Catheter-directed embolization is one of the cornerstones and defining procedures of interventional radiology. Armed with a plethora of agents and devices, interventionalists can acutely stop flow, usually of a blood vessel, to effectively treat pathologies from head to toe. The list of pathologies treatable by embolization is extensive and diverse, including life-threatening hemorrhage, aneurysms, lymphatic duct leaks, varices, pelvic congestion syndrome, benign prostatic hyperplasia, uterine fibroids, and cancer.
Coils are arguably one of the most important embolization agents due to their ease of use, visibility on fluoroscopy, and ready availability. Coils are made from steel or platinum and offer permanent occlusion when deployed. They come in a wide range of shapes, sizes, coating materials, and deployment methods which can be used for effective target embolization in a variety of scenarios. The aim of this article is to provide a general back-to-the-basics overview of coil embolization. We discuss the historical development of coils as an embolization material, the numerous various coil types and their respective advantages/disadvantages, as well as general clinical and technical considerations when utilizing coils for embolization.
History
One of the first reported techniques using metal coils to induce thrombosis was performed by Dr. Sean Mullan in 1974. 1 After performing a craniotomy, copper coils were inserted into a giant aneurysm through an external puncture. Shortly after, in 1975, Italian American physician and spear header of interventional radiology, Dr. Cesare Gianturco, described the use of endovascular “coils” for embolization by employing a 5-cm curled segment from a 0.038-in guidewire. 2 Wool strands were later attached to the original coil to increase efficacy and thrombogenicity ( Fig. 1 ). Unlike the variety of coil options available today, the first coils were compatible only with a thin-walled, Teflon-coated 7F system. By 1978, detachable and smaller coils were developed which could be delivered through a 5-Fr system. Advancement in coil technology quickly evolved and by the 1990s, a variety of pushable, injectable, and detachable coils in all shapes and sizes became options for embolization ( Table 1 ).
Fig. 1.

Types of coil materials and coverings. ( a ) Bare platinum coil. ( b ) Fibered helical coil with nylon covering. ( c ) Terumo Azur hydrogel-coated coil preexpansion. ( d ) Hydrogel coil after exposure to blood and expansion of hydrogel covering (∼20 minutes after deployment). (Images used with permissions from Terumo Interventional Systems.)
Table 1. Advantages, disadvantages, and cost of various coil specifications.
| Advantages | Disadvantages | Cost 18 19 | |
|---|---|---|---|
| Material | |||
| Steel | Less expensive | Largely have been replaced by their platinum counterparts in recent years. Once advanced into catheter, can no longer be retrieved without removing entire catheter. Severe local artifact on MRI |
$ |
| Platinum | Softer, more malleable, more radiopaque on fluoroscopy, results in less severe local artifacts on MRI | More expensive. Still produces artifact on imaging (as opposed to other embolic agents such as gel-foam, glue, etc.) |
$$ |
| Coil deployment method | |||
| Pushable | Easy to use, rapid deployment, widely available | Can become jammed, especially in tortuous vessels with acute turns. Cannot be repositioned. Prone to catheter reflux and retraction upon deployment. Not ideal if very little purchase within the target vessel or if nontarget embolization would be catastrophic. |
$ |
| Injectable | Very rapid deployment, easy to use, widely available | Similar to pushable coils—more prone to reflux and catheter retraction upon deployment resulting in nontarget embolization | $ |
| Detachable | Precise and controlled deployment, allows for repositioning and reconfiguration of the coil | Expensive—oftentimes more expensive than the pushable counterpart. Less readily available and less variety of sizes/shapes. More intricate mechanisms for deployment and more dependent on operator experience |
$$$$ |
| Coil coatings | |||
| Fibered coils | Readily available, variable shapes and sizes, conform to target vessel anatomy. Fibered thrombogenic material facilitates embolization | Depends on thrombogenic factors from the patient and may not work in cases of severe coagulopathy | $ |
| Hydrogel-coated | Helpful in cases where mechanical occlusion is primary goal, or in patients with underlying coagulopathy. Expansion in volume can be helpful in aneurysm packing and may be more cost-effective than packing with helical coils. Not as affected by natural thrombolytic processes which result in recanalization | Detachable coil, more expensive. Takes up to 20 minutes to reach complete volume expansion of hydrogel polymer. Coil may become stuck in catheter. Requires more time as the coil should be prepared in warm saline before deployment (1–5 min) |
$$$ |
General Coil Technology
Coils offer permeant embolization when deployed by inducing thrombosis and complete occlusion of the target vessel. This is primarily achieved through three synergistic mechanisms: (1) slowing of flow through the vessel due to mechanical blockage, (2) acting as a thrombogenic scaffold for clot formation, and (3) inducing vessel wall damage that results in the release of thrombogenic factors. 3 Typically, thrombosis occurs within 5 minutes after deployment, although this timeframe may vary depending on the type of coil used, size of vessel embolized, and the degree of flow through the structure embolized. Over time, the acute coil–thrombus complex becomes organized thrombus with development of neointimal hyperplasia and fibrosis of the underlying vessel ( Fig. 2 ). 4
Fig. 2.
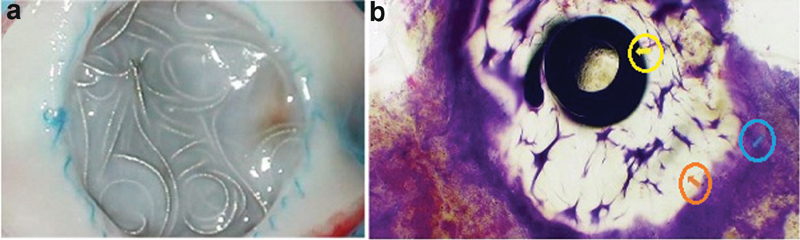
Neointimal proliferation, thrombus formation, and vessel occlusion from coil deployment. ( a ) Gross specimen demonstrating tissue proliferation at the site of hydrogel coil deployment at ∼3 months postintervention. ( b ) Microscopy at 72 days demonstrating neointimal hyperplasia and thrombus formation (blue arrow) adjacent to hydrogel (orange arrow) and platinum coil (yellow arrow). (Images used with permissions from Terumo Interventional Systems.)
Patient Selection
Coils are typically used for occlusion of vessels and cause complete occlusion analogous to surgical ligation. As the coil acts as a thrombogenic agent and depends on the patient's coagulation cascade to induce thrombosis, they may not result in complete occlusion in markedly coagulopathic patients, for example, those with severe thrombocytopenia, platelet dysfunction, or coagulation-cascade impairments. 5
Types of Available Coils
Materials
Coils are generally constructed from either platinum or stainless steel. Previous studies on the treatment of arteriovenous malformations (AVMs) have demonstrated no difference in efficacy between platinum and steel. 6 Platinum coils, while more expensive than stainless steel, are softer, more malleable, and offer better visualization on fluoroscopy as they are more radiopaque ( Fig. 1 ). Additionally, steel results in severe local artifact on magnetic resonance imaging, which is less pronounced with platinum coils. Because of these properties, micro- and most modern-day coils are usually made from platinum wire.
Coils can be bare or fibered with various materials to increase thrombogenicity. Example materials used to fiber-coat coils include Dacron, nylon, polyester, silk, polyvinyl alcohol, or wool ( Fig. 1 ). Coils can also be covered with a hydrogel polymer, which allow the coil to expand to four times their size (ninefold in volume) within 20 minutes of deployment ( Fig. 1 ). This allows for an enhanced mechanical vascular occlusion effect, as opposed to a more prothrombotic effect initiated by fibered coils ( Fig. 2 ). Because of this, hydrogel coils depend less on thrombus formation and may be suited in patients with underlying coagulopathy. Also, unlike other coils, hydrogel coils are less affected by innate thrombolytic processes which may result in recanalization of the embolized segment. 7
Coils are classified into multiple stiffness levels including ultrasoft, soft, standard, firm, and stretch resistant. Softer coils are more pliable, allowing for better tractability through turns and denser coil packing. 8 Stiffer coils in contrast may provide a higher radial force and are ideal anchoring or scaffolding coils (see section “General Technical Considerations” later).
Liquid coils are ultrasoft platinum coils without fibering that are delivered hydrostatically with forceful injection of contrast through the catheter. Despite being made of metal, liquid coils earned their name through their ability to be more-easily tracked through tortuous vessels and acute turns, ultimately conforming into the space they are deployed, akin to a liquid embolic.
Shapes and Sizes
Coils are available in a wide range of diameters, lengths, and shapes. Size is typically designated in three numbers, where the first number is the diameter of wire used to make the coil (usually in inches), the second number is the length in centimeters, and the third number is the diameter of the coil complex in millimeters. Coil wires range in size from 0.008 to 0.052 inches, with lengths from 1 to 300 mm and diameters from 1 to 27 mm. Examples of different coil shapes include helical, conical, tornado, straight, J-shaped, C-shaped, or S-shaped and three-dimensional shapes.
Deployment Method
The three main filter types by deployment method are pushable, injectable, or detachable. Pushable coils are deployed with a guidewire which mechanically pushes the coil into the desired position. Injectable coils are pushable coils that can be deployed with saline injection through the catheter. Detachable coils are coils which are attached to an introducer wire and then deployed with a specific detachment mechanism at the operator's discretion. The advantages and disadvantages of each coil deployment type are discussed later.
Pushable Coils
Pushable coils are the most common and most often used type of coil considering their relatively low cost, ease and speed of use, and availability in most angiography suites. Pushable coils, however, can become lodged within the catheter, in particular when making acute turns in tortuous vessels. Once deployed, they cannot be repositioned. Pushable coils can also reflux/kick-back the catheter upon deployment resulting in nontarget embolization, especially when placing an oversized coil or when there is minimal purchase of the catheter within the target vessel.
Injectable Coils
Injectable coils are rapidly deployed, as a pusher wire does not need to be used. This allows for multiple coils to be placed in a rapid fashion. However, this results in less precise embolization and is highly prone to reflux and retraction of the catheter. Therefore, use of injectable coils often requires operator experience and secure purchase within the target vessel to avoid nontarget embolization.
Detachable Coils
Detachable coils, which are attached to an introducer wire, can be deployed with control and precision by either a mechanical, hydrostatic, or electrolytic mechanism. Mechanical detachable coils use either screw release or interlocking mechanisms ( Fig. 3 ). Screw-release coils are attached to the introducer wire by clockwise rotation, and using a mandrill, the coil can be detached by counterclockwise rotation. One possible complication of screw-release coils is that the coil can flip with inadvertent rotation of the wire and result in unintended nontarget embolization. Interlocking mechanical detachable coils are fastened by overlapping interlocking “bead hinges” to the introducer. Once the coil is released from the catheter, these bead hinges separate and no longer interlock, allowing for coil deployment. This deployment method can often be difficult to deliver through tortuous vasculature or acute turns due to significant friction between the coil and the catheter. Hydrostatic deployment uses a microballoon that attaches the coil at the end of the delivery system. When the microballoon is expanded with an operator-controlled inflator, the coil is released. Similar limitations exist for hydrostatic detachment systems as the previously described mechanical detachable coils. Electrolytic detachment involves using a coil which is welded to the introducer system and a very small electrical current is used to detach the coil at the weld point. The major advantage of this design is that it allows the operator to easily reposition or change the configuration of the coil before deployment. Furthermore, unlike mechanical detachable coils, no motion (such as wire rotation or system advancement) is involved in the detachment, and therefore one can achieve very precise and controlled deployment. The major disadvantage of these coils is that they are expensive and require an extensive setup before delivery. Although a rare complication, the coil can fail to detach, possibly due to melting of the catheter tip by the electrical current. 9 10 Nonetheless, these coils have become an important part of the interventional armamentarium in cases where precision is essential and where downstream nontarget embolization would be catastrophic.
Fig. 3.
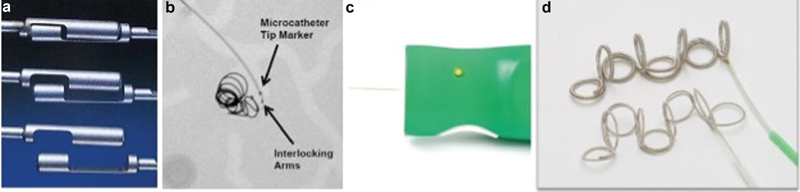
Detachable coil systems. ( a ) Boston scientific detachable interlock and IDC embolization coil via a coupling mechanism. ( b ) Fluoroscopy demonstrating interlocking coil microcatheter and deployed coils. ( c ) Terumo Azur detachable coil remote with coil detachment at push of a button. ( d ) Detachable Azur coils and deployment catheter. (Images used with permissions from Boston Scientific and Terumo Interventional Systems.)
General Technical Considerations
Preprocedural Planning, Inventory, and Catheter Selection
As with any other intervention, preprocedural planning is paramount to achieving technical success with embolization. An interventionalist should have an idea of what delivery system they are planning to employ, and whether these tools and correlating embolics are available in inventory.
In general, an introducer sheath should always be used for arterial access embolization cases, as if the coil becomes lodged irreversibly within the catheter, both the catheter and coil can be removed from the patient as a unit and arterial access will be maintained. A catheter with side holes should never be used at embolization, as the coil can become wedged within the side hole.
Each coil has its own catheter size requirement and ensuring that a compatible catheter is available and used will save any potential missteps during the procedure. For example, regularly sized coils are available in both 0.038- and 0.035-inch thickness; however, a 0.038-inch coil would become jammed in a 0.035-inch catheter. Inventory wise, it may be more practical to keep stock of only 0.035-inch coils that fit through both 0.035- and 0.038-inch catheters to avoid this possible complication altogether. Similar issues arise if microcoils are not deployed using the correctly sized corresponding catheter. If a micro coil is attempted to be deployed through a standard-sized catheter, it may coil within the catheter itself and cause a complete catheter occlusion. When using a larger microcatheter (e.g., a 0.027-inch), 0.018-inch microcoils may become jammed if the pushing wire passes side by side over the proximal end of the coil. For this reason, 0.018-inch microcoils should be deployed through a 0.021-inch microcatheter when possible.
Oversizing
Coils should generally be sized 20 to 30% larger than the size of the target vessel on predeployment angiography, although the exact degree of oversizing is dependent on the specific coil used. This prevents unintended distal embolization and coil migration and helps ensure the coil remains seated in the desired location. However, placement of a coil that is “too oversized” could result in incomplete or poor coil formation or may reflux/kick-back the catheter out of position on deployment. Softer coils can be oversized 20 to 30%, while stiffer coils should only be oversized by approximately 1 mm to avoid inappropriate shaping on deployment. 11 Oversizing is often device specific, and interventionalists should be familiar with the specific product they are deploying.
Scaffold and Anchoring Techniques
Scaffold technique for coil deployment involves using a larger coil with a higher radial force first as a “scaffold,” followed by several softer coils ( Fig. 4 ). This is often helpful to ensure that the coil pack is positioned appropriately, as using softer coils first may result in distal migration or an unexpected shape of the deployed coil. Anchoring technique involves using a distal coil to “anchor” within a branch vessel, and then additional coils are packed proximally ( Fig. 5 ). These techniques prevent nontarget embolization and result in a higher density coil pack. Several additional techniques, such as packing coils behind balloons or vascular occlusion devices, can also be used to prevent distal embolization, and are particularly useful in wide-necked aneurysms or high-flow systems such as AVMs. 12
Fig. 4.
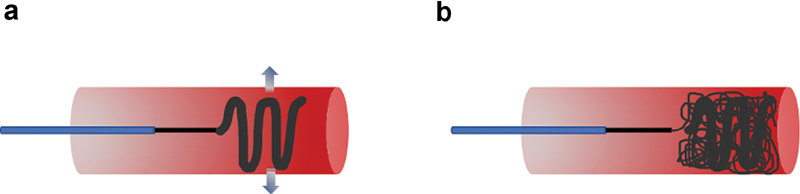
Scaffolding technique for coil deployment. ( a ) An oversized, high radial force (arrows) coil is first deployed into the target vessel to act as a scaffold and to prevent distal coil migration. ( b ) Several softer, smaller coils can then be deployed to achieve complete vessel occlusion.
Fig. 5.
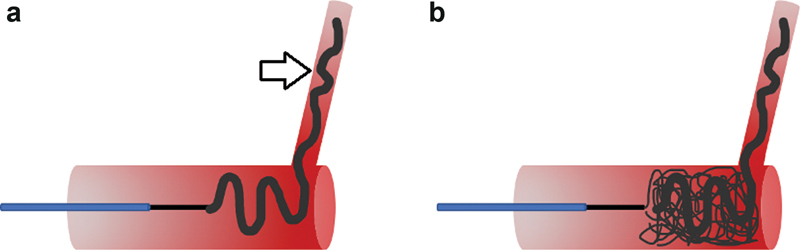
Anchoring technique for coil deployment. ( a ) A coil is first deployed partially into a side branch (arrow), as well as within the target vessel, acting as an anchor for all subsequent coils. ( b ) Several additional coils can then be densely packed to achieve complete vessel occlusion.
Aneurysm Packing
When treating aneurysms with coils, the aneurysm sac is frequently densely packed to ensure thrombosis and exclusion. The sac itself must be sufficiently packed with coils to achieve durable technical success. Packing density is defined as the number of coils multiplied by coil volume divided by aneurysm volume. Several easily accessible calculators are now available to quickly calculate the packing density during a procedure. Previous studies have shown that packing densities less than 24% are associated with higher rates of recanalization and coil compaction. 13 In cases with large aneurysms, hydrogel-coated coils which expand on deployment may be helpful (and cost-effective) to achieve more complete aneurysm filling.
Stability and Catheter Reflux/Kick Back
Ensuring that there is stable access before coil deployment is of utmost importance. This not only prevents inadvertent nontarget embolization but also ensures that dense coil packing can be performed without reflux or kick-back of the delivery catheter ( Fig. 6 ). In cases where there is dubious stability of the support system, a triaxial system with a long guiding catheter or sheath can be used. A “wire-test,” when a wire is advanced a few centimeters into the coil target area to simulate coil deployment, can be performed to assess the stability of the system and any potential kick-back. If sufficient stability cannot be achieved, a detachable coil system may ultimately have to be used, especially in a vascular bed where nontarget embolization would be catastrophic.
Fig. 6.
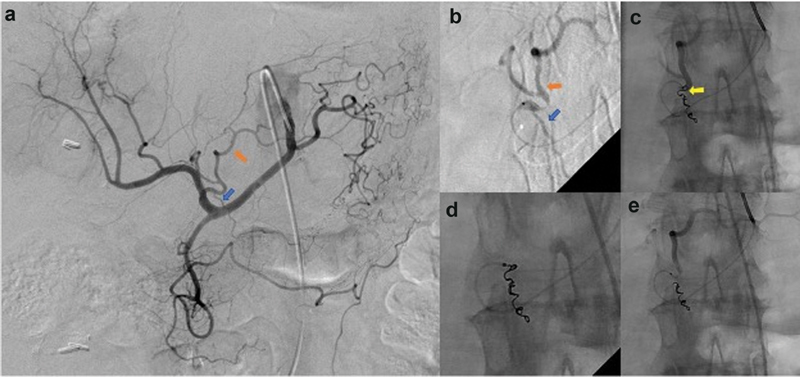
Off-target embolization from catheter reflux and kick-back. ( a ) Celiac artery angiography demonstrates a right gastric artery (blue arrow) arising from the proximal left hepatic artery (orange arrow). Embolization of the right gastric artery is attempted to avoid off-target delivery of radio-embolics to the stomach. ( b ) A microcatheter is used to catheterize the right gastric artery (blue arrow) arising from the left hepatic artery (orange arrow). ( c ) The microcatheter refluxes out of the target right gastric artery with deployment of the last embolization coil (yellow arrow), which appears to be within the left hepatic artery. ( d ) A snare is used to retrieve the unintentional coil deployment within the left hepatic artery. ( e ) Completion angiography demonstrating complete occlusion of the right gastric artery and appropriate contrast-related flow within the left hepatic artery.
Complications of Coil Embolization
Complications of coil embolization include nontarget embolization, coil migration, dissection, and perforation ( Figs. 6 and 7 ). In very rare circumstances, coils may become superinfected or result in a hypersensitivity/allergic reaction. 14 15 Adverse events related to nontarget embolization and/or coil migration can range from no clinical consequence to major complications including stroke, spinal cord ischemia, pulmonary embolism, myocardial infarction, and even death. Vessel dissection and perforation are relatively less common, and the risk can be mitigated in part by using softer coils.
Fig. 7.
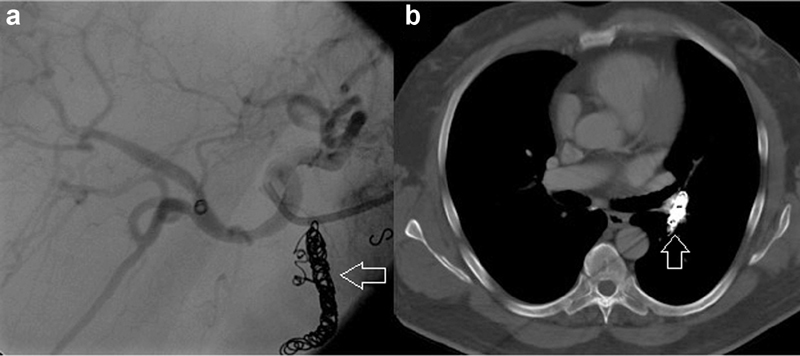
Migration of coil pack. ( a ) Visualization of coil pack in gastroduodenal artery (arrow) pre-radioembolization of the hepatic artery distribution. ( b ) The coil pack (arrow) has migrated to the pulmonary artery.
The “Perfect” Coil
The perfect coil is one which is perfectly suited for the task at hand. While detachable coils are undeniably useful for their precise and controlled deployment, this is often not necessary in most embolization cases. For instance, in a decompensating hypotensive patient with active hemorrhage, a simple pushable or injectable coil will not only be more cost-effective but also quicker to deploy and possibly clinically superior if it stops the hemorrhage sooner. While no one coil device is perfect in its own right, an interventionalist familiar with the unique advantages and disadvantages of their full armament of tools can certainly find a coil that is “perfect” for achieving the desired goal at hand.
Future Coil Applications
Modern coils are predominantly used in the vascular space, specifically the arterial vasculature. Coil design has been optimized for this usage. However, coils can be employed in other spaces as well, such as the veins, lymphatics, and even the ureters. 16 17 As the physiology for both acute and chronic thrombosis is different in veins versus their arterial counterparts, there may be opportunities to design coils which are more effective in the venous system. In theory, coils can also be specially designed and optimized to treat lymphatic duct or ureteral pathologies.
Conclusion
Embolization coils are one of the most widely used embolization agents and are available in a variety of specifications, making them suitable for a wide range of procedures. Familiarity with the numerous types of coils and their specific technical considerations is paramount for optimizing technical success in embolic therapy.
Disclosures/Conflicts of Interest
None declared.
References
- 1.Mullan S. Experiences with surgical thrombosis of intracranial berry aneurysms and carotid cavernous fistulas. J Neurosurg. 1974;41(06):657–670. doi: 10.3171/jns.1974.41.6.0657. [DOI] [PubMed] [Google Scholar]
- 2.Gianturco C, Anderson J H, Wallace S. Mechanical devices for arterial occlusion. Am J Roentgenol Radium Ther Nucl Med. 1975;124(03):428–435. doi: 10.2214/ajr.124.3.428. [DOI] [PubMed] [Google Scholar]
- 3.Henkes H, Brew S, Miloslavski E, Fischer S, Tavrovski I, Kühne D. The underlying mechanisms of endovascular exclusion of intracranial aneurysms by coils. How important is electrothrombosis? Interv Neuroradiol. 2003;9(02):127–140. doi: 10.1177/159101990300900202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki I, Lee D, Murayama Y. Thrombus organization and healing in an experimental aneurysm model. Part II. The effect of various types of bioactive bioabsorbable polymeric coils. J Neurosurg. 2007;107(01):109–120. doi: 10.3171/JNS-07/07/0109. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar D S, Schechtman D W, Thomas S B. Endovascular embolization techniques in a novel swine model of fatal uncontrolled solid organ hemorrhage and coagulopathy. Ann Vasc Surg. 2021;70:143–151. doi: 10.1016/j.avsg.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Prasad V, Chan R P, Faughnan M E.Embolotherapy of pulmonary arteriovenous malformations: efficacy of platinum versus stainless steel coils J Vasc Interv Radiol 200415(2, Pt 1):153–160. [DOI] [PubMed] [Google Scholar]
- 7.Ferral H. Hydrogel-coated coils: product description and clinical applications. Semin Intervent Radiol. 2015;32(04):343–348. doi: 10.1055/s-0035-1564809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White J B, Ken C G, Cloft H J, Kallmes D F. Coils in a nutshell: a review of coil physical properties. AJNR Am J Neuroradiol. 2008;29(07):1242–1246. doi: 10.3174/ajnr.A1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C Y, Yim M B, Benndorf G.Mechanical detachment of Guglielmi detachable coils after failed electrolytic detachment: rescue from a technical complicationNeurosurgery 2008;63(4, Suppl 2):293–294, discussion 294 [DOI] [PubMed]
- 10.Pickett G E, Cora A. Electrothermal coil detachment failure in flow diverter-assisted coiling of a small blister aneurysm: technical considerations and possible solutions. Neurointervention. 2021;16(02):171–174. doi: 10.5469/neuroint.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopera J E. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010;27(01):14–28. doi: 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson P K, Levy D I. Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. AJNR Am J Neuroradiol. 2001;22(01):19–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Yasumoto T, Osuga K, Yamamoto H. Long-term outcomes of coil packing for visceral aneurysms: correlation between packing density and incidence of coil compaction or recanalization. J Vasc Interv Radiol. 2013;24(12):1798–1807. doi: 10.1016/j.jvir.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Falagas M E, Nikou S A, Siempos I I. Infections related to coils used for embolization of arteries: review of the published evidence. J Vasc Interv Radiol. 2007;18(06):697–701. doi: 10.1016/j.jvir.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Uwatoko T, Tsumoto T, Wada N. Dermatitis caused by metal allergy after coil embolization for unruptured cerebral aneurysm. J Neurointerv Surg. 2016;8(10):e42. doi: 10.1136/neurintsurg-2015-011981.rep. [DOI] [PubMed] [Google Scholar]
- 16.Shindel A W, Zhu H, Hovsepian D M, Brandes S B. Ureteric embolization with stainless-steel coils for managing refractory lower urinary tract fistula: a 12-year experience. BJU Int. 2007;99(02):364–368. doi: 10.1111/j.1464-410X.2006.06569.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28(01):63–74. doi: 10.1055/s-0031-1273941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhoke G S, Pandya Y K, Jadhav A P. Cost of coils for intracranial aneurysms: clinical decision analysis for implementation of a capitation model. J Neurosurg. 2018;128(06):1792–1798. doi: 10.3171/2017.3.JNS163149. [DOI] [PubMed] [Google Scholar]
- 19.Simon S D, Reig A S, James R F, Reddy P, Mericle R A. Relative cost comparison of embolic materials used for treatment of wide-necked intracranial aneurysms. J Neurointerv Surg. 2010;2(02):163–167. doi: 10.1136/jnis.2009.001719. [DOI] [PubMed] [Google Scholar]


