Abstract
The glycosphingolipid disialoganglioside GD2 is a cell-surface associated antigen expressed on tumors of neuroectodermal origin that serves as a target of immunotherapy in select cancer types. Information about the expression of GD2 in breast cancer is limited. In the present study we investigate the utility of GD2 as a potential biomarker for targeted treatment. The study cohort consists of 386 breast carcinomas of several histologic types. GD2 expression was assessed in both whole tumor sections and tissue microarrays with anti-GD2 3F8 monoclonal antibody immunohistochemistry and correlated with clinicopathologic features and survival outcomes. A total of 134 (35%) breast carcinomas were positive for GD2, with a median H-score of 100. 3F8 staining displayed granular and predominantly cytoplasmic or perinuclear patterns, which was confined to the neoplastic tissue in nearly all cases. GD2 positivity was significantly associated with tumor histologic type (P = 0.0015), low grade (P <0.0001), ER-positivity (P <0.0001), low stage (P = 0.0014), and multifocality (P = 0.022). Event-free and overall survival of patients with GD2-positive and GD2-negative tumors were not significantly different. Our results support further assessment of GD2 using the 3F8 antibody as a predictive and prognostic biomarker in breast cancer.
Keywords: 3F8, breast cancer, disialoganglioside, GD2, glycosphingolipid
INTRODUCTION
Gangliosides are glycosphingolipids comprised of sialic acids linked to a sugar chain (1). Their surface localization in the cell membrane makes them accessible to antibody-based targeted therapies. Different gangliosides can be distinguished based on the number of sialic acids. The glycosphingolipid disialoganglioside GD2 is synthesized in the endoplasmic reticulum and Golgi apparatus from its precursor GD3 via the activity of GD2 synthase (2). It is primarily expressed on the cell membrane where it plays a role in cell-cell adhesion and signal transduction (3). The antigen is highly expressed in the nervous system and skin melanocytes as well as in tumors of neuroectodermal origin, such as neuroblastomas, and melanomas (4–7). Few studies have also identified GD2 in breast carcinomas.
The anti-GD2 monoclonal antibodies dinutuximab (chimeric 14.18) and naxitamab (humanized 3F8) were FDA approved for the treatment of high-risk neuroblastomas in pediatric patients. Recently there has been interest in exploring the potential applications of this biomarker and therapeutic antibodies in other tumor types. At present, little is known about the in-situ presence of GD2 in carcinomas. Prior immunohistochemistry (IHC) studies looking at ganglioside expression tested a small number of cases because of the difficulty encountered using paraffin sections; the requirement for frozen sections has severely limited any large scale efforts. Although preclinical studies in breast cancer models have shown promise for GD2 as a biomarker or therapeutic target (8–10), few have assessed GD2 expression by IHC on paraffin sections in a large cohort of clinical breast cancer specimens. Analysis on a limited number of clinical samples have demonstrated associations with advanced and triple-negative breast cancer in older patients (11,12). The utility of GD2 as a prognostic and/or predictive marker in breast carcinoma using standardized IHC methodology remains largely unexplored.
In the present study, we analyzed a cohort of 386 breast carcinomas consisting of both whole tumor sections and tissue microarrays (TMAs) for the presence of GD2 by immunohistochemistry (IHC) on paraffin sections. We correlated expression with clinicopathologic features and survival outcomes of the study cohort.
MATERIALS AND METHODS
Case Selection and Clinicopathologic Data Collection
This study was conducted under institutional review board approval. All tissues were retrieved from the archives of the Department of Pathology at MSKCC. Representative tumor blocks as well as TMAs were employed for the study. The TMAs included a total of 359 breast carcinomas (96 consecutive cases from 2004, 69 ER-positive cases from 2009, and 194 triple-negative cases from 2002–2007). The TMAs were constructed from paraffin blocks with triplicate 7 mm tumor cores. The whole section tumor blocks included 27 primary invasive breast carcinomas collected between 2004 and 2019. The tumor histologic subtype, grade (Nottingham combined histologic grade), size, multifocality and receptor status were extracted from the pathology reports. Immunohistochemistry (IHC) for Estrogen Receptor (ER) and Progesterone Receptor (PR) and IHC and/or fluorescence in situ hybridization (FISH) for Human Epidermal Growth Factor Receptor 2 (HER2) were performed at the time of diagnosis and reported according to the ASCO/CAP guideline recommendations (13,14). Relevant clinical information, including patient demographics, date of diagnosis, stage, and clinical outcomes were obtained from the electronic medical records. Event-free survival (EFS) was defined as the time from date of diagnosis until detection of recurrence, metastasis, or death.
Immunohistochemistry
Clone 3F8 was used for the immunohistochemical detection of GD2 in formalin-fixed paraffin embedded (FFPE) tissues (15). Clone 3F8 is the murine version of the therapeutic reagent naxitamab, which we optimized for use in IHC on FFPE tissues. All assays were performed on a Leica Bond-III (Leica, Buffalo Grove, IL) automated staining platform. An IHC protocol employing monoclonal antibody (mAb) 3F8 was previously developed. 3F8 worked best at a concentration of 1.25ug/ml employing a heat-based antigen high pH buffer (ER2, Leica) for 30 minutes (99°C); primary incubation time was 30 minutes. A polymeric secondary kit was used for the detection of the primary antibody (Refine, Leica). DAB was used as a chromogen.
Staining was evaluated in both tumor and normal cells. The intensity of cell staining was classified into four categories: 0 (negative), 1 (weak), 2 (moderate), 3 (strong). The histochemical score (H-score) was calculated by the sum of products of the percentage of cells staining with each intensity level multiplied by the intensity level. A tumor was considered GD2-positive when the H-score was greater than or equal to the median positive H-score for the cohort (H-score ≥ 20). Scoring was performed by two pathologists (EZ, DSR).
Statistical Analysis
Statistical analysis was performed in R for Windows, version 4.0.3. Association between categorical variables was assessed using Chi-square or Fisher’s exact test. Association between continuous variables was assessed using Spearman’s correlation. Survival curves were generated using the Kaplan-Meier method and comparisons between groups were performed using a log-rank test. P-values less than 0.05 were considered significant.
RESULTS
Clinicopathologic Features
The study cohort consists of 386 cases from 378 patients (Table 1). All patients were female, with a median age of 54 years at diagnosis (range 29–90 years) and median tumor size of 2.0 cm (range 0.6–12.7 cm). The carcinoma histologic subtypes include: 318 invasive ductal carcinomas of no special type (IDC NST), 31 invasive lobular carcinomas (ILC), 11 mammary carcinomas with mixed ductal and lobular features, 19 metaplastic carcinomas, and 7 others (including carcinomas of other special types such as adenoid cystic, mucinous, and small cell carcinoma) (Figure 1). The ILC consisted of 20 classic type, including 6 with solid and alveolar growth patterns, 6 pleomorphic type, and 5 with pleomorphic features. Among the metaplastic carcinomas, there were 7 matrix-producing, 7 squamous, and 5 spindle cell type. A total of 145 (38%) were ER/PR-positive and HER2-negative, 22 (6%) were triple-positive, and 218 (57%) were triple-negative. No patient received neoadjuvant chemotherapy. Follow-up was available for all patients. In a median follow-up of 132 months, 3 patients (0.8%) experienced local recurrence, 38 (10%) developed distant metastases, and 112 (29%) died.
Table 1.
Clinicopathological features of breast carcinomas and association with GD2.
| Clinical-pathological parameters | Total number (%) | GD2 positive (%) | GD2 negative (%) | P value |
|---|---|---|---|---|
| Total | 386 | 134 (35) | 252 (65) | |
| Patient age | 0.73 | |||
| ≤54 | 195 (50) | 70 (36) | 125 (64) | |
| >54 | 191 (50) | 64 (34) | 127 (66) | |
| Tumor types | 0.0015 | |||
| IDC | 318 (82) | 108 (34) | 210 (66) | |
| ILC | 31 (8) | 20 (65) | 11 (35) | |
| Mixed IDC + ILC | 11 (3) | 2 (18) | 9 (82) | |
| Metaplastic | 19 (5) | 3 (16) | 16 (84) | |
| Other | 7 (2) | 1 (14) | 6 (86) | |
| Histological grade | <0.0001 | |||
| G1 or G2 | 123 (45) | 68 (55) | 55 (45) | |
| G3 | 251 (55) | 61 (24) | 190 (76) | |
| Tumor size (cm) | 0.83 | |||
| ≤2.0 | 195 (50) | 69 (35) | 126 (65) | |
| >2.0 | 191 (50) | 65 (34) | 126 (66) | |
| Receptor status | <0.0001 | |||
| ER/PR +, HER2 − | 145 (38) | 78 (54) | 67 (46) | |
| ER/PR +, HER2 + | 22 (6) | 16 (73) | 6 (27) | |
| ER/PR −, HER2 − | 218 (57) | 39 (18) | 179 (82) | |
| Lymph node involvement | 0.052 | |||
| N0 | 205 (54) | 59 (29) | 146 (71) | |
| N1 | 111 (30) | 47 (42) | 64 (58) | |
| N2 | 40 (11) | 18 (45) | 22 (55) | |
| N3 | 20 (5) | 7 (35) | 13 (65) | |
| Stage | 0.0014 | |||
| I | 201 (53) | 85 (42) | 116 (58) | |
| II | 77 (20) | 19 (25) | 58 (75) | |
| III | 63 (17) | 12 (19) | 51 (81) | |
| IV | 38 (10) | 14 (37) | 24 (63) | |
| Distant metastasis | 0.95 | |||
| Yes | 38 (10) | 14 (37) | 24 (63) | |
| No | 328 (90) | 114 (35) | 214 (65) | |
| Multifocal | 0.022 | |||
| Yes | 75 (19) | 35 (47) | 40 (53) | |
| No | 311 (81) | 99 (32) | 212 (68) | |
| Lymphovascular invasion | 0.29 | |||
| Yes | 162 (42) | 51 (31) | 111 (69) | |
| No | 224 (58) | 83 (37) | 141 (63) | |
| Overall survival | 0.56 | |||
| Alive | 274 (71) | 98 (36) | 176 (64) | |
| Dead | 112 (29) | 36 (32) | 76 (68) |
Figure 1.
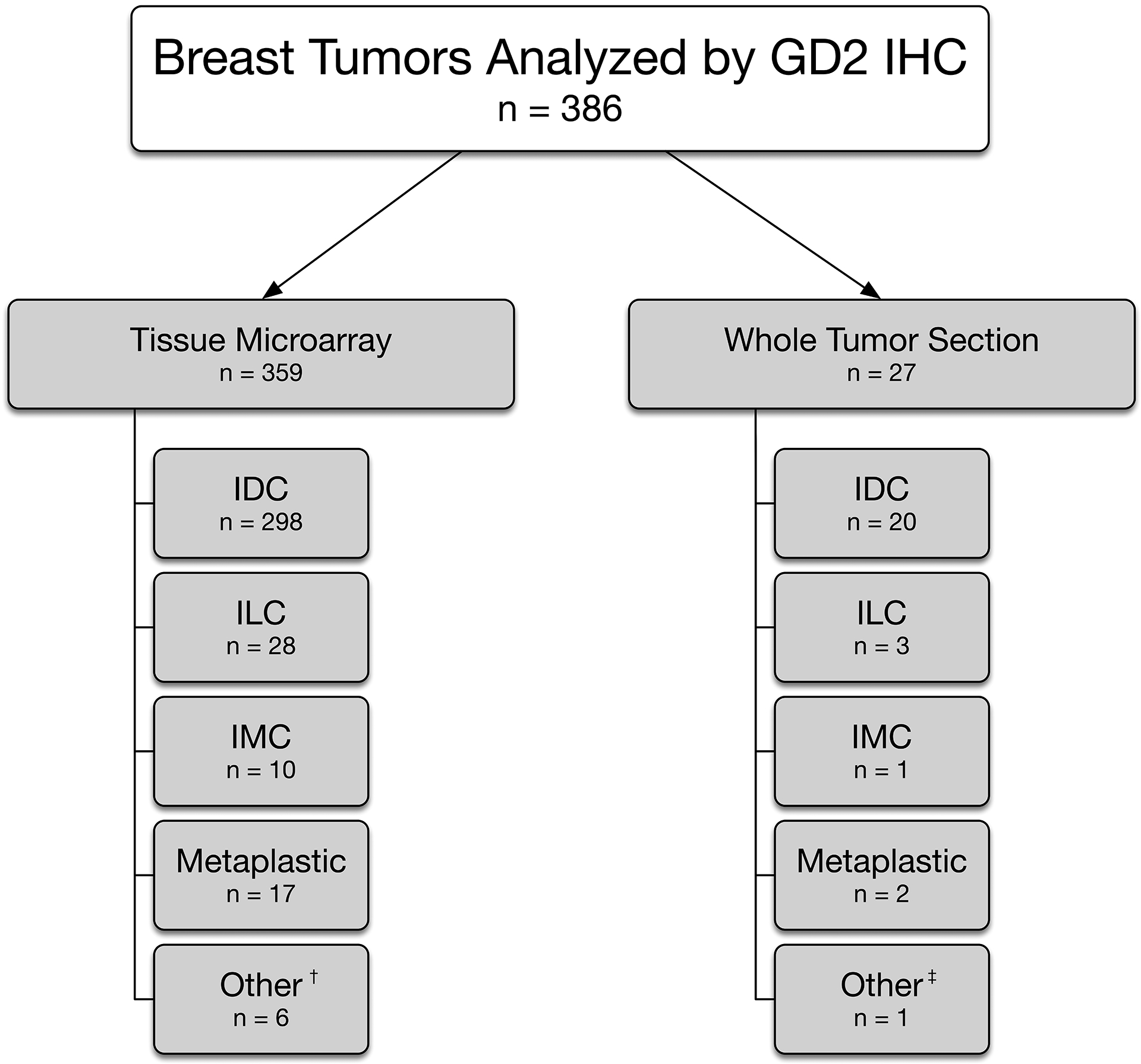
Breast tumors analyzed by GD2 immunohistochemistry.
IHC, Immunohistochemistry; IDC, Invasive ductal carcinoma; ILC, Invasive lobular carcinoma; IMC, Invasive mammary carcinoma with mixed ductal and lobular features.
†Other category encompasses cases of invasive carcinoma with special histologic features, including mucinous, apocrine, and papillary, small cell carcinoma, adenoid cystic carcinoma, and mammary carcinoma with skin adnexal and adenosquamous features.
‡Other category encompasses a poorly differentiated invasive carcinoma
GD2 Immunohistochemistry: Staining patterns
The 3F8 antibody staining displayed granular and predominantly cytoplasmic or perinuclear patterns (Figure 2). In the whole section tumors, the majority of cases displayed staining confined to the neoplastic tissue. However, isolated cases exhibited off-target staining outside of invasive carcinoma: rare benign stromal cells, one case of tumor infiltrating lymphocytes (diffuse, nuclear), one case of atypical lobular hyperplasia (perinuclear), and one case in benign lobules (membranous) (Figure 3).
Figure 2.
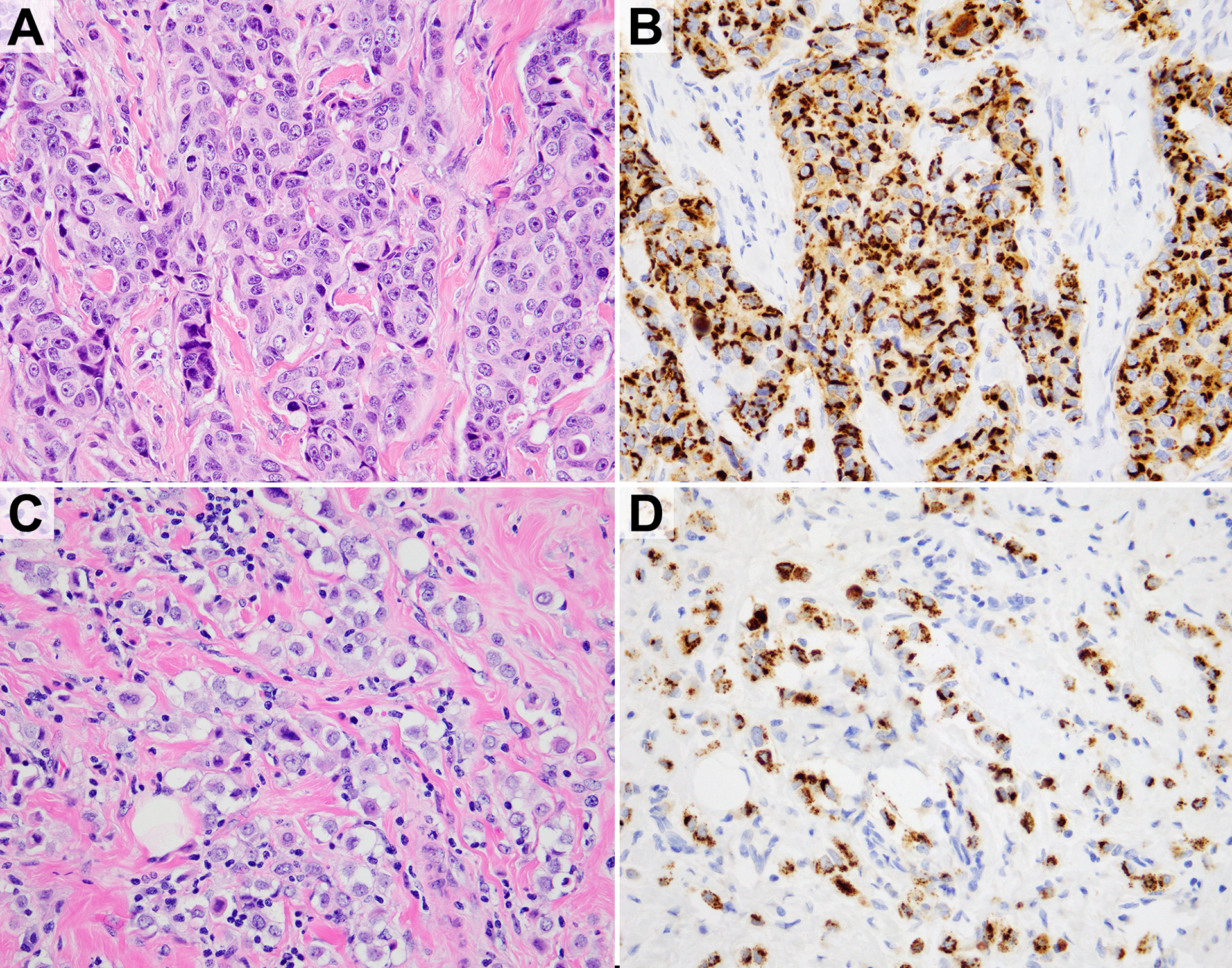
H&E and anti-GD2 3F8 immunohistochemistry. A,B) Invasive ductal carcinoma. C,D) Invasive lobular carcinoma with pleomorphic features.
Figure 3.
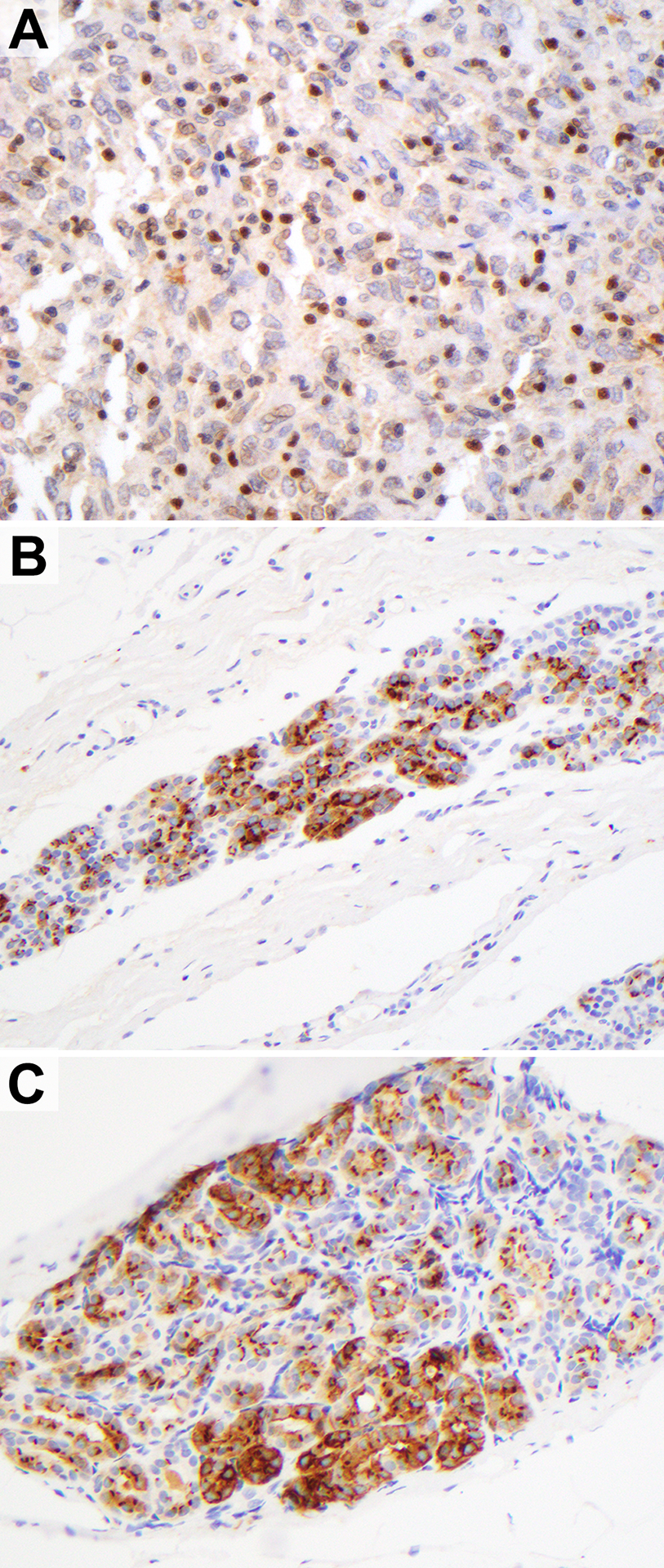
Rare off-target staining outside of invasive carcinoma with anti-GD2 3F8 immunohistochemistry. A) Nuclear staining in tumor infiltrating lymphocytes. B) Perinuclear staining in atypical lobular hyperplasia. C) Predominantly membranous staining in a normal lobule.
GD2 Immunohistochemistry: Staining in breast carcinoma
A total of 134 (35%) carcinomas were positive for GD2. The median H-score among positive cases was 100. Ductal carcinoma in situ frequently stained similarly to the accompanying invasive carcinoma. GD2 positivity was significantly associated with tumor histologic subtype (P = 0.0015), grade 1 or 2 (P <0.0001), ER-positive status (P <0.0001), low stage (P = 0.0014), and multifocality (P = 0.022). The histologic subtype with the highest proportion of GD2-positivity was ILC, of which 65% (20/31) were positive. 34% (108/318) of IDC NST were GD2-positive, as were 16% (3/19) of metaplastic carcinomas. 54% of ER-positive/HER2-negative and 73% of triple-positive tumors were GD2-positive, while only 18% of triple-negative carcinomas were positive. The clinical stages with the highest rate of GD2-positivity were stage I (42%) and IV (37%). Multifocal tumors had a somewhat higher positive rate than unifocal tumors (47% vs 32%). There was no association between GD2 expression and patient age at diagnosis, tumor size, lymph node involvement, presence of distant metastasis, or presence of lymphovascular invasion. There was no difference between GD2-positive and GD2-negative cases in EFS or overall survival (P = 0.76 and 0.64, respectively), including when subgrouped by hormone receptor status.
DISCUSSION
Few prior studies have investigated the expression of GD2 in breast cancer, and variable antibodies and scoring systems were used. Battula et al. analyzed a total of 12 primary breast tumor dissociated samples by IHC with monoclonal anti-GD2 clone 14G2a (2Q549, Abcam, Cambridge, MA) and found expression in all of them albeit in a variable degree ranging from 0.5% to 36%, with half of the cases displaying GD2 expression in less than 5% of the tumor (8). They employed an operational cutoff of 5% of cells which was close to their median of 4.35%. The investigators went on to stain 89 frozen samples of triple-negative breast carcinomas and observed detectable levels of GD2 staining in 67%, which ranged in extent from 0% to 45% of tumor cells. Low GD2 expression (below 10.378%) was associated with superior overall survival in this study (16). Orsi et al., using an anti-GD2 rabbit polyclonal antibody (Matreya LLC, Pleasant Gap, PA) studied 63 breast cancer cases. The authors considered any staining of any intensity to be positive. GD2 expression was detected in 59% of tumors, including 79% (11/14) of spindle cell metaplastic carcinomas. In their study, univariate logistic regression (but not multivariate or categorical analysis) demonstrated correlations between GD2 and triple-negative phenotype and age >78 years at diagnosis (11). Mansoori et al. stained 168 breast carcinomas also with monoclonal anti-GD2 clone 14G2a (sc-53,831, Santa Cruz Biotechnology Inc., Dallas, TX) using TMAs and observed higher GD2 expression in tumors of high grade, larger size (>4 cm), lymph node metastasis, and older age (>47 years). One of 2 metaplastic carcinomas showed high expression (12). They selected the mean (98) as the cutoff. Hormone receptor and HER2 status was not available for these samples. We employed a similar scoring strategy as Mansoori et al., although our cutoff was lower, likely due to case selection. As others have articulated, the optimal and most clinically relevant method of scoring GD2 IHC has not been standardized in a large cohort and it still warrants further investigation.
The present study constitutes the largest analysis of GD2 IHC in breast tumors to date, using whole tumor sections and TMAs. It is the first published analysis of 3F8 IHC on FFPE breast tissue. We observed associations between GD2 and histologic subtype (with highest prevalence in ILC), low grade, hormone receptor positivity, prognostic stage, and multifocality. A minority of metaplastic carcinomas were GD2-positive. These findings suggest that carcinomas of more favorable prognosis are more likely to express GD2, with some exceptions, suggesting potential treatment options for patients with recurrent or metastatic disease progressing on standard therapies. Our results contrast with the aforementioned published findings of higher GD2 expression in triple-negative and otherwise more aggressive subsets of breast carcinoma (11,12,16). This is likely attributable to differences in scoring, antibodies, and case selection in these relatively small cohorts.
The humanized version of the murine antibody used in our study, 3F8, is now available for the treatment of metastatic neuroblastoma (7). The IHC protocol set up for analysis at our institution was based on analysis of neuroblastomas and paired samples of frozen and FFPE samples. Chimeric 14.18 is the human-mouse chimera of the mouse antibody 14.18, from which the class switch variant 14G2a was derived (17,18). Previous IHC studies for GD2 in human neuroblastoma or sarcomas have relied on frozen sections and IHC on paraffin sections has been difficult and not well standardized. Since gangliosides are lipid anchored oligosaccharides, deparaffinization processes could affect their content and distribution, and if not standardized could make comparison or validation substantially more difficult.
There have been multiple studies linking GD2 expression in tumors with epithelial-mesenchymal transition (EMT). It is thought that GD2 and related gangliosides play a role in the immune evasion and EMT critical for tumor metastasis (19,20). EMT-inducing factors such as ZEB1 upregulate GD3 in glioblastoma (21). Induction of EMT in breast epithelial cell lines using Snail or Twist resulted in mesenchymal morphology and increased expression of GD2 (8). Inhibition of GD3 synthase was also shown to prevent metastasis, inhibit EMT, and reduce mesenchymal characteristics in breast cancer models (8,9,22). It is interesting, therefore, that we observed GD2 expression in a relatively low proportion of the 18 metaplastic carcinomas included in this study, a tumor type associated with EMT (23–25). Notably, only 2 of the 18 metaplastic carcinomas were assessed on whole tumor sections, and the limited sample in TMAs may contribute to underestimation of GD2 expression. A higher rate of positivity was observed in ILC, an entity whose discohesive growth pattern, canonical loss of E-cadherin expression, and high Twist expression had been suggested to indicate EMT (26–29). However meta-analysis has not supported a defining role for EMT in ILC (21,30,31). Metastatic breast carcinomas are known to show transcriptomic evidence of EMT (32). Comparison of GD2 expression in metastatic breast carcinomas with their paired primary tumors would be an interesting area of future study.
While this is the largest study of GD2 IHC in breast carcinoma yet published, it remains a limited sample, and due to the exploratory nature of the analysis it was not powered for many subgroup comparisons. As alluded to above, the reliance on TMA samples, a feature also of previous studies on the subject, may lead to underestimation of GD2 expression, as most tumors were not diffusely positive. The heterogeneous staining is consistent with evidence that GD2 is a marker and functional regulator of breast cancer stem cells, which comprise a minor subset of tumors (9). Subsequent IHC analysis should prioritize whole tumor sections. Finally, although GD2 is known to be a primarily membrane-bound antigen, staining was mostly observed in the cytoplasm and perinuclear region, a finding also reported by Orsi et al. (11). Of note, this is in contrast to the strong, diffuse, and typically membranous staining reported in other cancer types (33–35). This may represent reactivity with GD2 precursors in the endoplasmic reticulum and Golgi apparatus, tumor specific variation in localization, or it could possibly represent an artifact introduced by tissue processing
In conclusion, we demonstrate the presence of GD2 in approximately one third of breast cancers in our cohort. This finding suggests that at least a proportion of this tumor type may be susceptible to therapeutic targeted interventions based on already available agents including anti-GD2 antibody-based therapies and GD2-GD3 vaccines (36,37). Further studies are necessary to analyze the presence of GD2 in various breast cancer types and its biological processes.
FUNDING
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
NKC reports past commercial research grants from Y-mAbs Therapeutics for hu3F8, 8H9, bispecific antibodies for CD33 and GD2, and a grant for SADA technology transfer; NKC has ownership interest/equity in Y-mAbs Therapeutics Inc., Abpro-Labs, and Eureka Therapeutics. NKC is the inventor and owner of issued patents licensed by MSK to Y-mAbs Therapeutics, Biotec Pharmacon, and Abpro-labs. Hu3F8, 8H9, bispecific antibodies against CD33 and GD2 were licensed by MSK to Y-mAbs Therapeutics. NKC is an advisory board member of Eureka Therapeutics. MSK has financial interest in Y-mabs.
Footnotes
CONFLICTS OF INTEREST
The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Sipione S, Monyror J, Galleguiollos D et al. Gangliosides in the brain: physiology, pathophysiology and therapeutic applications. Neurosci 2020;14:572965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishman PH, Brady RO. Biosynthesis and function of gangliosides. Science 1976;194:906–915. [DOI] [PubMed] [Google Scholar]
- 3.Yu RK, Tsai YT, Ariga T et al. Structures, biosynthesis, and functions of gangliosides -- an overview. J Oleo Sci 2011;60:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz G, Cheresh DA, Varki NM et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res 1984;44:5914–5920. [PubMed] [Google Scholar]
- 5.Lloyd KO, Old LJ. Human monoclonal antibodies to glycolipids and other carbohydrate antigens: dissection of the humoral immune response in cancer patients. Cancer Res 1989;49:3445–3451. [PubMed] [Google Scholar]
- 6.Hakomori S Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res 1996;56:5309–5318. [PubMed] [Google Scholar]
- 7.Dobrenkov K and Cheung N. GD2-targeted immunotherapy and radioimmunotherapy. Semin Oncol 2014;41:589–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battula VL. Shi Y Evans KW et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Invest 2012;122:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y Ding Y. Levery SB et al. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. PNAS 2013;110:4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seitz CM, Schroeder S, Knopf P et al. GD2-targeted chimeric antigen receptor T cells prevent metastasis formation by elimination of breast cancer stem-like cells. Oncoimmunology 2019;9:1683345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orsi G, Barbolini M, Ficarra G et al. GD2 expression in breast cancer. Oncotarget 2017;8:31592–31600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoori M, Roudi R, Abbasi A et al. High GD2 expression defines breast cancer cells with enhanced invasiveness. Exp Mol Pathol 2019;109:25–35. [DOI] [PubMed] [Google Scholar]
- 13.Allison KH, Hammond MEH, Dowsett M et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 2020;38:1346–1366. [DOI] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond MEH, Allison KH et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 2018;36:2105–2122. [DOI] [PubMed] [Google Scholar]
- 15.Cheung NV, Saarinen UM, Neely JE et al. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res 1985;45:2642–2649. [PubMed] [Google Scholar]
- 16.Ly S, Anand V, El-Dana F et al. Anti-GD2 antibody dinutuximab inhibits triple-negative breast tumor growth by targeting GD2+ breast cancer stem-like cells. J Immunother Cancer 2021;9:e001197. doi: 10.1136/jitc-2020-001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JL, Cunningham JE, Brewer H et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neurectodermal tumors. J Clin Oncol 1994;12:184–193. [DOI] [PubMed] [Google Scholar]
- 18.Frost JD, Hank JA, Reaman GH et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer 1997;80:317–333. [DOI] [PubMed] [Google Scholar]
- 19.Biswas K, Richmond A, Rayman P et al. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res 2006;66:6816–6825. [DOI] [PubMed] [Google Scholar]
- 20.Sa G, Das Y, Moon C et al. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res 2009;69:3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dae HM, Kwon HY, Kang NY et al. Isolation and functional analysis of the human glioblastoma-specific promoter region of the human GD3 synthase (hST8Sia I) gene. Acta Biochim Biophys Sin (Shanghai) 2009;41:237–245. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar TR, Battula VL, Werden SJ et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene 2015;34:2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarrio D, Rodriguez-Pinilla SM, Hardisson D et al. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 2008;68:989–997. [DOI] [PubMed] [Google Scholar]
- 24.Taube JH, Herschkowitz JI, Komurov K et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 2010;107:15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Toy KA, Kleer CG. Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol 2012;25:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Mani SA, Donaher JL et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927–939. [DOI] [PubMed] [Google Scholar]
- 27.Zou D, Yoon HS, Anjomshoaa A et al. Increased levels of active c-Src distinguish invasive from in situ lobular lesions. Breast Cancer Res 2009;11:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrogh M, Andrade VP, Giri D et al. Cadherin-catenin complex dissociation in lobular neoplasia of the breast. Breast Cancer Res Treat 2012;132:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis Genes Dev. 2013;27:2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollestelle A, Peeters JK, Smid M et al. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res Treat 2013;138:47–59. [DOI] [PubMed] [Google Scholar]
- 31.McCart Reed AE, Kutasovic JR, Vargas AC et al. An epithelial to mesenchymal transition programme does not usually drive the phenotype of invasive lobular carcinomas. J Pathol 2016;238:489–494. [DOI] [PubMed] [Google Scholar]
- 32.Weigelt B, Ng CK, Shen R et al. Metastatic breast carcinomas display genomic and transcriptomic heterogeneity. Mod Pathol 2015;28:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sariola H, Terävä H, Rapola J et al. Cell-surface ganglioside GD2 in the immunohistochemical detection and differential diagnosis of neuroblastoma. Am J Clin Pathol 1991;96:248–252. [DOI] [PubMed] [Google Scholar]
- 34.Kailayangiri S, Altvater B, Meltzer J et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer 2012;106:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziebarth AJ, Felder MA, Harter J et al. Uterine leiomyosarcoma diffusely express disialoganglioside GD2 and bind the therapeutic immunocytokine 14.18-IL2: implications for immunotherapy. Cancer Immunol Immunother 2012;61:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JA, Cheung NV. Targets and antibody formats for immunotherapy of neuroblastoma. J Clin Oncol 2020;38:1836–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung IY, Cheung NV, Modak S et al. Survival impact of anti-GD2 antibody response in a phase II ganglioside vaccine trial among patients with high-risk neuroblastoma with prior disease progression. J Clin Oncol 2021;39:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


