Abstract
Since the first successful application of messenger ribonucleic acid (mRNA) as a vaccine agent in a preclinical study nearly 30 years ago, numerous advances have been made in the field of mRNA therapeutic technologies. This research uncovered the unique favorable characteristics of mRNA vaccines, including their ability to give rise to non-toxic, potent immune responses and the potential to design and upscale them rapidly, making them excellent vaccine candidates during the coronavirus disease 2019 (COVID-19) pandemic. Indeed, the first two vaccines against COVID-19 to receive accelerated regulatory authorization were nucleoside-modified mRNA vaccines, which showed more than 90% protective efficacy against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection alongside tolerable safety profiles in the pivotal phase III clinical trials. Real-world evidence following the deployment of global vaccination campaigns utilizing mRNA vaccines has bolstered clinical trial evidence and further illustrated that this technology can be used safely and effectively to combat COVID-19. This unprecedented success also emphasized the broader potential of this new drug class, not only for other infectious diseases, but also for other indications, such as cancer and inherited diseases. This review presents a brief history and the current status of development of four mRNA vaccine platforms, nucleoside-modified and unmodified mRNA, circular RNA, and self-amplifying RNA, as well as an overview of the recent progress and status of COVID-19 mRNA vaccines. We also discuss the current and anticipated challenges of these technologies, which may be important for future research endeavors and clinical applications.
Keywords: COVID-19, mRNA vaccines, nucleoside-modified mRNA, mRNA vaccine platforms, first mRNA vaccine approval
Graphical abstract
The COVID-19 pandemic has highlighted the powerful clinical potential of mRNA vaccines. The authors give an overview of the recent status of COVID-19 vaccine candidates on the different mRNA platforms, including the approved nucleoside-modified mRNA vaccines, and briefly discuss the challenges and the future of this technology.
Introduction
Vaccines are important tools to prevent, control, and/or eradicate infectious diseases and are fundamental components of public health programs worldwide.1 The development and approval of effective coronavirus disease 2019 (COVID-19) vaccines represented a significant milestone during the ongoing pandemic. A vast number of parallel vaccine development projects were launched to combat the disease caused by a previously unknown pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of January 14, 2022, there were 333 vaccine candidates in development, of which 139 have entered the clinical phase according to the World Health Organization's vaccine tracker.2
Rapid development and highly efficient immune responses brought messenger ribonucleic acid (mRNA) technologies to the forefront of the COVID-19 vaccine race, mainly due to the fact that the technology requires a sequenced viral genome rather than live virus and takes merely a couple of days to design.3 Furthermore, the upscaling of cell-free vaccine production is relatively straightforward, thereby providing a cutting-edge tool for rapid response in epidemics and pandemics.4, 5, 6, 7 Of the COVID-19 vaccines that have entered clinical trials as of this writing, 23 (17%) are mRNA-based candidates.2 Moreover, the first two vaccines to receive conditional marketing authorization (CMA) from the European Medicines Agency or emergency use authorization (EUA) from the US Food and Drug Administration (FDA) were nucleoside-modified mRNA vaccines from BioNTech/Pfizer and Moderna, respectively, and showed over 90% protective efficacy against symptomatic SARS-CoV-2 infection in their phase III clinical trials,8,9 surpassing previous expectations.10
In this review, we summarize the preclinical and clinical data of the vaccine candidates, which fall under four mRNA vaccine platforms: non-replicating linear nucleoside-modified and unmodified mRNA, circular RNA (circRNA), and self-amplifying RNA (saRNA). In addition, we discuss potential challenges that may hinder future vaccine development programs.
The path to COVID-19 mRNA vaccine development
Compared with traditional vaccines, which are relatively slow and laborious to develop,1,11,12 mRNA-based vaccines have features that allow them to be rapidly designed and upscaled while still being highly potent and low cost.6 Although mRNA vaccines were already being investigated in clinical trials for other diseases, e.g., cancer, their far-reaching potential was not realized until the COVID-19 pandemic, where mRNA vaccine candidates were some of the first to enter clinical trials and obtain accelerated regulatory approvals.13,14 Without the extensive research and technological advances over the past three decades, this achievement would not have been possible. This section highlights some of the most important breakthroughs that ultimately carved the way to COVID-19 mRNA vaccine development and approval.
Following the discovery of mRNA,15,16 research in this area surged and gave rise to multiple scientific breakthroughs ultimately leading to the development of RNA-based vaccines. Examination of the properties of mRNA was previously hampered by the minimal cellular uptake of naked mRNA. However, the development of protective lipid-based formulations, which were first successfully used in 1978 in a study where rabbit reticulocyte 9S mRNA was introduced into mouse lymphocytes, resulting in globin synthesis,17 made subsequent mRNA research pursuits less complex. In the same year, protein expression was also induced in human cells after liposomal mRNA transport.18 Later, the efficacy of transfection was further enhanced with the incorporation of a synthetic cationic lipid into liposomes for mRNA delivery.19
The identification of deoxyribonucleic acid (DNA)-dependent RNA polymerase enzymes was a crucial step leading to in vitro mRNA transcription (IVT) using DNA templates. First published in 1984, IVT made the transcription of a selected functional mRNA from a template in the desired quantity possible.20,21 It was not until 1993 that mRNA was used as a vaccine for the first time to elicit a specific immune response against the encoded pathogenic antigen in a preclinical setting using lipid-based delivery.22 Another 20 years later, mRNA vaccines against infectious disease were investigated in phase I, proof-of-concept, clinical trials.23,24 Based on the potential toxicity of liposomes in clinical application,25,26 the success of the first approved small interfering RNA-lipid nanoparticle (LNP) therapeutic27 and mRNA COVID-19 vaccines originates only from their delivery using ionizable lipid-containing LNPs,28, 29, 30 which are known to have significantly higher delivery efficiency in hepatocytes after intravenous (i.v.) injection31 or in muscle cells after intramuscular (i.m.) injection.32 Moreover, it was recently found that LNPs possess a potent adjuvant function, which further demonstrates their beneficial effects in vaccine application.33
Lack of mRNA stability and innate immune activation were important concerns for many years in the development of mRNA as a drug substance. Uridine-containing mRNA stimulates the innate immune response and has an established adjuvant function when used as a vaccine.34,35 However, the incorporation of modified nucleosides into mRNA can significantly improve the biological stability and translational capacity of the mRNA while decreasing the innate immune response.36, 37, 38 Further improvement of mRNA quality can be made with purification of IVT mRNA using cellulose,39 high-pressure liquid chromatography (HPLC),40 fast protein liquid chromatography (FPLC),41 oligo(dT) purification,3 or tangential flow filtration (TFF).42,43 Details on the existing mRNA vaccines are not disclosed and hence can only be speculated.
In combination with LNPs, modified mRNA is now the basis for the separate mRNA vaccine platform41,44 that has been proven as the most successful based on its optimal immune response originating from the compromise between LNP adjuvant function33 and modified mRNA, allowing improved efficacy and safety. Despite the multiple advantages offered by the mRNA-LNP platform, there is still room for improvement, and we will likely see further iterations of this technology. Recently, limitations have been found when predicting clinical outcome based on data generated in murine models regarding downstream effects of systemic inflammation induced by stimulation of toll-like receptor (TLR) 7/8 by mRNA lipoplexes.45 It has been reported that humans secrete pro-inflammatory interleukin (IL)-1β, whereas mice upregulate the induction of the IL-1 receptor antagonist to control inflammation. Another challenge in this area is the fact that the lipid components themselves may activate immune responses that may differ depending on the type of component and formulation. In an elegant study comparing LNPs and lipoplexes, differences were found in cytokine induction, which indicate that the ionizable lipid of the LNP formulation is likely responsible.45 With this in mind, future research on mRNA immunological downstream effects after application, which could affect the safety profile of the mRNA, needs to be cautiously investigated, and the type as well as the composition of an mRNA formulation needs to be selected carefully depending on the disease.
In addition to linear mRNA, other mRNA vaccine platforms have received attention. circRNA was first identified in 197646 and was later detected in human cells.47 Although it was initially thought not to serve as a translational template, later reports contradicted this hypothesis, triggering further research.48 Given its enhanced biostability compared with linear RNA due to the lack of terminals preventing degradation,49 circRNA could be a promising research avenue. Another approach to reducing dose levels is the use of an mRNA platform that is able to self-amplify.50 Subsequent viral biology research led to the insertion of an RNA-dependent RNA polymerase (RdRp) sequence next to the antigen-encoding sequence, leading to the amplification of the antigen of interest in the cytoplasm.51 Along with the intracellular RNA replication of the antigen of interest, saRNA can lead to high antigen production at a low dose.52
The mechanism of immunization with mRNA vaccines and selection of the SARS-CoV-2 antigen
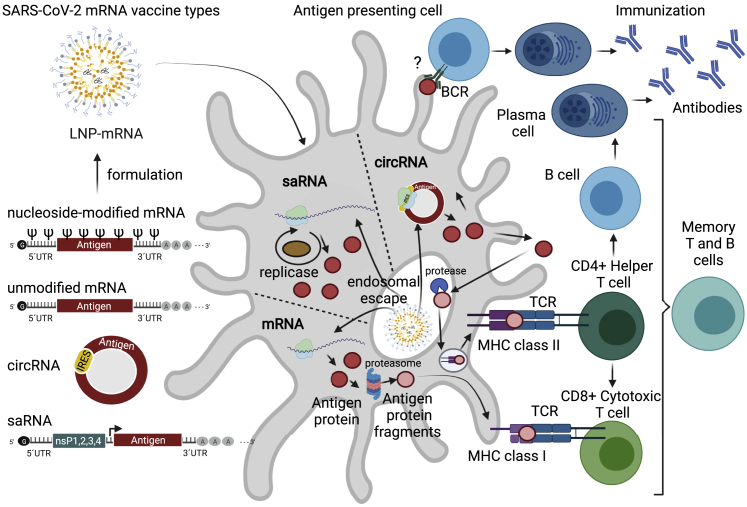
Immunization with mRNA vaccines requires an antigen-encoding mRNA transcript formulated into LNPs that is delivered to antigen-presenting cells (APCs) (Figure 1). LNP-mRNA is endocytosed and released through the process of endosomal escape to the cytoplasm, where the antigen of interest is produced and presented as a membrane-bound antigen by transfected cells, including muscle cells and APCs, resulting in activation of B cells, CD4+ helper T cells, and CD8+ cytotoxic T cell responses (Figure 1). The germinal center B cell response and its regulation by CD4+ T follicular helper (Tfh) cells are of key importance for high-affinity neutralization antibody titers and long-lasting B cell responses.53,54 Tfh cells recognize antigens on the APC surface and help to activate B cells, which in turn produce high-affinity virus-neutralizing antibodies.6,55, 56, 57 Recently, it was found that the LNP component of LNP-mRNA vaccines has adjuvant activity, which is dependent on its ionizable lipid component and IL-6 cytokine induction.33 This LNP-driven adjuvant activity leads to induction of strong Tfh cell responses and humoral immunity, thus enhancing the efficacy of mRNA-based vaccines.33 Tfh cells further help activate CD8+ cytotoxic T cells that may specifically recognize and eliminate virus-infected cells (Figure 1).6,55, 56, 57 Indeed, a persistent antigen-specific germinal center B cell response and plasmablast response in blood and draining lymph nodes are elicited after vaccination with SARS-CoV-2 LNP-mRNA in humans, leading to the development of a robust and prolonged humoral immunity.53,58
Figure 1.
Immunization against COVID-19 with mRNA vaccines
Immunization with mRNA vaccines requires an antigen-encoding mRNA transcript. The linear non-replicating mRNAs consist of a sequence encoding an antigen (e.g., the S protein for SARS-CoV-2) flanked by 5′ and 3′ UTRs, with a cap structure at the 5′ end and a poly(A) tail at the 3′ end.57 Depending on the use of native or modified nucleosides during IVT, unmodified or modified mRNAs are produced. saRNA consists of the same sequence organization, but in addition contains: (1) a sequence encoding four non-structural proteins (nsP1–4), which form a replicase responsible for amplification of the saRNA, and (2) a subgenomic promoter (black arrow) of viral origin that initiates transcription of antigens.50 circRNA for vaccine application consists of a covalently closed single-stranded RNA that contains antigen sequence and an IRES that allows initiation of antigen translation.49,59,60 Antigen-encoding mRNAs are formulated into LNPs, endocytosed, and released through the process of endosomal escape to the cytoplasm. The S protein is produced by the translational machinery of the APCs (red circles), degraded by proteasomes (pink circles), and presented on MHC class I (pink circles), leading to a specific CD8+ cytotoxic T cell response against SARS-CoV-2. Antigens can also be anchored to the membrane of the APC and directly recognized by BCRs leading to B cell responses; however, such a path and its contribution to antibody production is currently under debate. Finally, the antigen protein can be exported from the cell and endocytosed back to the same or another APC, degraded by endosomal proteases, and presented on MHC II structures resulting in a CD4+ helper T cell response. Immunization progresses with CD4+ helper T cells further helping in (1) activation of B cells that produce SARS-CoV-2 neutralizing antibodies and (2) activation of CD8+ cytotoxic T cells that may specifically recognize and eliminate virus-infected cells. APC, antigen-presenting cell; BCR, B cell receptor; circRNA, circular ribonucleic acid; IRES, internal ribosome entry site; IVT, in vitro translation; LNP, lipid nanoparticle; MHC, major histocompatibility complex; mRNA, messenger ribonucleic acid; saRNA, self-amplifying ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S protein, spike protein; TCR, T cell receptor; UTR, untranslated region. Figure was created with BioRender.com.
The entire surface spike (S) glycoprotein of SARS-CoV-2 or the receptor binding domain (RBD) of the S protein, which is critical for viral entry into the host cell,61 represent the most widely selected and suitable antigen target sites in COVID-19 vaccine development. Both the full-length S protein and the RBD itself are immunogenic and induce a strong protective neutralizing antibody response after recognition by the immune system.62,63 Immune responses are improved by introducing two consecutive proline residues (2P), which are known to retain the full-length S protein in the prefusion conformation.64,65 The S protein is cleaved by the host cell protease furin into subunits (S1 and S2), which support cellular entry.66 Interestingly, the C-terminal motif of the cleaved S1 subunit can bind to neuropillin-1 (NRP-1),67,68 which negatively influences T cell memory.69 Consequently, a loss-of-function modification or deletion of the furin cleavage site of the S protein in vaccine development can be beneficial.70,71
The efficacy of a viral antigen-encoding mRNA vaccine is influenced by codon optimization of the coding sequence,72 but not just in relation to stability and translatability. A recent study revealed that cryptic epitopes, originating from out-of-frame open reading frame translation during SARS-CoV-2 infection,73 can be modified or lost during codon optimization, leading to either enhanced or diminished immunogenic responses.
Nucleoside-modified mRNA vaccines
Numerous vaccine candidates were being investigated in the early stages of the COVID-19 pandemic, including nucleoside-modified LNP-mRNA vaccines, which were the first to enter clinical trials74 and receive EUAs, CMAs, and later full regulatory approvals.75, 76, 77, 78, 79 At present, there are a total of eight nucleoside-modified LNP-mRNA vaccine candidates in clinical trials, all of which share the key feature of enabling the replacement of all uridines in the mRNA with 1-methylpseudouridine (m1Ψ) (Table 1).
Table 1.
Nucleoside-modified COVID-19 LNP-mRNA vaccines in clinical trials
| Name | Developer | Clinical phase, trial identifier | Dose, regimen | Antigen coding sequence | Formulation | Clinical outcome | Regulatory authority approval date |
|---|---|---|---|---|---|---|---|
| BNT162b2 | BioNTech/Pfizer | phase I/II/III (IV), and phase III booster dose NCT04368728 | 30 μg (10, 20, 30 μg tested in phase I); p-b in 3 weeks | full-length S prefusion-stabilized; optimized, GC-rich | Acuitas LNP | 95% protection against symptomatic COVID-19 in participants 16 years or older8 and 100% efficacy in participants 12 to 15 years of age80 | EUA by FDA, December 11, 2020 |
| CMA by EMA, December 21, 2020 | |||||||
| Full approval by FDA, August 23, 2021 | |||||||
| EUA by FDA for those ≥12 years of age, May 10, 2021; under CMA, by EMA, May 28, 2021 | |||||||
| phase II booster dose, NCT05004181, NCT04955626 | 30 μg (booster dose); p-b in 3 weeks | single booster dose under EUA, by FDA, September 22, 2021; under CMA, by EMA, October 4, 2021 | |||||
| phase II/III, NCT04816643 | 10 μg dose for 5- to 11-year-olds; p-b in 3 weeks | 90.7% efficacy in participants 5 to 11 years of age96 | EUA by FDA for 5- to 11-year-olds. October 29, 2021; under CMA, by EMA, November 25, 2021 | ||||
| BNT162b1 | BioNTech/Pfizer | phase I/II/III, NCT04368728 | 10, 20, 30, 100 μg tested in phase I; p-b in 3 weeks | RBD, secreted | Acuitas LNP | positive clinical data, BNT162 was selected for a pivotal efficacy study based on favorable safety data81,82 | |
| BioNTech/Fosun | phase I, NCT04523571 | acceptable safety profile and high levels of humoral and T cell responses in younger (ages 18–55 years) and older adults (ages 65–85 years) in an Asian population83 | |||||
| BNT162b3 | BioNTech/Pfizer | phase I/II NCT04537949, EUCTR2020-003267-26-DE | undisclosed, dose escalation study, p-b | RBD trans-membrane | Acuitas LNP | not published | |
| mRNA-1273 | Moderna/NIAID/BARDA | phase III (IV) NCT04811664, phase III, NCT04470427 | 100 μg (25, 100, 250 μg tested in phase I); p-b in 4 weeks | full-length S prefusion-stabilized | Moderna LNP | 94.1% efficacy at preventing COVID-19 illness, including severe disease in participants 18 years or older9 95% efficacy in 12- to 17-year olds84 |
EUA by FDA, December 18, 2020 |
| CMA by EMA, January 6, 2021 | |||||||
| full approval by FDA, January 31, 202285 | |||||||
| single booster dose under EUA, by FDA, October 20, 2021; under CMA, by EMA, October 25, 2021 | |||||||
| mRNA-1273.211 | Moderna | phase II/III, NCT04927065 booster dose against variants study | 50, 100 μg; booster | full-length S prefusion-stabilized | Moderna LNP | boosters increased neutralization titers against key variants86 | |
| mRNA-1273.351 | Moderna | phase II, NCT04405076 | 20, 50 μg; booster | full-length S prefusion-stabilized against B.1.351 | Moderna LNP | not published | |
| booster dose against variants study (B.1.351) | |||||||
| TAK-919 | Takeda/Moderna | phase I/II, NCT04677660 | N/A μg; p-b in 4 weeks | full-length S prefusion-stabilized | Moderna LNP | not published | PMDA, Japan, May 21, 2021 |
| ChulaCov19 | Chulalongkorn University | phase II, NCT04566276 | 10, 25, 50 μg; p-b in 3 weeks | full-length S | Genevant LNP | not published |
EMA, European Medicines Agency; EUA, emergency use authorization; CMA, conditional marketing authorization; FDA, US Food and Drug Administration; GC, guanine-cytosine; LNP, lipid nanoparticle; p-b, prime-boost regimen; PMDA, Pharmaceuticals and Medical Devices Agency; S, spike protein; RBD, receptor binding domain.
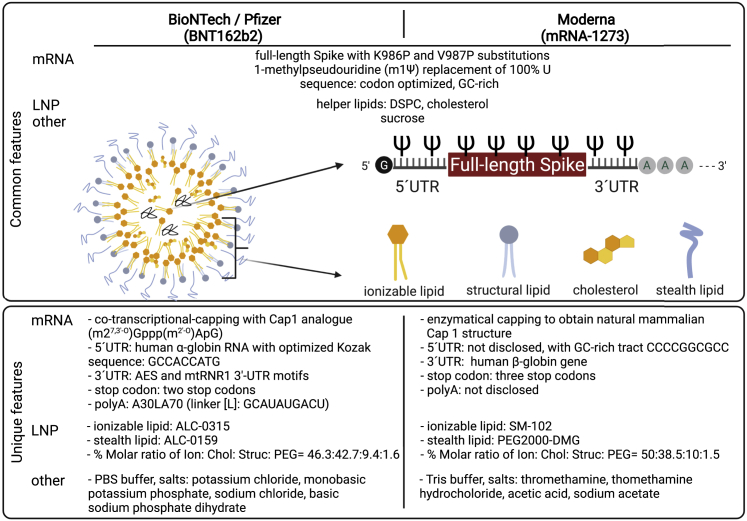
mRNA and LNP features of nucleoside-modified vaccines have been extensively summarized elsewhere.55,56,87, 88, 89, 90 Here, we highlight the main disclosed differences in the characteristics of the mRNA constructs (Figure 2). BioNTech/Pfizer's modRNA platform RNA sequence (used for BNT162b2) consists of human α-globin 5′ untranslated region (UTR), amino-terminal enhancer of split (AES), and mtRNR1 3′ UTR motifs91 and a poly(A) tail consisting of A30LA70 (linker [L]: GCAUAUGACU),81 while the mRNA sequences developed by Moderna (mRNA-1273, mRNA-1273.211, mRNA-1273.351), Takeda (TAK-919), and Chulalongkorn University (ChulaCov19) are not fully disclosed. Capping of BNT162b2 was performed co-transcriptionally using a trinucleotide cap1 analog ((m27,3′-O)Gppp(m2′-O)ApG) (TriLink),81,92 while Moderna used enzymatic capping to obtain cap1 for their preclinical mRNA-1273.3 All eight nucleoside-modified mRNAs are formulated using one of three types of LNP originating from Acuitas, Moderna, and Genevant, which consist of four components: ionizable lipids, structural lipids, stealth lipids, and cholesterol.
Figure 2.
Widely used mRNA-based COVID-19 vaccines: Comparison of ingredients
BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna) are composed of 1-methylpseudouridine-modified full-length spike mRNA, with proline substitutions, that is GC rich, codon optimized, and composed of standard mRNA components: cap, 5′ UTR, coding sequence, 3′ UTR, and a poly(A) tail. BNT162b2 is co-transcriptionally capped with ((m27,3′-O)Gppp(m2′-O)ApG) cap1 and has human α-globin 5′ UTR, AES, and mtRNR1 3′ UTR motifs; two stop codons; and a poly(A) tail consisting of A30LA70.91,92 mRNA-1273 is enzymatically capped and has an undisclosed 5′ UTR and a human β-globin gene-based 3′ UTR, three stop codons, and a poly(A) tail of undisclosed length.93 In both cases, the mRNA is formulated using LNPs consisting of ionizable, structural, and stealth lipids and cholesterol. The LNPs of both mRNA vaccines contain DSPC and cholesterol. Unique features of BNT162b2 and mRNA-1273 LNP formulations are the use of ALC-0315 and SM-102 ionizable lipids and ALC-0159 and PEG2000-DMG, PEG-based stealth lipids, respectively.88,90,94,95 Lipids are integrated into the LNPs under specific molar ratios.88,90,94,95 In addition to the mRNA and LNP components, the only ingredients are salts (PBS and Tris buffers for BNT162b2 and mRNA-1273, respectively) and 10% sucrose that is used as a cryoprotectant for both mRNA vaccines.88,90. ALC-0159, 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; K, lysine; mRNA, messenger ribonucleic acid; LNP, lipid nanoparticle; P, proline; PEG2000-DMG, 1,2-dimyristoyl-sn-glycero-3-methoxypolyethylene glycol; UTR, untranslated region; V, valine; AES, amino-terminal enhancer of split; mtRNR1, mitochondrially encoded 12S rRNA; ALC-3015, ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis; SM-102, 9-heptadecanyl 8-{(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino}octanoate. Figure was created with BioRender.com.
The design and preclinical testing of nucleoside-modified mRNA vaccines is based on years of research; thus, at the start of the COVID-19 pandemic they were well placed for rapid adjustments and application to SARS-CoV-2. However, one of the bottlenecks that required significant innovation was manufacturing, process development, and scale-up methods to meet supply demands for large-scale clinical trials and later worldwide marketing. The details of the manufacturing processes are proprietary to the companies producing nucleoside-modified mRNA vaccines and not disclosed in literature.
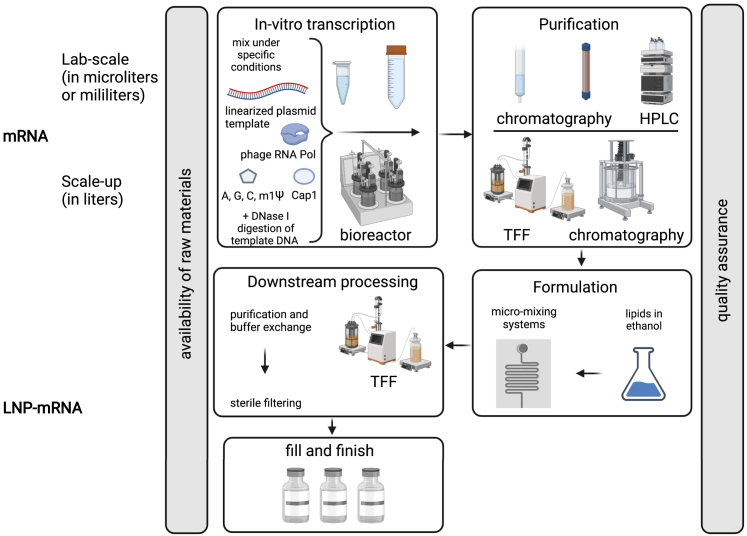
Figure 3 outlines the basic steps of this manufacturing and scale-up: IVT, mRNA purification, formulation process, and downstream steps up to fill and finish. One of the key steps in this process is mRNA purification, which allows depletion of double-stranded RNA (dsRNA) contaminants. Through recognition of TLR3 in endosomes or retinoic acid-inducible gene I, melanoma differentiation-associated protein 5, and inflammasomes in the cytoplasm,96, 97, 98, 99 depending on the amount, dsRNAs might overactivate innate immunity and lead to adverse events. The exact purification processes performed for BNT162b2 and mRNA-1273 used on a large manufacturing scale are undisclosed. HPLC is a gold standard for mRNA purification in a lab setting;100 however, it presents challenges when scaling up, and thus it can only be speculated that certain adjustments had to be made between the mRNA vaccine purity and the manufacturing process that allows the scale necessary to produce enough vaccine to meet global requirements during a pandemic. Another possibility is innovation on the IVT level (e.g., by using engineered RNA polymerases and specific reaction conditions)101,102 that could lead to a significant decrease in dsRNA formation during the IVT reaction, allowing achievement of excellent purity with standard industry purification methods. A thermostable RNA polymerase has been recently used for mRNA IVT and to prevent formation of dsRNA, allowing a lower immune response to such mRNA without the purification step.102 In 2021, Moderna provided details on their T7 RNA polymerase, which is able to minimize dsRNA formation;103 however, its potential use in mRNA-1273 vaccine production is currently not publicly disclosed and can only be speculated.
Figure 3.
Manufacturing and scale-up of nucleoside-modified mRNA vaccines
The first step in nucleoside-modified mRNA vaccine production consists of an IVT reaction. This reaction, which is conducted under specific conditions, is based on mixing linearized plasmid template, phage RNA polymerase, nucleoside-triphosphates (including m1Ψ), and the Cap1 structure when a co-transcriptional capping process is used.57 The IVT reaction can be performed at different scales and is typically followed by DNase I digestion, which allows DNA template depletion. Purification of mRNA is a process that allows depletion of unwanted IVT reaction by-products and other impurities. Depletion of dsRNA formed during IVT reactions by diverse types of chromatography, such as HPLC or TFF techniques, means that mRNA vaccine-triggered adverse events caused by systemic innate immune system responses are kept to a minimum. Purified mRNA is diluted in an appropriate buffer and then formulated with lipid components, which are dissolved in ethanol by a micro-mixing technology.104, 105, 106 Downstream processes include further purification, buffer exchange, and sterile filtering prior to fill and finish.106 Availability of raw materials is of key importance for continual large-scale production when demands are high, such as during a pandemic. The process is tightly controlled by numerous quality assessments at the LNP, mRNA, and LNP-mRNA levels. HPLC, high-performance liquid chromatography; LNP, lipid nanoparticle; m1Ψ, 1-methylpseudouridine; mRNA, messenger ribonucleic acid; RNA Pol, RNA polymerase; TFF, tangential flow filtration. Figure was created with BioRender.com.
The dosing of nucleoside-modified LNP-mRNAs against COVID-19 applied in clinical trials ranged between 10 and 250 μg RNA (Table 1). BNT162b2 was granted EUA by the FDA on December 11, 2020, for a 30 μg dose for individuals 16 years or older for the prevention of COVID-19, and the EUA for mRNA-1273 was granted shortly after on December 18, 2020, for individuals 18 years of age and older at a dose of 100 μg. Full approval by the FDA was granted for BNT162b2 for individuals 16 years and older and mRNA-1273 for individuals 18 years and older on August 23, 2021, and on January 31, 2022, respectively. All nucleoside-modified LNP-mRNAs referred to in Table 1 are administered i.m. in a prime-boost (p-b) regimen with a 3 week interval for BNT162b2 and ChulaCov19, or a 4 week interval for mRNA-1273 and TAK-919.
In preclinical studies, one i.m. injection of BNT162b1 or BNT162b2 was sufficient to elicit an inhibitory antibody response with high titers as well as CD4+ helper and CD8+ cytotoxic T cell responses.107 Two injections of BNT162b2 administered to rhesus macaques elicited SARS-CoV-2 neutralizing antibody titers 8.2- to 18.2-fold higher than in convalescent human sera.107 The safety and immunogenicity data of the phase I/II trials of BNT162b1 and BNT162b2 supported the selection of BNT162b2 for a phase II/III trial based on milder systemic reactions82 and strong adaptive humoral and poly-specific cellular immune responses against epitopes conserved in diverse SARS-CoV-2 virus variants.81 In the BNT162b2 phase II/III trial, with a total of 43,548 participants 16 years of age or older, two doses of BNT162b2 resulted in confirmation of the favorable safety profile and 95% vaccine efficacy (prevention of COVID-19).8 In 12- to 15-year-olds, two 30 μg doses of BNT162b2 showed a favorable safety profile and resulted in 100% vaccine efficacy.80 Recently, as a part of the clinical trial examining BNT162b2 in 5- to 11-year-olds (NCT04816643), two 10 μg doses of BNT162b2 were found to give rise to 90.7% vaccine efficacy (prevention of COVID-19).108 Owing to the reduced efficacy of LNP-mRNA vaccines over time and the emergence of highly transmissible SARS-CoV-2 variants such as delta (B.1.617.2) and omicron (B.1.1.529),109,110 the effects of booster vaccination are being examined. An Israeli study looking at the population ≥60 years of age receiving a single booster of BNT162b2 given at least 5 months after completion of the p-b primary regimen demonstrated 95% fewer reports of severe illness and significantly lower rate of confirmed COVID-19 cases.111 Recently, multiple trials have investigated vaccine-induced immunity against the delta (B.1.617.2) and omicron (B.1.1.529) variants.110,112,113 In all cases, neutralizing antibody titers after the second dose of mRNA-based vaccines were highly reduced for omicron (B.1.1.529) compared with delta (B.1.617.2) and especially compared with the wild-type variant, while the third dose significantly improved antibody neutralization titers.110,112,113 This suggests that application of a booster dose of mRNA vaccines may protect against these highly transmissible variants and supports booster vaccine doses, which are now being rolled out globally. A booster dose of BNT162b2 obtained EUA by the FDA on September 22, 2021, for individuals ≥65 years of age or ≥18 years at high risk of severe COVID-19.114 Currently, clinical trials with authorized vaccines in specific population groups, including 6-month- to 4-year-old children, pregnant women, immunocompromised individuals, and cancer patients, are ongoing (Table 2).
Table 2.
Clinical development of BioNTech/Pfizer and Moderna COVID-19 vaccines in specific population groups
| Name | Sponsor of clinical trial | Clinical phase, identifier | Population type | Population | Age | Estimated primary completion date |
|---|---|---|---|---|---|---|
| BNT162b2 | BioNTech SE | phase II/III, NCT04816643 | healthy | healthy individuals | 6 months to 18 years | June 18, 2024 |
| BioNTech SE | phase III, NCT04754594 | healthy | pregnant women | ≥18 years | October 15, 2022 | |
| BioNTech SE | phase II, NCT04895982 | immunocompromised | immunocompromised | ≥12 years | February 11, 2023 | |
| Centre Hospitalier Régional d'Orléans | phase IV, NCT04952766 | immunocompromised | kidney transplant, myeloma, cancer, hematologic malignancy, multiple sclerosis, hypergammaglobulinemia, malignant tumors, HIV, diabetes mellitus type 2 | ≥18 years | March 2022 | |
| University of Liege | phase III, NCT04951323 | immunocompromised | allogeneic stem cell recipients | 18 to 100 years | December 1, 2021 | |
| Humanity & Health Medical Group Ltd. | phase IV, NCT04775069 | immunocompromised | chronic liver disease | ≥18 years | December 31, 2021 | |
| National Institute of Allergy and Infectious Diseases (NIAID) | phase II, NCT04761822 | immunocompromised | high-allergy/mast cell disorder | ≥12 years | October 2021 | |
| Assistance Publique-Hôpitaux de Paris | Phase I/II, NCT04969601 | cancer | acute leukemia | ≥12 years | April 2022 | |
| Moderna | phase III, NCT04470427 | healthy | healthy individuals | ≥18 years | October 27, 2022 | |
| mRNA-1273 | Moderna | phase III, NCT04649151 | healthy | healthy individuals | 12 to 17 years | June 30, 2022 |
| Moderna | phase III, NCT04796896 | healthy | healthy individuals | 6 months to 11 years | June 12, 2023 | |
| Moderna | phase III, NCT04860297 | immunocompromised | solid organ transplant recipients | ≥18 years | March 31, 2023 | |
| McGill University Health Centre/Research Institute of the McGill University Health Centre | phase III, NCT04806113 | immunocompromised | rheumatic diseases, rheumatoid arthritis, systemic lupus erythematosus | ≥18 years | June 13, 2021 | |
| NIAID | phase II, NCT04761822 | immunocompromised | high-allergy/mast cell disorder | ≥12 years | October 2021 | |
| National Cancer Institute (NCI) | phase II, NCT04847050 | cancer | solid tumor malignancies, hematologic malignancies, lymphoma, multiple myeloma | ≥18 years | January 1, 2022 |
HIV, human immunodeficiency virus.
mRNA-1273 went through a clinical development process similar to that of BNT162b2,3 and a favorable safety profile and 94.1% vaccine efficacy (protection in preventing COVID-19 illness, including severe disease) for those ≥18 years of age was demonstrated in a pivotal clinical phase II/III trial.9 In an interim analysis of a single booster dose following p-b vaccination, Moderna tested mRNA-1273 and multiple variant-modified versions and found high safety and tolerability, as well as increased neutralization titers against key variants of concern and variants of interest of SARS-CoV-2, including beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529).86 In 12- to 17-year-olds, mRNA-1273 showed 95% vaccine efficacy and had an acceptable safety profile.84 A single booster dose of mRNA-1273 obtained EUA by the FDA on October 20, 2021, while numerous clinical trials for specific population groups are currently recruiting (Table 2).
While initial clinical trials included healthy volunteers, an important aspect is the safety and efficacy in pregnant women, who were not included. A recent observational study in a cohort of 10,861 pregnant women showed that BNT162b2 had 96% effectiveness in the prevention of COVID-19.115 In a smaller patient cohort with 131 individuals, BNT162b2 and mRNA-1273 were studied in pregnant or lactating women, and both vaccines led to robust humoral immunity with no significant differences in postvaccination reactogenicity between pregnant and non-pregnant women.116 Importantly, functional neutralizing antibodies against SARS-CoV-2 were found in infant cord blood and in the breast milk of vaccinated pregnant women, suggesting that there is a protective effect for newborns.116, 117, 118 In January 2022, the European Medicine Agency's COVID-19 task force finalized a detailed review of multiple studies in pregnant woman, including about 65,000 pregnancies. Based on the results, the use of mRNA-based COVID-19 vaccines during pregnancy is encouraged, since no increased risk of pregnancy complications for expectant mothers and their unborn babies was found, and effectiveness was not compromised compared with non-pregnant women.119
Unmodified mRNA vaccines
The optimization of mRNA constructs, including the coding sequence, can regulate immunogenicity without incorporation of modified nucleosides. Uridine depletion to reduce counterproductive immune reactions can be achieved by the replacement of uridine by codon optimization, but this must be balanced with regard to transfer RNA abundance.120, 121, 122 Currently, there are no marketed unmodified mRNA vaccines, but candidates are being investigated in clinical trials (Table 3).
Table 3.
Unmodified mRNA COVID-19 vaccine candidates being tested in clinical trials
| Name | Developer | Clinical phase, trial identifier | Dose, regimen | Antigen coding sequence | Formulation | Clinical outcome |
|---|---|---|---|---|---|---|
| CVnCoV | CureVac | phase IIb/III, NCT04652102 phase I, II, NCT04449276, NCT04515147 | 12 μg, p-b, 4 weeks | full-length S, 2P | Acuitas LNP | 48% efficacy for the prevention of COVID-19 in age group 18–60 years123 |
| MRT5500 (VAW00001) | Translate Bio/Sanofi | phase I/II, NCT04798027 | 7.5 μg, p-b, 3 weeks | full-length S 2P, modified furin cleavage site | LNP | not published |
| ARCoV | AMS/Walvax/Suzhou | phase II, ChiCTR2100041855 | 15 μg, p-b, 2 to 4 weeks | RBD | LNP | not published |
| phase III, NCT04847102 | ||||||
| PTX-COVID19B | Providence Therapeutics | phase I (II), NCT04765436 | 16, 40, 100 μg, p-b, 4 weeks; 40 μg was selected for phase II | full-length S | LNP | well tolerated in seronegative 18- to 64-year-old individuals, strong IgG antibody response124 |
| phase II, NCT05175742 comparison with BNT162b2 | 60, 80 μg, p-b, 4 weeks | not published | ||||
| DS5670 | Daiichi Sankyo | phase I/II, NCT04821674 | dose not disclosed, p-b | not disclosed | LNP | neutralizing activity without any safety concerns in both age groups (20–64 and 65–74 years)125 |
| SW-0123 | Stemirna Therapeutics/Shanghai East Hospital | phase I, ChiCTR2100045984 | 10, 30, 60, 100 μg, p-b | full-length S | LPP | not published |
| EG-COVID | eyeGENE | phase I/IIa | 50, 100, 200 μg, p-b, 3 weeks | full-length S | cationic liposome | not published |
2P, two consecutive proline residues; AMS, Academy of Military Science of the Chinese People’s Liberation Army; IgG, immunoglobulin G; LNP, lipid nanoparticle; LPP, core-shell structured lipopolyplex; p-b, prime-boost regimen; RBD, receptor binding domain; S, spike protein.
The favorable safety profile of the CVnCoV vaccine candidate126,127 using the RNActive mRNA vaccine platform developed by CureVac128 was confirmed at up to a 12 μg dose,129 but later in the phase IIb/III trial, using a p-b regimen with 12 μg 4 weeks apart, only 48% efficacy was disclosed for the prevention of COVID-19 at any severity in all age groups among 39,680 participants,130 which finally led to its withdrawal from the regulatory approval process. The inherent limitations of unmodified mRNA vaccines against COVID-19 are yet to be overcome. These limitations are based on the intrinsic immunogenicity of unmodified mRNA due to intracellular detection131,132 and type I interferon (IFN) production,34 which in turn is responsible for the inhibition of translation, the reduction of CD8+ T cell activation, and the inhibition of T helper cell generation. These alterations possibly lead to a blunted specific immune response.37,133, 134, 135 However, CureVac further engineered the UTRs of the mRNA and showed a superior immunological profile in preclinical tests,136,137 and subsequently, in collaboration with GlaxoSmithKline, CureVac plans to investigate this second-generation candidate in a clinical program.138 MRT5500, developed by Translate Bio and Sanofi, was examined in a phase I/II trial where different doses (15, 45, or 135 μg) were administered with a 3 week interval without safety or tolerability concerns. Interestingly, the MRT5500 vaccine candidate was designed using the full-length S protein with a loss-of-function modified furin cleavage site (RRAR682-685GSAS)-encoding sequence. However, the developers decided not to proceed with a phase III clinical trial for the vaccine candidate.139 BioNTech/Pfizer started COVID-19 vaccine development in three different mRNA vaccine platforms, including one utilizing unmodified mRNA. BNT162a2, a full-length S protein encoding unmodified linear mRNA, was not selected for later-stage clinical trials. The ArCoV vaccine candidate, developed through the cooperation of Walvax Biotechnology, Suzhou Abogen Biosciences, and the Academy of Military Science of the Chinese People's Liberation Army, is registered for entering late-stage clinical assessment. The investigational product is an RBD-encoding, LNP-mRNA, which is administered as a 15 μg dose in a p-b regimen. The developers emphasize thermostability, as their vaccine can be stored at room temperature for at least 1 week, which was confirmed in preclinical studies.140,141 PTX-COVID19-B, developed by Providence Therapeutics, entered a phase II clinical trial142 following preclinical testing143 and a successful phase I trial, which showed that it had a tolerable safety profile at a dose of up to 100 μg among seronegative individuals, and induced high neutralizing antibody levels. In January 2022, a clinical trial was started to compare PTX-COVID19-B with the authorized BNT162b2 COVID-19 vaccine.144 DS5670, developed by Daiichi Sankyo, is being tested in four different doses up to 100 μg in a Japanese population focusing on age-specific immunogenic responses in the phase I/II clinical trial. Recently, the company disclosed that no relevant safety concerns have been observed in different age groups, with appropriate immunogenic response.125 In a phase I clinical trial, Stemirna Therapeutics in cooperation with Shanghai East Hospital are testing their SW-0123 vaccine candidate. The mRNA encodes the natural full-length S protein rather than a prefusion-stabilized form, and is formulated into a core-shell structured lipopolyplex (LPP), making it highly selective to dendritic cells, according to preclinical study results.145 EyeGene developed a proprietary formulation using cationic liposome to avoid the use of polyethylene glycol and to facilitate freeze-drying during production. Their candidate is scheduled to enter testing in a phase I/IIa trial in Korea in late 2021.146,147
Furthermore, numerous development projects in preclinical phases aim to create an effective COVID-19 vaccine using an unmodified linear mRNA platform, including the Max Planck Institute of Colloids and Interfaces (Germany), Selçuk University (Turkey), CanSino (China) in collaboration with Precision NanoSystems, BIOCAD (Russia), RNAimmune (USA), Greenlight Biosciences (USA), IDIBAPS (Spain), Cell Tech Pharmed (Iran), ReNAP (Iran), and Globe Biotech Ltd. (Bangladesh).148 In particular, a unique formulation developed by eTheRNA makes their candidate suitable for intranasal administration, which could prove advantageous in vaccination against SARS-CoV-2 by activation of mucosal immunity.149 A consortium of RNACure BioPharma, Fudan University, and Shanghai Jiao Tong University is developing three candidates, including one that encodes membrane (M) and envelope (N) proteins in addition to S protein, resulting in the production of virus-like particles,150 marking a different approach with regard to the chosen antigen and in contrast to other mRNA vaccine candidates.
circRNA vaccines
circRNA (also called endless RNA) can be translated in eukaryotic cells48 and, due to its closed-ring structure, is significantly more resistant to exonuclease-mediated degradation compared with linear mRNA.151 Despite these potential benefits, there is only one circRNA COVID-19 vaccine candidate under development, which is produced using a group I ribozyme autocatalysis strategy49 but, as of January 31, 2022, has not yet entered clinical trials. The RBD antigen-encoding sequence of this circRNA, which is encapsulated in LNPs, was fused with signal peptide sequences to ensure secretion of antigens and to improve binding capacity. To drive translation, an undisclosed internal ribosome entry site (IRES) was positioned in front of the antigen-encoding sequence. In the preclinical study, the candidate was administered to mice i.m. at two different doses (10 and 50 μg) in a p-b regimen 2 weeks apart, which elicited a high titer of neutralizing antibodies in a dose-dependent manner, with Th1-biased T cell responses.59
saRNA vaccines
Similar to other mRNA vaccine platforms, saRNA facilitates rapid candidate design and development, as well as cell-free molecule synthesis.152 Moreover, owing to its self-replicative nature, in which the encoded replicase enables multiple copies of the antigen-encoding RNA, lower doses seem to be required to reach the same translational level compared with non-replicative mRNAs.153,154 saRNA vaccines are inherently self-adjuvanting, as their replicase activity leads to dsRNA and replicon intermediates during transcription mimic a viral infection. This triggers a broader immune response, which was also considered to have potential to be effective with a single-dose inoculation.155,156
Several clinical trials investigating the safety and efficacy of saRNA vaccines against COVID-19 are currently ongoing (Table 4). The front-running and disclosed candidates are designed with Venezuelan equine encephalitis virus (VEEV)-derived replicase in one RNA molecule with the antigen coding sequence predominantly full-length S protein. Most of the clinical trials are using p-b immunization or comparing a single dose with a p-b regimen. Only one candidate, EXG5003, is undergoing testing with only a single-dose administration. One of the main obstacles concerning the platform is the large size of the saRNA, as it contains the non-structural protein encoding sequence derived from the alphavirus genome necessary for replicative activity. The length of the RNA substantially influences the stability and ease of delivery. However, there is no saRNA COVID-19 vaccine candidate that was developed using the previously successfully applied size reduction method.157,158
Table 4.
Self-amplifying mRNA vaccines in clinical trials
| Name | Developer | Clinical phase, trial identifier | Dose, regimen | Antigen coding sequence | LNP features | Clinical outcome |
|---|---|---|---|---|---|---|
| LNP-nCoVsaRNA | Imperial College London | phase I/II, ISRCTN17072692 | 0.1 to 10 μg, p-b, 4 weeks | full-length S, prefusion-stabilized | LNP | dose-dependent immunological effect up to 5 μg, seroconversion 8%–61% by ELISA, 46%–87% by immunoblot assay159 |
| LNP-nCOV saRNA-02 | Imperial College London/MRC/UVRI/LSHTM/Uganda Research Unit | phase I, NCT04934111 | 5 μg, p-b, 4 weeks | full-length S, prefusion-stabilized | LNP | not published |
| EXG5003 | Elixirgen Therapeutics | phase I/II, NCT04863131 | N/A μg, one dose | RBD | LNP | not published |
| ARCT 021 ARCT 154 ARCT 165 |
Arcturus Therapeutics/Duke-NUS Medical School | phase II/III, NCT05012943 phase I/II, NCT04480957 phase II, NCT04728347 phase I/II NCT05037097 |
0.2 to 10 μg ARCT 021: 5 μg, one dose or p-b, 4 weeks | full-length S, prefusion-stabilized | LUNAR | dose-dependent binding and neutralizing antibody responses in interim data160 |
| CoV2 SAM LNP | GSK | phase I, NCT04758962 | 1 μg, p-b | full-length S | LNP | not published |
| HDT301 (repRNA-CoV2S; HGCO19) | SENAI Cimatec/HDT/Gennova/Quratis | phase I, NCT04844268 | 1, 5, 25 μg, one dose or p-b in 4 or 8 weeks | full-length S | LION | not published |
| VLPCOV-01 | VLP Therapeutics Japan | phase I, jRCT2071210067 | N/A | N/A | N/A | not published |
| SAM-SARS-CoV-2 | Gritstone | phase I | 10 μg, one dose as booster | full-length S, perfusion stabilized, mutated furin cleavage site | LNP | not published |
GSK, GlaxoSmithKline; LION, lipid inorganic nanoparticles; LNP, lipid nanoparticle; LSHTM, London School of Hygiene and Tropical Medicine; LUNAR, lipid-enabled nucleic acid delivery reagent; MRC, Medical Research Council; N/A, not applicable/not disclosed; p-b, prime-boost regimen; RBD, receptor binding domain; S, spike protein; UVRI, Uganda Virus Research Institute.
After a preclinical in vivo experiment demonstrating proof of principle for an effective saRNA candidate against COVID-19,161 Imperial College London entered LNP-nCoVsaRNA into a phase I/II clinical trial.162 Doses of 0.1–10 μg showed a dose-dependent immune response; however, there was no seroconversion in a significant portion of the trial participants, all of whom were seronegative at inclusion for SARS-CoV-2 infection159 and, accordingly, the clinical development process was halted. The self-adjuvant effect of saRNA is partly related to the activation of types I and III IFN,153 which has the potential to induce a negative feedback loop and inhibit translation. Accordingly, despite lower doses, the use of saRNA vaccines lead to higher reactogenicity.50,157
To avoid this, a candidate with an undisclosed redesigned saRNA backbone to dampen IFN production is being studied in the phase I COVAC-Uganda trial (LNP-nCOV saRNA-02), which assesses immune response in SARS-CoV-2 antibody seronegative and seropositive individuals, but is not directly testing efficacy against COVID-19.163 One of the first saRNA vaccine candidates to enter the clinical phase, BNT162c2, was developed and tested by BioNTech; however, this vaccine format was not selected for further clinical investigation. Arcturus Therapeutics, in collaboration with Duke-NUS Medical School, is studying three candidates (ARCT-021, ARCT-154, ARCT-165) using their STARR mRNA saRNA platform. The company's first candidate, ARCT-021, was tested in a phase I/II trial with a single-dose regimen and p-b dosing in the range of 1–10 μg. Currently, a single-dose regimen is being investigated in an ongoing phase II trial. ARCT-154 has started a phase I/II/III trial in Vietnam using a modified antigen-encoding sequence focusing on the recent alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and delta (B.1.617.2) virus variants. Addressing the question of the boost application of saRNA, all three candidates will be directly compared in a phase I/II trial in previously vaccinated and unvaccinated individuals in the United States and in Singapore. First results have been released from 24 participants, in which a 5 μg booster dose of ARCT-154 or ARCT-165 5 months after immunization with BNT162b2 resulted in a robust increase in neutralizing antibody responses against the omicron variant (B.1.1.529) after booster injection.164
All saRNA candidates that are being tested in the clinic were designed for i.m. administration, with the exception of EXG-5003 (Elixirgen Therapeutics), which is formulated for intradermal administration, rendering it effective only at the injection site in a temperature-sensitive manner.165 In an extensive collaboration of SENAI Cimatec, Gennova Biopharmaceuticals, HDT Biotech, and Quratis, another candidate, HDT301 (also called repRNA-CoV2S or HGCO19), is being tested in a phase I/II trial. In the preclinical setting, the p-b regimen has been shown to induce a more potent T cell response in comparison with the administration of a single dose;166 however, in the ongoing clinical trial, a one-shot regimen is also being tested. Data from a phase I trial in India demonstrated that Gennova's COVID-19 RNA vaccine candidate HGCO19 has an acceptable safety and tolerability profile.167
Recently, another important question has emerged regarding the boost potential of different vaccine modalities, specifically, the heterologous administration of two different products. In the preclinical setting, a heterologous vaccine regimen of an adenoviral vectored vaccine (ChAdOx1 nCoV-19/AZD1222) followed by saRNA was tested, which resulted in improved antigen-specific antibody responses and a higher neutralization effect compared with the p-b homologous vaccine regimen.168 Similarly, Gritstone Bio has an saRNA vaccine candidate (SAM-SARS-CoV-2), which is being investigated in a phase I clinical trial among individuals previously immunized with an adenoviral-vectored vaccine169. SAM-SARS-CoV-2 is designed to elicit immune responses against S and the nucleoprotein, as well as the membrane protein and open reading frame 3, which may bolster its efficacy against variants of concern170.
There are several other vaccine candidates on the horizon that will enter clinical trials in the immediate future, such those developed by Ziphius Vaccines with Ghent University and by Amyris (USA) in collaboration with the Infectious Disease Research Institute, which has promising preclinical data justifying a clinical development program.171
Conclusion and outlook
The COVID-19 pandemic led to the fastest vaccine development in history.172 Although the therapeutic potential of mRNA had previously been investigated for many decades without being available as a marketed medical treatment, the time was now ripe to harness its unique features compared with conventional vaccine approaches. In this review, we aimed to give an overview of the approved COVID-19 mRNA vaccines and vaccine candidates as well as their different molecular biology approaches in this fast-moving field.
If a suitable antigen sequence is available to target, mRNA vaccine technology facilitates rapid design and production because it does not involve pathogens or growing the vaccine by specific cell culture processes or fermentation. In contrast, these challenges make traditional vaccine research, development, and production processes more complex and lengthy.173
The two vaccines against COVID-19 to first gain accelerated approvals and meet the required safety and efficacy standards were mRNA vaccines.8,81,82 Nucleoside-modified mRNA in LNPs seems to be the superior choice over unmodified candidates, as demonstrated by the clinical efficacy and safety data. While relatively similar dose levels of unmodified mRNA and saRNA vaccine candidates were tested in phase I trials, unmodified mRNA vaccines that proceeded to later stages of clinical testing were used at lower doses compared with nucleoside-modified mRNA vaccines. Since none of the unmodified mRNA and saRNA candidates have made it to an approval stage as of this writing, it may be speculated that this is due to intrinsic adverse effects caused by the unmodified nucleosides or immunostimulatory by-products. circRNA represents a favorable concept, but it seems that further improvement is necessary prior to continuing their development as drug candidates. The circRNA platform is theoretically compatible with the replacement of uridine; however, to our knowledge, the functionality of an IRES element in combination with modified nucleosides is yet to be demonstrated.
The flexibility of LNP-mRNA-based vaccine design and scalability of manufacturing, in contrast to conventional technologies, may allow for a rapid response to other novel SARS-CoV-2 variants in the case where the efficacy of the vaccine designed for the original strain is lower. Nowadays, different approaches are in the pipeline with the use of mRNA platforms that aim to have a variant-specific response, including modified, adjusted coding sequences for new strains or heterologous vaccine administration. In January 2022, BioNTech and Pfizer announced that a new clinical trial has started to test an omicron (B.1.1.529)-specific mRNA vaccine in healthy adults.174 Similarly, Moderna started a phase II study to test their omicron-specific booster candidate (mRNA-1273.529).175
The success of mRNA vaccines in the COVID-19 vaccine race emphasized the promising potential of this technology in a wide variety of future applications,176,177 including other infectious diseases,4 cancer therapy,178,179 and protein replacement therapies,180,181 which is reflected in the current pipelines of the developers.
However, it must be considered that, in addition to the success of the first mRNA vaccines, the proof of a versatile future usage of this technology must be delivered. Profound immunogenicity data for therapeutic usage in non-inflammatory environments is still missing. It is also expected that the world will have to deal more frequently with new pandemics, and, in terms of COVID-19, novel virus variants of concern may arise and manifest in the population. Here, the mRNA technology could prove its suggested supremacy regarding speed of design and scale-up and cost efficiency when adapting to new sequences or even other diseases. Israel, a country with extensive real-world data on COVID-19 vaccine effectiveness due to its rapid vaccine rollout, reported a drop in neutralizing antibody titer to less than 10% of the initial titer after vaccination.182 This raises concerns regarding additional booster doses of the highly similar mRNA vaccine and if it may cause host tolerance, which is particularly pertinent if the vaccine needs to be administered several times a year or seasonally. Although immune escape of the omicron (B.1.1.529) variant has been reported,183 data from Israel have shown that there is a significantly lower rate of SARS-CoV-2 infection after a booster-immunization compared with after the primary two-dose series.184 Ultimately, the world will need multiple adaptable vaccine technologies in parallel to cover supply demands. In terms of mRNA vaccine use for other diseases, the fundamentals of a therapeutic mRNA vaccine for the induction of antigen-specific tolerization in the treatment of experimental autoimmune encephalomyelitis, a mouse model for multiple sclerosis, have been developed.185 Some of the future applications of mRNA technology include individualized cancer vaccines,186,187 co-stimulatory ligand receptors, cytokines, immunomodulators, and mRNA-encoded antibodies, as well as chimeric antigen receptors (CARs), and are extensively reviewed elsewhere.179 Despite the current hype of mRNA technology, many researchers are already pursuing novel methods to improve mRNA composition and expansion into their fields of application.
The administration routes of mRNA therapies are mainly limited to i.m. and i.v.; however, there are encouraging results addressing different modes of delivery. It seems beneficial to reach the respiratory tract epithelium in airborne diseases in order to prime lung-resident memory T cells.188 In addition to different indications,189,190 a COVID-19 vaccine candidate for intranasal delivery is being investigated.149 For enteral use, an interesting approach is the milli-injector capsule with nanoparticle formulations of nucleic acids, which is able to deliver to the gastric epithelia.191 Furthermore, norovirus and SARS-CoV-2 sequences have been combined to generate an orally delivered saRNA vaccine.192 Recombinant murine cytomegalovirus was successfully used in mice as a vector-based vaccine against influenza and SARS-CoV-2.193 Others pursue DNA-based vaccine approaches to fight drug resistance in cancer.194 Adenoviral-based drug delivery is an option to deliver nucleic acids (reviewed by Sakurai et al.195); however, repeated administration could fail to induce or greatly reduce the desired immune response due to induction of anti-adenoviral antibodies by the host.196
The new mRNA vaccines must still overcome several challenges to meet the world market needs. The further development of an adjustable formulation platform resulting in even lower or no adverse effects is crucial, especially in terms of choice of lipids and their charge and biodegradability. Hurdles for continuous production could be the availability and scalability of essential reagents. Furthermore, low-income countries should have equitable access to these new vaccines, which will be instrumental in tackling the pandemic. BioNTech and Pfizer have designed a three-step price system depending on the income level of a country, of which the lowest price is a non-profit price, financed by the higher price for high-income countries, such as the United States or the European Union.197 Similarly, Moderna states that they are “aiming to provide effective and affordable vaccines and therapeutics to all populations.”198
In summary, mRNA-based therapeutics have key advantages in terms of their versatility and adaptability, which could lead to promising therapeutics not only for infectious diseases but for other indications such as cancer, and thus, further research and investment into this technology is warranted. Given the success of the technology thus far, the expectations are high and so could be the reward.
Source of the data
References published as of January 31, 2022, about mRNA vaccine candidates were selected from the PubMed online library and from publications without finalized peer review available on the medRxiv and bioRxiv preprint servers. In addition, announcements, communications, and press releases from pharmaceutical companies and health care agencies were cited to enable a timely review. Relevant information about ongoing clinical trials was identified using ClinicalTrials.gov and the Chinese Clinical Trial Registry websites.
Acknowledgments
We would like to express our special thanks to Andrew Finlayson, PhD, and Camilla West, PhD, of BioNTech SE for scientific and medical writing support. We owe special thanks to Katalin Karikó for her advice and discussion of literature choice.
Declaration of interests
G.T.S., A.J.M., and I.V. are all full-time employees at BioNTech SE, Mainz, Germany, and may hold shares from BioNTech SE.
References
- 1.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Vaccine tracker: vaccines as of 14th January. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 3.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alameh M.-G., Weissman D., Pardi N. Messenger RNA-based vaccines against infectious diseases. Curr. Top. Microbiol. Immunol. 2020 doi: 10.1007/82_2020_202. [DOI] [PubMed] [Google Scholar]
- 5.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardi N., Hogan M.J., Weissman D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020;65:14–20. doi: 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N., Weissman D. Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol. Biol. 2017;1499:109–121. doi: 10.1007/978-1-4939-6481-9_6. [DOI] [PubMed] [Google Scholar]
- 8.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO WHO target product profiles for COVID-19 vaccines. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines Updated April 9, 2020.
- 11.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H.J.H.M., Osterhaus A.D.M.E. Risk in vaccine research and development quantified. PLoS One. 2013;8:e57755. doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotkin S. History of vaccination. Proc. Natl. Acad. Sci. U S A. 2014;111:12283–12287. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EMA EMA recommends first COVID-19 vaccine for authorisation in the EU. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu Updated December 21, 2020.
- 14.FDA FDA approves first COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine Updated August 23, 2021.
- 15.Brenner S., Jacob F., Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190:576–581. doi: 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- 16.Gros F., Hiatt H., Gilbert W., Kurland C.G., Risebrough R.W., Watson J.D. Unstable ribonucleic acid revealed by pulse labelling of Escherichia coli. Nature. 1961;190:581–585. doi: 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- 17.Dimitriadis G.J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature. 1978;274:923–924. doi: 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- 18.Ostro M.J., Giacomoni D., Lavelle D., Paxton W., Dray S. Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line. Nature. 1978;274:921–923. doi: 10.1038/274921a0. [DOI] [PubMed] [Google Scholar]
- 19.Malone R.W., Felgner P.L., Verma I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. U S A. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melton D.A., Krieg P.A., Rebagliati M.R., Maniatis T., Zinn K., Green M.R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg P.A., Melton D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., Lévy J.P., Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 23.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 24.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson Ö., Thompson J., et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv H., Zhang S., Wang B., Cui S., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Filion M.C., Phillips N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 27.FDA NDA approval of Onpattro. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210922Orig1s000Approv.pdf Updated January 26, 2022.
- 28.Leung A.K.K., Tam Y.Y.C., Cullis P.R. Lipid nanoparticles for short interfering RNA delivery. Adv. Genet. 2014;88:71–110. doi: 10.1016/B978-0-12-800148-6.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E., et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 30.Geall A.J., Verma A., Otten G.R., Shaw C.A., Hekele A., Banerjee K., Cu Y., Beard C.W., Brito L.A., Krucker T., et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. U S A. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X., Hope M.J., Madden T.D., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 32.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alameh M.-G., Tombácz I., Bettini E., Lederer K., Sittplangkoon C., Wilmore J.R., Gaudette B.T., Soliman O.Y., Pine M., Hicks P., et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021 doi: 10.1016/j.immuni.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devoldere J., Dewitte H., Smedt S.C.D., Remaut K. Evading innate immunity in nonviral mRNA delivery: don't shoot the messenger. Drug Discov. Today. 2016;21:11–25. doi: 10.1016/j.drudis.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 35.van Lint S., Renmans D., Broos K., Dewitte H., Lentacker I., Heirman C., Breckpot K., Thielemans K. The ReNAissanCe of mRNA-based cancer therapy. Expert Rev. Vaccin. 2015;14:235–251. doi: 10.1586/14760584.2015.957685. [DOI] [PubMed] [Google Scholar]
- 36.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karikó K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Karikó K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman D., Pardi N., Muramatsu H., Karikó K. HPLC purification of in vitro transcribed long RNA. Methods Mol. Biol. 2013;969:43–54. doi: 10.1007/978-1-62703-260-5_3. [DOI] [PubMed] [Google Scholar]
- 41.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa S.S., Prazeres D.M.F., Azevedo A.M., Marques M.P.C. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39:2190–2200. doi: 10.1016/j.vaccine.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heartlein M., DeRosa F., Dias A., Karve S. Methods for purification of messenger RNA: Patent WO/2014/152966. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014152966
- 44.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C., et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168:1114–1125.e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellman I., Tahtinen S., Tong A.-J., Himmels P., Oh J., Paler-Martinez A., Kim L., Wichner S., Oei Y., McCarron M., et al. 2021. IL-1β and IL-1ra are key regulators of the inflammatory response to RNA vaccines. [DOI] [PubMed] [Google Scholar]
- 46.Sanger H.L., Klotz G., Riesner D., Gross H.J., Kleinschmidt A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu M.T., Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 48.Chen C.Y., Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 49.Wesselhoeft R.A., Kowalski P.S., Anderson D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blakney A.K., Ip S., Geall A.J. An update on self-amplifying mRNA vaccine development. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perri S., Greer C.E., Thudium K., Doe B., Legg H., Liu H., Romero R.E., Tang Z., Bin Q., Dubensky T.W., et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003;77:10394–10403. doi: 10.1128/jvi.77.19.10394-10403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleeton M.N., Chen M., Berglund P., Rhodes G., Parker S.E., Murphy M., Atkins G.J., Liljeström P. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J. Infect. Dis. 2001;183:1395–1398. doi: 10.1086/319857. [DOI] [PubMed] [Google Scholar]
- 53.Lederer K., Castaño D., Gómez Atria D., Oguin T.H., Wang S., Manzoni T.B., Muramatsu H., Hogan M.J., Amanat F., Cherubin P., et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verbeke R., Lentacker I., Smedt S.C.D., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J. Control Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J., Eygeris Y., Gupta M., Sahay G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 58.Turner J.S., O'Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu L., Yi Z., Shen Y., Xu Y., Wu Z., Tang H., Xiao X., Dong X., Guo L., Yisimayi A., et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants: Qu et al CircRNA COVID-19 vaccine. https://www.biorxiv.org/content/10.1101/2021.03.16.435594v1 [DOI] [PMC free article] [PubMed]
- 60.Yang Y., Wang Z. IRES-mediated cap-independent translation, a path leading to hidden proteome. J. Mol. Cell Biol. 2019;11:911–919. doi: 10.1093/jmcb/mjz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1128/mBio.02281-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U S A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bestle D., Heindl M.R., Limburg H., van Lam van T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teesalu T., Sugahara K.N., Kotamraju V.R., Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. U S A. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C., Somasundaram A., Manne S., Gocher A.M., Szymczak-Workman A.L., Vignali K.M., Scott E.N., Normolle D.P., John Wherry E., Lipson E.J., et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 2020;21:1010–1021. doi: 10.1038/s41590-020-0733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalnin K.V., Plitnik T., Kishko M., Zhang J., Zhang D., Beauvais A., Anosova N.G., Tibbitts T., DiNapoli J., Ulinski G., et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines. 2021;6:61. doi: 10.1038/s41541-021-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wrobel A.G., Benton D.J., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020;27:763–767. doi: 10.1107/S2052252519007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weingarten-Gabbay S., Klaeger S., Sarkizova S., Pearlman L.R., Chen D.-Y., Gallagher K.M.E., Bauer M.R., Taylor H.B., Dunn W.A., Tarr C., et al. Profiling SARS-CoV-2 HLA-I peptidome reveals T cell epitopes from out-of-frame ORFs. Cell. 2021;184:3962–3980.e17. doi: 10.1016/j.cell.2021.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moderna Moderna announces first participant dosed in NIH-led phase 1 study of mRNA vaccine (mRNA-1273) against novel coronavirus: announcment of mRNA 1273 in phase 1. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-nih-led-phase-1-study Updated March 16, 2020.
- 75.EMA An overview of Comirnaty and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/comirnaty-epar-medicine-overview_en.pdf Updated November 2021.
- 76.EMA An overview of Spikevax and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/spikevax-previously-covid-19-vaccine-moderna-epar-medicine-overview_en.pdf Updated December 2021.
- 77.FDA BNT162b2 EUA review document. https://www.fda.gov/media/144416/download
- 78.FDA BNT162b2 FDA BLA document. https://www.fda.gov/media/151710/download
- 79.FDA Moderna COVID19 mRNA vaccine EUA review. https://www.fda.gov/media/144673/download
- 80.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R., et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N. Engl. J. Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 82.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Hui A., Zhang X., Yang Y., Tang R., Ye H., Ji R., Lin M., Zhu Z., Türeci Ö., et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 2021;27:1062–1070. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PubMed] [Google Scholar]
- 84.Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronado-Voges M., Ding B., Dooley J., Girard B., Hillebrand W., et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N. Engl. J. Med. 2021;385:2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.FDA News Release Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine Updated January 31, 2022.
- 86.Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., Nunna N., Huang W., Oestreicher J., Colpitts T., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 2021 doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., Crommelin D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021 doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kon E., Elia U., Peer D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2021;73:329–336. doi: 10.1016/j.copbio.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orlandini von Niessen A.G., Poleganov M.A., Rechner C., Plaschke A., Kranz L.M., Fesser S., Diken M., Löwer M., Vallazza B., Beissert T., et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3' UTRs identified by cellular library screening. Mol. Ther. 2019;27:824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 93.Xia X. Detailed dissection and critical evaluation of the Pfizer/BioNTech and Moderna mRNA vaccines. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.FDA Pfizer-BioNTech COVID-19 vaccine emergency use authorization review memorandum. https://www.fda.gov/media/144416/download
- 95.Moderna A phase 3, randomized, stratified, observer-blind, placebo-controlled study to Evaluate the efficacy, safety, and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine in adults aged 18 years and older: ModernaTX, Inc. Protocol mRNA-1273-P301, amendment 3. https://www.modernatx.com/sites/default/files/mRNA-1273-P301-Protocol.pdf
- 96.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 97.Goubau D., Schlee M., Deddouche S., Pruijssers A.J., Zillinger T., Goldeck M., Schuberth C., van der Veen A.G., Fujimura T., Rehwinkel J., et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5'-diphosphates. Nature. 2014;514:372–375. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S., et al. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bauernfried S., Scherr M.J., Pichlmair A., Duderstadt K.E., Hornung V. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021:371. doi: 10.1126/science.abd0811. [DOI] [PubMed] [Google Scholar]
- 100.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mu X., Greenwald E., Ahmad S., Hur S. An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 2018;46:5239–5249. doi: 10.1093/nar/gky177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu M.Z., Asahara H., Tzertzinis G., Roy B. Synthesis of low immunogenicity RNA with high-temperature in vitro transcription. RNA. 2020;26:345–360. doi: 10.1261/rna.073858.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moderna Investor day presentation: September 9, 2021. https://investors.modernatx.com/Events--Presentations/events/default.aspx
- 104.Belliveau N.M., Huft J., Lin P.J., Chen S., Leung A.K., Leaver T.J., Wild A.W., Lee J.B., Taylor R.J., Tam Y.K., et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids. 2012;1:e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leung A.K.K., Tam Y.Y.C., Chen S., Hafez I.M., Cullis P.R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B. 2015;119:8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 106.Evonik From formulation to manufacturing. https://www.ondrugdelivery.com/wp-content/uploads/2020/08/110_Aug_2020_Evonik.pdf
- 107.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Güler A., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 108.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., Barnett E.D., Muñoz F.M., Maldonado Y., Pahud B.A., et al. Evaluation of the BNT162b2 covid-19 vaccine in children 5 to 11 Years of age. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022 doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pérez-Then E., Lucas C., Monteiro V.S., Miric M., Brache V., Cochon L., Vogels C.B.F., Malik A.A., La Cruz E.D., Jorge A., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022 doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muik A., Lui B.G., Wallisch A.-K., Bacher M., Mühl J., Reinholz J., Ozhelvaci O., Beckmann N., La Güimil Garcia RdC., Poran A., et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022:eabn7591. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.FDA FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations: press release 22 September 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations Updated September 22, 2021.
- 115.Dagan N., Barda N., Biron-Shental T., Makov-Assif M., Key C., Kohane I.S., Hernán M.A., Lipsitch M., Hernandez-Diaz S., Reis B.Y., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021;27:1693–1695. doi: 10.1001/jama.2021.5782. [DOI] [PubMed] [Google Scholar]
- 116.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., Baez A.M., Shook L.L., Cvrk D., James K., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collier A.-R.Y., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., Chandrashekar A., Patel S., Apraku Bondzie E., Sellers D., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perl S.H., Uzan-Yulzari A., Klainer H., Asiskovich L., Youngster M., Rinott E., Youngster I. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.EMA COVID-19: latest safety data provide reassurance about use of mRNA vaccines during pregnancy. https://www.ema.europa.eu/en/news/covid-19-latest-safety-data-provide-reassurance-about-use-mrna-vaccines-during-pregnancy
- 120.Linares-Fernández S., Lacroix C., Exposito J.-Y., Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Gustafsson C., Govindarajan S., Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 123.CureVac CureVac final data from phase 2b/3 trial of first-generation COVID-19 vaccine candidate, CVnCoV, demonstrates protection in age group of 18 to 60: CureVac CVnCoV 2b3 trial data. https://www.curevac.com/en/2021/06/30/curevac-final-data-from-phase-2b-3-trial-of-first-generation-covid-19-vaccine-candidate-cvncov-demonstrates-protection-in-age-group-of-18-to-60/ Updated Junius 30, 2021.
- 124.Röltgen K., Nielsen S.C.A., Arunachalam P.S., Yang F., Hoh R.A., Wirz O.F., Lee A.S., Gao F., Mallajosyula V., Li C., et al. mRNA vaccination compared to infection elicits an IgG-predominant response with greater SARS-CoV-2 specificity and similar decrease in variant spike recognition. medRxiv. 2021 doi: 10.1101/2021.04.05.21254952. Preprint at. [DOI] [Google Scholar]
- 125.Daichii Development progress of mRNA COVID19 vaccine in Japan: press release. https://www.daiichisankyo.com/files/news/pressrelease/pdf/202110/20211021_E.pdf Updated October 21, 2021.
- 126.Rauch S., Gooch K., Hall Y., Salguero F.J., Dennis M.J., Gleeson F.V., Harris D., Ho C., Humphries H.E., Longet S., et al. mRNA vaccine CVnCoV protects non-human primates from SARS-CoV-2 challenge infection. [DOI]
- 127.Rauch S., Roth N., Schwendt K., Fotin-Mleczek M., Mueller S.O., Petsch B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines. 2021;6:57. doi: 10.1038/s41541-021-00311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rauch S., Lutz J., Kowalczyk A., Schlake T., Heidenreich R. RNActive® technology: generation and testing of stable and immunogenic mRNA vaccines. Methods Mol. Biol. 2017;1499:89–107. doi: 10.1007/978-1-4939-6481-9_5. [DOI] [PubMed] [Google Scholar]
- 129.Kremsner P.G., Mann P., Kroidl A., Leroux-Roels I., Schindler C., Gabor J.J., Schunk M., Leroux-Roels G., Bosch J.J., Fendel R., et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: a phase 1 randomized clinical trial. Wien. Klin. Wochenschr. 2021;133:931–941. doi: 10.1007/s00508-021-01922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kremsner P., Guerrero R.A.A., Arana E., Martinez G.J.A., Bonten M.J.M., Chandler R., Corral G., De Block E.J.L., Ecker L., Gabor J.J., et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate: results from Herald, a phase 2b/3, randomised, observer-blinded, placebo-controlled clinical trial in ten countries in Europe and Latin America: Herald study preprint data. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3911826 [DOI] [PMC free article] [PubMed]
- 131.Chen N., Xia P., Li S., Zhang T., Wang T.T., Zhu J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 2017;69:297–304. doi: 10.1002/iub.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tatematsu M., Funami K., Seya T., Matsumoto M. Extracellular RNA sensing by pattern recognition receptors. J. Innate Immun. 2018;10:398–406. doi: 10.1159/000494034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beuckelaer A.D., Pollard C., van Lint S., Roose K., van Hoecke L., Naessens T., Udhayakumar V.K., Smet M., Sanders N., Lienenklaus S., et al. Type I interferons interfere with the capacity of mRNA lipoplex vaccines to elicit cytolytic T cell responses. Mol. Ther. 2016;24:2012–2020. doi: 10.1038/mt.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pollard C., Rejman J., Haes W.D., Verrier B., van Gulck E., Naessens T., Smedt S.D., Bogaert P., Grooten J., Vanham G., et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013;21:251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anderson B.R., Muramatsu H., Jha B.K., Silverman R.H., Weissman D., Karikó K. Nucleoside modifications in RNA limit activation of 2'-5'-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39:9329–9338. doi: 10.1093/nar/gkr586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gebre M.S., Rauch S., Roth N., Yu J., Chandrashekar A., Mercado N.B., He X., Liu J., McMahan K., Martinot A., et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature. 2021 doi: 10.1038/s41586-021-04231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roth N., Schön J., Hoffmann D., Thran M., Thess A., Mueller S.O., Petsch B., Rauch S. CV2CoV, an enhanced mRNA-based SARS-CoV-2 vaccine candidate, supports higher protein expression and improved immunogenicity in rats. 2021. [DOI]
- 138.CureVac & GSK Press Release CureVac to shift focus of COVID-19 vaccine development to second-generation mRNA technology: second generation mRNA technology for COVID 19 vaccine. https://www.curevac.com/en/2021/10/12/curevac-to-shift-focus-of-covid-19-vaccine-development-to-second-generation-mrna-technology/ Updated October 12, 2021.
- 139.Sanofi Positive phase 1/2 study interim results for its first mRNA-based vaccine candidate: press release. https://www.sanofi.com/en/media-room/press-releases/2021/2021-09-28-08-00-00-2304069 Updated September 28, 2021.
- 140.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., Huang W.-J., Gao P., Zhou C., Zhang R.-R., et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283.e16. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao H., Wang T.-C., Li X.-F., Zhang N.-N., Li L., Zhou C., Deng Y.-Q., Cao T.-S., Yang G., Li R.-T., et al. Long-term stability and protection efficacy of the RBD-targeting COVID-19 mRNA vaccine in nonhuman primates. Signal Transduct. Target. Ther. 2021;6:438. doi: 10.1038/s41392-021-00861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Providence Therapeutics Providence therapeutics reports PTX-COVID19-B, its mRNA vaccine for COVID-19, neutralizes SARS-CoV-2 and variants of concern, including delta: press release September 13, 2021. https://providencetherapeutics.com/article-details/providence-therapeutics-reports-ptx-covid19-b-its-mrna-vaccine-for-covid-19-neutralizes-sars-cov-2-and-variants-of-concern-including-delta.html Updated September 13, 2021.
- 143.Liu J., Budylowski P., Samson R., Griffin B.D., Babuadze G., Rathod B., Colwill K., Abioye J.A., Schwartz J.A., Law R., et al. Preclinical evaluation of a SARS-CoV-2 mRNA vaccine PTX-COVID19-B. Sci. Adv. 2022;8:eabj9815. doi: 10.1126/sciadv.abj9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. NCT05175742 PTX-COVID19-B, an mRNA humoral vaccine, intended for prevention of COVID-19 in a general population. This study is designed to demonstrate the safety, tolerability, and immunogenicity of PTX-COVID19-B in comparison to the Pfizer-BioNTech COVID-19 vaccine. https://clinicaltrials.gov/ct2/show/NCT05175742 Updated January 4, 2022.
- 145.Yang R., Deng Y., Huang B., Huang L., Lin A., Li Y., Wang W., Liu J., Lu S., Zhan Z., et al. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct. Target. Ther. 2021;6:213. doi: 10.1038/s41392-021-00634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Korea Biomedical Review Eyegene wins approval for trial of mRNA Covid-19 vaccine. https://www.koreabiomed.com/news/articleView.html?idxno=12040 Updated September 1, 2021.
- 147.Contract Pharma TriLink extends EyeGene partnership for COVID-19 vaccine development: CleanCap technology to be used in the production of EG-COVID mRNA COVID-19 vaccine for clinical trials. https://www.contractpharma.com/contents/view_breaking-news/2021-10-05/trilink-extends-eyegene-partnership-for-covid-19-vaccine-development/ Updated October 5, 2021.
- 148.Nag K., Chandra Baray J., Rahman Khan M., Mahmud A., Islam J., Myti S., Ali R., Haq Sarker E., Kumar S., Hossain Chowdhury M., et al. An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose. Vaccine. 2021;39:3745–3755. doi: 10.1016/j.vaccine.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.eTheRNA Press Release eTheRNA launches an international consortium and starts development of cross-strain protective CoV-2 mRNA vaccine for high risk populations. https://epivax.com/news/press-release-etherna-consortium-cov-2-mrna-vaccine-for-high-risk-populations Updated Marcius 24, 2020.
- 150.Lu J., Lu G., Tan S., Xia J., Xiong H., Yu X., Qi Q., Yu X., Li L., Yu H., et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020;30:936–939. doi: 10.1038/s41422-020-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 152.Bloom K., van den Berg F., Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28:117–129. doi: 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pepini T., Pulichino A.-M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J., et al. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J. Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jensen S., Thomsen A.R. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012;86:2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H., et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blakney A.K., McKay P.F., Shattock R.J. Structural components for amplification of positive and negative strand VEEV splitzicons. Front. Mol. Biosci. 2018;5:71. doi: 10.3389/fmolb.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beissert T., Perkovic M., Vogel A., Erbar S., Walzer K.C., Hempel T., Brill S., Haefner E., Becker R., Türeci Ö., et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther. 2020;28:119–128. doi: 10.1016/j.ymthe.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pollock K.M., Cheeseman H.M., Szubert A.J., Libri V., Boffito M., Owen D., Bern H., O’Hara J., McFarlane L.R., Lemm N.-M., et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial: COVAC1 human phase I study. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3859294 [DOI] [PMC free article] [PubMed]
- 160.BioPharma Dealmakers Arcturus—a clinical-stage mRNA therapeutics and vaccines company: clinical results. https://www.nature.com/articles/d43747-021-00073-3
- 161.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., Zhou J., Bouton C.R., Rogers P., Polra K., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:3523. doi: 10.4049/jimmunol.1300120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McKay P.F., Hu K., Blakney A.K., Samnuan K., Bouton C.R., Rogers P., Polra K., Lin P.J.C., Barbosa C., Tam Y., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine induces equivalent preclinical antibody titers and viral neutralization to recovered COVID-19 patients. https://www.biorxiv.org/content/10.1101/2020.04.22.055608v1
- 163. NCT04934111 Safety and immunogenicity of LNP-nCOV saRNA-02 vaccine against SARS-CoV-2, the causative agent of COVID-19 (COVAC-Uganda) https://www.clinicaltrials.gov/ct2/show/NCT04934111
- 164.Arcturus Press Release Arcturus therapeutics reports new data demonstrating neutralizing antibody immune response to the SARS-CoV-2 omicron variant from ARCT-154 and ARCT-165 booster clinical trial. https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-therapeutics-reports-new-data-demonstrating
- 165.Elixirgen Elixirgen therapeutics concludes Pre-IND meeting with FDA for its COVID-19 vaccine candidate EXG-5003: press release. https://elixirgentherapeutics.com/news/fda/elixirgen-therapeutics-concludes-pre-ind-meeting-with-fda-for-its-covid-19-vaccine-candidate-exg-5003 Updated June 4, 2020.
- 166.Erasmus J.H., Khandhar A.P., O'Connor M.A., Walls A.C., Hemann E.A., Murapa P., Archer J., Leventhal S., Fuller J.T., Lewis T.B., et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Businesswire HDT Bio corp and Gennova complete phase 1 trial of COVID-19 RNA vaccine in India. https://www.businesswire.com/news/home/20210831005246/en/HDT-Bio-Corp-and-Gennova-Complete-Phase-1-Trial-of-COVID-19-RNA-Vaccine-in-India Updated August 31, 2021.
- 168.Spencer A.J., McKay P.F., Belij-Rammerstorfer S., Ulaszewska M., Bissett C.D., Hu K., Samnuan K., Blakney A.K., Wright D., Sharpe H.R., et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021;12:2893. doi: 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gritstone Gritstone announces positive clinical results from first cohort of a phase 1 study (CORAL-BOOST) evaluating a T cell-enhanced self-amplifying mRNA (samRNA) vaccine against COVID-19: press release January 4, 2022. https://ir.gritstonebio.com/news-releases/news-release-details/gritstone-announces-positive-clinical-results-first-cohort-phase
- 170.Rappaport A.R., Hong S.-J., Scallan C.D., Gitlin L., Akoopie A., Boucher G.R., Egorova M., Espinosa J.A., Fidanza M., Kachura M.A., et al. A self-amplifying mRNA COVID-19 vaccine drives potent and broad immune responses at low doses that protects non-human primates against SARS-CoV-2. bioRxiv. 2021 doi: 10.1101/2021.11.08.467773. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zyphius COVID-19 update: Ziphuis announces promising preclinical data.: ZIP-1642. https://www.ziphius.org/200615-press-release Updated June 15, 2020.
- 172.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 173.Moderna White Paper mRNA vaccines: disruptive innovation in vaccination. https://www.modernatx.com/sites/default/files/RNA_Vaccines_White_Paper_Moderna_050317_v8_4.pdf
- 174.BioNTech Pfizer Press Release Pfizer and BioNTech initiate study to evaluate omicron-based COVID-19 vaccine in adults 18 to 55 Years of age. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-initiate-study-evaluate-omicron-based-covid
- 175.Moderna Moderna announces first participant dosed in phase 2 study of omicron-specific booster candidate: omicron booster study phase II. https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participant-Dosed-in-Phase-2-Study-of-Omicron-Specific-Booster-Candidate-and-Publication-of-Data-on-Booster-Durability-Against-Omicron-Variant/default.aspx Updated January 31, 2022.
- 176.Dolgin E. How COVID unlocked the power of RNA vaccines. Nature. 2021;589:189–191. doi: 10.1038/d41586-021-00019-w. [DOI] [PubMed] [Google Scholar]
- 177.Dolgin E. mRNA flu shots move into trials. Nat. Rev. Drug Discov. 2021;20:801–803. doi: 10.1038/d41573-021-00176-7. [DOI] [PubMed] [Google Scholar]
- 178.Goldberg M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 179.Beck J.D., Reidenbach D., Salomon N., Sahin U., Türeci Ö., Vormehr M., Kranz L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer. 2021;20:69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weng Y., Li C., Yang T., Hu B., Zhang M., Guo S., Xiao H., Liang X.-J., Huang Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020;40:107534. doi: 10.1016/j.biotechadv.2020.107534. [DOI] [PubMed] [Google Scholar]
- 181.Vlatkovic I. Non-Immunotherapy application of LNP-mRNA: maximizing efficacy and safety. Biomedicines. 2021;9 doi: 10.3390/biomedicines9050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.CDC Research Effectiveness of mRNA BNT162b2 vaccine 6 months after vaccination among patients in large health maintenance organization, Israel. https://wwwnc.cdc.gov/eid/article/28/2/21-1834_article [DOI] [PMC free article] [PubMed]
- 183.Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.-H., Porrot F., Staropoli I., Lemoine F., et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. 2021 doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 184.Spitzer A., Angel Y., Marudi O., Zeltser D., Saiag E., Goldshmidt H., Goldiner I., Stark M., Halutz O., Gamzu R., et al. Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA. 2022 doi: 10.1001/jama.2021.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Krienke C., Kolb L., Diken E., Streuber M., Kirchhoff S., Bukur T., Akilli-Öztürk Ö., Kranz L.M., Berger H., Petschenka J., et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 186.Vormehr M., Türeci Ö., Sahin U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 2019;70:395–407. doi: 10.1146/annurev-med-042617-101816. [DOI] [PubMed] [Google Scholar]
- 187.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 188.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41586-020-2312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Legere R.M., Cohen N.D., Poveda C., Bray J.M., Barhoumi R., Szule J.A., La Concha-Bermejillo A.D., Bordin A.I., Pollet J. Safe and effective aerosolization of in vitro transcribed mRNA to the respiratory tract epithelium of horses without a transfection agent. Sci. Rep. 2021;11:371. doi: 10.1038/s41598-020-79855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qiu Y., Man R.C.H., Liao Q., Kung K.L.K., Chow M.Y.T., Lam J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 191.Abramson A., Kirtane A.R., Shi Y., Zhong G., Collins J.E., Tamang S., Ishida K., Hayward A., Wainer J., Rajesh N.U., et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter. 2022;87:46. doi: 10.1016/j.matt.2021.12.022. [DOI] [Google Scholar]
- 192.Keikha R., Hashemi-Shahri S.M., Jebali A. The evaluation of novel oral vaccines based on self-amplifying RNA lipid nanparticles (saRNA LNPs), saRNA transfected Lactobacillus plantarum LNPs, and saRNA transfected Lactobacillus plantarum to neutralize SARS-CoV-2 variants alpha and delta. Sci. Rep. 2021;11:21308. doi: 10.1038/s41598-021-00830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim Y., Zheng X., Eschke K., Chaudhry M.Z., Bertoglio F., Tomić A., Krmpotić A., Hoffmann M., Bar-On Y., Boehme J., et al. MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination. Cell. Mol. Immunol. 2022 doi: 10.1038/s41423-021-00814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sun Y., Zhang X., Yang S., Hu C., Pan J., Liu T., Ding J., Han C., Huang Y., Yang K. Preparation of antibodies against TXR1 and construction of a new DNA tumor vaccine. Int. Immunopharmacol. 2022;103:108505. doi: 10.1016/j.intimp.2021.108505. [DOI] [PubMed] [Google Scholar]
- 195.Sakurai F., Tachibana M., Mizuguchi H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2021;42:100432. doi: 10.1016/j.dmpk.2021.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Atasheva S., Yao J., Shayakhmetov D.M. Innate immunity to adenovirus: lessons from mice. FEBS Lett. 2019;593:3461–3483. doi: 10.1002/1873-3468.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pfizer Supporting manufacturing, trade and equitable global access to COVID-19 vaccines. https://cdn.pfizer.com/pfizercom/140122_PfizerFactsheet.pdf
- 198.Moderna Website Moderna’s mission is to deliver on the promise of mRNA science to create a new generation of transformative medicines for patients. https://www.modernatx.com/responsibility/medicines-patients