Abstract
Platelet-rich plasma (PRP) is a widely accepted treatment approach and has heightened the quality of care among physicians. PRP has been used over the last decade to boost clinical results of plastic therapies, periodontal surgery and intra-bony defects. According to certain research, elevated levels of PRP growth factors that could promote tissue repair and have the potential for PRP to be beneficial in regenerating processes that Maxillofacial and Oral Surgeons, Veterinary Officers, Athletic medicine specialists and Dermatologists have long admired. PRP is an autologous whole blood fraction that has a heavy amount of a variety of growth factors such as epidermal growth factor (EGF), Vascular Endothelial Growth Factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factors (FGFs), transforming growth factor beta-1 (TGF-b), insulin-like growth factor-I (IGF-I) and platelet-derived growth factor (PDGF) which can facilitate repair and regeneration. Moreover, a clinical trial of PRP in severe angina patients has shown its excellent safety profile. However, PRP is a very complex biological substance with an array of active biomolecules, its functions are yet to be fully clarified. In-addition, there was insufficient work assessing possible cardiovascular tissue benefits from PRP. Thus, it still remains necessary to identify the most clinically important cardiovascular applications and further research in clinical scenario need to be validated.
Keywords: Platelet-rich plasma, Cardiac injury, Cell repair and regeneration
Abbreviations: ADMSC, adipose-derived mesenchymal stem cells; BMSCs, bone marrow-derived mesenchymal stem cells; EGF, epidermal growth factor; FDPs, fibrin degradation products; FGFs, fibroblast growth factors; HGF, hepatocyte growth factor; IGF-I, insulin-like growth factor-I; IRI, ischemic reperfusion injury; ISO, Isoproterenol; LP-PRP, leukocyte-poor PRP; LR-PRP, leukocyte-rich PRP; MH, Manuka honey; MI, myocardial infarction; MRI, magnetic resonance imaging; nsPEF, nanosecond pulsed electric fields; PDGF, platelet-derived growth factor; PRP, platelet-rich plasma; P-PRF, pure platelet-rich fibrin; ROS, reactive oxygen species; TGF-b, transforming growth factor beta; VEGF, vascular endothelial growth factor
1. Introduction
The use of autologous haematological components in a variety of fields of medical research has been a particularly desirable resource for the treatment of different deficits. For example, PRP is a widely accepted treatment approach and has heightened the quality of care among physicians. PRP has been used over the last decade to boost clinical results of plastic therapies, periodontal surgery and intra-bony defects. Currently, PRP treatments are viable therapy alternatives with shown clinical advantages and favorable patient outcomes. PRP growth factors have been found to be raised in certain trials, which may help with tissue repair [1] and have positive potential of PRP in regenerative processes that Maxillofacial Surgeons, Veterinary Officers, Skin and Athletic drug Specialists have long admired [2].
Furthermore, in the developed countries, Myocardial infarction is the leading cause of death, despite progress in preventative, pharmacological, and surgical procedures. Researchers intend to look at possible biological treatments for the treatment of injured myocardial tissue. Since stem cell therapies are a promising subject of research right now, cell choice, mode and treatment as well as cell survival are challenging. PRP is an interesting biological method in regenerative medicine that has arisen as a type of cell platelet treatment. Its advantages include access to point of treatment, easy autologous planning and no chances of rejection. PRP is an autologous whole blood fraction that has a heavy amount of a variety of growth factors such as VEGF, HGF, EGF FGF, TGF-b, IGF-1 and PDGF which can facilitate repair and regeneration [3,4] which can aid in the healing and regeneration of damaged tissues [5,6] (Table 1). These growth factors promote the migration of undifferentiated cells to the site of injury, commencing the healing process. An accelerated neovascularization, increasing blood flow and nutrition in surrounding cells is possible with a PRP treatment strategy. This is important for cellular regeneration and tissue repairing. In addition, PRP may enhance other biological processes, including cell recruitment, proliferation and differentiation, which collectively aid restore [7,8]. In 2017 Ahmed et al. [9] discovered that the autologous PRP gel treatment can significantly improve healing rates by accelerating wound closure in chronic diabetic foot ulcers by releasing essential growth factors. In the same manner, Gonchar and his collaborators [10] have explored the regeneration potential of PRP and combinations to increase diabetic foot ulcer treatments. Moreover, a clinical trial of PRP in severe angina patients has shown its excellent safety profile [11]. However, PRP is a very complex biological substance with an array of active biomolecules, its functions are yet to be fully clarified. In addition, differences in patient results and ongoing studies in the practicality of PRP clinical applications have been questioned. In-addition, there was insufficient work assessing possible cardiovascular tissue benefits from PRP. Thus, it still remains necessary to identify the most clinically important cardiovascular applications and further research in the clinical scenario need to be validated (Table 1).
Table 1.
List of PRP-based growth factors and cytokines with cell source.
| Name of Growth Factors and Cytokines | Abbreviation | Cell Sources | Biological Function and Effects |
|---|---|---|---|
| Epidermal growth factor | EGF | Platelets, monocytes, macrophages | Promotes cytokine release by mesenchymal and epithelial cells; stimulates epithelial cell proliferation and differentiation. |
| Platelet-derived growth factor | PDGF | Platelets, macrophages,<!--Soft-enter Run-on-- > smooth muscle cells, endothelial cells, | Increases collagen expression, cell proliferation, chemotaxis of fibroblasts and proliferation activities; activation of macrophages. |
| Fibroblast growth factor | FGF | Platelets, mesenchymal cells, chondrocytes, osteoblasts, macrophages, | Promotes chondrocyte and osteoblast development, proliferation & differentiation, and increases mesenchymal cell proliferation, regulates cellular survival and migration, |
| Transforming growth factor-β | TGF-β | Macrophages, keratinocytes, T lymphocytes, |
Stimulates angiogenesis and chemotaxis of immune cells, inhibits osteoclast formation and bone resorption, regulates collagen type 1 synthesis and collagenase secretion, regulates mitogenic effects of other growth factors, stimulates endothelial chemotaxis and angiogenesis, inhibits macrophage and lymphocyte proliferation |
| Hepatocyte growth factor | HGF | Platelets, mesenchymal cells | Regulates epithelial/endothelial cell growth and motility, promotes mitogenesis, cell motility, and matrix invasion, promoting epithelial repair and neovascularization during wound healing. |
| Insulin-like growth factor-1 | IGF-1 | Platelets, fibroblasts, plasma, osteoblasts, endothelial cells, bone matrix | Chemotactic for fibroblasts and boosts protein synthesis, resulting in anabolic effects and cellular development. Additionally, it promotes bone formation through osteoblast proliferation and differentiation. |
| Vascular endothelial growth factor | VEGF | Platelets, endothelial cells macrophages, keratinocytes, | Triggers angiogenesis, macrophage and neutrophil chemotaxis, endothelial cell migration and mitosis, and enhances blood vessel permeability. |
| Tumor necrosis factor | TNF | Macrophages, T-lymphocytes, mast cells | Monocyte migration, fibroblast proliferation, macrophage activation, and angiogenesis are all regulated. |
2. Selection of literature review
Mendeley/Springer link/ScienceDirect/PubMed/Medline/Public library of science and Google Scholar were used to locate possibly relevant studies. Numerous keywords, both alone and in combination, were utilized to conduct the literature search. The following keywords were used to conduct the literature search: ‘Definition myocardial infraction’, ‘Epidemiology cardiac injury’, ‘early stage cardiac injury associated inflammation’, ‘Pathogenesis of cardiac injury’, ‘Involvement of inflammation in early stage cardiac injury’, ‘Cytokine activation mediated biological response in cardiac injury’, ‘Separation of platelet rich plasma from blood’, ‘Evidence based effectivity of platelet rich plasma in various myocardial problems ‘, ‘Reactive oxygen species mediated myocardial defect, ‘Role of platelet rich plasma in early-stage cardiac injury’, ‘Mechanism of platelet rich plasma in early-stage cardiac repair and regeneration. The current study included only publications written in the English language. The reference lists of the articles found have also been examined for articles which have not been discovered by an initial search strategy.
3. Preparation of platelet rich plasma
While numerous techniques are in place to prepare PRP, most need of them two centrifuges with accurate time and centrifuge force (g) parameters [12]. The cell components in the blood are separated into several density gradients for the PRP. Following the initial centrifugation, the densest ions in the suspension, erythrocytes, gets separated from the plasma and settles to the lowest layer. A fragile coating of leukocytes (1% of total blood) forms directly above the erythrocytes, referred to as the “buffy coat.” By contrast, platelets are concentrated in the mixture's superior layers, right above the buffy coat. Plasma is obtained and then subjected to a final centrifugation to maximize platelet concentration [13,14]. If automated techniques are not utilized, the final component concentration in a PRP package may vary depending on the commercial kit utilized. Or the skill level required to perform the process manually [12]. Additional factors, such as individual patient characteristics, can also affect the finished product. These include, but are not limited to, advancing age, comorbidities, and circulation [15,16].
Several categorization methods for different PRP formulations have been developed based on PRP terminology and product descriptions. Unfortunately, there is no consensus on a categorization system for PRP or other autologous blood and blood-derived products. A categorization system should ideally concentrate on the different PRP properties, definitions, and suitable terminology that are significant for therapeutic decision-making in the treatment of patient-specific diseases. PRP is now classified into three classes by ortho-biological applications: pure platelet-rich fibrin (P-PRF), leukocyte-rich PRP (LR-PRP), and leukocyte-poor PRP (LP-PRP) [17,18]. The LR-PRP and LP-PRP categories are considerably deficient in clarity regarding the leukocyte composition, although being more precise than a generic PRP product classification. Because of their immunological and host-defense systems, leukocytes have a significant influence on the intrinsic biology of chronic tissue lesions. As a result, PRP biological preparations including particular leukocytes can play an important role in immune regulation as well as tissue repair and regeneration. Lymphocytes, in particular, are plentiful in PRP, generating insulin-like growth factors and assisting tissue remodeling [19,20]. Immunomodulatory activities and tissue healing mechanisms rely heavily on monocytes and macrophages. It's not known how important neutrophils are in PRP. LP-PRP was selected as the optimum PRP formulation for achieving successful treatment results for joint OA in systematic evaluations. Lana et al. on the other hand, were against using LP-PRP in the treatment of knee OA, claiming that certain leukocytes play a crucial role in the inflammatory process preceding tissue regeneration since they produce both pro and anti-inflammatory chemicals. They discovered that combining neutrophils and activated platelets may have a beneficial rather than harmful effect on tissue healing. They also suggested that monocyte plasticity is critical for the non-inflammatory and reparative functions of monocytes in tissue healing [21,22].
The majority of published researches do not include the PRP preparation procedures essential for protocol repeatability, and the reporting of PRP preparation protocols in clinical investigations has been very uneven. There is no apparent consensus among therapeutic indications, making comparisons of PRP products and their associated therapy outcomes challenging. Even for the same therapeutic reason, platelet concentrate treatments are all bundled under the label “PRP” in the majority of reported cases. Progress has been made in understanding how differences in PRP formulations, distribution methods, platelet function, and other PRP ingredients influence tissue repair and regeneration in several medical sectors (e.g., OA and tendinopathies). However, further study is needed to build an agreement on PRP terminologies connected to PRP bioformulations so that particular diseases and disorders may be treated effectively and safely [[23], [24], [25]].
4. Overview of cardiac injury
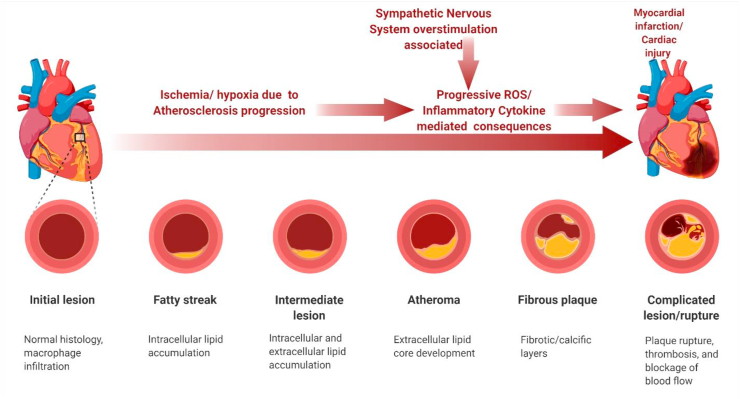
Cardiovascular disorders are globally the primary cause of illness and death. Cardiac arrest with high prevalence and broad socioeconomic effect is the largest source of myocardial infarction (MI) [26]. MI means that myocardial damage is permanently caused by hypoxic ischemia and subsequently by reperfusion for certain periods [27]. Ischemic heart disease is actually the most prevalent cardiovascular disease which seriously jeopardises human wellbeing, and MI [28]. Reperfusion is now a routine therapy for MI, although it might lead to a malfunctioning in cardiomyocytes known as ischemic reperfusion injury (IRI) [29]. Although great progress has been made in identifying prospective pharmacological targets, no particular therapy is yet available for myocardial injuries in MI patients [30, 31]. Appropriate atherosclerotic coronary artery pharmacomechanical revascularization reduces acute MI mortality. In this process, myocardial ischemia creates over generations of free radicals that can lead to oxidative and apoptotic damage [32,33]. However, the secretion of myocardial proteins such as cardiac troponins does not necessarily entail the death of myocardial cells. In the absence of cell death, experiments suggest a variety of mechanisms for protein extrusion from reversibly damaged cardiomyocytes, include cell wounds that produce transient alterations in permeability, the formation of microparticles and their release [[34], [35], [36]]. In Petri dishes or animal models, cardiomyocytes that appear to be viable exchange macromolecules via the plasma membrane [37,38]. Reventing research implies that oxidative damage, inflammation and activation of neurohumoral contribute to IRI. Catecholamine synthesis generated by excessive adrenergic stimulation, in particular, is critical during stress-related cardiac dysfunction [32,39]. External stresses prolong cardiomyocyte contractions, which are stimulated by beta-adrenergic agonists, or are exposed to acute ischaemia, this macromolecular exchange is accelerated [38]. Abnormally high plasma catecholamine levels disturb lipid metabolism in the heart and cause cardiomyocyte apoptosis or necrosis, leading to serious myocardial damage [40]. Isoproterenol (ISO) is a synthetic and β-adrenergic agonist that is capable of inducing cardiac lesions in animals. ISO exposure leads to serious cardiomyocyte stress, through oxygen deficiency, calcium influx, and free radical overproduction, this can lead to myocardial function loss [40]. Moreover, evidence of ISO toxicity as a factor to atherosclerotic plaques between fatty components from the artery wall and calcium precipitation has been explored and reported [41,42]. In the meantime, new treatment using inhibitors of angiotensin-conversion and the development of post–MI cardiac failure or arrest is nearly entirely prevented by β-blockers. But the inability to treat myocardial infarction survivors effectively and uncomplicated has caused a high incidence of cardiac defects [43,44] Dystrophin complexes appear to stabilize the membrane by connecting the sarcomere to the extracellular matrix [45]. In a mechanism called cell injury repair, cells are remarkably durable and repair membrane holes greater than 10 μm [34] within seconds. This procedure is also vague in its molecular detail [45]. In comparison, a morphological ‘point of no return’ for cell damage, i. e, a necrosis, is thought to signify the fragmentation and sarcolemmal interference with consequent massive release of intracellular molecules into the interstitial space. Any heart injury results in some degree of hemorrhage, ranging from hemorrhage into the myocardium as a result of contusion to exsanguinating hemorrhage into the thoracic cavity or into the cavity as a result of penetrating or perforating wounds. In myocardial damage, acute myocardial infarction is significant for oxidative stress and inflammatory cytokines [46]. The heart protection is strengthened by the endogenous antioxidant activity and the oxidative stress is recognized as typical of heart disease. HO-1 is a heat-shock protein group which catalyses heme breakdown into biliverdine, carbon-monoxide and free iron. Different stimuli, including oxidative stresses, can activate the HO-1 and is a significant preventive factor for cardiovascular damage. For example, activation of pharmacological HO-1 decreases myocardial ischemia and/or reperfusion damage [47,48], Reduces myocardial damage caused by endotoxin [49], Optimises the time window for therapy in MI [50]. Furthermore, the Nrf2 controls a number of phase II detoxifying enzymes, including HO-1. In rats following myocardial ischemia, activating Nrf2/HO-1 signalling displayed antiarrhythmic benefits [51]. On the other hand, inflammation is a reaction normally occurring during heart attacks in heart tissue [52]. Postischemia, molecules released from the death cells warn against immunity and activate an inflammatory response, including neutrophil and macrophage infusion, inflammatory cytokines and cell mediated immunity [53,54]. The synthesis of inflammatory substances and cytokine, in reality, helps promotes the healing of wounds and the remodeling of the heart following a myocardial infarction. Excess inflammation can, however, result in more damages, infarct expansion and adverse ventricular remodeling [55] Numerous studies demonstrate that isoproterenol-induced myocardial infarction raises the levels of pro-inflammatory cytokines in the blood and heart tissue, and in patients with stable angina, serum TNF levels are linked to the degree of coronary stenosis [[56], [57], [58]]. TNF-α released by cardiomyocytes contributed most to inflammation, implying that TNF-α is required for the initiation of pathophysiological responses following MI [59] (Fig. 1).
Fig. 1.
Pathological factor associated progressive chronic inflammation associated cardiac cell injury and myocardial Infraction.
5. Complimentary wound healing stage
As noted earlier, wounds establish a prominent pro-inflammatory micro-environment that interferes with the repair process. Additionally, the increased protease activity diminishes the typical growth factor effects to make matters more complicated. Additionally, PRP is a good source of numerous growth factors, in addition to its mitogenic, Angiogenic and Chemotherapy capabilities. These biomolecules have the potential to mitigate the detrimental effects in inflamed tissues by reducing aggravated inflammation and creating pro-anabolic stimuli. With these features in mind, researchers may discover significant promise for treating a variety of difficult ailments. This section outlines the established processes through which PRP promotes wound healing.
PRP is increasingly being used in people and animals, and its ability to cure various organ ailments has been shown in several clinical and experimental trials including dogs [60], horses [61], humans [62], and other species [63]. Platelets are critical in the wound healing process due to their hemostatic properties and high concentrations of growth factors and cytokines. Increased growth factor concentrations increase epithelial and endothelial cell regeneration, boost angiogenesis and collagen deposition, and speed the healing process. The first clinical use of platelet-rich preparation was in chronic leg ulcers, where it was shown that it stimulated the creation of vascularised connective tissue [64].
In human clinical trials, PRP is primarily used to treat chronic conditions such as diabetic ulcers and cardiac injury that are characterised by persistent inflammation caused by an imbalance of pro-inflammatory and anti-inflammatory cytokines, low growth factor concentrations, or even excessive reactive oxygen species. Growth factors and cytokines are important in this regard, since they aid in the management of oxidative damage [65]. The ability of GFs in PRP to regenerate tissue aids in the reduction of healing time after wounds and heart damage in animals. According to the aforementioned remark, Babaei et al. noticed the creation of healthy granulation tissue and the early full healing of all wounds in 150 patients with diabetic foot ulcers after topical PRP administration. Non-healing ulcers of various etiologies were treated with subcutaneous autologous PRP injections and topical PRP gel. The results demonstrated the potential safety and efficacy of autologous PRP for chronic non-healing ulcers, with a significant reduction in wound size in all treated patients without adverse effects, as well as a reduction in pain and inflammation at the site of injury due to cytokine suppression [66]. Similar favourable effects have been shown in secondary wounds caused by necrotizing soft tissue infections after topical application of autologous PRP, as well as in AIDS patients with chronic wounds where increased neovascularization and reepithelialization have been observed [67]. Additionally, a research conducted by Man et al. revealed that topically treating cutaneous flaps with autologous PRP improved wound healing quantitatively. As seen, various trials in human medicine have been undertaken to treat chronic wounds and have demonstrated some degree of improvement as measured by wound area, volume, and closure [68]. Other researchers achieved similar findings in randomised, prospective, and retrospective trials. Thus, the usage of platelets seems to result in a more rapid healing process as compared to conventional treatments. A meta-analysis of the use of PRP in wounds vs control wound care shown that PRP dramatically improved wound healing and ulcers in tiny, difficult-to-heal acute and chronic wounds, and that PRP also had antibacterial action against Staphylococcus aureus and Escherichia coli [69].
The therapeutic effects of PRP are based on the fact that platelets contain a diverse array of growth factors with critical healing capabilities that aid in tissue regeneration. The use of PRP in animal models remains the gold standard for evaluating innovative regenerative treatments, which are thus applicable in both veterinary and human medicine. Numerous in vivo studies in dogs and horses have been conducted on the utility of PRP in injury treatment. Farghali et al. investigated the impact of perilesional subcutaneous autologous PRP infiltration in dogs with full-thickness wounds. Significantly higher wound contraction and re-epithelization rate percentages were observed throughout their clinical examination. Additionally, increased collagen deposition, accelerated granulation tissue maturation, and decreased scar formation were seen in comparison to control wounds due to well-organized collagen fibres [70]. PRP accelerates wound healing by stimulating type I collagen, matrix metalloproteinase I, and cell cycle regulators. Additionally, a recent research conducted in dogs examined the efficiency of PRP injections intralesionally into wounds. PRP-treated wounds healed more rapidly than control groups, both macroscopically and microscopically, with increased angiogenesis and upper granulation development at day 7, as well as increased collagen deposition, quicker re-epithelialization, and epithelial differentiation [71]. On the other hand, Kim et al. investigated the therapeutic impact of autologous PRP on a skin defect and heart damage in a dog, with extremely encouraging findings [72].
With regards to the experimental topical application of PRP in rabbits and other small research models, Ostvar et al. conducted a study on full-thickness cutaneous wounds in rabbits and obtained significant results such as faster wound healing rates and adequate granulation tissue formation in PRP-treated wounds. Additionally, PRP gel was shown to promote angiogenesis, as seen by a considerable increase in vascular density, an abundance of fibroblasts, and well-organized collagen bundles in PRP treated wounds [73]. Lee et al. discovered similar findings in rabbit full-thickness wounds, with enhanced epithelialization and angiogenesis, as well as regression of the initial inflammatory phase and creation of granulation tissue [63]. Additionally, Molina-Miano et al. noticed a considerable acceleration of the reepithelialization process and a significant decrease in inflammation in the damaged region when the PRGF System was used. Additionally, similar findings were seen after PRGF injection into experimental tongue wounds. Beneficial results have also been documented in rabbits when heterologous blood was used to prepare PRP, with no deleterious consequences [74].
PRP has been implicated in tissue growth by increasing cell proliferation, collagen formation, and neovascularization. Additionally, it has been claimed that PRP may be used in conjunction with other therapies to improve outcomes. In accordance with this, Lian et al. demonstrated a synergistic impact when PRP and bone marrow-derived mesenchymal stem cells (BMSCs) were combined [75]. Similarly, Park et al. demonstrated that PRP + hydrogel treatment improved wound healing in mice when compared to control and separately treated wounds, resulting in a substantial reduction in healing time and increased angiogenesis [76]. Manuka honey (MH) is also well-known for its inherent wound healing capacity. An in vitro study was conducted to determine the response of fibroblasts, endothelial cells, and macrophages to culture media supplemented with MH, PRGF, or a combination of the two. A greater increase in cellular activity was observed in the presence of PRGF + MH, with fibroblasts being the most positively responsive cells [77].
6. Modulation of inflammatory process
Many conditions, particularly those of the musculoskeletal system, depend substantially on biodegrading products, such as PRP for osteoarthritis. PRP is well-known for its capacity to influence tissue turnover by releasing growth factors from platelet α -granules, which also has analgesic properties [78]. Indeed, stopping the current inflammatory and catabolic microenvironment is one of the primary goals of PRP treatment and stimulate the change into early heart injury repair and regeneration. Several other authors have demonstrated that stimulation of PRP by thrombin promotes biomolecular release [79] including TNF-α, HGF, TGF-β1, EGF, VEGF. Additional research reveals that PRP promotes the expression of type-II collagen and aggrecan mRNA while decreasing their inhibition by the pro-inflammatory cytokine interleukin beta-1 [80]. In the anti-inflammatory action attributed to PRP, HGF seems to play a crucial function. This powerful anti-inflammatory cytokine reduces inflammation by inhibiting the NF- κB signalling mechanism [81].
PRP has been proven to be important in regulating tissue repair and inflammatory damage. Anti-inflammatory cytokines are a class of biological substances that help activated macrophages moderate the pro-inflammatory response of cytokines [82]. Anti-inflammatory cytokines control inflammation through interactions with soluble cytokine receptors and cytokine inhibitors. IL -1 receptor antagonist, IL -4, IL -10, IL -11, and IL -13 are the most prevalent anti-inflammatory cytokines. Certain cytokines, such as interferon-alpha, leukaemia inhibitory factor, TGF- β1, and IL-6, can have pro- or anti-inflammatory effects, depending on the kind of wound. TNF- α, IL-1, and IL -18 all have cytokine receptors that may act as an inhibitor of other proteins' pro-inflammatory activities(82). IL -10, a potent anti-inflammatory cytokine, inhibits pro-inflammatory cytokine production such as IL-1, IL-6, and TNF-α, while increasing the production of anti-inflammatory agents [83]. These negative feedback systems are critical for the synthesis and function of pro-inflammatory cytokines [84]. Additionally, particular cytokines can activate distinct signaling pathways that promote fibroblasts, which are crucial for tissue healing(83). TGFβ1, IL-1, IL-6, IL-13, and IL-33 are pro-inflammatory cytokines that induce fibroblast differentiation into myofibroblasts, therefore ameliorating ECM(83). TGF, IL-1, IL-33, CXC, and CC chemokines are released sequentially by fibroblasts, which activate and attract immune cells like macrophages, promoting pro-inflammatory reactions [84]. At the wound site, these inflammatory cells play a variety of roles, including wound debridement and the production of growth factors, metabolites and chemokines, all of which are required for tissue regeneration [85]. As a pro-angiogenic stimulator the VEGF, TGF-β and PDGF play a major role. Evidence demonstrates that PDGF can promote differentiation of endothelial cells as an arteriogenic agent [86,87]. Post-ischemia neovascularization is known to cause VEGF [88], and cell mitosis is enhanced by TGF-β [89]. However, several growth factors such as TGF-β and PDGF have also been shown in other papers that they affect the angiogenic potential of FGF [90]. The angiogenic effects were assessed using mixed growth factors solutions. PDGF permits blood vessels to expand functionally within growth factors [91]. According to this, the development of blood vessels would be enhanced by many releases of prostaglandin F2 alpha metabolite and FGF. Mixed VEGF and PDGF releases have also been shown to increase the ripening of the newly developed blood vessels alone versus VEGF release [92]. Other researchers [93] have proposed that fibrin degradation products (FDPs) may operate as molecular mediators that stimulate tissue healing, leading from the deposition of fibrins and elimination all the way to angiogenesis that is needed for the treatment of the wound, have previously been appreciated for fibrinolytic reactions. The cytokines contained in PRP therefore play a significant role in activating the cell types in mediating immunological responses, leading to resolution of the inflammatory phase. Indeed, this mechanism has been named “regenerative inflammation” by some investigators [94]. In fact, the inflammatory phase is an essential stage to successfully complete the tissue healing process while discomforting the patient, given that the inflammatory phase stimulates cellular plasticity processes [95].
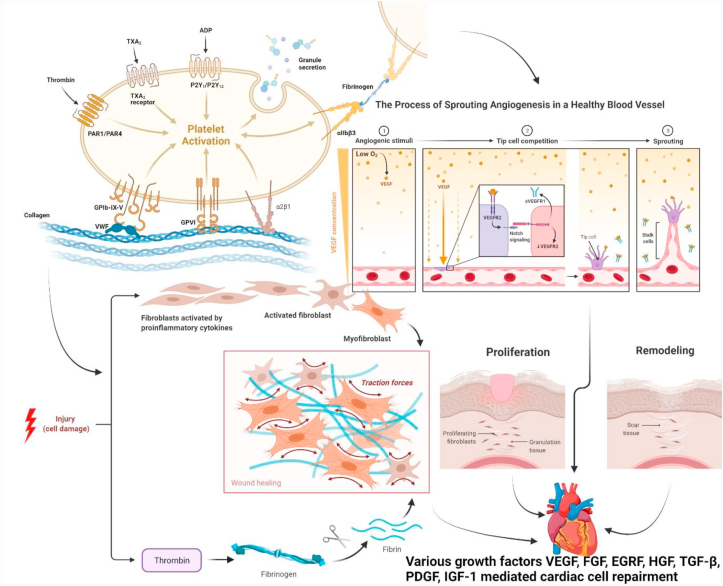
The left ventricle results from Hargrave et al. [96] study showed that the contraction/relaxation rate in the left ventricle was increased and the infarct size was decreased in cardiac treatments for PRP compared to saline. PRP-treated cells lowered their generation of mitochondrial depolarization and reactive oxygen species (ROS). These findings suggest that PRP helps to protect the heart by stabilizing mitochondria and lowering the creation of ROS of the ischaemic heart. Yu et al. [97] conducted a research to examine influence on ventricular remodeling, cardiac function and myocardial infusion in rats of the direct myocardial injection of PRP. In the PRP group, EF was much greater and myocardial perfusion improved dramatically. Histological investigations have shown that PRP can reduce infarct size, enhance wall thickness of the ventricle, and enhance cardiac performance. Furthermore, Li et al. [98] have shown that a paracrine mechanism mediated by plateletes can speed the healing process following early-stage myocardial injury in mice. The insertion of thrombin-activated PRP into ischemic myocardium improved ventricular remodeling according to this experimental technique, as evidenced by ventricular extension limitation, neovascularisation facilitation, infarct arteriogenesis and noninfarct myocardial mitigation. In a platelet-rich fibrin scaffold (PRF), sun et al. [99] found that adipose-derived mesenchymal stem cells (ADMSC) had superior to ADMSC direct injection in the improvement of LV function and decrease LV remodeling in a post–MI model (Fig. 2).
Fig. 2.
Platelet rich plasma mediated repair and regeneration of cell in early stage of cardiac injury.
7. PRP studies on cardiac injuries
Despite a vast body of research supporting PRP's efficacy, only a small amount of work has been done using PRP in the myocardium.
Gallo et al. looked studied the effects of PRP injection on the histology and morphology of ischemic sheep myocardium. The creation of new blood vessels in hematoxylin-eosin-stained sections and the presence of factor VIII in PRGF-treated myocardia were noteworthy. The implantation of platelet growth factors in previously infarcted sheep hearts increased neovascularization, according to this study [100].
In order to assess the efficacy of a regimen comprising the in vivo treatment of ischemia and reperfused myocardium cells in culture with PRP in rabbits, Hargrave et al. used the technology of nanosecond pulsed electric fields (nsPEF). When comparing PRP-treated hearts to saline-treated hearts, the left ventricular contraction/relaxation rate was quicker, and the infarct size was less. PRP-treated cells had less mitochondrial depolarization and produced less ROS. These findings demonstrate that PRP protects the heart by stabilizing mitochondria and lowering ROS production in the ischemic-reperfused heart [96]. In order to see if PRP improves cardiac function in an ischemia-reperfusion scenario as determined by left ventricular ejection fraction [101].
PRP-treated mice had a greater LVEF after ischemia than PBS controls, while PRP-treated animals who underwent ischemia-reperfusion had a higher LVEF after ischemia than PBS controls. The control group had more granulation than the PRP group, according to histology. At the same time, magnetic resonance imaging (MRI) showed that PRP improved left ventricular function in both the ligation and ischemia/reperfusion mouse models. In a pig model, Vu et colleagues explored a translational, large-scale restorative yet minimally invasive strategy, intending to both structurally stabilize the LV wall and improve function after ischemia damage.
A combination of PRP, anti-oxidant, and anti-inflammatory substances, along with intramyocardial injection of hydrogel, has the ability to structurally and functionally improve the wounded heart muscle while reducing unfavorable cardiac remodeling following an acute myocardial infarction in this investigation [102].
8. Discussion
Patients frequently suffer first chest discomfort when blockage occurred in one of the coronary arteries in cardiac ischemia. Patients are subsequently received thrombolytic or percutaneous heart treatment on arriving in the hospital in order to enable heart reperfusion. Cardiomyocytes still undergo cell death due to strong inflammation even after oxygenation is restored during reperfusion. The adult mammalian heart is of extremely low regenerating capabilities, which leads to collagen-based scars. The fundamental task of the scar is to replace the deceased cardiomyocytes, hence preserving the left ventricles' structural integrity. However, new investigations have demonstrated that the immune cells attracted are macrophages, monocytes and derivatives in particular, release cytokines and proteases in healthy cardiomyocytes which are apoptotic. As more cardiomyocytes die, the quantity of scar tissue increases, leading to cardiac fibrosis, which is characterized by a loss of cardiac muscle elasticity and, eventually, heart failure.
In recent years, research on the utilization of biological therapies in cardiovascular disease has gotten a lot of attention in combination with conventional procedures for repairing or preserving myocardium damaged. Adult stem cell treatments, such as transplantation of mononuclear bone marrow cells, mesenchymal bone marrow cells and skeletal myoblast, among these new biotreats, have been promising, based on studies showing an increased EF and/or lower infarct size [103,104]. There is compelling evidence that skeletal muscle myocytes can die in response to skeletal muscle stress [105,106]. These cells tend to be replaced by resident myoblasts that rebuild the injured skeletal muscle [106], which is believed to be one way to cause improved muscle strength. Unlike skeletal muscle, however, it has long been considered that the heart is a post-mitotic organ that cannot regenerate or restore injured cells. Thus, protein extrusion from cardiomyocytes has long been seen as a sign of cell necrosis [107]. However, complete functional recovery following myocardial infarction has been proven in the newborn human heart [108], and recent years have also seen signs of continuing partial cardiac regeneration in the adult human heart [109,110]. Adult human cardiomyocytes undergo mitosis at a rate of approximately 0.5–1% each year in a normal heart, and the percent of cardiomyocytes are never exchanged(109). While preliminary data on the regeneration of human cardiomyocytes from local and extracardiac stem/progenitor cells have been described, they could not be verified reliably(110). Nonetheless, cardiomyocyte turnover and renewal tend to be reduced in humans under stable and diseased circumstances, most cardiomyocyte proliferation occurs during life, and the rate of cardiomyocyte renewal following damage may be greater [110].
Platelets have been demonstrated to interact with the surfaces of attracted monocytes in individuals with MI, platelet-monocyte aggregates have been used as an early detection biomarker and to track the progression of the illness [111]. PRP products have been developed for decades in the field of study in biomaterials and pharmaceutical research in order to encourage tissue healing and regeneration. Although the biological importance of the interaction between platelets and monocytes is unknown, it's been proposed that it might help PRP-mediated tissue repair. and regeneration. In view of the knowledge that PRP actively manipulates healing and inflammatory pathways, thus a comparison cascade has to be added. The cascade of tissue repair involves several components, such as platelets and cytokine granules, leucocytes, fibrin matrix and many more synergistic cytokines. During this cascade, a complicated coagulation process involves platelet activation and subsequent release of dense and α-platelet granules contents, fibrinogen polymerisation (platelet activation, or plasma discharge), and platelet plug formation in a fibrin mesh [112]. The treatment method is mainly subdivided into four phases: haemostasis process, inflammatory stage activation, Activation of Cellular matrix proliferation and finally process of wound remodeling [113].
9. Conclusions
Platelet-rich products can be produced by collecting and centrifuging whole blood from a person, separating it into different layers including plasma, platelets, leukocytes, and erythrocytes. A higher platelet level over the basal value allows for the rapid regeneration of soft tissue with few adverse effects. Autologous PRP products are an emerging biotechnology with promising outcomes in stimulating and improving a range of tissue damage. Previous studies have contributed significantly to our understanding of PRP and in the context of regenerative medicine, there is a wide spectrum of impacts. For many medical practitioners, accelerated recovery is a highly sought goal, and PRP is a biological technique that continues to demonstrate promise in initiating and directing the cascade of regenerative processes. Furthermore, growth factor-induced angiogenesis is essential for organ regeneration following ischemia. Combining cells with platelet-rich plasma-derived growth factors within biomaterials has opened new opportunities for myocardial infarction therapy, according to recent tissue engineering study. Many cases had favorable results. There are However, despite multiple animal research’ encouraging outcomes, well-controlled human research is missing. Additional study is required to completely comprehend this therapeutic method in heart cell repair and regeneration at an early stage.
Author contributions
Conceptualization, I.K.; writing manuscript original draft and investigation, M.A.; methodology, M.M.G., F.A.A-.A., S.H., S.S.I. Review & editing, S.S.I., M.S.N., G.G., S.I.A., A.A. and S.A.
Funding
This work was funded by the Deanship of Scientific Research at Jouf University, Saudi Arabia under the grant number (DSR-2021-01-0312).
Data availability
No datasets were generated or analyzed during the current study.
Declaration of competing interest
The authors have no conflict of interests.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Jouf University, Saudi Arabia under the grant number (DSR-2021-01-0312).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Muhammad Afzal, Email: afzalgufran@ju.edu.sa.
Imran Kazmi, Email: ikazmi@kau.edu.sa.
References
- 1.Camargo P.M., Lekovic V., Weinlaender M., Vasilic N., Madzarevic M., Kenney E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37(4):300–306. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 2.Blood plasma derivatives for tissue engineering and regenerative medicine therapies. Tissue Eng Part B Rev. 2018;24(6):454–462. doi: 10.1089/ten.TEB.2018.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weibrich G., Kleis W.K.G., Hafner G., Hitzler W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Cranio-Maxillofacial Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 4.Duran A., Yasar S., Aytekin S., Gunes P., Adaleti R., Duran A. Clinical and histopathological evaluation of the effects of platelet rich plasma, platelet poor plasma and topical serum physiologic treatment on wound healing caused by radiofrequency electrosurgery. Deri Hastaliklari ve Frengi Arsivi. 2018;52:44–50. [Google Scholar]
- 5.Koriyama Y., Homma K., Sugitani K., Higuchi Y., Matsukawa T., Murayama D., et al. Upregulation of IGF-I in the goldfish retinal ganglion cells during the early stage of optic nerve regeneration. Neurochem Int. 2007;50(5):749–756. doi: 10.1016/j.neuint.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Lin G., Shindel A.W., Fandel T.M., Bella A.J., Lin C.-S., Lue T.F. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010;105(1):114–120. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma:from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 8.Chellappan D.K., Sivam N.S., Teoh K.X., Leong W.P., Fui T.Z., Chooi K., et al. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–1200. doi: 10.1016/j.biopha.2018.09.138. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M., Reffat S.A., Hassan A., Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017;38:206–211. doi: 10.1016/j.avsg.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Gonchar I.V., Lipunov A.R., Afanasov I.M., Larina V., Faller A.P., Kibardin A.V. Platelet rich plasma and growth factors cocktails for diabetic foot ulcers treatment: state of art developments and future prospects. Diabetes Metabolic Syndrome: Clin Res Rev. 2018;12(2):189–194. doi: 10.1016/j.dsx.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Wehberg K.E., Answini G., Wood D., Todd J., Julian J., Ogburn N., et al. Intramyocardial injection of autologous platelet-rich plasma combined with transmyocardial revascularization. Cell Transplant. 2009;18(3):353–360. doi: 10.3727/096368909788534988. [DOI] [PubMed] [Google Scholar]
- 12.Lana J.F.S.D., Purita J., Paulus C., Huber S.C., Rodrigues B.L., Rodrigues A.A., et al. Contributions for classification of platelet rich plasma – proposal of a new classification: MARSPILL. Regen Med. 2017;12(5):565–574. doi: 10.2217/rme-2017-0042. [DOI] [PubMed] [Google Scholar]
- 13.Boswell S.G., Cole B.J., Sundman E.A., Karas V., Fortier L.A. Platelet-rich plasma: a milieu of bioactive factors. Arthrosc J Arthrosc Relat Surg. 2012;28(3):429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Chellappan D.K., Yap W.S., Na B.A.S., Gupta G., Dua K. Current therapies and targets for type 2 diabetes mellitus. Panminerva Med. 2018;60(3):117–131. doi: 10.23736/S0031-0808.18.03455-9. [DOI] [PubMed] [Google Scholar]
- 15.Pavlovic V., Ciric M., Jovanovic V., Stojanovic P. Platelet Rich Plasma: a short overview of certain bioactive components. Open Med. 2016;11(1):242–247. doi: 10.1515/med-2016-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur M., Vyas G. Role of nanoparticles for production of smart herbal drug-An overview. Indian J Nat Produc Resour. 2013;4:329–338. [Google Scholar]
- 17.van Gils J.M., Zwaginga J.J., Hordijk P.L. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85(2):195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 18.Dua K., Gupta G., Chellappan D.K., Shukla S., Hansbro P.M. Targeting bacterial biofilms in pulmonary diseases in pediatric population. Minerva Pediatr. 2019;71(3):309–310. doi: 10.23736/S0026-4946.18.05256-8. [DOI] [PubMed] [Google Scholar]
- 19.Haunschild E.D., Huddleston H.P., Chahla J., Gilat R., Cole B.J., Yanke A.B. Platelet-rich plasma augmentation in meniscal repair surgery: a systematic review of comparative studies. Arthrosc J Arthrosc Relat Surg : Off Pub Arthroscopy Assoc North Am Int Arthroscopy Assoc. 2020;36(6):1765–1774. doi: 10.1016/j.arthro.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Vyas G., Mathur M., Patel N.A., Patel R.P. Aphrodisiac Efficacy of Blepharis sindica seeds: a comparative assessment using different solvent types. Indian J Biochem Biophys. 2017;54:223–230. [Google Scholar]
- 21.Mariani E., Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21(4):1328. doi: 10.3390/ijms21041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta G., Dahiya R., Singh M., Tiwari J., Sah S., Ashwathanarayana M., et al. Role of liraglutide in a major complication of diabetes: a critical review of clinical studies. Bull Pharmaceut Res. 2018;8(1):155–164. [Google Scholar]
- 23.Yuan T., Zhang C.-Q., Wang J.H.C. Augmenting tendon and ligament repair with platelet-rich plasma (PRP) Muscles Ligaments Tendons J. 2013;3(3):139–149. [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta G., Kazmi I., Afzal M., Rahman M., Saleem S., Ashraf M.S., et al. Sedative, antiepileptic and antipsychotic effects of Viscum album L.(Loranthaceae) in mice and rats. J Ethnopharmacol. 2012;141(3):810–816. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian V.B., Katari N.K., Ponnam V., Konduru N., Dongala T., Marisetti V.M., et al. Stability-indicating reversed-phase-HPLC method development and validation for sacubitril/valsartan complex in the presence of impurities and degradation products: robustness by quality-by-design approach. Biomed Chromatogr : BMC (Biomed Chromatogr) 2022;36(1) doi: 10.1002/bmc.5240. [DOI] [PubMed] [Google Scholar]
- 26.Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381(9866):510–511. doi: 10.1016/S0140-6736(13)60058-6. [DOI] [PubMed] [Google Scholar]
- 27.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 28.Kaski J.-C., Crea F., Gersh B.J., Camici P.G. Reappraisal of ischemic heart disease. Circulation. 2018;138(14):1463–1480. doi: 10.1161/CIRCULATIONAHA.118.031373. [DOI] [PubMed] [Google Scholar]
- 29.Caccioppo A., Franchin L., Grosso A., Angelini F., D'Ascenzo F., Brizzi M.F. Ischemia reperfusion injury: mechanisms of damage/protection and novel strategies for cardiac recovery/regeneration. Int J Mol Sci. 2019;20(20):5024. doi: 10.3390/ijms20205024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downey J.M., Cohen M.V. Why do we still not have cardioprotective drugs? Circ J : Off J Japanese Circ Soc. 2009;73(7):1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 31.Gupta G., Singh Y., Tiwari J., Raizaday A., Alharbi K.S., Al-Abbasi F.A., et al. Beta-catenin non-canonical pathway: a potential target for inflammatory and hyperproliferative state via expression of transglutaminase 2 in psoriatic skin keratinocyte. Dermatol Ther. 2020;33(6) doi: 10.1111/dth.14209. [DOI] [PubMed] [Google Scholar]
- 32.Adameova A.D., Bhullar S.K., Elimban V., Dhalla N.S. Activation of β(1)-adrenoceptors may not be involved in arrhythmogenesis in ischemic heart disease. Rev Cardiovasc Med. 2018;19(3):97–101. doi: 10.31083/j.rcm.2018.03.3181. [DOI] [PubMed] [Google Scholar]
- 33.Gupta G., Singhvi G., Chellappan D.K., Sharma S., Mishra A., Dahiya R., et al. Peroxisome proliferator-activated receptor gamma: promising target in glioblastoma. Panminerva Med. 2018;60(3):109–116. doi: 10.23736/S0031-0808.18.03462-6. [DOI] [PubMed] [Google Scholar]
- 34.Takemura G., Kanoh M., Minatoguchi S., Fujiwara H. Cardiomyocyte apoptosis in the failing heart--a critical review from definition and classification of cell death. Int J Cardiol. 2013;167(6):2373–2386. doi: 10.1016/j.ijcard.2013.01.163. [DOI] [PubMed] [Google Scholar]
- 35.Pradhan R., Singhvi G., Dubey S.K., Gupta G., Dua K. Future Science Ltd London; UK: 2019. MAPK pathway: a potential target for the treatment of non-small-cell lung carcinoma. [DOI] [PubMed] [Google Scholar]
- 36.Muchakayala S.K., Katari N.K., Dongala T., Marisetti V.M., Vyas G., Vegesna R.V.K. Eco-friendly and green chromatographic method for the simultaneous determination of chlorocresol and betamethasone dipropionate in topical formulations using Box–Behnken design. J Iran Chem Soc. 2021 doi: 10.1007/s13738-021-02388-5. [DOI] [Google Scholar]
- 37.Hoffstein S., Gennaro D.E., Fox A.C., Hirsch J., Streuli F., Weissmann G. Colloidal lanthanum as a marker for impaired plasma membrane permeability in ischemic dog myocardium. Am J Pathol. 1975;79(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- 38.Page E., Upshaw-Earley J., Goings G. Permeability of rat atrial endocardium, epicardium, and myocardium to large molecules. Stretch-dependent effects. Circ Res. 1992;71(1):159–173. doi: 10.1161/01.res.71.1.159. [DOI] [PubMed] [Google Scholar]
- 39.Valluri V., Katari N., Khatri C., Vyas G., Polagani S. Highly sensitive liquid chromatography–tandem mass spectrometry assay for the determination of azathioprine in presence of mercaptopurine and its application to a human pharmacokinetic study. Separ Sci. 2021:4. [Google Scholar]
- 40.Radhiga T., Rajamanickam C., Sundaresan A., Ezhumalai M., Pugalendi K.V. Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie. 2012;94(5):1135–1142. doi: 10.1016/j.biochi.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Sage A.P., Tintut Y., Demer L.L. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7(9):528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta M., Sharma N., Vyas M., Khurana N., Maurya P.K., Singh H., et al. Interactions with the macrophages: an emerging targeted approach using novel drug delivery systems in respiratory diseases. Chem Biol Interact. 2019;304:10–19. doi: 10.1016/j.cbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Braunwald E., Domanski M.J., Fowler S.E., Geller N.L., Gersh B.J., Hsia J., et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh Y., Gupta G., Gilhotra R.M., Singh S.K., Prasher P., Krishnan A., et al. Emerging prospects of vitamin D3 in metabolic syndrome: a proof of concept (POC) approach targeting inflammation. EXCLI J. 2020;19:1512. doi: 10.17179/excli2020-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil P.L., Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6(6):499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 46.Neri M., Fineschi V., Di Paolo M., Pomara C., Riezzo I., Turillazzi E., et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13(1):26–36. doi: 10.2174/15701611113119990003. [DOI] [PubMed] [Google Scholar]
- 47.Hwa J.S., Jin Y.C., Lee Y.S., Ko Y.S., Kim Y.M., Shi L.Y., et al. 2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J Ethnopharmacol. 2012;139(2):605–615. doi: 10.1016/j.jep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Samuel V.P., Dahiya R., Singh Y., Gupta G., Sah S.K., Gubbiyappa S.K., et al. Metformin: a salutary candidate for colorectal cancer treatment in patients with diabetes. J Environ Pathol Toxicol Oncol. 2019;38(2) doi: 10.1615/JEnvironPatholToxicolOncol.2019029388. [DOI] [PubMed] [Google Scholar]
- 49.Hao E., Lang F., Chen Y., Zhang H., Cong X., Shen X., et al. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei B., Li W.W., Ji J., Hu Q.H., Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis. 2014;235(2):318–327. doi: 10.1016/j.atherosclerosis.2014.05.924. [DOI] [PubMed] [Google Scholar]
- 51.Lee T.-M., Lin S.-Z., Chang N.-C. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO-1 signaling. Free Radic Biol Med. 2014;77:71–81. doi: 10.1016/j.freeradbiomed.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Mehta J.L., Li D.Y. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43(2):291–299. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 53.Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh Y., Gupta G., Sharma R., Matta Y., Mishra A., Pinto TdJA., et al. Embarking effect of ACE2-angiotensin 1–7/mas receptor Axis in benign prostate hyperplasia. Crit Rev Eukaryot Gene Expr. 2018;28(2) doi: 10.1615/CritRevEukaryotGeneExpr.2018021364. [DOI] [PubMed] [Google Scholar]
- 55.Frangogiannis N.G. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagheri B., Sohrabi B., Movassaghpur A., Mashayekhi S., Garjani A., Shokri M., et al. Association of monoctye expression of Toll-like receptor 4 and its related cytokines with coronary luminal stenosis. Adv Biosci Biotechnol. 2013;4(7):7. [Google Scholar]
- 57.Soraya H., Farajnia S., Khani S., Rameshrad M., Khorrami A., Banani A., et al. Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: are AMPK and TLRs connected? Int Immunopharm. 2012;14(4):785–791. doi: 10.1016/j.intimp.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Tew X.N., Lau N.J.X., Chellappan D.K., Madheswaran T., Zeeshan F., Tambuwala M.M., et al. Immunological axis of berberine in managing inflammation underlying chronic respiratory inflammatory diseases. Chem Biol Interact. 2020;317:108947. doi: 10.1016/j.cbi.2020.108947. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Pat B., Zheng J., Cain L., Powell P., Shi K., et al. Tumor necrosis factor-α produced in cardiomyocytes mediates a predominant myocardial inflammatory response to stretch in early volume overload. J Mol Cell Cardiol. 2010;49(1):70–78. doi: 10.1016/j.yjmcc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jee C.H., Eom N.Y., Jang H.M., Jung H.W., Choi E.S., Won J.H., et al. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J Vet Sci. 2016;17(1):79–87. doi: 10.4142/jvs.2016.17.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carter C.A., Jolly D.G., Worden C.E., Sr, Hendren D.G., Kane C.J. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74(3):244–255. doi: 10.1016/s0014-4800(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 62.Suthar M., Gupta S., Bukhari S., Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24(1):16. doi: 10.1186/s12929-017-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H.W., Reddy M.S., Geurs N., Palcanis K.G., Lemons J.E., Rahemtulla F.G., et al. Efficacy of platelet-rich plasma on wound healing in rabbits. J Periodontol. 2008;79(4):691–696. doi: 10.1902/jop.2008.070449. [DOI] [PubMed] [Google Scholar]
- 64.Krupski W.C., Reilly L.M., Perez S., Moss K.M., Crombleholme P.A., Rapp J.H. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg. 1991;14(4):526–532. ; discussion 32-6. [PubMed] [Google Scholar]
- 65.Lacci K.M., Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 66.Babaei V., Afradi H., Gohardani H.Z., Nasseri F., Azarafza M., Teimourian S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J Wound Care. 2017;26(12):784–787. doi: 10.12968/jowc.2017.26.12.784. [DOI] [PubMed] [Google Scholar]
- 67.Hersant B., SidAhmed-Mezi M., Bosc R., Meningaud J.P. Autologous platelet-rich plasma/thrombin gel combined with split-thickness skin graft to manage postinfectious skin defects: a randomized controlled study. Adv Skin Wound Care. 2017;30(11):502–508. doi: 10.1097/01.ASW.0000524399.74460.87. [DOI] [PubMed] [Google Scholar]
- 68.Man D., Plosker H., Winland-Brown J.E. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107(1):229–237. doi: 10.1097/00006534-200101000-00037. ; discussion 38-9. [DOI] [PubMed] [Google Scholar]
- 69.Frykberg R.G., Driver V.R., Carman D., Lucero B., Borris-Hale C., Fylling C.P., et al. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy/Wound Manag. 2010;56(6):36–44. [PubMed] [Google Scholar]
- 70.Farghali H.A., AbdElKader N.A., Khattab M.S., AbuBakr H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci Rep. 2017;37(2) doi: 10.1042/BSR20160503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho J.W., Kim S.A., Lee K.S. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29(1):32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- 72.Kim J.H., Park C., Park H.M. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet Dermatol. 2009;20(2):123–126. doi: 10.1111/j.1365-3164.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- 73.Ostvar O., Shadvar S., Yahaghi E., Azma K., Fayyaz A.F., Ahmadi K., et al. Effect of platelet-rich plasma on the healing of cutaneous defects exposed to acute to chronic wounds: a clinico-histopathologic study in rabbits. Diagn Pathol. 2015;10:85. doi: 10.1186/s13000-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.López-Jornet P., Camacho-Alonso F., Molina-Miñano F., Vicente-Ortega V. Effects of plasma rich in growth factors on wound healing of the tongue. Experimental study on rabbits. Med Oral, Patol Oral Cirugía Bucal. 2009;14(9):e425–e428. [PubMed] [Google Scholar]
- 75.Lian Z., Yin X., Li H., Jia L., He X., Yan Y., et al. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol. 2014;26(1):1–10. doi: 10.5021/ad.2014.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park Y.G., Lee I.H., Park E.S., Kim J.Y. Hydrogel and platelet-rich plasma combined treatment to accelerate wound healing in a nude mouse model. Arch Plastic Surgery. 2017;44(3):194–201. doi: 10.5999/aps.2017.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sell S.A., Wolfe P.S., Spence A.J., Rodriguez I.A., McCool J.M., Petrella R.L., et al. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int J Biomater. 2012;2012:313781. doi: 10.1155/2012/313781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kon E., Filardo G., Di Martino A., Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):516–527. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 79.Huber S.C., Cunha Júnior J.L.R., Montalvão S., da Silva L.Q., Paffaro A.U., da Silva F.A.R., et al. In vitro study of the role of thrombin in platelet rich plasma (PRP) preparation: utility for gel formation and impact in growth factors release. J Stem Cells Regen Med. 2016;12(1):2–9. doi: 10.46582/jsrm.1201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie X., Wang Y., Zhao C., Guo S., Liu S., Jia W., et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–7018. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 81.Giannopoulou M., Dai C., Tan X., Wen X., Michalopoulos G.K., Liu Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κb signaling. Am J Pathol. 2008;173(1):30–41. doi: 10.2353/ajpath.2008.070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saxena A., Khosraviani S., Noel S., Mohan D., Donner T., Hamad A.R.A. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J.-M., An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5(123) doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eming S.A., Wynn T.A., Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 86.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 87.Persson A.B., Buschmann I.R. Vascular growth in health and disease. Front Mol Neurosci. 2011;4:14. doi: 10.3389/fnmol.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baggott R.R., Alfranca A., López-Maderuelo D., Mohamed T.M., Escolano A., Oller J., et al. Plasma membrane calcium ATPase isoform 4 inhibits vascular endothelial growth factor-mediated angiogenesis through interaction with calcineurin. Arterioscler Thromb Vasc Biol. 2014;34(10):2310–2320. doi: 10.1161/ATVBAHA.114.304363. [DOI] [PubMed] [Google Scholar]
- 89.Schultz G.S., Grant M.B. Neovascular growth factors. Eye. 1991;5(Pt 2):170–180. doi: 10.1038/eye.1991.31. [DOI] [PubMed] [Google Scholar]
- 90.Tengood J.E., Ridenour R., Brodsky R., Russell A.J., Little S.R. Sequential delivery of basic fibroblast growth factor and platelet-derived growth factor for angiogenesis. Tissue Eng. 2011;17(9–10):1181–1189. doi: 10.1089/ten.tea.2010.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zymek P., Bujak M., Chatila K., Cieslak A., Thakker G., Entman M.L., et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48(11):2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 92.Matsui M., Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012;8(5):1792–1801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 93.Lana J.F., da Fonseca L.F., Azzini G., Santos G., Braga M., Cardoso Junior A.M., et al. Bone marrow aspirate matrix: a convenient ally in regenerative medicine. Int J Mol Sci. 2021;22(5) doi: 10.3390/ijms22052762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karin M., Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooke J.P. Inflammation and its role in regeneration and repair. Circ Res. 2019;124(8):1166–1168. doi: 10.1161/CIRCRESAHA.118.314669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hargrave B., Li F. Nanosecond pulse electric field activation of platelet-rich plasma reduces myocardial infarct size and improves left ventricular mechanical function in the rabbit heart. J Extra Corpor Technol. 2012;44(4):198–204. [PMC free article] [PubMed] [Google Scholar]
- 97.Yu F.X., Zhang Y., Tran N., Fu Y., Liao B., Shi Y.K. [Effects of myocardial platelet rich plasma injection on rats with acute myocardial infarction:(99)Tc(m)-MIBI gated SPECT imaging evaluation results] Zhonghua Xinxueguanbing Zazhi. 2012;40(5):392–396. [PubMed] [Google Scholar]
- 98.Li X.H., Zhou X., Zeng S., Ye F., Yun J.L., Huang T.G., et al. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron Artery Dis. 2008;19(5):363–370. doi: 10.1097/MCA.0b013e3282fc6165. [DOI] [PubMed] [Google Scholar]
- 99.Sun C.K., Zhen Y.Y., Leu S., Tsai T.H., Chang L.T., Sheu J.J., et al. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int J Cardiol. 2014;173(3):410–423. doi: 10.1016/j.ijcard.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 100.Gallo I., Sáenz A., Arévalo A., Roussel S., Pérez-Moreiras I., Artiñano E., et al. [Effect of autologous platelet-rich plasma on heart infarction in sheep] Arch Cardiol Mex. 2013;83(3):154–158. doi: 10.1016/j.acmx.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Mishra A., Velotta J., Brinton T.J., Wang X., Chang S., Palmer O., et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc Revascularization Med : Mol Interv. 2011;12(3):158–163. doi: 10.1016/j.carrev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Vu T.D., Pal S.N., Ti L.K., Martinez E.C., Rufaihah A.J., Ling L.H., et al. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: a translational approach: Vu and Pal "Myocardial Repair: PRP, Hydrogel and Supplements. Biomaterials. 2015;45:27–35. doi: 10.1016/j.biomaterials.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 103.Assmus B., Schächinger V., Teupe C., Britten M., Lehmann R., Döbert N., et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 104.Wollert K.C., Meyer G.P., Lotz J., Ringes Lichtenberg S., Lippolt P., Breidenbach C., et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 105.Sorichter S., Puschendorf B., Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev. 1999;5:5–21. [PubMed] [Google Scholar]
- 106.Shi X., Garry D.J. Muscle stem cells in development, regeneration, and disease. Gene Dev. 2006;20(13):1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 107.Mair J. Tissue release of cardiac markers: from physiology to clinical applications. Clin Chem Lab Med. 1999;37(11–12):1077–1084. doi: 10.1515/CCLM.1999.157. [DOI] [PubMed] [Google Scholar]
- 108.Haubner B.J., Schneider J., Schweigmann U., Schuetz T., Dichtl W., Velik-Salchner C., et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res. 2016;118(2):216–221. doi: 10.1161/CIRCRESAHA.115.307017. [DOI] [PubMed] [Google Scholar]
- 109.Bergmann O., Zdunek S., Felker A., Salehpour M., Alkass K., Bernard S., et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161(7):1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 110.Eschenhagen T., Bolli R., Braun T., Field L.J., Fleischmann B.K., Frisén J., et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136(7):680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furman M.I., Barnard M.R., Krueger L.A., Fox M.L., Shilale E.A., Lessard D.M., et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38(4):1002–1006. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 112.Mosesson M.W., Siebenlist K.R., Meh D.A. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 113.Middleton K.K., Barro V., Muller B., Terada S., Fu F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.