Abstract
Background
South Africa reported a notable increase in COVID-19 cases from mid-November, 2021, onwards, starting in Tshwane District, which coincided with the rapid community spread of the SARS-CoV-2 omicron (B.1.1.529) variant. This increased infection rate coincided with a rapid increase in paediatric COVID-19-associated admissions to hospital (hereafter referred to as hospitalisations).
Methods
The Tshwane Maternal-Child COVID-19 study is a multicentre observational study in which we investigated the clinical manifestations and outcomes of paediatric patients (aged ≤19 years) who had tested positive for SARS-CoV-2 and were admitted to hospital for any reason in Tshwane District during a 6-week period at the beginning of the fourth wave of the COVID-19 epidemic in South Africa. We used five data sources, which were: (1) COVID-19 line lists; (2) collated SARS-CoV-2 testing data; (3) SARS-CoV-2 genomic sequencing data; (4) COVID-19 hospitalisation surveillance; and (5) clinical data of public sector COVID-19-associated hospitalisations among children aged 13 years and younger.
Findings
Between Oct 31 and Dec 11, 2021, 6287 children and adolescents in Tshwane District were recorded as having COVID-19. During this period, 2550 people with COVID-19 were hospitalised, of whom 462 (18%) were aged 19 years or younger. The number of paediatric cases was higher than in the three previous SARS-CoV-2 waves, uncharacteristically increasing ahead of adult hospitalisations. 75 viral samples from adults and children in the district were sequenced, of which 74 (99%) were of the omicron variant. Detailed clinical notes were available for 138 (75%) of 183 children aged ≤13 years with COVID-19 who were hospitalised. 87 (63%) of 138 children were aged 0–4 years. In 61 (44%) of 138 cases COVID-19 was the primary diagnosis, among whom symptoms included fever (37 [61%] of 61), cough (35 [57%]), shortness of breath (19 [31%]), seizures (19 [31%]), vomiting (16 [26%]), and diarrhoea (15 [25%]). Median length of hospital stay was 2 days [IQR 1–3]). 122 (88%) of 138 children with available data needed standard ward care and 27 (20%) needed oxygen therapy. Seven (5%) of 138 children were ventilated and four (3%) died during the study period, all related to complex underlying copathologies. All children and 77 (92%) of 84 parents or guardians with available data were unvaccinated to COVID-19.
Interpretation
Rapid increases in paediatric COVID-19 cases and hospitalisations mirror high community transmission of the SARS-CoV-2 omicron variant in Tshwane District, South Africa. Continued monitoring is needed to understand the long-term effect of the omicron variant on children and adolescents.
Funding
South African Medical Research Council, South African Department of Science & Innovation, G7 Global Health Fund.
Introduction
On Nov 24, 2021, the Network for Genomic Surveillance in South Africa (NGS-SA) announced a new variant of SARS-CoV-2, which was first detected in a sample collected on Nov 9, 2021; this variant was later designated by the WHO Technical Advisory Group on SARS-CoV-2 Evolution as omicron (B.1.1.529).1, 2, 3, 4 This announcement by the NGS-SA coincided with a noticeable increase in new SARS-CoV-2 infections in the Gauteng Province of South Africa, heralding the start of country's fourth wave of the COVID-19 epidemic. The virus genome of the omicron variant differs substantially from the delta (B.1.617.2) variant, which dominated the third COVID-19 wave in South Africa. The omicron viral genomic sequence includes 26–32 spike protein changes and 45–52 amino acid changes in total compared with the original strain, causing worldwide concern regarding possible immune evasion.5
Clinical characteristics and disease profiles of paediatric (ie, aged 0–19 years) COVID-19 cases in South Africa in the first three waves of the epidemic—namely, between June and September, 2020 (first wave); November, 2020, and February, 2021 (second wave; beta [B.1.351] variant predominance); and June and October, 2021 (third wave; delta variant predominance)—have been broadly similar to descriptions in international literature, ranging from asymptomatic to mild-to-moderate disease in most children.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Research in context.
Evidence before this study
The SARS-CoV-2 variant omicron (B.1.1.529) was first reported on Nov 24, 2021. Clinical characteristics and disease profiles of children with COVID-19 disease of previous variants have been described in the literature.
Added value of this study
Here we describe trends in paediatric SARS-CoV-2 test positivity rates and paediatric COVID-19-associated hospitalisations, together with clinical characteristics, in Tshwane District, Gauteng Province, South Africa—one of the first known epicentres of the SARS-CoV-2 omicron outbreak—providing an overview on how this variant affected a paediatric population in a middle-income country at the beginning of the fourth wave of the COVID-19 epidemic.
Implications of all the available evidence
Our data suggest that the SARS-CoV-2 omicron variant is highly transmissible but mostly causes mild-to-moderate disease in children (aged ≤13 years). We provide novel paediatric data in the context of SARS-CoV-2 omicron variant, to assist with global preparation towards mitigating the impact of this variant across paediatric settings.
Therefore, an increase in paediatric admissions to hospital due to COVID-19, starting from mid-November, 2021, in the Tshwane District (an urban district in the Gauteng Province) was of substantial concern. As of 2020, the district has an estimated population of 3 552 452 people and a population density of 527 people per km2.19 There are nine public sector general hospitals serving the population, of whom an estimated 73% are uninsured, including two central, one academic, one regional, and five district hospitals. Additionally, the insured population has access to networks of private hospitals within the district.
We aimed to describe the incidence of paediatric hospital admissions (hereafter referred to as hospitalisations) due to COVID-19 in 38 hospitals in the Tshwane District of South Africa at the start of the fourth COVID-19 wave, with a particular focus on detailed clinical manifestations and outcomes in the subgroup of hospitalised children (aged ≤13 years) with COVID-19 admitted to public sector hospitals.
Methods
Study design and population
In this multicentre observational study (The Tshwane Maternal-Child COVID-19 study), we collated paediatric COVID-19-related data (ie, for children aged ≤19 years) during the early stages of the fourth COVID-19 wave in South Africa. Our key population of interest was children aged ≤13 years who had been admitted to any of 38 hospitals in Tshwane District during this period. We also collected data for adolescents aged 14 to 19 years, and adults older than 19 years, for comparison.
The Tshwane Maternal-Child COVID-19 research study received permission from the ethics committees of both Health Sciences Faculties in Tshwane (University of Pretoria [reference number 822/2020] and Sefako Makgatho Health Sciences University [reference number SMUREC/M/54/2021:IR]), together with the Tshwane District Research Committee. Additionally, three large public sector hospitals (Steve Biko Academic Hospital, Kalafong Academic Hospital, and George Mukhari Academic Hospital) are part of the SA COVID KIDS study and have received ethics approval from the South African Medical Research Council (reference number EC048-11/2020). Ethics approval was also obtained for sequencing of COVID-19 samples (University of Pretoria Ethics Committee, reference number 101/2017). Individual patient consent was not needed for the genome samples at is part of ongoing routine surveilance.
Data sources and definitions
We extracted data relevant to the Tshwane District from the following sources: district-based COVID-19 line lists for contact tracing activities (totals, with age breakdowns); SARS-CoV-2 testing data collated by the National Institute for Communicable Diseases (NICD); SARS-CoV-2 genomic sequencing data from samples obtained within the district through the Zoonotic Arbo and Respiratory Virus Research Group (ZARV) at the Department of Medical Virology, National Health Laboratory Services (NHLS), University of Pretoria (Pretoria, South Africa) and from the NGS-SA from the global reference database for SARS-CoV-2 viral genomes, Global Initiative on Sharing Avian Influenza Data (GISAID); COVID-19 hospitalisation data (DATCOV hospital surveillance system, collated by the NICD); and clinical data of public sector paediatric (ie, aged ≤13 years) hospitalisations due to COVID-19, collected for the SA COVID Kids study and for local planning of paediatric clinical services.
SARS-CoV-2 testing data were extracted from the NICD for adults, adolescents, and children for the period March 1, 2020, to Dec 5, 2020. A laboratory-confirmed COVID-19 case was defined as any person who tested positive for SARS-CoV-2 on either a real-time RT-PCR (rRT-PCR) or an antigen test using samples obtained from nasopharyngeal or oropharyngeal swabs, with testing done at NHLS laboratories located in four public sector hospitals in the Tshwane District. Access to SARS-CoV-2 testing was not substantially constrained; increased access to rapid antigen testing for enhanced speed of diagnosis was the most important change in COVID-19 testing practices since Oct 5, 2021.
For genomic sequencing, we used clinical samples from adults and children from public sector clinics and hospitals in Tshwane District submitted to NHLS for SARS-CoV-2 rRT-PCR testing. Positive cases among adult and paediatric patients from Nov 7 to 29, 2021 (epidemiological weeks 45–48), were collected by ZARV staff for genome sequencing. SARS-CoV-2-positive samples with crossing point threshold values (ct) of 30 or less were sent for next-generation sequencing at the Research Innovation and Sequencing Platform at the University of KwaZulu-Natal (Durban, South Africa), as part of the NGS-SA initiative. Sequences were submitted to GISAID and assigned to lineages.
Regarding the DATCOV surveillance system, 38 hospitals in Tshwane District were submitting hospitalisation data during our study period, including all nine public sector hospitals and 25 private sector hospitals (as of Dec 11, 2021). Due to restructuring during the COVID-19 pandemic, one public sector district hospital was closely linked to its adjacent public sector central hospital and subsequently counted as one academic hospital complex for the purposes of this study. The dataset includes information on patient numbers, age groups, sex, level of hospital care, length of stay, and patient outcomes. Although the DATCOV system provides overall disease surveillance, it does not provide detailed clinical data. Therefore, we collated clinical data from treating clinicians and hospital files to supplement and verify the DATCOV data, including clinical presentation, diagnoses, management, and outcomes. Symptoms were analysed in a subgroup of children (aged ≤13 years) with primary SARS-CoV-2 infection.
A COVID-19-associated hospitalisation was defined by DATCOV as any person who tested positive for SARS-CoV-2 and was admitted to hospital, regardless of the reason for hospitalisation. Hospitals in the South African public health sector are divided into different levels of care; however, for geographical reasons, care is often first accessed at the closest hospital and not necessarily linked to disease severity. For descriptive purposes, we grouped public sector hospitals into central and academic hospitals (n=3) and regional and district hospitals (n=5) with high care and intensive care services available at central and academic hospitals, and with standard inpatient care (including oxygen therapy, fluid management, and antibiotics) available at all levels. Children who were referred from another hospital were classed as being at the higher level hospital, irrespective of the reason for referral, to avoid being included twice in the dataset.
Statistical analysis
The DATCOV surveillance system reports on children and adolescents aged 19 years and younger. We used descriptive statistics, including mean (SD), ranges, median (IQR), and counts and proportions to describe demographic characteristics for tests, cases, and hospitalisations, and further stratified these data by age group (<1 year; 1–4 years; 5–9 years; 10–14 years; 15–19 years). We calculated the crude admission rate as the number of admissions in different age groups as a proportion of the population (as per Statistics South Africa mid-year population estimates for 2020),19 and present these data as admissions per 1 000 000 people, by age and week of admission. For our in-depth analysis of clinical features, we assessed data for all paediatric patients (aged ≤13 years) admitted to public sector hospitals who had detailed clinical notes from their treated physician. We also did subgroup analyses within this population by primary diagnosis on admission.
Role of the funding source
The funders did not have a role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
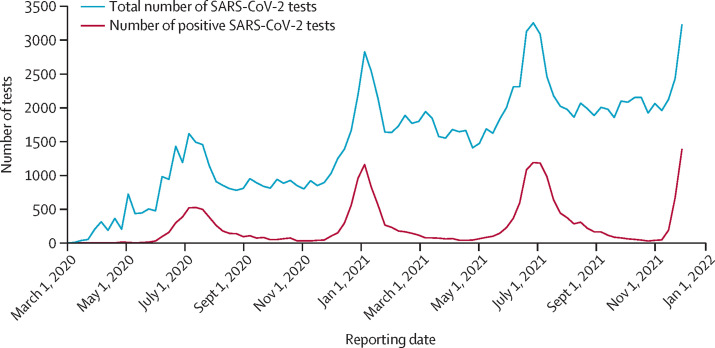
In adults, adolescents, and children, since March, 2020, SARS-CoV-2 testing volumes have substantially increased, especially since the second wave and between waves (figure 1 ). During each wave of the epidemic, the number of positive tests increased sharply, as expected, together with the proportion test positivity, with a more pronounced increase in test positivity seen during the fourth wave (appendix p 1).
Figure 1.
Total weekly SARS CoV-2 testing numbers at four public sector hospital-based laboratories in Tshwane District, March 1, 2020, to Dec 15, 2020
Data are for adults, adolescents, and children. SARS-CoV-2 testing was done with either real-time RT-PCR or antigen tests.
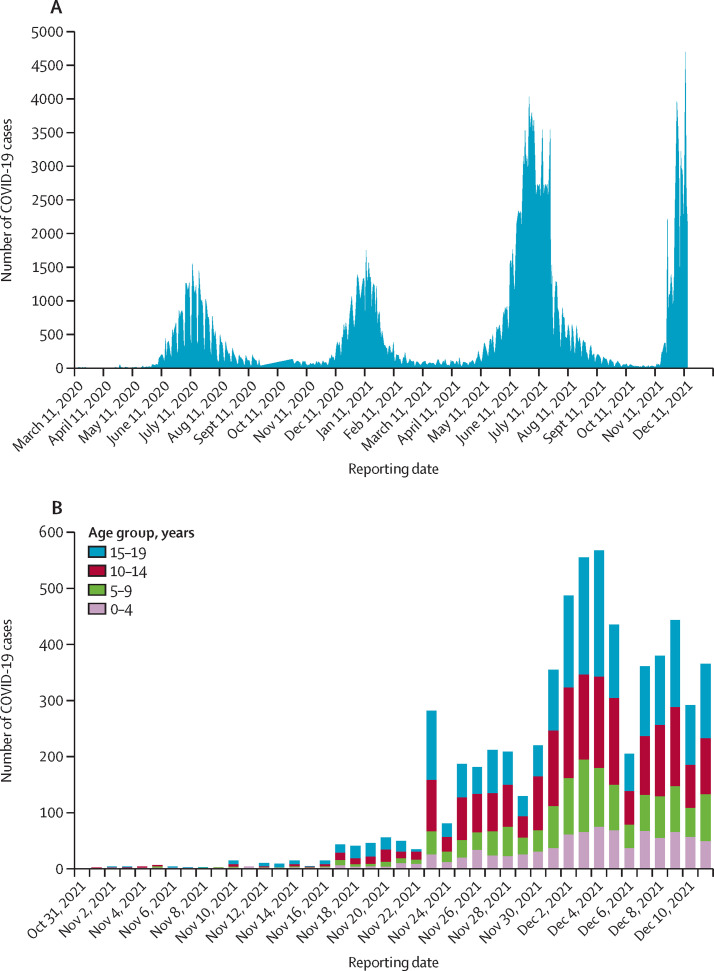
The weekly SARS-CoV-2 test numbers, laboratory-confirmed COVID-19 cases, and COVID-19-associated hospitalisations among residents in Gauteng Province, where Tshwane District is located, started increasing rapidly from week 45 (Nov 7, 2021), 8 weeks after the end of the third wave (week 37; Oct 5, 2021; appendix p 2). As of Dec 11, 2021, the district had recorded 202 548 cumulative COVID-19 cases, with 36 152 COVID-19-associated hospital admissions and 7132 COVID-19-associated deaths across the district's four epidemic curves since March, 2020 (figure 2A ). The period from Nov 7 to Dec 15, 2021, is considered as the fourth wave, and is characterised by the steepest increase in percentage test positivity of all the waves, increasing rapidly to above 40% (appendix p 1).
Figure 2.
Daily COVID-19 cases in the Tshwane District for the total population (March 11, 2020, to Dec 11, 2021; A) and for children and adolescents (aged ≤19 years; Oct 31 to Dec 11, 2021; B)
Data are from the Tshwane District COVID-19 line list.
Between Oct 31 and Dec 11, 2021 (ie, epidemiological weeks 44–49), 6287 paediatric laboratory-confirmed COVID-19 cases were recorded on the Tshwane District COVID-19 line list (ages 0–4 years: 869 cases; 5–9 years: 1231 cases; 10–14 years: 2023 cases; and 15–19 years: 2164 cases; figure 2B).
Genomic sequencing was done on 75 SARS-CoV-2-positive samples from adult and paediatric patients from public sector hospitals and clinics in Tshwane District taken between Nov 7 and 29, 2021 (weeks 45–48). 74 (99%) cases were determined to be due to the omicron variant and one (1%) was determined to be due to the delta variant. This finding suggests that the rapid increase in cases in the region was probably driven by the omicron variant. The earliest detection of omicron in the district was on Nov 12, 2021.
COVID-19-associated hospitalisations increased rapidly alongside the notable increase in cases in the district (appendix p 2). Although overall COVID-19-associated hospitalisation numbers have, as of Dec 11, 2021, been lower than in the previous three waves (appendix p 2), the number of COVID-19-associated hospitalisations in children and adolescents (aged ≤19 years) has increased beyond what was seen in the first three waves (appendix p 2). The overall number of COVID-19-associated hospital admissions between Oct 31 and Dec 11, 2021, was 2550, of whom 462 (18%) were aged 19 years or younger. The increase in paediatric COVID-19-associated hospitalisations coincided with the increase in SARS-CoV-2 tests and test positivity, with both occurring in week 46. This increase in paediatric COVID-19-associated hospitalisations (aged ≤13 years) was seen in both the private and public sector hospitals (appendix p 2), with 183 such admissions reported in the public sector and 281 in the private sector during the 6-week study period. The number of COVID-19-associated hospitalisations (overall and among those aged ≤19 years) showed a substantial decrease in the last week of the study (week 49; Dec 5–11, 2021; appendix p 2).
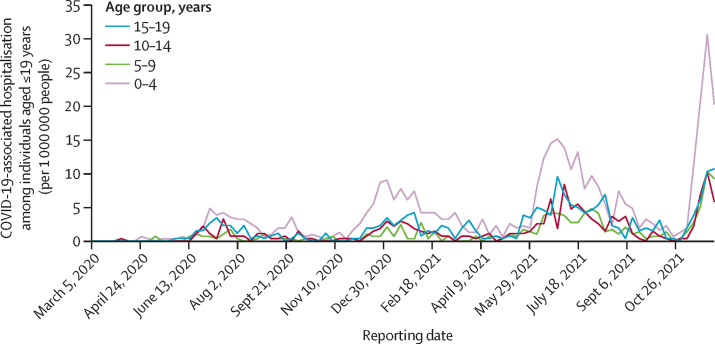
Further analysis of the incidence of COVID-19-associated hospital admissions showed that, although in the first three waves paediatric admissions (aged ≤19 years) lagged adult admissions (figure 3 ; appendix p 3), this pattern was reversed in the fourth wave, particularly in the age 0–4 years group. We also found that the incidence of hospital admissions among children aged 10–19 years were lower than among those aged 0–9 years despite the corresponding higher number of COVID-19 cases among this age group than among the younger age groups.
Figure 3.
Weekly COVID-19-associated hospitalisation incidence for children and adolescents (ie, aged ≤19 years), in the Tshwane District, by age group, March 1, 2020, to Dec 11, 2021
Data are from the 38 hospitals in the district.
Table 1 summarises data from both DATCOV and the verified clinical information for the 183 paediatric COVID-19-associated hospitalisations (aged ≤13 years) to general public sector hospitals. These data shows a rapid increase in weekly hospitalisations from Nov 14, 2021, with numbers tapering off in the last week under review. These data indicate that the peak in paediatric COVID-19-associated hospitalisations had likely been reached in the district by this timepoint.
Table 1.
Hospital admissions among children (aged ≤13 years) with COVID-19 in general public sector hospitals in Tshwane District, by week, Oct 31 to Dec 11, 2021
| Week 44: Oct 31 to Nov 6, 2021 | Week 45: Nov 7–13, 2021 | Week 46: Nov 14–20, 2021 | Week 47: Nov 21–27, 2021 | Week 48: Nov 28 to Dec 4, 2021 | Week 49: Dec 5–11, 2021 | Total | |
|---|---|---|---|---|---|---|---|
| Central and academic hospitals | |||||||
| Hospital 1 | 1 | 1 | 8 | 18 | 15 | 9 | 52 |
| Hospital 2 | 0 | 1 | 3 | 5 | 11 | 13 | 33 |
| Hospital 3 | 0 | 0 | 2 | 13 | 12 | 7 | 34 |
| Regional and district hospitals | |||||||
| Hospital 1 | 1 | 1 | 0 | 5 | 3 | 5 | 15 |
| Hospital 2 | 0 | 0 | 9 | 7 | 5 | 1 | 22 |
| Hospital 3 | 2 | 0 | 0 | 3 | 6 | 0 | 11 |
| Hospital 4 | 0 | 0 | 2 | 2 | 3 | 1 | 8 |
| Hospital 5 | 0 | 0 | 0 | 2 | 1 | 5 | 8 |
| Total | 4 | 3 | 24 | 55 | 56 | 41 | 183 |
In-depth clinical information was available for 138 (75%) of 183 children who were admitted to the public sector hospitals in the 6-week period under review, including all children who died or had received high care or intensive care (table 2 ). COVID-19 disease was deemed by clinicians to be the primary diagnosis in 61 (44%) of 138 children, while in 27 (20%) patients it was deemed to be a contributory diagnosis and in 49 patients (36%) it was an incidental diagnosis. Patients for whom COVID-19 was an incidental diagnosis included patients undergoing surgery with orthopaedic conditions, hydrocephalus, and appendicitis. No child was diagnosed with multi-system inflammatory syndrome in children (MIS-C), as per WHO case definition.
Table 2.
Clinical features of children (≤13 years) with COVID-19 who were admitted to general public sector hospitals in the Tshwane District, Oct 31 to Dec 11, 2021
| Children (n=138) | ||
|---|---|---|
| Age, years | ||
| Mean | 4·2 (4·1) | |
| Median | 2·8 (0·5–7·8) | |
| <1 | 48 (35%) | |
| 1 to 4 | 39 (28%) | |
| 5 to 9 | 34 (25%) | |
| 10 to ≤13* | 17 (12%) | |
| Sex | ||
| Female | 61 (44%) | |
| Male | 77 (56%) | |
| Ethnicity | ||
| Black | 123 (89%) | |
| Indian | 5 (4%) | |
| White | 8 (6%) | |
| Mixed race | 2 (1%) | |
| Presenting features† | ||
| Fever | 58/125 (46%) | |
| Cough | 50/125 (40%) | |
| Vomiting | 30/125 (24%) | |
| Shortness of breath or difficulty breathing | 28/125 (22%) | |
| Diarrhoea | 25/125 (20%) | |
| Seizures | 25/125 (20%) | |
| Headache | 7/125 (6%) | |
| Skin rash | 4/125 (3%) | |
| Other (eg, body aches and painful joints) | 4/125 (3%) | |
| Duration of symptoms, days‡ | ||
| Mean | 1·7 (2·5) | |
| Range | 1–14 | |
| Median | 1 (0–2) | |
| Comorbidities§ | ||
| None | 45/114 (39%) | |
| Haematological or oncological disease | 7/114 (6%) | |
| Perinatal HIV exposure | 6/114 (5%) | |
| Type 1 diabetes | 6/114 (5%) | |
| Cardiac disease | 4/114 (4%) | |
| HIV infection | 4/114 (4%) | |
| Cerebral palsy | 2/114 (2%) | |
| Asthma | 1/114 (1%) | |
| Other¶ | 39/114 (34%) | |
| Missing | 5/114 (4%) | |
| Laboratory results (on admission)‖ | ||
| Haemoglobin, g/dL | 12·5 (2·1; 8·3–20·6) | |
| Platelets, 109/L | 318·0 (122·6; 38·0–795·0) | |
| White cell count, 109/L | 10·5 (5·3; 0·1–29·0) | |
| Neutrophil count, 109/L | 5·6 (4.0; <0·1–20·4) | |
| Lymphocyte count, 109/L | 3·8 (3·1; 0·1–16·4) | |
| C-reactive protein, mg/L | 40·3 (82·4; 0–502·0) | |
| Length of hospital stay, days** | ||
| Mean | 3·2 (4·5) | |
| Range | 0–30 | |
| Median | 2 (1–3) | |
| Highest level of in-hospital care | ||
| Standard ward care | 122 (88%) | |
| High care†† | 4 (3%) | |
| Intensive care‡‡ | 7 (5%) | |
| Missing | 5 (4%) | |
| Highest category of oxygen supplementation§§ | ||
| None | 91/133 (68%) | |
| Received any oxygen therapy | 27/133 (20%) | |
| Nasal prong oxygen | 19/133 (14%) | |
| High-flow oxygen | 1/133 (1%) | |
| Ventilation | 7/133 (5%) | |
| Outcome (as of Dec 15, 2021) | ||
| Died | 4 (3%) | |
| Discharged | 102 (74%) | |
| Still in hospital | 32 (23%) | |
Data are n (%), n/N(%), mean (SD), mean (SD; range), or median (IQR), unless otherwise stated.
The category of children included those aged up to 13 years and 11 months.
Multiple presenting features were possible; n=125.
n=125.
n=114.
Included neonatal jaundice and sepsis, epilepsy, tuberculosis, neurosurgical conditions, burn wounds, and other paediatric surgical and orthopaedic conditions.
n=83.
n=104.
High care includes patients who might need closer monitoring and inotropic support but are not venitlated.
Intensive care services are allocated for critically ill children who require assisted, or invasive ventilation or intensive fluid monitoring and interventions for blood pressure stabilisation, or both.
n=133.
The mean age of these children was 4·2 years (SD 4·1), ranging from newborn to 13 years and 11 months, with most admissions being in the younger than 1 year age group, and two-thirds of children being aged 0–4 years (table 2). 77 (56%) of 138 children were male and 61 (44%) were female. Only nine (7%) children had been diagnosed using a rapid SARS-CoV-2 antigen test, all others had been diagnosed via rRT-PCR testing.
Clinical symptoms on presentation varied, with fever and cough being noted as the two main symptoms, followed by vomiting, difficulty in breathing, diarrhoea, and seizures (table 2). When this analysis was restricted to the 61 children with a primary diagnosis of COVID-19, fever (37 [61%] of 61) and cough (35 [57%]) were even more frequently seen as the presenting symptoms, as were shortness of breath (19 [31%]), seizures (19 [31%]), vomiting (16 [26%]), and diarrhoea (15 [25%]). Overall, length of hospital stay was short (median 2 days [IQR 1–3]), with the longest stay being 30 days. Only four children stayed in hospital for more than 14 days, all related to diagnoses other than COVID-19, including burn wounds, severe malnutrition, and tuberculosis. Of 114 patients with available data, 45 (39%) had no underlying comorbidities and among the 69 (61%) with comorbidities, no single comorbid condition was found to be particularly common.
The most frequent clinical diagnoses linked to hospitalisation were seizures (25 [20%] of 125 children with available data) and acute gastroenteritis (25 [20%]), followed by respiratory tract infections—namely, upper respiratory infection (18 [14%]) and bronchopneumonia (19 [15%]; appendix p 3). Seizures were diagnosed clinically, and in only four of the 25 children a relevant copathology was recorded (epilepsy n=1, cerebral palsy n=1, hypoglycaemia n=1, and severe electrolyte derangement n=1). For 19 (76%) of 25 children who had seizures, COVID-19 was the primary diagnosis for hospital admission. 21 (84%) of 25 children had uncomplicated seizures, of whom 17 (81%) of 21 were suspected to have simple febrile convulsions and four (19%) were not in the age range for febrile seizures (ie, age <1 year or >5 years). All 25 children who had seizures were discharged, and none had a diagnosis of bacterial meningitis.
Among children with data available, 122 (88%) of 138 received standard ward care and 27 (20%) received oxygen therapy. Only one child (1%) of 133 received high-flow oxygen therapy and seven children (5%) were ventilated. Reasons for ventilation included nosocomial sepsis (n=2), near-drowning (n=1), perforated appendicitis with septic shock (n=1), croup grade four (n=1), aspiration pneumonia (n=1), and presumed COVID-19 pneumonia (n=1). The child who had presumed COVID-19 pneumonia was born at 29 weeks with bronchopulmonary dysplasia and a new-onset pneumonia, with other viral pathogens and pertussis excluded as possible causes.
The clinical presentations, diagnoses, and management were similar at the central and academic hospitals as at the regional and district hospitals, apart from the few children who required high care (four [3%] of 138) or intensive care (seven [5%]). Seven (5%) children were referred to central and academic hospitals for higher level care, including two COVID-19-related referrals and five referrals unrelated to COVID-19 disease. At time of analysis (Dec 15, 2021), 102 (74%) of 138 children had been discharged and 32 (23%) were still in hospital.
Among 138 children admitted to public sector hospitals, four (2%) COVID-19-associated deaths were recorded during the study period, all occurring at one central and academic hospital (hospital 1) with intensive care facilities, one occurred in week 48 (Nov 28 to Dec 4, 2021) and three occurred in week 49 (Dec 5–11, 2021). All four children who died had presented with complex pathology and other clinically significant diagnoses: an 8-year-old who died due to accidental injury; an 8-year-old HIV-infected and severely emaciated child who had chronic diarrhoea and severe electrolyte derangements leading to fatal seizures; a 10-year-old in septic shock due to a ruptured appendix who died 2 h after presenting at hospital, and a 4-month-old infant with hydrocephalus and Pierre Robin Sequence who died of nosocomial sepsis. The in-hospital case–fatality rate was 2·2% for COVID-19-associated hospitalisations, but 0% if attributed to COVID-19 as primary diagnosis.
During the study period, in South Africa, only children older than 12 years were eligible for COVID-19 vaccination and among the 121 children with data on vaccination status, none were vaccinated. Furthermore, of the 84 parents or guardians of children for whom COVID-19 vaccination data were available, 77 (92%) were unvaccinated and only seven (8%) were partially or fully vaccinated.
Discussion
We found a rapid increase in SARS-CoV-2 positivity and associated COVID-19 hospitalisations among children and adolescents aged 19 years and younger in Tshwane District, South Africa, linked with accelerated displacement of the delta variant by omicron, and high community transmission from mid-November, 2021, onwards.3, 20 The omicron variant of SARS-CoV-2 has been associated with lower antibody neutralisation, higher infectivity, and lower vaccine effectiveness than the delta variant and an increased risk for reinfection, as has been observed by the South African COVID-19 Modelling Consortium via their epidemic explorer.2 The South African paediatric population is largely unvaccinated against COVID-19, with only those aged 12 years and older being eligible for vaccination as of October, 2021. At the start of the omicron variant outbreak in Tshwane District, 32% of adults in the district had received partial and 27% had received full COVID-19 vaccination.20 During the study period, no boosters after the primary COVID-19 vaccination were being given in South Africa. We attempted to ascertain the COVID-19 vaccination status of the parents or guardians of children in our study, but unfortunately this was not well documented in clinical notes, and individual-level vaccination data from the national electronic vaccination data system were not available. Although in our analysis many parents and guardians of children who had available data were unvaccinated, they might have been partially protected via previous SARS-CoV-2 infection.
The fourth COVID-19 wave started during a period of low SARS-CoV-2 infection in the Tshwane District, with evidence of very low levels of SARS-CoV-2 transmission (low numbers of positive SARS-CoV-2 test results despite continued baseline testing, low test positivity rates, low COVID-19-associated hospitalisation numbers). Paediatric hospital wards noticed substantial increases in admissions of children and adolescents with COVID-19 from mid-November, 2021, onwards (week 46), at much higher levels than in the previous three COVID-19 waves and uncharacteristically ahead of COVID-19-related admissions for adults. This finding supports hospital-based clinicians' anecdotal reports of the unexpected rapid increase in paediatric COVID-19-associated hospitalisations before increasing adult hospitalisations. The increased numbers of paediatric admissions, and rapid upward trajectory, created local logistical challenges because few paediatric COVID-19 hospital beds were available, as per experiences from the previous three COVID-19 waves. Coupled with acute staff shortages due to COVID-19-related isolation and quarantine, this paucity of hospital beds created a challenging environment in which to admit the unexpectedly large number of paediatric patients with COVID-19.
Increases in SARS-CoV-2 testing and test positivity rates coincided with increased paediatric COVID-19-associated hospitalisations, which supports the high infectivity of the omicron variant. Reassuringly, 36% of COVID-19 diagnoses among hospitalised children were deemed incidental, with an additional 20% being a contributory diagnosis (not the primary diagnosis), indicating the rapid community spread of the virus. National statistics from a private laboratory (Lancet laboratories) showed that the prevalence of most other seasonal respiratory viruses started to return to pre-pandemic levels in November, 2021.21 Nationally, influenza A virus was the most prevalent virus in all 4 weeks of November, 2021, followed by rhinovirus (22%) and adenovirus (12%), suggesting that the omicron variant of SARS-CoV-2 had not yet displaced these viruses as the most prevalent.21 We hypothesise that the high infectivity of the omicron variant of SARS-CoV-2, with its short doubling time and high immune evasion, the absence of vaccination availability for children, and higher rate of previous infection and hence higher natural immunity in adults than in children, coupled with the fact that children wear facemasks less frequently than do adults, could explain the unusual increase in infections and COVID-19-assoicated hospitalisations among children ahead of adults during the summer months in South Africa. COVID-19 lockdown regulations, including varying levels of intermittent school and preschool closures over the past 18 months, might also have restricted children's exposure and natural immunity to common childhood illnesses.7
The clinical picture of children hospitalised due to COVID-19, as described in this Article, was varied and overlapped with other childhood illnesses, but our most important finding was that 92% of children with available data needed only standard ward care. Seven (5%) needed intensive care or ventilation, and four (3%) needed high care. 74% of children had been discharged at the time of database lock. The severity of disease was mostly mild-to-moderate, with few children (27 [20%] of 133) needing oxygen therapy and only one child with comorbidities being ventilated for presumed COVID-19 pneumonia. The median length of hospital stay was also short (2 days). Some previously unfamiliar clinical presentations were observed, including children presenting with seizures, also in children outside the expected age range for simple febrile convulsions, raising the possibility of an underlying encephalitis in these children. However, from these data we cannot determine whether SARS-CoV-2 infection was the primary cause of the seizures. No MIS-C cases were recorded but MIS-C is a late-onset and mostly post-COVID-19 phenomenon so cases might yet occur as the fourth wave matures.
An analysis by the NICD from the pre-omicron period (March 1, 2020, to Aug 28, 2021) showed that of the 17 184 COVID-19-associated hospital admissions in South Africa among individuals aged 19 years and younger (419 [2·4%]) were ventilated during their stay in hospital. However, the hospitals included were mostly specialised, and by design only admit children with more severe disease. Furthermore, the NICD described an in-hospital case–fatality risk of 3·6% (565 of 15891) in the pre-omicron era from COVID-19, slightly higher than the 2·2% in our study, with our analysis covering multiple public sector hospitals at all levels of care.17 Additionally, of note, in our study no child died primarily due to COVID-19 disease.
Further research into the rapid spread of the omicron variant of SARS-CoV-2 will be crucial. Possible mechanisms for increased transmissibility of any airborne virus includes higher or longer viral shedding than other variants, change in tropism, improved environmental survival, need for a lower infectious dose with more efficient viral entry than other variants, evasion of either innate or adaptive immune responses, the ability to infect a new niche population (eg, young children), or a combination of these factors. All these possibilities require further investigation to determine their relative contribution to the increased paediatric cases and hospitalisations during the fourth wave of the pandemic ahead of increased adult COVID-19-related hospitalisations. Notably, adult SARS-CoV-2 seropositivity from previous infection or COVID-19 vaccination was estimated to be 65–80% in South Africa in October, 2021, with the lowest estimates being in children aged 0–14 years (around 50%).20 When overall population immunity is low, then viral variants with high transmissibility will spread more easily, whereas in populations with higher immunity, the immune evasion properties of a virus become more important for facilitation of viral spread, with the immune evasion being more likely in the current South African fourth wave than in previous waves, with its rapidly increasing number of COVID-19 cases.
Our analysis had several limitations. We used routinely collected datasets that have a lag in reporting and some incompleteness. We were unable to verify COVID-19 vaccination status for any parents or patients, which were all based on self-report, and this data element was not well captured in clinical notes. Other common childhood coinfections remained unconfirmed. Further limitations are that detailed clinical records were only available for 138 (75%) of 183 children (aged ≤13 years) who were admitted to public sector hospitals in the district, although it is very unlikely that severely ill children were missed because referrals for more intensive or high care and deaths would have been noted on the hospital surveillance system. Whether a child had primary, contributory, or incidental COVID-19 disease was based on clinicians' judgement, with no formal disease severity scoring system in place. However, all clinicians at the large hospitals were paediatricians who were participating in a national COVID-19 paediatric consortium, sharing perspectives monthly.23 The change in testing strategy during the fourth wave of the epidemic, with wider use of rapid SARS-CoV-2 testing, could have affected comparisons with previous waves. Such comparisons might also be affected by less than optimal capturing of rapid test results on the COVID-19 line lists and DATCOV surveillance system. However, these factors are unlikely to have had a major effect on our study results in terms of identifying the rapid increase in paediatric COVID-19-associated hospitalisations, because only nine (7%) of 138 children in the detailed clinical analysis were diagnosed via rapid testing.
Our study also has several strengths. Multiple data sources were used, including surveillance systems with good local and national coverage, together with detailed clinical data from a multidisciplinary research team including clinicians, district-level health officials, epidemiologists, and laboratory-based scientists. Although our findings to date do not suggest an increased severity of COVID-19 disease among children admitted to hospital during this fourth wave of the epidemic, further monitoring is needed. Encouragingly, a large proportion of COVID-19 diagnoses were incidental, and most children had mild-to-moderate disease. However, the rapid increase in the number of cases among children suggests that hospitals need to be prepared for highly infectious SARS-CoV-2 variants that might cause rapid increases in paediatric cases and hospitalisations, given that this population remains unvaccinated in many countries.
For the NGS-SA website see https://www.ngs-sa.org/ngs-sa_network_for_genomic_surveillance_south_africa/
For the GISAID website see https://www.gisaid.org
Data sharing
All de-identified individual participant data will be made available, as well as the study protocol, statistical analysis plan, informed consent form, and clinical study report, immediately after publication of this Article, with no end date. Anyone wishing to request these data and study materials should contact the corresponding author, and will need to sign a data access agreement. There will be no limitations on who can request access, for what it will be used, and for how long it will be available.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Tshwane District management team; the health-care workers at public sector hospitals in Tshwane District (especially L Chumba, N Singh, M Maharaj, J Talma, D Kutumela, and V Zulu); the staff at Tshwane District Health Services responsible for COVID-19 surveillance (especially M Moshime-Shabangu); Tshwane District Clinical Specialist Team members (especially R Skhosana); DATCOV team (especially R Welch); South African Medical Research Council research staff (especially C Chabalala); the laboratory teams at the ZARV, Department of Medical Virology, University of Pretoria (especially M Davids, A Mendes, and A Strydom); and the National Health Laboratory Service Tshwane Academic division, Department Medical Virology, University of Pretoria and members of NGS-SA for sequencing information. The South African Medical Research Council funds the SA COVID Kids study and staff time (AG). The ZARV programme is funded through the African Network for improved Diagnostics, Epidemiology and Management of Common Infectious Agents, COVID-19 fund, G7 global Health Fund. NGS-SA is funded by the Department of Science and Innovation and the South African Medical Research Council.
Contributors
JC contributed to data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. UF contributed to conceptualisation, data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. AK contributed to data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. MM contributed to data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. NMdP contributed to data curation, formal analysis, investigation, methodology, and review and editing of the manuscript. DM contributed to data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. MT contributed to data curation, investigation, methodology, and review and editing of the manuscript. TM contributed to data curation, investigation, methodology, resources, and review and editing of the manuscript. LK contributed to data curation, investigation, methodology, and review and editing of the manuscript. MV contributed to data curation, formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. WJ contributed to data curation, formal analysis, investigation, methodology, resources, and review and editing of the manuscript. AG contributed to formal analysis, investigation, methodology, and writing the original draft and review and editing of the manuscript. JC and UF accessed and verified the underlying study data.
Supplementary Material
References
- 1.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; 2021. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern#:~:text=Based%20on%20the%20evidence%20presented,as%20a%20VOC%2C%20named%20Omicron [Google Scholar]
- 3.Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600:21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022 doi: 10.1038/s41586-022-04411-y. published online Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 7.Devulapalli CS. COVID-19 is milder in children possibly due to cross-immunity. Acta Paediatr. 2020;109 doi: 10.1111/apa.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandão MB. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020;55:1892–1899. doi: 10.1002/ppul.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciuca IM. COVID-19 in children: an ample review. Risk Manag Healthc Policy. 2020;13:661–669. doi: 10.2147/RMHP.S257180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. 2020;55:2565–2575. doi: 10.1002/ppul.24991. [DOI] [PubMed] [Google Scholar]
- 11.De Luca CD, Esposito E, Cristiani L, et al. COVID-19 in children: a brief overview after three months experience. Paediatr Respir Rev. 2020;35:9–14. doi: 10.1016/j.prrv.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zare-Zardini H, Soltaninejad H, Ferdosian F, Hamidieh AA, Memarpoor-Yazdi M. Coronavirus disease 2019 (COVID-19) in children: prevalence, diagnosis, clinical symptoms, and treatment. Int J Gen Med. 2020;13:477–482. doi: 10.2147/IJGM.S262098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146:1–11. doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 14.Sankar J, Dhochak N, Kabra SK, Lodha R. COVID-19 in children: clinical approach and management. Indian J Pediatr. 2020;87:433–442. doi: 10.1007/s12098-020-03292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha IP, Harwood R, Semple MG, et al. COVID-19 infection in children. Lancet Respir Med. 2020;8:446–447. doi: 10.1016/S2213-2600(20)30152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray DM, Davies MA, Githinji L, et al. COVID-19 and pediatric lung disease: a South African tertiary center experience. Front Pediatr. 2021;8 doi: 10.3389/fped.2020.614076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Communicable Diseases Epidemiology and clinical characteristics of laboratory confirmed COVID-19 among individuals aged ≤19 years, South Africa, March 1, 2020–August 28, 2021. https://www.nicd.ac.za/wp-content/uploads/2021/09/COVID-19-in-children-surveillance-report.pdf
- 18.National Institute of Communicable Diseases COVID-19 weekly epidemiological update. https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/national-covid-19-daily-report/
- 19.Department of Statistics South Africa Mid-year population estimates, 2020. Statistical release PO302, Pretoria. 2021. http://www.statssa.gov.za/publications/P0302/P03022020.pdf
- 20.South Africa Department of Health COVID-19 public dashboard. 2021. https://sacoronavirus.co.za/latest-vaccine-statistics/
- 21.Grabowski F, Kochańczyk M, Lipniacki T. Omicron strain spreads with the doubling time of 3.2–3.6 days in South Africa province of Gauteng that achieved herd immunity to delta variant. medRxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.12.08.21267494v1 published online Dec 9. (preprint). [Google Scholar]
- 23.South African Medical Research Council COVID-19 in children. https://www.samrc.ac.za/intramural-research-units/covid-19-children
Uncited References
- 22.Lancet Laboratories Respiratory virus statistics. November, 2021. https://www.lancet.co.za/respiratory-virus-statistics-for-november-2021/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All de-identified individual participant data will be made available, as well as the study protocol, statistical analysis plan, informed consent form, and clinical study report, immediately after publication of this Article, with no end date. Anyone wishing to request these data and study materials should contact the corresponding author, and will need to sign a data access agreement. There will be no limitations on who can request access, for what it will be used, and for how long it will be available.