Abstract
Cytomegalovirus (CMV) DNA quantitation in clinical specimens is progressively becoming a cornerstone in the diagnosis and management of CMV infection in the immunocompromised host. We evaluated two automated and reproducible PCR tests, the LightCycler (Roche Molecular Biochemicals, Indianapolis, Ind.) and the COBAS AMPLICOR CMV Monitor (Roche Diagnostics, Pleasanton, Calif.), for the detection of CMV DNA in blood samples from transplant recipients with CMV infection as determined by shell vial culture. Following a log transformation analysis, the mean CMV DNA in plasma (PL), whole blood (WB), peripheral blood leukocytes (PBL), and peripheral blood mononuclear cells (PBMC) using the LightCycler was 6.79 copies per ml, 7.23 copies per ml, 6.38 copies per 2 × 106 cells, and 6.27 copies per 2 × 106 cells, respectively. This compares to 7.86 copies per ml, 8.37 copies per ml, 7.59 copies per 2 × 106 cells, and 7.44 copies per 2 × 106 cells, respectively, using COBAS AMPLICOR CMV Monitor. While higher CMV DNA levels were observed for the various blood compartments analyzed using COBAS AMPLICOR CMV Monitor, a high degree of correlation was evident between the two automated systems (jackknife correlation r = PL 0.77 [95% confidence interval (CI); 0.64, 0.90], WB 0.77 [95% CI; 0.62, 0.92], PBL 0.77 [95% CI; 0.67, 0.88], and PBMC 0.81 [95% CI; 0.72, 0.89], all P < 0.001). Therefore, we conclude that either automated diagnostic system is accurate for CMV DNA quantitation.
Cytomegalovirus (CMV), a betaherpesvirus, remains latent following primary infection by integration within the host cell chromosome or under a low level of replication controlled by an effective anti-CMV immune system. Both reactivation from latency and increased replication are commonly observed following organ transplantation (15). In this population of immunocompromised patients, CMV causes morbidity either directly (e.g., CMV syndrome, hepatitis, pneumonitis, encephalitis, and colitis) or indirectly through its immune-modulating properties (e.g., increased bacterial and fungal infections, or allograft dysfunction) (14).
In recent years, considerable progress has been made in the control of CMV infection among transplant recipients with the use of effective prophylactic and treatment strategies. Despite these, the virus continues to significantly impact transplant outcomes as a result of the use of more aggressive immunosuppression (15). Thus, constant vigilance in the field of CMV diagnosis is again emphasized as this potentially devastating infection continues to remain a critical hurdle to successful organ transplantation. A significant advance in the field of CMV diagnosis in the past decade has been the application of PCR-based assays as rapid, accurate, sensitive, and specific methods for detection of CMV DNA. Because of high sensitivity, the PCR-based assays detect viral replication even prior to the onset of clinical symptoms. The main drawback of using qualitative PCR assays is the inability to differentiate between latency and higher levels of replication (2). In contrast, quantitative methods may be more clinically useful, as higher CMV DNA load predictably correlates with CMV disease (16, 18, 19). Thus, quantitative PCR assays are increasingly being utilized in the clinical setting to (i) identify individuals at risk of developing CMV disease, (ii) provide rapid diagnosis of established CMV disease, (iii) monitor viral response to antiviral treatment, (iv) predict individuals at risk of virologic and clinical relapse, and (v) serve as an early indicator of antiviral resistance (1, 3, 5, 6, 9, 12, 16, 18).
The COBAS AMPLICOR CMV Monitor (COBAS; Roche Diagnostics, Pleasanton, Calif.) is an automated quantitative PCR system that amplifies a sequence of ∼365 bp within the CMV DNA polymerase gene UL54 (4, 11, 17). Its potential clinical utility has been previously assessed (11, 17). We have also developed a quantitative CMV PCR assay using the LightCycler (LC; Roche Molecular Biochemicals, Indianapolis, Ind.) instrument, a system that uses fluorescence resonance energy transfer technology (7, 8). Our assay was optimized in the detection and quantitation of CMV DNA by using primers directed at a highly conserved HindIII-X fragment of CMV (GenBank accession no. X04650, previously shown by our group to confer high sensitivity) (13). Therefore, taking advantage of these two potentially useful, high-throughput, automated, and reproducible quantitative assays, we have analyzed the CMV DNA load in whole blood (WB), plasma (PL), peripheral blood leukocytes (PBL), and peripheral blood mononuclear cells (PBMC) that were simultaneously collected from transplant recipients with CMV viremia prior to and during antiviral treatment.
MATERIALS AND METHODS
Patients and clinical samples.
Sixteen solid organ transplant recipients (9 male and 7 female; including 10 liver, 3 kidney, 2 heart, and 1 pancreas transplant recipients) with CMV viremia defined by the isolation of CMV using shell vial blood cultures (10) were studied between June 1999 and July 2000. The median age of the patients was 49 years (mean, 46; range, 28 to 60). Seventeen episodes of CMV viremia from these 16 patients were included in this study. Following the diagnosis of CMV infection, all patients were immediately treated with intravenous ganciclovir (i.v. GCV), 5 mg/kg of body weight every 12 h for 14 days (adjusted to renal function). Diagnosis of CMV infection prompted the immediate collection of 10 ml of blood (obtained prior to the administration of i.v. GCV) and then weekly collection while patients were on the 2-week i.v. GCV treatment and weekly collection for up to 4 weeks after i.v. GCV treatment. These samples were transported immediately to the research laboratory where they were processed into WB, PL, PBL, and PBMC, as follows.
Two milliliters of WB was divided and stored in two 1-ml conical tubes. The PL was obtained from 3 ml of WB following centrifugation at 660 × g for 20 min. PBL was isolated from 2.5 ml of WB using the Ficoll-Paque method (Ficollpaque; Fine Chemicals, Uppsala, Sweden), counted, and aliquoted at 106 per vial. The PBMC sample was isolated from 2.5 ml of WB using the Histopaque Leukocyte Separation Method 1119 (Histopaque 1119; Sigma, St. Louis, Mo.), counted, and aliquoted at 106 per vial. All samples were stored at −70°C prior to nucleic acid extraction and PCR testing.
A total of 323 samples (82 PL, 75 WB, 84 PBI, and 82 PBMC) from 17 episodes of CMV viremia among the 16 patients were assayed for CMV DNA using COBAS compared to 270 samples (72 PL, 57 WB, 73 PBL, and 68 PBMC) subjected to LC. Of these, there were 266 pairs of samples (72 PL, 56 WB, 72 PBL, and 66 PBMC) that were available for direct comparison between LC and COBAS during all time points of the study.
COBAS AMPLICOR CMV Monitor test.
Nucleic acid was extracted from 200 μl of the WB sample using the QIAamp Midi kit (Qiagen, Inc., Valencia, Calif.). The resulting pellet was resuspended in 200 μl of eluent according to the manufacturer's instructions. This was further subjected to the COBAS specimen preparation step, simultaneously with 200 μl of PL, 4 × 105 PBL, and 4 × 105 PBMC. This preparation step entailed the addition to 600 μl of guanidinium thiocyanate lysis reagent, to which dextran blue and an internal quantitation standard (DNA copy number of 269 copies/ml) had been added. Following this step, the DNA was precipitated with 800 μl of isopropanol by centrifugation, washed once with 1 ml of 70% ethanol, and rehydrated in 400 μl of sample diluent.
From this, 50 μl was added to an equal volume of amplification MasterMix containing deoxynucleoside triphosphate, Taq DNA polymerase, magnesium, uracil-N-glycosylase, biotinylated CMV-specific primers (LC383 and LC342), and salts. An automated amplification system ensued, followed by denaturation to form single-stranded DNA containing biotin-labeled amplified products that were captured by the amplicon-specific oligonucleotide probes. Detection of the hybridization products using the avidin-horseradish peroxidase-tetramethylbenzidine-H2O2 colorimetric reaction was indicated by a blue complex measured at a wavelength of 660 nm. The measured absorbance was compared to the quantitation standard present in the amplification reaction mixture.
The results were reported as a numerical concentration in number of CMV DNA copies per milliliter of WB and PL samples. For the PBL and PBMC, the results of the PCR assay were adjusted as the number of CMV DNA copies per 2 × 106 cells. Three controls (negative, low-positive, and high-positive controls) were simultaneously analyzed with each batch of specimens tested. The assay results for the low-positive control were between 3.6 × 103 and 6.4 × 103 copies per ml, while the high-positive control recorded a target range of 2.5 × 104 to 9.1 × 104 copies per ml. The maximum number of patient samples to be tested per run is 21. All samples per patient were analyzed simultaneously to eliminate interassay variability.
LightCycler CMV DNA PCR assay.
Nucleic acid extraction was performed using the IsoQuick nucleic acid extraction kit (ORCA Research Inc., Bothell, Wash.) on 200 μl of WB, 200 μl of PL, 6 × 105 PBL, and 6 × 105 PBMC following the manufacturer's guidelines. The resulting pellets were rehydrated in 100 μl of RNase- and DNase-free water.
From this, a 5-μl aliquot was added to 15 μl of amplification MasterMix in a cuvette and was subjected to the automated PCR assay using the LC (7, 8). The CMV MasterMix contained 0.05 μM primers, 0.2 μM fluorescein probe, 0.4 μM Red 640 probe, 0.03 U of platinum Taq per μl, 0.2 nM deoxynucleoside triphosphates, 0.2% uracil-N-glycosylase, 0.025% bovine serum albumin, 4% dimethyl sulfoxide, and 4 mM MgCl2. The primers consisted of 291 bp contained at positions 2500 to 2791 of the CMV genome (GGA CGT ATC CAC CTC AGG TAC ACA and TAC GTT ACG AAA CTG AGC TCC CAC), and the probes consisted of 57 bp contained at positions 2565 to 2622 (CGT GTT TCA CAA ACT GCA CCA GTA CCA C-Fluor probe, and Red 640-TAG AGG AAT GTC AGG TAG CGT CTC TCC G-Phos probe). All cuvettes were amplified using the following protocol: 37°C for 5 min, 95°C for 3 min for one cycle followed by denaturation at 95°C, 12 s of annealing at 62°C, and 12 s of primer extension at 72°C for 45 cycles. After amplification, melting curve analysis was performed. The temperature in the thermal chamber was raised to 95°C, rapidly lowered to 54°C, and then slowly raised to 95°C, and the fluorescence was measured every 0.2°C. Analysis of the amplification and melting curve of the PCR products was accomplished using the LC software, version 3.0.
The results of the LC were adjusted by conversion factor to report values in copies per milliliter of WB and PL and copies per 2 × 106 PBL or PBMC. Four controls were analyzed simultaneously during the PCR run. The negative control contained only a 15-μl aliquot of the PCR MasterMix solution. Three quantitation standards using plasmid-positive controls designed to detect copies ranging from 101 to 103 copies were simultaneously analyzed in separate cuvettes during each test run. The maximum number of patient samples that can be tested per run is 28. All samples per patient were analyzed simultaneously to eliminate interassay variability.
Statistical methods.
Our direct comparative analysis was performed only on clinical samples that were tested by both automated systems. Thus, a pair of samples denotes the availability of a sample for CMV DNA detection using both the LC and COBAS. A sample was considered positive (positive sample) if CMV DNA was detected at any level by either LC or COBAS. A negative sample denotes the absence of CMV DNA using both the LC and COBAS.
Data are presented as mean or median values. The correlation between the automated systems was analyzed using the jackknife correlation analysis on log-transformed results.
RESULTS
Of the 266 pairs of samples, there were 192 (72.2%) with detectable CMV DNA (positive samples) by either or both assays. Among the positive samples, CMV DNA was detected by the LC in 170 samples (88.5%) compared to 183 (95.3%) by the COBAS. Both automated assays were congruent in 157 of 192 (81.7%) positive samples. Thirty-five samples (6 PL, 10 WB, 12 PBL, and 7 PBMC) were positive by COBAS test but were negative by LC assay, while 12 samples (4 PL, 1 WB, 3 PBL, and 4 PBMC) were positive by LC but were negative by COBAS. Among these discrepant samples, low levels of CMV DNA were observed: mean CMV DNA load was 546 copies per ml of PL, 523 copies per ml of WB, 90 copies per 2 × 106 PBL, and 37 copies per 2 × 106 PBMC using the LC compared to 1,309 copies per ml of PL, 1,521 copies per ml of WB, 7,774 copies per 2 × 106 PBL, and 1,001 copies per 2 × 106 PBMC using the COBAS.
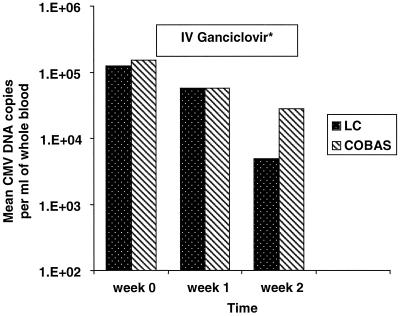
Predictably, we observed a decline in the CMV DNA levels in all patients following the institution of i.v. GCV treatment. The mean CMV DNA levels in WB during the course of CMV disease and its treatment as assessed by the two automated systems are presented in Fig. 1. The CMV DNA levels continued to decline even after the completion of i.v. GCV treatment except in two patients who subsequently relapsed. In these two patients who had relapse, the CMV DNA levels started to increase 1 to 2 weeks after the completion of i.v. GCV treatment and became clinically evident 6 weeks thereafter. Both patients responded to a second course of i.v. GCV therapy.
FIG. 1.
Comparison between the LightCycler and COBAS AMPLICOR CMV Monitor mean CMV DNA levels in WB during CMV infection and/or disease and its treatment. Note: i.v. GCV was administered immediately following the collection of the first blood sample for CMV DNA quantitation and for a duration of 14 days.
Overall, higher CMV DNA levels were observed for samples analyzed using COBAS. Following a log transformation, the mean PL CMV DNA load was 6.79 copies per ml (standard deviation [SD], ±, 2.17) with LC compared to 7.86 copies per ml (SD, ±, 2.53) using the COBAS. The mean WB CMV DNA for all samples was 7.23 copies per ml (SD, ±, 2.58) using LC and 8.37 copies per ml (SD, ±, 2.61) using COBAS. The mean PBL CMV DNA load was 6.38 copies per 2 × 106 cells (SD, ±, 1.91) and 7.59 copies per 2 × 106 cells (SD, ±, 2.42) for LC and COBAS, respectively, while the mean PBMC CMV DNA was 6.27 copies per 2 × 106 cells (SD, ±, 1.89) and 7.44 copies per 2 × 106 cells (SD, ±, 2.40) using LC and COBAS, respectively (Fig. 2).
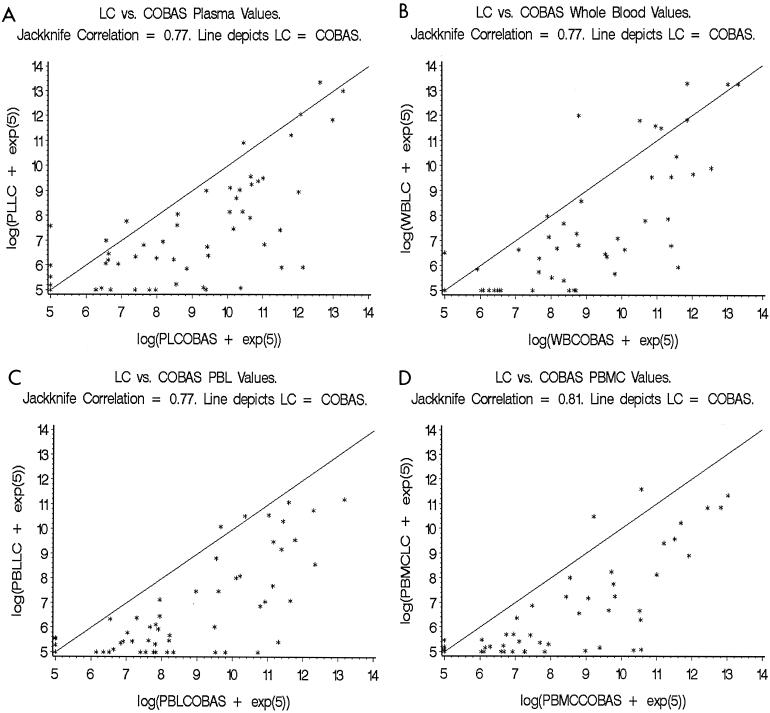
FIG. 2.
Jackknife analysis of correlation of the CMV DNA levels in different blood compartments (PL [A], WB [B], PBL [C], and PBMC [D]) as assessed by the LightCycler and COBAS AMPLICOR CMV Monitor. Note: Each point in the graphs represents an individual sample that was tested for CMV DNA using both the LightCycler (LC) and COBAS AMPLICOR CMV Monitor (COBAS). The x axis represents the log values of CMV DNA per milliliter of WB or PL and per 2 × 106 PBL or PBMC as assessed by the COBAS, and the y axis represents the log values of CMV DNA per milliliter of WB or PL and per 2 × 106 PBL or PBMC as assessed by the LC.
Using the jackknife analysis of correlation, a comparison of the CMV DNA levels of all the individual samples showed a high degree of correlation between the results of the LC and COBAS (jackknife correlation r = PL 0.77 [95% confidence interval (CI), 0.64, 0.90], WB 0.77 [CI, 0.62, 0.92], PBL 0.77 [CI, 0.67, 0.88], and PBMC 0.81 [CI, 0.72, 0.89]; all P < 0.001) (Fig. 2).
DISCUSSION
Using two high-throughput automated assays that were developed for the quantitation of CMV DNA in a series of blood samples obtained from solid organ transplant recipients with CMV infection, our study observed a high degree of correlation between the two assays, the COBAS and the LC. Using these tests, we corroborated the following information: (i) the presence at high levels of CMV DNA among patients with CMV disease and/or infection, (ii) the decline in CMV DNA levels following i.v. GCV therapy, (iii) the direct correlation between the baseline CMV DNA level and its decline (i.e., higher CMV DNA levels at initiation of treatment correlated with longer time to PCR negativity), and (iv) the utility of the quantitative assays in monitoring response to treatment and in predicting relapse. These observations, which confirm and expand previous findings by our group and other investigators using a variety of individual laboratory-designed PCR assays (1–3, 6, 9, 12, 16–19), provide the basis for utilizing these two automated, quantitative, and reproducible assays in the clinical management of CMV infection in immunocompromised patients.
We hypothesize that the difference in the CMV DNA levels detected between the two systems may be explained by several factors. This could be a reflection of a more efficient method of nucleic acid extraction used in the COBAS. We attempted to standardize our PCR input using spectrophotometric readings. However, this was not technically feasible because of the required addition of quantitation standards during the process of extraction of the samples to be analyzed using the COBAS. This will certainly cause a pseudoelevation in the spectrophotometric readings of the COBAS samples. We also note the difference in the number of cells subjected to nucleic acid extraction (4 × 105 for COBAS compared to 6 × 105 for the LC). This is a variable that should provide higher CMV DNA levels for LC since the estimated number of cells extracted by IsoQuick is more than that for the COBAS by a ratio of 3:2. To offset this limitation, we utilized a simple formula to standardize the reporting of genomic copies based on the amount of sample or cell equivalents in the PCR input. Thus, even if the PCR inputs between the two assays were different, their results were converted and expressed similarly (i.e., CMV DNA copies per 2 × 106 cells). Furthermore, the efficiency of amplification could also explain this discrepancy in CMV DNA copies between the two assays. We utilized 45 cycles of amplification using the LC assay. In contrast, the COBAS amplified the targets at a predetermined but not published number of cycles set by the manufacturer (4, 11, 17). In addition, the different efficiencies of the primer sets between the two systems may also account for this variation of CMV DNA levels (2, 13).
Despite these differences (reagents, extraction methods, and instrumentation), however, we observed that the results of the two automated systems were highly comparable. Both are in agreement in 82% of all positive samples. While the LC did not detect 12% of COBAS-positive samples and the COBAS did not detect 5% of LC-positive samples, analysis of these discrepant samples revealed low copy levels of CMV DNA. The clinical relevance of these findings is controversial and beyond the analytical nature of our study. However, applying the observations previously reported by our group and other investigators, these discrepant values may not be of immediate clinical relevance (2, 18, 19).
The selection of an optimal diagnostic assay for use in clinical virology laboratories depends upon several factors. Ideally, the test should possess high sensitivity and specificity and should offer a cost-effective method with convenience and short turnaround time. The PCR assays have proven their superior sensitivity and turnaround time compared to conventional culture assays (19). While conventional cultures would require days before a result is reported, these two PCR assays could give the clinician information about CMV DNA levels within hours from the time of specimen collection. With a very dynamic CMV replication (in vitro doubling time, ∼1 day) (5, 6) and a rapid exponential CMV decline following i.v. GCV treatment, these reliable and rapid methods for CMV DNA detection are certainly advantageous in improving our ability to identify patients who are at high risk of developing disease or who may fail therapy even before it clinically manifests (2, 5, 6, 16).
Contrasting the two automated assays, we determined that, based on the parameters used in the performance of these tests in this study, the LC has a shorter turnaround time from nucleic acid extraction until completion of the full run (240 min compared to 460 min). While this is certainly a theoretical advantage, our study was not designed to detect if this would translate into clinical significance. Additionally, the LC can accommodate more samples in a single PCR run than can COBAS (32 versus 24, respectively). These characteristics would offer benefit to laboratories that process high numbers of samples at any single time.
In summary, we found a high degree of correlation between the results of the LC and COBAS automated systems for the detection of CMV DNA from several compartments of blood obtained from solid organ transplant patients with CMV infection. Thus, we recommend the use of either system in the clinical management of CMV disease in immunocompromised hosts.
REFERENCES
- 1.Abecassis M M, Koffron A J, Kaplan B, Buckingham M, Muldoon J P, Cribbins A J, Kaufman D B, Fryer J P, Stuart J, Stuart F P. The role of PCR in the diagnosis and management of CMV in solid organ recipients: what is the predictive value for the development of disease and should PCR be used to guide antiviral therapy? Transplantation. 1997;63:275–279. doi: 10.1097/00007890-199701270-00017. [DOI] [PubMed] [Google Scholar]
- 2.Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin G, Quirk M R, Kringstad B A, Germain M, Jordan M C. Early effects of ganciclovir therapy on the quantity of cytomegalovirus DNA in leukocytes of immunocompromised patients. Antimicrob Agents Chemother. 1997;41:860–862. doi: 10.1128/aac.41.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiDomenico N, Link H, Knobel R, Caratsch T, Weschler W, Loewy Z G, Rosenstraus M. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin Chem. 1996;42:1915–1923. [PubMed] [Google Scholar]
- 5.Emery V C, Griffiths P D. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc Natl Acad Sci USA. 2000;97:8039–8044. doi: 10.1073/pnas.140123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery V C, Sabin C A, Cope A V, Gor D, Hassan-Walker A F, Griffiths P D. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 7.Espy M J, Ross T K, Teo R, Svien K A, Wold A D, Uhl J R, Smith T F. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol. 2000;38:3116–3118. doi: 10.1128/jcm.38.8.3116-3118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espy M J, Uhl J R, Mitchell P S, Thorvilson J N, Svien K A, Wold A D, Smith T F. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:795–799. doi: 10.1128/jcm.38.2.795-799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerna G, Furione M, Baldanti F, Sarasini A. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J Clin Microbiol. 1994;32:2709–2717. doi: 10.1128/jcm.32.11.2709-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleaves C A, Smith T F, Shuster E A, Pearson G R. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1985;21:217–212. doi: 10.1128/jcm.21.2.217-221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiyoshi M, Tagawa S, Takubo T, Tanaka K, Nakao T, Higeno Y, Tamura K, Shimaoka M, Fujii A, Higashihata M, Yasui Y, Kim T, Hiraoka A, Tatsumi N. Evaluation of the AMPLICOR CMV test for direct detection of cytomegalovirus in plasma specimens. J Clin Microbiol. 1997;35:2692–2694. doi: 10.1128/jcm.35.10.2692-2694.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humar A, Gregson D, Caliendo A M, McGeer A, Malkan G, Krajden M, Corey P, Greig P, Walmsley S, Levy G, Mazzulli T. Clinical utility of quantitative cytomegalovirus viral load determination for predicting cytomegalovirus disease in liver transplant recipients. Transplantation. 1999;68:1305–1311. doi: 10.1097/00007890-199911150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Mendez J C, Espy M J, Smith T F, Wilson J A, Paya C V. Evaluation of PCR primers for early diagnosis of cytomegalovirus infection following liver transplantation. J Clin Microbiol. 1998;36:526–530. doi: 10.1128/jcm.36.2.526-530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel R, Paya C V. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paya C. Prevention of cytomegalovirus disease in recipients of solid-organ transplants. Clin Infect Dis. 2001;32:596–603. doi: 10.1086/318724. [DOI] [PubMed] [Google Scholar]
- 16.Roberts T C, Brennan D C, Buller R S, Gaudreault-Keener M, Schnitzler M A, Sternhell K E, Garlock K A, Singer G G, Storch G A. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J Infect Dis. 1998;178:626–635. doi: 10.1086/515383. [DOI] [PubMed] [Google Scholar]
- 17.Sia I G, Wilson J A, Espy M J, Paya C V, Smith T F. Evaluation of the COBAS AMPLICOR CMV MONITOR test for detection of viral DNA in specimens taken from patients after liver transplantation. J Clin Microbiol. 2000;38:600–606. doi: 10.1128/jcm.38.2.600-606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia I G, Wilson J A, Groettum C M, Espy M J, Smith T F, Paya C V. Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis. 2000;181:717–720. doi: 10.1086/315242. [DOI] [PubMed] [Google Scholar]
- 19.Spector S A, Hsia K, Wolf D, Shinkai M, Smith I. Molecular detection of human cytomegalovirus and determination of genotypic ganciclovir resistance in clinical specimens. Clin Infect Dis. 1995;21(Suppl. 2):S170–S173. doi: 10.1093/clinids/21.supplement_2.s170. [DOI] [PubMed] [Google Scholar]