Abstract
Background:
We sought to compare self-reported alcohol consumption using Timeline Followback (TLFB) to biomarker-based evidence of significant alcohol use (phosphatidylethanol (PEth) >20 ng/mL). Using data from patients with HIV (PWH) entering a clinical trial, we asked whether TLFB could predict PEth >20 ng/mL and assessed the magnitude of association between TLFB and PEth level.
Methods:
We defined unhealthy alcohol use as any alcohol use in the presence of liver disease, at-risk drinking, or alcohol use disorder. Self-reported alcohol use obtained from TLFB interview was assessed as mean number of drinks/day and number of heavy drinking days over the past 21 days. Dried blood spot samples for PEth were collected at the interview. We used logistic regression to predict PEth >20ng/mL and Spearman correlation to quantify the association with PEth, both as a function of TLFB.
Results:
Among 282 individuals (99% men) in the analytic sample, approximately two-thirds (69%) of individuals had PEth >20 ng/mL. The proportion with PEth >20ng/mL increased with increasing levels of self-reported alcohol use; of the 190 patients with either at-risk drinking or alcohol use disorder based on self-report, 82% had PEth >20 ng/mL. Discrimination was better with number of drinks/day than heavy drinking days (AUC: 0.80 [95%CI: 0.74–0.85] vs. 0.74 [95%CI: 0.68–0.80]). The number of drinks/day and PEth were significantly and positively correlated across all levels of alcohol use (Spearman’s R ranged from 0.29 to 0.56, all p values <0.01).
Conclusions:
In this sample of PWH entering a clinical trial, mean numbers of drinks/day discriminated individuals with evidence of significant alcohol use by PEth. PEth complements self-report to improve identification of self-reported unhealthy alcohol use among PWH.
Keywords: Alcohol use, Biomarker, Timeline Followback, TLFB, Phosphatidylethanol, PEth, HIV
INTRODUCTION
Unhealthy alcohol use extends across a spectrum that ranges from low-risk drinking in the presence of a health problem caused or exacerbated by alcohol (e.g. liver disease) to alcohol use disorder; its negative consequences on individual and public health is well established (World Health Organization (WHO), 2014, Saitz, 2005). Despite the growing body of evidence suggesting that individuals with HIV may be at greater risk of harm from unhealthy alcohol consumption compared to their uninfected counterparts (Justice et al., 2016, Williams et al., 2016, Conigliaro et al., 2003), unhealthy alcohol use remains common among this population. Among patients with HIV (PWH), unhealthy alcohol use is associated with worse outcomes at each stage of the HIV care continuum and independently increases risk of death (Williams et al., 2019, Justice et al., 2016, Rentsch et al., 2016, Braithwaite and Bryant, 2010, Samet et al., 2007, Saitz, 2005, Conigliaro et al., 2003), as well as risky sexual behaviors and ongoing HIV transmission (Cook et al., 2006).
Given the health consequences associated with unhealthy alcohol use, it is important that alcohol exposure is accurately assessed. This is particularly critical in clinical settings – where care is provided and individuals may be recruited for clinical trial studies, to ensure that those at risk of harm are identified and offered appropriate interventions, including counseling and medications. Accurate measurement of unhealthy alcohol use is challenging (Justice et al., 2016) and alcohol consumption may be inconsistently reported due to social desirability bias, recall bias, and/or misinformation related to social norms. Under-reporting may be common especially among PWH or those with hepatitis C virus (HCV) infection for whom alcohol use may be discouraged by clinicians for reasons including concerns about viral disease progression, liver injury, and risk behaviors (Justice et al., 2016, Pellowski et al., 2016, Kalichman et al., 2012). Accordingly, self-report of alcohol consumption used to assess intervention eligibility and efficacy may be biased. While self-reported calendar based methods, such as those employed with the Alcohol Timeline Followback (TLFB) (Sobell et al., 1996), are the gold standard for assessing alcohol use among patients in clinical trials (Witkiewitz et al., 2015), biomarkers of alcohol, such as phosphatidylethanol (PEth), may serve to improve confidence in the accuracy of patient-reported alcohol consumption.
PEth is a direct metabolite of alcohol use and has high sensitivity, with near perfect specificity for assessing any alcohol use in the recent past (Hahn et al., 2012a, Aradottir et al., 2006). PEth can detect alcohol exposure up to 21 days after consumption (Hahn et al., 2016, Wurst et al., 2015, Winkler et al., 2013). PEth >20 ng/mL reflects significant alcohol use ranging from an average of 2 to 4 drinks per day for several days a week to 4 or more drinks per day several days a week (Ulwelling and Smith, 2018, Piano et al., 2015, Stewart et al., 2009). PEth levels can vary between persons consuming the same amount of alcohol likely because of individual differences in alcohol metabolism (Javors et al., 2016). PEth does not differentiate alcohol consumption patterns (e.g. heavy episodic drinking vs. constant daily ingestion). However, its performance does not appear related to demographic factors such as age and sex, or other ingested substances (Viel et al., 2012). It has been demonstrated to be superior to other alcohol biomarkers (Walther et al., 2015, Wurst et al., 2015, Viel et al., 2012, Aradottir et al., 2006). Although PEth is an objective measure and may improve assessment of unhealthy alcohol use, data on the correlation between PEth and self-reported alcohol use among PWH have been mixed. While some studies have shown evidence of strong correlation (Bajunirwe et al., 2014, Hahn et al., 2012a, Aradottir et al., 2006), others have not (Wang et al., 2018, Littlefield et al., 2017, Papas et al., 2016). Explanations which have been proposed for these mixed results point to a number of PEth related characteristics that may vary across studies including individual differences in PEth formation and elimination (Javors et al., 2016, Stewart et al., 2010); differences in PEth measurement and cutoffs in studies (Papas et al., 2016, Hahn et al., 2012a, Isaksson et al., 2011); imperfect overlap of window of alcohol detectability by self-report and PEth (Bajunirwe et al., 2014); as well as the heterogeneity of the type and level of alcohol consumed (Aradottir et al., 2006). In addition to these, we suspect the mixed results are likely also a reflection of the validity of self-reported alcohol measures (Littlefield et al., 2017), which could be under-reported or over-reported depending on the context of the study.
Therefore, in the context of the Starting Treatment for Ethanol in Primary care Trials (STEP Trials), where PWH were eligible for study enrollment across the spectrum of unhealthy alcohol use, we sought to test the degree of consistency between self-reported alcohol consumption and PEth >20 ng/mL. Specifically, we were interested in the magnitude of association between self-reported alcohol consumption by TLFB and PEth >20 ng/mL and whether TLFB could be used to predict PEth >20 ng/ml. Given that PEth may not be readily accessible across all settings due to cost or other feasibility issues, the latter objective of this study seeks to address the uncertainty around the use of self-report measures in clinical research settings and thus increase our understanding of how well self-report measures predict clinically relevant alcohol use.
METHODS
Study Design and Setting
This study uses baseline data collected as part of the STEP trials, described in detail elsewhere (Edelman et al., 2019a, Edelman et al., 2019b, Edelman et al., 2017). Briefly, the STEP Trials consists of three multi-site, parallel, randomized controlled trials designed to evaluate the effectiveness of integrated stepped alcohol treatment compared to treatment as usual to address unhealthy alcohol use (see criteria below) among individuals with HIV. Ethics approval was received from the institutional review boards of the coordinating center at Yale University, New Haven, CT, the Veterans Affairs (VA) Connecticut Healthcare System, West Haven, CT, and from the participating clinical sites. The study is registered at www.clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT01410123).
Study Participants
Participants were recruited at VA Infectious Disease clinics in Washington, DC; Atlanta, GA; Brooklyn/Manhattan, NY; Dallas and Houston, TX. Potentially eligible patients were identified based on the Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) with score >0 and then assessed for eligibility. Eligible participants had to be: 1) HIV-positive adults; 2) a patient at one of the five clinics; 3) English speaking and able to provide written informed consent; and 4) with unhealthy alcohol use based on established criteria and recommendations (National Institute on Alcohol Abuse and Alcoholism, 2005, American Psychiatric Association, 2013). Each of the three trials focused on a specific type of unhealthy alcohol use which were defined in increasing order of alcohol use severity as: 1) moderate alcohol use with liver disease [MALD] trial, defined as report of any alcohol consumption in the past 30 days and are either HCV coinfected, confirmed by HCV antibody and a detectable HCV RNA viral load, or have evidence of liver fibrosis, defined as a FIB-4 score >1.45 (Lim et al., 2014, Mendeni et al., 2011, Sterling et al., 2006), and who do not meet criteria for at-risk drinking or alcohol use disorder; 2) at-risk drinking trial, defined as alcohol consumption that is 14 or more drinks per week or 4 or more drinks per occasion in men younger than or equal to 65 years old or 7 or more drinks per week or 3 or more drinks per occasion in women or men older than 65 years old (National Institute on Alcohol Abuse and Alcoholism, 2005); and 3) alcohol use disorder trial, defined as meeting Diagnostic and Statistical Manual (DSM)-IV TR criteria for alcohol abuse or dependence (as DSM-5 was released after initiation of the trial) (American Psychiatric Association, 2013). TLFB was used to assess alcohol use pattern and frequency; participants were assessed for presence of alcohol use disorder based on their AUDIT-C score (>4 for men; >3 for women and men over 65 years old).
Participants were excluded if: 1) suicidal; 2) unable to provide informed consent or participate in counseling; 3) currently enrolled in treatment for unhealthy alcohol use excluding self or mutual-help groups; 4) had any medical condition(s) that would preclude completing the study or cause harm during the course of the study; or 5) a woman who is pregnant, nursing or does not agree to use birth control.
Self-reported alcohol use
Self-reported alcohol use was measured at baseline using the TLFB during face-to-face, in-person interview by a research assistant trained in TLFB methods. TLFB is a validated, calendar-based, self-report method of assessing daily alcohol consumption in the past month and has good psychometric properties (Sobell and Sobell, 1996). Research assistants were trained to use probes, such as birthdays, holidays and weekends with use of a printed calendar, to improve recall. Our primary analysis considered two key measures of alcohol use at baseline, namely: (i) mean number of drinks per day in the past 21 days; and (ii) heavy drinking days, defined as ≥ 5 drinks in a given day, over the past 21 days. We used the last 21 days TLFB measure for this analysis to align with the 21-days PEth detection window. Additional measures included AUDIT-C score, collected at baseline screening for eligibility.
PEth
To quantify PEth, dried blood spots obtained by fingerstick on the same day as TLFB were collected by the same research assistant or as needed by phlebotomist, and sent to United States Drug Testing Laboratories (USDTL), in Des Plaines, IL, and assayed using a previously described method (Jones et al., 2011). The order in which PEth and TLFB were collected was determined on a participant-participant basis to optimize data collection and minimize logistical hurdles. As no single PEth cutoff uniformly correlates with the spectrum of alcohol use (Gnann et al., 2012, Stewart et al., 2010, Aradottir et al., 2006), several different PEth thresholds have been used in previous studies (Eyawo et al., 2018, Asiimwe et al., 2015, Bajunirwe et al., 2014, Hahn et al., 2012a). Whereas PEth ≥ 8 ng/mL – excess of the lower limit of quantitation – is frequently used as an indicator of any alcohol use in the past 21 days, a PEth value < 8 ng/mL is consistent with alcohol abstinence (Asiimwe et al., 2015, Bajunirwe et al., 2014, Stewart et al., 2014). Given our interest in alcohol use that impacts health rather than detecting any alcohol use, and coupled with our desire to minimize risk of false positive results (Hahn et al., 2016), we selected a PEth cutoff of 20 ng/ml, as values above reflect clinically significant alcohol use (Ulwelling and Smith, 2018). PEth results were not used to determine study eligibility.
Sociodemographic and other clinical characteristics
Additional information on sociodemographic and biomarkers specific to HIV were also collected at baseline and included age, sex, race, HIV viral load, CD4 cell count, and the VACS Index score. The VACS Index is a validated, composite measure of morbidity and mortality that incorporates weighted values of routinely collected laboratory test results and measures of organ system injury, including age, CD4 cell count, HIV viral load, hemoglobin, platelets, aspartate transaminase, alanine transaminase, creatinine, and hepatitis C serostatus (Justice et al., 2013, Tate et al., 2013). The VACS Index is a reliable predictor of hospitalization and mortality with higher scores indicating higher risks (Eyawo et al., 2018, Akgun et al., 2013, Justice et al., 2013, Tate et al., 2013). We categorized HIV viral load as detectable (≥ 50 copies/mL) vs. undetectable (<50 copies/mL).
Statistical Analysis
Baseline characteristics of participants were evaluated using descriptive statistics and compared according to PEth (≤ 20 ng/mL vs. >20 ng/mL) using Chi-square or Fisher’s exact test for categorical variables and t test or Wilcoxon rank sum test for continuous variables, as appropriate. To summarize mean number of drinks per day in the past 21 days, we constructed dot plots by PEth status. As no sociodemographic or clinical variables satisfied the criteria of a confounder, we did not include any of these variables in subsequent models. We examined the association between PEth >20 ng/mL and TLFB self-report as the independent variable using logistic regression. Discrimination was assessed using receiver operating characteristics (ROC) curves. Area under the curve (AUC) and corresponding 95% confidence intervals were calculated to evaluate discrimination. We used spearman correlation to explore the relationship between mean number of drinks per day and PEth as a continuous value. We performed these analyses for the whole sample, as well as individually for the three defined subgroups of unhealthy alcohol use (MALD, at-risk drinking, and alcohol use disorder). In sensitivity analyses we used a TLFB time window covering the past 14 and 7 days (Fiellin et al., 2013). We also repeated all analyses using a less conservative PEth cutoff of 8 ng/mL. All p-values were 2-tailed and were considered statistically significant if less than 0.05. All analyses were performed using SAS version 9.4 (SAS, Cary, North Carolina, USA).
RESULTS
Sample Characteristics
Of 315 participants randomized into one of the three STEP trials, 33 were missing baseline PEth data as they were not collected or unable to be processed. This resulted in a total sample of 282 (MALD: 92, at-risk drinking: 85, and alcohol use disorder:105). Characteristics of the study participants, overall and stratified by baseline PEth (≤20 ng/mL vs. >20 ng/mL) are presented in Table 1 and Figure 1. Most study participants were male (99%), African-American (81%) with median age of 59 years (interquartile range [IQR]: 53, 63). Compared to those with PEth ≤20 ng/mL, participants with baseline PEth >20 ng/mL were more likely to have a lower VACS Index score (median [IQR]: 28 [18, 40] vs. 30 [21, 48], p = 0.021); higher AUDIT-C score (mean [SD]: 6.3 [2.7] vs. 3.9 [2.4], p < 0.001); less likely to be in MALD group (20% vs. 61%, p < 0.001); and were more likely to fall into the more severe spectrum of unhealthy alcohol consumption, namely; in the at-risk drinking (35% vs. 19%, p < 0.001) and alcohol use disorder groups (45% vs. 19%, p < 0.001) (Table 1).
Table 1.
Baseline characteristics of the study participants, overall and by PEth
| Characteristic, n (%) | Overall sample | PEth (ng/mL) | P-value | |
|---|---|---|---|---|
| ≤ 20 | > 20 | |||
| Sample size | 282 | 88 | 194 | |
| Age, median (IQR) | 59 (53, 63) | 59 (54, 65) | 58 (53, 63) | 0.26 |
| Sex | 1.00 | |||
| Female | 4 (1.4) | 1 (1.1) | 3 (1.6) | |
| Male | 278 (98.6) | 87 (98.9) | 191 (98.5) | |
| Race | 0.24 | |||
| White | 48 (17.0) | 17 (19.3) | 31 (16.0) | |
| African-American | 227 (80.5) | 67 (76.1) | 160 (82.5) | |
| Other | 7 (2.5) | 4 (4.6) | 3 (1.6) | |
| Undetectable viral load (≤ 50 copies/mL) | 186 (66.2) | 54 (62.1) | 132 (68.0) | 0.33 |
| CD4 cell count, median (IQR) | 534 (373, 750) | 510 (370, 749) | 543 (379, 760) | 0.41 |
| VACS index score, median (IQR) | 29 (18, 43) | 30 (21, 48) | 28 (18, 40) | 0.021 |
| Unhealthy alcohol use (trial groups) | < 0.001 | |||
| MALD | 92 (32.6) | 54 (61.4) | 38 (19.6) | |
| At-risk drinking | 85 (30.1) | 17 (19.3) | 68 (35.1) | |
| Alcohol use disorder | 105 (37.2) | 17 (19.3) | 88 (45.4) | |
| Self-reported alcohol use, * mean (SD) | ||||
| AUDIT-C score | 5.53 (2.8) | 3.85 (2.4) | 6.29 (2.7) | < 0.001 |
| Drinks per day, past 21 days | 2.91 (3.6) | 1.13 (1.6) | 3.73 (3.9) | < 0.001 |
| Drinks per day, past 14 days | 2.89 (3.5) | 1.14 (1.6) | 3.70 (3.8) | < 0.001 |
| Drinks per day, past 7 days | 2.86 (3.6) | 1.13 (1.8) | 3.65 (4.0) | < 0.001 |
| Heavy drinking days, past 21 days | 4.48 (6.0) | 1.61 (3.2) | 5.80 (6.5) | < 0.001 |
| Heavy drinking days, past 14 days | 3.03 (4.1) | 1.13 (2.2) | 3.91 (4.4) | < 0.001 |
| Heavy drinking days, past 7 days | 1.50 (2.1) | 0.52 (1.1) | 1.95 (2.2) | < 0.001 |
assessed using Alcohol Timeline Follow back (TLFB) except for AUDIT-C score;
IQR, Interquartile range; MALD, Moderate alcohol use with liver disease; SD, Standard deviation
Note: several participants had missing data for a small number of variables as follows: 4 for VACS index score; 1 for viral load status; and 2 participants with missing TLFB measures data, respectively
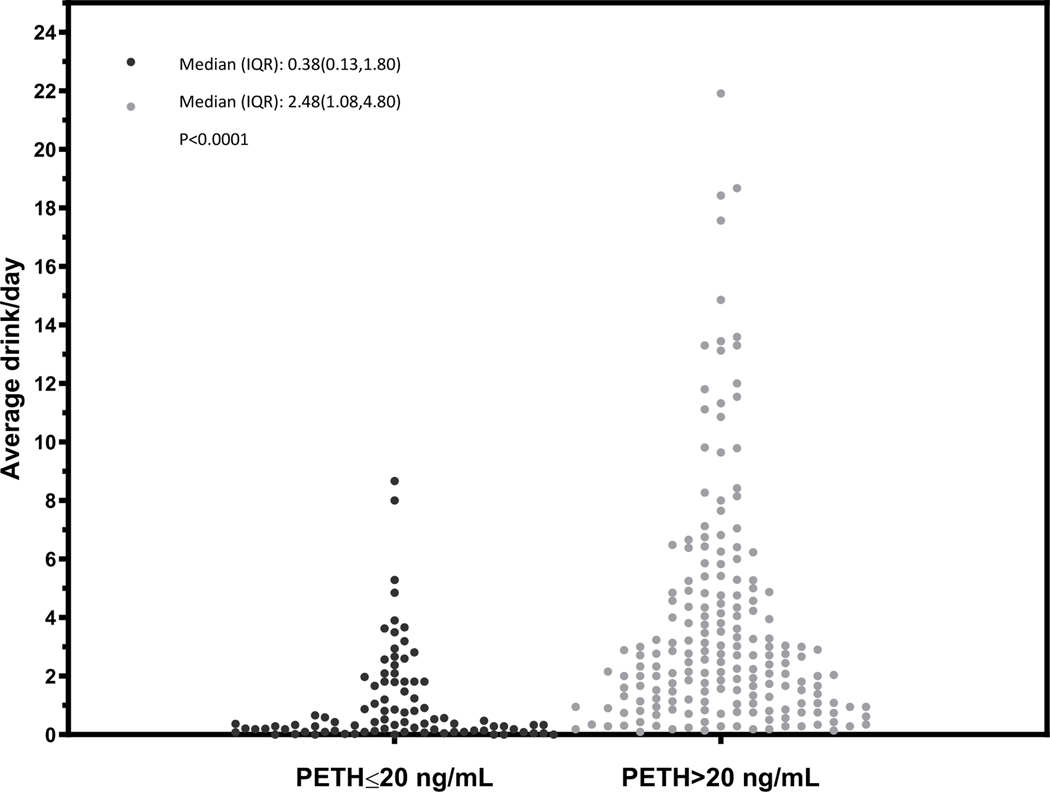
Figure 1.
Dot plot characterizing the mean number of drinks per day over the prior 21 days before the PEth test for overall sample, stratified by PEth ≤ 20 or >20 ng/mL
Characterization of alcohol exposure by PEth compared to TLFB self-report
Approximately two-thirds (69%, 194/282) of individuals had PEth >20 ng/mL. Of these, 20% were in the MALD, 35% in at-risk drinking, and 45% in the alcohol use disorder group (Table 1). Accordingly, the percent of participants with PEth >20 ng/mL increased with severity of unhealthy alcohol use across the three subgroups (Table 1, Figures 2 to 4). Among the 190 patients with either at-risk drinking or alcohol use disorder (i.e. the upper spectrum of unhealthy alcohol use), approximately 82% had PEth >20 ng/mL. In sensitivity analyses using a cutoff of 8 ng/mL, 77% of participants had PEth >8 ng/mL. The percent of participants with PEth >8 ng/mL also increased with severity of unhealthy alcohol use (48% of MALD, 87% of at-risk drinking, and 93% of alcohol use disorder). Of 190 patients with either at-risk drinking or alcohol use disorder, 91% had PEth >8 ng/mL.
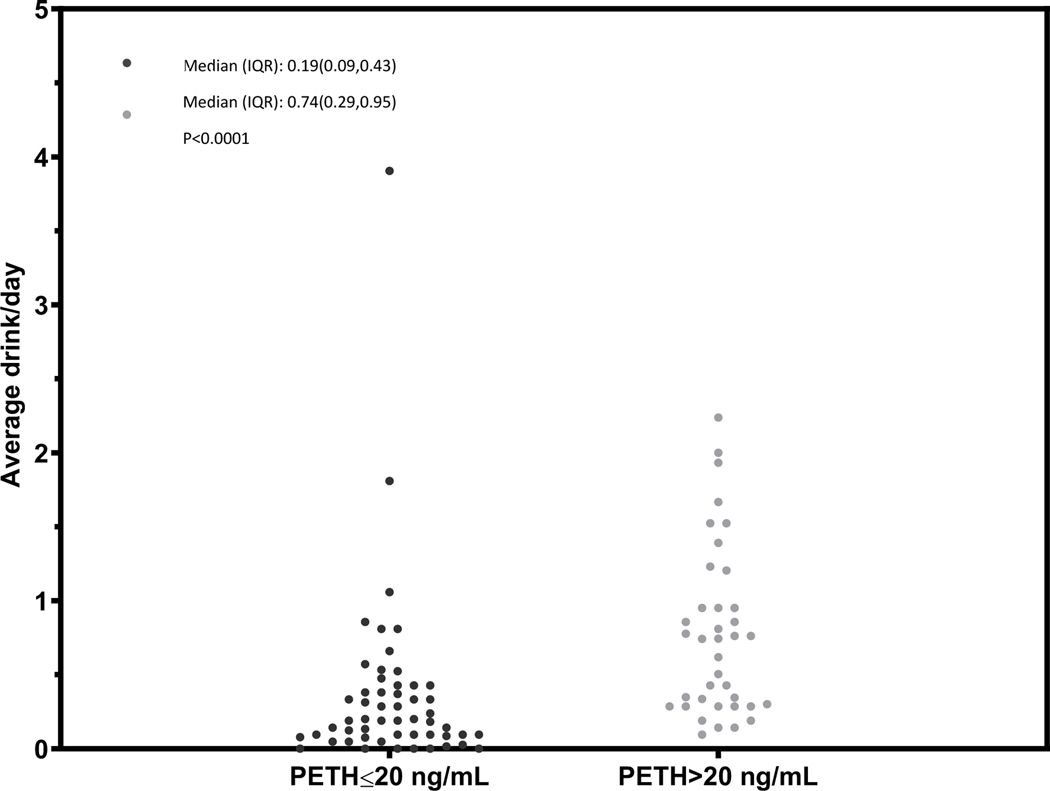
Figure 2.
Dot plot characterizing the mean number of drinks per day over the prior 21 days before the PEth test among participants with MALD, stratified by PEth ≤ 20 or >20 ng/mL
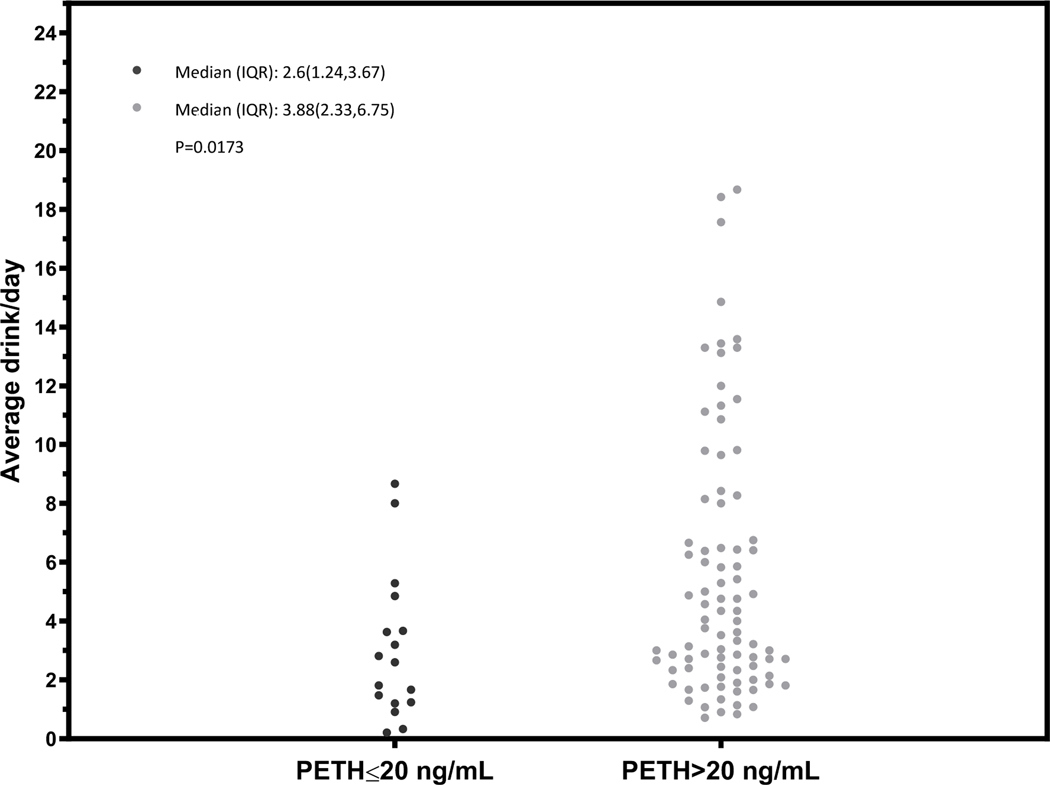
Figure 4.
Dot plot characterizing the mean number of drinks per day over the prior 21 days before the PEth test among participants with alcohol use disorder, stratified by PEth ≤ 20 or >20 ng/mL
Among 176 participants reporting at least one drink per day in the prior 21 days, 84% had baseline PEth >20 ng/mL compared to 42% of 104 participants who reported less than one drink per day (p < 0.0001). Irrespective of the TLFB look-back window (21 vs. 14 vs. 7 days), we observed that participants with baseline PEth >20 ng/mL had on average two extra drinks per day more than the daily amount consumed by those with PEth ≤20 ng/mL (Table 1). Similarly, the mean number of heavy drinking days based on TLFB were higher among those with a PEth >20 ng/mL, regardless of the look-back window. For example, the mean number of heavy drinking days in the prior 21 days was 5.8 among participants with a baseline PEth >20 ng/mL compared to 1.6 among those with a PEth ≤20 ng/mL (p < 0.0001, Table 1).
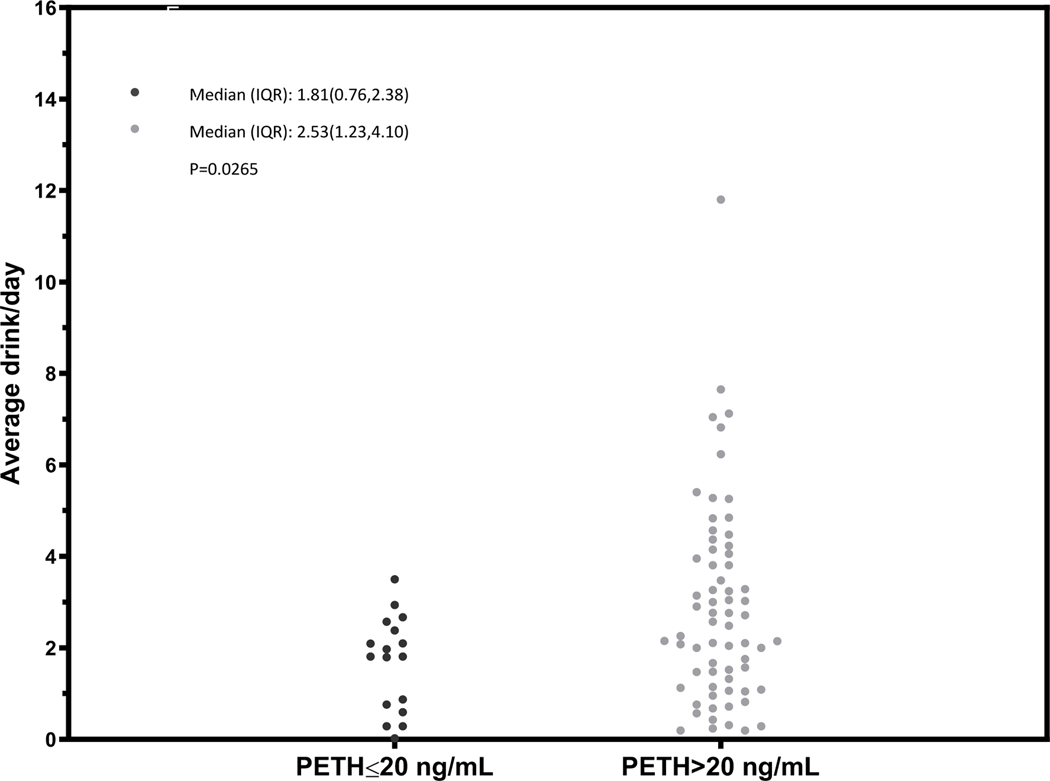
In general, we observed differences in the distribution of the mean number of drinks per day among those with a PEth ≤20 ng/mL compared to PEth >20 ng/mL (Figures 1 – 4) and no individual with a PEth ≤20 ng/mL reported 10 or more drinks per day (Figure 1). Among MALD or at-risk drinking subgroups, no individual with a PEth ≤20 ng/mL reported 4 or more drinks per day (Figures 2 and 3); among those in the alcohol use disorder subgroup, no individual with a PEth ≤20 ng/mL reported 9 or more drinks per day (Figure 4).
Figure 3.
Dot plot characterizing the mean number of drinks per day over the prior 21 days before the PEth test among participants with at-risk drinking, stratified by PEth ≤ 20 or >20 ng/mL
Associations between PEth >20 ng/mL and self-report TLFB measures
We found strong associations between self-reported alcohol use and PEth >20 ng/mL (or PEth >8 ng/mL – data not shown). The odds of a PEth >20 ng/mL was 1.73 (95% CI: 1.41, 2.11) with each unit increase in the mean number of drinks per day (Table 2). This varied by subgroup from 4.31 (95% CI: 1.61, 11.54) for MALD, 1.57 (95% CI: 1.04, 2.36) for at-risk drinking and 1.26 (95% CI: 1.01, 1.57) for alcohol use disorder (Table 2). The association was similar in sensitivity analyses using TLFB look-back windows of 14 and 7 days (Table 2). Here, for each unit increase in the mean number of drinks per day in the past 14 or 7 days, the odds ratio of having a PEth >20 ng/mL was 1.66 (95% CI: 1.37, 2.00) and 1.60 (95% CI: 1.34, 1.91) respectively. We observed similar results when the analysis was repeated using the mean number of heavy drinking days; each unit change was associated with odds ratio of 1.24 (95% CI: 1.13, 1.36) for having PEth > 20 ng/mL.
Table 2.
Logistic regression analysis and area under the curve of the ROC curves predicting PEth > 20ng/mL based on self-reported aclohol use by TLFB
| Self-reported alcohol use** | Odds ratio (95% CI)* | P-value | Area under the curve (95% CI) |
|---|---|---|---|
| Mean number of drinks per day, past 21 days | |||
| • Overall sample | 1.73 (1.41, 2.11) | <0.0001 | 0.80 (0.74, 0.85) |
| • Individual trial samples | |||
| ⚪ MALD | 4.31 (1.61, 11.54) | 0.004 | 0.78 (0.69, 0.88) |
| ⚪ At-risk drinking | 1.57 (1.04, 2.36) | 0.035 | 0.68 (0.55, 0.80) |
| ⚪ Alcohol use disorder | 1.26 (1.01, 1.57) | 0.04 | 0.68 (0.54, 0.82) |
|
| |||
|
Sensitivity analyses (among overall sample only)
|
|||
| Mean number of drinks per day, past 14 days | 1.66 (1.37, 2.00) | <0.0001 | 0.79 (0.73, 0.85) |
| Mean number of drinks per day, past 7 days | 1.60 (1.34, 1.91) | <0.0001 | 0.78 (0.72, 0.84) |
| Mean number of heavy drinking days, past 21 days | 1.24 (1.13, 1.36) | <0.0001 | 0.74 (0.68, 0.80) |
CI, Confidence interval; ROC, Receiver operating characteristics curve; TLFB, Alcohol Timeline Followback;
this is the OR of PEth > 20ng/mL. The OR are calculated based on 1 unit increase in each of the TLFB measures
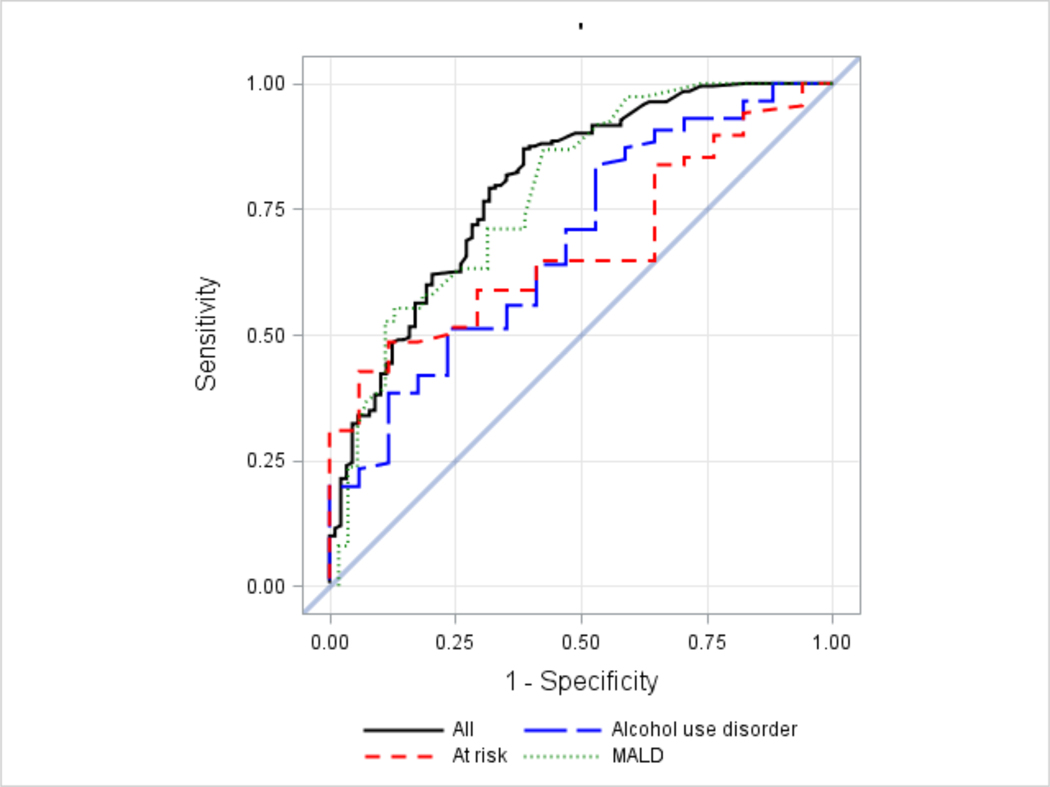
These results were also confirmed in the ROC curves (Figure 5) and corresponding AUC (Table 2). ROC curves and AUC estimates demonstrate that both mean number of drinks per day and heavy drinking days may be used to distinguish individuals with a PEth >20 ng/mL from those with a PEth ≤20ng/mL. However, based on AUC our results suggest mean number of drinks per day has better discriminatory ability (AUC: 0.80, 95% CI: 0.74, 0.85) compared to mean number of heavy drinking days (AUC: 0.74, 95% CI: 0.68, 0.80). Mean number of drinks per day also performed well in sensitivity analysis using a 14- or 7-day look back window with AUC of 0.79 (95% CI: 0.73, 0.85) and 0.78 (95% CI: 0.72, 0.84), respectively. Considering the subgroups separately, the highest AUC, and thus the greatest discriminatory ability was observed among participants in MALD with AUC of 0.78 (95% CI: 0.69, 0.88).
Figure 5.
ROC curves for predicting PEth > 20 ng/mL in the overall (all), MALD, at-risk and alcohol use disorder sample (note: the diagonal line represents chance level, or probability of 0.5)
Association between self-reported alcohol exposure and PEth
In the overall sample, there was a moderate but statistically significant positive correlation between the mean number of drinks per day and PEth (Spearman’s R = 0.55, p < 0.0001). Within subgroups, moderate and positive correlation was observed among participants in MALD (Spearman’s R = 0.56, p < 0.0001) and at-risk (Spearman’s R = 0.41, p < 0.0001). Weak but statistically significant positive relationship was observed among participants with alcohol use disorder (Spearman’s R = 0.29, p = 0.003) (Table 3). This was unattenuated in sensitivity analyses where we limited the time window to the past 14 and 7 days (Table 3). We also observed a significant positive correlation with PEth when estimated using mean number of heavy drinking days instead of mean number of drinks per day (Table 3).
Table 3.
Spearman correlation for the relationship between TLFB self-reported alcohol use by TLFB and PEth on a continuous scale
| Self-reported alcohol use | Spearman Correlation (95% CI)* | P-value for Ho: Rho=0 |
|---|---|---|
| Mean number of drinks per day, past 21 days | ||
| • Overall sample | 0.55 (0.46, 0.63) | <0.0001 |
| • Individual trial samples | ||
| ⚪ MALD | 0.56 (0.40, 0.68) | <0.0001 |
| ⚪ At-risk drinking | 0.41 (0.22, 0.58) | <0.0001 |
| ⚪ Alcohol use disorder | 0.29 (0.10, 0.45) | 0.0034 |
|
| ||
|
Sensitivity analyses (among overall sample only)
|
||
| Mean number of drinks per day, past 14 days | 0.53 (0.44, 0.61) | <0.0001 |
| Mean number of drinks per day, past 7 days | 0.52 (0.43, 0.60) | <0.0001 |
| Mean number of heavy drinking days, past 21 days | 0.44 (0.34, 0.530) | <0.0001 |
CI, Confidence interval; TLFB, Alcohol Timeline Followback
DISCUSSION
Using baseline data collected among PWH with unhealthy alcohol use entering alcohol trials in a clinical setting, we examined the association between self-report alcohol use and PEth and whether self-report alcohol use data could predict clinically significant alcohol use as defined by a PEth >20 ng/mL. Several key findings emerged. First, our results underscore the potential for misclassification of unhealthy alcohol use based on self-report data alone. Among those that met the criteria for at-risk drinking and alcohol use disorder based on established criteria, only 82% had PEth >20 ng/mL, and 91% had PEth >8 ng/mL. Second, we found a low to moderate positive correlation between self-report mean number of drinks per day or heavy drinking days and PEth >20 ng/mL. Third, our results suggest that better discrimination is found with self-reported mean number of drinks per day than number of heavy drinking. These results highlighting the association between self-report and PEth are strengthened by the fact that our 21 days look-back window for the measurement of self-report alcohol use by TLFB coincide with the 21-days window of detectable PEth, and the results remained robust in sensitivity analyses using shorter look-back windows of 14 and 7 days to minimize the effect of recall bias in self-reports. Taken together, these findings highlight the potential role of alcohol biomarkers such as PEth as a complementary measure to improve the assessment of alcohol consumption in clinical settings (e.g., use of biomarker feedback to patients) and patients entering clinical trials.
Although under-reporting of alcohol consumption based on self-report may occur in the setting of HIV due to social desirability bias, social norms, misinformation (Papas et al., 2016, Bajunirwe et al., 2014, Hahn et al., 2012b) and among patients with liver disease, we found a substantial proportion of the participants self-reporting levels consistent with at-risk drinking and alcohol use disorder and yet, did not have a PEth >20 ng/mL. Our observation suggestive of self-reported over-reporting of alcohol use in the context of baseline assessments for a clinical trial may be due to several potential reasons. Although issues of recall bias are common in settings where retrospective assessment of behaviors such as drinking is performed (Greenfield and Kerr, 2008), the structural features of TLFB including calendar and other memory aids to improve recall may have minimized this issue. It is not improbable that our mode of administration of TLFB via face to face interview with participants (instead of self-administration) may have contributed to the observed trend, although the direction of such an effect should have been more towards an under-reporting as opposed to over-reporting (Bradley et al., 2011). It is also possible that some participants exaggerated their drinking due to perceived social norms (Chung and Rimal, 2016) or to qualify for the alcohol treatment trials given potential reimbursements, but have had low- to moderate-level drinking in the recent past. A different but somewhat related reason was reported from a study conducted in Uganda, where participants overreported drinking for secondary gain of transportation reimbursement (Papas et al., 2016), although our study did not provide such incentives. To reduce demand characteristics that might lead to overreporting during participant recruitment or administration of the TLFB, our recruitment materials did not specify any particular level of drinking to be eligible for study participation; participants were eligible across a range of alcohol use. Furthermore, the research assistants were trained to competence to collect TLFB and were not involved in participants’ medical care, thereby minimizing any perceived incentive to disclose. Another potential reason for the self-reported over-reporting of alcohol use may have to do with our choice of PEth cutoff. Although we chose a cutoff of 20 ng/mL to reflect clinically significant alcohol exposure, previous studies have used lower cutoffs of 8 ng/mL (Asiimwe et al., 2015, Bajunirwe et al., 2014) including suggesting that drinking at PEth cutoffs ≥8 ng/mL may be associated with some harms (Eyawo et al., 2018). The extent of the observed overreporting was cut by half when we used a less conservative cutoff of 8 ng/mL in sensitivity analyses thereby improving the concordance between PEth and self-report. In the study by Asiiwe and colleagues that used a PEth cutoff of 8 ng/mL (Asiimwe et al., 2015), only 10% of self-reported unhealthy drinkers had a PEth at or below the 8 ng/mL cutoff, compared to 9% of individuals with at-risk drinking and alcohol use disorder in our sensitivity analysis that used a 8 ng/mL cutoff. We also observed that having a PEth >20 ng/mL appeared to be directly related to the severity of unhealthy alcohol use based on self-report. This corroborates previously reported findings from other studies in terms of the correlation between self-report alcohol intake and PEth (Aradottir et al., 2006). Prior research has demonstrated the association between increasing severity of unhealthy alcohol use and adverse health outcomes including mortality (Eyawo et al., 2018, Justice et al., 2016).
A key objective of our study was to investigate whether self-reported alcohol use data could predict clinically relevant use, resulting in a PEth >20 ng/mL. Our findings that unit increases in the self-reported mean number of drinks per day in the past 21 days has better discriminatory ability for predicting PEth >20 ng/mL than use of heavy drinking days may likely be related to the mechanism of formation and elimination of PEth. Findings from previous studies have shown that there is inter-individual variability in the absorption and elimination of PEth, both of which have an impact on the PEth concentration that is measured at a given point in time (Javors et al., 2016, Stewart et al., 2010). With this in mind, it makes sense that the mean number of drinks per day has better discriminatory power, as it more closely reflects changes in daily alcohol concentration in the blood compared to heavy drinking days that may be episodic and less regular in frequency. Although the mean number of drinks per day in the past 21 days appears to have better discriminatory ability over the heavy drinking days, it does not necessarily mean that the latter is not useful. Given the fair discriminatory power of PEth for heavy drinking days, it could also be used in settings where information on the mean number of drinks per day in the past 21 days are unavailable.
We also found the mean number of drinks per day in the past 21 days to have greater discriminatory power for predicting PEth >20 ng/mL among individuals meeting the criteria for MALD (AUC: 0.78, 95% CI: 0.69, 0.88), compared to the other subgroups. Given that these individuals are co-infected with HCV or have evidence of liver fibrosis, it is possible that their underlying health status may have directly or indirectly impacted how sensitive they are to changes in blood alcohol levels (Stewart et al., 2014). We have previously demonstrated that individuals co-infected with HCV may be at particularly high risk of mortality (Eyawo et al., 2018). Nevertheless, at least one study has suggested that liver disease severity does not modify the effect of alcohol use on PEth concentration and thus the validity of PEth in the presence of liver disease (Stewart et al., 2014). However, given the small sample size of that study and that all patients included in the analysis had liver disease, confirmatory analyses are needed. It would be interesting to examine whether factors such as FIB-4 score might impact the correlation between TLFB and PEth among individuals with unhealthy alcohol use and liver disease. This will be particularly relevant for patients with clinically important alcohol exposure that could harm the liver or exacerbate other liver-related conditions. Furthermore, given the current limited knowledge of inter-individual differences in PEth synthesis and elimination, additional research is needed to better understand this and improve our understanding of the relationship between self-reported alcohol use and PEth concentrations. Additionally, given that liver disease is associated with shortened red cell survival (Bhattacharjee et al., 2018), our findings that self-reported alcohol use was more strongly associated with PEth among the MALD participants may be explained by the fact that PEth reflects alcohol use over shorter period of time among patients with HIV and comorbid liver disease and would thus be less subject to recall bias.
Our study has some limitations. Most importantly, we were only able to evaluate PEth among those reporting at least some alcohol use. Given social desirability bias, PEth may be particularly important for detecting harmful alcohol use among those reporting abstinence. Approximately 99% of the participants in the study were men. As such, the findings are not generalizable to women. Furthermore, our sample included only individuals who were currently receiving HIV care. It is possible that this sample is systematically different from those that are not in care. Furthermore, the results presented are based on data from a relatively small sample of 282 participants, therefore it would be interesting to replicate these analyses in a larger sample with more women. Despite these limitations, our study has shed light on some important relationship between alcohol self-report and PEth.
In summary, our study demonstrated self-reported alcohol use overestimates alcohol consumption compared to PEth in approximately 9% to 18% of HIV-positive individuals entering alcohol clinical trials supporting the role of confirming self-report with PEth, irrespective of the PEth cutoff used (8 ng/mL or 20 ng/mL). Specific to PEth > 20 ng/mL – a level that reflects clinically significant alcohol use, our results suggest that better discrimination is found with self-reported mean number of drinks per day than number of heavy drinking. Using a biomarker such as PEth to complement self-report measures could improve the measurement of alcohol use and be an effective way to accurately measure unhealthy alcohol use in clinical settings and trials.
ACKNOWLEDGMENTS
Funding
This work was support by grants from the US National Institute on Alcohol Abuse and Alcoholism (grant #U01AA020795, U01AA020790, U24AA020794). EJE was supported as a Yale-Drug Abuse, HIV and Addiction Research Scholar (NIDA grant #K12DA033312) during the conduct of this work.
Footnotes
Conflicts of interest
None.
References
- AKGUN KM, GORDON K, PISANI M, FRIED T, MCGINNIS KA, TATE JP, BUTT AA, GIBERT CL, HUANG L, RODRIGUEZ-BARRADAS MC, RIMLAND D, JUSTICE AC & CROTHERS K. 2013. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Defic Syndr, 62, 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed.,. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- ARADOTTIR S, ASANOVSKA G, GJERSS S, HANSSON P. & ALLING C. 2006. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol, 41, 431–7. [DOI] [PubMed] [Google Scholar]
- ASIIMWE SB, FATCH R, EMENYONU NI, MUYINDIKE WR, KEKIBIINA A, SANTOS GM, GREENFIELD TK & HAHN JA 2015. Comparison of Traditional and Novel Self-Report Measures to an Alcohol Biomarker for Quantifying Alcohol Consumption Among HIV-Infected Adults in Sub-Saharan Africa. Alcohol Clin Exp Res, 39, 1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJUNIRWE F, HABERER JE, BOUM Y 2ND, HUNT P, MOCELLO R, MARTIN JN, BANGSBERG DR & HAHN JA. 2014. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One, 9, e113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTACHARJEE D, VRACAR S, ROUND RA, NIGHTINGALE PG, WILLIAMS JA, GKOUTOS GV, STRATTON IM, PARKER R, LUZIO SD, WEBBER J, MANLEY SE, ROBERTS GA & GHOSH S. 2018. Utility of HbA1c assessment in people with diabetes awaiting liver transplantation. Diabet Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY KA, LAPHAM GT, HAWKINS EJ, ACHTMEYER CE, WILLIAMS EC, THOMAS RM & KIVLAHAN DR 2011. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med, 26, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAITHWAITE RS & BRYANT KJ 2010. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health, 33, 280–7. [PMC free article] [PubMed] [Google Scholar]
- CHUNG A. & RIMAL RN 2016. Social Norms: A Review. Review of Communication Research, 4, 1–29. [Google Scholar]
- CONIGLIARO J, GORDON AJ, MCGINNIS KA, RABENECK L, JUSTICE AC & VETERANS AGING COHORT 3-SITE S. 2003. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr, 33, 521–5. [DOI] [PubMed] [Google Scholar]
- COOK RL, MCGINNIS KA, KRAEMER KL, GORDON AJ, CONIGLIARO J, MAISTO SA, SAMET JH, CRYSTAL S, RIMLAND D, BRYANT KJ, BRAITHWAITE RS & JUSTICE AC 2006. Intoxication before intercourse and risky sexual behavior in male veterans with and without human immunodeficiency virus infection. Med Care, 44, S31–6. [DOI] [PubMed] [Google Scholar]
- EDELMAN EJ, MAISTO SA, HANSEN NB, CUTTER CJ, DZIURA J, DENG Y, FIELLIN LE, O’CONNOR PG, BEDIMO R, GIBERT CL, MARCONI VC, RIMLAND D, RODRIGUEZ-BARRADAS MC, SIMBERKOFF MS, TATE JP, JUSTICE AC, BRYANT KJ & FIELLIN DA 2019a. Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. Lancet HIV, 6, e509–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN EJ, MAISTO SA, HANSEN NB, CUTTER CJ, DZIURA J, DENG Y, FIELLIN LE, O’CONNOR PG, BEDIMO R, GIBERT CL, MARCONI VC, RIMLAND D, RODRIGUEZ-BARRADAS MC, SIMBERKOFF MS, TATE JP, JUSTICE AC, BRYANT KJ & FIELLIN DA 2019b. Integrated stepped alcohol treatment for patients with HIV and liver disease: A randomized trial. J Subst Abuse Treat, 106, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN EJ, MAISTO SA, HANSEN NB, CUTTER CJ, DZIURA J, FIELLIN LE, O’CONNOR PG, BEDIMO R, GIBERT C, MARCONI VC, RIMLAND D, RODRIGUEZ-BARRADAS MC, SIMBERKOFF MS, JUSTICE AC, BRYANT KJ & FIELLIN DA 2017. The Starting Treatment for Ethanol in Primary care Trials (STEP Trials): Protocol for Three Parallel Multi-Site Stepped Care Effectiveness Studies for Unhealthy Alcohol Use in HIV-Positive Patients. Contemp Clin Trials, 52, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYAWO O, MCGINNIS KA, JUSTICE AC, FIELLIN DA, HAHN JA, WILLIAMS EC, GORDON AJ, MARSHALL BDL, KRAEMER KL, CRYSTAL S, GAITHER JR, EDELMAN EJ, BRYANT KJ, TATE JP & TEAM VP 2018. Alcohol and Mortality: Combining Self-Reported (AUDIT-C) and Biomarker Detected (PEth) Alcohol Measures Among HIV Infected and Uninfected. J Acquir Immune Defic Syndr, 77, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELLIN DA, MCGINNIS KA, MAISTO SA, JUSTICE AC & BRYANT K. 2013. Measuring alcohol consumption using Timeline Followback in non-treatment-seeking medical clinic patients with and without HIV infection: 7-, 14-, or 30-day recall. J Stud Alcohol Drugs, 74, 500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GNANN H, WEINMANN W. & THIERAUF A. 2012. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res, 36, 1507–11. [DOI] [PubMed] [Google Scholar]
- GREENFIELD TK & KERR WC 2008. Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction, 103, 1082–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, ANTON RF & JAVORS MA 2016. The Formation, Elimination, Interpretation, and Future Research Needs of Phosphatidylethanol for Research Studies and Clinical Practice. Alcohol Clin Exp Res, 40, 2292–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, DOBKIN LM, MAYANJA B, EMENYONU NI, KIGOZI IM, SHIBOSKI S, BANGSBERG DR, GNANN H, WEINMANN W. & WURST FM 2012a. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res, 36, 854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, FATCH R, KABAMI J, MAYANJA B, EMENYONU NI, MARTIN J. & BANGSBERG DR 2012b. Self-Report of Alcohol Use Increases When Specimens for Alcohol Biomarkers Are Collected in Persons With HIV in Uganda. J Acquir Immune Defic Syndr, 61, e63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAKSSON A, WALTHER L, HANSSON T, ANDERSSON A. & ALLING C. 2011. Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal, 3, 195–200. [DOI] [PubMed] [Google Scholar]
- JAVORS MA, HILL-KAPTURCZAK N, ROACHE JD, KARNS-WRIGHT TE & DOUGHERTY DM 2016. Characterization of the Pharmacokinetics of Phosphatidylethanol 16:0/18:1 and 16:0/18:2 in Human Whole Blood After Alcohol Consumption in a Clinical Laboratory Study. Alcohol Clin Exp Res, 40, 1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES J, JONES M, PLATE C. & LEWIS D. 2011. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods, 3, 1101–1106. [Google Scholar]
- JUSTICE AC, MCGINNIS KA, TATE JP, BRAITHWAITE RS, BRYANT KJ, COOK RL, EDELMAN EJ, FIELLIN LE, FREIBERG MS, GORDON AJ, KRAEMER KL, MARSHALL BD, WILLIAMS EC & FIELLIN DA 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend, 161, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUSTICE AC, MODUR SP, TATE JP, ALTHOFF KN, JACOBSON LP, GEBO KA, KITAHATA MM, HORBERG MA, BROOKS JT, BUCHACZ K, ROURKE SB, RACHLIS A, NAPRAVNIK S, ERON J, WILLIG JH, MOORE R, KIRK GD, BOSCH R, RODRIGUEZ B, HOGG RS, THORNE J, GOEDERT JJ, KLEIN M, GILL J, DEEKS S, STERLING TR, ANASTOS K, GANGE SJ, NA A. & TEAMS VP 2013. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr, 62, 149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALICHMAN SC, AMARAL CM, WHITE D, SWETSZE C, KALICHMAN MO, CHERRY C. & EATON L. 2012. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care, 23, 511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM JK, TATE JP, FULTZ SL, GOULET JL, CONIGLIARO J, BRYANT KJ, GORDON AJ, GIBERT C, RIMLAND D, GOETZ MB, KLEIN MB, FIELLIN DA, JUSTICE AC & LO RE V 3RD 2014. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis, 58, 1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD AK, BROWN JL, DICLEMENTE RJ, SAFONOVA P, SALES JM, ROSE ES, BELYAKOV N. & RASSOKHIN VV 2017. Phosphatidylethanol (PEth) as a Biomarker of Alcohol Consumption in HIV-Infected Young Russian Women: Comparison to Self-Report Assessments of Alcohol Use. AIDS Behav, 21, 1938–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDENI M, FOCA E, GOTTI D, LADISA N, ANGARANO G, ALBINI L, CASTELNUOVO F, CAROSI G, QUIROS-ROLDAN E. & TORTI C. 2011. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis, 52, 1164–73. [DOI] [PubMed] [Google Scholar]
- NATIONAL INSTITUTE ON ALCOHOL ABUSE AND ALCOHOLISM 2005. Helping patients who drink too much: a clinician’s guide. In: DEPARTMENT OF HEALTH US & HUMAN SERVICES; (ed.). [Google Scholar]
- PAPAS RK, GAKINYA BN, MWANIKI MM, KETER AK, LEE H, LOXLEY MP, KLEIN DA, SIDLE JE, MARTINO S, BALIDDAWA JB, SCHLAUDT KL & MAISTO SA 2016. Associations Between the Phosphatidylethanol Alcohol Biomarker and Self-Reported Alcohol Use in a Sample of HIV-Infected Outpatient Drinkers in Western Kenya. Alcohol Clin Exp Res, 40, 1779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLOWSKI JA, KALICHMAN SC, KALICHMAN MO & CHERRY C. 2016. Alcohol-antiretroviral therapy interactive toxicity beliefs and daily medication adherence and alcohol use among people living with HIV. AIDS Care, 28, 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIANO MR, TIWARI S, NEVORAL L. & PHILLIPS SA 2015. Phosphatidylethanol Levels Are Elevated and Correlate Strongly with AUDIT Scores in Young Adult Binge Drinkers. Alcohol Alcohol, 50, 519–25. [DOI] [PubMed] [Google Scholar]
- RENTSCH C, TATE JP, AKGUN KM, CRYSTAL S, WANG KH, RYAN GREYSEN S, WANG EA, BRYANT KJ, FIELLIN DA, JUSTICE AC & RIMLAND D. 2016. Alcohol-Related Diagnoses and All-Cause Hospitalization Among HIV-Infected and Uninfected Patients: A Longitudinal Analysis of United States Veterans from 1997 to 2011. AIDS Behav, 20, 555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITZ R. 2005. Clinical practice. Unhealthy alcohol use. N Engl J Med, 352, 596–607. [DOI] [PubMed] [Google Scholar]
- SAMET JH, CHENG DM, LIBMAN H, NUNES DP, ALPEREN JK & SAITZ R. 2007. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr, 46, 194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBELL LC, BROWN J, LEO GI & SOBELL MB 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend, 42, 49–54. [DOI] [PubMed] [Google Scholar]
- SOBELL LC & SOBELL SM 1996. Alcohol Timeline Followback (TLFB). Handbook of Psychiatric Measures Washighton, D.C.: American Psychiatric Association. [Google Scholar]
- STERLING RK, LISSEN E, CLUMECK N, SOLA R, CORREA MC, MONTANER J, TORRIANI M,SS, DIETERICH FJ, THOMAS DT, MESSINGER DL, NELSON, M D. & INVESTIGATORS AC. 2006. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology, 43, 1317–25. [DOI] [PubMed] [Google Scholar]
- STEWART SH, KOCH DG, WILLNER IR, ANTON RF & REUBEN A. 2014. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res, 38, 1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, LAW TL, RANDALL PK & NEWMAN R. 2010. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res, 34, 488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, REUBEN A, BRZEZINSKI WA, KOCH DG, BASILE J, RANDALL PK & MILLER PM 2009. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol, 44, 464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATE JP, JUSTICE AC, HUGHES MD, BONNET F, REISS P, MOCROFT A, NATTERMANN J, LAMPE FC, BUCHER HC, STERLING TR, CRANE HM, KITAHATA MM, MAY M. & STERNE JA 2013. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS, 27, 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULWELLING W. & SMITH K. 2018. The PEth Blood Test in the Security Environment: What it is; Why it is Important; and Interpretative Guidelines. J Forensic Sci, 63, 1634–1640. [DOI] [PubMed] [Google Scholar]
- VIEL G, BOSCOLO-BERTO R, CECCHETTO G, FAIS P, NALESSO A. & FERRARA SD 2012. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci, 13, 14788–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTHER L, DE BEJCZY A, LOF E, HANSSON T, ANDERSSON A, GUTERSTAM J, HAMMARBERG A, ASANOVSKA G, FRANCK J, SODERPALM B. & ISAKSSON A. 2015. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and gamma-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res, 39, 2200–8. [DOI] [PubMed] [Google Scholar]
- WANG Y, CHEN X, HAHN JA, BRUMBACK B, ZHOU Z, MIGUEZ MJ & COOK RL 2018. Phosphatidylethanol in Comparison to Self-Reported Alcohol Consumption Among HIV-Infected Women in a Randomized Controlled Trial of Naltrexone for Reducing Hazardous Drinking. Alcohol Clin Exp Res, 42, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS EC, HAHN JA, SAITZ R, BRYANT K, LIRA MC & SAMET JH 2016. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res, 40, 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS EC, MCGINNIS KA, EDELMAN EJ, MATSON TE, GORDON AJ, MARSHALL BDL, BRYANT KJ, RUBINSKY AD, LAPHAM GT, SATRE DD, RICHARDS JE, CATZ SL, FIELLIN DA, JUSTICE AC & BRADLEY KA 2019. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS Behav, 23, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKLER M, SKOPP G, ALT A, MILTNER E, JOCHUM T, DAENHARDT C, SPORKERT F, GNANN H, WEINMANN W. & THIERAUF A. 2013. Comparison of direct and indirect alcohol markers with PEth in blood and urine in alcohol dependent inpatients during detoxication. Int J Legal Med, 127, 761–8. [DOI] [PubMed] [Google Scholar]
- WITKIEWITZ K, FINNEY JW, HARRIS AH, KIVLAHAN DR & KRANZLER HR 2015. Guidelines for the Reporting of Treatment Trials for Alcohol Use Disorders. Alcohol Clin Exp Res, 39, 1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION (WHO). 2014. Global status report on alcohol and health [Online]. Geneva: World Health Organization (WHO). Available: http://www.who.int/substance_abuse/publications/alcohol/en/ [Accessed August 15, 2018. [Google Scholar]
- WURST FM, THON N, YEGLES M, SCHRUCK A, PREUSS UW & WEINMANN W. 2015. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res, 39, 2060–72. [DOI] [PubMed] [Google Scholar]