Abstract
Evidence supports the observational associations of gut microbiota with a variety of psychiatric disorders, but the causal nature of such associations remains obscure. Aiming to comprehensively investigate their causal relationship and to identify specific causal microbe taxa for psychiatric diseases, we conducted a two-sample Mendelian randomization (MR) analysis of gut microbiome with 15 psychiatric diseases. Specifically, the microbiome genome-wide association study (GWAS) in 18,473 individuals from the MiBioGen study was used as exposure sample, and the GWAS for 15 psychiatric diseases was used as outcome samples. One-hundred ninety bacterial taxa from six levels were available for analysis. At a multiple-testing corrected significance level (phylum P < 5.56 × 10–3, class P < 3.33 × 10–3, order P < 2.63 × 10–3, family P < 1.67 × 10–3, genus P < 4.90 × 10–4, and species P < 3.33 × 10–3), the following eight causal associations from seven bacterial features (one phylum + three classes + one order + one family + one species) were identified: family Prevotellaceae with autism spectrum disorder (P = 5.31 × 10–4), class Betaproteobacteria with bipolar disorder (P = 1.53 × 10–3), class Actinobacteria with schizophrenia (P = 1.33 × 10–3), class Bacteroidia and order Bacteroidales with Tourette syndrome (P = 2.51 × 10–3 and 2.51 × 10–3), phylum Actinobacteria and class Actinobacteria with extroversion (P = 8.22 × 10–4 and 1.09 × 10–3), and species Clostridium innocuum with neuroticism (P = 8.92 × 10–4). Sensitivity analysis showed no evidence of reverse causality, pleiotropy, and heterogeneity. Our findings offered novel insights into the gut microbiota–mediated development mechanism of psychiatric disorders.
Keywords: Mendelian randomization (MR), gut microbiota (GM), psychiatric disorders, causal relationship, species Clostridium innocuum
Introduction
Psychiatric disorders are a cluster of complex psychological syndromes in cognition, behavior, or emotion regulation, representing the second leading cause of disability and premature death worldwide (Whiteford et al., 2013). Epidemiological research has shown that the global lifetime incidence of psychiatric disorders in adults ranges between 12.2 and 48.6%, with the overall prevalence varying from 4.3 to 26.4% (Demyttenaere et al., 2004). Certain mental diseases, such as depression, attention deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and schizophrenia (SCZ), account for approximately 12% of the global disease burden (Ochoa-Reparaz et al., 2020). Because they frequently require long-term treatment, their burden was estimated to be $8.5 trillion in 2010 and continuously raised by 41% between 1990 and 2010 (Patel et al., 2016). Thus, there is an urgent need to identify potential causal risk factors for various psychiatric disorders.
The etiologies of psychiatric disorders are largely multi-factorial, including psychological, genetic, and environmental factors. Recently, growing evidence has suggested that the gut microbiota is closely related to host health and is involved in the etiology of a variety of human complex diseases including psychiatric disorders (Chow et al., 2010; Clemente et al., 2012; Cryan et al., 2019). The gut microbiota is a dynamic and complex community of ecological microbes, inhabiting the human intestine, even called a “forgotten organ” (O’Hara and Shanahan, 2006). The microbiota and central nervous system might communicate with each other via the microbiota–gut–brain (MGB) axis, which includes diverse routes including the immune response, the vagus and enteric nerve, and microbiota-derived molecules or metabolites (Cryan et al., 2019). A variety of observational studies have shown that the gut microbiome differs between healthy controls and psychiatric patients (Wang et al., 2011; Strati et al., 2017; Valles-Colomer et al., 2019; Hua et al., 2020). Altered compositions and function of intestinal microbiota were observed in ASD or depression patients (Strati et al., 2017; Valles-Colomer et al., 2019). Further experimental studies also demonstrated the importance of microbiota in the development of psychiatric disorders. For example, fecal microbiota transplantation (FMT) from human donors with ASD into murine induced exacerbated corresponding symptoms (Sharon et al., 2019). However, the causal association between the gut microbiota and psychiatric disorders remains unclear.
Conventionally, the gold standard for inferring a causal association is randomized controlled trials (RCTs). Whereas a RCT is difficult to implement or sometimes even impossible due to ethic restriction. As an alternative, Mendelian randomization (MR) is an efficient method to statistically assess causality from an exposure to an outcome, utilizing genetic variants as instrumental variables (IVs) (Katan, 1986; Smith and Ebrahim, 2003). Because a random assortment of genetic variants occurs during meiosis yielding according to the Mendel’s second law, the selected genetic variants avoid social economic confounding (Emdin et al., 2017). The MR approach is conceptually similar to the RCT study, with only one difference that patients are allocated according to their DNA genotypes. MR analysis relies on three important assumptions: (i) IV is strongly associated with exposure; (ii) IV should be independent of any observed and unobserved confounders of exposure–outcome association; (iii) IV–outcome association is only mediated via exposure rather than any other pathway. In a recent study, utilizing MR, Sanna et al. (2019) identified that propionate, one type of fecal short-chain fatty acid (SCFA), increases the risk of type 2 diabetes, demonstrating the efficacy of microbiota-oriented causal inference via MR analysis.
Two-sample MR analysis can utilize single-nucleotide polymorphism (SNP)–exposure and SNP–outcome associations from independent GWAS analyses and combine them into a single causal estimate. As the number of genome-wide association studies (GWASs) in gut microbiota and psychiatric disorders has increased rapidly, large-scale summary statistics have become more widely available (Demontis et al., 2019; Grove et al., 2019; Howard et al., 2019; Stahl et al., 2019; Kurilshikov et al., 2021), allowing for two-sample MR analysis with significantly improved statistical power.
In this study, we applied a systematic two-sample MR analysis to comprehensively explore whether gut microbiota components have a causal effect on various psychiatric disorders and to identify specific causal bacterial taxa. Specifically, summary statistics of gut microbiota and 15 common psychiatric disorders/traits were derived from large-scale GWAS or genetic consortia.
Materials and Methods
Data Sources
Genome-wide association study summary-level statistics for gut microbiota and 15 common psychiatric disorders/traits were obtained from previous studies or consortia. All studies were approved by their respective institutional review boards (IRBs). No new IRB approval was required. A flowchart briefly presents the whole procedure in Figure 1.
FIGURE 1.
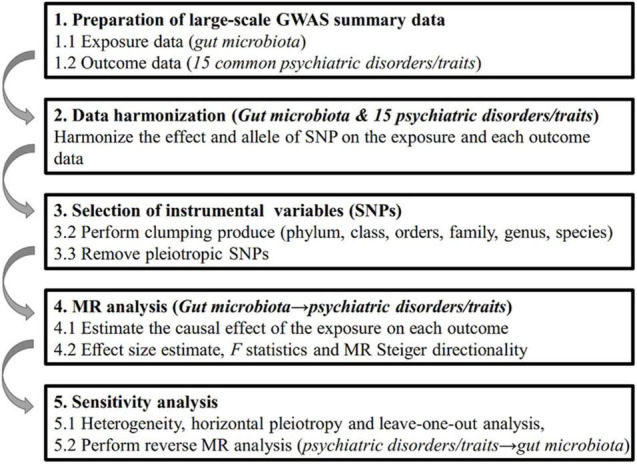
Diagrammatic description of the whole workflow in MR analysis. A flowchart of the whole MR analysis was displayed in this figure.
Genome-wide association study summary statistics of gut microbiota was assessed from the MiBioGen study (Kurilshikov et al., 2021) (1, as of June 28, 2020), which is the largest, multi-ethnic, genome-wide meta-analysis of the gut microbiome to date. Briefly, the MiBioGen study coordinated 16S rRNA gene sequencing profiles and whole-genome genotyping data from 18,473 individuals (25 cohorts) as described elsewhere (Kurilshikov et al., 2021). The microbial composition of distinct cohorts was profiled by targeting three different variable regions of the 16S rRNA gene: V4, V3–V4, and V1–V2, and all microbiome datasets were rarefied to 10,000 reads per cohort. The majority of cohorts used similar imputation procedures using the Michigan Imputation Server or IMPUTE2 software and the Haplotype Reference Consortium 1.0 or 1.1 reference panel. Then, microbiome trait loci mapping was performed to identify genetic loci that affect the relative abundance of microbial taxa. The cutoffs mapping included at least 3,000 effective samples in the presence of at least three cohorts. In total, all available GWAS summary statistics of 190 bacterial taxa were eventually included in the MR analysis.
Genome-wide association study summary statistics for psychiatric disorders were generated from large-scale GWAS or their meta-analysis. We collected as many psychiatric disorders as possible, resulting in 15 disorders (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; van den Berg et al., 2016; International Obsessive Compulsive Disorder Foundation Genetics Collaborative (Iocdf-Gc) and Ocd Collaborative Genetics Association Studies (OCGAS), 2018; Nagel et al., 2018; Pasman et al., 2018; Demontis et al., 2019; Grove et al., 2019; Howard et al., 2019; Meier et al., 2019; Nievergelt et al., 2019; Sanchez-Roige et al., 2019; Stahl et al., 2019; Watson et al., 2019; Yu et al., 2019; Erlangsen et al., 2020). Criteria to define these disorders are listed in Supplementary Table 1. For each disorder, summary statistics from the largest GWAS were assessed. Detailed descriptions of GWAS for 15 common psychiatric disorders/traits, including the ethnicity, genotyping platform, imputation reference panel, and consortium, are presented in Table 1.
TABLE 1.
Characteristics of included genome-wide association studies for psychiatric disorders.
| Psychiatric disorders/traits | Ethnicity | N | No. SNP | Data type | Genotyping platform and SNP panel | References | Study |
| Depression | European | 500,199 | 8,483,301 | Binary | Affymetrix United Kingdom Biobank/BiLEVE Axiom array, IMPUTE4, HRC and UK10K; PGC Ricopili (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) | Howard et al. (2019) | UKB, PGC |
| ADHD | Multi-ancestry | 55,374 | 8,047,421 | Binary | Illumina PsychChip; 1KGP Phase 3; PGC Ricopili | Demontis et al. (2019) | iPSYCH, PGC |
| ASD | European | 46,351 | 9,112,386 | Binary | PsychChip array; 1KGP Phase 3; PGC Ricopili | Grove et al. (2019) | iPSYCH, PGC |
| BD | European | 51,710 | 9,372,253 | Binary | PGC Ricopili, 1KGP | Stahl et al. (2019) | PGC |
| SCZ | Multi-ancestry | 152,805 | 9,444,230 | Binary | PGC Ricopili, 1KGP | Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) | PGC |
| AUDIT | European | 121,604 | 16,213,998 | Continuous | Affymetrix United Kingdom Biobank/BiLEVE Axiom array, IMPUTE4, HRC | Sanchez-Roige et al. (2019) | UKB |
| CUD | European | 162,082 | 11,535,592 | Continuous | Affymetrix United Kingdom Biobank/BiLEVE Axiom array, IMPUTE4, HRC; various, 1KGP Phase 1 | Pasman et al. (2018) | UKB, ICC |
| AN | European | 72,517 | 82,191,012 | Binary | Affymetrix United Kingdom Biobank/BiLEVE Axiom array, IMPUTE4, HRC; PGC Ricopili, 1KGP Phase 3; various | Watson et al. (2019) | UKB, PGC, ANGI, GCAN/WTCCC3 |
| TS | European | 14,307 | 8,265,318 | Binary | Illumina HumanOmniExpress 8/12v1, IMPUTE v2, 1KGP Phase 1 | Yu et al. (2019) | PGC |
| OCD | Multi-ancestry | 9,725 | 8,409,516 | Binary | Illumina Human610-Quadv1_B, Illumina HumanOmniExpress, IMPUTE2, 1KGP Phase 1 | International Obsessive Compulsive Disorder Foundation Genetics Collaborative (Iocdf-Gc) and Ocd Collaborative Genetics Association Studies (OCGAS) (2018) | IOCDF-GC, OCGAS |
| Extroversion | European | 63,030 | 6,941,603 | Continuous | Illumina/Affymetrix, IMPUTE, 1KGP Phase 1 | van den Berg et al. (2016) | GPC |
| NEU | European | 390,278 | 10,849,319 | Continuous | Affymetrix United Kingdom Biobank/BiLEVE Axiom array, HRC and UK10K; Illumina/Affymetrix, IMPUTE, 1KGP Phase 1 | Nagel et al. (2018) | UKB, GPC |
| AD | European | 23,809 | 9,029,716 | Binary | Illumina PsychChip, SHAPEIT and IMPUTE2, PGC Ricopili, 1KGP phase 3 | Meier et al. (2019) | iPSYCH |
| SD | European | 29,056 | 8,047,611 | Binary | Illumina PsychChip, SHAPEIT and IMPUTE2, PGC Ricopili, 1KGP phase 3 | Meier et al. (2019) | iPSYCH |
| PTSD | Multi-ancestry | 206,655 | 9,788,621 | Binary | Affymetrix Axiom array; Illumina genotyping arrays, PGC Ricopili, IMPUTE2, 1KGP phase 3 | Nievergelt et al. (2019) | UKB, PGC |
| SA | European | 50,264 | 8,017,027 | Binary | Infinium PsychChip v1.0 array, IMPUTE2, 1KGP phase 3 | Erlangsen et al. (2020) | iPSYCH |
No. SNP is the total number of SNPs released from the summary data of GWAS.
“Various” refers to more details on genotyping platform as described elsewhere previously.
ADHD, attention deficit/hyperactivity disorder; ASD, autism spectrum disorder; BD, bipolar disorder; SCZ, schizophrenia; AUDIT, alcohol use disorder identification test; CUD, cannabis use disorder; AN, anorexia nervosa; TS, Tourette syndrome; OCD, obsessive-compulsive disorder; NEU, neuroticism; AD, anxiety-related disorder; SD, stress-related disorder; PTSD, posttraumatic stress disorder; SA, suicide attempts; HRC, Haplotype Reference Consortium; PGC, Psychiatric Genomics Consortium; Ricopili, Rapid Imputation Consortium Pipeline; UKB, United Kingdom Biobank; 1KGP, 1000 Genomes Project; iPSYCH, Integrative Psychiatric Research; ICC, International Cannabis Consortium; ANGI, Anorexia Nervosa Genetics Initiative GCAN/WTCCC3, Genetic Consortium for Anorexia Nervosa/Wellcome Trust Case Control Consortium-3; IOCDF-GC, International Obsessive Compulsive Disorder Foundation Genetics Collaborative; OCGAS, Collaborative Genetics Association Studies; GPC: Genetics of Personality Consortium.
Instrumental Variable Selection
Bacterial taxa were analyzed at six levels (phylum, class, order, family, genus, and species). A distinct taxon was defined as a feature. Candidate IVs for each feature were selected at the P < 1.0 × 10–5 significance in accordance with the study of Sanna et al. (2019). Then, SNPs associated with each feature were clumped with PLINK (v1.9) to retain only independent SNPs. The linkage disequilibrium (LD) threshold was set to be r2< 0.1, with a clumping window of 500 kb. The 1,000 Genomes Project sequencing data (phase 3) was used to estimate LD.
The horizontal pleiotropy effect, that is, the confounding effect caused by other diseases, is a severe problem and may violate the second assumption in MR analysis. We applied the MR-PRESSO test and the MR-Egger regression test to monitor potential horizontal pleiotropy effect. The MR-PRESSO Outlier test calculates for each SNP a P-value for its pleiotropy significance, whereas the MR-PRESSO Global test calculates a P-value for overall horizontal pleiotropy. SNPs were sorted in an ascending order in terms of their MR-PRESSO Outlier test P-values and were then removed one by one. Each time a SNP was removed from the list, the MR-PRESSO Global test was performed on the remaining SNPs. The recursion was repeated until P-value for the Global test was unsignificant (P > 0.05). The list of the remaining SNPs after removing pleiotropic ones was used for subsequent MR analysis. The significant intercept item of MR-Egger implies the existence of pleiotropy.
To avoid distortion of strand orientation or allele coding, we deleted palindromic SNPs (e.g., with A/T or G/C alleles). In the harmonization process, we aligned alleles to the human genome reference sequence (build 37) and removed ambiguous and duplicated SNPs.
Effect Size Estimate
The GWAS summary statistics of gut microbiota and psychiatric disorders/traits were derived from a standardized phenotype (i.e., mean 0 and variance 1). Therefore, we could estimate the proportion of phenotypic variance explained by SNP from summary statistics with the formula 2f(1−f)β2, where f is the effect allele frequency and β is the regression coefficient for gut microbiota and psychiatric disorders/traits.
Mendelian Randomization Analysis
We performed MR analysis to investigate the causal relationship between microbiome features and the 15 common psychiatric disorders/traits. For features containing only one IV, the Wald ratio test was used to estimate the association between the identified IV and each psychiatric disorder/trait (Burgess et al., 2017). For features containing multiple IVs, five popular MR methods were used: the inverse-variance weighted (IVW) test (Burgess et al., 2013), the maximum likelihood estimator (MLE) (Pierce and Burgess, 2013), the MR-Egger regression (Bowden et al., 2015), the weighted median estimator (WME) (Bowden et al., 2016), and the MR-PRESSO (Verbanck et al., 2018). Each statistical method has its own model assumption, and any violation of the assumption may make the method inferior or even completely invalid. Specific to the five methods investigated: 1) The IVW method assumes no horizontal pleiotropy (Burgess et al., 2013); 2) the MLE (Pierce and Burgess, 2013) assumes the linear correlation of outcome and exposure with jointly normal distribution and allows for uncertainty in both gene–exposure and gene–outcome associations (Burgess et al., 2015); 3) the MR-Egger assumes the presence of pleiotropy in > 50% SNPs (Bowden et al., 2015); 4) the WME assumes the presence of pleiotropy in < 50% SNPs (Bowden et al., 2016); and 5) the MR-PRESSO assumes the presence of pleiotropy but will remove pleiotropic SNPs intrinsically (Verbanck et al., 2018). The IVW method is reported to be slightly more powerful than the others under certain conditions (Bowden et al., 2016). Therefore, the results were mainly based on the IVW method, with the other four methods serving as its complements. Additionally, we established a multiple-testing significance threshold at each feature level (phylum, class, order, family, genus, and species) defined as P < 0.05/n (where n is the effective number of independent bacterial taxa on the corresponding taxonomic level).
To assess robustness of significant results, we performed several sensitivity analyses. The potential heterogeneity was examined by the Q test in the IVW test and the MR-Egger regression. Meanwhile, the leave-one-out analysis was performed to determine whether the causal signal was driven by one SNP. To infer causal direction, we used the MR Steiger directionality (Hemani et al., 2017) test to examine whether the exposure was directionally causal for the outcome. This approach compares the variance explained by IVs for both exposure and outcome. If the IVs explain a greater variance in the exposure than the outcome, then the identified causal association could be considered directionally credible. Furthermore, we calculated F statistics (Burgess and Thompson, 2011) to evaluate the weak instrument bias using the following formula:
where n, k, and R2 are sample size, number of IVs, and the variance explained by IVs, respectively. An F-value less than 10 indicates weak instrument.
Bidirectional Mendelian Randomization Analysis
We performed an additional reverse MR analysis to explore reverse causality. Significant reverse MR analysis indicates reverse causality from psychiatric disorders/traits (as exposure) to microbiota features (as outcome). The reverse MR analysis procedure was the same as the above MR analysis.
All of the analyses, including MR analyses and sensitivity analyses, were performed with the R packages TwoSampleMR2 (Hemani et al., 2018) and MRPRESSO3 (Verbanck et al., 2018).
Results
After removing palindromic SNPs, we identified 937, 1,576, 1,583, 2,390, 6,525, and 739 SNPs associated with gut microbiota in the phylum, class, order, family, genus, and species levels at the suggestive significance level P < 1.0 × 10–5, respectively (Supplementary Table 2). After clumping and harmonization, the number of IVs associated with each psychiatric disorder varies from 3 to 28. For instance, a total of 2,411 IVs are associated with SCZ, and these IVs are categorized into nine bacteria phyla (123 SNPs), 15 classes (208 SNPs), 19 orders (251 SNPs), 30 families (397 SNPs), 102 genera (1,285 SNPs), and 15 species (177 SNPs), respectively. For SCZ, the genus with the largest number of SNPs is Bifidobacterium (26 SNPs), followed by Roseburia (24 SNPs) and genus with the least number is Senegalimassilia (four SNPs). There is no feature containing only one SNP at any level.
The horizontal pleiotropy effect was evaluated at each taxonomic level. For SCZ, only one of 16 IVs for the order Coriobacteriales was detected as outlier using the MR-PRESSO outlier test. Similarly, at the family level, two out of 15 IVs in family Desulfovibrionaceae and one out of 16 IVs in family Streptococcaceae were identified as outliers. After removing pleiotropic SNPs identified by the MR-PRESSO outlier test and the MR-Egger regression, there is no evidence of horizontal pleiotropy of the remaining IVs (both MR-PRESSO Global test P > 0.05 and MR-Egger regression P > 0.05) (Supplementary Table 3).
Mendelian Randomization Analysis
Causal association between each pair of bacterial taxon and psychiatric disorder is tested by five MR methods. To take into account multiple-testing correction, the significance threshold for various taxa levels was set to the following: phylum P = 5.56 × 10–3 (0.05/9), class P = 3.33 × 10–3 (0.05/15), order P = 2.63 × 10–3 (0.05/19), family P = 1.67 × 10–3 (0.05/30), genus P = 4.90 × 10–4 (0.05/102), and species P = 3.33 × 10–3 (0.05/15).
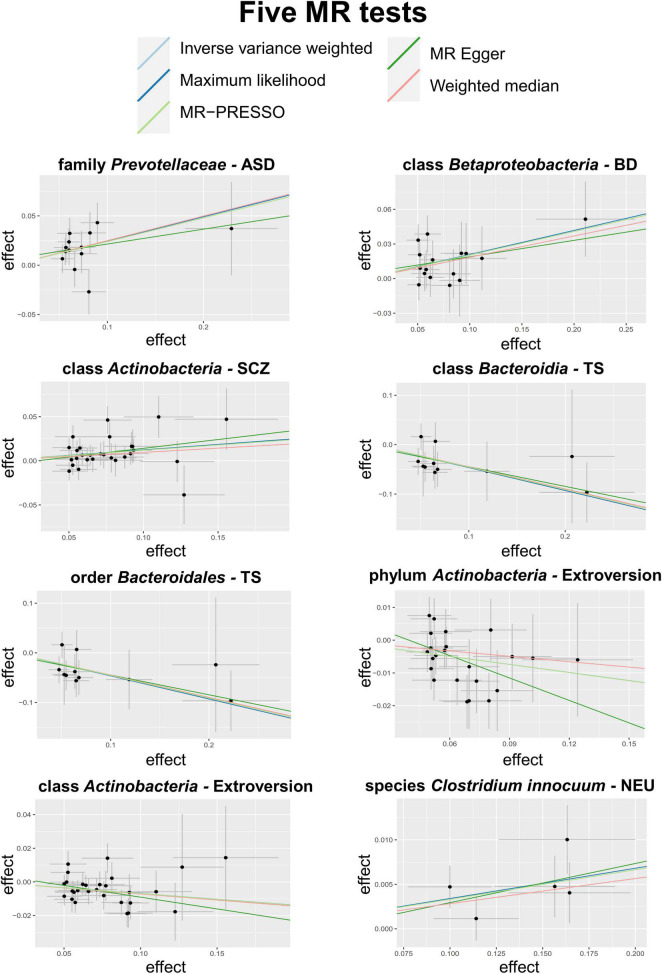
A total of eight causal associations from seven bacterial features to six psychiatric disorders/traits were identified by the IVW method (Table 2), including family Prevotellaceae with ASD (PIVW = 5.31 × 10–4), class Betaproteobacteria with bipolar disorder (BD) (PIVW = 1.53 × 10–3), class Actinobacteria with SCZ (PIVW = 1.33 × 10–3), class Bacteroidia (PIVW = 2.51 × 10–3), and order Bacteroidales (PIVW = 2.51 × 10–3) with Tourette syndrome (TS), phylum Actinobacteria (PIVW = 8.22 × 10–4) and class Actinobacteria (PIVW = 1.09 × 10–3) with extroversion, and species Clostridium innocuum with neuroticism (NEU) (PIVW = 8.92 × 10–4). Scatter plots across various tests are displayed in Figure 2 and Supplementary Figure 1. In total, 234 SNPs are included as IVs of gut microbiota to calculate the causal relationship with psychiatric disorders/traits (Supplementary Table 4). Seven of the eight causal associations are cross-validated by more than two MR tests, demonstrating the robustness of our results. All MR methods produced consistent direction of effect estimates, which strengthens the confidence toward true association (Table 2). Specifically, four bacterial features showed positive causal direction with ASD, BD, SCZ, and NEU, with regression coefficients ranging from 0.03 to 0.24. A total of four bacterial features showed a negative causal direction with TS and extroversion, whose regression coefficients are between −0.46 and −0.07. Of note, genus is a sub-category of family; therefore, the sets of SNPs contained in genus and its relevant family may heavily overlap. For instance, in SCZ, the SNPs of genus Bacteroides are within the family Bacteroidaceae. Besides, 10 more bacterial features were identified by only one of the five MR tests, as listed in Supplementary Table 5.
TABLE 2.
Causal estimations of gut microbiota on psychiatric disorders in the MR analysis.
| Bacterial taxa (exposure) | Psychiatric disorder/traits (outcome) | No. SNP | R2 | F | IVW |
MLE |
MR-Egger |
WME |
MR-PRESSO |
|||||
| bxy | P | bxy | P | bxy | P | bxy | P | bxy | P | |||||
| Family Prevotellaceae | ASD | 13 | 2.61% | 37.99 | 0.24 | 5.31 × 10–4 | 0.25 | 6.31 × 10–4 | 0.15 | 0.55 | 0.25 | 7.81 × 10–3 | 0.24 | 1.84 × 10–3 |
| Class Betaproteobacteria | BD | 16 | 3.39% | 40.45 | 0.20 | 1.53 × 10–3 | 0.21 | 1.56 × 10–3 | 0.14 | 0.44 | 0.18 | 0.043 | 0.20 | 1.50 × 10–3 |
| Class Actinobacteria | SCZ | 28 | 5.54% | 38.60 | 0.12 | 1.33 × 10–3 | 0.12 | 1.12 × 10–3 | 0.20 | 0.18 | 0.10 | 0.06 | 0.12 | 2.02 × 10–3 |
| Class Bacteroidia | TS | 11 | 2.66% | 45.82 | −0.46 | 2.51 × 10–3 | −0.46 | 3.11 × 10–3 | −0.40 | 0.25 | −0.45 | 0.03 | –0.46 | 1.75 × 10–3 |
| Order Bacteroidales | 11 | 2.66% | 45.82 | −0.46 | 2.51 × 10–3 | −0.46 | 3.11 × 10–3 | −0.40 | 0.25 | −0.45 | 0.03 | –0.46 | 1.75 × 10–3 | |
| Phylum Actinobacteria | Extroversion | 23 | 3.36% | 27.86 | −0.08 | 8.22 × 10–4 | −0.08 | 1.22 × 10–3 | −0.23 | 0.063 | −0.05 | 0.12 | –0.08 | 2.78 × 10–3 |
| Class Actinobacteria | 28 | 5.41% | 37.68 | −0.07 | 1.09 × 10–3 | −0.07 | 1.46 × 10–3 | −0.14 | 0.13 | −0.07 | 0.02 | –0.07 | 1.52 × 10–3 | |
| Species Clostridium innocuum | NEU | 5 | 1.95% | 73.33 | 0.03 | 8.92 × 10–4 | 0.03 | 1.36 × 10–3 | 0.04 | 0.43 | 0.03 | 0.03 | 0.03 | 0.02 |
No. SNP is the number of SNPs being used as IVs.
R2 is the proportion of phenotypic variation explained by used SNPs.
F is the value of F statistics to examine the weak instrument bias.
bxy is the estimated effect coefficient.
s.e. is standard error of estimate coefficient.
Significant P-values were marked in bold after multiple-testing correction [phylum P = 5.56 × 10–3 (0.05/9), class P = 3.33 × 10–3 (0.05/15), order P = 2.63 × 10–3 (0.05/19), family P = 1.67 × 10–3 (0.05/30), genus P = 4.90 × 10–4 (0.05/102) and species P = 3.33 × 10–3 (0.05/15)].
IVW, the inverse-variance weighted test; MLE, the maximum likelihood estimator; WME, the weighted median estimator; ADHD, attention deficit/hyperactivity disorder; ASD, autism spectrum disorder; BD, bipolar disorder; SCZ, schizophrenia; OCD, obsessive-compulsive disorder; TS, Tourette syndrome; NEU, neuroticism; SA, suicide attempt.
FIGURE 2.
Scatter plots of the 5 MR tests in 8 causal associations from 7 bacterial features to 6 psychiatric disorders/traits. SNP effects were plotted into lines for the inverse-variance weighted test (light blue line), MR-Egger regression (green line), weighted median estimator (red line), MR-PRESSO (light green line) and maximum likelihood estimator (blue line). The slope of the line corresponded to the causal estimation.
Instrumental variables for each identified features can explain 1.95–5.54% of the variance in each feature and 0.003–0.53% of the variance in the corresponding psychiatric disorders/traits, respectively. The F statistics for all IVs are larger than 10, indicating no evidence of weak instrument bias. Furthermore, the MR Steiger directionality test revealed that the variances explained by included SNPs of bacterial exposure are larger than psychiatric outcome, implying the true causal associations directionally (Supplementary Table 3). Q statistics of the IVW test and the MR-Egger regression showed no evidence of heterogeneity at the identified results (Supplementary Table 3). Forest plots of causal effects using single SNP showed that none of them is extremely significant for association with psychiatric disorders/traits (Supplementary Figures 2A, 3A), and the leave-one-out sensitivity analysis demonstrated no single SNP driving the causal association signal (Supplementary Figures 2B, 3B).
The results of the reverse MR analysis, as listed in the Supplementary Table 6, showed no evidence of causal effect from psychiatric disorders/traits to identified bacterial features after multiple-testing correction (P < 0.05/18 = 2.78 × 10–3).
Some factors, such as chronic bowel diseases, may affect the association between gut microbiota and psychiatric disorders. To check the potential influence of confounding factors, we identified several sub-types of chronic bowel disease, including irritable bowel syndrome, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, bowel problem, other non-infective gastroenteritis and colitis, and other functional intestinal disorders categories. We retrieved the associations of the identified IVs with each type in the United Kingdom Biobank summary statistics through the GeneATLAS website4. After multiple-testing correction, the results showed that none of the associations is significant, indicating limited confounding effect of chronic bowel diseases, as listed in the Supplementary Table 7. We further excluded IVs associated with any one of the chronic bowel diseases at a nominal level (P < 0.05) and re-perform the MR analysis using the remaining IVs. The results remain significant at the eight identified causal associations. Meanwhile, neither the MR-PRESSO test nor the MR-Egger regression test showed evidence of horizontal pleiotropy (both P > 0.05). Together, these results implied that the identified causal associations were unlikely to be mediated by chronic bowel disease.
Discussion
In the current study, we conducted MR analyses to evaluate the potential causality between the gut microbiota and 15 psychiatric disorders/traits. Using large-scale summary statistics from microbiome GWAS and 15 psychiatric disorders/traits GWAS, we identified seven bacterial features that were causally associated with six psychiatric disorders/traits.
The positive association between family Prevotellaceae and ASD is in line with previous findings (Qiao et al., 2018; Dan et al., 2020). Prevotellaceae is characterized as a propionate-producing bacterium. Previous studies have found that propionate, as an enteric metabolite produced by gut microbiota, induced social abnormalities, cognitive impairments, sensorimotor dysfunction, and exacerbated ASD symptoms after intracerebroventricular injection (Shultz et al., 2015). Other studies have also manifested the consistent inference that changes in brain tissue after propionate administration result in conditions similar to ASD patients, such as reactive astrogliosis and oxidative stress (Thomas et al., 2012).
In accordance with the previous studies, class Actinobacteria, as a gram-positive bacterium, showed a positive causal association with SCZ, and class Betaproteobacteria has a positive direction on BD. For instance, patients with SCZ and other psychotic disorders have a higher abundance of Actinobacteria (Zheng et al., 2016; Li et al., 2020; Vindegaard et al., 2020), which is also supported by animal models (Dunphy-Doherty et al., 2018). Members of Betaproteobacteria were found to be more abundant in a mouse model of psychiatric diseases, whereas they were strongly correlated with increased gut permeability and intestinal chronic inflammation in humans, which may affect mental health or brain development via the MGB axis (Bauerl et al., 2018; Chen et al., 2018).
Consistent with previous literature, our result showed that class Bacteroidia and its child taxon, order Bacteroidales, have a negative effect on TS. Both Bacteroidia and Bacteroidales are correlated with the tryptophan hydroxylase-II (TPH2) serotonin pathway (Liu et al., 2020). In animal experiments, deficits and excess of TPH2 activity may induce significant behavioral disturbances and catalepsy, whereas the human TPH2 gene is related to psychiatric disorders (Kulikova and Kulikov, 2019). Moreover, serotonin, one of the main brain neurotransmitters, plays an important role in promoting immunity and reducing inflammation in mucosal infections (Gao J. et al., 2018). Thus, decreased serotonin concentrations in the brain and cerebrospinal fluid in Tourette patients might be implicated in the pathogenesis of TS (Mossner et al., 2007). In a FMT study, the abundance of Bacteroides coprocola was reduced in TS patients, but its restoration could improve tic symptoms (Zhao et al., 2020). Personality traits, such as extroversion and NEU, affect fundamental behavior patterns and have been related to mental disorders. In the present study, phylum Actinobacteria and its child taxon, order Actinobacteria, both have a negative effect direction on extroversion, whereas species Clostridium innocuum has a positive effect on NEU.
In addition to the above causal associations identified by IVW test, several intriguing results were identified by other MR tests, including families Christensenellaceae and Methanobacteriaceae, both of which have negative effects on OCD and SA. These associations are broadly supported by previous studies. For instance, it is accepted that SCFA butyrate might suppress the inflammation and oxidative damages in colon and brain, alleviating cognitive impairments, behavioral disorders, and gastrointestinal disorders (Peruzzotti-Jametti and Pluchino, 2018). Family Christensenellaceae, as a gram-negative, strictly anaerobic, and SCFA-producing taxon (Waters and Ley, 2019), could increase the concentration of butyrate in colon, potentially alleviating colitis-related OCD behaviors by diet in humans (Nagpal et al., 2019). It was also found to be positively correlated with cognitive ability in mice (Gao L. et al., 2018). Whereas Methanobacteriaceae, a dominant methanogenic archaeon, can increase levels of SCFAs in the colon (Samuel and Gordon, 2006) and likely has beneficial psychological effects via SCFAs such as migraine reduction in elderly women, which could partly explain the protective effect of this taxon against suicide (Chen et al., 2019).
There are many population-based observational studies and their meta-analyses for the association of gut microbiota with psychiatric disorders (Strati et al., 2017; Ma et al., 2019; Valles-Colomer et al., 2019; Hua et al., 2020; Iglesias-Vazquez et al., 2020; Nikolova et al., 2021). Among them, Nikolova et al. (2021) meta-analyzed 34 case-control studies in a total of 1,519 psychiatric patients versus 1,429 normal controls and found no difference in the diversity of gut microbiota. The meta-analysis attempts to resolve a controversial scientific question such as if an association between two conditions exists, whereas the causal nature of such association is unknown. Fundamentally different from meta-analysis, MR analysis, on the other hand, is an approach statistically inferring the causal nature of an association observed in a cross-sectional study.
This study has advantages in several aspects. First, the identified causal relationship may provide candidate bacteria for subsequent functional studies. Second, we comprehensively studied up to 15 common psychiatric disorders. In a previous study, Zhuang et al. (2020) studied three psychiatric disorders and revealed that order Enterobacteriales and family Enterobacteriaceae were causally associated with a higher risk of schizophrenia, and increased class Bacilli was causally associated with a higher risk of major depressive disorder.
There are also certain limitations in this study. First, gut microbiota GWAS is still in its infancy in terms of sample size; therefore, the number of associated loci is relatively small compared with that for psychiatric disorders. Second, because of the small sample size and insufficient power for microbiome GWAS, there may not be enough IVs for certain bacterial features at genus or species level. As a compromise, we analyzed features at a higher level (phylum, class, order, or family). When microbiome GWAS will eventually be equipped with sufficient sample size, these more specific features will hopefully be identified at a finer resolution (Thomas, 2019). Third, to maximize sample size and statistical power, GWAS of gut microbiota and psychiatric disorders/traits analyzed in this study might originate from multi-ancestry samples. Thus, the results should be interpreted with caution.
In conclusion, we comprehensively assessed the potential causal association between gut microbiota and a series of psychiatric disorders/traits. Four bacterial features showed positive causal direction with ASD, BD, SCZ, and NEU, whereas another four bacterial features showed a negative causal direction with TS and extroversion. This study may be useful in providing new insights into the development mechanism of microbiota-mediated psychiatric disorders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by all studies were approved by respective institutional review boards (IRBs). No new IRB approval was required. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
LZ and Y-FP designed the study. LZ, QX, and J-JN collected the data. J-JN and QX analyzed the data. S-SY, B-XH, HZ, X-TW, G-JF, Y-FP, MZ, QX, and J-JN performed the literature search. J-JN drafted the early version of the manuscript. LZ and Y-FP jointly supervised the study. All authors were involved in writing the manuscript and had final approval of the submitted and published versions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate all the volunteers who participated in this study. We would like to thank the MiBioGen study for releasing the gut microbiota GWAS summary statistics and large-scale consortia or studies including HRC, PGC, United Kingdom Biobank, iPSYCH, ICC, ANGI, GCAN/WTCCC3, IOCDF-GC, OCGAS, and GPC for releasing psychiatric disorders/traits GWAS summary statistics.
Abbreviations
- ADHD
attention deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- SCZ
schizophrenia
- FMT
fecal microbiota transplantation
- MGB
microbiota–gut–brain
- RCT
randomized controlled trial
- MR
Mendelian randomization
- IV
instrumental variable
- SNP
nucleotide polymorphism
- SCFA
short-chain fatty acid
- GWAS
genome-wide association study
- IRB
institutional review board
- LD
linkage disequilibrium
- IVW
inverse-variance weighted
- MLE
maximum likelihood estimator
- WME
weighted median estimator
- BD
bipolar disorder
- TS
Tourette syndrome
- NEU
neuroticism
- OCD
obsessive-compulsive disorder
- SA
suicide attempt
- TPH2
tryptophan hydroxylase-II.
Footnotes
Funding
Y-FP and LZ were partially supported by the funding from the National Natural Science Foundation of China (31771417 and 31571291) and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu higher education institutions. The numerical calculations in this manuscript have been done on the supercomputing system of the National Supercomputing Center in Changsha.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.737197/full#supplementary-material
References
- Bauerl C., Collado M. C., Diaz Cuevas A., Vina J., Perez Martinez G. (2018). Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 66 464–471. 10.1111/lam.12882 [DOI] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G., Burgess S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44 512–525. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G., Haycock P. C., Burgess S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 304–314. 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Thompson S. G. (2011). Bias in causal estimates from mendelian randomization studies with weak instruments. Stat. Med. 30 1312–1323. 10.1002/sim.4197 [DOI] [PubMed] [Google Scholar]
- Burgess S., Butterworth A., Thompson S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 658–665. 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Scott R. A., Timpson N. J., Davey Smith G., Thompson S. G., Consortium E.-I. (2015). Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30 543–552. 10.1007/s10654-015-0011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Small D. S., Thompson S. G. (2017). A review of instrumental variable estimators for mendelian randomization. Stat. Methods Med. Res. 26 2333–2355. 10.1177/0962280215597579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Q., Wang A., Lin Z. (2019). Structural and functional characterization of the gut microbiota in elderly women with migraine. Front. Cell Infect. Microbiol. 9:470. 10.3389/fcimb.2019.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Wu H., Wu S. D., Lu N., Wang Y. T., Liu H. N., et al. (2018). Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 33 1844–1852. 10.1111/jgh.14281 [DOI] [PubMed] [Google Scholar]
- Chow J., Lee S. M., Shen Y., Khosravi A., Mazmanian S. K. (2010). Host-bacterial symbiosis in health and disease. Adv. Immunol. 107 243–274. 10.1016/B978-0-12-381300-8.00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente J. C., Ursell L. K., Parfrey L. W., Knight R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148 1258–1270. 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J. F., O’Riordan K. J., Cowan C. S. M., Sandhu K. V., Bastiaanssen T. F. S., Boehme M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99 1877–2013. [DOI] [PubMed] [Google Scholar]
- Dan Z., Mao X., Liu Q., Guo M., Zhuang Y., Liu Z., et al. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut. Microbes. 11 1246–1267. 10.1080/19490976.2020.1747329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D., Walters R. K., Martin J., Mattheisen M., Als T. D., Agerbo E., et al. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K., Bruffaerts R., Posada-Villa J., Gasquet I., Kovess V., Lepine J. P., et al. (2004). Prevalence, severity, and unmet need for treatment of mental disorders in the world health organization world mental health surveys. JAMA. 291 2581–2590. 10.1001/jama.291.21.2581 [DOI] [PubMed] [Google Scholar]
- Dunphy-Doherty F., O’Mahony S. M., Peterson V. L., O’Sullivan O., Crispie F., Cotter P. D., et al. (2018). Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 68 261–273. 10.1016/j.bbi.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Emdin C. A., Khera A. V., Kathiresan S. (2017). Mendelian randomization. JAMA. 318 1925–1926. [DOI] [PubMed] [Google Scholar]
- Erlangsen A., Appadurai V., Wang Y., Turecki G., Mors O., Werge T., et al. (2020). Genetics of suicide attempts in individuals with and without mental disorders: a population-based genome-wide association study. Mol. Psychiatry 25 2410–2421. 10.1038/s41380-018-0218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., et al. (2018). Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 8:13. 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Li J., Zhou Y., Huang X., Qin X., Du G. (2018). Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem. Neurosci. 9 1714–1724. 10.1021/acschemneuro.8b00074 [DOI] [PubMed] [Google Scholar]
- Grove J., Ripke S., Als T. D., Mattheisen M., Walters R. K., Won H., et al. (2019). Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51 431–444. 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G., Tilling K., Davey Smith G. (2017). Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13:e1007081. 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G., Zheng J., Elsworth B., Wade K. H., Haberland V., Baird D., et al. (2018). The MR-base platform supports systematic causal inference across the human phenome. Elife 7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. M., Adams M. J., Clarke T. K., Hafferty J. D., Gibson J., Shirali M., et al. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22 343–352. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Zhu J., Yang T., Guo M., Li Q., Chen J., et al. (2020). The gut microbiota and associated metabolites are altered in sleep disorder of children with autism spectrum disorders. Front. Psychiatry 11:855. 10.3389/fpsyt.2020.00855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Vazquez L., Van Ginkel Riba G., Arija V., Canals J. (2020). Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients 12:792. 10.3390/nu12030792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Obsessive Compulsive Disorder Foundation Genetics Collaborative (Iocdf-Gc) and Ocd Collaborative Genetics Association Studies (OCGAS) (2018). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23 1181–1188. 10.1038/mp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M. B. (1986). Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1 507–508. 10.1016/s0140-6736(86)92972-7 [DOI] [PubMed] [Google Scholar]
- Kulikova E. A., Kulikov A. V. (2019). Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: focus on animal models. Exp. Opin. Ther. Targets 23 655–667. 10.1080/14728222.2019.1634691 [DOI] [PubMed] [Google Scholar]
- Kurilshikov A., Medina-Gomez C., Bacigalupe R., Radjabzadeh D., Wang J., Demirkan A., et al. (2021). Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53 156–165. 10.1038/s41588-020-00763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhuo M., Huang X., Huang Y., Zhou J., Xiong D., et al. (2020). Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ 8:e9574. 10.7717/peerj.9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Chong H. X., Chung F. Y., Li Y., Liong M. T. (2020). Lactobacillus plantarum DR7 modulated bowel movement and gut microbiota associated with dopamine and serotonin pathways in stressed adults. Int. J. Mol. Sci. 21:4608. 10.3390/ijms21134608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Liang J., Dai M., Wang J., Luo J., Zhang Z., et al. (2019). Altered gut microbiota in chinese children with autism spectrum disorders. Front. Cell Infect. Microbiol. 9:40. 10.3389/fcimb.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S. M., Trontti K., Purves K. L., Als T. D., Grove J., Laine M., et al. (2019). Genetic variants associated with anxiety and stress-related disorders: a genome-wide association study and mouse-model study. JAMA Psychiatry 76 924–932. 10.1001/jamapsychiatry.2019.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R., Muller-Vahl K. R., Doring N., Stuhrmann M. (2007). Role of the novel tryptophan hydroxylase-2 gene in tourette syndrome. Mol. Psychiatry 12 617–619. 10.1038/sj.mp.4002004 [DOI] [PubMed] [Google Scholar]
- Nagel M., Jansen P. R., Stringer S., Watanabe K., de Leeuw C. A., Bryois J., et al. (2018). Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 50 920–927. 10.1038/s41588-018-0151-7 [DOI] [PubMed] [Google Scholar]
- Nagpal R., Neth B. J., Wang S., Craft S., Yadav H. (2019). Modified mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47 529–542. 10.1016/j.ebiom.2019.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt C. M., Maihofer A. X., Klengel T., Atkinson E. G., Chen C. Y., Choi K. W., et al. (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10:4558. 10.1038/s41467-019-12576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova V. L., Smith M. R. B., Hall L. J., Cleare A. J., Stone J. M., Young A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78 1343–1354. 10.1001/jamapsychiatry.2021.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J., Ramelow C. C., Kasper L. H. A. (2020). gut feeling: the importance of the intestinal microbiota in psychiatric disorders. Front. Immunol. 11:510113. 10.3389/fimmu.2020.510113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara A. M., Shanahan F. (2006). The gut flora as a forgotten organ. EMBO Rep. 7 688–693. 10.1038/sj.embor.7400731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman J. A., Verweij K. J. H., Gerring Z., Stringer S., Sanchez-Roige S., Treur J. L., et al. (2018). GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat. Neurosci. 21 1161–1170. 10.1038/s41593-018-0206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Chisholm D., Parikh R., Charlson F. J., Degenhardt L., Dua T., et al. (2016). Addressing the burden of mental, neurological, and substance use disorders: key messages from disease control priorities, 3rd edition. Lancet 387 1672–1685. 10.1016/S0140-6736(15)00390-6 [DOI] [PubMed] [Google Scholar]
- Peruzzotti-Jametti L., Pluchino S. (2018). Targeting mitochondrial metabolism in neuroinflammation: towards a therapy for progressive multiple sclerosis. Trends Mol. Med. 24 838–855. 10.1016/j.molmed.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Pierce B. L., Burgess S. (2013). Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178 1177–1184. 10.1093/aje/kwt084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Wu M., Feng Y., Zhou Z., Chen L., Chen F. (2018). Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 8:1597. 10.1038/s41598-018-19982-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B. S., Gordon J. I. (2006). A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. U.S.A. 103 10011–10016. 10.1073/pnas.0602187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S., Palmer A. A., Fontanillas P., Elson S. L., The 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium. Adams M. J., et al. (2019). Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. Am. J. Psychiatry 176 107–118. 10.1176/appi.ajp.2018.18040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna S., van Zuydam N. R., Mahajan A., Kurilshikov A., Vich Vila A., Vosa U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51 600–605. 10.1038/s41588-019-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G., Cruz N. J., Kang D. W., Gandal M. J., Wang B., Kim Y. M., et al. (2019). Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177 1600–1618.e17. 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S. R., Aziz N. A., Yang L., Sun M., MacFabe D. F., O’Brien T. J. (2015). Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behav. Brain Res. 278 542–548. 10.1016/j.bbr.2014.10.050 [DOI] [PubMed] [Google Scholar]
- Smith G. D., Ebrahim S. (2003). ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32 1–22. 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- Stahl E. A., Breen G., Forstner A. J., McQuillin A., Ripke S., Trubetskoy V., et al. (2019). Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51 793–803. 10.1038/s41588-019-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F., Cavalieri D., Albanese D., De Felice C., Donati C., Hayek J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. (2019). Mendelian randomization reveals causal effects of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16 198–199. 10.1038/s41575-019-0133-y [DOI] [PubMed] [Google Scholar]
- Thomas R. H., Meeking M. M., Mepham J. R., Tichenoff L., Possmayer F., Liu S., et al. (2012). The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J. Neuroinflam. 9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E. F., Wang J., Tito R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4 623–632. 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- van den Berg S. M., de Moor M. H., Verweij K. J., Krueger R. F., Luciano M., Arias Vasquez A., et al. (2016). Meta-analysis of genome-wide association studies for extraversion: findings from the genetics of personality consortium. Behav. Genet. 46 170–182. 10.1007/s10519-015-9735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck M., Chen C. Y., Neale B., Do R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 50 693–698. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard N., Speyer H., Nordentoft M., Rasmussen S., Benros M. E. (2020). Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res. 234 1–10. [DOI] [PubMed] [Google Scholar]
- Wang L., Christophersen C. T., Sorich M. J., Gerber J. P., Angley M. T., Conlon M. A. (2011). Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 77 6718–6721. 10.1128/AEM.05212-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. L., Ley R. E. (2019). The human gut bacteria christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 17:83. 10.1186/s12915-019-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. J., Yilmaz Z., Thornton L. M., Hubel C., Coleman J. R. I., Gaspar H. A., et al. (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford H. A., Degenhardt L., Rehm J., Baxter A. J., Ferrari A. J., Erskine H. E., et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382 1575–1586. 10.1016/s0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Yu D., Sul J. H., Tsetsos F., Nawaz M. S., Huang A. Y., Zelaya I., et al. (2019). Interrogating the genetic determinants of tourette’s syndrome and other tic disorders through genome-wide association studies. Am. J. Psychiatry 176 217–227. 10.1176/appi.ajp.2018.18070857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. J., Luo X., Shi Y. C., Li J. F., Pan F., Ren R. R., et al. (2020). The efficacy of fecal microbiota transplantation for children with tourette syndrome: a preliminary study. Front. Psychiatry 11:554441. 10.3389/fpsyt.2020.554441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21 786–796. 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zhuang Z., Yang R., Wang W., Qi L., Huang T. (2020). Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflam. 17:288. 10.1186/s12974-020-01961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.