Abstract
Purpose
The previous analysis of systematic reviews and meta-analyses have illustrated that obstructive sleep apnea (OSA) is correlated with multiple health outcomes. In the present research, our main aim was to execute an umbrella review to assess the available evidence for the associations between OSA and health outcomes.
Methods
Herein, a meta-analysis of previous observational investigations that have reported associations between OSA and health outcomes in all human populations and settings was performed. We used these studies to execute an umbrella review of available meta-analyses and systematic reviews.
Results
Sixty-six articles comprising 136 unique outcomes were enrolled in this analysis. Of the 136 unique outcomes, 111 unique outcomes had significant associations (p < 0.05). Only 7 outcomes (coronary revascularization after PCI, postoperative respiratory failure, steatosis, alaninetrans aminase (ALT) elevation, metabolic syndrome (MS), psoriasis, and Parkinson’s disease) had a high quality of evidence. Twenty-four outcomes had a moderate quality of evidence, and the remaining 80 outcomes had a weak quality of evidence. Sixty-nine outcomes exhibited significant heterogeneity. Twenty-five outcomes exhibited publication bias. Sixty-three (95%) studies showed critically low methodological quality.
Conclusion
Among the 66 meta-analyses exploring 136 unique outcomes, only 7 statistically significant outcomes were rated as high quality of evidence. OSA may correlate with an increased risk of coronary revascularization after PCI, postoperative respiratory failure, steatosis, ALT elevation, MS, psoriasis, and Parkinson’s disease.
Keywords: Obstructive sleep apnea, Health, Umbrella review, Meta-analysis
Introduction
Obstructive sleep apnea (OSA) is a prevalent but treatable chronic sleep disorder that is determined through episodes of sleep apnea and hypopnea during sleep and results in recurrent episodes of hypercapnia and hypoxemia [1–3]. OSA has a prevalence of between 5 and 20% depending on the population surveyed and the definition utilized [4, 5]. The prevalence is also increasing due to an increase in body mass index which is one of its major predisposing factors. Apart from causing uncomfortable symptoms such as headache [6] and attention deficit [7], earlier studies indicated that OSA also contributed to the advancement of several diseases including hypertension [8], cardiovascular disease [9, 10], and diabetes [11]. Recent studies have drawn consistent conclusions [12–14]. Recently, a great number of researches have explored the correlation between OSA and other diseases. Multiple investigations and meta-analyses have illustrated that OSA poses a threat to human health because it increases the risk of various diseases, including cancers [15–17], depression [18], laryngopharyngeal reflux disease [19], metabolic disease [20], Parkinson’s disease [21], and chronickidney disease (CKD) [22].
These studies suggest a possible causal relationship between OSA and different health outcomes, indicating that OSA has a bad influence on human health. However, several factors are known to decrease the validity and strength of reported evidence including publication bias, protocol design flaws, or inconsistencies of studies. Currently, there have been no systematic reviews that have accurately summarized and critically appraised existing studies. In the current study, an umbrella review was executed to comprehensively evaluate published systematic reviews and meta-analyses of observational researches that reported associations between OSA and health information. This work can provide important guidance in the diagnosis and treatment of OSA.
Materials and methods
The protocol of the research was registered with PROSPERO (registration number: CRD42020220015) before the umbrella review began. A systematic exploration of the literature search was accomplished in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols [23].
Literature search
From initiation until November 23, 2020, literature searches were performed using online databases such as Embase, PubMed, the Cochrane Database of Systematic Reviews, and the Web of Science. Literature searches were independently conducted by two researchers (CZ and LG). The search terms applied were (“obstructive sleep apnea” OR “obstructive sleep apnea–hypopnea” OR “OSA” OR “OSAH”) AND (Meta-Analysis[ptyp] OR metaanaly*[tiab] OR meta-analy*[tiab] OR Systematic review [ptyp] OR “systematic review”[tiab]). The references were manually screened to identify eligible articles to be included in the study. The article titles, abstracts, and the complete manuscripts of the identified paper were then further assessed. A discussion was used to resolve potential discrepancies; ST acted as an arbiter to deal with discrepancies that could not be resolved by discussion among the investigators.
Eligibility criteria and exclusion criteria
The eligibility of articles was based on a systematic search by the authors to identify the most pertinent studies. Only systematic reviews or meta-analyses on the basis of the epidemiological studies performed in humans were considered in the analysis. Diagnostic trials and meta-analyses of interventional trials were not performed as part of the current study. Furthermore, the abstracts of the conference on review questions were not included in the final analysis. The final systematic reviews and meta-analyses that were analyzed had to include the data of pooled summary effects(i.e., relative risks (RRs); odds ratios (ORs); hazard ratios (HRs); mean difference (MD); weighted mean difference (WMD); standard mean difference (SMD); and their 95% confidence intervals (CIs)), number of included researches, number of participants and cases, heterogeneity, and publication bias. Whenever more than one meta-analysis was executed using on the basis of the same outcome, the agreement with the main conclusions reported in the study were verified. When the reported conclusions were conflicting, the meta-analysis with the greatest number of investigations was considered.
Data extraction
For investigations to be eligible for inclusion in the meta-analysis, two researchers (WC and YL) independently extracted data from the articles. This included the first author, the number of included investigations, the year of publication, the study design, the whole numbers of cases, and participants. The reported relative summary risk evaluates (ORs, RRs, HRs, SMD, WMD, or MD) and the corresponding 95% CIs were extracted, for each eligible systematic review and meta-analysis. The values of p for the total pooled effects, Cochran Q measurement, Egger’s measurement, and I2 were extracted. Discrepancies in the analyses were resolved by discussion among the investigators.
Assessment of methodological quality
Two investigators (WC and YL) independently assessed the quality of the methods reported in the studies. This was performed using a 16-criteria checklist included in AMSTAR 2 [24]. AMSTAR 2 is a fundamental revision of the original instrument of AMSTAR which was devised to evaluate systematic reviews that included randomized controlled experiments. The AMSTAR 2 score is categorized as high in studies that have no or one noncritical weakness, moderate in surveys with more than one noncritical weakness, low when the study has only one serious flaw without or with noncritical weaknesses, and seriously low when a study has more than one serious flaw without or with nonserious weaknesses. Discrepancies between the AMSTARS 2 scores for the articles were resolved by discussion between the investigators.
Assessment of the evidence quality
Two investigators (WC and YL) independently evaluated the quality of the evidence conforming to the parameters that have previously been applied in various fields [25–28]. Discrepancies were resolved by discussion. First, p value for the estimate < 0.001 [29, 30] and more than 1000 cases of the disease, which indicated fewer false-positive results. Second, I2 < 50% and p value for Cochran Q test > 0.10, which indicated consistency of results. Third, p value for Egger’s test > 0.10, which exhibited no evidence of small-study impacts. When all of the above criteria were satisfied, the strength of the epidemiologic evidence was rated as high. When 1 of the criterion was not satisfied and the p value for the estimate was < 0.001, the strength of the epidemiologic evidence was rated as moderate. Then, the rest was defined as weak (p < 0.05). The value of p for the evaluation can be assessed from the 95% confidence interval of the pooled impact estimate utilizing an established method [31] if it was not directly reported in the article.
Data analysis
From each of the published studies, the outcome data of the available meta-analyses was extracted along with the estimated summary effect at the corresponding 95% CI. The total impacts of the pooled meta-analysis were considered significant when the p-value was < 0.05. Heterogeneity was appraised by the I2 test and Q test, publication bias was estimated by utilizing Egger’s test, and both were considered significant at p < 0.1. Studies that did not have the heterogeneity or publication bias results were reanalyzed if raw data were available.
Results
Characteristics of the meta-analyses
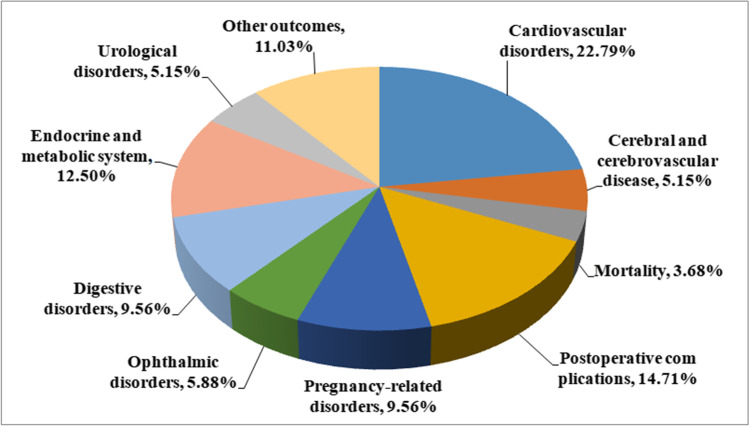
The outcomes of the systematic investigation and the selection of eligible investigations are summarized in Fig. 1. Overall, 1972 articles were searched from which 66 meta-analyses of observational investigations were identified that had 136 unique outcomes [21, 22, 32–95]. The 66 eligible non-overlapping meta-analyses had publication dates ranging from 2009 to 2020 and are summarized in Table 1. The median number of primary investigations per evidence synthesis was 7 (range 2–64). Furthermore, 1 meta-analysis [54] lacked the data of both participants and cases, and 2 meta-analyses [52, 95] lacked the data of cases. Among the meta-analyses identified in this study, the median number of cases was 900 (88–3,117,496) and the median number of participants was 2962 (170–56,746,100). An extensive range of data were reported such as cardiovascular disorders (n = 31), cerebral and cerebrovascular disease (n = 7), mortality (n = 5), postoperative complications (n = 20), pregnancy-related disorders (n = 13), ophthalmic disorders (n = 8), digestive disorders (n = 13), endocrine and metabolic system disorders(n = 17), urological disorders (n = 7), and other data (n = 15) (Fig. 2).
Fig. 1.
Flowchart of the selection procedure
Table 1.
Associations between OSA and multiple heath outcomes
| Outcomes | Publication | Number of studies | Number of participants | Number of cases | Type of metric | Relative risk (95% CI) | P value* | P value # | I2 (%) | P value※ | Whether exist publication bias | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular disorders | |||||||||||||||||||||||||||
| Aortic dissection | Xiushi Zhou (2018) | 1 cohort study, 2 case–control studies | 55,911 | 16,019 | OR | 1.60 (1.01–2.53) | 0.04 | 0.44 | 0 | 0.58 | No | ||||||||||||||||
| Cardiovascular disease(CVD) | Xia Wang (2013) | 11 cohort studies | 25,594 | 2628 | RR | 1.79 (1.47–2.18) | < 0.001 | 0.131 | 31.5 | 0.028 | Yes | ||||||||||||||||
| Stroke | Min Li (2014) | 10 cohort studies | 18,609 | 678 | RR | 2.10 (1.50–2.93) | < 0.001 | 0.04 | 47.5 | 0.288& | No | ||||||||||||||||
| Ischemic heart disease(IHD) | Wuxiang Xie (2014) | 6 cohort studies | 1083 | 625 | RR | 1.83 (1.15–2.93) | 0.011 | 0.111 | 44.2 | 0.006 | Yes | ||||||||||||||||
| Coronary heart disease(CHD) | Chengjuan Xie (2017) | 6 cohort studies | 18,022 | 15,562 | RR | 1.63 (1.18–2.26) | 0.003 | 0.061& | 52.7& | 0.145& | No | ||||||||||||||||
| Major adverse cardiac events (MACEs) | Chengjuan Xie (2017) | 9 cohort studies | 18,022 | 15,562 | RR | 2.04 (1.56–2.66) | < 0.001 | 0.021 | 55.7 | 0.132 | No | ||||||||||||||||
| Atrial fibrillation | Irini Youssef (2018) | 4 cross-sectional studies, 5 cohort studies | 19,837 | 12,255 | OR | 2.12 (1.84–2.43) | < 0.001 | 0.004 | 64.42 | 0.097& | Yes | ||||||||||||||||
| Resistant hypertension | Haifeng Hou (2018) | 6 case -control studies | 1465 | 925 | OR | 2.84 (1.70–3.98) | < 0.001 | 0.816 | 0 | 0.187& | No | ||||||||||||||||
| Essential hypertension | Haifeng Hou (2018) | 2 case–control studies, 5 cohort studies | 7102 | 4513 | OR | 1.80 (1.54–2.06) | < 0.001 | 0.221 | 26 | 0.0526& | Yes | ||||||||||||||||
| Atrial fibrillation recurrence after catheter ablation | Chee Yuan Ng (2011) | 6 observational studies | 3995 | 958 | RR | 1.25 (1.08–1.45) | 0.003 | 0.008 | 49 | 0.879& | No | ||||||||||||||||
| major adverse cardiovascular event (MACE) after PCI | Xiao Wang (2018) | 9 observational studies | 2755 | 1581 | RR | 1.96 (1.36–2.81) | < 0.001 | 0.02 | 54 | 0.002 | Yes | ||||||||||||||||
| Stroke after PCI | Xiao Wang (2018) | 6 observational studies | 2110 | 1254 | RR | 1.55 (0.90–2.67) | 0.11 | 0.62 | 0 | 0.149& | No | ||||||||||||||||
| Myocardial infarction (MI) after PCI | Hua Qu (2018) | 6 observational studies | 2342 | 1112 | OR | 1.59 (1.14–2.23) | 0.007 | 0.32 | 15 | 0.655& | No | ||||||||||||||||
| Coronary revascularization after PCI | Hua Qu (2018) | 7 observational studies | 2415 | 1163 | OR | 1.57 (1.23–2.01) | < 0.001 | 0.7 | 0 | 0.483& | No | ||||||||||||||||
| Re-admission for heart failure after PCI | Hua Qu (2018) | 4 observational studies | 1774 | 793 | OR | 1.71 (0.99–2.96) | 0.06 | 0.86 | 0 | 0.254& | No | ||||||||||||||||
| Left ventricular hypertrophy (LVH) | Cesare Cuspidi (2020) | 9 observational studies | 3244 | 1802 | OR | 1.70 (1.44–2.00) | < 0.001 | < 0.001 | 60 | 0.0876& | Yes | ||||||||||||||||
| Left ventricular diastolic diameter (LVEDD) | LeiYu (2019) | 13 observational studies | 882 | 563 | WMD | 1.24 (0.68, 1.80) | < 0.001 | 0.658 | 0 | 0.431 | No | ||||||||||||||||
| Left ventricular systolic diameter (LVESD) | LeiYu (2019) | 11 observational studies | 630 | 396 | WMD | 1.14 (0.47, 1.81) | 0.001 | 0.696 | 0 | 0.722 | No | ||||||||||||||||
| Left ventricular mass(LVM) | LeiYu (2019) | 6 observational studies | 432 | 304 | WMD | 35.34 (20.67, 50.00) | < 0.001 | < 0.001 | 79.1 | 0.914 | No | ||||||||||||||||
| Leftventricular ejection fraction (LVEF) | LeiYu (2019) | 15 observational studies | 1104 | 710 | WMD | − 3.01 (− 1.90, − 0.79) | 0.001 | < 0.001 | 64.7 | 0.048 | Yes | ||||||||||||||||
| Left atrial diameter (LAD) | LeiYu (2019) | 7 observational studies | 468 | 311 | WMD | 2.13 (1.48, 2.77) | < 0.001 | 0.408 | 2.2 | 0.072 | Yes | ||||||||||||||||
| Left atrial diameter volume index (LAVI) | LeiYu (2019) | 3 observational studies | 228 | 159 | WMD | 3.96 (3.32, 4.61) | < 0.001 | 0.445 | 0 | 0.735 | No | ||||||||||||||||
| Right ventricular internal diameter (RVID) | Abdirashit Maripov (2017) | 16 observational studies | 1498 | 902 | WMD | 2.49 (1.62, 3.37) | < 0.001 | < 0.001 | 96.8 | 0.001 | Yes | ||||||||||||||||
| Right ventricular free wall thickness (RVWT) | Abdirashit Maripov (2017) | 9 observational studies | 976 | 579 | WMD | 0.82 (0.51, 1.13) | < 0.001 | < 0.001 | 95.6 | 0.671 | No | ||||||||||||||||
| Right ventricular myocardial performance index(RV MPI) | Abdirashit Maripov (2017) | 14 observational studies | 1298 | 864 | WMD | 0.08 (0.06, 0.10) | < 0.001 | < 0.001 | 84.1 | 0.15 | No | ||||||||||||||||
| Tricuspid annular systolic velocity (RV S′) | Abdirashit Maripov (2017) | 14 observational studies | 1030 | 639 | WMD | − 0.95 (− 0.32, − 1.59) | 0.003 | < 0.001 | 88.4 | 0.347 | No | ||||||||||||||||
| Tricuspid annular plane systolic excursion (TAPSE) | Abdirashit Maripov (2017) | 11 observational studies | 1033 | 655 | WMD | − 1.76 (− 0.78, − 2.73) | < 0.001 | < 0.001 | 89.3 | 0.462 | No | ||||||||||||||||
| Right ventricular fractional area change (RA FAC) | Abdirashit Maripov (2017) | 6 observational studies | 661 | 422 | WMD | − 3.16 (− 0.73, − 5.60) | 0.011 | < 0.001 | 80.2 | 0.006 | Yes | ||||||||||||||||
| Epicardial adipose tissue (EAT) thickness | Guang Song (2020) | 9 observational studies | 1178 | 898 | WMD | 0.95 (0.73, 1.16) | < 0.001 | < 0.001 | 64.7 | 0.549 | No | ||||||||||||||||
| Coronary flow reserve (CFR) | Rui-Heng Zhang (2020) | 1 case–control study, 4 cross-sectional studies | 1336 | 829 | WMD | ’ − 0.78 (− 0.32, − 1.25) | < 0.001 | < 0.001 | 84.4 | 0.49 | No | ||||||||||||||||
| Systolic blood pressure (SBP) | De-Lei Kong (2016) | 2 cross-sectional studies, 3 cohort studies, 1 case–control studies | 1046 | 534 | SMD | 0.56 (0.40, 0.71) | < 0.001 | 0.132 | 41.03 | NA | NA | ||||||||||||||||
| Cerebral and cerebrovascular disease | |||||||||||||||||||||||||||
| Cerebral white matter changes | Bo-Lin Ho (2018) | 10 observational studies | 1582 | 818 | OR | 2.06 (1.52–2.80) | < 0.001 | 0.025 | 48.5 | 0.338 | No | ||||||||||||||||
| Cerebrovascular (CV) disease | Zesheng Wu (2018) | 15 cohort studies | 3,120,368 | 3,117,496 | HR | 1.94 (1.31–2.89) | 0.001 | < 0.001 | 90.3 | > 0.05 | No | ||||||||||||||||
| White matter hyperintensities (WMH) | Yuhong Huang (2019) | 11 cross-sectional studies, 2 case–control studies | 4412 | 2065 | OR | 2.23 (1.53–3.25) | < 0.001 | < 0.001 | 80.3 | < 0.01 | Yes | ||||||||||||||||
| Silent brain infarction (SBI) | Yuhong Huang (2019) | 9 cross-sectional studies, 2 case–control studies, 1 cohort study | 3353 | 1893 | OR | 1.54 (1.06–2.23) | 0.023 | 0.018 | 52 | 0.605 | No | ||||||||||||||||
| Cerebral microbleeds (CMBs) | Yuhong Huang (2019) | 3 cross-sectional studies | 342 | 271 | OR | 2.17 (0.61–7.73) | 0.234 | < 0.01 | 60.2 | NA | Unclear | ||||||||||||||||
| Perivascular spaces (PVS) | Yuhong Huang (2019) | 2 cross-sectional studies | 267 | 152 | OR | 1.56 (0.28–8.57) | 0.623 | < 0.01 | 69.5 | NA | NA | ||||||||||||||||
| Asymptomatic lacunar infarction (ALI) | AnthipaChokesuwattanaskul (2019) | 6 cross-sectional studies, 1 cohort study | 1756 | 713 | OR | 1.78 (1.06–3.01) | 0.03 | 0.128& | 41 | 0.43 | No | ||||||||||||||||
| Mortality | |||||||||||||||||||||||||||
| All-cause mortality | Lei Pan (2016) | 12 cohort studies | 34,382 | 18,139 | HR | 1.26 (1.09–1.43) | 0.001 | < 0.001 | 70.4 | 0.003 | Yes | ||||||||||||||||
| Cardiovascular mortality | Xiahui Ge (2013) | 4 cohort studies | 5228 | 239 | RR | 2.21 (1.61–3.04) | < 0.001 | 0.418 | 0 | 0.448 | No | ||||||||||||||||
| All-cause death after PCI | Xiao Wang (2018) | 4 cohort studies | 1919 | 1154 | RR | 1.70 (1.05–2.77) | 0.03 | 0.71 | 0 | 0.176& | No | ||||||||||||||||
| Cardiac death after PCI | Hua Qu (2018) | 7 cohort studies | 2465 | 1187 | OR | 2.05 (1.15–3.65) | 0.01 | 0.96 | 0 | 0.828& | No | ||||||||||||||||
| Cancer mortality | Xiaobin Zhang (2017) | 3 cohort studies | 7346 | 179 | HR | 1.38 (0.79–2.41) | 0.257 | 0.004 | 66.1 | 0.205 | No | ||||||||||||||||
| Postoperative complications | |||||||||||||||||||||||||||
| Postoperative respiratory failure | Faizi Hai BA (2013) | 12 cohort studies | 5611 | 2390 | OR | 2.42 (1.53–3.84) | < 0.001 | 0.39 | 5 | 0.28 | No | ||||||||||||||||
| Postoperative cardiac events | Faizi Hai BA (2013) | 11 cohort studies | 3781 | 2109 | OR | 1.63 (1.16–2.29) | 0.005 | 0.7 | 0 | 0.187& | No | ||||||||||||||||
| Postoperative desaturation | R. Kaw (2012) | 11 cohort studies | 3645 | 1764 | OR | 2.27 (1.20–4.26) | 0.01 | < 0.001 | 68 | 0.04& | Yes | ||||||||||||||||
| Postoperative ICU transfer | R. Kaw (2012) | 9 cohort studies | 5743 | 2062 | OR | 2.81 (1.46–5.43) | 0.002 | 0.02 | 57 | 0.033& | Yes | ||||||||||||||||
| Postoperative composite endpoints of postoperative cardiac or cerebrovascular complications | Ka Ting Ng (2020) | 12 observational studies | 2,003,694 | 126,027 | OR | 1.44 (1.17–1.78) | < 0.001 | NA | 89 | NA | Unclear | ||||||||||||||||
| Postoperative myocardial infarction | Ka Ting Ng (2020) | 8 observational studies | 714,650 | NA | OR | 1.37 (1.19–1.59) | < 0.001 | NA | 36 | NA | Unclear | ||||||||||||||||
| Postoperative congestive cardiac failure | Ka Ting Ng (2020) | 3 observational studies | 2104 | NA | OR | 3.16 (1.02–9.81) | 0.05 | NA | 0 | NA | Unclear | ||||||||||||||||
| Postoperative atrial fibrillation | Ka Ting Ng (2020) | 6 observational studies | 1,463,449 | NA | OR | 1.50 (1.30–1.73) | < 0.001 | NA | 87 | NA | Unclear | ||||||||||||||||
| Postoperative cerebrovascular accident | Ka Ting Ng (2020) | 5 observational studies | 1,641,495 | NA | OR | 1.09 (0.75–1.60) | 0.65 | NA | 61 | NA | Unclear | ||||||||||||||||
| Postoperative composite endpoints of pulmonary complications | Ka Ting Ng (2020) | 8 observational studies | 1,983,748 | NA | OR | 2.52 (1.92–3.31) | < 0.001 | NA | 96 | NA | Unclear | ||||||||||||||||
| Postoperative pneumonia | Ka Ting Ng (2020) | 10 observational studies | 2,675,205 | NA | OR | 1.66 (1.17–2.35) | 0.004 | NA | 96 | NA | Unclear | ||||||||||||||||
| Postoperative reintubation | Ka Ting Ng (2020) | 9 observational studies | 2,061,268 | NA | OR | 2.29 (0.90–5.82) | 0.08 | NA | 99 | NA | Unclear | ||||||||||||||||
| Postoperative in-hospital mortality | Ka Ting Ng (2020) | 6 observational studies | 2,497,794 | NA | OR | 0.86 (0.42–1.76) | 0.68 | NA | 94 | NA | Unclear | ||||||||||||||||
| Postoperative 30-day mortality | Ka Ting Ng (2020) | 6 observational studies | 616,754 | NA | OR | 1.27 (1.03–1.57) | 0.02 | NA | 0 | NA | Unclear | ||||||||||||||||
| Postoperative acute kidney injury | Ka Ting Ng (2020) | 5 observational studies | 1,724,932 | NA | OR | 2.41 (1.93–3.02) | < 0.001 | NA | 92 | NA | Unclear | ||||||||||||||||
| Postoperative delirium | Ka Ting Ng (2020) | 6 observational studies | 2346 | NA | OR | 2.45 (1.50–4.01) | < 0.001 | NA | 2 | NA | Unclear | ||||||||||||||||
| Postoperative venoembolism | Ka Ting Ng (2020) | 10 observational studies | 2,100,013 | NA | OR | 1.63 (1.17–2.27) | 0.004 | NA | 94 | NA | Unclear | ||||||||||||||||
| Postoperative surgical site infection | Ka Ting Ng (2020) | 5 observational studies | 2962 | NA | OR | 1.30 (0.93–1.83) | 0.13 | NA | 0 | NA | Unclear | ||||||||||||||||
| Postoperative bleeding | Ka Ting Ng (2020) | 3 observational studies | 18,712 | NA | OR | 1.10 (0.40–3.01) | 0.85 | NA | 63 | NA | Unclear | ||||||||||||||||
| Postoperative length of hospital stay | Ka Ting Ng (2020) | 15 observational studies | 1,569,278 | NA | MD | 0.09 (0.00–0.17) | 0.04 | NA | 96 | NA | Unclear | ||||||||||||||||
| Pregnancy-related disorders | |||||||||||||||||||||||||||
| Gestational diabetes mellitus (GDM) | Xinge Zhang (2020 | 6 cohort studies | 2,522,547 | 139,559 | RR | 1.60 (1.21–2.12) | 0.004 | 0.003 | 69.2 | 0.4829 | No | ||||||||||||||||
| C-section | Lina Liu (2019) | 6 observational studies | NA | NA | OR | 1.42 (1.12–1.79) | < 0.001 | < 0.001 | 86.5 | NA | Unclear | ||||||||||||||||
| Pregnancy-related prolonged hospital stay | Lina Liu (2019) | 3 observational studies | NA | NA | OR | 1.94 (0.88–4.28) | 0.1 | < 0.001 | 98.6 | NA | Unclear | ||||||||||||||||
| Pregnancy-related wound complication | Lina Liu (2019) | 3 observational studies | NA | NA | OR | 1.87 (1.56–2.24) | < 0.001 | 0.883 | 0 | NA | Unclear | ||||||||||||||||
| Pregnancy-related pulmonary edema | Lina Liu (2019) | 3 observational studies | NA | NA | OR | 6.35 (4.25–9.50) | < 0.001 | 0.294 | 18.2 | NA | Unclear | ||||||||||||||||
| Small for gestational age | Lina Liu (2019) | 4 observational studies | NA | NA | OR | 1.26 (0.80–2.01) | 0.321 | 0.01 | 73.8 | NA | Unclear | ||||||||||||||||
| Stillbirth | Lina Liu (2019) | 3 observational studies | NA | NA | OR | 1.12 (0.85–1.49) | 0.413 | 0.572 | 0 | NA | Unclear | ||||||||||||||||
| Poor fetal growth | Lina Liu (2019) | 4 observational studies | NA | NA | OR | 1.15 (0.98–1.34) | 0.091 | 0.266 | 24.3 | NA | Unclear | ||||||||||||||||
| Gestational hypertension | Liwen Li (2018) | 4 cross-sectional studies, 7 cohort studies | 56,731,077 | 19,047 | OR | 1.80 (1.28–2.52) | 0.001 | 0.72 | 0 | 0.649& | No | ||||||||||||||||
| Preeclampsia | Liwen Li (2018) | 2 cross-sectional studies, 7 cohort studies | 56,097,993 | 19,776 | OR | 2.63 (1.87–3.70) | < 0.001 | < 0.01 | 78 | 0.797& | No | ||||||||||||||||
| Preterm birth | Liwen Li (2018) | 2 cross-sectional studies, 3 cohort studies | 56,746,100 | 18,337 | OR | 1.75 (1.21–2.55) | 0.003 | < 0.01 | 90 | 0.931& | No | ||||||||||||||||
| Birth weight | Liwen Li (2018) | 4 cohort studies | 4311 | 1387 | WMD | − 47.46 (− 242.09, 147.16) | 0.281 | < 0.01 | 93 | NA | No$ | ||||||||||||||||
| Neonatal intensive care unit (NICU) admission | Ting Xu (2014) | 4 cohort studies | 757 | 177 | RR | 2.65 (1.86–3.76) | < 0.001 | 0.235 | 29.6 | 0.063& | Yes | ||||||||||||||||
| Ophthalmic disorders | |||||||||||||||||||||||||||
| Diabetic retinopathy (DR) | Zhenliu Zhu (2017) | 6 case -control studies | 1092 | 608 | OR | 2.01 (1.49–2.72) | < 0.001 | 0.062 | 52.4 | 0.112& | No | ||||||||||||||||
| Keratoconus | Marco Pellegrini (2020) | 4 case–control studies, 1 cohort study | 33,844 | 16,922 | OR | 1.84 (1.16–2.91) | 0.009 | 0.003 | 74.6 | 0.07 | Yes | ||||||||||||||||
| Glaucoma | Xinhua Wu (2015) | 12 observational studies | 36,909 | 11,765 | OR | 1.65 (1.44–1.88) | < 0.001 | 0.06 | 43 | 0.335 | No | ||||||||||||||||
| Floppy eyelid syndrome (FES) | Leh-Kiong Huon (2016) | 7 cross-sectional studies | 902 | 337 | OR | 4.70 (2.98–7.41) | < 0.001 | 0.129& | 39.3& | 0.379& | No | ||||||||||||||||
| Nonarteritic anterior ischemic optic neuropathy (NAION) | Yong Wu (2015) | 4 cohort studies, 1 case–control study | 5916 | 164 | OR | 6.18 (2.00–19.11) | 0.002 | 0.002 | 77 | 0.35 | No | ||||||||||||||||
| Central serous chorioretinopathy (CSCR) | Chris Y.Wu (2018) | 6 case–control studies | 7238 | 1479 | OR | 1.56 (1.16–2.10) | 0.003 | 0.237 | 26.3 | 0.281 | No | ||||||||||||||||
| retinal nerve fiber layer (RNFL) thickness | Cheng-Lin Sun (2016) | 8 case–control studies | 1237 | 763 | WMD | − 2.92 (− 4.61, − 1.24) | 0.001 | 0.017 | 59.1 | 0.929 | No | ||||||||||||||||
| Choroidal thickness | Chris Y.Wu (2018) | 9 case–control studies | 778 | 514 | WMD | 25.52 (− 78.79, − 27.76) | 0.824 | 0.001 | 98.6 | 0.137 | No | ||||||||||||||||
| Digestive disorders | |||||||||||||||||||||||||||
| Gastroesophageal reflux disease | Zeng-Hong Wu (2019) | 1 case–control study, 6 cross-sectional studies | 2699 | 1452 | OR | 1.75 (1.18–2.59) | 0.006 | 0.04 | 54 | 0.052 | Yes | ||||||||||||||||
| Steatosis | Shanshan Jin (2018) | 3 cohort studies, 1 cross-sectional study | 1635 | 1375 | OR | 3.19 (2.34–4.34) | < 0.001 | 0.677 | 0 | 0.89 | No | ||||||||||||||||
| Lobular inflammation | Shanshan Jin (2018) | 3 cohort studies | 350 | 205 | OR | 2.85 (1.8–-4.49) | < 0.001 | 0.994 | 0 | 0.469 | No | ||||||||||||||||
| Ballooning degeneration | Shanshan Jin (2018) | 3 cohort studies | 350 | 205 | OR | 2.29 (1.36–3.84) | 0.002 | 0.774 | 0 | 0.888 | No | ||||||||||||||||
| NAFLD activity score(NAS) | Shanshan Jin (2018) | 3 cohort studies | 350 | 205 | OR | 1.63 (0.68–3.86) | 0.271 | 0.259 | 25.9 | 0.839 | No | ||||||||||||||||
| NAFLD defined by liver histology | G. Musso (2013) | 8 cross-sectional studies | 994 | 537 | OR | 2.01 (1.36–2.97) | < 0.001 | 0.4 | 4 | 0.303& | No | ||||||||||||||||
| NAFLD defined by radiology | G. Musso (2013) | 6 cross-sectional studies | 561 | 269 | OR | 2.99 (1.79–4.99) | < 0.001 | 0.33 | 13 | 0.433& | No | ||||||||||||||||
| NAFLD defined by AST elevation | G. Musso (2013) | 11 cross-sectional studies | 746 | 368 | OR | 2.36 (1.46–3.82) | < 0.001 | 0.99 | 0 | 0.65& | No | ||||||||||||||||
| NAFLD defined by ALT elevation | G. Musso (2013) | 14 cross-sectional studies | 1833 | 938 | OR | 2.60 (1.88–3.61) | < 0.001 | 0.74 | 0 | 0.179& | No | ||||||||||||||||
| Nonalcoholic steatohepatitis(NASH) | G. Musso (2013) | 10 cross-sectional studies | 1114 | 589 | OR | 2.37 (1.59–3.51) | < 0.001 | 0.81 | 0 | 0.404& | No | ||||||||||||||||
| Fibrosis | G. Musso (2013) | 10 cross-sectional studies | 1114 | 589 | OR | 2.16 (1.45–3.20) | < 0.001 | 0.67 | 0 | 0.778& | No | ||||||||||||||||
| Alanine transaminase (ALT) | Shanshan Jin (2018) | 7 cohort studies, 1 cross-sectional study | 2059 | 1684 | SMD | 0.21 (0.11, 0.31) | < 0.001 | 0.672 | 0 | 0.468 | No | ||||||||||||||||
| Aspartate transaminase (AST) | Shanshan Jin (2018) | 7 cohort studies, 1 cross-sectional study | 2059 | 1684 | SMD | 0.07 (− 0.03, 0.17) | 0.152 | 0.918 | 0 | < 0.05 | Yes | ||||||||||||||||
| Endocrine and metabolic system disorders | |||||||||||||||||||||||||||
| Type 2 diabetes (T2DM) | Ranran Qie (2020) | 16 cohort studies | 338,912 | 19,355 | RR | 1.40 (1.32–1.48) | < 0.001 | 0.045 | 40.8 | 0.221& | No | ||||||||||||||||
| Metabolic syndrome (MS) | Shaoyong Xu (2015) | 15 cross-sectional studies | 4161 | 2457 | OR | 2.87 (2.41–3.42) | < 0.001 | 0.23 | 20 | 0.232 | No | ||||||||||||||||
| Fasting blood glucose (FBG) | De-Lei Kong (2016) | 3 cross-sectional studies, 5 cohort studies, 2 case–control studies | 2053 | 1296 | SMD | 0.35 (0.18, 0.53) | < 0.001 | 0.008 | 59.69 | NA | No$ | ||||||||||||||||
| Total cholesterol (TC) | Rashid Nadeem (2014) | 63 observational studies | 18,111 | NA | SMD | 0.267 (0.146, 0.389) | 0.001 | NA | NA | NA | No$ | ||||||||||||||||
| Low-density lipoprotein (LDL) | Rashid Nadeem (2014) | 50 observational studies | 13,894 | NA | SMD | 0.296 (0.156, 0.436) | 0.001 | NA | NA | NA | No$ | ||||||||||||||||
| High-density lipoprotein (HDL) | Rashid Nadeem (2014) | 64 observational studies | 18,116 | NA | SMD | − 0.433 (− 0.604, − 0.262) | < 0.001 | NA | NA | NA | No$ | ||||||||||||||||
| Triglyceride (TG) | Rashid Nadeem (2014) | 62 observational studies | 17,831 | NA | SMD | 0.603 (0.431, 0.775) | < 0.001 | NA | NA | NA | No$ | ||||||||||||||||
| Adiponectin | Mi Lu (2019) | 20 case–control studies | 1356 | 878 | SMD | ′ − 0.71 (− 0.92, − 0.49) | < 0.001 | < 0.01 | 73 | 0.09 | Yes | ||||||||||||||||
| Oxidized low-density lipoprotein (Ox-LDL) | Reza Fadaei (2020) | 8 case -control studies | 623 | 391 | SMD | 0.95 (0.24, 1.67) | 0.009 | < 0.001 | 94.1 | < 0.161 | No | ||||||||||||||||
| Fibrinogen | Fang Lu (2019) | 25 observational studies | 3792 | 1480 | WMD | 0.38 (0.29, 0.47) | < 0.001 | < 0.001 | 80.3 | 0.208 | No | ||||||||||||||||
| Homocysteine | Kun Li (2017) | 10 observational studies | 773 | 457 | MD | 2.40 (0.60, 4.20) | 0.009 | < 0.001 | 96 | 0.947 | No | ||||||||||||||||
| Advanced glycation end products (AGEs) | Xingyu Wu (2018) | 5 cross-sectional studies | 670 | 323 | SMD | 0.98 (0.69, 1.27) | < 0.001 | 0.08 | 51 | NA | No$ | ||||||||||||||||
| Plasma renin activity(PRA) | Ze-Ning Jin (2016) | 5 case–control studies | 300 | 180 | MD | 0.17 (− 0.22, 0.55) | 0.4 | < 0.001 | 82 | NA | Unclear | ||||||||||||||||
| Plasma renin concentration(PRC) | Ze-Ning Jin (2016) | 5 case–control studies | 170 | 101 | MD | 0.95 (− 0.58, 2.48) | 0.23 | 0.001 | 78 | NA | Unclear | ||||||||||||||||
| Angiotensin II(AngII) | Ze-Ning Jin (2016) | 7 case–control studies | 384 | 207 | MD | 3.39 (2.00, 4.79) | < 0.001 | < 0.001 | 95 | 0.167 | No | ||||||||||||||||
| Aldosterone | Ze-Ning Jin (2016) | 9 case–control studies | 474 | 265 | MD | 0.95 (− 0.16, 2.07) | 0.09 | < 0.001 | 78 | 0.622 | No | ||||||||||||||||
| Serum vitamin D | Xiaoyan Li (2020) | 6 case–control studies, 21 cross-sectional studies, 2 cohort studies | 6298 | 4209 | SMD | ′ − 0.84(− 1.14, − 0.54) | < 0.001 | < 0.001 | 95 | NA | No$ | ||||||||||||||||
| Urological disorders | |||||||||||||||||||||||||||
| Diabetic kidney disease (DKD) | Wen Bun Leong (2016) | 7 cross-sectional studies | 1877 | 1159 | OR | 1.59 (1.16–2.18) | 0.004 | 0.224& | 26.8 | 0.684& | No | ||||||||||||||||
| Microalbuminuria | Tongtong Liu (2020) | 4 cross-sectional studies | 667 | 415 | RR | 2.32 (1.48–3.62) | < 0.001 | 0.578 | 0 | 0.55 | No | ||||||||||||||||
| Chronic kidney disease (CKD) | Der-Wei Hwu (2017) | 2 cohort studies, 16 cross-sectional studies | 7090 | 3720 | OR | 1.77 (1.37–2.29) | < 0.001 | < 0.001& | 87.2& | 0.011& | Yes | ||||||||||||||||
| Serum uric acid level | Tingting Shi (2019) | 14 observational studies | 5219 | 2656 | WMD | 50.25 (36.16,64.33) | < 0.001 | < 0.001 | 91.2 | 0.001 | Yes | ||||||||||||||||
| Serum cystatin C | Tongtong Liu (2020) | 7 cross-sectional studies | 1412 | 274 | SMD | 0.53 (0.42,0.64) | < 0.001 | 0.16 | 33.7 | 0.111 | No | ||||||||||||||||
| Estimated glomerular filtration rate (eGFR) | Tongtong Liu (2020) | 13 cross-sectional studies | 3344 | 657 | SMD | − 0.19 (− 0.27, − 0.12) | 0.001 | 0.057 | 33.1 | 0.516 | No | ||||||||||||||||
| Albumin/creatinine ratio(ACR) | Tongtong Liu (2020) | 3 cross-sectional studies | 740 | 88 | WMD | 0.71 (0.58, 0.84) | < 0.001 | 0.003 | 69.2 | 0.574 | No | ||||||||||||||||
| Other outcomes | |||||||||||||||||||||||||||
| Diabetic neuropathy | Xiandong Gu (2018) | 11 case -control studies | 1842 | 840 | OR | 1.84 (1.18–2.87) | 0.007 | < 0.01 | 68.6 | 0.13 | No | ||||||||||||||||
| Psoriasis | Tzong-Yun Ger (2020) | 3 cohort studies | 5,544,674 | 42,656 | RR | 2.52 (1.89–3.36) | < 0.001 | 0.95 | 0 | 0.545 | No | ||||||||||||||||
| Nocturia | Jiatong Zhou (2019) | 3 cohort studies, 8 case–control studies, 2 cross-sectional studies | 9924 | 406 | RR | 1.41 (1.26–1.59) | < 0.001 | 0.001 | 63.3 | 0.076 | Yes | ||||||||||||||||
| Allergic rhinitis | Yuan Cao (2018) | 1 cross-sectional study, 2 case–control studies, 1 cohort study | 1283 | 371 | OR | 1.73 (0.94–3.20) | 0.078 | 0.023 | 64.8 | 0.977 | No | ||||||||||||||||
| Parkinson’s disease | A-Ping Sun (2020) | 4 cohort studies, 1 case–control study | 83,449 | 26,070 | HR | 1.59 (1.36–1.85) | < 0.001 | 0.17 | 40 | 0.186 | No | ||||||||||||||||
| Erectile dysfunction | Luhao Liu (2015) | 1 cohort study, 3 case–control studies, 1 cross-sectional study | 834 | 532 | RR | 1.82 (1.12–2.97) | 0.016 | 0.002 | 76.5 | 0.077 | Yes | ||||||||||||||||
| Female sexual dysfunction | Luhao Liu (2015) | 2 case–control studies, 2 cohort studies | 438 | 149 | RR | 2.0 (1.29–3.08) | 0.002 | 0.194 | 36.4 | 0.327 | No | ||||||||||||||||
| Sexual dysfunction | Luhao Liu (2015) | 3 cohort studies, 5 case–control studies, 1 cross-sectional study | 1272 | 681 | RR | 1.87 (1.35–2.58) | < 0.001 | 0.001 | 70.1 | 0.692 | No | ||||||||||||||||
| Osteoporosis | Sikarin Upala (2016) | 2 cohort studies, 2 cross-sectional studies | 113,922 | 3141 | OR | 1.13 (0.60–2.14) | 0.703 | < 0.001 | 89.1 | 0.608& | No | ||||||||||||||||
| Gout | Tingting Shi (2019) | 3 cohort studies | 154,455 | 30,109 | HR | 1.25 (0.91–1.70) | 0.162 | < 0.001 | 91 | 0.876 | No | ||||||||||||||||
| Cancer incidence | Ghanshyam Palamaner Subash Shantha (2015) | 5 cohort studies | 112,226 | 904 | RR | 1.40 (1.01–1.95) | 0.04 | 0.04 | 60 | 0.069 | Yes | ||||||||||||||||
| Depression | Cass Edwards (2020) | 5 cohort studies | 45,056 | 10,983 | RR | 2.18 (1.47–2.88) | < 0.001 | 0.005 | 72.8 | 0.667& | No | ||||||||||||||||
| Crash risk | Stephen Tregear (2009) | 10 observational studies | 10,846 | 2214 | RR | 2.43 (1.21–4.89) | 0.013 | < 0.001 | 89 | 0.838& | No | ||||||||||||||||
| Work accidents | Sergio Garbarino (2016) | 7 cross-sectional studies | 8819 | 2738 | OR | 2.18 (1.53–3.10) | < 0.001 | 0.02 | 61 | 0.61 | No | ||||||||||||||||
| Carotid intima-media thickness (CIMT) | Min Zhou (2016) | 10 case–control studies, 8 case-sectional studies | 1896 | 1247 | SMD | 0.88 (0.65, 1.12) | < 0.001 | < 0.001 | 81 | 0.94 | No | ||||||||||||||||
*p value of significance level
#p value of Q test
※p value for Egger’s test
$The publication bias was assessed using funnel plot
&The result was reanalyzed
Fig. 2.
Map of achievements related to OSA
Summary effect size
A brief explanation of the effects of the included meta-analysis is given in Table 1. Overall, 111 (82%) of the 136 data reported significant summary outcomes (p < 0.05). These associations relate to the outcomes of the following different systems: 29 meta-analyses in cardiovascular disorders, 5 in cerebral and cerebrovascular disease, 4 in mortality, 14 in postoperative complications, 8 in pregnancy-related disorders, 7 in ophthalmic disorders, 11 in digestive disorders, 14 in endocrine and metabolic system, 7 in urological disorders, and 12 in other outcomes. Therefore, it can be concluded that OSA can enhance the risk of disease and have adverse effects on human health.
Heterogeneity and publication bias
For heterogeneity, 5 results in 5 articles were reanalyzed owing to that they did not exhibit the outcomes of heterogeneity [22, 36, 46, 59, 64]. Among the 136 outcomes including the reanalyzed articles, 47 outcomes showed no heterogeneity between researches (p ≥ 0.1 of Q test), whereas 69 indicated significant heterogeneity (p < 0.1 of Q test). However, there were still 20 results in 2 articles that could not be reanalyzed due to the lack of raw data [52, 95], so we could not evaluate their heterogeneity. For publication bias, 76 outcomes demonstrated no statistical evidence on publication bias (p ≥ 0.1 of Egger’s test), whereas 25 outcomes presented publication bias (p < 0.1 of Egger’s test). There were still 35 results in 9 articles that could not be reanalyzed due to the lack of raw data [45, 52, 54, 55, 87, 92–95], so we could not evaluate their publication bias.
AMSTAR 2 and summary of evidence
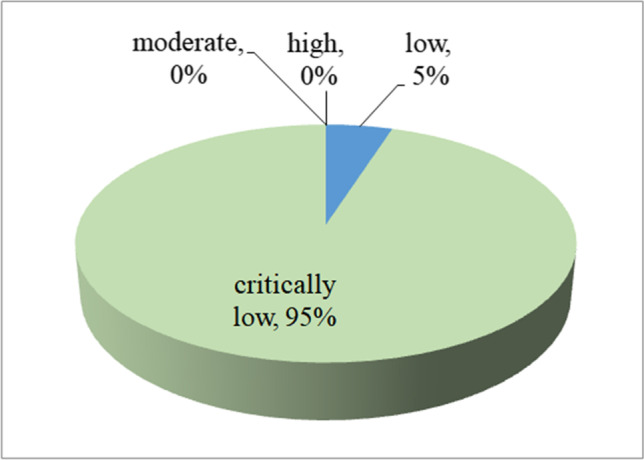
The results for the evaluation of the methodological qualities of the 66 included articles are shown in Table 2. Only 3 (5%) studies were determined to be low; the remaining 63 (95%) studies were determined to be critically low (Fig. 3). Based on the AMSTAR 2 criteria, none of the investigations were graded as moderate or high quality.
Table 2.
Assessments of AMSTAR 2 scores
| Reference | AMSTAR 2 checklist | Overall assessment quality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 | No. 13 | No. 14 | No. 15 | No. 16 | ||
| Xiushi Zhou (2018) | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xia Wang (2013) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Min Li (2014) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | No | No | No | No | No | Critically low |
| Wuxiang Xie (2014) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Chengjuan Xie (2017) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Irini Youssef (2018) | Yes | No | No | Partial yes | No | No | Partial yes | No | No | No | Yes | No | No | No | No | No | Critically low |
| Haifeng Hou (2018) | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Chee Yuan Ng (2011) | Yes | No | Yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Xiao Wang (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Hua Qu (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Cesare Cuspidi (2020) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | Yes | No | Yes | Critically low |
| Bo-Lin Ho (2018) | Yes | No | No | Partial yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Zesheng Wu (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Yuhong Huang (2019) | Yes | No | No | Partial yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Anthipa Chokesuwattanaskul (2019) | Yes | No | Yes | Partial yes | No | No | Partial yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Lei Pan (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xiahui Ge (2013) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xiaobin Zhang (2017) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Faizi Hai BA (2013) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| R. Kaw (2012) | Yes | No | Yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Ka Ting Ng (2020) | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Xinge Zhang (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Lina Liu (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Liwen Li (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | Critically low |
| Ting Xu (2014) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Marco Pellegrini (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xinhua Wu (2015) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Leh-Kiong Huon (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | No | No | Yes | Critically low |
| Yong Wu (2015) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Chris Y.Wu (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Ranran Qie (2020) | Yes | No | Yes | Partial yes | No | No | Partial yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Xiandong Gu (2018) | Yes | No | Yes | Partial yes | No | No | Partial yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Wen Bun Leong (2016) | Yes | Yes | Yes | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Zhenliu Zhu (2017) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Zeng-Hong Wu (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Shanshan Jin (2018) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| G. Musso (2013) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Tzong-Yun Ger (2020) | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | No | No | Yes | Critically low |
| Jiatong Zhou (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Yuan Cao (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | No | No | No | No | Yes | Critically low |
| A-Ping Sun (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Luhao Liu (2015) | Yes | No | Yes | Partial yes | Yes | Yes | No | Yes | No | No | Yes | No | No | No | Yes | Yes | Critically low |
| Sikarin Upala (2016) | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tingting Shi (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | No | Yes | Yes | Critically low |
| Tongtong Liu (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | No | No | No | Yes | Yes | Critically low |
| Der-Wei Hwu (2017) | Yes | Yes | Yes | Partial yes | Yes | Yes | Yes | Partial yes | No | No | Yes | No | No | No | No | Yes | Critically low |
| Ghanshyam Palamaner Subash Shantha (2015) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Shaoyong Xu (2015) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Cass Edwards (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Stephen Tregear (2009) | Yes | No | No | Yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Sergio Garbarino (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Cheng-Lin Sun (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Min Zhou (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Guang Song (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| LeiYu (2019) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Abdirashit Maripov (2017) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Rui-Heng Zhang (2020) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| De-Lei Kong (2016) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Rashid Nadeem (2014) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Mi Lu (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Reza Fadaei (2020) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Fang Lu (2019) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Kun Li (2017) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xingyu Wu (2018) | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Ze-Ning Jin (2016) | Yes | No | No | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Xiaoyan Li (2020) | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
Fig. 3.
Map of results of AMSTAR 2
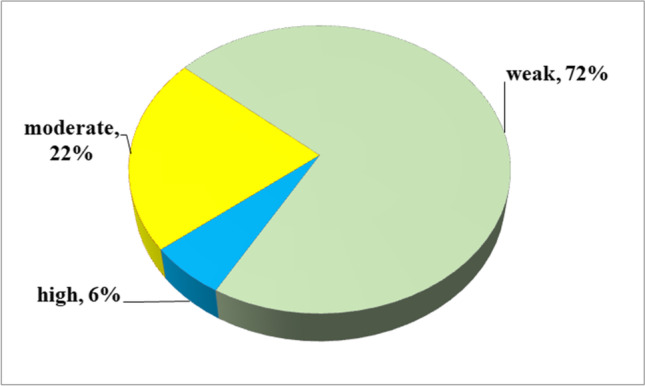
The outcomes of the evidence measurement are shown in Table 3. When a study did not present the result of heterogeneity and publication bias, the corresponding criteria were considered to be not satisfied. Among the 111 statistically significant outcomes, 7 (6%) showed high epidemiologic evidence, 24 (22%) showed moderate epidemiologic evidence, and the remaining 80 (72%) were rated as weak (Fig. 4).
Table 3.
Detail of results for evidence quality assessing
| Outcomes | Reference | Precision of the estimate | Consistency of results | No evidence of small-study effects | Grade | |
|---|---|---|---|---|---|---|
| > 1000 disease cases | P < 0.001 | (I2 < 50% and Cochran Q test P > 0.10) | (P > 0.10) | |||
| Cardiovascular disorders | ||||||
| Aortic dissection | Xiushi Zhou (2018) | Yes | No | Yes | Yes | Weak |
| Cardiovascular disease (CVD) | Xia Wang (2013) | Yes | Yes | Yes | No | Moderate |
| Stroke | Min Li (2014) | No | Yes | No | Yes | Weak |
| Ischemic heart disease (IHD) | Wuxiang Xie (2014) | No | No | Yes | No | Weak |
| Coronary heart disease (CHD) | Chengjuan Xie (2017) | Yes | No | No | Yes | Weak |
| Major adverse cardiac events (MACEs) | Chengjuan Xie (2017) | Yes | Yes | No | Yes | Moderate |
| Atrial fibrillation | Irini Youssef (2018) | Yes | Yes | No | No | Weak |
| Resistant hypertension | Haifeng Hou (2018) | No | Yes | Yes | Yes | Moderate |
| Essential hypertension | Haifeng Hou (2018) | Yes | Yes | Yes | No | Moderate |
| Atrial fibrillation recurrence after catheter ablation | Chee Yuan Ng (2011) | No | No | No | Yes | Weak |
| Major adverse cardiovascular event (MACE) after PCI | Xiao Wang (2018) | Yes | Yes | No | No | Weak |
| Myocardial infarction(MI) after PCI | Hua Qu (2018) | Yes | No | Yes | Yes | Weak |
| Coronary revascularization after PCI | Hua Qu (2018) | Yes | Yes | Yes | Yes | High |
| Left ventricular hypertrophy (LVH) | Cesare Cuspidi (2020) | Yes | Yes | No | No | Weak |
| Left ventricular diastolic diameter (LVEDD) | LeiYu (2019) | No | Yes | Yes | Yes | Moderate |
| Left ventricular systolic diameter (LVESD) | LeiYu (2019) | No | No | Yes | Yes | Weak |
| Left ventricular mass (LVM) | LeiYu (2019) | No | Yes | No | Yes | Weak |
| Left ventricular ejection fraction (LVEF) | LeiYu (2019) | No | No | No | No | Weak |
| Left atrial diameter (LAD) | LeiYu (2019) | No | Yes | Yes | No | Weak |
| Left atrial diameter volume index (LAVI) | LeiYu (2019) | No | Yes | Yes | Yes | Moderate |
| Right ventricular internal diameter (RVID) | Abdirashit Maripov (2017) | No | Yes | No | No | Weak |
| Right ventricular free wall thickness (RVWT) | Abdirashit Maripov (2017) | No | Yes | No | Yes | Weak |
| Right ventricular myocardial performance index (RV MPI) | Abdirashit Maripov (2017) | No | Yes | No | Yes | Weak |
| Tricuspid annular systolic velocity (RV S′) | Abdirashit Maripov (2017) | No | No | No | Yes | Weak |
| Tricuspid annular plane systolic excursion (TAPSE) | Abdirashit Maripov (2017) | No | Yes | No | Yes | Weak |
| Right ventricular fractional area change (RA FAC) | Abdirashit Maripov (2017) | No | No | No | No | Weak |
| Epicardial adipose tissue (EAT) thickness | Guang Song (2020) | No | Yes | No | Yes | Weak |
| Coronary flow reserve (CFR) | Rui-Heng Zhang (2020) | No | Yes | No | Yes | Weak |
| Systolic blood pressure (SBP) | De-Lei Kong (2016) | No | Yes | Yes | NA | Weak |
| Cerebral and cerebrovascular disease | ||||||
| Cerebral white matter changes | Bo-Lin Ho (2018) | No | Yes | No | Yes | Weak |
| Cerebrovascular (CV) disease | Zesheng Wu (2018) | Yes | No | No | No | Weak |
| White matter hyperintensities (WMH) | Yuhong Huang (2019) | Yes | Yes | No | No | Weak |
| Silent brain infarction (SBI) | Yuhong Huang (2019) | Yes | No | No | Yes | Weak |
| Asymptomatic lacunar infarction (ALI) | Anthipa Chokesuwattanaskul (2019) | No | No | Yes | Yes | Weak |
| Mortality | ||||||
| All-cause mortality | Lei Pan (2016) | Yes | No | No | No | Weak |
| Cardiovascular mortality | Xiahui Ge (2013) | No | Yes | Yes | Yes | Moderate |
| All-cause death after PCI | Xiao Wang (2018) | Yes | No | Yes | Yes | Weak |
| Cardiac death after PCI | Hua Qu (2018) | Yes | No | Yes | Yes | Weak |
| Postoperative complications | ||||||
| Postoperative respiratory failure | Faizi Hai BA (2013) | Yes | Yes | Yes | Yes | High |
| Postoperative cardiac events | Faizi Hai BA (2013) | Yes | No | Yes | Yes | Weak |
| Postoperative desaturation | R. Kaw (2012) | Yes | No | No | No | Weak |
| Postoperative ICU transfer | R. Kaw (2012) | Yes | No | No | No | Weak |
| Postoperative composite endpoints of postoperative cardiac or cerebrovascular complications | Ka Ting Ng (2020) | Yes | Yes | No | NA | Weak |
| Postoperative myocardial infarction | Ka Ting Ng (2020) | NA | Yes | Yes | NA | Weak |
| Postoperative atrial fibrillation | Ka Ting Ng (2020) | NA | Yes | No | NA | Weak |
| Postoperative composite endpoints of pulmonary complications | Ka Ting Ng (2020) | NA | Yes | No | NA | Weak |
| Postoperative pneumonia | Ka Ting Ng (2020) | NA | No | No | NA | Weak |
| Postoperative 30-day mortality | Ka Ting Ng (2020) | NA | No | Yes | NA | Weak |
| Postoperative acute kidney injury | Ka Ting Ng (2020) | NA | Yes | No | NA | Weak |
| Postoperative delirium | Ka Ting Ng (2020) | NA | Yes | Yes | NA | Weak |
| Postoperative venoembolism | Ka Ting Ng (2020) | NA | No | No | NA | Weak |
| Postoperative length of hospital stay (days) | Ka Ting Ng (2020) | NA | No | No | NA | Weak |
| Pregnancy-related disorders | ||||||
| Gestational diabetes mellitus (GDM) | Xinge Zhang (2020) | Yes | No | No | Yes | Weak |
| C-section | Lina Liu (2019) | NA | Yes | No | NA | Weak |
| Pregnancy-related wound complication | Lina Liu (2019) | NA | Yes | Yes | NA | Weak |
| Pregnancy-related pulmonary edema | Lina Liu (2019) | NA | Yes | Yes | NA | Weak |
| Gestational hypertension | Liwen Li (2018) | Yes | No | Yes | Yes | Weak |
| Preeclampsia | Liwen Li (2018) | Yes | Yes | No | Yes | Moderate |
| Preterm birth | Liwen Li (2018) | Yes | No | No | Yes | Weak |
| Neonatal intensive care unit (NICU) admission | Ting Xu (2014) | No | Yes | No | No | Weak |
| Ophthalmic disorders | ||||||
| Diabetic retinopathy (DR) | Zhenliu Zhu (2017) | No | Yes | No | Yes | Weak |
| Keratoconus | Marco Pellegrini (2020) | Yes | No | Yes | No | Weak |
| Glaucoma | Xinhua Wu (2015) | Yes | Yes | No | Yes | Moderate |
| Floppy eyelid syndrome (FES) | Leh-Kiong Huon (2016) | No | Yes | Yes | Yes | Moderate |
| Nonarteritic anterior ischemic optic neuropathy (NAION) | Yong Wu (2015) | No | No | No | Yes | Weak |
| Central serous chorioretinopathy (CSCR) | Chris Y.Wu (2018) | Yes | No | Yes | Yes | Weak |
| Retinal nerve fiber layer (RNFL) thickness | Cheng-Lin Sun (2016) | No | No | No | Yes | Weak |
| Digestive disorders | ||||||
| Gastroesophageal reflux disease | Zeng-Hong Wu (2019) | Yes | No | No | No | Weak |
| Steatosis | Shanshan Jin (2018) | Yes | Yes | Yes | Yes | High |
| Lobular inflammation | Shanshan Jin (2018) | No | Yes | Yes | Yes | Moderate |
| Ballooning degeneration | Shanshan Jin (2018) | No | No | Yes | Yes | Weak |
| NAFLD defined by liver histology | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| NAFLD defined by radiology | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| NAFLD defined by AST elevation | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| NAFLD defined by ALT elevation | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| Nonalcoholic steatohepatitis (NASH) | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| Fibrosis | G. Musso (2013) | No | Yes | Yes | Yes | Moderate |
| Alanine transaminase (ALT) | Shanshan Jin (2018) | Yes | Yes | Yes | Yes | High |
| Endocrine and metabolic system disorders | ||||||
| Type 2 diabetes (T2DM) | Ranran Qie (2020) | Yes | Yes | No | Yes | Moderate |
| Metabolic syndrome (MS) | Shaoyong Xu (2015) | Yes | Yes | Yes | Yes | High |
| Fasting blood glucose (FBG) | De-Lei Kong (2016) | Yes | Yes | No | NA | Meak |
| Total cholesterol (TC) | Rashid Nadeem (2014) | NA | No | NA | NA | Weak |
| Low-density lipoprotein (LDL) | Rashid Nadeem (2014) | NA | No | NA | NA | Weak |
| High-density lipoprotein (HDL) | Rashid Nadeem (2014) | NA | Yes | NA | NA | Weak |
| Triglyceride (TG) | Rashid Nadeem (2014) | NA | Yes | NA | NA | Weak |
| Adiponectin | Mi Lu (2019) | No | Yes | No | No | Weak |
| Oxidized low-density lipoprotein (Ox-LDL) | Reza Fadaei (2020) | No | No | No | Yes | Weak |
| Fibrinogen | Fang Lu (2019) | Yes | Yes | No | Yes | Moderate |
| Homocysteine | Kun Li (2017) | No | No | No | Yes | Weak |
| Advanced glycation end products (AGEs) | Xingyu Wu (2018) | No | Yes | No | NA | Weak |
| Angiotensin II (AngII) | Ze-Ning Jin (2016) | No | Yes | No | Yes | Weak |
| Serum vitamin D | Xiaoyan Li (2020) | Yes | Yes | No | NA | Weak |
| Urological disorders | ||||||
| Diabetic kidney disease (DKD) | Wen Bun Leong (2016) | Yes | No | Yes | Yes | Weak |
| Microalbuminuria | Tongtong Liu (2020) | No | Yes | Yes | Yes | Moderate |
| Chronic kidney disease (CKD) | Der-Wei Hwu (2017) | Yes | Yes | No | No | Weak |
| Serum uric acid level | Tingting Shi (2019) | Yes | Yes | No | No | Weak |
| Serum cystatin C | Tongtong Liu (2020) | No | Yes | Yes | Yes | Moderate |
| Estimated glomerular filtration rate (eGFR) | Tongtong Liu (2020) | No | No | No | Yes | Weak |
| Albumin/creatinine ratio (ACR) | Tongtong Liu (2020) | No | Yes | No | Yes | Weak |
| Other outcomes | ||||||
| Diabetic neuropathy | Xiandong Gu (2018) | No | No | No | Yes | Weak |
| Psoriasis | Tzong-Yun Ger (2020) | Yes | Yes | Yes | Yes | High |
| Nocturia | Jiatong Zhou (2019) | No | Yes | No | No | Weak |
| Parkinson’s disease | A-Ping Sun (2020) | Yes | Yes | Yes | Yes | High |
| Erectile dysfunction | Luhao Liu (2015) | No | No | No | No | Weak |
| Female sexual dysfunction | Luhao Liu (2015) | No | No | Yes | Yes | Weak |
| Sexual dysfunction | Luhao Liu (2015) | No | Yes | No | Yes | Weak |
| Cancer incidence | Ghanshyam Palamaner Subash Shantha (2015) | No | No | No | No | Weak |
| Depression | Cass Edwards (2020) | Yes | Yes | No | Yes | Moderate |
| Crash risk | Stephen Tregear (2009) | Yes | No | No | Yes | Weak |
| Work accidents | Sergio Garbarino (2016) | Yes | Yes | No | Yes | Moderate |
| Carotid intima-media thickness (CIMT) | Min Zhou (2016) | Yes | Yes | No | Yes | Moderate |
Fig. 4.
Map of results of evidence assessment
Discussion
In the current umbrella review, we identified 66 meta-analyses of observational studies and evaluated the current evidence supporting an association between OSA and various health outcomes. Also, we provide an extensive overview of the available evidence and critically evaluate the methodological quality of the meta-analyses and the quality of evidence for all the reported associations. OSA increased the risk of 111 health outcomes, including cardiovascular disorders, cerebral and cerebrovascular disease, mortality, postoperative complications, pregnancy-related disorders, ophthalmic disorders, digestive disorders, endocrine and metabolic system disorders, urological disorders, and other outcomes. The evidence quality was graded as high only for coronary revascularization after PCI, postoperative respiratory failure, steatosis, ALT elevation, MS, psoriasis, and Parkinson’s disease. The evidence quality was either moderate or low for the other associations. Furthermore, this umbrella review showed there were no considerable associations between OSA and 25 health outcomes.
Among the 111 outcomes, 54 outcomes had serious heterogeneity between studies. These possible confounding parameters (e.g., sex, body mass index, age, method of assessing OSA, OSA severity, smoking, alcohol drinking, the region of study, and follow-up period) may be the cause of heterogeneity. Substantial heterogeneity led to unreliable results. Of the 111 health outcomes, 23 outcomes possessed a remarkable publication bias, demonstrating that some negative achievements were not presented. Several reasons were leading to publication bias. First, when people start a study, they tend to assume that a positive result may ensure their work complies with the hypothesis during publication. Second, positive results have a higher probability of being published compared to negative results. Third, the study population is only a small fraction of the actual population with the disease. According to AMSTAR 2 criteria, 95% of the studies included in this umbrella analysis had “critically low” methodological quality. The critical flaws considered the absence of a registered protocol, the absence of the risk of bias in the considered investigations, and the absence of consideration of the risk of bias in the included investigations when interpreting or discussing the achieved outcomes of each study. Moreover, none of the meta-analyses in this study explained details of the funding source that had supported the work. The majority of the evaluated meta-analyses had considerable heterogeneity and small-study impacts; these were the main reasons for the evidence rating downgrade.
An umbrella review is a more beneficial method compared to a normal systematic review or meta-analysis due to it representing an overall illustration of achievements for phenomena or special questions [96]. To our knowledge, we are the first to use this method to present a comprehensive critical literature appraisal on published associations between OSA and diverse health information. Also, our two authors systematically searched four scientific databases using a strong search strategy with clearly defined eligibility criteria and data extraction parameters. The quality of included systematic reviews was also evaluated through AMSTAR 2. This is a benchmark methodological quality measurement that is utilized to assessing the quality of the methods utilized for meta-analyses. Furthermore, we graded the epidemiologic evidence conforming to established, prespecified criteria. Its criteria included an assessment of heterogeneity, publication bias, and precision of the estimate, which is more objective than the GRADE system criteria.
There are some limitations in our umbrella review. First, in this analysis, we explained associations evaluated through the meta-analyses of observational investigations. In doing so, we may have missed other health outcomes that have not yet been investigated by meta-analyses. Second, this umbrella analysis included systematic reviews and meta-analyses that were only published in English. The potential missing information in other languages could influence the assessment outcomes. Third, the majority of the meta-analyses had heterogeneity; observational researches are susceptible to uncertainty and confounding bias.
Conclusions
The associations between OSA and an extensive range of health information have been broadly reported in many meta-analyses. Based on our umbrella review, 66 meta-analyses explored 136 unique outcomes, only 7 outcomes showed a high level of epidemiologic evidence with statistical significance. OSA could be associated with the enhanced risk of coronary revascularization after PCI, postoperative respiratory failure, steatosis, ALT elevation, MS, psoriasis, and Parkinson’s disease. Overall, OSA is harmful to human health but will need further exploration on this topic with high-quality prospective studies.
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Author contribution
Idea and design: TSH, CWW. Literature search: ZCX, GLLZ. Data extraction and analysis:CWW, LYT. Manuscript writing: CWW. Manuscript revision: TSH, CWW. All authors read and approved the version of the manuscript to be published. All authors take responsibility for appropriate content.
Data availability
The data used to support the findings of this study are included within the article. The primary data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval
All analyses were based on published studies and no ethical approval was required.
Conflict of interest
The authors declare no competing interests.
Footnotes
Weiwei Chen and Yuting Li contributed equally to this work and should be considered co-first authors
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Investig. 2014;124(4):1454–1457. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355–360. [PubMed] [Google Scholar]
- 3.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet (London, England) 2014;383(9918):736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 6.Russell MB, Kristiansen HA, Kværner KJ. Headache in sleep apnea syndrome: epidemiology and pathophysiology. Cephalalgia. 2014;34(10):752–755. doi: 10.1177/0333102414538551. [DOI] [PubMed] [Google Scholar]
- 7.Youssef NA, Ege M, Angly SS, Strauss JL, Marx CE. Is obstructive sleep apnea associated with ADHD? Ann Clin Psychiatry. 2011;23(3):213–224. [PubMed] [Google Scholar]
- 8.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 9.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 10.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet (London, England) 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 11.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 12.Dredla BK, Castillo PR. Cardiovascular consequences of obstructive sleep apnea. Curr Cardiol Rep. 2019;21(11):137. doi: 10.1007/s11886-019-1228-3. [DOI] [PubMed] [Google Scholar]
- 13.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig. 2018;9(5):991–997. doi: 10.1111/jdi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strausz S, Havulinna AS, Tuomi T, Bachour A, Groop L, Mäkitie A, Koskinen S, Salomaa V, Palotie A, Ripatti S, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open. 2018;8(10):e022752. doi: 10.1136/bmjopen-2018-022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JH, Lee JY, Han KD, Lim YC, Cho JH. Association between obstructive sleep apnoea and breast cancer: the Korean National Health Insurance Service Data 2007–2014. Sci Rep. 2019;9(1):19044. doi: 10.1038/s41598-019-55551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seijo LM, Pérez-Warnisher MT, Giraldo-Cadavid LF, Oliveros H, Cabezas E, Troncoso MF, Gómez T, Melchor R, Pinillos EJ, El Hachem A, et al. Obstructive sleep apnea and nocturnal hypoxemia are associated with an increased risk of lung cancer. Sleep Med. 2019;63:41–45. doi: 10.1016/j.sleep.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Brenner R, Kivity S, Peker M, Reinhorn D, Keinan-Boker L, Silverman B, Liphsitz I, Kolitz T, Levy C, Shlomi D et al (2019) Increased risk for cancer in young patients with severe obstructive sleep apnea. Respiration 97(1):15–23 [DOI] [PubMed]
- 18.Hobzova M, Prasko J, Vanek J, Ociskova M, Genzor S, Holubova M, Grambal A, Latalova K. Depression and obstructive sleep apnea. Neuro Endocrinol Lett. 2017;38(5):343–352. [PubMed] [Google Scholar]
- 19.Gouveia CJ, Yalamanchili A, Ghadersohi S, Price CPE, Bove M, Attarian HP, Tan BK. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients? Laryngoscope. 2019;129(5):1244–1249. doi: 10.1002/lary.27557. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Li X, Lu Y. Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology. 2018;159(7):2670–2675. doi: 10.1210/en.2018-00248. [DOI] [PubMed] [Google Scholar]
- 21.Sun AP, Liu N, Zhang YS, Zhao HY, Liu XL (2020) The relationship between obstructive sleep apnea and Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci 41(5):1153–1162 [DOI] [PubMed]
- 22.Hwu DW, Lin KD, Lin KC, Lee YJ, Chang YH. The association of obstructive sleep apnea and renal outcomes-a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):313. doi: 10.1186/s12882-017-0731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systems Control Found Appl. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ (Clinical research ed) 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ (Clinical research ed) 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 27.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–273. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 28.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647–659.e644. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Johnson VE. Revised standards for statistical evidence. Proc Natl Acad Sci USA. 2013;110(48):19313–19317. doi: 10.1073/pnas.1313476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. 2011;22(4):450–456. doi: 10.1097/EDE.0b013e31821b506e. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ (Clinical research ed) 2011;343:d2090. doi: 10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Liu F, Zhang W, Wang G, Guo D, Fu W, Wang L. Obstructive sleep apnea and risk of aortic dissection: a meta-analysis of observational studies. Vascular. 2018;26(5):515–523. doi: 10.1177/1708538118766102. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol. 2014;172(2):466–469. doi: 10.1016/j.ijcard.2013.12.230. [DOI] [PubMed] [Google Scholar]
- 35.Xie W, Zheng F, Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2014;93(29):e336. doi: 10.1097/MD.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie C, Zhu R, Tian Y, Wang K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: a meta-analysis. BMJ Open. 2017;7(12):e013983. doi: 10.1136/bmjopen-2016-013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar S, Kane J, Hoffner H, Salifu M, McFarlane SI. Obstructive sleep apnea as a risk factor for atrial fibrillation: a meta-analysis. Journal of Sleep Disorders & Therapy. 2018;7(1):282. doi: 10.4172/2167-0277.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, Xing W, Wang W. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. doi: 10.7189/jogh.08.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Fan JY, Zhang Y, Nie SP, Wei YX. Association of obstructive sleep apnea with cardiovascular outcomes after percutaneous coronary intervention: a systematic review and meta-analysis. Medicine. 2018;97(17):e0621. doi: 10.1097/MD.0000000000010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu H, Guo M, Zhang Y, Shi DZ. Obstructive sleep apnea increases the risk of cardiac events after percutaneous coronary intervention: a meta-analysis of prospective cohort studies. Sleep Breath. 2018;22(1):33–40. doi: 10.1007/s11325-017-1503-8. [DOI] [PubMed] [Google Scholar]
- 42.Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Obstructive sleep apnoea syndrome and left ventricular hypertrophy: a meta-analysis of echocardiographic studies. J Hypertens. 2020;38(9):1640–1649. doi: 10.1097/HJH.0000000000002435. [DOI] [PubMed] [Google Scholar]
- 43.Ho BL, Tseng PT, Lai CL, Wu MN, Tsai MJ, Hsieh CF, Chen TY, Hsu CY. Obstructive sleep apnea and cerebral white matter change: a systematic review and meta-analysis. J Neurol. 2018;265(7):1643–1653. doi: 10.1007/s00415-018-8895-7. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Chen F, Yu F, Wang Y, Guo Z. A meta-analysis of obstructive sleep apnea in patients with cerebrovascular disease. Sleep Breath. 2018;22(3):729–742. doi: 10.1007/s11325-017-1604-4. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Yang C, Yuan R, Liu M, Hao Z. Association of obstructive sleep apnea and cerebral small vessel disease: a systematic review and meta-analysis. Sleep. 2020;43(4):zsz264. doi: 10.1093/sleep/zsz264. [DOI] [PubMed] [Google Scholar]
- 46.Chokesuwattanaskul A, Lertjitbanjong P, Thongprayoon C, Bathini T, Sharma K, Mao MA, Cheungpasitporn W, Chokesuwattanaskul R. Impact of obstructive sleep apnea on silent cerebral small vessel disease: a systematic review and meta-analysis. Sleep Med. 2020;68:80–88. doi: 10.1016/j.sleep.2019.11.1262. [DOI] [PubMed] [Google Scholar]
- 47.Pan L, Xie X, Liu D, Ren D, Guo Y. Obstructive sleep apnoea and risks of all-cause mortality: preliminary evidence from prospective cohort studies. Sleep Breath. 2016;20(1):345–353. doi: 10.1007/s11325-015-1295-7. [DOI] [PubMed] [Google Scholar]
- 48.Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, Guo X. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLoS One. 2013;8(7):e69432. doi: 10.1371/journal.pone.0069432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang XB, Peng LH, Lyu Z, Jiang XT, Du YP (2017) Obstructive sleep apnoea and the incidence and mortality of cancer: a meta-analysis. Eur J Cancer Care 26(2) [DOI] [PubMed]
- 50.Hai F, Porhomayon J, Vermont L, Frydrych L, Jaoude P, El-Solh AA. Postoperative complications in patients with obstructive sleep apnea: a meta-analysis. J Clin Anesth. 2014;26(8):591–600. doi: 10.1016/j.jclinane.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 52.Liu T, Zhan Y, Wang Y, Li Q, Mao H (2021) Obstructive sleep apnea syndrome and risk of renal impairment: a systematic review and meta-analysis with trial sequential analysis. Sleep Breath 25(1):17–27 [DOI] [PMC free article] [PubMed]
- 53.Zhang X, Zhang R, Cheng L, Wang Y, Ding X, Fu J, Dang J, Moore J, Li R. The effect of sleep impairment on gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sleep Med. 2020;74:267–277. doi: 10.1016/j.sleep.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Su G, Wang S, Zhu B. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis. Sleep Breath. 2019;23(2):399–412. doi: 10.1007/s11325-018-1714-7. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Zhao K, Hua J, Li S. Association between sleep-disordered breathing during pregnancy and maternal and fetal outcomes: an updated systematic review and meta-analysis. Front Neurol. 2018;9:91. doi: 10.3389/fneur.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu T, Feng Y, Peng H, Guo D, Li T. Obstructive sleep apnea and the risk of perinatal outcomes: a meta-analysis of cohort studies. Sci Rep. 2014;4:6982. doi: 10.1038/srep06982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pellegrini M, Bernabei F, Friehmann A, Giannaccare G (2020) Obstructive sleep apnea and keratoconus: a systematic review and meta-analysis. Optom Vis Sci 97(1):9–14 [DOI] [PubMed]
- 58.Wu X, Liu H. Obstructive sleep apnea/hypopnea syndrome increases glaucoma risk: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(1):297–303. [PMC free article] [PubMed] [Google Scholar]
- 59.Huon LK, Liu SY, Camacho M, Guilleminault C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20(4):1145–1154. doi: 10.1007/s11325-016-1358-4. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Zhou LM, Lou H, Cheng JW, Wei RL. The association between obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Curr Eye Res. 2016;41(7):987–992. doi: 10.3109/02713683.2015.1075221. [DOI] [PubMed] [Google Scholar]
- 61.Wu CY, Riangwiwat T, Rattanawong P, Nesmith BLW, Deobhakta A. Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis. Retina (Philadelphia, Pa) 2018;38(9):1642–1651. doi: 10.1097/IAE.0000000000002117. [DOI] [PubMed] [Google Scholar]
- 62.Qie R, Zhang D, Liu L, Ren Y, Zhao Y, Liu D, Liu F, Chen X, Cheng C, Guo C, et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of cohort studies. J Diabetes. 2020;12(6):455–464. doi: 10.1111/1753-0407.13017. [DOI] [PubMed] [Google Scholar]
- 63.Gu X, Luo X, Wang X, Tang J, Yang W, Cai Z. The correlation between obstructive sleep apnea and diabetic neuropathy: a meta-analysis. Prim Care Diabetes. 2018;12(5):460–466. doi: 10.1016/j.pcd.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Leong WB, Jadhakhan F, Taheri S, Thomas GN, Adab P. The association between obstructive sleep apnea on diabetic kidney disease: a systematic review and meta-analysis. Sleep. 2016;39(2):301–308. doi: 10.5665/sleep.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Z, Zhang F, Liu Y, Yang S, Li C, Niu Q, Niu J. Relationship of obstructive sleep apnoea with diabetic retinopathy: a meta-analysis. Biomed Res Int. 2017;2017:4737064. doi: 10.1155/2017/4737064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu ZH, Yang XP, Niu X, Xiao XY, Chen X. The relationship between obstructive sleep apnea hypopnea syndrome and gastroesophageal reflux disease: a meta-analysis. Sleep Breath. 2019;23(2):389–397. doi: 10.1007/s11325-018-1691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):841–851. doi: 10.1007/s11325-018-1625-7. [DOI] [PubMed] [Google Scholar]
- 68.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R (2013) Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev 14(5):417–431 [DOI] [PubMed]
- 69.Ger TY, Fu Y, Chi CC. Bidirectional association between psoriasis and obstructive sleep apnea: a systematic review and meta-analysis. Sci Rep. 2020;10(1):5931. doi: 10.1038/s41598-020-62834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou J, Xia S, Li T, Liu R. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020;24:1293–1298. doi: 10.1007/s11325-019-01981-6. [DOI] [PubMed] [Google Scholar]
- 71.Cao Y, Wu S, Zhang L, Yang Y, Cao S, Li Q. Association of allergic rhinitis with obstructive sleep apnea: a meta-analysis. Medicine. 2018;97(51):e13783. doi: 10.1097/MD.0000000000013783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L, Kang R, Zhao S, Zhang T, Zhu W, Li E, Li F, Wan S, Zhao Z. Sexual dysfunction in patients with obstructive sleep apnea: a systematic review and meta-analysis. J Sex Med. 2015;12(10):1992–2003. doi: 10.1111/jsm.12983. [DOI] [PubMed] [Google Scholar]
- 73.Upala S, Sanguankeo A, Congrete S. Association between obstructive sleep apnea and osteoporosis: a systematic review and meta-analysis. International journal of endocrinology and metabolism. 2016;14(3):e36317. doi: 10.5812/ijem.36317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi T, Min M, Sun C, Cheng C, Zhang Y, Liang M, Rizeq FK, Sun Y. A meta-analysis of the association between gout, serum uric acid level, and obstructive sleep apnea. Sleep Breath. 2019;23(4):1047–1057. doi: 10.1007/s11325-019-01827-1. [DOI] [PubMed] [Google Scholar]
- 75.Liu T, Zhan Y, Wang Y, Li Q, Mao H (2021) Obstructive sleep apnea syndrome and risk of renal impairment: a systematic review and meta-analysis with trial sequential analysis. Sleep Breath 25(1):17–27 [DOI] [PMC free article] [PubMed]
- 76.Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, Pancholy SB. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015;16(10):1289–1294. doi: 10.1016/j.sleep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, Ji Q. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15:105. doi: 10.1186/s12890-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards C, Almeida OP, Ford AH. Obstructive sleep apnea and depression: a systematic review and meta-analysis. Maturitas. 2020;142:45–54. doi: 10.1016/j.maturitas.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(6):573–581. [PMC free article] [PubMed] [Google Scholar]
- 80.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2016;39(6):1211–1218. doi: 10.5665/sleep.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun CL, Zhou LX, Dang Y, Huo YP, Shi L, Chang YJ. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome: a meta-analysis. Medicine. 2016;95(32):e4499. doi: 10.1097/MD.0000000000004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The association between obstructive sleep apnea and carotid intima-media thickness: a systematic review and meta-analysis. Angiology. 2017;68(7):575–583. doi: 10.1177/0003319716665985. [DOI] [PubMed] [Google Scholar]
- 83.Song G, Sun F, Wu D, Bi W. Association of epicardial adipose tissues with obstructive sleep apnea and its severity: a meta-analysis study. Nutr Metab Cardiovasc Dis. 2020;30(7):1115–1120. doi: 10.1016/j.numecd.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Yu L, Li H, Liu X, Fan J, Zhu Q, Li J, Jiang J, Wang J (2020) Left ventricular remodeling and dysfunction in obstructive sleep apnea : Systematic review and meta-analysis. Herz 45(8):726–738 [DOI] [PMC free article] [PubMed]
- 85.Maripov A, Mamazhakypov A, Sartmyrzaeva M, Akunov A, Muratali Uulu K, Duishobaev M, Cholponbaeva M, Sydykov A, Sarybaev A. Right ventricular remodeling and dysfunction in obstructive sleep apnea: a systematic review of the literature and meta-analysis. Can Respir J. 2017;2017:1587865. doi: 10.1155/2017/1587865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang RH, Zhao W, Shu LP, Wang N, Cai YH, Yang JK, Zhou JB, Qi L. Obstructive sleep apnea is associated with coronary microvascular dysfunction: a systematic review from a clinical perspective. J Sleep Res. 2020;29:e13046. doi: 10.1111/jsr.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kong DL, Qin Z, Wang W, Pan Y, Kang J, Pang J. Association between obstructive sleep apnea and metabolic syndrome: a meta-analysis. Clin Invest Med. 2016;39(5):E161–e172. doi: 10.25011/cim.v39i5.27148. [DOI] [PubMed] [Google Scholar]
- 88.Lu M, Fang F, Wang Z, Wei P, Hu C, Wei Y. Association between serum/plasma levels of adiponectin and obstructive sleep apnea hypopnea syndrome: a meta-analysis. Lipids Health Dis. 2019;18(1):30. doi: 10.1186/s12944-019-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fadaei R, Safari-Faramani R, Rezaei M, Ahmadi R, Rostampour M, Moradi N, Khazaie H. Circulating levels of oxidized low-density lipoprotein in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2020;24(3):809–815. doi: 10.1007/s11325-020-02089-y. [DOI] [PubMed] [Google Scholar]
- 90.Lu F, Jiang T, Wang W, Hu S, Shi Y, Lin Y. Circulating fibrinogen levels are elevated in patients with obstructive sleep apnea: a systemic review and meta-analysis. Sleep Med. 2020;68:115–123. doi: 10.1016/j.sleep.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Li K, Zhang J, Qin Y, Wei YX. Association between serum homocysteine level and obstructive sleep apnea: a meta-analysis. Biomed Res Int. 2017;2017:7234528. doi: 10.1155/2017/7234528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu X, She W, Niu X, Chen X. Association between serum level of advanced glycation end products and obstructive sleep apnea-hypopnea syndrome: a meta-analysis. J Int Med Res. 2018;46(11):4377–4385. doi: 10.1177/0300060518786906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. Journal of Geriatric Cardiology : JGC. 2016;13(4):333–343. doi: 10.11909/j.issn.1671-5411.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, He J, Yun J. The association between serum vitamin D and obstructive sleep apnea: an updated meta-analysis. Respir Res. 2020;21(1):294. doi: 10.1186/s12931-020-01554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, Naseem J, Champeau D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(5):475–489. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article. The primary data used to support the findings of this study are available from the corresponding author upon request.