Precis:
Uterine corpus cancer mortality is now similar to that for ovarian cancer, and the disproportionate burden among Black women is widening.
Introduction:
Uterine corpus (hereafter uterine) and ovarian cancers are the most common gynecologic malignancies in the United States. While ovarian cancer mortality is steadily declining, rates for uterine cancer, which has among the largest racial disparity in cancer, increased by 2% annually from 2008–2018.1 How recent trends have shifted the gynecologic cancer burden and inequalities is unknown. We analyzed mortality for uterine and ovarian cancer contemporaneously during 1990–2019 by race and ethnicity.
Methods:
This study used government-issued de-identified data exempt from institutional review board approval based on National Human Research Protections Advisory Committee guidelines. Uterine and ovarian cancer death rates, age-standardized to the 2000 US-standard population and expressed per 100,000 person-years, during 1990–2019 were obtained from SEER*Stat (National Cancer Institute) version 8.3.9, as reported by the National Center for Health Statistics. Rates were stratified by mutually exclusive racial and ethnic categories (Asian or Pacific Islander, Black, Hispanic, or White) as reported on death certificates. The average annual percent change during the most recent decade (2010–2019) was calculated using Joinpoint Regression Program (National Cancer Institute) version 4.9.0.0 and described as increasing/decreasing when statistically significantly different from zero (P<0.05) using a 2-sided test. Mortality rate ratios (MRRs) comparing uterine and ovarian cancer and racial and ethnic subpopulations to White people were calculated using the Tiwari Method.
Results:
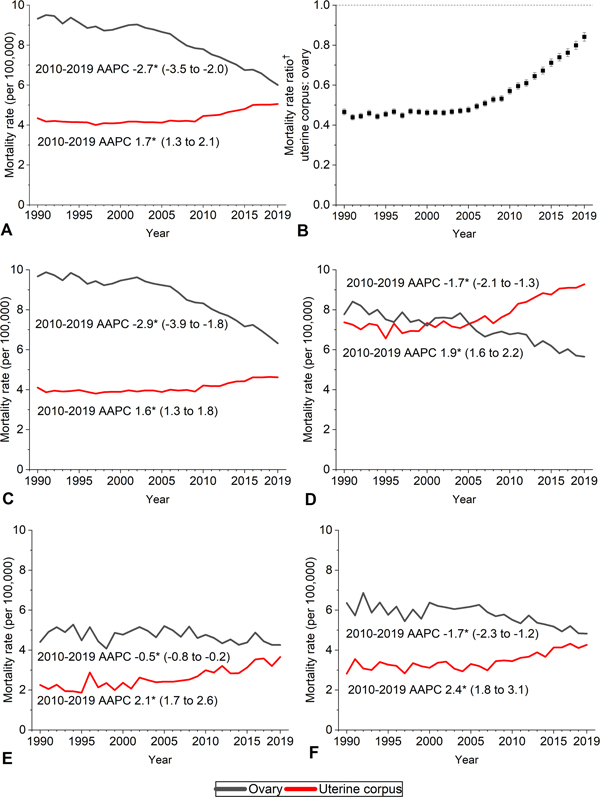
There were 232,957 deaths from uterine cancer and 419,085 deaths from ovarian cancer during 1990–2019, including 11,556 and 13,445, respectively, in 2019. Ovarian cancer mortality rates/100,000 decreased from 9.3 in 1990 to 6.0 in 2019 (2010–2019 average annual percent change, −2.7%;95%CI −3.5 – −2.0%) whereas uterine cancer mortality decreased from 4.3 in 1990 to 4.0 in 1997, then decreased to 5.1 in 2019 (2010–2019 average annual percent change 1.7%;95%CI, 1.3% – 1.8%) (Figure 1). Consequently, excess deaths from ovarian cancer reduced from approximately 5.0/100,000 women during the early 1990s to 0.9/100,000 in 2019 (uterine versus ovarian cancer MRR, 0.47;95%CI, 0.45–0.48 versus 0.84;95%CI, 0.82–0.87).
Figure 1.
Trends in uterine corpus and ovarian cancer mortality rates by race and ethnicity in the United States, 1990–2019. Deaths were classified according to International Classification of Diseases, Ninth Revision (ICD-9) codes 179 and 182 for uterus and 183.0 for ovary during years 1990–1998 and ICD-10 codes C54 and C55 for uterus and C56 for ovary during 1999–2019. All races and ethnicities (A), uterus:ovary rate ratio (B), non-Hispanic White (C), non-Hispanic Black (D), non-Hispanic Asian/Pacific Islander (E), Hispanic (F). American Indian/Alaskan Native rates not shown due to sparse data. Rates were age-adjusted to the 2000 U.S. standard population; data shown in C–F exclude Louisiana, New Hampshire, and Oklahoma due to incomplete ethnicity information. *The average annual percent change (average annual percent change) was calculated using Joinpoint version 4.9.0.0, based on up to 5 Joinpoints, and all were statistically significantly different from zero (2-sided P<.05). †Mortality rate ratio calculated using Tiwari Method.
Similar patterns for these cancers occurred in racial and ethnic subgroups except Black women, among whom mortality was historically similar and converged in 2005, with rates in 2019 3.6/100,000 higher for uterine (9.3) than ovarian cancer (5.7). Concomitantly, the uterine cancer Black compared with White MRR increased from 1.83 (95% CI, 1.77–1.89) in 1990–1994 to 1.98 (95% CI, 1.93–2.02) in 2015–2019 (p<0.001) (Figure 2).
Figure 2.
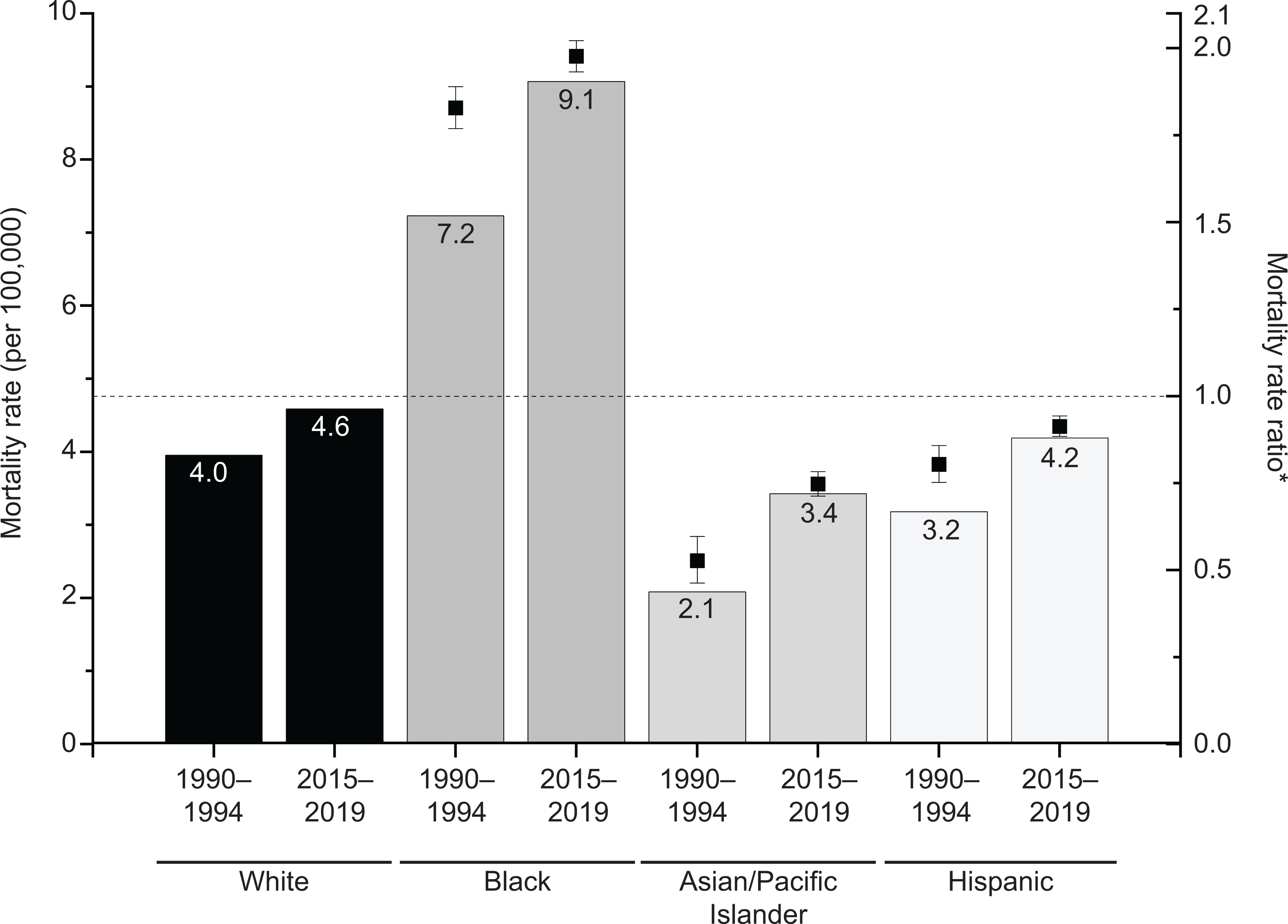
Uterine cancer mortality rates and rate ratios by race and ethnicity in the United States, 1990–1994 vs. 2015–2019. Rates were age-adjusted to the 2000 U.S. standard population and exclude Louisiana, New Hampshire, and Oklahoma due to incomplete ethnicity data. American Indian/Alaskan Native rates not shown due to sparse data. *Mortality rate ratios were calculated using the Tiwari method with non-Hispanic Whites as the referent group and tested for statistical significance between 1990–1994 and 2015–2019 with SAS 9.4 Wald Chi-Square test. All rate ratios were statistically significantly different (P<.001).
Discussion:
The twofold higher risk of death from ovarian versus uterine cancer in the early-1990s has been virtually eliminated by oppositional mortality trends. Among Black women, the crossover has already occurred and the uterine cancer disparity widened, with mortality now twofold higher than White women (9.1/100,000 versus 4.6/100,000) despite similar incidence.2
Mortality trends reflect changing disease risk and improved treatment. Rising obesity and physical inactivity have a much larger effect on occurrence of uterine cancer (70% of cases) than ovarian cancer (4% of cases).3 Additionally, major treatment advances, including poly (ADP-ribose) polymerase (PARP) inhibitors, have extended ovarian cancer survival,4 while uterine cancer survival has remained stagnant for 40 years.1 Lack of progress for uterine cancer may relate to deficits in research investment.5 For example, NCI funding for uterine cancer in 2018 was one-seventh that for ovarian cancer,6 despite comparable fatality projected for 2021 (12,940 vs 13,770, respectively) and profound racial disparities.1, 2 Increased opportunistic salpingectomy, as well as prophylactic bilateral salpingo-oophorectomy for women with predisposing high-risk germline mutations, such as BRCA1/2, may also contribute to ovarian cancer mortality declines.
The alarming Black versus White disparity in uterine cancer mortality reported herein is an underestimate of the true excess burden because rates were uncorrected for higher hysterectomy prevalence among Black women.2 Nevertheless, the gynecologic cancer landscape is changing rapidly and the risk of death from uterine cancer is now similar to that for ovarian cancer among women overall and approximately 60% higher among Black women based on national mortality statistics.
Supplementary Material
Funding/Support:
NIH SPORE in Uterine Cancer P50 CA098258 (Russell R. Broaddus).
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Contributor Information
Angela N. Giaquinto, Surveillance & Health Equity Science Department, American Cancer Society.
Russell R. Broaddus, Department of Pathology & Laboratory Medicine, University of North Carolina School of Medicine.
Ahmedin Jemal, Surveillance & Health Equity Science Department, American Cancer Society.
Rebecca L. Siegel, Surveillance & Health Equity Science Department, American Cancer Society.
References:
- 1.Siegel RL; Miller KD; Fuchs HE; Jemal A, Cancer Statistics, 2021. CA: a Cancer Journal for Clinicians 2021, 71 (1), 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MA; Devesa SS; Harvey SV; Wentzensen N, Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. Journal of Clinical Oncology 2019, 37 (22), 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islami F; Goding Sauer A; Miller KD; Siegel RL; Fedewa SA; Jacobs EJ; McCullough ML; Patel AV; Ma J; Soerjomataram I; Flanders WD; Brawley OW; Gapstur SM; Jemal A, Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018, 68 (1), 31–54. [DOI] [PubMed] [Google Scholar]
- 4.González-Martín A; Pothuri B; Vergote I; DePont Christensen R; Graybill W; Mirza MR; McCormick C; Lorusso D; Hoskins P; Freyer G; Baumann K; Jardon K; Redondo A; Moore RG; Vulsteke C; O’Cearbhaill RE; Lund B; Backes F; Barretina-Ginesta P; Haggerty AF; Rubio-Pérez MJ; Shahin MS; Mangili G; Bradley WH; Bruchim I; Sun K; Malinowska IA; Li Y; Gupta D; Monk BJ, Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. New England Journal of Medicine 2019, 381 (25), 2391–2402. [DOI] [PubMed] [Google Scholar]
- 5.Spencer RJ; Rice LW; Ye C; Woo K; Uppal S, Disparities in the allocation of research funding to gynecologic cancers by Funding to Lethality scores. Gynecol Oncol 2019, 152 (1), 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FY 2018 Research Funding by Cancer Type. https://fundedresearch.cancer.gov/nciportfolio/search/funded?fy=PUB2018&type=site (accessed April 08).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.