Abstract
Diabetic retinopathy (DR) is a devastating disease leading to blindness among majority of working adults around the globe. Nonetheless, an effective treatment or cure for the disease is still to be achieved. This is because the cellular and molecular mechanisms of DR are complex and not fully understood yet. In this article, we describe how high glucose induced TXNIP upregulation and associated redox stress may cause mitochondrial dysfunction, mitophagy, ferritinophagy (iron release by autophagy) and lysosome destabilization. Labile irons react with hydrogen peroxide (H2O2) to generate hydroxyl radicals (.OH) by the Fenton reaction and cause membrane phospholipid peroxidation due to reduction in glutathione (GSH) level and glutathione peroxidase 4 (GPX4) activity, which cause ferroptosis, a recently identified non-apoptotic cell death mechanism. We used in this study a retinal pigment epithelial cell line, ARPE- 19 and exposed it to high glucose in in vitro cultures to highlight some of the intricacies of these cellular processes, which may be relevant to the pathogenesis of DR and age-related retinal neurodegenerative diseases, such as age-related macular degeneration, AMD.
Keywords: TXNIP, Oxidative stress, Mitophagy, Ferritinophagy, Ferroptosis, RPE, Diabetic retinopathy
Introduction
The retina is a complex organ/tissue, a part of the central nervous system, involved in visual perception. It consumes large amounts of glucose and oxygen to generate ATP needed for its phototransduction activity via oxidative phosphorylation in the mitochondrial electron transport chain. Nonetheless, during the process of ATP production, reactive oxygen radicals are produced, which can damage mitochondrial membranes, proteins and mtDNA. Therefore, antioxidant systems are present in mitochondria to neutralize the harmful reactive oxygen species by glutathione (GSH), thioredoxin 2 (Trx2), superoxide dismutase 2 (SOD2) and others [1]. However, under chronic stress and diseases, such as diabetic retinopathy (DR), the reactive oxygen/nitrogen species (ROS/RNS) overwhelm the antioxidant system causing mitochondrial stress and damage. Damaged mitochondria produce less ATP but continues to generate ROS, therefore, needed to remove by a specific process of autophagy, called mitophagy [2]. To begin mitophagy, the damage part of the mitochondrion is separated by fission involving dynamin-related protein 1 (DRP-1) and mitochondrial fission protein 1 fis1. Then, the isolated damaged mitochondria are marked with ubiquitin by the Pink1-Parkin pathway [3]. Parkin is an E3 ubiquitin ligase, which adds ubiquitin to mitochondrial outer membrane proteins, including VDAC1 and Mfn2. Pink1 is an inner mitochondrial membrane protein, which is imported inside mitochondria under normal physiology and degraded by PARL, a member of the rhomboid intramembrane protease family [4]. However, when the mitochondrion is damaged or depolarized, Pink1 accumulates on the outer membrane and phosphorylates ubiquitin and other mitochondrial outer membrane proteins, which are then recognized by Parkin. Once ubiquitinated by Parkin, the damaged mitochondria bind to its adaptors, such as optineurin and p62 sequestosome 1 and the double-membrane LC3BII auto phagophore, then engulfs inside [5]. The mito-autophagosome subsequently fuses with lysosomes to form autolysosome and the contents are digested by lysosomal acidic proteases and enzymes and recycle as nutrients [6]. Mitophagy is important, as a quality control process, in fully differentiated non-dividing cells, including retinal neurons and pigment epithelial cells (RPE). These cells cannot redistribute damaged mitochondria to daughter cells. Therefore, mitophagy reduces cellular stress and inflammatory processes as damaged mitochondria release mtROS and oxidized mtDNA that are recognized by innate immune platforms, such as membrane toll-like receptors (TLRs) and cytosolic NLRP3 inflammasome [7].
To balance mitochondrial number and maintain bioenergetics, synthesis of new mitochondria is important. For this to occur, the activation of transcription factors PGC1α and TFAM are required to synthesize nuclear-encoded mitochondria-targeted genes and mitochondria genes, respectively [8]. Furthermore, several mitochondrial enzymes involved in TCA cycles, such as aconitase 2 and mitochondrial electron transport complexes (I, II and III) contain iron-sulfur complex/cluster (4Fe-4S) that is required for holoenzyme assembly and function [9]. Cellular iron is stored as ferric iron (Fe3+) in ferritin cages containing ferritin heavy and light chains since labile ferrous iron Fe2+ is redox active and reacts with hydrogen peroxide (H2O2) to generate highly reactive hydroxyl radicals (·OH), which attack on membrane lipids, proteins and DNA [10,11]. Each ferritin cage may contain up to 24 ferritin subunits (heavy and light chains) and stores up to 4500 ferric iron molecules [12]. Therefore, iron supply for mitochondrial iron-sulfur complex assembly comes from the autophagic degradation of ferritin cages in lysosomes (ferritinophagy), which releases a pool of labile Fe2+ in the cytosol [13,14]. These cytosolic labile irons are then transferred into the mitochondrion via mitochondrial membrane iron transporters, namely, mitoferrin 1 and 2, where they are assembled into iron-sulfur complexes into mitochondrial TCA enzymes and electron transport complexes by the iron-sulfur cluster synthesis machinery [15]. In addition, free iron (Fe2+) is also required for several cytosolic enzymes, including 5-lipoxygenase (ALOX-5) and nuclear DNA repair and replication enzymes as cofactors for their activity [16]. Circulating ferric iron in transferrin complex (Tf-Fe3+) is taken up by cell surface transferrin receptors (TfR1 and TfR2) via endocytosis. Ferric iron is then reduced to ferrous iron and releases into the cytosol by endosomal divalent metal transporter DMT1 [17]. Then, the free iron (Fe2+) is trapped into ferritin cages and converted to Fe3+ inside for storage [18]. As mentioned above, utilization of iron requires ferritinophagy, autophagic degradation of ferritin and acidic reduction of ferric to ferrous iron inside the lysosome. During chronic disease and oxidative stress, mitochondrial stress and damage occur, which are removed by mitophagy, and biosynthesis of new mitochondria require ferritinophagy. Therefore, continuous, or excess mitophagic and ferritinophagic flux to lysosomes will cause lysosomal iron accumulation, oxidative stress, increased pH, lipoprotein aggregation, lysosome enlargement and lysosomal membrane permeabilization (LMP) [19,20]. Therefore, released ferrous iron will produce reactive hydroxyl radicals, which results in membrane lipid peroxidation, including the lysosomal, mitochondrial and plasma membrane [21]. In addition, free iron also activates ALOX5, further enhancing lipid peroxidation [22]. One of the enzymes that detoxify lipid peroxidation is glutathione peroxidase 4 (GPX4), which uses 2 molecules of glutathione (GSH) [23]. However, in diabetes and under high glucose, we have shown that a protein called thioredoxin-interacting protein (TXNIP) is strongly induced in most cells examined, including retinal and renal cells [24–27]. TXNIP causes cellular oxidative stress and inflammation by binding to and inhibiting antioxidant thioredoxin (Trx) [26–28]. Trx1 is present in the cytosol and nucleus while Trx2 is the mitochondrial isoform. TXNIP is present in all cellular organelles, including mitochondria. Therefore, sustained TXNIP upregulation under high glucose environment causes cellular redox stress, mitochondrial dysfunction, and premature cell death [26,29]. Because cellular and mitochondrial stress depletes antioxidants, including GSH, the activity of GPX4 and other redox enzymes are inactivated. Cell death due to iron accumulation and lipid peroxidation due to GPX4 inactivation was recently termed as ferroptosis [30]. Mitophagy, ferritinophagy and lysosome destabilization may play critical roles in this type of ferroptotic cell death. In the present article, we propose a plausible mechanism for TXNIP and associated redox stress in mitochondrial dysfunction, mitophagy and ferritinophagy in ferroptosis in retinal RPE cells under sustained high glucose environment in culture as seen in DR. RPE cells form the outer blood-retinal-barrier (oBRB) between the neuroretina and fenestrated choriocapillaris. RPE dysfunction leads to photoreceptor death in DR and age-related retinal disease [31,32].
Materials and Methods
The reagents, experimental procedures and assays performed in this study with human retinal pigment epithelial cell line, ARPE-19, in in vitro culture under high glucose (HG, 25mM) or low glucose (LG, 5.5mM) are like those described in our recent publications [33,34]. The assays include Western blots, QPCR, LDH leakage measurement, and Immunofluorescence staining. Ad-CMV-mt-Keima and Ad-CMV- LAMP1-mCherry were produced by Vector Biosystems, Inc., Malvern, USA. A summary of reagents used are shown in Table 1.
Table 1:
Reagents and Chemicals.
| Name | Company | Cat # |
|---|---|---|
| DMEM medium | Mediatech Inc (Manassas, VA) | 10-014-CM |
| F-12 medium | HyClone (Logan, UT) | SH30026.01 |
| Antibiotics and Trypsin | HyClone | |
| FBS | Corning (Corning, NY) | MT35010CV |
| Glucose | Sigma (St. Louis, MO) | G8270 |
| Azaserine | Sigma | A-1164 |
| Hydrogen peroxide | Sigma | 95321 |
| Mounting medium | Molecular Probes (Eugene, OR) | P36935 FeSO4, Iron (II) sulfate heptahydrate) Sigma 7782-63-0 |
| Ferrostatin 1 | Sigma | SML0583 |
| Deferasirox (DFX) | Sigma | SML2673 |
| Zileuton | Sigma | Z4277 |
| Selenium | Sigma | 229865 |
| Primary Antibodies | Dilution | Species | Company | Cat # (WB) |
|---|---|---|---|---|
| TXNIP | 1:1000 | Rabbit | Cell Signaling (Danvers, MA) | 14714S |
| SOD1 | 1:1000 | Rabbit | Proteintech (Tucson, AR) | 10269-1-AP |
| Trx1 | 1:500 | Rabbit | Santa Cruz | SC-20146 |
| ZO-1 | 1:1000 | Rabbit | Cell Signaling | #13663 |
| Tubulin | 1:1000 | Rabbit | Cell Signaling | 2144S |
| Aco1 | 1:1000 | Mouse | Proteintech | 12406-1- AP |
| NCOA4 | 1:1000 | Rabbit | Cell Signaling | 66849S0 |
| Ferritin L | 1:3000 | Rabbit | Proteintech | 10727-1-AP |
| LC3B | 1:3000 | Rabbit | Pierce (Rockville, IL) | L10382 |
| Secondary Antibodies | Dilution | C Company | Cat # |
|---|---|---|---|
| Anti-mouse 2°ry antibody HRP | 1:5000 (WB) | S Sigma | A5278 |
| Anti-rabbit 2°ry antibody HRP | 1:5000 (WB) | S Sigma | A6154 |
| Anti-goat 2°ry antibody HRP | 1:5000 (WB) | S Santa Cruz | sc-2020 |
| IF: | |||||
|---|---|---|---|---|---|
| ZO-1 Primary Ab | 1:200 | (IF) | Rabbit | Cell Signaling | #13663 |
| Anti-rabbit 2°ry Ab | 1:400 | (IF) | Goat | Jackson | 111-585-176 |
| Alexa Fluor 594 | (West Grove, PA) |
Results and Discussion
High glucose alters redox and tight junction protein expression in ARPE-19. We maintained ARPE- 19 cells under LG or HG for 5 days as previously described [33]. As results, on Western blots, we observe that HG significantly increases TXNIP expression, a pro-oxidant protein, when compared to LG while that of the antioxidant enzymes Cu/Zn superoxide dismutase 1 (SOD1) and Trx1 are reduced, indicating cellular redox stress (Figure 1A & 1B). Similarly, the level of tight junction protein, zona occludens 1 (ZO-1), is also reduced under HG both in protein and mRNA levels (Figure 1C–1E). Similar observation is also seen in immunofluorescence staining of ZO-1 in plasma membranes under HG versus LG (Figure 1F, upper panel). Interestingly, azaserine, an inhibitor of the hexosamine biosynthesis pathway and of TXNIP [25], restore ZO-1 plasma membrane staining under HG (Figure 1F, lower panel), indicating that TXNIP may play a role in RPE tight junction leakage and oBRB dysfunction in DR (Figure 1).
Figure 1:
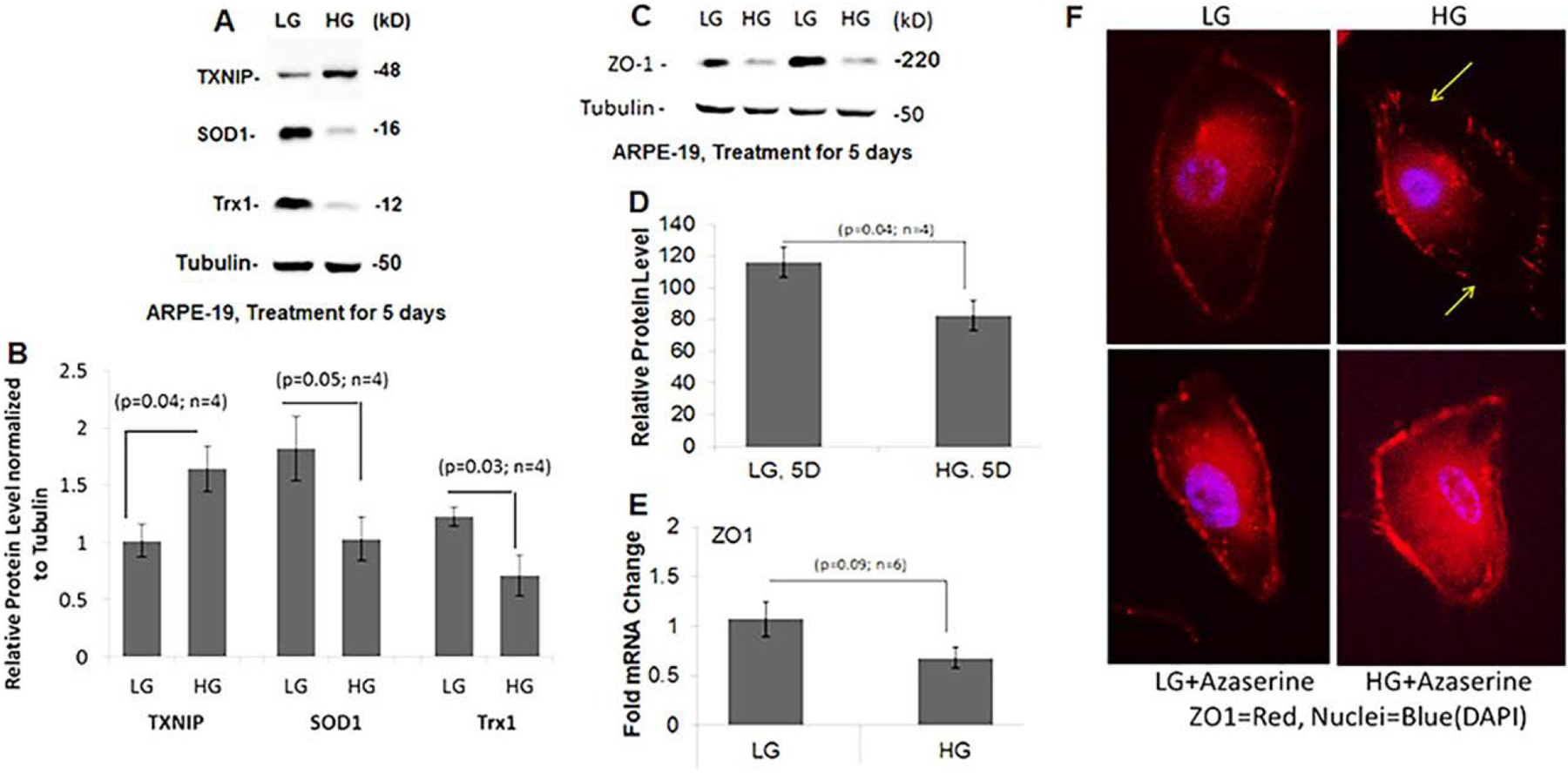
High glucose mediates aberrant redox and tight junction protein expression in APRE-19.
ARPE- 19 cells were maintained in DMEM/F-12 medium containing 2% serum with antibiotics containing 5.5mM glucose (LG) or 25mM glucose (HG) for 5 days like those described previously (33). Western blotting and QPCR detected protein and mRNA levels. Data are presented as SEM+/−SE and p value of <0.05 is considered significant when compared between LG and HG using Student’s t-test.
(A-B) TXNIP level is increased in HG compared to LG (p<0.04) while that SOD1 and Trx1 are down regulated significantly. Tubulin is used as a control protein.
(C-E) Protein and mRNA levels of ZO-1 also decrease in HG compared to LG.
(F) Furthermore, ZO-1 immunostaining (red) is also disrupted at the plasma membrane under HG (yellow arrows) when compared to LG. Furthermore, addition of 2μM azaserine (an inhibitor of TXNIP, ref. 25) in the last 24h of the experiment restores ZO-1 plasma membrane staining in HG. A representative of n=3 is shown here.
High glucose induces mitophagic flux and lysosomal enlargement in ARPE-19. We have previously shown that HG causes mitochondrial dysfunction and mitophagic flux in ARPE-19 and primary human HRPE in confocal live cell imaging using the mitochondrial matrix targeted mitophagic probe, mt-Keima [33,34]. Mt-Keima is a coral protein, which emits green light in mitochondrial matrix (neutral or alkaline pH) while it emits red light in acidic lysosomes (pH 4.5–5) after mitophgic flux [35,36]. However, mt-Keima red emission loses if the cells are fixed because the lysosomal acidic environment is disturbed, therefore, emits green. Hence, in this study, we employed co-transduction of adenoviral vectors bearing mt-Keima and LAMP1-mCherry (lysosomal membrane probe, red) in ARPE-19 cells under LG or HG for 5 days. After that, the cells were fixed and imaged in a confocal microscopy for green and red signals. Mt-Keima in fixed cells emits green both in mitochondria and lysosomes while LAMP1-mCherry emits red in lysosomes [33]; therefore, auto/mitolysosomes appear yellow (combination of green and red) while free mitochondria and lysosomes show green and red, respectively. Using this approach, we demonstrate that ARPE-19 under HG, show a greater number of yellow and enlarged autolysosomes than in LG (Figure 2 inset - arrowheads and arrows), suggesting that HG causes mitophagic flux and lysosomal enlargement in RPE cells. This dual labelling of mt-Keima and LAMP1-mCherry may employ in fixed retinal tissues in in vivo studies.
Figure 2:
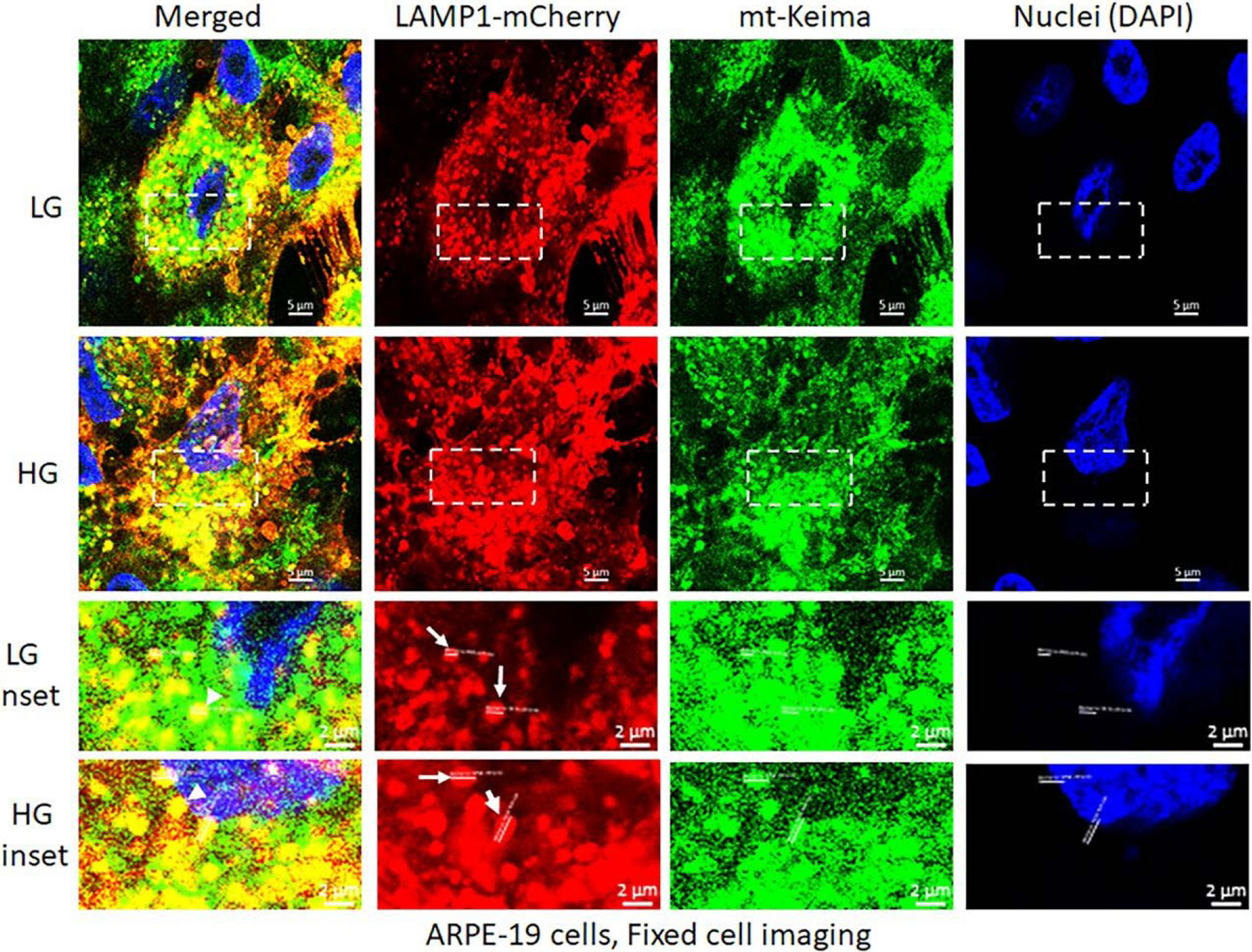
High glucose induces mitophagic flux and lysosome enlargement in ARPE-19 cells. Transduction of mt-Keima and LAMP1-mCherry bearing adenovirus vectors in APRE-19 cells were described previously [33]. Mt-Keima and LAMP1-mCherry vectors were transfected together in ARPE-19 cells and incubated with LG (5.5mM) or HG (25mM glucose) for 5 days, then the cells were fixed, mounted on DAPI containing mounting medium to stain nuclei and imaged with a Zeiss Confocal microscopy at 630x magnification. The images were analyzed by Zen 3.0 blue software and compile in Adobe Photoshop. Under these experimental conditions, mt-Keima emits green both in mitochondria and lysosomes while LAMP1-mCherry emits red in lysosomes. With HG treatment, lysosome sizes are ~1.5- to 2-folds larger than in LG (mCherry, white arrows in insets) while there are also more yellow mito-lysosomes in HG versus LG (green and red combination, arrowheads in insets). A representative of three experiments is shown here.
Ferritinophagy and ferroptosis occur under HG and iron overloading in ARPE-19. We previously showed that HG-induced TXNIP up-regulation or redox stress by auranofin, which inhibits thioredoxin reductases TrxR1 and TrxR2, in ARPE-19 cells induces mitophagic flux, lysosomal destabilization and NLRP3 inflammasome activation and pyroptosis [34]. Here, we observed that HG causes a reduction in cytosolic aconitase 1 (Aco1), ferritinophagy adaptor NCOA4, and ferritin L levels in ARPE-19, suggesting ferritinophagic flux (Figure 3A). Similar observation is seen with H2O2 treated ARPE-19, indicating free labile iron may be released under high glucose and oxidative stress environment. To examine if ARPE- 19 undergoes ferroptosis, we treated these cells with 1mM FeSO4 for 24h and examine cell death by LDH release in media. The results show that FeSO4 causes LDH leakage in ARPE-19, indicating iron-dependent cell death by ferroptosis while pre-incubation (2h before FeSO4) ferrostain 1 (Fer-1, 2μM), an inhibitor of ferroptosis, reduces LDH leakage (Figure 3B). In addition, iron chelator deferasirox (DFX, 25μM), Zileuton, ALOX5 inhibitor (Zel, 100nM), and antioxidant Selenium (Se, 160nM) also reduce FeSO4-mediated LDH leakage, suggesting several enzymes may participate in this ferroptotic process (Figure 3C). ALOX5 (arachidonate 5-lipoxygenase) is a free iron-containing dioxygenase that catalyzes peroxidation of arachidonic acid, a polyunsaturated fatty acid (PUFA) [22]. Selenium is an antioxidant that is required for the synthesis of selenoproteins (containing Se-cysteine amino acid), such as GPX4, TrxR1 and TrxR2 [37]. Interestingly, Aco1, which is a 4Fe-4S containing enzyme, catalyzes citrate to isocitrate via cis-aconitase in the cytosol like Aco2 in the mitochondrion [38]. However, under oxidative stress, after losing the 4Fe-4S complex, Aco1 acts as an Iron regulatory protein 1 (IRP1), which binds to the 3’-hairpin loops of TfR1 mRNA and stabilizes to increase TfR1 translation and iron uptake [38,39]. The fact that Aco1 level is down by HG in our study may suggest a protective cellular response from free iron increases in the cytosol via mitophagic and ferrritinophagic flux to lysosomes. These results suggest that RPE cells could undergo ferroptotic cell death under chronic hyperglycemia, oxidative stress, and iron overload due to defects in iron metabolic enzymes, transport and ferritinophagy/mitophagy.
Figure 3:
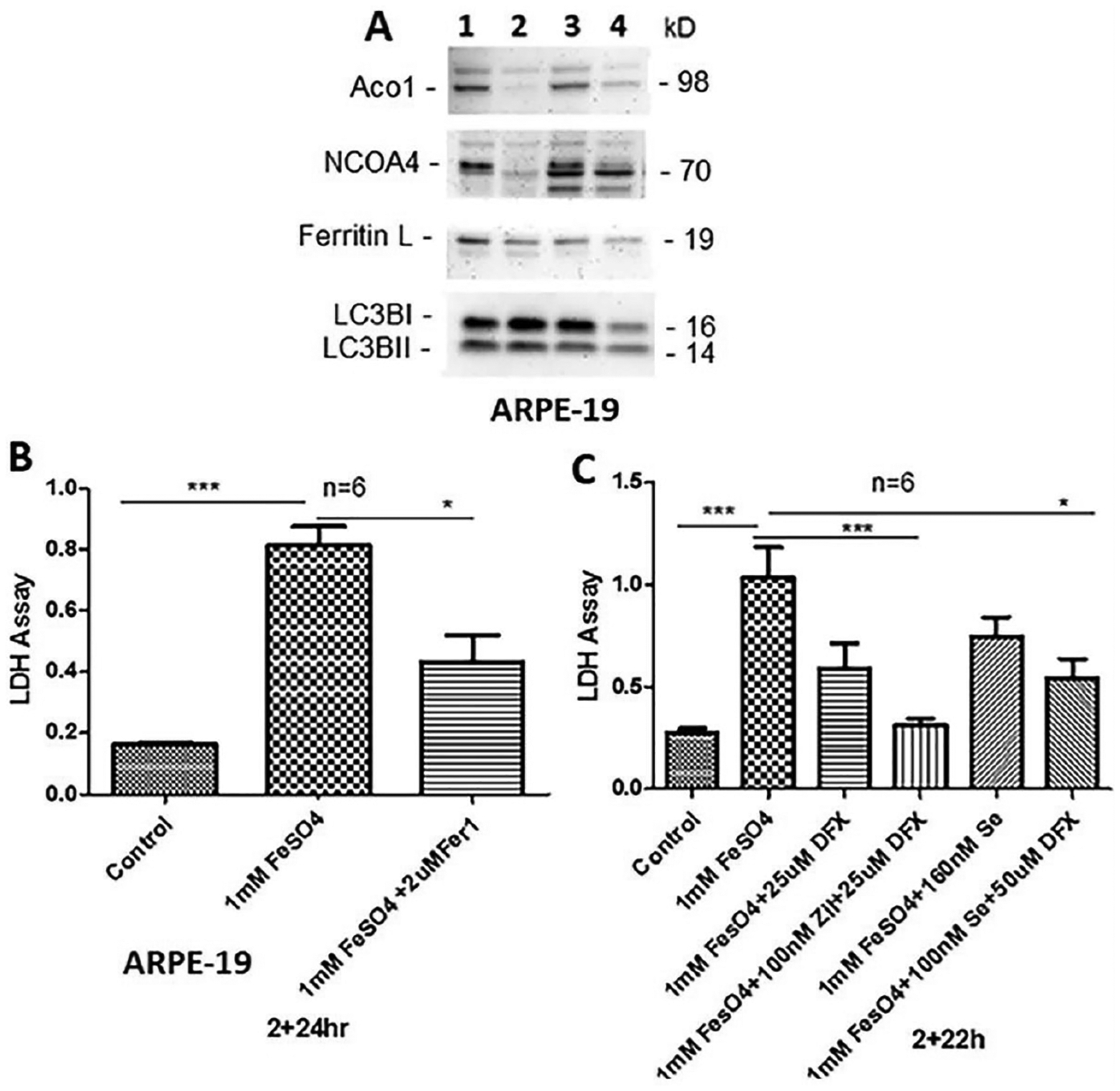
Ferritinophagy and ferroptosis induction by HG and iron-sulfate in ARPE-19.
(A) ARPE-19 cells were cultured in LG (1) or HG (2) for 5 days and LG (3) or HG (4) 5 days with 0.5mM hydrogen peroxide (H2O2) in the last 24h of experiment, cells harvested and proteins (30mg) were detected by Western blotting as previously described [33,34]. Cytosolic aconitase (Aco1), ferritinophagy adaptor - nuclear receptor coactivator 4 (NCOA4), and ferritin light chain (Ferritin L) are reduced in HG and H2O2 indicating ferritinophagy induction. HG increases LC3BI, however, H2O2 reduces both LC3BI and II. The blot is a representative n=2.
(B) Cell death assay by LDH leakage was performed as described before [34]. Fifty microliters of the medium were assayed for the LDH activity using a commercial LDH assay kit from Pierce (Cat# 88953), according to Manufacturer’s instructions. Incubation of ARPE-19 cells with 1mM FeSO4 increases LDH activity ~5 folds (p<0.05) in media from HG than from LG. Preincubation with ferrostatin 1 (Fer-1, a ferroptosis inhibitor) 2h before adding FeSO4 and present throughout the duration of the experiment, reduces LDH leakage significantly, suggesting ferroptotic cell death in ARPE-19.
(C) Similarly, iron chelator deferasirox (25μM DFX), a combination of ALOX-5 inhibitor Zileuton (Zil, 100nM) and DFX (25μM), antioxidant Selenium (Se, 160nM) or a combination of 100nM Se and 50μM DFX reduces LDH activity significantly in ARPE-19 cells, indicating that ferroptosis mechanism may involve various signaling pathways. One-way ANOVA and Bonferroni post hoc test determined differences among means in multiple sets of experiments. Data are presented as SEM+/−SE and p value of <0.05 is considered significant, *<0.01 and ***<0.0001.
Potential pathways for mitophagy, ferritinophagy and ferroptosis interaction in RPE cells under high glucose environment and oxidative stress:
RPE is a single layer of fully differentiated cells that separate the neuroretina from the underlying fenestrated choriocapillaries, forming the oBRB [31,32]. RPE supplies glucose, oxygen and nutrients to photoreceptors while recycling vitamin A (visual pigment). It also phagocytoses photoreceptor outer segment (POS) daily and degrade in lysosomes [40]. Therefore, dysregulation of RPE causes photoreceptor dysfunction and death leading to blindness in various age-related retinal diseases, including DR and AMD [31,32,41]. In this study, we show that (i) high glucose-induced aberrant redox protein expression and oxidative stress in ARPE-19 cause the mitochondrial-lysosomal axis dysregulation and tight junction protein ZO-1 downregulation. (ii) Flux of damaged mitochondria to lysosomes via mitophagy together with activation of ferritinophagy, ferritin autophagy in lysosomes, will result in lysosomal enlargement and subsequent pH increase, lysosomal membrane permeabilization (LMP) and leaking out lysosomal hydrolytic enzymes and undigested materials to the cytosol. One additional event is the release of free labile iron (ferrous Fe2+) from lysosomes due to excess mitophagic flux (carrying mitochondrial Fe-S clusters/heme moieties and mtFerritin, mitochondrial iron storage) and from ferritinophagic flux (degradation of ferritin-Fe3+ cage in lysosomes). These free Fe2+ irons react with H2O2 to generate highly reactive hydroxyl radicals (·OH) via. Fenton reaction [42]. These highly reactive hydroxyl radicals attack membrane lipids by peroxidation, particularly the polyunsaturated fatty acids (PUFA-OOH), thereby damaging membrane integrity and leaking. The only enzyme that can detoxify phospholipid hydroperoxide to hydroxyl moiety is the redox enzyme GPX4 using 2 GSH molecules. GSH itself is one of the major antioxidants in cells, including mitochondrial matrix, which reduces oxidized proteins and scavenge oxygen radicals. Therefore, TXNIP upregulation in RPE cells under high glucose and downregulation of antioxidant proteins ((SOD1, Trx1) (Figure 1)) will lead to generation of oxidized GSSG and depletion of GSH. Glutathione biosynthesis (a tripeptide of γ-L-glutamyl-L-cysteinyl-glycine) by glutathione synthetase requires cysteine amino acid, which is imported intracellularly as cystine (cysteine-cysteine) via the plasma membrane glutamate- cystine exchanger, xCT, which is down-regulated in DR [43,44]. Along with this, mitochondrial dysfunction, mitophagic flux, ferritinophagy and lysosome destabilization will increase ROS generation and labile iron accumulation within the cell and ferroptosis, which is depicted in Figure 4.
Figure 4:
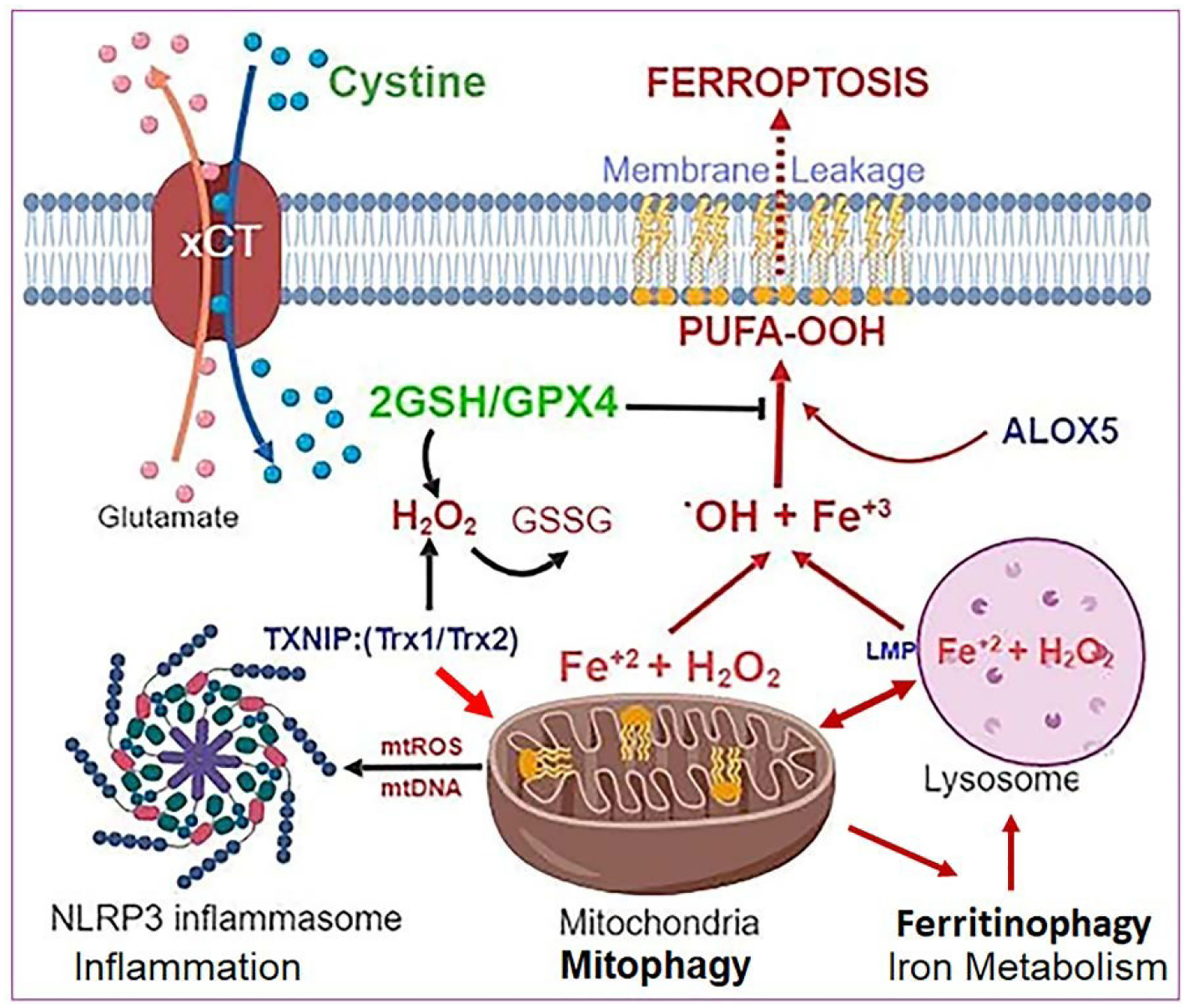
Relationship among Mitophagy, Ferritinophagy and Ferroptosis under oxidative stress. Hyperglycemia-induced TXNIP upregulation and oxidative/nitrosative stress cause mitochondrial damage, ROS generation and mitophagic flux to lysosomes for degradation. A feedback mechanism to replenish mitochondrial mass and bioenergetics may activate ferritinophagy, an autophagic process of degradation and release of free iron Fe2+ from ferritin cages in lysosomes for using in the synthesis of mitochondrial Iron-sulfur cluster and heme moeity. However, the released ferrous iron also reacts with H2O2 via Fenton reaction to generate highly reactive hydroxyl radicals (.OH), and biproducts of hydroxide (OH−) and ferric iron (Fe3+). These .OH radicals react with cell membranes rich in polyunsaturated fatty acids (PUFA) to mediate lipid peroxidation (PUFA-OOH), including the mitochondria, lysosome and plasma membranes. PUFA-OOH in membranes disturb fluidity and membrane integrity. Free Fe2+ may also activate arachidonic 5-lipoxygenase (ALOX5), which generates lipid peroxides (L- OOH). The only enzyme that can remove and prevent lipid peroxidation is GPX4, a selenocysteine protein, using 2 GSH molecules. However, under oxidative stress, GSH level and biosynthesis are down-regulated leading to accumulation of oxidized GSSG. Extracellular cystine (di-cysteine) is transported into cells through plasma membrane glutamate-cystine exchanger (xCT). Down-regulation of xCT, which occurs in DR, will limit cystine import and, therefore, cysteine available for GSH synthesis. Under these conditions, iron-dependent PUFA-OOH due to inhibition of GPX4 activity causes cell death by ferroptosis, a recently identified non-apoptotic cell death. Ferroptosis itself induces an inflammatory response, which assembles the NLRP3 inflammasome, activating caspase-1, IL- 1β/IL-18 and gasdermin-D to cause pyroptotic cell death as well. These events, if unchecked, eventually will sustain a chronic low-grade inflammation, oxidative stress and premature retinal cell death leading to disease initiation and progression of DR and age-related retinal diseases. [Image created with BioRender.com.].
Ferroptosis is a newly defined oxidative cell death mechanism due to iron accumulation and membrane lipid peroxidation due to reduced GSH/GPX4 activity [30]. Ferroptosis itself also is an inflammatory process due to release of cytosolic and nuclear components, such as mitochondrial DNA, single-stranded RNA, mitochondrial heat shock protein HSP60, nuclear HMGB1 and other materials within the cell and extracellularly [45]. Therefore, these cytosolic and nuclear components are recognized by membrane and cytosolic innate immune platforms, such as toll-like receptors (TLR4, TLR7, TLR9, etc.) and inflammasomes, particularly NOD-like NLRP3 and DNA sensing AIM2 [46]. Subsequent activation of caspase-1, IL-1β/IL-18 and gasdermin-D influences oxidative stress and inflammatory cell death by pyroptosis [46,47]. In fact, we demonstrated that auranofin-induced redox stress and mitochondrial dysfunction in ARPE-19 cells cause NLRP3 inflammasome activation and pyroptosis [34]. Moreover, triple combination drug treatment comprising of amlexanox (TBK1/IKKα inhibitor), SS-31 (mitochondria-targeted antioxidant) and tranilast (an inhibitor of NLRP3) reduces redox stress-mediated mitophagic flux, lysosome enlargement and cell death [34]. Therefore, we further propose that targeting TXNIP and the mitochondrial-lysosomal axis dysfunction will also prevent high glucose-induced redox stress, mitochondrial dysfunction, and cell death. To support this hypothesis, we recently found that a triple combination treatment using TXNIP-IN1 (TXNIP-Trx interaction inhibitor), mito-TEMPO (mitochondrial antioxidant) and ML-SA1 (lysosomal transient calcium release channel Mucolipin 1 (MCOLN1/TRPML1), which activates calcineurin phosphatase-lysosomal transcription factor TFEB, prevents high glucose- induced mitochondrial dysfunction, mitophagic flux and lysosome destabilization in a rat retinal Müller cell line, rMC1 (data not presented). TFEB is important for lysosomal biogenesis, autophagic gene expression and mitogenesis via activation of PGC1α expression, which encompasses the Coordinated Lysosomal Expression and Regulation (CLEAR) gene network [48,49].
In conclusion, a slow mitophagy will cause accumulation of damaged mitochondria, which produce less ATP but generate ROS while too fast a mitophagic flux will result in deficiencies in mitochondrial number, bioenergetics and lysosomal overloading and reduced digestive capacity. Therefore, future studies will require identifying an optimal mitophagic flux, which also maintains ferritinophagy and mitochondrial and lysosomal biosynthesis [34,50]. Working out the potential roles of mitophagy, ferritinophagy and lysosome destabilization and organelle communication in retinal cell death by ferroptosis/pyroptosis (including photoreceptor and RPE in DR and age-related retinal diseases) will be helpful in developing combination and organelle targeted drug and gene therapy modalities.
Acknowledgement
Funding for this work was supported by the grants NIH/NEI EY023992 (LPS), Research to Prevent Blindness (RPB to OVAS), and Core Grant P30 EY004068 (OVAS).
References
- 1.Mailloux RJ (2018) Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxid Med Cell Longev 7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickles S, Pierre Vigié P, Youle RJ (2018) Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol. 28(4): R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank M, Duvezin Caubet S, Koob S, Occhipinti A, Jagasia R, et al. (2012) Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 1823(12): 2297–2310. [DOI] [PubMed] [Google Scholar]
- 4.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, et al. (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191(5): 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong YC, Holzbaur EL (2014) Optineurin is an autophagy receptor for damaged mitochondria in parkin- mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA 111(42): E4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, et al. (2016) Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol 215(6): 857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman LC, Ting JP (2016) The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem 136 Suppl 1: 29–38. [DOI] [PubMed] [Google Scholar]
- 8.Thounaojam MC, Jadeja RN, Warren M, Powell FL, Raju R, et al. (2019) MicroRNA-34a (miR-34a) Mediates Retinal Endothelial Cell Premature Senescence through mitochondrial Dysfunction and Loss of Antioxidant Activities. Antioxidants (Basel) 8(9):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maio N, Rouault TA (2020) Outlining the Complex Pathway of Mammalian Fe-S Cluster Biogenesis. Trends Biochem Sci 45(5): 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theil EC (2013) Ferritin: the protein nanocage and iron biomineral in health and in disease. Inorg Chem 52(21): 12223–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn P, Dentchev T, Qian Y, Rouault T, Harris ZL, et al. (2004) Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol Vis 10: 598–607. [PubMed] [Google Scholar]
- 12.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509(7498): 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimaki M, Furuya N, Saiki S, Amo T, Imamichi Y, et al. (2019) Iron Supply via NCOA4-Mediated Ferritin Degradation Maintains Mitochondrial Functions. Mol Cell Biol 39(14): e00010–00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seguin A, Jia X, Earl AM, Li L, Wallace J, et al. (2020) The mitochondrial metal transporters mitoferrin1 and mitoferrin2 are required for liver regeneration and cell proliferation in mice. J Biol Chem. 295(32): 11002–11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzollo F, More S, Vangheluwe P, Agostinis P (2021) The lysosome as a master regulator of iron metabolism. Trends Biochem Sci S0968-0004(21): 00159–00160. [DOI] [PubMed] [Google Scholar]
- 16.Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS (2008) 5-Lipoxygenase, but not 12/15- lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 57(5): 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard E, Daruich A, Youale J, Courtois Y, Behar-Cohen F (2020) From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology. Cells 9(3): 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finazzi D, Arosio P (2014) Biology of ferritin in mammals: an update on iron storage, oxidative damage, and neurodegeneration. Arch Toxicol, pp. 1787–1802. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi T, Kuwahara T, Sakurai M, Komori T, Fujimoto T, et al. (2018) LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc Natl Acad Sci USA 115(39): E9115–E9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorojod RM, Alaimo A, Porte Alcon S, Pomilio C, Saravia F, et al. (2015) The autophagic-lysosomal pathway determines the fate of glial cells under manganese- induced oxidative stress conditions. Free Radic Biol Med 87: 237–251. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Kim WK, Bae KH, Lee SC, Lee EW (2021) Lipid Metabolism and Ferroptosis. Biology (Basel) 10(3): 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun QY, Zhou HH, Mao XY (2019) Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid Med Cell Longev 2749173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, Chen Z, Zhang H, Chen C, Zeng M, et al. (2021) Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol 22(9): 1127–1139. [DOI] [PubMed] [Google Scholar]
- 24.Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP (2009) Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol 221(1): 262–272. [DOI] [PubMed] [Google Scholar]
- 25.Perrone L, Devi TS, Hosoya Kl, Terasaki T, Singh LP (2010) Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis 1: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh LP (2013) Thioredoxin Interacting Protein (TXNIP) and Pathogenesis of Diabetic Retinopathy. J Clin Exp Ophthalmol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng DW, Jiang Y, Shalev A, Kowluru R, Crook ED, et al. (2006) An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem 112: 189–218. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, et al. (2014) Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 4: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devi TS, Hosoya K, Terasaki T, Singh LP (2013) Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res 319: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang D, Kroemer G (2020) Ferroptosis. Curr Biol 30(21): R1292–R1297. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Xu CL, Breazzano MP, Tanaka AJ, Ryu J, et al. (2020) Progressive RPE atrophy and photoreceptor death in KIZ-associated autosomal recessive retinitis pigmentosa. Ophthalmic Genet 41(1): 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarchick MJ, Cutler AH, Trobenter TD, Kozlowski MR, Makowski ER, et al. (2019) Endogenous insulin signaling in the RPE contributes to the maintenance of rod photoreceptor function in diabetes. Exp Eye Res 180: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devi TS, Yumnamcha T, Yao F, Somayajulu M, Kowluru RA, et al. (2019) TXNIP mediates high glucose- induced mitophagic flux and lysosome enlargement in human retinal pigment epithelial cells. Biol. Open 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yumnamcha T, Devi TS, Singh LP (2019) Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci 13: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh LP, Devi TS, Yumnamcha T (2017) The Role of Txnip in Mitophagy Dysregulation and Inflammasome Activation in Diabetic Retinopathy: A New Perspective. JOJ Ophthalmol 4: 555643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun N, Malide D, Liu J, Rovira II, Combs CA, et al. (2017) A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-Keima. Nat Protoc 12(8):1576–1587. [DOI] [PubMed] [Google Scholar]
- 37.Schweizer U, Bohleber S, Zhao W, Fradejas Villar N (2021) The Neurobiology of Selenium: Looking Back and to the Future. Front Neurosci 15:652099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khodagholi F, Shaerzadeh F, Montazeri F (2018) Mitochondrial Aconitase in Neurodegenerative Disorders: Role of a Metabolism-related Molecule in Neurodegeneration. Curr Drug Targets 19(8):973–985. [DOI] [PubMed] [Google Scholar]
- 39.Rouault TA, Maio N Biogenesis, and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J Biol Chem 292(31): 12744–12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storm T, Burgoyne T, Futter CE (2017) Membrane trafficking in the retinal pigment epithelium at a glance. J Cell Sci 133(16): jcs238279. [DOI] [PubMed] [Google Scholar]
- 41.Tonade D, Kern TS (2021) Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog Retin Eye Res 83:100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordan R, Wongjaikam S, Gwathmey JK, Chattipakorn N, Chattipakorn SC, et al. (2018) Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update. Heart Fail Rev 23(5): 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Shanmugam A, Markand S, Zorrilla E, Ganapathy V, et al. (2015) 1 receptor regulates the oxidative stress response in primary retinal Muller glial cells via NRF2 signaling and system xc(−), the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med 86: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpi-Santos R, Ferreira MJ, Pereira Netto AD, Giestal-de-Araujo E, Ventura ALM, et al. (2016) Early changes in glutamate-cystine exchanger system [Formula: see text] and glutathione in the retina of diabetic rats. Exp Eye Res 146: 35–42. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Li Y, Wu Z, Wu Z, Fang H (2021) Diabetic ferroptosis plays an important role in triggering on inflammation in diabetic wound. Am J Physiol Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18(9): 2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al Mamun A, Mimi AA, Zaeem M, Wu Y, Monalisa I, et al. (2021) Role of pyroptosis in diabetic retinopathy and its therapeutic implications. Eur J Pharmacol 904: 174166. [DOI] [PubMed] [Google Scholar]
- 48.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, et al. (2009) A gene network regulating lysosomal biogenesis and function. Science 325(5939):473–477. [DOI] [PubMed] [Google Scholar]
- 49.Pan HY, Alamri AH, Valapala M (2019) Nutrient deprivation and lysosomal stress induce activation of TFEB in retinal pigment epithelial cells. Cell Mol Biol Lett 24: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Li J, Kang R, Tang D (2020) Interplay between lipid metabolism and autophagy. Front Cell Dev Biol 8: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]


