Abstract
Purpose
Breast cancer is the most common type of cancer found in women in Sweden and worldwide. Treatment leads to increased survival of patients, but they are at risk to experience psychological distress, including anxiety and depressive symptoms, and decreased health-related quality of life (HRQoL). This study investigated the relationship between psychological distress and HRQoL and related factors among women with breast cancer in Sweden.
Methods
This descriptive cross-sectional study was conducted in Sweden. A total of 481 women with breast cancer answered voluntarily a questionnaire about sociodemographic and support factors, psychological distress, and HRQoL. Data were subjected to Pearson’s correlation and linear regression analyses.
Results
Psychological distress was related to HRQoL in terms of body image, future perspective, side effects of systemic therapy, breast symptoms, arm symptoms, and hair loss. Women with lower age were associated with increased symptoms of anxiety, while those having undergone breast reconstruction were associated with increased symptoms of depression. Breast reconstruction and chemotherapy worsened body image, low support from partner decreased sexual functioning and enjoyment, and low support from physicians and nurses worsened future perspective, side effects of systemic therapy, breast symptoms, and indignation about hair loss.
Conclusions
Psychological distress was correlated with the HRQoL. Increased support from physicians, nurses, and husband/partner may increase the HRQoL among women with breast cancer. Breast cancer treatments such as breast reconstruction and chemotherapy were factors that decreased the psychological distress and increased the HRQoL.
Keywords: Anxiety, Depressive symptoms, Health-related quality of life, Support, Women with breast cancer
Background
Worldwide, cancer is a major cause of morbidity and mortality; about 15 million new cases were found in 2012, and eight million people died from diseases related to cancer [1]. Breast cancer is the most common type of cancer among women, and more than 12 percent of women are diagnosed with breast cancer [2]. In 2018, Sweden had an age-adjusted rate of breast cancer of 89.8 per 100,000 [3, 4]. Although improved diagnostics and treatments lead to an increased survival rate among patients with breast cancer [5], 1,400 Swedish women died due to breast cancer in 2006 [4]. After diagnosis, women with breast cancer are at high risk to experience psychological distress [6, 7] and may have decreased health-related quality of life (HRQoL) [8].
Psychological distress is a state of emotional suffering commonly characterized by symptoms of depression and anxiety [9]. More than 25% of women with breast cancer suffer from such symptoms [7, 10]. They are more likely to have suicidal thoughts than the general population [11]. Some factors are related to the psychological distress among them. Some factors are related to the psychological distress among them. Women with breast cancer living in a rural area, being Christian, or having traits of anxiety at the time of diagnosis are associated with psychological distress [7, 12]. Psychological distress may be a forerunner to mental, physical, and emotional exhaustion in a country with high a incidence rate of breast cancer like Sweden [9, 13]. There is a need of an investigation of factors that help to avoid mental, physical, and emotional chaos in patients with breast cancer [9].
HRQoL refers to an individual’s perception of his or her position in life, covering independence; physical, psychological, and social relations; and environmental and spiritual dimensions [14]. HRQoL has been acknowledged as an important outcome for patients with cancer [15]. HRQoL among women with breast cancer is often poorer in comparison with women in the general population regarding social and emotional functioning [16, 17]. Sociodemographic characteristics, including low age, low education, financial problems, and occupation, could be factors associated with low HRQoL [10, 13, 18, 19]. Chemotherapy treatment, time since diagnosis, and lack of support from family and friends are also associated with lower HRQoL among women with breast cancer [8, 10, 13, 18]. Lack of emotional support from professional counselors in hospitals leads to psychological distress among cancer patients, who need support also from family and friends [20].
In addition, psychological distress and stress are correlated with lower HRQoL [8, 19, 21]. Although important, these factors are often neglected or under-recognized [22]. Therefore, psychological distress and the HRQoL among women with breast cancer need to be investigated [22]. The aim of this study is to investigate the relationship between psychological distress and the HRQoL among women with breast cancer in Sweden. It is also to investigate different factors that affect psychological distress and the HRQoL such as sociodemography, treatments, and support (e.g., from healthcare personnel in hospitals, husband/partner, family, and friends).
Materials and methods
Study setting and design
A descriptive cross-sectional study was carried out in three cities in Sweden: Uppsala, Gävle, and Falun.
Participants
Based on the Regional Cancer Centre (RCC) in Uppsala and Örebro, registered women were invited to participate in the study. The inclusion criteria were: Women who (1) had been diagnosed with breast cancer at least 1 year before data collection to ensure unchanged diagnosis; (2) were at least 18 years old; (3) lived in Uppsala, Gävle, or Falun; and (4) were willing to participate. Women who reported a history of mental disorder or dementia were excluded. In total, 481 out of 975 eligible women with breast cancer agreed to participate in the study.
Instruments
The use was made of a questionnaire containing four parts: (1) sociodemographic characteristics, (2) support, (3) psychological distress, and (4) HRQoL. Sociodemographic characteristics concerned age, marital status, education, religion, belonging to a cultural/ethnic minority, having an underlying disease, duration of diagnosed breast cancer, methods of treatment (i.e., chemotherapy, radiotherapy, Herceptin, and hormone therapy).
The part support concerned six sources of support, viz., physicians, nurses, the Internet, husband/partner, family members and friends, and the patient’s institution. It was created by PCL. Each source comprised nine questions, each of which gave a score of zero if the answer was “no” and one if the answer was “yes.” Therefore, each source could give a total score ranging from zero to nine, and a higher total score indicated more support. This part had a Cronbach’s alpha coefficient of 0.89.
The part psychological distress comprised of anxiety and depressive symptoms. Anxiety and depressive symptoms were measured by use of the Hospital Anxiety and Depression Scale [23]. The scale had 14 items divided into 2 subscales: one measured anxiety (HADS-A) and the other measured depressive symptoms (HADS-D). Each subscale had seven items with a four-Likert scale. The total possible score for each subscale ranged from zero to 21, and a higher score indicated more symptoms. HADS-A and HADS-D had Cronbach’s alpha coefficients of 0.89 and 0.84, respectively, for Swedish women with breast cancer [13].
HRQoL was measured using the European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire (QLQ-BR23) [24]. It is a disease-specific questionnaire with 23 questions, each of which had four options assigned by a number (not at all = 1, a little = 2, quite a bit = 3, and very much = 4). It assessed eight dimensions: body image (BRBI), sexual functioning (BRSEF), sexual enjoyment (BRSEE), future perspective (BRFU), side effects of systemic therapy (BRST), breast symptoms (BRBS), arm symptoms (BRAS), and indignation by hair loss (BRHL) [25]. All dimensions were transformed to 100-percent scores, and higher scores indicated the lower quality of life. This questionnaire was translated to Swedish and tested before data collection among other breast cancer patients with an acceptable Cronbach’s alpha score in each sub-scale [13].
Procedure
The heads and nurses of clinics of surgery/oncology and plastic surgery in Uppsala, Gävle, and Falun were informed about the study by one of the researchers (PCL). They asked questions until they found that everything about the study was clear. In this way, the nurses became able to answer questions if the participants would ask. Finally, the heads of the clinics gave permission to conduct the study. Written information about the study and its purpose was sent by ordinary mail together with a consent letter and a questionnaire to the eligible women. They were assured of their anonymity and of confidentiality, and they were told that they could drop out at any time. The Declaration of Helsinki for medical research was fulfilled. The women who agreed to participate in the study signed a consent letter, responded to the questionnaire, and returned these documents in a stamped envelope. Women who did not wish to participate in the study returned the documents without filling in any information. A reminder was sent twice by post (after two weeks and one month) to women who had not returned the envelope in due time.
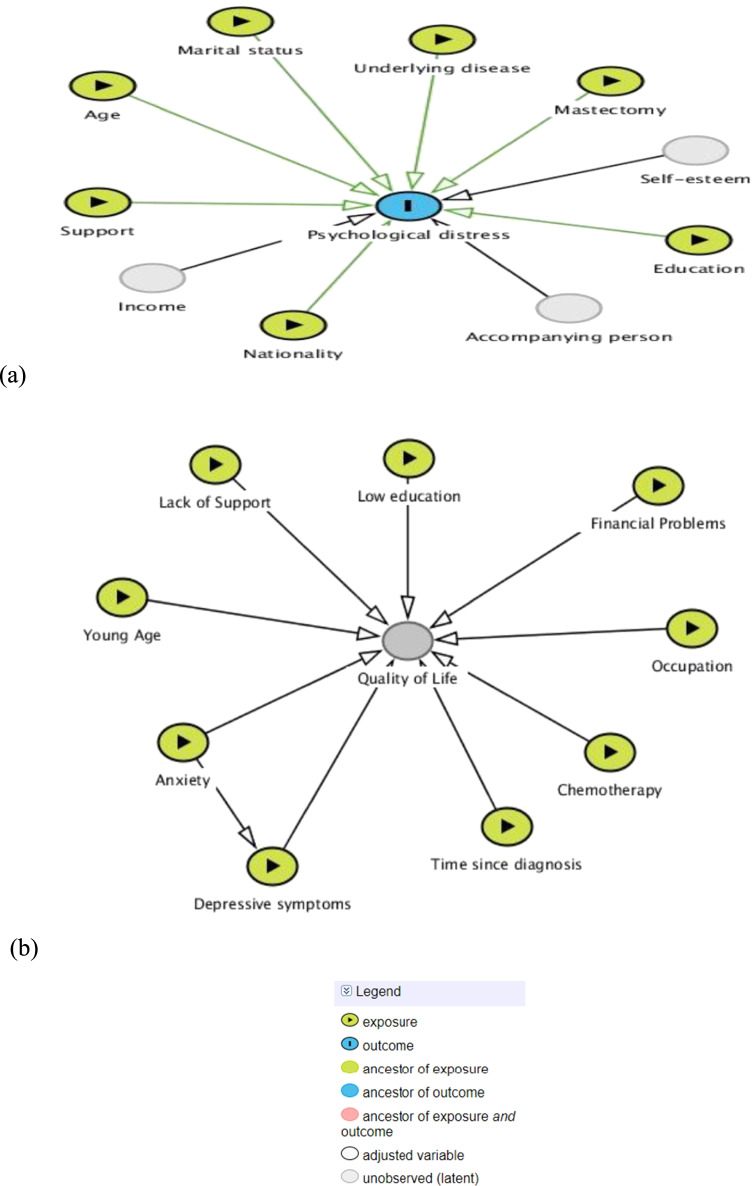
Directed acyclic graphs (DAGs) [26] were constructed based on previous studies in order to demonstrate what factors were associated with psychological distress [27–29] and with HRQoL [13]. See Fig. 1a and b.
Fig. 1.
Directed acyclic graphs for possible risk factors associated with a psychological distress and b quality of life among cancer patients
Analyses
We analyzed data using descriptive and inferential statistics. Descriptive statistics summarized sociodemographic characteristics, social support, HADS-A scores, HADS-D scores, and QLQ-BR23 scores for participants in terms such as frequency, mean, and standard deviation (SD). Inferential statistics applied Pearson’s correlation and linear regression analyzes.
Pearson’s correlation was used to determine the correlation between the scores of the HADS-A, HADS-D, and each dimension of QLQ-BR23, and linear regression analyses were performed to determine relationships between sociodemographic factors, support factors, treatments, and outcome variables.
In multiple linear regression analyses, outcome variables were the scores of psychological distress, and QLQ-BR23, all of which were continuous variables. Sociodemographic and support factors were considered independent variables. Age, duration of diagnosed breast cancer, and each support was a continuous variable. Dummy variables (categorization to zero and one) were marital status (married/lived together = 0, the others = 1), education (high school or above = 0, secondary school/others = 1), belonging to a cultural/ethnic minority (no = 0, yes = 1), having an underlying disease (no = 0, yes = 1), and methods of treatments (no = 0, yes = 1). Religion was excluded because of a low number in its subgroup. Assumptions were satisfied before the analyses (i.e., autocorrelation, multicollinearity, homoscedasticity, linearity, and multivariate normality). First, we inserted each independent variable into a simple linear regression analysis for each outcome variable. Significant independent variables from the simple analyses remained in multiple linear regression analyses using the stepwise selection method (alpha-to-enter of 0.05, alpha-to-remove of 0.10). We provided adjusted R2 and a standardized partial regression coefficient (β) and 95% confidence interval (CI) to demonstrate the fitness and strength of association of each outcome variable. The level of statistical significance for all analyses was set at p < 0.05.
Results
The mean age (and SD) of participants was 62.7 (12.35), while the average number of year (and SD) from diagnosis was 2.9 (3.97). Approximately 60% of the participants had no underlying diseases, and 20% of them underwent breast reconstruction. See Table 1.
Table 1.
Number and percentage of sociodemographic and treatment characteristics among participants (n = 481)
| Characteristics | n (%) | |
|---|---|---|
| Age (years)a | ||
| Mean = 62.7, SD = 12.35, Min = 31, Max = 93 | ||
| Civil statusa | ||
| Married/ Live together | 321 (67.1) | |
| Single/alone | 157 (32.9) | |
| Education levela | ||
| Secondary school/unidentified | 226 (47.4) | |
| High school or university | 251 (52.6) | |
| Religiona | ||
| Christian | 422 (90.4) | |
| Non-Christian | 45 (9.6) | |
| Cultural/Ethnic minoritya | ||
| No | 455 (96.8) | |
| Yes | 15 (3.2) | |
| Having an underlying diseasea | ||
| No | 277 (59.3) | |
| Yes | 190 (40.7) | |
| Duration from diagnosis (year)a | ||
| Mean = 2.92, SD = 3.97, Min = 1, Max = 44 | ||
| Chemotherapy treatmenta | ||
| Yes | 234 (49.6) | |
| No | 238 (50.4) | |
| Radiation therapy treatmenta | ||
| Yes | 227 (52.6) | |
| No | 252 (47.4) | |
| Hormone therapy treatmenta | ||
| Yes | 293 (62.7) | |
| No | 174 (37.3) | |
| Herceptin treatmenta | ||
| Yes | 82 (18.7) | |
| No | 357 (81.3) | |
| Breast reconstructiona | ||
| Yes | 93 (19.7) | |
| No | 380 (80.3) | |
| Total score of support from physiciansa | ||
| Mean = 6.36, SD = 2.78, Min = 0, Max = 9 | ||
| Total score of support from nursesa | ||
| Mean = 4.60, SD = 3.22, Min = 0, Max = 9 | ||
| Total score of support from interneta | ||
| Mean = 0.75, SD = 1.71, Min = 0, Max = 9 | ||
| Total score of support from husband/partnera | ||
| Mean = 0.79, SD = 1.65, Min = 0, Max = 9 | ||
| Total score of support from family and friendsa | ||
| Mean = 0.77, SD = 1.56, Min = 0, Max = 9 | ||
| Total score of support from patient institutiona | ||
| Mean = 0.79, SD = 1.65, Min = 0, Max = 9 | ||
| HADS-Aa | ||
| Mean = 7.07, SD = 3.38, Min = 2, Max = 19 | ||
| HADS-Da | ||
| Mean = 14.10, SD = 2.14, Min = 7, Max = 19 | ||
aObtained number < 481; Hospital Anxiety and Depression Scale – Anxiety sub-scale (HADS-A); Hospital Anxiety and Depression Scale – Depression sub-scale (HADS-D)
The correlation analyses demonstrated that the scores of HADS-A correlated significantly with all dimensions of the HRQoL except BRSEF and BRSEE, while HADS-D correlated significantly with all dimensions of the HRQoL. HADS-A had its strongest correlation 0.619 with BRFU, while HADS-D had its strongest correlation 0.325 with BRBI. See Table 2.
Table 2.
Correlation between the scores of the Hospital Anxiety and Depression Scale – Anxiety sub-scale (HADS-A) and Depression sub-scale (HADS-D) and all dimensions of the Breast Cancer-Specific Quality of Life Questionnaire
| Variables | BRBI | BRSEF | BRSEE | BRFU | BRST | BRBS | BRAS | BRHL |
|---|---|---|---|---|---|---|---|---|
| HADS-A | 0.490* | 0.067 | 0.130 | 0.619* | 0.428* | 0.330* | 0.276* | 0.171* |
| HADS-D | 0.325* | 0.159* | 0.280* | 0.316* | 0.269* | 0.256* | 0.177* | 0.104* |
*Correlations were significant at 0.05 level
Body image (BRBI); sexual functioning (BRSEF); sexual enjoyment (BRSEE); future perspective (BRFU); systemic therapy side effects (BRST); breast symptoms (BRBS); arm symptoms (BRAS); and upset by hair loss (BRHL)
According to Table 3, breast cancer patients who were younger (β = − 0.230, 95% CI − 0.180, − 0.279, p < 0.001) had an underlying disease (β = 0.219, 95% CI 0.118, 0.319, p < 0.001) and had received less support from physicians (β = − 0.142, 95% CI − 0.212, − 0.071, p = 0.003) were likely to get increased symptoms of anxiety. Those who had an underlying disease (β = 0.116, 95% CI 0.072, 0.163, p = 0.015) and had undergone breast reconstruction (β = 0.116, 95% CI 0.061, 0.182, p = 0.013) were likely to get increased symptoms of depression.
Table 3.
Multivariate linear regression analysis results of the scores of the HADS-A, HADS-D, and HRQoL
| Variables | Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|---|
| B | Standard error | 95% CI | Beta (Descending) | t | p | |
| HADS-Aa | ||||||
| Constant | 9.447 | 1.008 | 7.466, 11.428 | 9.374 | < .001* | |
| Age | − 0.065 | .014 | − .092, − .038 | − 0.230 | − 4.692 | < .001* |
| Having an underlying disease | 1.526 | .332 | .874, 2.178 | 0.219 | 4.599 | < .001* |
| Support from physicians | − 0.175 | .059 | − .291, − .059 | − 0.142 | − 2.965 | .003* |
| HADS-D | ||||||
| Constant | 14.443 | 0.143 | 14.162, 14.723 | 101.289 | < .001* | |
| Breast reconstruction | 0.626 | 0.252 | .130, 1.121 | 0.116 | 2.482 | .013* |
| Having an underlying disease | 0.501 | 0.115 | .099, .903 | 0.115 | 2.451 | .015* |
| BRBIa | ||||||
| Constant | 59.467 | 6.742 | 46.211, 72.722 | 8.820 | < .001* | |
| Chemotherapy | 8.027 | 2.301 | 3.503, 12.552 | 0.180 | 3.488 | .001* |
| Having an underlying disease | 7.761 | 2.278 | 3.283, 12.239 | 0.169 | 3.407 | .001* |
| Age | − 0.303 | .099 | − .498, − .108 | − 0.166 | − 3.059 | .002* |
| Breast reconstruction | 5.589 | 2.758 | .166, 11.011 | 0.102 | 2.026 | .043* |
| BRSEFa | ||||||
| Constant | 57.389 | 4.227 | 49.079, 65.699 | 5.987 | < .001* | |
| Age | 0.403 | .067 | .271, .536 | 0.281 | 5.987 | < .001* |
| Having an underlying disease | 5.530 | 1.643 | 2.299, 8.760 | 0.156 | 3.365 | .001* |
| Support from husband/partner | − 1.532 | .490 | − 2.494, − .569 | − 0.143 | − 3.129 | .002* |
| Belonging to a culture/ethnic minority | 13.531 | 4.694 | 4.303, 22.759 | 0.131 | 2.882 | .004* |
| BRSEEa | ||||||
| Constant/ | 25.446 | 8.902 | 7.885, 43.006 | 2.859 | .005* | |
| Age | .535 | 0.161 | .217, .853 | 0.247 | 3.316 | .001* |
| Education | 9.005 | 3.521 | 2.059, 15.951 | 0.191 | 2.558 | .011* |
| Support from husband/partner | − 1.703 | .789 | − 3.259, − .147 | − 0.145 | − 2.159 | .032* |
| BRFUa | ||||||
| Constant | 77.786 | 7.304 | 63.428, 92.144 | 10.649 | < .001* | |
| Age | − 0.548 | 0.097 | − 0.739, − 0.357 | − 0.272 | − 5.639 | < .001* |
| Having an underlying disease | 11.497 | 2.251 | 7.072, 15.922 | 0.230 | 5.107 | < .001* |
| Support from physicians | − 1.503 | .402 | − 2.293, − .713 | − 0.171 | − 3.741 | < .001* |
| Chemotherapy | 6.229 | 2.291 | 1.726, 10.732 | 0.127 | 2.719 | .007* |
| BRSTa | ||||||
| Constant | 28.431 | 1.578 | 25.328, 31.533 | 18.012 | < .001* | |
| Having an underlying disease | 8.391 | 1.180 | 6.071, 10.711 | 0.316 | 7.110 | < .001* |
| Chemotherapy | 5.118 | 1.174 | 2.811, 7.424 | 0.197 | 4.361 | < .001* |
| Support from family and friends | − 1.111 | .366 | − 1.830, − .393 | − 0.135 | − 3.040 | .003* |
| Support from physicians | − .631 | .212 | − 1.048, − .215 | − 0.135 | − 2.979 | .003* |
| BRBSa | ||||||
| Constant | 43.823 | 3.785 | 36.383, 51.262 | 11.579 | < .001* | |
| Radiotherapy | 7.040 | 1.255 | 4.572, 9.507 | 0.252 | 5.607 | < .001* |
| Belonging to a culture/ethnic minority | 16.237 | 3.718 | 8.928, 23.545 | 0.195 | 4.367 | < .001* |
| Age | − 0.226 | 0.054 | − 0.332, − 0.119 | − 0.196 | − 4.159 | < .001* |
| Having an underlying disease | 4.680 | 1.304 | 2.118, 7.242 | 0.164 | 3.590 | .018* |
| Support from nurses | − .477 | .200 | − .870, − .084 | − 0.110 | − 2.384 | .018* |
| BRASa | ||||||
| Constant | 28.234 | 1.265 | 25.746, 30.721 | 22.311 | < .001* | |
| Radiotherapy | 8.455 | 1.661 | 5.190, 11.721 | 0.265 | 5.090 | < .001* |
| Having an underlying disease | 8.462 | 1.457 | 5.597, 11.326 | 0.260 | 5.808 | < .001* |
| Chemotherapy | 3.991 | 1.655 | 0.738, 7.244 | 0.125 | 2.411 | .016* |
| BRHL | ||||||
| Constant | 32.794 | 1.879 | 29,100, 36.487 | 17.449 | < .001* | |
| Support from physicians | − .706 | .271 | − 1.238, − .174 | − 0.123 | − 2.608 | .009* |
| Belonging to a culture/ethnic minority | 10.450 | 4.430 | 1.744, 19.157 | 0.111 | 2.359 | .019* |
*A level of significance of 0.05
aChemotherapy and support from nurses were significant only in univariate analyses for HADS-A; radiotherapy, Herceptin, support from physicians, and from nurses were significant only in univariate analyses for quality of life – body image. Breast reconstruction, civil status, education, and support from physicians were significant only in univariate analyses for BRSEF. Chemotherapy and support from internet were significant only in univariate analyses for BRSEE. Civil status and support from nurses were significant only in univariate analyses for BRFU. Age was significant only in univariate analysis for BRST. Chemotherapy, civil status, and support from physicians were significant only in univariate analyses for BRBS. Age and support from physicians were significant only in univariate analyses for BRAS
HADS-A, F = 17.551, p < .001, Adjusted R2 = 0.107
HADS-D, F = 5.585, p = .004, Adjusted R2 = 0.020
BRBI, F = 11.067, p < .001, Adjusted R2 = 0.093
BRSEF, F = 21.160, p < .001, Adjusted R2 = 0.164
BRSEE, F = 11.775, p < .001, Adjusted R2 = 0.146
BRFU, F = 24.916, p < .001, Adjusted R2 = 0.184
BRST, F = 23.228, p < .001, Adjusted R2 = 0.173
BRBS, F = 19.798, p < .001, Adjusted R2 = 0.185
BRAS, F = 27.954, p < .001, Adjusted R2 = 0.160
BRHL, F = 6.377, p = .002, Adjusted R2 = 0.024
Hospital, Anxiety, and Depression Scale – Anxiety subscale (HADS-A); Hospital, Anxiety, and Depression Scale – Depression subscale (HADS-D); Health-Related Quality of Life (HRQoL); Quality of life – body image (BRBI); Quality of life – sexual functioning (BRSEF); Quality of life – sexual enjoyment (BRSEE); Quality of life – future perspective (BRFU); Quality of life – systemic therapy side effects (BRST); Quality of life – breast symptoms (BRBS); Quality of life – arm symptoms (BRAS); and Quality of life – upset by hair loss (BRHL)
Patients with breast cancer who had been treated with chemotherapy (β = 0.180, 95% CI 0.145, 0.215, p = 0.001) and had an underlying disease (β = 0.169, 95% CI 0.136, 0.202, p = 0.001) were younger (β = − 0.166, 95% CI − 0.265, − 0.067, p = 0.002) were associated with decreased HRQoL in BRBI. Patients who were older (β = 0.403, 95% CI 0.336, 0.470, p < 0.001) and had an underlying disease (β = 0.156, 95% CI 0.068, 0.244, p = 0.001) were associated with decreased HRQoL in BRSEF. Patients who were older (β = 0.247, 95% CI 0.162, 0.332, p = 0.001) and had low education (β = 0.191, 95% CI 0.090, 0.284, p = 0.011) were associated with decreased HRQoL in BRSEE. Patients who were younger (β = − 0.272, 95% CI − 0.369, − 0.175, p < 0.001) and had an underlying disease (β = 0.230, 95% CI 0.196, 0.262, p < 0.001) were associated with decreased HRQoL in BRFU. Having an underlying disease (β = 0.316, 95% CI 0.282, 0.402, p < 0.001) and having undergone treatment with chemotherapy (β = 0.197, 95% CI 0.169, 0.232, p < 0.001) decreased HRQoL in BRST. Having received radiotherapy (β = 0.252, 95% CI 0.167, 0.336, p < 0.001) and belonging to an ethnic minority (β = 0.195, 95% CI 0.095, 0.289, p < 0.001) decreased HRQoL in BRBS. Having received radiotherapy (β = 0.265, 95% CI 0.180, 0.348, p < 0.001) and having an underlying disease (β = 0.260, 95% CI 0.174, 0.342, p < 0.001) were factors associated with decreased HRQoL in BRAS. Patients who had received increased support from physicians (β = 0.123, 95% CI 0.108, 0.138, p = 0.009) and belonged to an ethnic minority (β = 0.111, 95% CI 0.096, 0.127, p = 0.019) were associated with decreased HRQoL in BRHL.
Discussion
In this study, both anxiety and depressive symptoms demonstrated the highest correlation with HRQoL in the dimensions of future perspective and body image. Psychological distress is commonly diagnosed among patients with breast cancer [7, 30]. People living with psychological distress may experience an imbalance between their realities and their ideal wishes, resulting in a breakdown in their self-esteem and low well-being [9]. Moreover, psychological distress is recognized as associated with decreased HRQoL among patients [31]. Greater depressive symptoms are associated with more emotional suppression [12], and suicidal thoughts, and attempted suicide may occur among women with breast cancer suffering from depressive symptoms [11]. It might be useful to examine psychological distress and HRQoL along with treatment of psychologically vulnerable women, like women with breast cancer.
Our study revealed that participants who had an underlying disease were more likely to have psychological distress. Moreover, those having breast reconstruction might have more symptoms of psychological distress. People with psychological distress seem to have reduced capacity and lack of control of their everyday lives [9]. Although having an underlying disease is common, comorbidity can make life difficult for patients with breast cancer [32]. Also, after life change events like surgery, patients with breast cancer may experience psychological distress [12]. Therefore, a preventive intervention related to emotional awareness for such patients with breast cancer should be implemented. For instance, the mindfulness-based stress reduction program has potential to improve the mental health among women with breast cancer [2].
External sources, like support from healthcare professionals (HCPs), could reduce psychological distress [33], while poor support contributes to psychological distress [34]. The development of treatment plans by physicians and patients is essential [35]. Patients need to be involved in a person-centered dialogue with physicians to strengthen their own capacities for daily lives [9]. Therefore, our study suggests that HCPs should provide sufficient information to patients with breast cancer and include the patients in their planning. This may contribute to decreased psychological distress.
Several sources of support (e.g., physicians, nurses, and husband/partner) have been found to be related to decreased HRQoL for many dimensions. The participants needed support from their husbands/partners about their sexual functioning and enjoyment. After treatments of breast cancer, sexual dysfunction becomes a challenge for patients [35]. They need more support and tenderness from their partners [32] to maintain their HRQoL [35]. Patients with breast cancer in our study also thought about side effects of their therapy, e.g., breast symptoms and hair loss. Moreover, those treated with radiotherapy and chemotherapy needed information from HCPs about their current and future lives to increase their well-being. Cognitive behavioral therapy and supportive-expressive group therapy give positive effects on patients with breast cancer [36]. Thus, after treatments of patients with breast cancer, individual or group therapy may assist the improvement of the HRQoL among the patients.
Strengths and limitations
We constructed the DAGs from reviewed literature, which assisted data collection and analyses. Some confounders, such as age, had been adjusted by multiple linear regression analyses. The use of real scores from the questionnaire rather than categorized scores enhanced estimates [37]. In addition, all instruments used in this study had been tested before data collection with acceptable values of validity and reliability.
The cross-sectional character of the study limited cause-effect relationships. Therefore, subsequent longitudinal studies may more clearly explain factors associated with psychological distress and health-related quality of life. Some information bias could be seen because the participants self-reported. Moreover, some information that might be related to psychological distress and HRQoL (e.g., pathological stage and metastasis) had not been collected. In addition, the rate of participation was only 60% which may also have affected our findings.
Conclusions
Psychological distress was correlated with most dimensions of HRQoL. The strongest correlation was found for anxiety symptoms and future perspective and depressive symptoms and body image. Women with breast cancer who were younger were likely to get increased symptoms of anxiety, while those who had undergone breast reconstruction were likely to get increased symptoms of depression. Low support from HCPs decreased the HRQoL in terms of future perspective, systemic therapy side effects, breast symptoms, and indignation about hair loss. Support from husband/partner increased the HRQoL in terms of sexual functioning and enjoyment. Treatment with chemotherapy decreased the HRQoL in terms of body image, systemic therapy side effects, and arm symptoms. Women with breast cancer need support from many sources, in particular HCPs and their husbands/partners.
Acknowledgements
The authors thank all women with breast cancer who participated in this study.
Author contribution
PCL planned the study and collected data. NP analyzed the data and contributed in manuscript writing. Both authors read and approved the final manuscript.
Funding
Open access funding provided by Uppsala University. This study was supported by the Breast Cancer Association in Sweden.
Availability of data and materials
The datasets used in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee at Uppsala-Örebro, Sweden (dnr 2012/385). All participants received information about the purpose of the study and were assured anonymity and confidentiality before signing a consent form. Informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stewart BW, Wild CP (eds) (2014) World cancer report 2014. International Agency for Research on Cancer, Lyon
- 2.Christensen H, Marck DE. The efficacy of mindfulness based stress reduction (MBSR) for decreasing anxiety and depression among breast cancer survivors. Sch Physician Assist Stud. 2017;16:213. [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.The national board of health and welfare (2018) Statistics on cancer incidence 2017. Stockholm: The national board of health and welfare ISSN 1401–2016
- 5.Tryggcadoittir L, Gislum M, Bray F, Klint A, Hakulinen T, Storm HH, Engholm G. Trends in survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:624–631. doi: 10.3109/02841860903575323. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen L, Garne JP, Sogaard M, Laursen BS. The experience of distress in relation to surgical treatment and care for breast cancer: an interview study. Eur J Oncol Nurs. 2015;19(6):612–618. doi: 10.1016/j.ejon.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Tsaras K, Papathanasiou IV, Mitsi D, Veneti A, Kelesi M, Zyga S, Fradelos EC. Assessment of depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev. 2018;19(6):1661–1669. doi: 10.22034/APJCP.2018.19.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daldoul A, Khechine W, Bhiri H, Ammar N, Bouriga R, Krir MW, Soltani S, Zoular O, Rhim MS, Bouslah S, Dimassi S, Abbess I, Saidani Z, Zaied S. Factors predictive of quality of life among breast cancer patients. Asian Pac J Cancer Prev. 2018;19(6):1671–1675. doi: 10.22034/APJCP.2018.19.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvidsdotter T, Marklund B, Kylen S, Taft C, Ekman I. Understanding persons with psychological distress in primary health care. Scand J Caring Sci. 2016;30:687–694. doi: 10.1111/scs.12289. [DOI] [PubMed] [Google Scholar]
- 10.Knobf T. Psychological responses in breast cancer survivors. Semin Oncol Nurs. 2007;23:71–83. doi: 10.1016/j.soncn.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Walker J, Hansen CH, Martin P, Symeonides S, Ramessur R, Murray G, Sharpe M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1:343–350. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani Y, Iwamitsu Y, Kuranami M, Okazaki S, Yamamoto K, Watanabe M, Miyaoka H. Predictors of psychological distress in breast cancer patients after surgery. Kitasato Med J. 2013;43:49–56. [Google Scholar]
- 13.Hoyer M, Johansson B, Nordin K, Bergkvist L, Ahlgren J, Lidin-Lindqvist A, Lambe M, Lampic C. Health-related quality of life among women with breast cancer – a population-based study. Acta Oncol. 2011;50(7):1015–1026. doi: 10.3109/0284186X.2011.577446. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organisation Quality of Life Group . The development of the World Health OrganisationQuality of Life assessment instrument (The WHOQOL) In: Orley J, Kuyken W, editors. Quality of life assessment: international perspectives. Berlin: Springer-Verlag; 1994. pp. 41–60. [Google Scholar]
- 15.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review if the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;16:1141–1150. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debess J, Riis J, Pedersen L, Ewertz M. Cognitive function and quality of life after surgery for early breast cancer in North Jutland, Denmark. Acta Oncol. 2009;48:532–540. doi: 10.1080/02841860802600755. [DOI] [PubMed] [Google Scholar]
- 17.Disipio T, Hayes S, Newman B, Janda M. Health-related quality of life 18 months after breast cancer: comparison with the general population of Queensland, Australia. Support Care Cancer. 2008;16:1141–1150. doi: 10.1007/s00520-007-0392-y. [DOI] [PubMed] [Google Scholar]
- 18.Eaker S, Halmin M, Bellocco R, Bergkvist L, Ahlgren J, Holmberg L, Lambe M. Social differences in breast cancer survival in relation to patient management within a National Health Care System (SWEDEN) Int J Cancer. 2009;124:180–187. doi: 10.1002/ijc.23875. [DOI] [PubMed] [Google Scholar]
- 19.Sharma N, Purkayastha A. Factors affecting quality of life in breast cancer patients: a descriptive and cross-sectional study with review of literature. J Midlife Health. 2017;8(2):75–83. doi: 10.4103/jmh.JMH_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajah HDA, Chan CMH, Kong Y, Wong L, Bustaman RS, Ho G, Lai KM, Yip C, Bhoo-Pathy N. Insights on emotional distress following cancer, sources of support and the unmet needs in a setting with limited supportive care services for people living with cancer. Support Care Cancer. 2021;29:5811–5819. doi: 10.1007/s00520-021-06148-2. [DOI] [PubMed] [Google Scholar]
- 21.Papathanasiou IV, Kelepouris K, Valari C, Papagiannis D, Tzavella F, Kourkouta L, Tsaras K, Fradelos EC. Depression, anxiety and stress among patients with hematological malignancies and the association with quality of life: a cross-sectional study. Med Pharm Rep. 2020;93(1):62–68. doi: 10.15386/mpr-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng CG, Mohamed S, Kaur K, Sulaiman AH, Zainal NZ, Taib NA, MyBCC Study group Perceived distress and its association with depression and anxiety in breast cancer patients. Plos One. 2017;12:e0172975. doi: 10.1371/journal.pone.0172975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):360–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, Franzini L, Williams A, de Haes HC, Hopwood P, Cull A, Aaronson NK. The European Organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 25.Fayers PM, Machin D. Quality of life: assessment, analysis and interpretation. Chichester: J Wiley & Sons Ltd; 2000. [Google Scholar]
- 26.Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30:1418–1423. doi: 10.1093/ndt/gfu325. [DOI] [PubMed] [Google Scholar]
- 27.Boing L, Pereira GS, de Araujo CC, Sperandio FF, Loch MG, Bergmann A, Borgatto AF, Guimaraes AC (2019) Factors associated with depression symptoms in women after breast cancer. Revista de Saude Publica 53(30) [DOI] [PMC free article] [PubMed]
- 28.Akel R, Darsa HE, Anouti B, Mukherji D, Temraz S, Raslan R, Tfayli A, Assi H. Anxiety, Depression and quality of life in breast cancer patients in the Levant. Asian Pac J Cancer Prevent. 2017;18(10):2809–2816. doi: 10.22034/APJCP.2017.18.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava V, Ansari MA, Kumar A, Shah AG, Meena RK, Sevach P, Singh OP (2016) Study of anxiety and depression among breast cancer patients from north India. Clin Psychiatr 2:1(4)
- 30.Ando N, Iwamitsu Y, Kuranami M, Okazaki S, Nakatani Y, Yamamoto K, Watanabe M, Miyaoka H. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics. 2011;52:56–64. doi: 10.1016/j.psym.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Linden W, Vodermaier A, MacKenzie R, Grieg D. Anxiety and depression after cancer diagnosis: prevalence rate by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Bukovic D, Fajdic J, Hrgovic Z, Kaufmann M, Hojsak I, Stanceric T. Sexual dysfunction in breast cancer survivors. Onkologie. 2005;28(1):29–34. doi: 10.1159/000082115. [DOI] [PubMed] [Google Scholar]
- 33.Drapeau A, Merchand A, Beaulieu-Prevost D (2012) Epidemiology of psychological distress. In: LAbate PL. (ed.) Mental Illnesses – Understanding, Prediction, and Control. In Tech. Rijeka, pp 155–34
- 34.Marchand A, Demers A, Derand P. Do occupation and work conditions really matter? A longitudinal analysis of psychological distress experiences among Canadian workers. Soc Health Illness. 2015;27:602–627. doi: 10.1111/j.1467-9566.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 35.Boswell EN, Dizon DS. Breast cancer and sexual function. Transl Androl Urol. 2015;4(2):160–168. doi: 10.3978/j.issn.2223-4683.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas. 2011;70:343–348. doi: 10.1016/j.maturitas.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Poon W, Wang H. Analysis of ordinal categorical data with misclassification. British J Math Stat Psychol. 2010;63(1):17–42. doi: 10.1348/000711008X401314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.