Abstract
The SARS-CoV-2 virus continues to overwhelm health care systems impairing human to human social and economic interactions. Invasion or damage to the male reproductive system is one of the documented outcomes of viral infection. Existing studies have reported that SARS-CoV-2 may contribute to this loss in relation to inflammatory responses and the formation of cytokine storms in COVID-19 patients. Although direct infection of the testes and entry of SARS-CoV-2 into semen as well as subsequent consequences on the male reproductive system need to be studied more systematically, warnings from two organising ASRM and SART for prospective parents when infected with SARS-CoV-2 should be considered. In the context of an increasingly complex pandemic, this review provides preliminary examples of the potential impact of COVID-19 on male reproductive health and guidance for prospective parents currently infected with or recovering from SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, Male reproductive health, Testes, Semen
Introduction
Since the first patients were reported [1], the COVID-19 pandemic caused by the SARS-CoV-2 virus has spread to 222 countries and territories, with more than 265 million people infected and over 5.2 million deaths (accessed: 4 Dec 2021 https://coronavirus.jhu.edu/map.html). In particular, the appearance of highly transmissible strains of the SARS-CoV-2 virus, such as Alpha (B.1.1.7) and Delta (B.1.617) [2–4], and a sustained increase in the number of globally reported COVID-19 cases, governments and have prioritised the detection of new variants in essential (healthcare staff) and front-line workers.
As members of the Coronaviridae family, SARS-CoV-2 and SARS-CoV-1 are 79.6% genetically similar, with the latter strain responsible for [3] more than 8000 infections in 2003. Both of these viruses use transmembrane serine protease 2 (TMPRSS2) and the angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells [5]. In addition, ACE2 is abundantly expressed in testicular tissue, and the presence of SARS-CoV-2 in semen has been reported [6–8], suggesting the male reproductive system may be susceptible to infection [9]. Moreover, SARS-CoV-2 can also infect cells via the host cell receptor CD147 (basigin, BSG), a transmembrane glycoprotein [10] essential to the integrity of the blood–testis barrier (BTB) [11].
Recent analysis of RNA-Seq-associated proteins at the organ, tissue and cellular levels in both sexes showed elevated ACE-2 expression in thyroid, heart, breast, kidney, intestinal and leg muscle cells (https://www.proteinatlas.org/). Damage to these organs has been reported in patients with COVID-19 [12–14]. The differing levels of expression of ACE2–mRNA between men and women are highlighted in Fig. 1. Of note is ACE-2 expression in the adipose tissues (breast and thigh in women) and, to a lesser extent, the testes in men suggest that women may be more prone to severe disease than men. Yet male mortality rates from COVID-19 are remarkably higher than their female counterparts [15], suggesting a male-specific vulnerability to the disease. In addition, recent studies showed that plasma levels of cytokines, chemokines and interferon (IL-8 and 18, CCL5, IFNγ) were significantly different in male patients compared to female patients, with female patients mounting a more robust T cell response during the course of an infection [16]. Moreover, Cai et al. reported that kynurenic acid (KA) might underlie sex-specific immune responses in COVID-19 [17] by negatively regulating metabolite (glutamate) availability in older males, thereby impairing their immunological response.
Fig. 1.
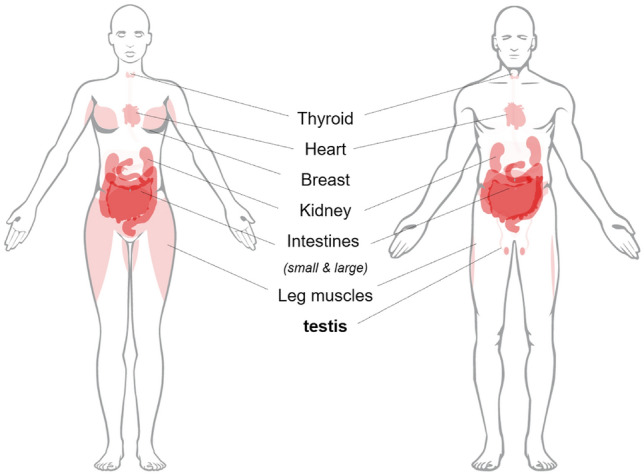
In addition to thyroid, heart, breast, kidney, intestinal, and leg muscle cells, ACE2-rich expression is observed in testis cells with SARS-CoV-2 infection
Due to necessary restrictions, almost all fertility centers have stopped their activities, resulting in the postponement of larger studies [18]. Thus, the impact of the virus on male fertility can only be assessed based on limited information from patients that have died or recovered from an infection.
In this review, we visit the known and potential modes of viral transmission, testicular immunity, male reproductive system, followed by allergic-asthma and hypoxia on their potential impact on male fertility.
Primary mode of transmission
Successful transmission of COV-2 primarily occurs by inhaling aerosols exhaled from infected individuals (vaccinated or unvaccinated) by other persons. Although masks offer some protection (via particle collapse) at distances exceeding 20 cm from infected individuals, masked persons still remain susceptible to infection in unventilated areas [19]. In such conditions, the virions remain unperturbed, making infection of type II alveolar cells (AT2) more likely [20, 21]. It could be argued that the progressive damage to AT2 cells during a symptomatic infection would limit surfactant production and aerosolisation; whether this could be said for asymptomatic (resting state) individuals requires further investigation. As well as alveoli surfactants, Xia et al. showed that the virus is transmitted through conjunctival fluids and tears secreted from mucous membranes [22]. Moreover, studies have shown that the SARS-CoV-2 can persist in saliva, implying that salivary glands may act as significant reservoirs and that enhanced oral hygiene practices in hospitals and schools should be considered [23]. SARS-CoV-2 has also been detected in specimens, such as feces, urine and blood [24, 25], posing additional risks (contamination) to clinical and industrial healthcare professionals.
With the emergence of Alpha, Beta, Delta and Omicron variants, the transmissibility of SARS-CoV-2 has increased several-fold, with experts suggesting the Omicron strain is between 5 and 8 times [26] more transmissible than the original. Variant spike proteins bind to the ACE2 receptor as previously reported; however [27], the three mutations in the receptor-binding domain (RBD) found in the Delta seem to improve its ability to bind to ACE2 as well as its internalisation [28]. In addition, the D614G S protein (NBD) mutation may further enhance Delta's transmissibility by preserving its functional spike density [29], suggesting a lower dose of the variant may be required for infection. For a thorough review regarding the emerging SARS-COV-2 variants of concern and the impact on viral transmissibility, the article by Khateeb et al. is suggested.
The male reproductive system and infertility
The testicles are composed of two distinct anatomical features: the seminiferous tubules and the interstitial spaces among the tubules. Residing in the interstitial spaces are various cell types (minor dendritic cells (DC), T lymphocytes (T) and macrophage) and the Leydig cells. Commonly known as interstitial cells, the Leydig cells occupy the interstitium of the testes and produce the male sex hormone testosterone in the presence of luteinising hormone (LH).
Under the influence of testosterone, non-motile spermatozoa are released into the seminiferous tubules' lumen before entering the epididymis for maturation and storage [30].
The tubules range from 150 to 300 µm in diameter to 30–80 cm in length, forming several layers of peritubular myoid cells (PMC) constituting a tubular wall and Sertoli cells comprising the seminiferous epithelium. The epithelium is separated into two compartments basal and adluminal. Sertoli cells and various junctions form the BTB and are located near the basal side. These cells provide physical and nutritional support to develop germ cells and eliminate apoptotic components generated during spermatogenesis. Timely elimination of these components via phagocytosis (apoptotic germs cells (AGC) & residual bodies (RB)) before their necrosis is necessary to prevent the release of autoantigens and a non-localised immune response [31].
Male infertility accounts for 50% of all infertility cases worldwide [32] and is defined as a “disease of the reproductive system” by the World Health Organization (WHO) (accessed on 9 Dec 2021: https://www.who.int/reproductivehealth/topics/infertility/perspective/en/). Approximately half of all male infertility cases result from some form of defective sperm production, such as low sperm count, abnormal motility or function and complete blockage of spermatogenesis [33]. In the male reproductive tract, two iso-forms of the angiotensin-converting enzyme can be found, germinal isoform (tACE), a marker of male fertility and the somatic isoform (sACE). sACE is a soluble form of the enzyme found in seminal plasma [34] and on the surface of Leydig cells and epididymal epithelial. Whilst, tACE is exclusively expressed in postmeiotic spermatids and spermatozoa [35]. Specifically, sperm motility was recently shown to be negatively correlated with both the percentage of tACE-positive spermatozoa and the number of tACE molecules per spermatozoon, which is consistent with ACE inactivation of bradykinin [33]. Thus, one would expect to see significant differences in sperm motility between infected and uninfected males and the possibility of angiotensinogen II upregulation of microcirculatory environments, a reduction in LH-stimulated testosterone synthesis, capillary leakage and mitochondrial damage in ACE2-rich regions of the male reproductive tract. To the best of our knowledge, no studies have been conducted on the impact of COVID-19 on tACE levels in semen.
The impact of microbial and viral infections on the male reproductive tract
The testis is an immunoprivileged protected site. The majority of that protection is afforded to germ cells via orchestrated active processes associated with Sertoli cells, peritubular cells, Leydig cells, tolerogenic antigen-presenting cells(APCs), T cells and the production of immune-regulatory factors, such as IL10, activin and TGFβ, [36]. Thus, ACE2-rich Sertoli cells may be prone to SAR-COV-2, potentially prolonging fertility problems (impaired phagocytosis, autoimmunity) post infection. In addition, many RNA viruses, such as Mumps (MuV) and HIV, via blood circulation predominantly affect Leydig and Sertoli cells in the testis [37]. Various microorganisms, including bacteria (E. coli), viruses (Human papillomavirus) and protozoa (Trichomonas vaginalis), can also infect the male reproductive tract and impair fertility [38].
Traditionally murine models have been used to study the adverse impact of microbes reducing the risk of patient infection. For example, murine models of experimental autoimmune orchitis (EAO) and Experimental autoimmune epididymo-orchitis (EAEO) induced by immunisation with testis homogenate and adjuvants have been employed in the study of acute and chronic testicular inflammation [39]. Whereas, for bacteria that preferentially infect the accessory glands and epididymis, a pathogen-induced (E. coli) inflammation (epididymo-orchitis) or an acute LPS stimulation may be used [40–42]. Biopsies from infertile patients, and murine models (EAO), show an increase in the number of mast cells(MC) within the walls of the seminiferous tubules and cells that express tryptase [43]. Furthermore, consistent with multiple EAO data sets [44, 45], focal inflammatory lesions in testes of infertile men show activated CD4+ and CD8+ T immune cells and non-resident MC and CD68+ macrophage elevations.
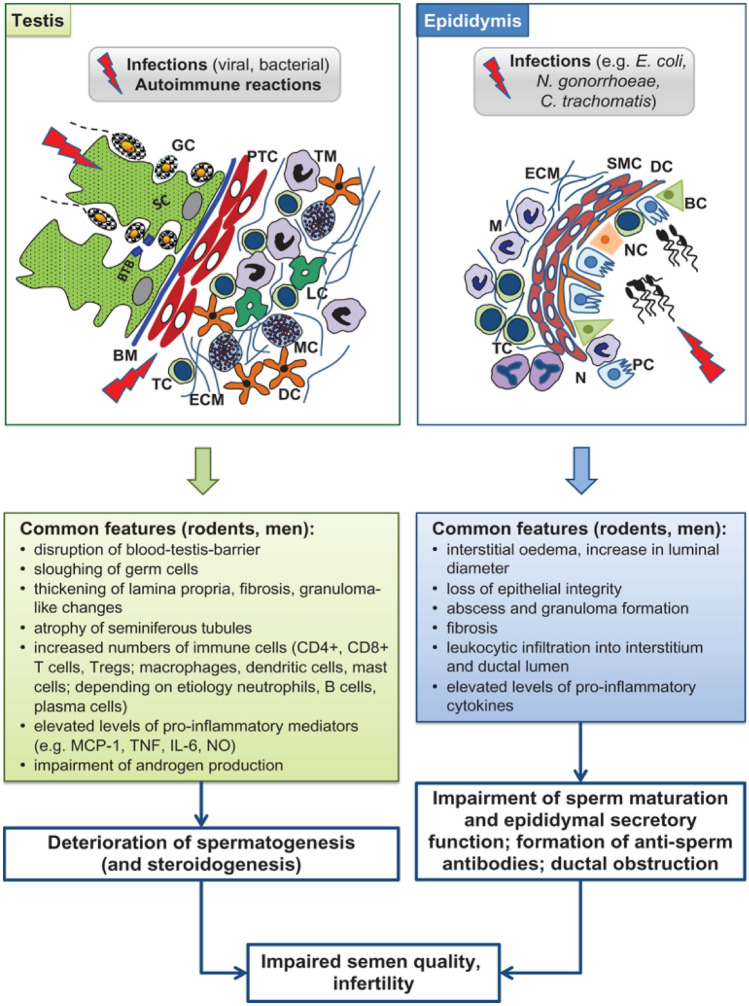
However, animal model phenotypes can differ significantly from what is reported in humans(Zika) [46]. According to Fijak et al. [47], Amongst all animal models related to inflammatory and/or infectious diseases of the epididymis and testis, the acute bacterial epididymitis model is the closest to the clinical situation. The lessons learnt from the different animal models of testicular and epididymal infection, and inflammation are summarised in Fig. 2.
Fig. 2.
Lessons learned from animal models of testicular and epididymal infection and inflammation. BC basal cell, BM basement membrane, BTB blood–testis barrier, DC dendritic cell, ECM extracellular matrix, GC germ cell, IL interleukin, LC Leydig cell, M macrophage, MC mast cell, MCP monocyte chemotactic protein, N neutrophils, NC narrow and clear cell, NO nitric oxide, PC principal cell, PTC peritubular cell, SC Sertoli cell, SMC smooth muscle cell, TC T cell, TM testicular macrophage, TNF tumor necrosis factor, Treg regulatory T cell.
Copyright © The Author(s) 2018. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology [47]
Perhaps one of the reasons for the discrepancies between animal models and humans is LPS–viral interaction. Human circulatory LPS values are connected with HIV and herpesvirus one (HSV-1) and two (HSV-2) infection severity [48, 49]. For example, LPS was reported to enhance human HSV-1 replication and IL-6 release in epithelial cells in vitro [50]. In addition, LPS also interacts with toll-like receptors (TLR4) and myeloid differentiation protein 2 (MD2), both necessary for HSV-2 infection of genital cells in vitro [51]. Furthermore, SARS-COV-2 S protein directly binds with LPS, increasing pro-inflammatory nuclear factor kappa B (NF-kB) and monocytic cell activation in vitro [52]. In addition, the combination of ultra-low levels of LPS and SARS-CoV-2 S protein also yields significant boosting of TNF-α and IL-6 leading to aggravated inflammation. Table 1 shows a compilation of viruses detected in the male genital tract, including SARS-CoV-2, and the effects on male reproductive health.
Table 1.
Viruses that are capable of causing on male reproductive health
| Virus | Family | Genus | Genome | Clinical presentation | Effect on male reproductive health |
|---|---|---|---|---|---|
| Ebola virus | Filoviridae | Ebolavirus | ssRNA ( −) | Hemorrhagic fever | Testis as an anatomic reservoir for persistence |
| Hepatitis B virus | Hepadnaviridae | Orthohepadnavirus | dsDNA (RT) | Hepatitis, cirrhosis, and hepatocellular carcinoma | Sperm parameter alteration and infertility |
| Hepatitis C virus | Flaviviridae | Hepacivirus | ssRNA ( +) | Hepatitis, cirrhosis, and hepatocellular carcinoma | Sperm parameter alteration and infertility |
| Herpes simplex virus type 1 | Herpesviridae | Simplexvirus | dsDNA | Herpes labialis and genital herpe | Prostatitis, epididymitis, infertility, and sperm parameter alteration |
| Herpes simplex virus type 2 | Herpesviridae | Simplexvirus | dsDNA | Genital herpes | Prostatitis, epididymitis, infertility, and sperm parameter alteration |
| Human immunodeficiency virus | Retroviridae | Lentivirus | ssRNA (RT) | AIDS | Orchitis, “Sertoli cell only” syndrome, and infertility |
| Human papillomavirus | Papillomaviridae | Alpha-, beta-, gamma-papillomavirus | dsDNA | Warts and preneoplastic lesions related to oropharyngeal genital and anal cancers | Subfertility and infertility |
| Influenza virus | Orthomyxoviridae | Influenzavirus | ssRNA ( −) | Systemic and respiratory symptoms | Sperm parameter alteration |
| Mumps virus | Paramyxoviridae | Rubulavirus | ssRNA ( −) | Swelling of the parotid glands, salivary glands, and other epithelial tissues | Epididymo-orchitis and infertility |
| SARS-CoV | Coronaviridae | Betacoronavirus | ssRNA ( +) | Severe acute respiratory syndrome | Orchitis |
| SARS-CoV-2 | Coronaviridae | Betacoronavirus | ssRNA ( +) | Severe acute respiratory syndrome | Orchitis |
| ZIKV | Flaviviridae | Flavivirus | ssRNA ( +) | Zika fever and congenital Zika | Orchitis, epididymo-orchitis, and infertility in mouse models. Sperm parameter alteration in men |
The blood–testis barrier and testicular immunity
The BTB is an ultrafine integral structure whose integrity is ensured by testosterone, nitric oxide (NO), cytokines and growth factors. BTB responds to the movement of preleptotene/leptotene spermatocytes to the adluminal compartment. Integrity can be compromised during a viral infection, leading to the infiltration of macrophages (Mϕ) and elevated secretion of pro-inflammatory TNFα, IL6, and NO.
Just like many other viruses, SARS-CoV-2 infections are associated with elevated cytokines, such as IL-6, TNF-α, and IL-12, also interferons, leading to impaired spermatogenesis and a reduced sperm count. High levels of IL-6 are observed in severe cases of COVID-19, and its receptors are highly expressed in testicular cells, which could result in testicular inflammation and the pathogenesis of autoimmune orchitis [53]. Indeed, Li et al. reported abnormal seminal leucocytes, decreased sperm concentration and increased concentration of CD68+ and CD3+ in the interstitial cells of testicular tissue in infected patients, which is consistent with experimental autoimmune orchitis reports [44, 45].
On the other hand, the cytokine IL4 is an anti-inflammatory polypeptide that weighs 15 KD with multiple effects on different cells and plays a role in normalising spermatogenesis and regulating testicular immunity [44]. Secreted by T helper type 2 (Th2) cells, the polypeptide, when administered early, increases the number of Th2 cells reducing symptoms in a few autoimmune diseases [54]. In addition, interleukin-4 (IL-4) and IL-13 can also regulate eosinophilic infiltration in allergic reactions. In COVID-19, T helper type 2 cells activate the IL-4 (through IgE and IgG) and decrease the level of ACE-2 [55].
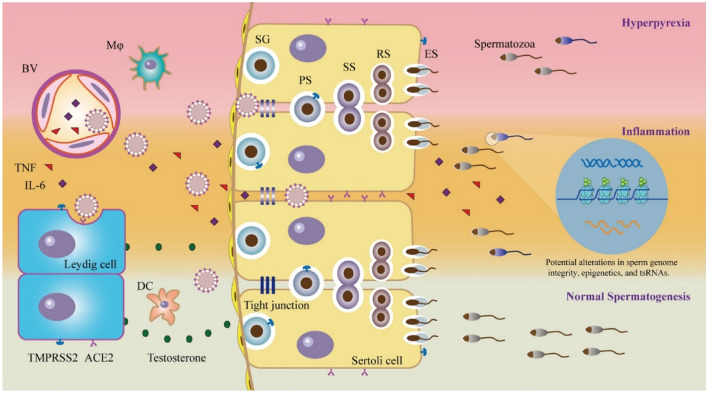
The potential factors in the body influencing the entry mechanism of SARS-CoV-2 into the male reproductive system are outlined in the schematic (Fig. 3) by Tian et al. [56]. The proposed infection routes are summarised as follows: (1) direct entry of the virus into germ cells and sexual transmission of the virus; (2) the virus affects the function of the male reproductive endocrine system; (3) an inflammatory reaction caused by a secondary infection that severely affects the testicles; and (4) fevers caused by viral infections that interfere with normal reproductive physiology. It should be noted, the sequence of the proposed routes may differ for individuals with underlying asymptomatic urinary tract infections.
Fig. 3.
Potential pathways of SARS-CoV-2 affecting the male reproductive system. SG spermatogenesis, PS primary spermatocytes, SS secondary chimeric cells, RS round-headed sperm, ES sperm elongation. Copyright:
© 2021 Society for Reproduction and Fertility 2021 [45]
Potential for sexual and vertical transmission
ACE-2 is expressed in the kidneys, testicular cells, bladder, and prostate; thus, the transmission of the virus in urine or semen seems likely. Indeed, in 2020 Giau et al. suggested analysing and diagnosing the presence of SARS-CoV-2 in semen and applying appropriate controls and prevention measures [6, 8]. However, studies regarding the presence of SARS-COV-2 in semen remain contradictory. For example, Song et al. found no detectable levels of viral RNA in semen samples from 12 male patients aged 22–38 years (11 mildly symptomatic, one asymptomatic) in the initial and convalescent stages of infection. The authors also examined the testicular tissue of a 67-year-old deceased patient, finding no viral RNA as well [57]. However, the researchers did not provide any pathological evidence to verify their conclusions. In addition, another study involving 34 Chinese male patients also confirmed the absence of viral RNA [58].
On the other hand, several studies [7, 59, 60] have reported high viral loads in the semen of COVID-19 patients. For example, semen samples analysed from 38 male patients with severe changes (12/38 comatose patients or about to die, dead and recovering) found six of them were positive for SARS-CoV-2 [7]. More recently, Ma et al. [61] examination of molecular features and pathophysiology of testes obtained from five COVID-19 patients at autopsy showed a massive loss in germ cell (GC) attachment to the seminiferous tubules in 4 patients. The number of DDX4 (a germ cell-marker)-positive cells in all specimens was reduced. Additional qPCR-based virus nucleic acid sample testing showed two out of the five samples were positive for SARS-CoV-2. Interestingly, transmission electron microscopy (TEM) analyses revealed intact coronavirus-like particles residing in the interstitial compartment of the testes. Unfortunately, the authors did not test the replicative capabilities of the particulates in cell culture or an animal model, which is the gold standard for confirming viral transmission [19]. Therefore reports that solely rely on PCR in determining infection potential and viral shedding in mild to severe patients are not definitive [62, 63].
As studies related to the sexual transmission of SARS-COV-2 via bodily fluids, such as blood, urine, and semen, are currently inconclusive, the transmissibility of other viruses, such as Zika, Ebola, HIV, human herpesvirus-8 and mumps, which are found in semen, may be considered. For example, previous reports showed that semen samples from Ebola virus disease survivors remained positive up to 272 days after the onset of symptoms [64]. In addition, Zika viruses can persist in male hosts, with detectable levels found in the semen of cured male patients for up to a year [65].
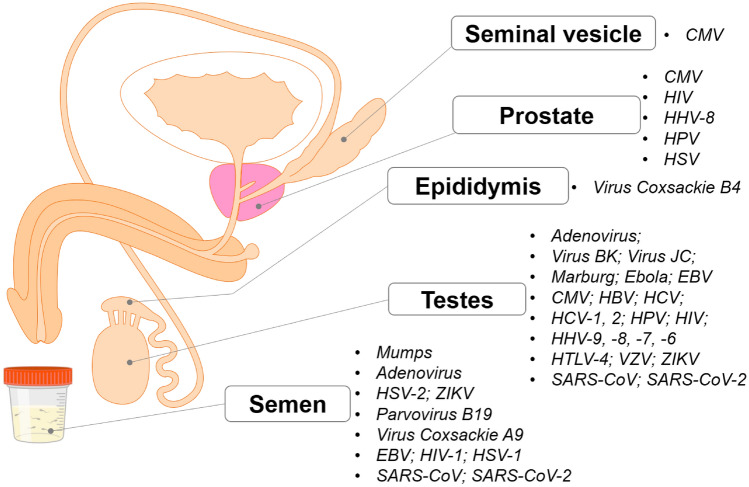
However, high viral loads in semen do not always equate to high transmissibility (Zika) [66]. The successful transmission of Ebola, HIV, Herpes and mumps partially rests in their abilities to dampen and evade the host's initial immunological response via extracellular vehicles (EVs). EVs are known to suppress or enhance the infectivity of sexually transmitted viruses in humans [67]. Whether EVs could sufficiently protect SARS-CoV-2 virions from the antimicrobial (seminal amyloid) environment of semen and a recipients' immuno-response requires investigation. In theory, the carriage of COV-2 EVs would allow the virus (RNA or virion) to enter multiple cell types with low ACE expression permitting vaccine escape as is the case with hepatitis C [68], increasing the likelihood of infection in un/vaccinated males. It should be noted that COV-2 can infect the lung, bronchus, nasopharynx, oesophagus, liver, and stomach of healthy individuals, even when the level of ACE2 expression is undetectable [69]. Suggesting the virus may also engage alternate receptor(s) such as BSG during the course of an infection [70]. The locations of the many viruses in the male reproductive tract utilising EVs to enhance their infectivity are shown in Fig. 4.
Fig. 4.
The viruses found in the male reproductive system. CMV cytomegalovirus, EBV Epstein–Barr virus, HBV Hepatitis B virus, HCV Hepatitis C virus, HHV Human herpesvirus 6, HIV human immunodeficiency virus, HPV Human papillomavirus, HSV Herpes simplex virus 1 and 2, HTLV human T-lymphotropic virus type 1, VZV Varicella zoster virus, ZIKV Zika virus, SARS-CoV severe acute respiratory syndrome-associated-coronavirus, SARS-CoV-2 severe acute respiratory syndrome-associated-coronavirus type 2
Single-cell transcriptomic analysis by Pique-Regi et al. [71] showed that ACE2 and TMPRSS2 were minimally expressed in the placenta throughout pregnancy, suggesting viral transmission from mother to fetus (vertical transmission) was unlikely. However, numerous studies [72, 73] have found high levels of SARS-CoV-2 viral RNA in the placenta and umbilical cord of SARS-CoV-2 from infected pregnant women. Of note was the study by Facchetti et al. [74], in which evidence of SARS-CoV-2 vertical transmission from an infected full-term pregnant woman to her newborn was found. Specifically, researchers detected viral RNA in several maternal–fetal interface cells, including Syncytiotrophoblast cells and confirmed the presence of infectious virions in fetal intravascular monocytes. For those readers seeking up to date opinions regarding the controversies surrounding the vertical transmission of SARS-CoV-2 in pregnant women, the review by Chaubey et al. is proposed [75].
Allergic-asthma and hypoxia
The prevalence of allergy among patients seeking infertility treatment is high compared with the general population [76]. Air-bone allergens can cause chronic airway inflammation, constriction and asthma. Allergen inhalation challenge remains a useful clinical model to examine allergen-induced airway responses and inflammation mechanisms. The bronchoconstrictor response to a challenge partially depends on IgE-facilitated allergen presentation mediated by the activation of allergen-specific memory TH2 cells and antigen-presenting cells [77]. However, significant negative correlations between ACE2–mRNA, epithelial IL-13 expression and immune type 2 (T2) biomarkers were reported by Jackson et al. in patients with allergies [78]. The researchers concluded that allergen exposures and T2 inflammation decreased ACE2 expression in the upper and lower airways. In addition, Shi et al. reported a lower rate of comorbid allergy in patients with COVID-19 than the prevalence of allergic diseases in the general population [79]. The authors suggesting significant elevations of T cells made the lymphocytes in the observation group less prone to viral attack.
Another hallmark of allergic respiratory inflammation is eosinophilia (higher than normal level of eosinophils), a condition most associated with parasitic disease [80]. CCL11/Eotaxin is an important eosinophil-specific chemokine associated with the recruitment of eosinophils into sites of inflammation [81]. CCL-11 is critical for allergen-induced eosinophilic gastrointestinal inflammation, can rapidly transverse the blood–brain barrier (BBB) and is recognised as an established marker for neurodegenerative diseases [81]. Elevated CCL-11 [82, 83] plasma levels were significantly higher in all COVID infected patient groups than healthy donors and have been reported in other studies [84]. A recent study [86] suggested eosinophilic (≥ 150 cells/μL of blood) asthmatic patients (Th2-phenotype) with elevated IL-13 levels infected with COVID-19 were associated with decreased hospital admissions and less severe outcomes. Furthermore, IL-13 was reported to reduce ACE2 expression and increase TMPRSS2 expression in bronchial epithelial cells ex vivo in asthmatic and nonasthmatic atopic groups [85]. Blood eosinophils are predominantly mediated by IL-5 and in part by IL-13, whereas fractional exhaled nitric oxide (FeNO) is mediated by IL-13. However, the function of elevated NO levels in asthma remains ambiguous, with both detrimental (exacerbation) as well as beneficial (antiviral) effects of airway NO being reported [86, 87]. For example, data from human eosinophils suggest direct antiviral binding capacity may be reduced in asthma and is shown to correlate with disease severity [88]. On the other hand, NO’s vasodilatory effects improve oxygenation, and its strong antiviral properties have been trialled in the treatment of SARS-COV and SARS-COV-2 [89]. Conversely, Camiolo et al. identified T2 low asthmatic phenotypes with reduced peripheral blood eosinophil counts (Eosinopenia) and increased ACE2 expression in the bronchial epithelium was more susceptible to an adverse outcome following infection [90]. Whether the benefits (shorter hospital stays, reductions in antibiotic usage) of NO treatments regarding mild/severe COVID outweigh the long-term costs (prolonged impairment of the alveolar-capillary membranes) requires further investigation.
Eosinophils accumulate in the airways of asthma patients during virus‐induced NO exacerbations suggesting that eosinophils may also be actively involved in the antiviral response [91, 92]. Yet influenza-exposed eosinophils have been shown to stimulate CD8+ T cell activation and proliferation in vitro, indicating a potential role for eosinophils as antigen-presenting cells in influenza infection during asthma [88]. Therefore it might be suggested that the protection afforded to Th-2 phenotypes against severe outcomes is a result of prolonged eosinophilia and partial cell-mediated immunity conferred from previous infections, such as Haemophilus influenzae, common cold viruses or SARS, as proposed by Bokatory et al. [93].
The paradoxical role of NO is not unique to asthma and COVID-19. NO can modulate sperm capacitation via protein S-nitrosylation and activate the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. Capacitation is a term used to define a complex and associated process that allows spermatozoa to complete their preparation to fertilise oocytes. Early clinical studies showed normozoospermic fertile men exhibited NO concentrations that were significantly lower than those of asthenozoospermic infertile men [94]. Most of the evidence supports the view that at levels exceeding physiologic concentrations, disruption of sperm function occurs, but that at low levels, NO is essential for sperm motility and capacitation [95]. In addition, many studies report that a fine regulation of protein phosphorylation is required for spermatozoa to achieve fertilising ability [96]. More recently, reported changes in the phosphorylation levels of sperm proteins further highlighted the effect of direct and indirect NO modulation on the type and degree of male infertility [97]. Thus sustained NO elevations, as observed in pathological settings, such as sepsis or sepsis-like situations, akin to the inflammatory response and severe SARS-CoV-2, could perturb capacitation. To our knowledge, the impact of NO elevations on seminal amyloid levels has not been reported.
Another feature of COVID-19 is hypoxia [90]. Hypoxia is a primary pathophysiologic feature and the leading cause of mortality in patients with severe COVID-19 and accompanies all the stages of the disease. In addition, severe hypoxia can diminish mitochondrial function (dysregulation, altered mitochondrial membrane permeability), leading to ATP insufficiency. Moreover, under the condition of hypoxia, studies show rise in testicular temperature, morphological changes, thinning of the seminiferous epithelium and a reduction in the number of spermatozoa [41, 98].
On the other hand, occasional exposure to a moderate hypoxic environment (akin to mild seasonal asthma) can induce an adaptation known as hypoxic conditioning, protecting cells and organs against hypoxia-induced damage. Moreover, hypoxic conditioning diminishes chronic inflammation and cytokine expression. The roles of hypoxia and hypoxia-inducible factor-1 (HIF-1α) in regulating cytokine expression have been controversial and depend upon various conditions (levels of reactive oxygen species and functional mitochondria upon infection) [99]. For example, HIF-1α is expressed by Leydig cells and is equally abundant in normoxic and hypoxic murine models [100]. Additionally, LPS-induced inflammation can increase testicular HIF-1 protein via the formation of neutrophil extracellular traps(NET’s), suggesting crosstalk between inflammatory and hypoxic responses in the testis and possible disruption of neutrophil apoptosis.
Additional factors
The ideal temperature for spermatogenesis is 29–36 °C. Long fever can increase testicular temperature and GC apoptosis. High fever (above 37.8 °C) is often seen in viral infections. In one study, a patient recovering from influenza produced abnormal sperm for 45 days’ post-fever [101]. In another, sperm count, motility, and genetic health were decreased for over two months after the patient's fever resolved [102]. Considering that one of the primary symptoms of COVID-19 is a high fever, it is reasonable to assume that men with COVID-19 early in the pandemic will also experience reduced fertility.
The basal levels of T vary widely in the population; therefore, the ratios of T/LH are used to evaluate the impact of infection on testicular function. Studies demonstrate COVID-19 may at least affect fertility hormones. Preliminary research revealed that men with novel coronavirus infections had elevated levels of luteinising hormone (LH) and decreased ratios of testosterone and follicle-stimulating hormone (FSH) compared to healthy men [103]. In another study, nearly 70% of men admitted to a German ICU with COVID-19 had clinically low testosterone levels [104]. In both cases, whether the hormone imbalances were caused by the COVID-19 infection or were a pre-existing factor in the severity of their illness is unknown. Although these studies may indicate that COVID-19 impacts male fertility, there have yet to be any follow-up studies to determine if the effect is reversible (as it is with influenza). Hence, the long-term impact of COVID-19 on male fertility is less clear.
Signs of genital tract pathologies in men are thought to be closely related to the severity of COVID-19 disease [105]; as such, the effects on male reproductive function post infection need more research attention. Unfortunately, to date, studies evaluating semen quality in the presence of SARS-CoV-2 in infected or recovered men include a limited number of participants.
Notably, one of the repercussions of COVID-19 is its impact on men's sexual health. COVID-19 infection would be associated with an increased likelihood of erectile dysfunction (ED) [106]. ED has been often considered a hallmark of endothelial dysfunction [107, 108], and as such, a potential association between ED and COVID-19 has also been postulated [109]. In addition, recent findings raise the question of whether erectile tissue in the penis, rich in endothelial-lined blood vessels, could result in wide-spread endothelial dysfunction due to COVID-19 [110].
Conclusion
The long-term threat to public health posed by COVID-19, and the effect of the infectious agent SARS-CoV-2 on male reproductive function, need to be systematically investigated. Perhaps most concerning was a 2021 report suggesting sperm and embryo quality [111] among mildly infected IVF couples is significantly reduced for several months post infection. The high expression of ACE2 and TMPRSS2 in the testes theoretically renders the organs of men with reproductive issues more susceptible to infection. In addition, the amplified inflammatory and hypoxic effects of LPS and SARS-COV-2 could potentially result in diminished testosterone output and changes in BTB permeability [112]. So far, such changes have only been reported in a small number of severe cases.
Although initial clinical study results are inconsistent, we cannot deny the potential risk of SARS-CoV-2 penetration into semen, even though it is a small but unacceptable probability in the context of many healthy couples undergoing infertility treatment. As a result, the American Society for Reproductive Medicine (ASRM) and the Society for Assisted Reproductive Technology (SART) have issued warnings to expectant parents, ART patients, gamete donors and pregnant women infected with SARS-CoV-2 must avoid becoming pregnant or not participate in any assisted reproductive programs [113]. In addition, based on current opinions and ASRM recommendations, recovering and long COVID-19 patients should have their fertility assessed [114]. However, a recent analysis of semen parameters in men recovered from COVID-19, 56 days after hospital discharge, showed adverse but potentially reversible consequence of COVID-19 on sperm quality [115]. Regarding Long haulers, emerging studies examining moderate COVID-19 infection on semen oxidative status at two weeks (the acute stage of infection) then three months [116] showed an increase in the total antioxidant capacity (TAC). On the other hand, persistently elevated C-reactive protein in some Long COVID-19 patients suggests there could be a prolonged impairment of testicular function for those individuals [117].
Therefore, the early diagnosis and detection of SARS-CoV-2 in semen have recently been proposed [6, 8], making fertility assessment and pre-pregnancy counselling essential for convalescent patients. Finally, the possible mechanism of SARS-CoV-2-induced orchitis and the potential transmission route of the virus is proposed, raising concerns about male reproductive health in the context of a large number of new cases.
While these studies may indicate that COVID-19 impacts male fertility, there have yet to be any follow-up studies to determine if the effect is reversible. Holtmann et al. reported changes in semen parameters in moderate and mild COVID-19 cases in men up to 54 days post infection, but a follow-up study would be necessary to understand if these changes persist [118]. In addition, there is currently an incomplete picture of whether semen quality is affected by SARS-CoV-2 infection as studies are often limited by the fact that no pre-infection control samples are available for direct comparison or prematurely concluded before long-term effects can be identified. Nevertheless, the few case–control studies published which compare semen quality infected with non-infected (control) individuals suggest that there may be a statistically significant alteration in sperm concentration and motility, although it is not clear whether this is linked to infection by the SARS-CoV-2 virus or simply a consequence of febrile illness and fever. Any long-term negative impact on male reproduction remains unexplored and an important future consideration.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
It is a review paper without the participation of people/humans/patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
J. Hulme, Email: johnhulme21@gmail.com
G. V. Vo, Email: vvgiau@medvnu.edu.vn
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss LF, Prete CA, Jr, Abrahim CMM, Mendrone A, Jr, Salomon T, de Almeida-Neto C, França RFO, Belotti MC, Carvalho M, Costa AG, et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Sci (New York, NY) 2021;371(6526):288–292. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moelling K (2021) Within-host and between-host evolution in SARS-CoV-2-new variant’s source. Viruses 13(5) [DOI] [PMC free article] [PubMed]
- 4.Singh M, Bansal V, Feschotte C. A single-cell RNA expression map of human coronavirus entry factors. Cell Rep. 2020;32(12):108175. doi: 10.1016/j.celrep.2020.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Vo G, Bagyinszky E, Park YS, Hulme J, An SSA. SARS-CoV-2 (COVID-19): beginning to understand a new virus. Adv Exp Med Biol. 2021;1321:3–19. doi: 10.1007/978-3-030-59261-5_1. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292–e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vo VG, Bagyinszky E, Shim K, Park YS, An SSA. Additional diagnostic testing of the 2019 novel coronavirus (SARS-CoV-2) Mol Cell Toxicol. 2020;16(4):355–357. doi: 10.1007/s13273-020-00096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta S, Sengupta P. SARS-CoV-2 and male infertility: possible multifaceted pathology. Reprod Sci (Thousand Oaks, Calif) 2021;28(1):23–26. doi: 10.1007/s43032-020-00261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, Zhang Y, Liao CG, Bian HJ, Jiang JL, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54(6):677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 11.Bi J, Li Y, Sun F, Saalbach A, Klein C, Miller DJ, Hess R, Nowak RA. Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and Sertoli cells. Dev Biol. 2013;380(2):145–156. doi: 10.1016/j.ydbio.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K et al (2020) Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 28 [DOI] [PMC free article] [PubMed]
- 13.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int Off J Int Assoc Study Liver. 2020;40(9):2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alviggi C, Esteves SC, Orvieto R, Conforti A, La Marca A, Fischer R, Andersen CY, Bühler K, Sunkara SK, Polyzos NP, et al. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 2020;18(1):45. doi: 10.1186/s12958-020-00605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis MC. Aerosol transmission of SARS-CoV-2: physical principles and implications. Front Public Health. 2020;8:590041. doi: 10.3389/fpubh.2020.590041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz Bezara ME, Thurman A, Pezzulo AA, Leidinger MR, Klesney-Tait JA, Karp PH, Tan P, Wohlford-Lenane C, McCray PB, Meyerholz DK (2020) Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. bioRxiv 2020.2004.2022.056127. [DOI] [PMC free article] [PubMed]
- 22.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J, Li Y, Huang X, Chen Z, Li Y, Liu C, Chen Z, Duan X. Systematic analysis of ACE2 and TMPRSS2 expression in salivary glands reveals underlying transmission mechanism caused by SARS-CoV-2. J Med Virol. 2020;92(11):2556–2566. doi: 10.1002/jmv.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H, Ma S, Chen X, Long B, Si G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600(7887):21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 27.Scudellari M. How the coronavirus infects cells—and why Delta is so dangerous. Nature. 2021;595(7869):640–644. doi: 10.1038/d41586-021-02039-y. [DOI] [PubMed] [Google Scholar]
- 28.Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Critical Care (London, England) 2021;25(1):244. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Jackson CB, Mou H, Ojha A, Peng H, Quinlan BD, Rangarajan ES, Pan A, Vanderheiden A, Suthar MS, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11(1):6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silber S. Histology of the testis and spermatogenesis. In: Silber S, editor. Fundamentals of male infertility. Cham: Springer International Publishing; 2018. pp. 29–37. [Google Scholar]
- 31.Wang F, Han D (2019) Sertoli cell phagocytosis: an essential event for spermatogenesis
- 32.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 33.Gianzo M, Urizar-Arenaza I, Muñoa-Hoyos I, Larreategui Z, Garrido N, Casis L, Irazusta J, Subirán N. Human sperm testicular angiotensin-converting enzyme helps determine human embryo quality. Asian J Androl. 2018;20(5):498–504. doi: 10.4103/aja.aja_25_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochem Biophys Acta. 1971;250(1):261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- 35.Pauls K, Metzger R, Steger K, Klonisch T, Danilov S, Franke FE. Isoforms of angiotensin I-converting enzyme in the development and differentiation of human testis and epididymis. Andrologia. 2003;35(1):32–43. doi: 10.1046/j.1439-0272.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 36.Arck P, Solano ME, Walecki M, Meinhardt A. The immune privilege of testis and gravid uterus: same difference? Mol Cell Endocrinol. 2014;382(1):509–520. doi: 10.1016/j.mce.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira TA, Oliveira YC, Bernardes FS, Kallas EG, Duarte-Neto AN, Esteves SC, Drevet JR, Hallak J. Viral infections and implications for male reproductive health. Asian J Androl. 2021;23(4):335–347. doi: 10.4103/aja.aja_82_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Bryan MK, Schlatt S, Phillips DJ, de Kretser DM, Hedger MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000;141(1):238–246. doi: 10.1210/endo.141.1.7240. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas N, Michel V, Bhushan S, Wahle E, Hayward S, Ludlow H, de Kretser DM, Loveland KL, Schuppe H-C, Meinhardt A, et al. Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo–orchitis in mice. Sci Rep. 2017;7(1):42391. doi: 10.1038/srep42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Fijak M, Hossain H, Markmann M, Nüsing RM, Lochnit G, Hartmann MF, Wudy SA, Zhang L, Gu H, et al. Characterization of the micro-environment of the testis that shapes the phenotype and function of testicular macrophages. J Immunol (Baltimore Md) 2017;198(11):4327–4340. doi: 10.4049/jimmunol.1700162. [DOI] [PubMed] [Google Scholar]
- 41.Klein B, Bhushan S, Günther S, Middendorff R, Loveland KL, Hedger MP, Meinhardt A. Differential tissue-specific damage caused by bacterial epididymo-orchitis in the mouse. Mol Hum Reprod. 2020;26(4):215–227. doi: 10.1093/molehr/gaaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerdprasert O, O'Bryan MK, Nikolic-Paterson DJ, Sebire K, de Kretser DM, Hedger MP. Expression of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor in normal and inflamed rat testis. Mol Hum Reprod. 2002;8(6):518–524. doi: 10.1093/molehr/8.6.518. [DOI] [PubMed] [Google Scholar]
- 43.Mayerhofer A, Walenta L, Mayer C, Eubler K, Welter H. Human testicular peritubular cells, mast cells and testicular inflammation. Andrologia. 2018;50(11):13055. doi: 10.1111/and.13055. [DOI] [PubMed] [Google Scholar]
- 44.Klein B, Haggeney T, Fietz D, Indumathy S, Loveland KL, Hedger M, Kliesch S, Weidner W, Bergmann M, Schuppe HC. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum Reprod (Oxf, Engl) 2016;31(10):2192–2202. doi: 10.1093/humrep/dew211. [DOI] [PubMed] [Google Scholar]
- 45.Schuppe H-C, Bergmann M. Inflammatory conditions of the testis. In: Ježek D, editor. Atlas on the human testis: normal morphology and pathology. London: Springer; 2013. pp. 113–121. [Google Scholar]
- 46.Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50(11):e13140. doi: 10.1111/and.13140. [DOI] [PubMed] [Google Scholar]
- 47.Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, Tung KSK, Schuppe HC, Meinhardt A. Infectious, inflammatory and 'autoimmune' male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update. 2018;24(4):416–441. doi: 10.1093/humupd/dmy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Weg CA, Koraka P, van Gorp EC, Mairuhu AT, Supriatna M, Soemantri A, van de Vijver DA, Osterhaus AD, Martina BE. Lipopolysaccharide levels are elevated in dengue virus infected patients and correlate with disease severity. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2012;53(1):38–42. doi: 10.1016/j.jcv.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science (New York, NY) 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 50.Zeng W, Zhang Y, Duan F, Lin T, Liu X, Li D, Wu K. Lipopolysaccharide enhances human herpesvirus 1 replication and IL-6 release in epithelial cells. Microb Pathog. 2020;140:103961. doi: 10.1016/j.micpath.2019.103961. [DOI] [PubMed] [Google Scholar]
- 51.Lv X, Wang H, Su A, Xu S, Chu Y. Herpes simplex virus type 2 infection triggers AP-1 transcription activity through TLR4 signaling in genital epithelial cells. Virol J. 2018;15(1):173. doi: 10.1186/s12985-018-1087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rival C, Theas MS, Guazzone VA, Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol. 2006;70(1–2):43–58. doi: 10.1016/j.jri.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Ren X, Wang S, Chen X, Wei X, Li G, Ren S, Zhang T, Zhang X, Lu Z, You Z, et al. Multiple expression assessments of ACE2 and TMPRSS2 SARS-CoV-2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID-19. Infect Drug Resist. 2020;13:3977–3990. doi: 10.2147/IDR.S270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol. 1998;113(3):317–319. doi: 10.1046/j.1365-2249.1998.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renu K, Subramaniam MD, Chakraborty R, Myakala H, Iyer M, Bharathi G, Siva K, Vellingiri B, Valsala Gopalakrishnan A. The role of Interleukin-4 in COVID-19 associated male infertility—a hypothesis. J Reprod Immunol. 2020;142:103213. doi: 10.1016/j.jri.2020.103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian Y, Zhou LQ. Evaluating the impact of COVID-19 on male reproduction. Reprod (Camb, Engl) 2021;161(2):R37–r44. doi: 10.1530/REP-20-0523. [DOI] [PubMed] [Google Scholar]
- 57.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, Wang W, Li C, Diao F, Hu Z, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients†. Biol Reprod. 2020;103(1):4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, Antonelli G, Lenzi A, Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43(12):1819–1822. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos LH, Henrique Jacomo R, Herinques Santa Rita T, Davis R. Presence of SARS-CoV-2 RNA in semen-cohort study in the United States COVID-19 positive patients. Infect Dis Rep. 2021;13(1):96–101. doi: 10.3390/idr13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, Ren W, Yuan Q, Zhang F, Kong F, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9(1):42–47. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma X, Guan C, Chen R, Wang Y, Feng S, Wang R, Qu G, Zhao S, Wang F, Wang X, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18(2):487–489. doi: 10.1038/s41423-020-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahadur G, Acharya S, Muneer A, Huirne J, Łukaszuk M, Doreski PA, Homburg R. SARS-CoV-2: diagnostic and design conundrums in the context of male factor infertility. Reprod Biomed Online. 2020;41(3):365–369. doi: 10.1016/j.rbmo.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deen GF, Broutet N, Xu W, Knust B, Sesay FR, McDonald SLR, Ervin E, Marrinan JE, Gaillard P, Habib N, et al. Ebola RNA persistence in semen of ebola virus disease survivors—final report. N Engl J Med. 2017;377(15):1428–1437. doi: 10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paoli D, Pallotti F, Turriziani O, Mazzuti L, Antonelli G, Lenzi A, Lombardo F. SARS-CoV-2 presence in seminal fluid: Myth or reality. Andrology. 2021;9(1):23–26. doi: 10.1111/andr.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23(11):1922–1924. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raab-Traub N, Dittmer DP. Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol. 2017;15(9):559–572. doi: 10.1038/nrmicro.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao F, Fofana I, Heydmann L, Barth H, Soulier E, Habersetzer F, Doffoël M, Bukh J, Patel AH, Zeisel MB, et al. Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS Pathog. 2014;10(5):e1004128–e1004128. doi: 10.1371/journal.ppat.1004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16(7):e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e1019. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, Leng Y, Hsu CD, Gomez-Lopez N (2020) Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 9 [DOI] [PMC free article] [PubMed]
- 72.Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, Yasumoto Y, Vogels CB, Casanovas-Massana A, Vijayakumar P, et al. SARS-CoV-2 infection of the placenta. J Clin Investig. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pulinx B, Kieffer D, Michiels I, Petermans S, Strybol D, Delvaux S, Baldewijns M, Raymaekers M, Cartuyvels R, Maurissen W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis. 2020;39(12):2441–2445. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Facchetti F, Bugatti M, Drera E, Tripodo C, Sartori E, Cancila V, Papaccio M, Castellani R, Casola S, Boniotti MB, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaubey I, Vignesh R, Babu H, Wagoner I, Govindaraj S, Velu V (2021) SARS-CoV-2 in Pregnant Women: Consequences of Vertical Transmission. Front Cell Infect Microbiol 11(848) [DOI] [PMC free article] [PubMed]
- 76.Esfandiari N, Nesbit C, Litzky J, Dela Cruz D, Gibson S, DeMars L, Esfandiari N. High prevalence of allergy in patients undergoing in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2020;37(2):311–320. doi: 10.1007/s10815-020-01691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, Melén E, Palomares O, Scadding GK, Togias A, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi: 10.1038/s41572-020-00227-0. [DOI] [PubMed] [Google Scholar]
- 78.Jackson DJ, Busse WW, Bacharier LB, Kattan M, O'Connor GT, Wood RA, Visness CM, Durham SR, Larson D, Esnault S, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e203. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi W, Gao Z, Ding Y, Zhu T, Zhang W, Xu Y. Clinical characteristics of COVID-19 patients combined with allergy. Allergy. 2020;75(9):2405–2408. doi: 10.1111/all.14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Z-S, Shu T, Kang L, Wu D, Zhou X, Liao B-W, Sun X-L, Zhou X, Wang Y-Y. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct Target Ther. 2020;5(1):100. doi: 10.1038/s41392-020-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xi X, Guo Y, Zhu M, Wei Y, Li G, Du B, Wang Y. Higher expression of monocyte chemotactic protein 1 in mild COVID-19 patients might be correlated with inhibition of Type I IFN signaling. Virol J. 2021;18(1):12. doi: 10.1186/s12985-020-01478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giau VV, Wu SY, Jamerlan A, An SSA, Kim SY, Hulme J (2018) Gut microbiota and their neuroinflammatory implications in Alzheimer's disease. Nutrients 10(11) [DOI] [PMC free article] [PubMed]
- 84.Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021;76(2):471–482. doi: 10.1111/all.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, Billheimer D, Kraft M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80–88.e88. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ritz T, Salsman ML, Young DA, Lippert AR, Khan DA, Ginty AT. Boosting nitric oxide in stress and respiratory infection: potential relevance for asthma and COVID-19. Brain Behav Immunity Health. 2021;14:100255. doi: 10.1016/j.bbih.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders SP. Nitric oxide in asthma. Am J Respir Cell Mol Biol. 1999;21(2):147–149. doi: 10.1165/ajrcmb.21.2.f158. [DOI] [PubMed] [Google Scholar]
- 88.Rich HE, Antos D, Melton NR, Alcorn JF, Manni ML. Insights into type I and III interferons in asthma and exacerbations. Front Immunol. 2020;11:574027–574027. doi: 10.3389/fimmu.2020.574027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikolaidis A, Kramer R, Ostojic S. Nitric oxide: the missing factor in COVID-19 severity? Med Sci. 2022;10(1):3. doi: 10.3390/medsci10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Camiolo M, Gauthier M, Kaminski N, Ray A, Wenzel SE. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146(2):315–324.e317. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Investig. 2016;126(9):3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabogal Piñeros YS, Bal SM, Dijkhuis A, Majoor CJ, Dierdorp BS, Dekker T, Hoefsmit EP, Bonta PI, Picavet D, van der Wel NN, et al. Eosinophils capture viruses, a capacity that is defective in asthma. Allergy. 2019;74(10):1898–1909. doi: 10.1111/all.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borkakoty B, Bali NK. Haemophilus influenzae and SARS-CoV-2: Is there a role for investigation? Indian J Med Microbiol. 2021;39(2):240–244. doi: 10.1016/j.ijmmb.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, Boscaro M, Ricciardo-Lamonica G, Mazzanti L. Role of nitric oxide concentrations on human sperm motility. J Androl. 2004;25(2):245–249. doi: 10.1002/j.1939-4640.2004.tb02784.x. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, He Q, Yan X, Cai Y, Chen J. Effect of exogenous nitric oxide on sperm motility in vitro. Biol Res. 2014;47(1):44–44. doi: 10.1186/0717-6287-47-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radical Biol Med. 2006;41(4):528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 97.Staicu F-D, Martínez-Soto JC, Canovas S, Matás C. Nitric oxide-targeted protein phosphorylation during human sperm capacitation. Sci Rep. 2021;11(1):20979. doi: 10.1038/s41598-021-00494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farías JG, Bustos-Obregón E, Reyes JG. Increase in testicular temperature and vascularization induced by hypobaric hypoxia in rats. J Androl. 2005;26(6):693–697. doi: 10.2164/jandrol.05013. [DOI] [PubMed] [Google Scholar]
- 99.Serebrovska ZO, Chong EY, Serebrovska TV, Tumanovska LV, Xi L. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin. 2020;41(12):1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palladino MA, Fasano GA, Patel D, Dugan C, London M. Effects of lipopolysaccharide-induced inflammation on hypoxia and inflammatory gene expression pathways of the rat testis. Basic Clin Androl. 2018;28:14–14. doi: 10.1186/s12610-018-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Evenson DP, Jost LK, Corzett M, Balhorn R. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J Androl. 2000;21(5):739–746. [PubMed] [Google Scholar]
- 102.Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88(4):970.e971–977. doi: 10.1016/j.fertnstert.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 103.Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M (2020) Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv 2020:2020.2003.2021.20037267
- 104.Schroeder M, Schaumburg B, Mueller Z, Parplys A, Jarczak D, Roedl K, Nierhaus A, de Heer G, Grensemann J, Schneider B, et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg Microbes Infect. 2021;10(1):1807–1818. doi: 10.1080/22221751.2021.1969869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, Pecoraro A, Manera A, Nicoletti R, Liaci A, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36(6):1520–1529. doi: 10.1093/humrep/deab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G, Jannini EA. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9(4):1053–1059. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guay AT. ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metab Clin N Am. 2007;36(2):453–463. doi: 10.1016/j.ecl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 108.Corona G, Jannini EA, Maggi M. Inventories for male and female sexual dysfunctions. Int J Impot Res. 2006;18(3):236–250. doi: 10.1038/sj.ijir.3901410. [DOI] [PubMed] [Google Scholar]
- 109.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 110.Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, Kryvenko ON, Almeida VW, Firdaus F, Hare JM, et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health. 2021;39(3):466–469. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mannur S, Jabeen T, Khader MA, Rao LSS. Post-COVID-19-associated decline in long-term male fertility and embryo quality during assisted reproductive technology. QJM Monthly J Assoc Phys. 2021;114(5):328–330. doi: 10.1093/qjmed/hcab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen JA, Diemer T, Janus P, Hales KH, Hales DB. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine. 2004;25(3):265–275. doi: 10.1385/ENDO:25:3:265. [DOI] [PubMed] [Google Scholar]
- 113.Cardona Maya WD, Du Plessis SS, Velilla PA. SARS-CoV-2 and the testis: similarity with other viruses and routes of infection. Reprod Biomed Online. 2020;40(6):763–764. doi: 10.1016/j.rbmo.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sansone A, Mollaioli D, Limoncin E, Ciocca G, Bắc NH, Cao TN, Hou G, Yuan J, Zitzmann M, Giraldi A, et al (2021) The sexual long COVID (SLC): erectile dysfunction as a biomarker of systemic complications for COVID-19 long haulers. Sexual Med Rev [DOI] [PMC free article] [PubMed]
- 115.Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23(5):479–483. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Falahieh FM, Zarabadipour M, Mirani M, Abdiyan M, Dinparvar M, Alizadeh H, Paktinat S, Hosseinirad H. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod Fertil Dev. 2021;33(12):683–690. doi: 10.1071/RD21153. [DOI] [PubMed] [Google Scholar]
- 117.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, Heightman M, Hillman TE, Jacob J, Jarvis HC, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114(2):233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]