Abstract
Chronic peritoneal dialysis (PD) is an underutilized renal replacement therapy in treating end-stage renal disease that has several advantages over hemodialysis. The success of continuous ambulatory PD is largely dependent on a functional long-term access to the peritoneal cavity. Several methods have been developed to place the PD catheter using both surgical and percutaneous techniques. The purpose of this article is to describe the percutaneous techniques using fluoroscopy guidance and peritoneoscope method. While fluoroscopic method uses fluoroscopy guidance and a guidewire to place the PD catheter, the peritoneoscopic technique utilizes a needlescope to directly visualize the peritoneal space to avoid adhesions and omentum during catheter placement. These percutaneous approaches are minimally invasive procedures that can be performed on an outpatient basis without the need for general anesthesia.
Keywords: peritoneal, dialysis, renal failure, percutaneous, image-guided, interventional, peritoneoscopic, interventional radiology
Peritoneal dialysis (PD) catheters are mainly used to perform dialysis in patients with end-stage renal disease (ESRD). However, these catheters can be utilized in a variety of clinical scenarios such as intraperitoneal chemotherapy and gene therapy for ovarian cancer, as well as drainage of uncontrolled refractory ascites. 1 2 The success of the PD as a renal replacement therapy is fundamentally dependent on a functional long-term durable access to the peritoneal cavity via the PD catheter. 3 It is well established that PD modality represents a cost-effective alternative to hemodialysis (HD) due to its comparable survival, lower cost, improved quality of life, and lower complication rates. 4 5 6
Several methods are available to place PD catheters including open surgical placement, laparoscopic placement, peritoneoscopic placement, and fluoroscopic placement. Both percutaneous fluoroscopically guided and peritoneoscopic PD catheter techniques are usually performed by interventional radiologists and interventional nephrologists and considered a minimally invasive procedure that can be performed on an outpatient basis without the need for general anesthesia. 2 7 8 Additional advantages include shorter procedure time, the ability to document the proper catheter placement by imaging, and timely placement, especially for urgent start PD. Currently, only approximately 5% of PD catheters are placed by interventional radiologists and interventional nephrologists. 9
In this article, we will discuss the technique of both percutaneous image-guided and peritoneoscopic PD placement, with particular attention to patient evaluation, catheter selection, and technique details.
Patient Evaluation and Selection
The success of PD therapy requires a thorough evaluation prior to proceeding with catheter placement. This includes a comprehensive history and physical exam along with a review of any absolute contraindications to insert the PD catheter such as ongoing infection, acute diverticulitis, uncontrolled coagulopathy, recent gastrostomy, or enterostomy placement. 10 11 12 Furthermore, other relative contraindications are also reviewed to decide the best course of action. In general, previous abdominal or pelvic surgery, peritoneal adhesions, chronic constipation, inflammatory bowel disease, abdominal wall hernia or abdominal mesh, and morbid obesity are considered relative contraindications and each case should be evaluated on individual basis. 10 13 Reviewing the past surgical history can offer valuable information about the degree of peritoneal membrane damage and scarring. Multiple abdominal surgeries with subsequent extensive scarring may render the peritoneal membrane ineffective for dialysis, irrespective of the method of placing the PD catheter. Additionally, the presence of organomegaly (polycystic kidney disease) or transplanted kidney should be kept in mind, as this may complicate the catheter placement. 12
Of equal importance, prior to proceeding with the procedure, a dedicated PD management team is crucial to evaluate the home environment, social support, and the patient's manual dexterity, vision, and physical ability. The managing nephrologist should approve the candidacy of the patient for PD therapy prior to catheter insertion. 12
Preprocedural Preparation
In the preparation for the PD catheter placement, several steps need to be addressed in a systematic way to assure good outcomes. First, the patient's preference, weight, and body habitus should be assessed to decide the best location of the PD catheter exit site, which could be below or above the belt line, or presternal. Second, bowel preparation is recommended to reduce the colonic distension and postoperative constipation that might affect the functionality of the PD catheter. This can be accomplished by administering an enema on the night prior to the procedure or by taking laxatives. Third, a basic metabolic panel, complete blood count, and coagulation profile should be obtained preoperatively to correct any coagulopathy, electrolyte abnormality, or thrombocytopenia. Moreover, anticoagulants should be held according to the general guidelines as recommended by the Society of Interventional Radiology. 14 Lastly, as the procedure is performed under moderate sedation, the patient should be fasting prior to procedure according to the institution's protocol. 2
Catheter Selection
Appropriate catheter design is chosen to allow for pelvic location of the distal catheter and an appropriate exit site is identified that is easily accessible by the patient, and away from belt line, skin creases and folds ( Fig. 1 ). 15 There is no consensus regarding the superiority of a particular design or length of the available catheters. Double cuff swan-neck Tenckhoff catheter design is commonly used in clinical practice. This catheter is made of silicon rubber; has a 16-cm coiled segment that contains 1-mm side holes; and its internal diameter measures 2.6 mm. 15 The deep (preperitoneal) cuff is anchored within the anterior rectus sheath (rectus abdominis muscle) to promote tissue ingrowth, which, in turn, prevents pericatheter hernia and leaks. The squamous epithelial cells of the parietal peritoneum reflect along the PD catheter and reach the deep cuff. On the other hand, the superficial (subcutaneous) cuff is placed within the subcutaneous tissue approximately 2 to 3 cm from the exit site to reduce cuff infection or extrusion. The stratified squamous epithelial cells of the skin follow the surface of PD catheter until reaching the superficial cuff ( Fig. 2 ). 15 The catheter is prepared by being placed into a surgical bowl filled with saline. The polyester fiber cuffs are manually compressed to extrude any trapped air to allow for tissue ingrowth.
Fig. 1.
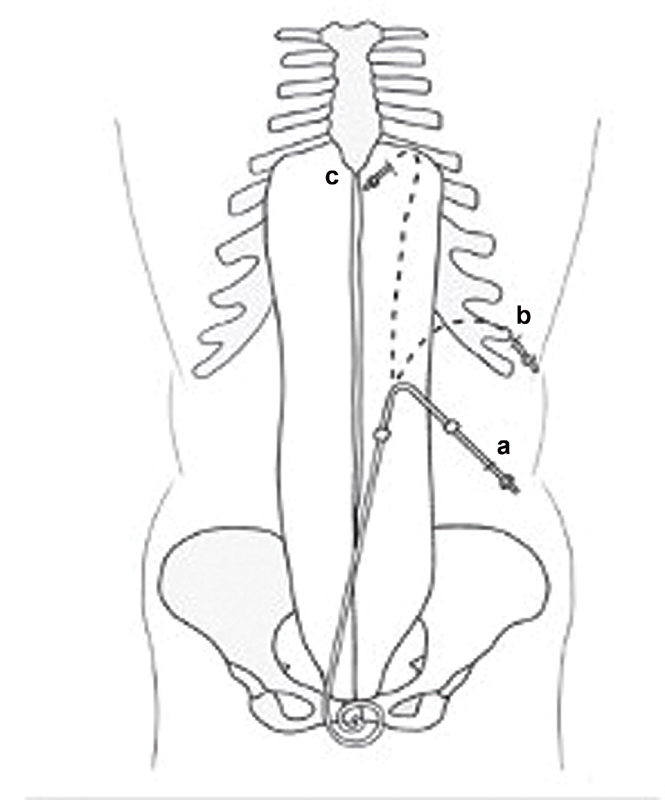
Peritoneal dialysis catheter exit sites: ( a ) below the belt; ( b ) above the belt; ( c ) presternal.
Fig. 2.
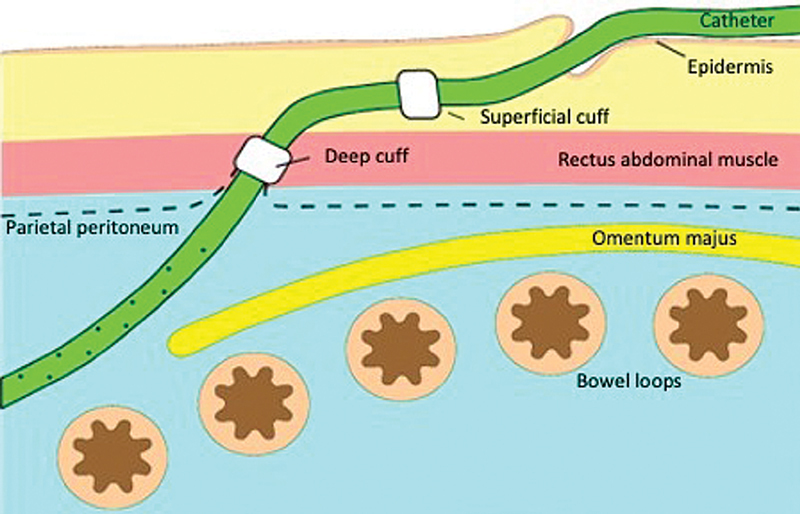
The location of both deep and superficial cuffs of the peritoneal dialysis catheter.
Upon Arrival to Procedure Suite
Before proceeding to the interventional suite, several steps are undertaken. The belt line is determined while the patient is fully dressed in both sitting and standing positions. Marking both the entry and exit sites for the catheter is performed using a nonsterile double-cuff PD catheter or a commercially available stencil. While the patient is in supine position, the upper border of the PD catheter curl is aligned to the upper border of the symphysis pubis. Then, the catheter length from the curled end to the deep rectus polyester fiber cuff determines the entry site location, which should be 2 to 3 cm lateral to the midline to prevent the injury of the inferior epigastric artery. 16 The inferior epigastric artery arises from the femoral artery and courses toward the umbilicus and is located behind rectus muscle approximately at its midline. The borders of the rectus muscle can be determined by using the ultrasound. The exit site is then marked such that the superficial subcutaneous cuff is at least 2 to 4 cm away from the exit site. 12
Signed consent of the procedure, its risks and benefits, and possible alternatives are obtained prior to administering any sedation and before proceeding to procedure. Furthermore, the urinary bladder should be emptied, and if the patient has bladder dysfunction, a Foley catheter can be placed. Preprocedural prophylactic antibiotics are usually administered perioperatively such as intravenous cefazolin. Vancomycin can be used if the patient is allergic to penicillin or cephalosporin antibiotics. 12 17 18
Percutaneous Fluoroscopy-Guided Technique
After placing the patient in supine position, a preliminary sonographic examination of the abdomen is performed prior to patient prepping to determine the safest puncture site ( Fig. 3a ). The abdomen is prepped, and the patient is draped in the standard sterile fashion. Moderate sedation is achieved using intravenous midazolam hydrochloride and fentanyl citrate with continuous monitoring of the vital signs and electrocardiography by the operating physician and a dedicated nurse. Rarely, general anesthesia is needed for patients who require continuous positive airway pressure ventilation or who are on chronic narcotics.
Fig. 3.
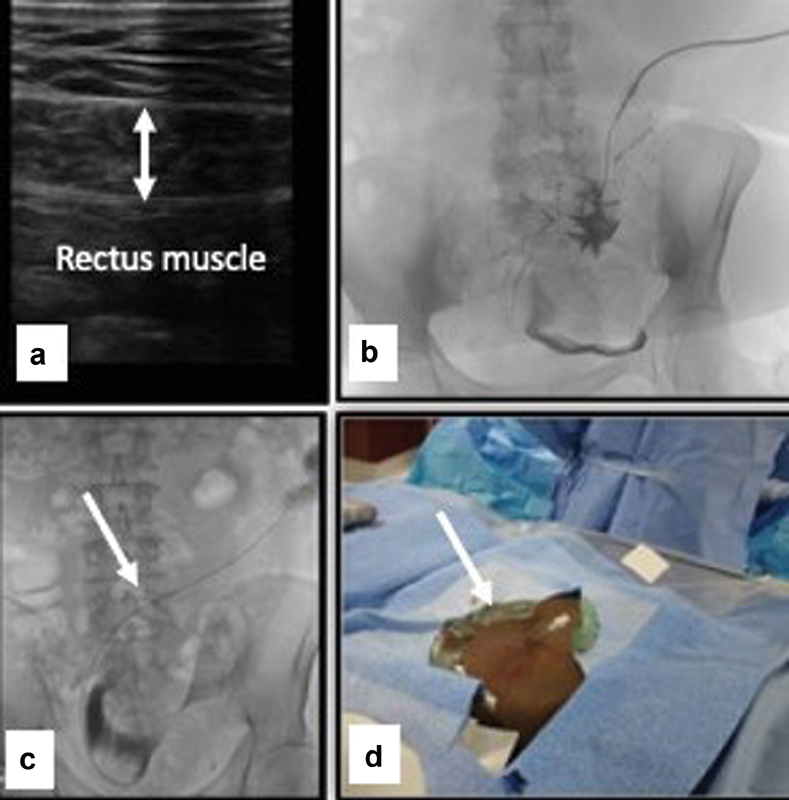
( a ) Screening ultrasound to determine the point of entry; ( b ) contrast injection to confirm peritoneal cavity entry; ( c ) guidewire (arrow) is negotiated toward the pelvis; ( d ) change to 5-Fr sheath (arrow).
Lidocaine 1 to 2% is infiltrated at the skin entry and incision sites as well as subcutaneous and deep tissues of the anterior abdominal wall to provide local anesthesia. Under sonographic guidance, a 21-gauge micro puncture needle (Stiffen microintroducer kit: Galt Medical Corp, Garland, TX) is advanced in a caudal direction toward the pelvis at 45-degree angle to enter the peritoneal space. 11 17 Once the peritoneal cavity is entered, a small amount of iodinated contrast (3–5 mL) can be injected under fluoroscopy guidance to confirm the peritoneal cavity entry ( Fig. 3b ). If small bowel mucosal folds or colonic haustra are outlined, inadvertent bowel puncture is confirmed. Once encountered, the procedure should be abandoned to avoid peritonitis and the procedure can be rescheduled in 1 to 2 weeks. Occasionally, a 1- to 2-week course of antibiotics can be administered in case of bowel puncture. 2 3 17
Full peritoneography can be performed using diluted iodinated contrast (100–200 mL) infused into the peritoneal space under fluoroscopy, which helps outline the retrovesical (retrouterine) space where the catheter is ideally placed. 3 13
After successful entry into the peritoneal cavity, a nitinol microwire (included in the microintroducer kit) is advanced into the peritoneal cavity. The needle is then exchanged over the nitinol wire for the 4- or 5-Fr micro sheath. The wire and inner stiffener of the sheath are then removed, and contrast is injected through the sheath to confirm positioning within the peritoneal cavity. Next, under fluoroscopic guidance, a 0.035-in. guide wire (Glide Wire Stiff Shaft: Terumo, Tokyo, Japan; Bentson Wire: Boston Scientific, Marlborough, MA) is advanced through the sheath and negotiated toward the pelvis ( Fig. 3c ). A 4- or 5-Fr Bernstein or KMP catheter (Slip-Cath Beacon Tip: Cook Medical) or C1 catheter (Cordis, Miami Lakes, FL) can be used along with the stiff guide wire to facilitate advancement toward the posterior pelvic cavity. Ipsilateral anterior oblique projections can help advancing the catheter and guidewire into the retrovesical (retrouterine) space. The micro sheath is then exchanged over the wire for a 6-Fr introducer sheath (Pinnacle: Terumo), and contrast is injected through the sidearm to confirm the positioning in the peritoneal cavity ( Fig. 3d ). A 2- to 4-cm incision (based on the abdominal wall thickness) is made at the entry site and blunt dissection down to the rectus abdominis muscle (anterior rectus sheath) is performed. It is worth mentioning that some operators prefer to make the incision after successful entry into the peritoneal cavity, while others make it prior to needle placement.
Next, a 500 to 1,000 mL of normal saline can be infused through the sidearm of the introducer sheath to separate bowel loops and facilitate catheter advancement. The sheath is then removed, and under fluoroscopic guidance the tract is serially dilated over the wire with an 8-, 12-, and 14-Fr dilator (hydrophilic coated dilator: Cook Medical; Fig. 4a–c ). The dilators should be directed caudally toward the pelvic cavity. Following this, a 16-Fr, 15-cm pull-apart sheath is advanced over the guidewire into the pelvis. After removing the inner dilator of the sheath, the curled end of the catheter is advanced over the guide wire within the pull-apart sheath until the deep cuff reaches the surface of the rectus abdominis muscle ( Fig. 4d, e ). At this point, the catheter and the sheath are advanced together as one unit for approximately 1 cm over the stiff guide wire to bury the deep cuff within the rectus abdominis muscle. 11 19 Next, the pull-apart sheath is split and pulled upward by the assistant, while the primary operator holds the cuff and applies downward pressure on the catheter toward the peritoneal cavity ( Fig. 4f–h ). The curl of the PD catheter should lie within the retrovesical (retrouterine) space that is confirmed with fluoroscopy ( Fig. 5a ). If urgent-start PD is planned, a purse-string absorbable suture should be placed through the deep cuff and around the deep rectus abdominis muscle to decrease the risk of dialysate leak. 10 13
Fig. 4.
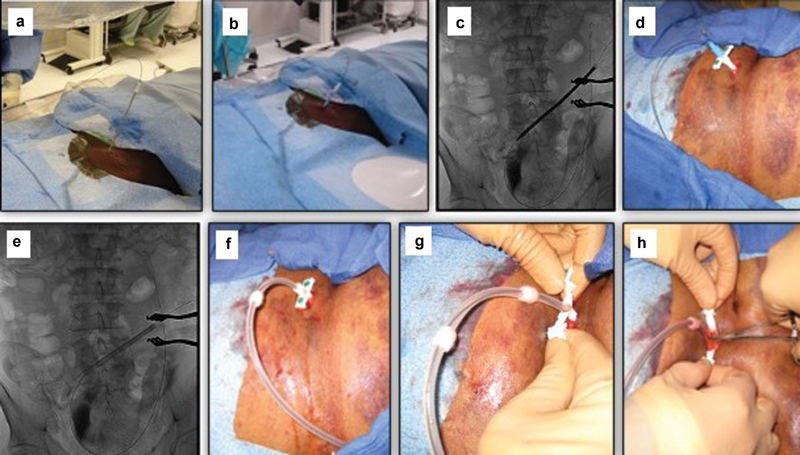
Serial dilatation of the tract ( a–c ) and inserting the peritoneal dialysis catheter using the pull-apart sheath ( d – h ).
Fig. 5.
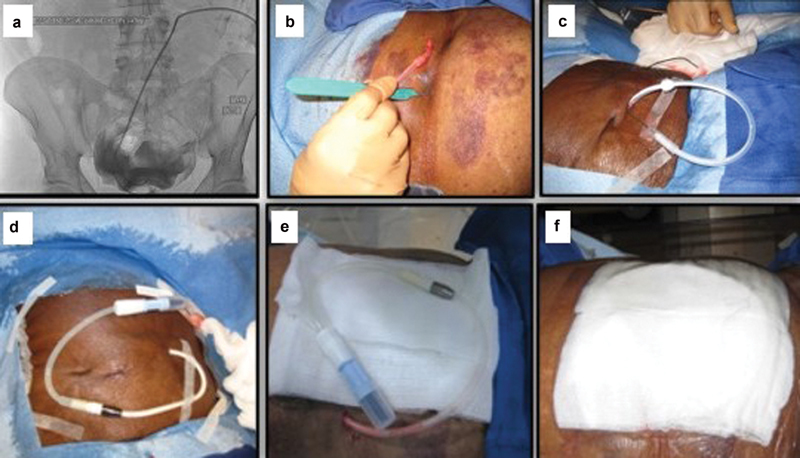
Catheter in the peritoneal space ( a ). Creating the exit site ( b – c ), connecting the connector and extending tube and dressing of the catheter ( d – f ).
The exit site should be located 4 cm lateral and inferior to the peritoneal entry site. 11 The lateral and downward direction minimizes the risk of exit site infection by preventing water and skin debris from pooling at the exit site. 12 17 To create the exit site, a local anesthesia with lidocaine 1% is used and a small 4- to 5-mm incision is made using a number 11 surgical blade ( Fig. 5b ). Now, the catheter's proximal end is then attached to a tunneler, and the catheter is tunneled in a lateral and inferior direction toward the exit site ( Fig. 5c ). Attention should be taken to prevent kinking of the catheter or displacement of the deep cuff from the rectus abdominis muscle while tunneling. The outer end of the catheter is connected to the titanium metallic Luer Lock adapter (included in the kit; Fig. 5d ), and 10 mL of nonionic contrast can be injected under fluoroscopic visualization to exclude any kink or twist in the catheter, and to confirm the proper positioning of the PD catheter coil within the posterior pelvis. Finally, the catheter and its extender tubing system are dressed in the standard fashion ( Fig. 5e, f ).
Peritoneoscopic PD Catheter Placement Technique
Peritoneoscopic placement of PD catheter is a safe nonsurgical technique that is performed in the interventional suite under conscious sedation. This technique uses 2.2-mm peritoneoscope (Y-TEC; Medigroup, Inc., Naperville, IL) to visualize the peritoneal cavity and decide the proper location for placing the PD catheter. The kit that is used in this technique contains a guide assembly, dilators, Cuff Implantor, and Tunnelor. The guide assembly has a cannula, trocar, and Quill guide (split sheath; Fig. 6 ).
Fig. 6.
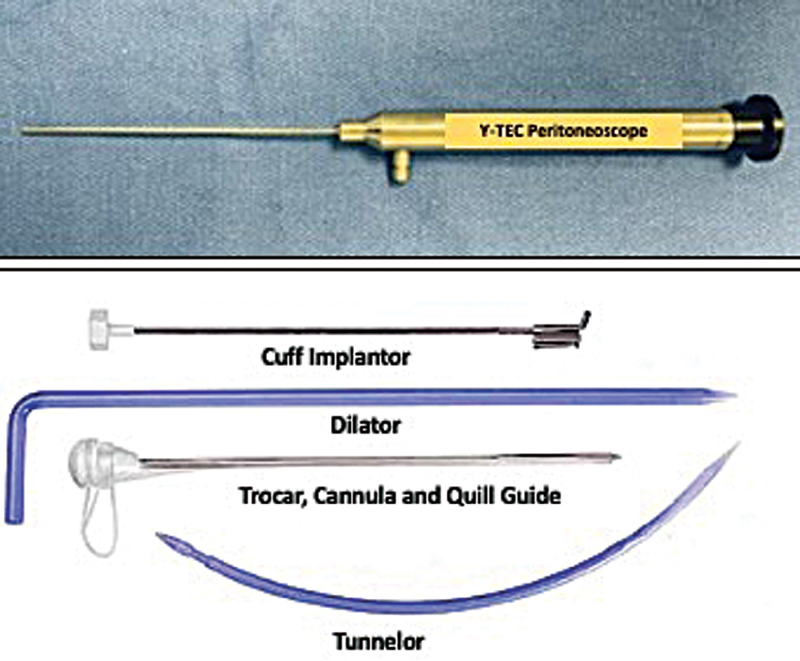
Peritoneoscope and Y-TEC kit.
After prepping the abdomen and draping the patient in the standard sterile fashion, the incision site is anesthetized using 1 to 2% lidocaine. A 3- to 5-cm horizontal (or vertical) skin incision is made ( Fig. 7a ) followed by blunt dissection to the anterior surface of the rectus sheath ( Fig. 7b ). 15 20 Bleeding, if encountered during dissection, can be controlled by using cauterization device, clips, or sutures. Intraprocedural ultrasound can be performed to visualize the best entry point ( Fig. 7c, d ). To facilitate the process of passing through the rectus sheath, a small incision is made ( Fig. 7e ), and then the guide assembly is inserted at a 45-degree angle, pointing toward the coccyx into the peritoneal space ( Fig. 7f ). 21 At this point, the trocar is removed and the Y-TEC peritoneoscope is inserted into the metallic cannula and locked together. Next, the patient is placed in Trendelenburg position and 700 to 1,200 mL of room air is insufflated into the abdominal cavity to create air-filled space between the peritoneal surface of the abdominal wall and bowel loops ( Fig. 7g ). 10 15 20 22 After air insufflation, the peritoneoscope is introduced again through the cannula and the peritoneum is visualized and examined directly to find the optimal space that is free of adhesions and omentum to place the PD catheter. 10 21 23 Now, both the peritoneoscope and cannula are removed leaving the split sheath in place, and the patient is returned to normal supine position ( Fig. 7h ). Importantly, the sheath is usually secured with a hemostat to prevent inadvertent advancement of the sheath into the abdominal cavity.
Fig. 7.
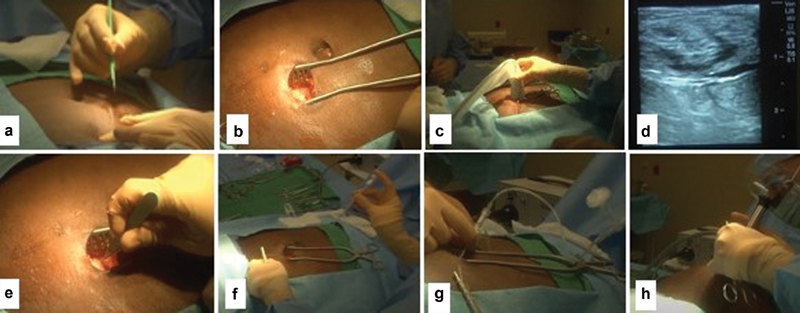
( a ) Skin incision; ( b ) dissection until reaching the surface of the rectus muscle; ( c and d ) intraoperative ultrasound. ( e ) Small incision within rectus muscle; ( f ) entering the peritoneal space using the Quill assembly; ( g ) air insufflation; ( h ) inspecting the peritoneal space using peritoneoscope.
The Quill sheath is then serially dilated with the provided dilators ( Fig. 8a ). 10 22 Next, the PD catheter is mounted on a rigid metallic rod (Stylette) using a lubricant or saline and introduced through the Quill sheath ( Fig. 8b, c ). Now, using a coordinated motion, the PD catheter is advanced into the peritoneal cavity while the Stylette is removed until the deep cuff reaches the rectus sheath ( Fig. 8d, e ). At this point, the Cuff Implantor is placed over the catheter and advanced until reaching the upper edge of the deep cuff. Next, both the catheter and Cuff Implantor are advanced simultaneously into the rectus muscle to facilitate implanting the deep cuff within the muscle ( Fig. 8f ). Then, both the Cuff Implantor and the Stylette are removed allowing the air to exit the peritoneal space. 21 22 23 The catheter is tested by infusing 500 to 1,000 mL of saline and observing the draining and its color.
Fig. 8.
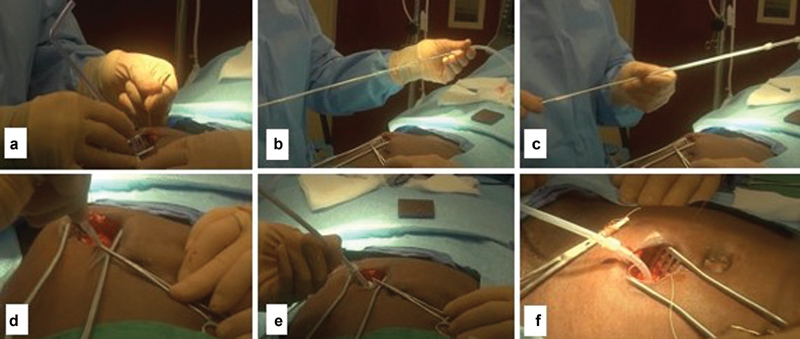
(a) serial dilation of entry site; (b, c) mounting the PD catheter over the Stylette; (d, e) inserting the PD catheter within peritoneal cavity; (f) Stylette is removed and the deep cuff is buried.
To tunnel the catheter, the exit-site location is anesthetized, and a small stab incision is made with a scalpel blade. The Tunnelor is then advanced from the exit site toward the primary insertion site; the catheter is attached to the Tunnelor and secured using sutures. The Tunnelor with the attached PD catheter is then retracted through the exit site ( Fig. 9a–c ). 15 22 24 Since most of the peritoneoscopic PD catheters are usually done in IR suites, a confirmatory imaging of the PD catheter with contrast injection can be done ( Fig. 9d ).
Fig. 9.
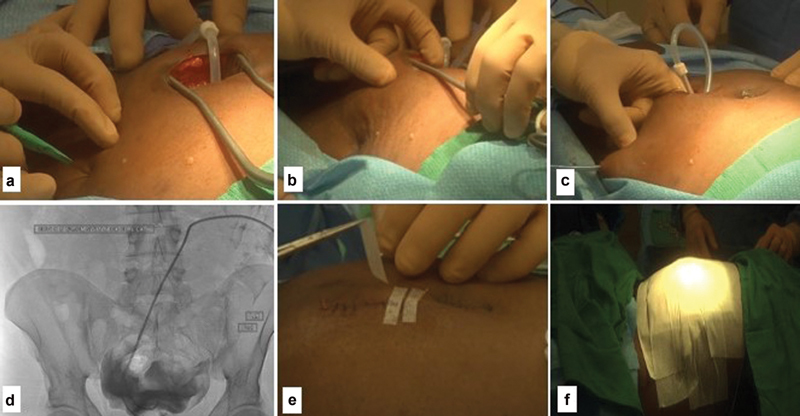
( a – c ) Tunneling the catheter; ( d ) closing the incision; ( e ) confirmation image of proper position of the peritoneal dialysis catheter; ( f ) final dressing.
Testing the Functionality of PD Catheter
A functional test to check catheter patency is performed by infusion of up to 1 L of normal saline to demonstrate free fluid inflow and drainage. Infusion rate greater than 100 mL/min with the saline bag 1.2 to 1.5 m above the patient indicates good infusion. A drainage rate greater than 80 mL/min with the bag on the floor indicates adequate drainage. 13
Incision Closure, Attaching the Tube Extender and Dressing
The entry-site incision is closed using a two-layer closure, where the subcutaneous tissue is closed with absorbable 2–0 Vicryl sutures, and the skin is closed using absorbable 4–0 Monocryl sutures. 25 A topical skin adhesive (Dermabond: Ethicon) can be used for skin closure as well. The adapter is then attached to an external extension catheter (PD Transfer Ser, Baxter Healthcare, Deerfield, IL), which serves as the transfer set for the dialysis solution provider and maintain sterile distal capping of the catheter. After both the catheter and transfer set are entirely covered with gauze, the final dressing can be achieved with a watertight sterile transparent semiocclusive dressing (Tegaderm: 3M Health Care, St. Paul, MN) or any other type of tape ( Figs. 5 and 9e, f ). The gauze is important to absorb any moisture and to prevent contact between the dressing and the catheter. Finally, a MiniCap Disconnect Cap with Povidone-Iodine (Baxter Health Care) is attached to the end of the extension catheter. 13
It is worth mentioning that if the plan is to use the PD catheter acutely, then the transfer set can be excluded from the dressing to allow for an easy access to the transfer set cap and to facilitate connection to the dialysate bag or cycler. 12
Post PD Catheter Insertion Care
Dressing change of the PD catheter should be performed after at least 7 days of insertion by a health care provider using a sterile technique. Earlier dressing change can be performed if there is an obvious hemorrhage or suspicion of infection. The new dressing should be kept in place for an additional 7 days. Only normal saline should be used to clean the exit site and skin incision. After 2 weeks, prophylaxis with mupirocin cream or gentamicin can be applied at the exit site. 12 26 27
Complete healing typically occurs after 21 days, and the exit site should be kept dry until then. Only sponge baths are permitted during this period. The use of the PD catheter for dialysis usually begins 3 to 4 weeks of placement. The patient should be instructed to avoid heavy lifting for 4 weeks following the procedure, to prevent dislodgment of the deep rectus abdominis cuff.
Complications
Several complications of percutaneous image-guided and peritoneoscopic PD catheter placement can be encountered. These include peritonitis, tunnel infection, dialysate leak, intestinal perforation, abdominal wall herniation, catheter cuff extrusion, and catheter dysfunction (due to external or internal occlusion, malposition, or kinking). The management of other complications is beyond the scope of this article and should follow the guidelines of International Society of Peritoneal Dialysis. 10
The bleeding from injury to the inferior epigastric artery or its branches can be encountered early after pacing the PD catheter. The clinical picture may include hypotension, tachycardia, abdominal pain, and severe drop in hemoglobin. While this complication is usually treated conservatively, it may require transarterial embolization.
Discussion
Several studies have shown that PD has similar or slightly better outcomes than HD with early survival advantages. 28 29 30 31 32 33 34 35 36 Furthermore, PD catheters can be placed by surgeons in the operating rooms or by interventional radiologists and interventional nephrologists in the angiography suites as an outpatient procedure using minimally invasive techniques. Initial percutaneous placement included the use of rigid trocar or a modified Seldinger technique, which was subsequently developed to include ultrasound guidance for needle placement and fluoroscopy guidance for guidewire and catheter positioning. 2 32
Percutaneous fluoroscopically guided PD catheter placement is a safe, expedient, and cost-effective procedure that can be performed on an outpatient basis, with reduced hospital costs, avoidance of operating room waiting, and anesthesia involvement. It represents a noninferior alternative to laparoscopic surgical PD catheter placement with similar or even less complication rates. 2 36 37 38 It also allows for urgent initiation of PD and avoidance of temporary vascular access catheters in late-referred ESRD patients, without significant increase in the complication rate in comparison to elective-start PD. 2 39 In a meta-analysis of 13 studies with a total of 2,681 patients, Boujelbane et al found no significant difference in catheter survival between percutaneous and surgical placement of PD catheters. 40 Furthermore, Abdel Aal et al found similar survival and complication rates between fluoroscopically inserted PD catheters and those which were inserted laparoscopically. 41
On the other hand, peritoneoscopic placement of PD catheters is associated with better survival and lower complication rate compared with surgically implanted catheters. 42 43 The survival rates of peritoneoscopically placed PD catheters at 12 and 36 months were 77.5 and 51.3% as compared with 62.5% ( p = 0.02) and 36% ( p = 0.04) in the surgically implanted catheters, respectively. 34 Furthermore, the rate of catheter leak and infection was less frequent in the peritoneoscopic group than its surgical counterpart. 34 To assess the role of insertion techniques on the risks of peritonitis, PD-related infections, and technique failure, Htay et al evaluated 42 studies with 3,144 participants and found that the use of different insertion techniques did not affect the rate of these complications. 44
In conclusion, both percutaneous fluoroscopically guided and peritoneoscopic PD insertion techniques are viable options for PD catheter placement with comparable outcomes to surgical and laparoscopic techniques and possibly better complication profile.
Acknowledgment
We are grateful to Dr. Shouwen Wang for providing some images.
Footnotes
Conflict of Interest A.K.A.-A. is a speaker for Baxter Healthcare. The other authors declare no conflicts of interest associated with this manuscript.
References
- 1.Golper T A, Guest S, Glickman J D, Turk J, Pulliam J P. Home dialysis in the new USA bundled payment plan: implications and impact. Perit Dial Int. 2011;31(01):12–16. doi: 10.3747/pdi.2010.00143. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Aal A K, Gaddikeri S, Saddekni S. Technique of peritoneal catheter placement under fluoroscopic guidance. Radiol Res Pract. 2011;2011:141707. doi: 10.1155/2011/141707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crabtree J H. Peritoneal dialysis catheter implantation: avoiding problems and optimizing outcomes. Semin Dial. 2015;28(01):12–15. doi: 10.1111/sdi.12299. [DOI] [PubMed] [Google Scholar]
- 4.Sinnakirouchenan R, Holley J L. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis. 2011;18(06):428–432. doi: 10.1053/j.ackd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Berger A, Edelsberg J, Inglese G W, Bhattacharyya S K, Oster G. Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease. Am J Manag Care. 2009;15(08):509–518. [PubMed] [Google Scholar]
- 6.Tokgoz B. Clinical advantages of peritoneal dialysis. Perit Dial Int. 2009;29 02:S59–S61. [PubMed] [Google Scholar]
- 7.Reddy C, Dybbro P E, Guest S. Fluoroscopically guided percutaneous peritoneal dialysis catheter placement: single center experience and review of the literature. Ren Fail. 2010;32(03):294–299. doi: 10.3109/08860220903548932. [DOI] [PubMed] [Google Scholar]
- 8.Savader S J. Percutaneous radiologic placement of peritoneal dialysis catheters. J Vasc Interv Radiol. 1999;10(03):249–256. doi: 10.1016/s1051-0443(99)70026-6. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree J H. Who should place peritoneal dialysis catheters? Perit Dial Int. 2010;30(02):142–150. doi: 10.3747/pdi.2009.00066. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree J H, Shrestha B M, Chow K M. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39(05):414–436. doi: 10.3747/pdi.2018.00232. [DOI] [PubMed] [Google Scholar]
- 11.Maya I D. Ultrasound/fluoroscopy-assisted placement of peritoneal dialysis catheters. Semin Dial. 2007;20(06):611–615. doi: 10.1111/j.1525-139X.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Aal A K, Dybbro P, Hathaway P, Guest S, Neuwirth M, Krishnamurthy V. Best practices consensus protocol for peritoneal dialysis catheter placement by interventional radiologists. Perit Dial Int. 2014;34(05):481–493. doi: 10.3747/pdi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris C S. Interventional radiology placement and management of tunneled peritoneal dialysis catheters: a pictorial review. Radiographics. 2020;40(06):1789–1806. doi: 10.1148/rg.2020200063. [DOI] [PubMed] [Google Scholar]
- 14.Davidson J C, Rahim S, Hanks S E. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions - Part I: Review of anticoagulation agents and clinical considerations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(08):1155–1167. doi: 10.1016/j.jvir.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Ash S R. Chronic peritoneal dialysis catheters: procedures for placement, maintenance, and removal. Semin Nephrol. 2002;22(03):221–236. doi: 10.1053/snep.2002.31710. [DOI] [PubMed] [Google Scholar]
- 16.Joy P, Prithishkumar I J, Isaac B. Clinical anatomy of the inferior epigastric artery with special relevance to invasive procedures of the anterior abdominal wall. J Minim Access Surg. 2017;13(01):18–21. doi: 10.4103/0972-9941.181331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree J H, Burchette R J. Prospective comparison of downward and lateral peritoneal dialysis catheter tunnel-tract and exit-site directions. Perit Dial Int. 2006;26(06):677–683. [PubMed] [Google Scholar]
- 18.Szeto C C, Li P K, Johnson D W. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37(02):141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 19.Vaux E C, Torrie P H, Barker L C, Naik R B, Gibson M R. Percutaneous fluoroscopically guided placement of peritoneal dialysis catheters – a 10-year experience. Semin Dial. 2008;21(05):459–465. doi: 10.1111/j.1525-139X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Asif A. Peritoneal dialysis catheter insertion. Minerva Chir. 2005;60(05):417–428. [PubMed] [Google Scholar]
- 21.Asif A. Peritoneal dialysis access-related procedures by nephrologists. Semin Dial. 2004;17(05):398–406. doi: 10.1111/j.0894-0959.2004.17355.x. [DOI] [PubMed] [Google Scholar]
- 22.Kelly J, McNamara K, May S. Peritoneoscopic peritoneal dialysis catheter insertion. Nephrology (Carlton) 2003;8(06):315–317. doi: 10.1111/j.1440-1797.2003.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Al Azzi Y, Zeldis E, Nadkarni G N, Schanzer H, Uribarri J. Outcomes of dialysis catheters placed by the Y-TEC peritoneoscopic technique: a single-center surgical experience. Clin Kidney J. 2016;9(01):158–161. doi: 10.1093/ckj/sfv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ash S R. Bedside peritoneoscopic peritoneal catheter placement of Tenckhoff and newer peritoneal catheters. Adv Perit Dial. 1998;14:75–79. [PubMed] [Google Scholar]
- 25.Zaman F, Pervez A, Atray N K, Murphy S, Work J, Abreo K D. Fluoroscopy-assisted placement of peritoneal dialysis catheters by nephrologists. Semin Dial. 2005;18(03):247–251. doi: 10.1111/j.1525-139X.2005.18321.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernardini J, Bender F, Florio T. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol. 2005;16(02):539–545. doi: 10.1681/ASN.2004090773. [DOI] [PubMed] [Google Scholar]
- 27.Piraino B, Bernardini J, Brown E. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31(06):614–630. doi: 10.3747/pdi.2011.00057. [DOI] [PubMed] [Google Scholar]
- 28.Yu A W, Chau K F, Ho Y W, Li P K. Development of the “peritoneal dialysis first” model in Hong Kong. Perit Dial Int. 2007;27 02:S53–S55. [PubMed] [Google Scholar]
- 29.Gregoor P J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 2005;67(06):2506. doi: 10.1111/j.1523-1755.2005.360_5.x. [DOI] [PubMed] [Google Scholar]
- 30.Heaf J G, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(01):112–117. doi: 10.1093/ndt/17.1.112. [DOI] [PubMed] [Google Scholar]
- 31.Jaar B G, Coresh J, Plantinga L C. Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med. 2005;143(03):174–183. doi: 10.7326/0003-4819-143-3-200508020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Weinhandl E D, Foley R N, Gilbertson D T, Arneson T J, Snyder J J, Collins A J. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21(03):499–506. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savader S J, Geschwind J F, Lund G B, Scheel P J. Percutaneous radiologic placement of peritoneal dialysis catheters: long-term results. J Vasc Interv Radiol. 2000;11(08):965–970. doi: 10.1016/s1051-0443(07)61323-2. [DOI] [PubMed] [Google Scholar]
- 34.Gadallah M F, Pervez A, el-Shahawy M A. Peritoneoscopic versus surgical placement of peritoneal dialysis catheters: a prospective randomized study on outcome. Am J Kidney Dis. 1999;33(01):118–122. doi: 10.1016/s0272-6386(99)70266-0. [DOI] [PubMed] [Google Scholar]
- 35.Ozener C, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant. 2001;16(09):1893–1899. doi: 10.1093/ndt/16.9.1893. [DOI] [PubMed] [Google Scholar]
- 36.Georgiades C S, Geschwind J F. Percutaneous peritoneal dialysis catheter placement for the management of end-stage renal disease: technique and comparison with the surgical approach. Tech Vasc Interv Radiol. 2002;5(02):103–107. doi: 10.1053/tvir.2002.36054. [DOI] [PubMed] [Google Scholar]
- 37.Latich I, Luciano R L, Mian A. Image-guided approach to peritoneal dialysis catheter placement. Tech Vasc Interv Radiol. 2017;20(01):75–81. doi: 10.1053/j.tvir.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Voss D, Hawkins S, Poole G, Marshall M. Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol Dial Transplant. 2012;27(11):4196–4204. doi: 10.1093/ndt/gfs305. [DOI] [PubMed] [Google Scholar]
- 39.Abdel Aal A K, Mahmoud K, Moustafa A S. Comparative study on the outcomes of elective-start versus urgent-start peritoneal dialysis catheter placement. Radiol Res Pract. 2020;2020:3.751827E6. doi: 10.1155/2020/3751827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boujelbane L, Fu N, Chapla K. Percutaneous versus surgical insertion of PD catheters in dialysis patients: a meta-analysis. J Vasc Access. 2015;16(06):498–505. doi: 10.5301/jva.5000439. [DOI] [PubMed] [Google Scholar]
- 41.Abdel Aal A K, Guest S S, Moawad S. Outcomes of fluoroscopic and ultrasound-guided placement versus laparoscopic placement of peritoneal dialysis catheters. Clin Kidney J. 2018;11(04):549–554. doi: 10.1093/ckj/sfx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copley J B, Lindberg J S, Back S N, Tapia N P. Peritoneoscopic placement of Swan neck peritoneal dialysis catheters. Perit Dial Int. 1996;16 01:S330–S332. [PubMed] [Google Scholar]
- 43.Pastan S, Gassensmith C, Manatunga A K, Copley J B, Smith E J, Hamburger R J. Prospective comparison of peritoneoscopic and surgical implantation of CAPD catheters. ASAIO Trans. 1991;37(03):M154–M156. [PubMed] [Google Scholar]
- 44.Htay H, Johnson D W, Craig J C. Catheter type, placement and insertion techniques for preventing catheter-related infections in chronic peritoneal dialysis patients. Cochrane Database Syst Rev. 2019;5(05):CD004680. doi: 10.1002/14651858.CD004680.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]


