Abstract
Objective:
In 2013 Utah enacted legislation requiring infants failing newborn hearing screening be tested for cytomegalovirus infection. As a result, cytomegalovirus-infected infants are being identified because of hearing deficits. The neuroimaging findings in this population have not been characterized.
Methods:
Retrospective medical record review was used to identify patients seen at the University of Utah and Primary Children’s Hospitals in Salt Lake City, Utah, who failed newborn hearing screens. A cohort of patients with congenital cytomegalovirus infection, brain MRI and sedated auditory brainstem response testing was studied.
Results:
Seventeen patients were identified; eleven (65%) were female. Confirmatory auditory brainstem response testing, performed at a median of 29 days old, showed profound hearing loss in eight subjects (47%), severe loss in two (12%), moderate loss in two (12%), and mild loss in three (18%); two (12%) had normal hearing. The diagnosis of cytomegalovirus infection was made at a median of 23 days old. Brain imaging was performed at a median of 65 days old. Ten subjects (59%) had one or more neuroimaging abnormality. White matter lesions were found in eight (47%), cysts in three (18%), and stroke in two (12%). Polymicrogyria was identified in two (12%). Seven (41%) had normal brain MRIs.
Interpretation:
These results indicate that the majority of infants whose CMV infections were identified after failing newborn hearing screening had abnormal brain MRIs. Our results suggest that brain MRIs should be considered in infants with congenital CMV infections who are identified through hearing screening programs.
Keywords: Congenital cytomegalovirus infection, Brain magnetic resonance imaging, Newborn hearing screen, Congenital CMV, hearing loss, brain MRI, Auditory brainstem response, White matter lesions
INTRODUCTION
Congenital cytomegalovirus (CMV) infection is the most common non-genetic cause of sensorineural hearing loss (SNHL) in children.1 In 2013 Utah passed legislation requiring that all infants who fail newborn hearing screening be tested for CMV infection.2 As a result, increasing numbers of CMV-infected infants are identified because of abnormal hearing screening.2 Infants with symptomatic congenital CMV infection often have intracranial calcifications, parenchymal cysts, white matter lesions, and cortical dysplasia, including polymicrogyria and brain clefting.3–6 Van der Knaap and others have shown that such infants have characteristic patterns of white matter abnormalities.5,7–9 In contrast to infants with symptomatic congenital CMV infection, infants with asymptomatic congenital CMV infections usually have normal neuroimaging results.10,11
The brain MRI findings in infants with CMV infections identified because of failed hearing screens have not been characterized to date. Such children may represent a unique subgroup of CMV-infected children, differing from those who are considered asymptomatic at birth and those who are overtly symptomatic with systemic or neurological findings. We report a cohort of such infants and children and describe their clinical characteristics, including hearing loss, and neuroimaging findings. Our results indicate infants with CMV-induced hearing loss who fail newborn hearing screening may represent a distinct subgroup of CMV-infected infants. We suggest that neuroimaging studies be considered in infants and children whose CMV infections are identified as a consequence of newborn hearing screening.
MATERIALS AND METHODS
Setting and study population
Approval for this study was obtained from the institutional review board of the University of Utah and Primary Children’s Hospital. Primary Children’s Hospital, operated by Intermountain Healthcare, and the University of Utah Health, both located in Salt Lake City, are the tertiary care centers for infants and children living in the Intermountain region, a large geographical area that includes Utah and portions of Colorado, Idaho, Wyoming, Montana, Arizona, and Nevada. Approximately 2 million children 18 years of age and younger live within the region served by University of Utah Health and Primary Children’s Hospital.
Selection criteria
The UUH billing records were used to identify children who met the following inclusion criteria: (1) evaluation by a University of Utah pediatric otolaryngologist between January 1, 1999, and December 1, 2015; (2) brain magnetic resonance imaging (MRI) studies available for review; and (3) failed newborn hearing screen that led to (4) testing for CMV and the diagnosis of congenital CMV infection (International Classification of Diseases-9 codes 777.1 or 078.5).
Microbiological studies
CMV infection was assessed by assay of urine, saliva or newborn blood spot using the polymerase chain reaction (PCR). CMV studies were performed by the Intermountain Healthcare microbiology laboratory or ARUP Laboratory, Inc., Salt Lake City, UT. Congenital infection was confirmed when CMV DNA was detected in clinical samples obtained within the first four weeks of life.
Neuroimaging studies
Magnetic resonance imaging was performed on a General Electric (GE Healthcare, Milwaukee, WI, USA) 1.5 Tesla or 3.0 Tesla MR imaging system. Many studies included dedicated sequences through the inner ear. Sedation for MRI, as needed, was managed by a nurse practitioner. The MRI images were reviewed independently by three authors (JH, GH and JFB). Final results were achieved by consensus review. All patients were assessed for white matter lesions, abnormal gyrification (polymicrogyria or other cortical dysplasias), and parenchymal cysts. These and any other abnormalities were recorded.
Hearing
Newborn hearing screening was performed by otoacoustic emissions testing in the immediate perinatal period. Infants who failed newborn screening underwent sedated auditory brainstem response (ABR) using standard protocols. Hearing loss was categorized according to the more severely impaired end of auditory range in the more severely impaired ear, using accepted hearing threshold classifications (mild: 25 to 40 dB; moderate: 40 to 70 dB; severe: 70 to 90 dB; and profound: >90 dB).12 Of note, some infants had conductive or mixed hearing loss on initial ABR.
Other Patient Characteristics
Medical records were reviewed to identify clinical features suggesting symptomatic congenital CMV infection [microcephaly (<3%ile orbitofrontal circumference after adjustment for prematurity), petechiae, thrombocytopenia, jaundice, or small for gestational age] prior to failing initial hearing screen. Abnormalities in transaminases and white blood cell count are additional characteristics but were not obtained in all infants in the study. Any subject with the above characteristics noted retrospectively was considered symptomatically infected. In all cases, failed hearing screen was the impetus for obtaining CMV testing.
Data Analysis
Data were entered into a RedCap databse (Vanderbilt University Medical Center, Nashville, TN, U.S.A) by one author (JH). Categorical data were compared using two-tailed Fisher’s exact test, and P values <0.05 were considered significant; analysis performed in Stata/IC 15 (StataCorp LLC, TX, USA).
Raw data are confidential and protected under the Health Insurance Portability and Accountability Act and are not available to be shared.
RESULTS
Study Population
Sixteen infants and children were identified; five (29%) were identified by targeted CMV testing secondary to hearing loss prior to enactment of the state statute. Eleven infants and children (65%) were female. The diagnosis of congenital CMV infection was made at a median age of 23 days old. One additional patient was included in the analysis, for a total of seventeen, who had failed the OAE, passed their initial confirmatory ABR testing, then later failed their second ABR and was confirmed to have CMV based on a newborn blood spot.
Hearing
The initial, confirmatory ABR testing or audiometry was performed at a median of 29 days old. Bone conduction, tympanometry and otoscopic examination were performed to determine whether any hearing loss was sensorineural, conductive or mixed. Hearing loss was mild in three (18%), moderate in two (12%), severe in two (12%) and profound in eight (47%). Two infants who failed newborn hearing screening (12%) had a normal ABR result (Table 1). Second confirmatory hearing testing results, available for 16 subjects, were obtained at a median of 240 days old and were either unchanged or showed increased impairment with the exception of one. One infant had mild conductive loss on the initial ABR and mild to moderate sensorineural hearing loss on a follow-up ABR.
Table 1:
Hearing outcomes in infants who failed newborn hearing screening and were identified as having CMV infection.
| No | % | ||
|---|---|---|---|
|
| |||
| Sensorineural Hearing* | |||
| Normal | 2 | 12 | |
| Mild Loss | 3 | 18 | |
| Moderate Loss | 2 | 12 | |
| Severe Loss | 2 | 12 | |
| Profound Loss | 8 | 47 | |
Results of initial sedated ABR testing. Category based on more- impaired ear, more-impaired end of hearing range
Neuroimaging
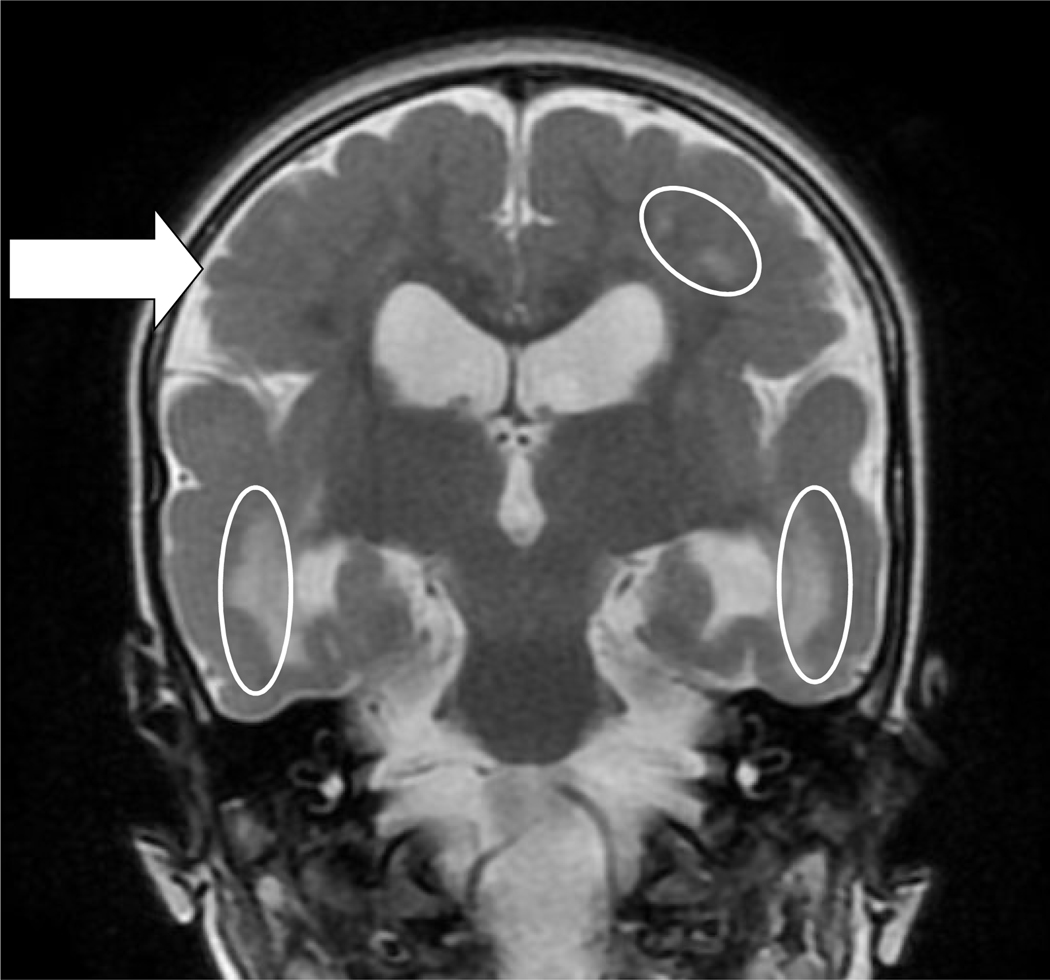
The subjects’ age at MRI ranged from 22 to 299 days old with a median of 65 days old. Ten of the 17 subjects (59%) had one or more MRI abnormality. White matter lesions were found in eight (47%) of the subjects; polymicrogyria (Figure 1) in two (12%), parenchymal cysts (typically in the temporal lobe) in three (18%), and stroke (a mixture of hemorrhagic and ischemic) in two (12%) (Table 2). Seven subjects had normal brain MRIs.
Figure 1:
Neuroimaging Findings.
T2 coronal sequence of two-year-old child with spastic quadriplegic cerebral palsy and global developmental delay. This patient had extensive, bilateral fronto-temporo-parietal polymicrogyria and bilateral abnormal white matter, especially biparietally. Here are indicated polymicrogyria (arrow) and white matter abnormalities (ovals) as well as mild enlargement of the lateral ventricles, more notable in the left temporal horn than the right.
Table 2:
Neuroimaging findings in CMV-infected infants identified due to failed newborn hearing screening.
| Most Abnormal Common Findings# | No | % |
|---|---|---|
|
| ||
| White matter changes | 8 | 47 |
| Cystic malformations | 3 | 18 |
| Polymicrogyria | 2 | 12 |
| Stroke - hemorrhagic or ischemic | 2 | 12 |
| None | 7 | 41 |
Some subjects had more than one imaging abnormality.
White matter lesions were more common in subjects with severe to profound hearing loss, occurring in 7 of 11 subjects (64%) versus 1 of 6 subjects (17%) with no, mild or moderate loss. Because of the small sample size, this difference was not significant with a two-tailed P value of 0.13 (Fisher’s exact test). One of eleven subjects with severe or profound hearing loss had polymicrogyria as well as one of the six subjects with none, mild or moderate hearing loss (not significant). Of the seven subjects with normal brain MRIs, five children had mild to moderate hearing loss and two had bilateral severe to profound hearing loss.
Clinical Features
One infant, who had white matter lesions on brain MRI, was retrospectively noted after failed infant hearing screen to have had petechiae, thrombocytopenia and low birth weight as a newborn, features consistent with symptomatic congenital CMV infection. The diagnosis of congenital CMV infection was not considered until the infant failed the newborn hearing screen. Outcome data were available for nine subjects (53%) at a median age of 246 days (range 0–556 days). Four of the nine (44%) were microcephalic, two (12%) had global developmental delay and three (18%) were speech delayed. Of the 4 microcephalic infants, 3 had neuroimaging abnormalities, consisting of bilateral caudothalamic groove cysts, small temporal cysts and white matter abnormalities for one, bilateral perisylvian and parietal polymicrogyria, periventricular cysts and white matter abnormalities in another, and only white matter abnormalities in a third.
CONCLUSIONS
Interpretation:
These results indicate that the majority of infants whose CMV infections were identified after failing newborn hearing screening had abnormal brain MRIs. Most authorities have traditionally categorized infants with congenital CMV infection as either symptomatic or asymptomatic, based upon the presence or absence of one or more clinical or laboratory abnormalities at birth, consisting of intrauterine growth retardation, microcephaly, hepatomegaly, splenomegaly, petechiae, jaundice, thrombocytopenia, hepatitis, or chorioretinitis, or neuroimaging findings, such as intracranial calcifications, white matter lesions or polymicrogyria. 11,13 Infants who are symptomatic at birth have much higher rates of neurodevelopmental sequelae, including epilepsy, cerebral palsy and cognitive impairment.8,13–15 Recent reviews suggest that approximately 50% of symptomatically-infected infants have permanent neurodevelopmental sequelae attributable to CMV.13,15 In addition, 30% to 50% of these infants have sensorineural hearing loss of variable severity.13
Recent imaging studies using ultrasound, head computed tomography (CT) or MRI, observed that 70–83 % of symptomatic infants have some type of intracranial abnormality, a figure similar to older studies relying on CT only.8,14,16–18 Noyola and colleagues observed that an abnormality on CT was a highly sensitive predictor of mental retardation, although head circumference at birth was more specific.14 Alarcon and colleagues attempted to incorporate MRI and ultrasound neuroimaging with head CT and observed similar results, noting, however, that developmental outcomes were difficult to predict in infants with mildly abnormal imaging.18
By contrast, infants with asymptomatic congenital CMV infections generally have normal developmental outcomes, although approximately 10% of these infants have sensorineural hearing loss, and some have cognitive impairment, possibly related to the severity and management of their hearing loss.13 A recent, comprehensive study of 89 children with asymptomatic congenital CMV infections followed through 18 years observed that those with sensorineural hearing loss had full scale intelligence quotients that were approximately seven points lower than controls, whereas children with normal hearing had similar intellectual outcomes.19 Thus, the dichotomous classification, symptomatic versus asymptomatic congenital CMV infection, has been useful historically to predict the neurodevelopmental outcome of infants with congenital CMV infection.
Although this categorization has clinical utility, most CMV experts have observed asymptomatically-infected infants with long term neurodevelopmental sequelae not explained by hearing loss.4,10,13,20 Boppana and colleagues estimated that approximately 5% of infants with asymptomatic congenital CMV infections have microcephaly or motor deficits and approximately 2% have chorioretinitis13 Townsend and colleagues observed neurological sequelae in 14% of the infants considered asymptomatically infected at birth.20 The explanations for such phenomena include the presence of subtle signs of CMV infection, such as mild petechiae, intrauterine growth retardation, jaundice or small head circumference, that were unrecognized in the newborn period or intracranial abnormalities that were not clinically apparent in early infancy. The latter explanation is supported by a recent, retrospective study suggesting that a substantial number of children with asymptomatic congenital CMV infections have intracranial abnormalities when studied by MRI.4 There may also be brain damage that is not apparent on imaging, as in one study of fetuses with congenital CMV examined at 21 week gestational age which showed that a little less than 43% of those with histological brain damage had abnormalities visualized on ultrasound.21 However, a further 11% of those with brain damage had inner ear tissue positive for CMV without associated ultrasound abnormality.
Based on our observations, we agree that a third category of congenital CMV infection is needed.11 The new category would encompass those infants who have confirmed CMV-induced sensorineural hearing loss in the absence of other signs of CMV infection. The terms asymptomatic and symptomatic do not adequately characterize this group of infants. The results of Lopez and colleagues indicate that some of these infants are at risk for cognitive impairment, and our outcome data, although limited by the small number of subjects and the short duration of follow-up, suggest that microcephaly and developmental delays are also possible. The occurrence of abnormal imaging studies in this current and other studies22 suggests that CMV-infected infants identified through newborn hearing screening programs should undergo a neurodiagnostic evaluation including a brain MRI, though our results do not answer the question of the most appropriate time to obtain such imaging.
ACKNOWLEDGEMENTS
The authors thank Karl White, PhD, for his leadership and inspiration.
Funding Sources: This investigation was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number U01DC014706
Footnotes
POTENTIAL CONFLICTS OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972–982. [DOI] [PubMed] [Google Scholar]
- 2.Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. Outcomes From a HearingTargeted Cytomegalovirus Screening Program. Pediatrics. 2017;139(2). [DOI] [PubMed] [Google Scholar]
- 3.Bale JF Jr., Bray PF, Bell WE. Neuroradiographic abnormalities in congenital cytomegalovirus infection. Pediatr. Neurol 1985;1(1):42–47. [DOI] [PubMed] [Google Scholar]
- 4.Uematsu M, Haginoya K, Kikuchi A, et al. Asymptomatic congenital cytomegalovirus infection with neurological sequelae: A retrospective study using umbilical cord. Brain Dev. 2016;38(9):819–826. [DOI] [PubMed] [Google Scholar]
- 5.Manara R, Balao L, Baracchini C, Drigo P, D’Elia R, Ruga EM. Brain magnetic resonance findings in symptomatic congenital cytomegalovirus infection. Pediatr. Radiol 2011;41(8):962–970. [DOI] [PubMed] [Google Scholar]
- 6.Fink KR, Thapa MM, Ishak GE, Pruthi S. Neuroimaging of pediatric central nervous system cytomegalovirus infection. Radiographics. 2010;30(7):1779–1796. [DOI] [PubMed] [Google Scholar]
- 7.White AL, Hedlund GL, Bale JF Jr., Congenital cytomegalovirus infection and brain clefting. Pediatr. Neurol 2014;50(3):218–223. [DOI] [PubMed] [Google Scholar]
- 8.Boppana SB, Fowler KB, Vaid Y, et al. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics. 1997;99(3):409–414. [DOI] [PubMed] [Google Scholar]
- 9.van der Knaap MS, Vermeulen G, Barkhof F, Hart AA, Loeber JG, Weel JF. Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology. 2004;230(2):529–536. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett AW, McMullan B, Rawlinson WD, Palasanthiran P. Hearing and neurodevelopmental outcomes for children with asymptomatic congenital cytomegalovirus infection: A systematic review. Rev. Med. Virol 2017. [DOI] [PubMed] [Google Scholar]
- 11.Luck SE, Wieringa JW, Blazquez-Gamero D, et al. Congenital Cytomegalovirus: A European Expert Consensus Statement on Diagnosis and Management. Pediatr. Infect. Dis. J 2017;36(12):1205–1213. [DOI] [PubMed] [Google Scholar]
- 12.Smith RJ, Bale JF Jr., White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–890. [DOI] [PubMed] [Google Scholar]
- 13.Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin. Infect. Dis 2013;57 Suppl 4:S178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noyola DE, Demmler GJ, Nelson CT, et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr 2001;138(3):325–331. [DOI] [PubMed] [Google Scholar]
- 15.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol 2007;17(5):355–363. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi A, Oh-Ishi T, Arai T, et al. Screening for seemingly healthy newborns with congenital cytomegalovirus infection by quantitative real-time polymerase chain reaction using newborn urine: an observational study. BMJ Open. 2017;7(1):e013810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alarcon A, Martinez-Biarge M, Cabanas F, Hernanz A, Quero J, Garcia-Alix A. Clinical, biochemical, and neuroimaging findings predict long-term neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr 2013;163(3):828–834 e821. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon A, Martinez-Biarge M, Cabanas F, Quero J, Garcia-Alix A. A Prognostic Neonatal Neuroimaging Scale for Symptomatic Congenital Cytomegalovirus Infection. Neonatology. 2016;110(4):277–285. [DOI] [PubMed] [Google Scholar]
- 19.Lopez AS, Lanzieri TM, Claussen AH, et al. Intelligence and Academic Achievement With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics. 2017;140(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Longterm outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin. Infect. Dis 2013;56(9):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrielli L, Bonasoni MP, Santini D, et al. Human fetal inner ear involvement in congenital cytomegalovirus infection. Acta Neuropathol Commun. 2013;1(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimani JW, Buchman CA, Booker JK, et al. Sensorineural hearing loss in a pediatric population: association of congenital cytomegalovirus infection with intracranial abnormalities. Arch. Otolaryngol. Head Neck Surg 2010;136(10):999–1004. [DOI] [PubMed] [Google Scholar]