Chir Plast Esthet. 2020;65:54–60. [DOI] [PubMed] [Google Scholar]
- 33.Bulla A, Bolletta A, Fiorot L, et al. Posterior tibial perforators relationship with superficial nerves and veins: a cadaver study. Microsurgery. 2019;39:241–246. [DOI] [PubMed] [Google Scholar]
- 34.Schaverien M, Saint-Cyr M. Perforators of the lower leg: analysis of perforator locations and clinical application for pedicled perforator flaps. Plast Reconstr Surg. 2008;122:161–170. [DOI] [PubMed] [Google Scholar]
- 35.Robotti E, Carminati M, Bonfirraro PP, et al. “On demand” posterior tibial artery perforator flaps: a versatile surgical procedure for reconstruction of soft tissue defects of the leg after tumor excision. Ann Plast Surg. 2010;64:202–209. [DOI] [PubMed] [Google Scholar]
- 36.Shin IS, Lee DW, Rah DK, et al. Reconstruction of pretibial defect using pedicled perforator flaps. Arch Plast Surg. 2012;39:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt K, Jakubietz M, Djalek S, et al. The distally based adipofascial sural artery flap: faster, safer, and easier? A long-term comparison of the fasciocutaneous and adipofascial method in a multimorbid patient population. Plast Reconstr Surg. 2012;130:360–368. [DOI] [PubMed] [Google Scholar]
- 38.Lee KJ, Lee SH, Kim MB, et al. Adipofascial fold-down flaps based on the posterior tibial artery perforator to cover the medial foot and ankle defects. J Plast Reconstr Aesthet Surg. 2016;69:e229–e237. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Chang SM, Du SC, et al. Distally based sural adipofascial turnover flap for coverage of complicated wound in the foot and ankle region. Ann Plast Surg. 2020;84:580–587. [DOI] [PubMed] [Google Scholar]
- 40.Losco L, Lo Torto F, Maruccia M, et al. Modified single pedicle reverse adipofascial flap for fingertip reconstruction. Microsurgery. 2019;39:221–227. [DOI] [PubMed] [Google Scholar]
- 41.Losco L, Ciamarra P, Cigna E. Comments on “Fenestrated adipofascial reverse flap for the reconstruction of fingertip amputations”. Microsurgery. 2020;40:282. [DOI] [PubMed] [Google Scholar]
- 42.Lin SD, Lai CS, Chou CK, et al. The distally based posterior tibial arterial adipofascial flap. Br J Plast Surg. 1992;45:284–287. [DOI] [PubMed] [Google Scholar]
- 43.Suliman MT. Distally based adipofascial flaps for dorsal foot and ankle soft tissue defects. J Foot Ankle Surg. 2007;46:464–469. [DOI] [PubMed] [Google Scholar]
- 44.Novak A, Khan WS, Palmer J. The evidence-based principles of negative pressure wound therapy in trauma & orthopedics. Open Orthop J. 2014;8:168–177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muangman P, Engrav LH, Heimbach DM, et al. Complex wound management utilizing an artificial dermal matrix. Ann Plast Surg. 2006;57:199–202. [DOI] [PubMed] [Google Scholar]
- 46.Cigna E, Pierazzi DM, Sereni S, et al. Lymphatico-venous anastomosis in chronic ulcer with venous insufficiency: a case report. Microsurgery. 2021;41:574–578. [DOI] [PubMed] [Google Scholar]
- 47.Schneider AM, Morykwas MJ, Argenta LC. A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg. 1998;102:1195–1198. [DOI] [PubMed] [Google Scholar]
- 48.Pontell ME, Saad N, Winters BS, et al. Reverse sural adipofascial flaps with acellular dermal matrix and negative-pressure wound therapy. Adv Skin Wound Care. 2018;31:612–617. [DOI] [PubMed] [Google Scholar]
- 49.Jeschke MG, Rose C, Angele P, et al. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg. 2004;113:525–530. [DOI] [PubMed] [Google Scholar]
- 50.Molnar JA, DeFranzo AJ, Hadaegh A, et al. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg. 2004;113:1339–1346. [DOI] [PubMed] [Google Scholar]
- 51.Diehm YF, Fischer S, Gazyakan E, et al. Negative pressure wound therapy as an accelerator and stabilizer for incorporation of artificial dermal skin substitutes – a retrospective, non-blinded, and non-randomized comparative study. J Plast Reconstr Aesthet Surg. 2021;74:357–363. [DOI] [PubMed] [Google Scholar]
- 52.Losco L, Cigna E. Aesthetic refinements in C-V flap: raising a perfect cylinder. Aesthet Surg J. 2018;38:NP26–NP28. [DOI] [PubMed] [Google Scholar]
- 53.Edsander-Nord A, Brandberg Y, Wickman M. Quality of life, patients’ satisfaction, and aesthetic outcome after pedicled or free TRAM flap breast surgery. Plast Reconstr Surg. 2001;107:1142–1154. [DOI] [PubMed] [Google Scholar]
- 54.Paolino G, Cardone M, Didona D, et al. Prognostic factors in head and neck melanoma according to facial aesthetic units. G Ital Dermatol Venereol. 2020;155:41–45. [DOI] [PubMed] [Google Scholar]
- 55.Posch NAS, Mureau MAM, Dumans AG, et al. Functional and aesthetic outcome and survival after double free flap reconstruction in advanced head and neck cancer patients. Plast Reconstr Surg. 2007;120:124–129. [DOI] [PubMed] [Google Scholar]