Abstract
Purpose
Casirivimab/imdevimab (REGN-COV), a cocktail of neutralizing antibodies against the receptor-binding domain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, was shown to be an effective treatment and post-exposure prophylaxis measure for coronavirus disease 2019 (COVID-19). We assessed the antibody titers among patients who received REGN-COV with the purpose of evaluating this therapeutic and prophylactic option from the serological point of view.
Methods
We collected serological data of patients with COVID-19 who were treated with REGN-COV 1200 mg (casirivimab 600 mg/imdevimab 600 mg). Antibody titers were assessed within 24 h before and within 48 h after the administration of REGN-COV using ARCHITECT SARS-CoV-2 immunoglobulin (Ig)G (IgGNC), which is against nucleocapsid protein, and ARCHITECT SARS-CoV-2 IgG II Quant (IgGSP), which is against spike protein.
Results
A total of nine patients were evaluated. IgGSP was elevated after REGN-COV administration with a median of 208,370 Arbitrary Units/mL while simultaneous IgGNC remained low. With the simple linear regression model, the IgGSP after the REGN-COV administration was correlated with the reciprocal of ideal body weight.
Conclusion
The high titer of IgGSP supports the clinical benefit of therapeutic and prophylactic use of REGN-COV from the serological point of view.
Keywords: COVID-19, SARS-CoV-2, REGN-COV, Casirivimab/imdevimab, Antibody titer
The pandemic of novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing threat globally. With the hypothesis that complications and death from COVID-19 emanate from the SARS-CoV-2 viral burden and that reducing this burden leads to clinical benefit, a clinical trial of casirivimab/imdevimab (REGN-COV), a cocktail of neutralizing antibodies targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein, was performed, which showed therapeutic benefit in reducing viral load and lowering the risk of hospitalization or death from COVID-19 [1]. Subcutaneous REGEN-COV was also reported to prevent symptomatic COVID-19 and asymptomatic SARS-CoV-2 infection in previously uninfected household contacts [2]. There are several serological assays for COVID-19, and they have been increasingly used to aid the diagnosis of SARS-CoV-2 infection and to evaluate the antibody response in individuals that have received the COVID-19 vaccine [3, 4]. We believe that to discuss the neutralizing antibody cocktail in comparison with other therapeutic and prophylactic options, common laboratory quantitative assays, such as antibody titer, are helpful. To our knowledge, however, there have been no reports of serological assessment of antibody cocktails among patients with COVID-19 in clinical settings. Since we have assessed patients’ immunological status by measuring antibody titers during the treatment of patients with COVID-19, we have accumulated data regarding antibody titers among patients with COVID-19, including those who were treated with REGN-COV. Herein, we present those data for a better understanding of this promising therapeutic and prophylactic option.
This study was approved by the ethics committee of Yokohama Municipal Citizen’s Hospital (no. 21-12-01) and conducted in accordance the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was waived due to the retrospective nature of this study and ethical consideration for the use of clinical information was made based on the institutional opt-out policy (https://yokohama-shiminhosp.jp/introduction/iryoukenkyu.html). We retrospectively collected the data of patients with COVID-19 who were admitted to Yokohama Municipal Citizen’s Hospital and were treated with REGN-COV. The dosing of REGN-COV was 1200 mg (casirivimab 600 mg/imdevimab 600 mg) in accordance with authorization by the Ministry of Health, Labour and Welfare of Japan. During hospitalization, we assessed patients’ immunological status using ARCHITECT SARS-CoV-2 IgG and ARCHITECT SARS-CoV-2 IgG II Quant (Abbott, IL, USA) with cutoff points of 1.4 sample result divided by the calibrator result (S/C) and 50 Arbitrary Units (AU)/mL, respectively, according to the recommendation by the manufacturer. The former kit identifies IgG against nucleocapsid (IgGNC), which was expected to be unaffected by REGN-COV administration and reflect the immune response to viral infection. The latter kit identifies IgG against the RBD in the spike protein of SARS-CoV-2 (IgGSP), which was expected to be affected with REGN-COV administration. We collected both antibody titers within 24 h before (pre-) and within 48 h after (post-) the administration of REGN-COV. With an assumption that post-IgGSP titers should be related to body mass with the same dose, we adopted the simple linear regression analysis to evaluate the correlation of post-IgGSP with the reciprocal of real and ideal body weight (IBW). Statistical analysis was done using SAS Institute JMP version 15.2.1 and p value < 0.05 was considered as statistically significant.
In total, post-IgGSP titers were available in nine patients, which were included in our observation (Table 1). The titers of pre- and post-IgGNC were low in all patients. The titers of pre-IgGSP were negative in seven patients. Two patients had positive pre-IgGSP. Both of them, whose pre-IgGSP titers were 231.2 AU/mL and 699.9 AU/mL, had already received two doses of the SARS-COV-2 vaccine (BNT162b2) with the first dose 3 months and 5 months prior to the admission, respectively. The post-IgGSP titers were positive in all patients. The exact titers level was unavailable in one patient, although it was confirmed to be more than 80,000 AU/mL. As for the other eight patients, the titers were ranging from 128,436 AU/mL to 332,710 AU/mL with a median of 208,370 AU/mL. With the simple linear regression analysis, post-IgGSP titers were correlated with the reciprocal of IBW with the predictive equation as follows (Fig. 1; p = 0.006, R2 = 0.743):
Table 1.
Baseline characteristics and results of cases
| Age/sex | Body height (cm) | Actual BW (kg) | Ideal BW (kg) | DOS | Pre-IgGNC (S/C) | Post-IgGNC (S/C) | Pre-IgGSP (AU/mL) | Post-IgGSP (AU/mL) | Follow-up IgGSP (AU/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 89/M | 170 | 54.8 | 63.6 | 1 | 0.02 | 0.02 | 231.2 | 145,364.0 | 27,203.9* |
| Case 2 | 89/F | 148 | 47 | 48.2 | 3 | 0.05 | 0.04 | 2.9 | 224,535.0 | |
| Case 3 | 79/F | 159 | 57 | 55.6 | 7 | 0.02 | 0.08 | 0.9 | 222,255.0 | |
| Case 4 | 78/F | 151 | 48 | 50.2 | 6 | N/D | 0.01 | N/D | 288,938.0 | |
| Case 5 | 62/M | 192 | 135 | 81.1 | 9 | 0.03 | 0.03 | 0.01 | 128,436.0 | |
| Case 6 | 57/F | 148 | 50 | 48.2 | 3 | 0.08 | 0.16 | 25.4 | 332,710.1 | |
| Case 7 | 53/M | 171 | 71 | 64.3 | 4 | 0.02 | N/D | 6 | > 80,000.0 | |
| Case 8 | 47/M | 165 | 72 | 59.9 | 5 | 0.02 | 0.1 | 0.01 | 194,485.0 | 78,706.2** |
| Case 9 | 45/M | 178 | 103 | 69.7 | 3 | 0.04 | 0.04 | 699.9 | 179,813.0 |
*Follow-up IgG was measured 42 days after the administration of REGN-COV in Case 1
**Follow-up IgG was measured 12 days after the administration of REGN-COV in Case 8
BW body weight, DOS days after the onset of symptoms of COVID-19, S/C sample result divided by the calibrator result, AU arbitrary units
Fig. 1.
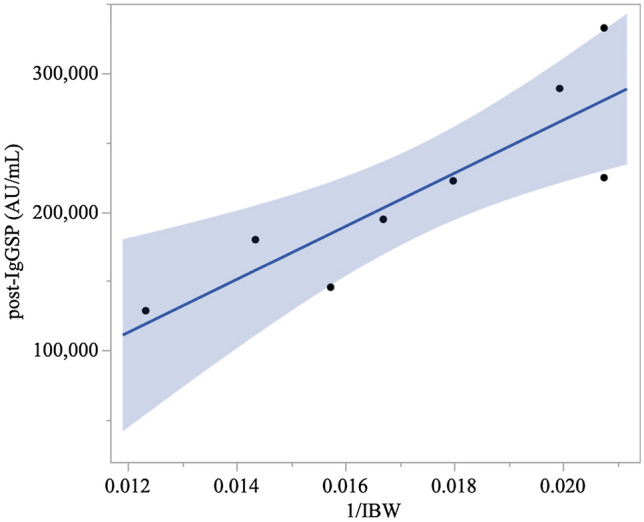
Distribution of the reciprocal of ideal body weight and post-IgGSP titers. The univariate linear regression model showed that post-IgGSP titer was significantly correlated with the reciprocal of ideal body weight (p = 0.012, R2 = 0.752) with the prediction equation: IgGSP (AU/mL) = 2.05 × 107 × 1/[ideal body weight (kg)] − 144,120. The blue area indicates 95% confidence interval
Post-IgGSP titers were not associated with the reciprocal of actual body weight (p = 0.080). In one patient (Case 8), IgGSP titers were followed 12 days after the REGN-COV administrations and showed a 60% decrease (from 194,485 AU/mL to 78,706.2 AU/mL). In another patient (Case 1), IgGSP titers were followed 42 days after the REGN-COV administrations and showed an 81% decrease (from 145,364 AU/mL to 27,203.9 AU/mL). No patients experienced adverse events related to REGN-COV infusion. No patients developed a severe condition and all patients recovered and were discharged from the hospital.
In our observation, the post-IgGSP titer was remarkably high. This titer could be regarded as that of passive immunity with REGN-COV administration rather than the patients’ immune reaction, because IgGNC titer, which starts to increase almost simultaneously with IgGSP titer [5], was not elevated at the time of post-IgGSP titer assessment. The post-IgGSP titer appeared to be higher than those in infected patients and vaccinated individuals based on the previous reports. For example, in the longitudinal assessment of the titer of IgGSP among COVID-19 inpatients, the mean titer increased to 59,396 AU/mL with a decline after 27-days post-COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) testing [3]. In our previous report on the IgGSP titer response after the COVID-19 vaccine (BNT162b2) among healthcare workers, the median titer of IgGSP was 23,489 AU/mL 35 days after the first dose of the vaccine [4].
Since correlation of anti-spike protein IgG and neutralizing antibody responses have already been shown [6–8], high titer of IgGSP among REGN-COV administered patients reflect their potent neutralizing capability, which, we believe, have clinical significance. The fact that the titer was higher in the patients with cocktail therapy than the infected patients might support the clinical efficacy of REGN-COV in contrast to convalescent plasma. In a randomized, multicenter single-blind trial, which enrolled a total of 511 patients, the administration of COVID-19 convalescent plasma with “a high titer of antibody against SARS-CoV-2” to high-risk outpatients within 1 week after the onset of symptoms of COVID-19 failed to prevent disease progression [9]. Although there are no data concerning the antibody titer level after administration of convalescent plasma, it was expected to be much lower than those with REGN-COV administration, since only one unit of plasma was administered and convalescent plasma itself likely had a lower titer in the first place.
In phase 3 clinical trial, subcutaneous REGN-COV prevented symptomatic COVID-19 and asymptomatic SARS-CoV-2 infection in a post-exposure prophylaxis setting [2]. It is reported that immune protection is highly predictable by the neutralization level [10]. Thus, the fact that the titer of IgGSP was higher in the patients with cocktail therapy than the vaccinated individual could reflect the superiority of REGN-COV in prophylaxis not only in rapidity but also strength, especially in unvaccinated patients or individuals with poor humoral response to vaccination, such as the Case 1 in our study.
One of the major limitations of this study is the small number of subjects, resulting in a wide 95% confidence interval of the predictive equation of the estimated IgGSP titer. Thus, the quantitatively of our study should be treated carefully and needs to be revised by analysis of a larger population. Another limitation is the lack of follow-up data. However, the above-mentioned clinical trial of REGN-COV [1] provided the pharmacokinetics parameters, which was linear and dose-proportional, suggesting the potential of measurement of initial IgGSP titer in predicting the duration of passive immunity. To prove this, a follow-up titer of IgGSP and clinical data are needed.
Acknowledgements
None.
Author contributions
All the authors meet the ICMJE authorship criteria. HS designed the study and wrote the manuscript. NM, NT, and YY commented on the manuscript and approved the final version.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
None.
Ethics approval
This study was approved by the ethics committee of Yokohama Municipal Citizen's Hospital (no. 21-12-01).
Consent to participate
Informed consent was waived due to the retrospective nature of this study and ethical consideration for the use of clinical information was made based on the institutional opt-out policy (https://yokohama-shiminhosp.jp/introduction/iryoukenkyu.html).
Consent to publish
Informed consent was waived due to the retrospective nature of this study and ethical consideration for the use of clinical information was made based on the institutional opt-out policy (https://yokohama-shiminhosp.jp/introduction/iryoukenkyu.html).
References
- 1.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 Spike-Specific Quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J Clin Microbiol. 2021;59:e0038821. doi: 10.1128/JCM.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura Y, Sasaki H, Miyata N, Miyazaki K, Tachikawa N. Antibody response after COVID-19 vaccine BNT162b2 on health care workers in Japan. J Infect Chemother. 2021;27:1713–1715. doi: 10.1016/j.jiac.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbelo PD, Riedo FX, Morishima C, Rawlings S, Smith D, Das S, et al. Detection of nucleocapsid antibody to SARS-CoV-2 is more sensitive than antibody to spike protein in COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.04.20.20071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poh CM, Carissimo G, Wang B, Amrun SN, Lee CY, Chee RS, et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat Commun. 2020;11:2806. doi: 10.1038/s41467-020-16638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolscheid-Pommerich R, Bartok E, Renn M, Kümmerer BM, Schulte B, Schmithausen RM, et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J Med Virol. 2022;94:388–392. doi: 10.1002/jmv.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]


